Abstract
Background:
Individuals with alcohol use disorder (AUD) have difficulty diverting attention away from alcohol-related stimuli and towards non-alcohol-related goals (i.e., alcohol-related attention interference). It remains unclear whether regulatory brain function differs during alcohol and non-alcohol-related interference. This study compares brain reactivity during the alcohol and classic Stroop and whether such brain function relates to AUD severity.
Methods:
46 participants with AUD completed alcohol and classic color-word Stroop tasks during fMRI. Brain activity was compared during alcohol and classic Stroop interference in the rostral and dorsal anterior cingulate cortices (rACC and dACC) and correlated with self-reported AUD severity. Exploratory whole-brain analyses were also conducted.
Results:
Behavioral interference (i.e., slower reaction times) was observed during alcohol and classic Stroop. rACC activity was significantly higher during the alcohol > neutral contrast versus the incongruent > congruent contrast. dACC activity did not differ between the Stroop tasks. dACC activity during incongruent > congruent was positively associated with AUD severity.
Conclusions:
Activity in ACC subregions differed during alcohol and non-alcohol interference. Increased alcohol-related activity in the rACC, a region linked to emotional conflict resolution, suggests an interfering effect of self-relevant alcohol cues on non-alcohol-related processing. AUD severity was related to greater dACC reactivity during classic Stroop interference, suggesting that non-drug-related cognitive control impairments are more pronounced in those with more problematic alcohol use.
Keywords: Alcohol use disorder, Stroop task, fMRI, Interference, Anterior Cingulate
Introduction
Individuals with alcohol use disorder (AUD) may have difficulty abstaining from alcohol because alcohol-related stimuli capture attention leading to enhanced motivation to use alcohol (Jones et al., 2013; Papachristou et al., 2012). Indeed, alcohol attentional bias, the implicit tendency to attend to alcohol-related cues, is well documented in AUD (Field and Cox, 2008). One potential mechanism for this bias is that alcohol use enhances the motivational salience of alcohol-related cues while negatively impacting brain regions responsible for executive function and self-regulation (Volkow et al., 2016). Thus, alcohol cues may be difficult to ignore due to increased alcohol salience and/or an impaired ability to deploy cognitive control resources to maintain non-alcohol-related goals.
It remains unclear whether cognitive interference due to alcohol- or non-alcohol-related stimuli share similar neurobiological underpinnings and whether such brain function relates to AUD severity. To address this gap, we employed the classic and an alcohol version of the Stroop task (Stroop, 1935; Williams et al., 1996). The classic Stroop task consists of congruent (i.e., word and font color match) and incongruent trials (i.e., word and font color do not match). To successfully respond to incongruent trials, participants must inhibit prepotent responses (i.e., word reading) to name the font color. During congruent trials, participants do not have to resolve conflicting word color/meaning, reducing inhibitory demands. Modifications to the Stroop have been used to study attentional bias and interference of alcohol cues in AUD. These studies indicate that problematic drinking is linked to greater alcohol cue-induced behavioral interference (Field et al., 2014). For example, relative to controls, adults (Mage=40.23) in treatment for AUD (Lusher et al., 2004) and non-treatment seeking heavy drinking adolescents (Field et al., 2007) show slower reaction times to alcohol versus neutral words. These findings suggest that for individuals with AUD, alcohol cues divert attention, leading to slowed behavioral performance.
Beyond behavioral Stroop performance, functional magnetic resonance imaging (fMRI) studies have identified the brain correlates of classic and affective Stroop interference. fMRI investigations of the classic Stroop identify the anterior cingulate (ACC) as a primary brain region recruited during interference (Laird et al., 2005). However, specific ACC subregions appear to be differentially involved in Stroop tasks with and without emotional stimuli. The dorsal ACC (dACC) shows increased reactivity during classic and emotional Stroop paradigms (Xu et al., 2016), whereas the rostral ACC (rACC) shows increased reactivity to emotional Stroop stimuli (Froeliger et al., 2012; Mohanty et al., 2007; Whalen et al., 1998) but not the classic color-word Stroop (Mohanty et al., 2007).
The differentiation of Stroop-related ACC activity is consistent with prior research demonstrating the different functions of the dACC and rACC. The dACC is engaged during conflict monitoring, irrespective of emotional valence (Egner et al., 2008), and facilitates general top-down cognitive control by monitoring relevant performance information and specifying the cognitive control allocation to downstream brain regions (Shenhav et al., 2016). Conversely, the rACC is recruited when resolving incongruent emotional stimuli (Egner et al., 2008; Etkin et al., 2006) and during self-focused reappraisal of emotional stimuli (Ochsner et al., 2004). In addiction, the rACC shows enhanced activation during inhibition trials of an alcohol go/no-go task in abstinent adults with AUD (Mage=51.21) versus controls (Czapla et al., 2017), and during exposure to drug cues in individuals with substance use disorders (SUD) (for review, Zhang and Volkow, 2019). In sum, the rACC is activated during emotion processing, including conflict monitoring, inhibition, and processing of self-relevant affective stimuli (i.e., drug cues), whereas the dACC is activated during tasks requiring cognitive control allocation, regardless of affective valence. However, it remains unclear whether the rACC and dACC will be differentially involved in alcohol and non-alcohol-related interference in AUD and whether ACC activity is associated with AUD severity.
The few studies that have evaluated Stroop-related brain function in addiction suggest that individuals with current or past AUD/SUD display differences in brain function during both classic and drug Stroop paradigms. A study using an alcohol Stroop paradigm found that relative to healthy controls, adults with remitted AUD (Mage=50.3) displayed reduced prefrontal, supplementary motor area/dACC activity to alcohol versus congruent words (Müller-Oehring et al., 2019). AUD severity was also linked to increased temporal and cerebellar activity during classic Stroop interference in treatment-seeking adults (Mage=39.7) (Wilcox et al., 2020). Several studies of participants with cocaine use disorder have found that Stroop-related brain reactivity was linked to clinical variables, including a positive correlation between rACC activity to drug > neutral trials and drug craving (Goldstein et al., 2009), between dACC activity to drug > neutral trials and post-treatment cocaine use (Marhe et al., 2013). Ventromedial PFC/ventral ACC and posterior cingulate activity to incongruent > congruent trials was also positively correlated with length of abstinence during treatment (Brewer et al., 2008). In one of the only studies to investigate both drug- and non-drug-related Stroop interference, individuals with stimulant use disorders had increased activity in the rostral and dorsal ACC to drug > neutral (and other regions including the posterior cingulate and cerebellum) but not incongruent > neutral words (Ersche et al., 2010). Finally, versus those without SUD, incarcerated persons with SUD displayed reduced dACC-nucleus accumbens functional connectivity, which in turn predicted worse performance on a behavioral classic Stroop task (Motzkin et al., 2014). Collectively, prior research suggests that individuals with SUDs, including AUD, have impairments in key affective and inhibitory control regions during alcohol and non-alcohol Stroop interference.
Several gaps remain in our knowledge of alcohol and non-alcohol-related attentional bias in AUD, as there are few studies in this area and considerable sample and methodological variability. First, the prior fMRI research using the Stroop in individuals with AUD has only investigated the classic or the alcohol Stroop paradigm. This limitation makes it difficult to differentiate whether alcohol and non-alcohol-related interference have similar or distinct patterns of brain reactivity. It may be that individuals with AUD experience both alcohol attentional bias and general difficulties in cognitive control, but that these processes are linked to different patterns of brain function and/or vary by AUD severity. Directly comparing brain reactivity to alcohol- and non-alcohol-related Stroop interference may identify mechanisms of alcohol attentional bias and links between AUD severity and brain function. Moreover, despite research indicating a functional distinction between the rACC and dACC for Stroop tasks with and without an emotional component (Mohanty et al., 2007), it remains unclear if these regions will show different associations with AUD severity during the alcohol or classic Stroop. Based on research linking rACC activity to drug cues and emotional conflict, we expect increased rACC activity during the alcohol Stroop relative to the classic Stroop. Finally, a major gap in the AUD literature is that most prior research of Stroop-related brain function focused on samples that are not generalizable to most individuals with current AUD (i.e., incarcerated individuals with SUD/AUD or individuals with remitted AUD). One strategy to address these gaps is to examine associations between AUD, behavioral interference, and brain reactivity during classic and alcohol Stroop paradigms in the same sample.
Current study
The current study addresses the above-discussed gaps by investigating brain and behavioral correlates of cognitive and alcohol-related interference in treatment-seeking adults with a range of AUD severity (from mild to substantial clinical impairment). During both tasks, we measured brain reactivity in the rACC and dACC due to their involvement in emotional regulation and general top-down control, respectively. Finally, we examined whether brain activity or behavioral performance on classic and alcohol Stroop is associated with AUD severity. We hypothesized that individuals with more severe AUD would show more ACC activation to complete the Stroop tasks. However, since prior research has not tested links between AUD and classic and alcohol Stroop interference in the same sample, we did not make specific predictions regarding whether these effects would be present for the alcohol or classic Stroop or in which ACC subregion. We also conducted exploratory whole-brain analysis to characterize the brain responses to the alcohol and classic Stroop in our sample. Using the classic and alcohol Stroop tasks within the same sample, the current study clarifies how core regulatory regions (i.e., rACC and dACC) are recruited during alcohol- and non-alcohol-related interference in AUD. Moreover, testing whether AUD severity is linked to brain reactivity during alcohol and non-alcohol-related interference may clarify brain regulatory mechanisms most impacted by problematic alcohol consumption.
Method
Participants
46 participants (32 male, 13 female, 1 transgender; mean age [SD] = 40.8 [11.0]) were recruited for an ongoing double-blind, randomized controlled treatment study for AUD. The current analysis focused on data collected during the pre-treatment baseline assessment. The sample was majority white (95.7%), and no participants identified as Hispanic or Latino (76% non-Hispanic/Latino; 11% did not report ethnicity; 13% reported other European ancestries, including “Irish” and “German”). Most of the sample (63%) reported an annual family income >$75,000. Participants were recruited based on meeting DSM-5 criteria for moderate-severe current AUD (via the Anxiety and Related Disorders Interview Schedule for DSM-5; Brown and Barlow, 2014), and over the last 90 days reported drinking an average >14 alcoholic drinks per week for males, or >7 alcoholic drinks per week for females. Participants were excluded if they reported a lifetime history of bipolar disorder or schizophrenia, a current diagnosis of bulimia, anorexia, dementia, or SUD (except for nicotine, cannabis, caffeine), current suicide risk, and significant neurological conditions or MRI or medical contraindications. Three subjects had SUDs, including tobacco (n=2) and cannabis and caffeine (n=1). The Boston University Charles River Institutional Review Board approved all procedures.
Procedure
During the initial study visit, participants completed self-report questionnaires, clinical diagnostic interviews, physical and neurocognitive exams, and health and drug toxicology bloodwork. Following the initial visit, eligible participants completed an MRI scan. At the MRI scanning facility, participants were screened for pregnancy and illicit drug use via urinalysis and recent alcohol use via a breathalyzer.
Assessment of AUD
Participants completed the Alcohol Dependence Scale (ADS) during the initial study visit (Skinner and Horn, 1984). The ADS is a 25-item self-report measure of severity of alcohol dependence, including withdrawal, impaired control over drinking, compulsion to drink, tolerance, and alcohol-seeking behavior. Sum scores ranged from 2–23 (mean [SD]= 13.96 [5.71]), indicating mild-to-moderate AUD severity.
Stroop Task
Participants completed an fMRI Stroop paradigm designed to include both the classic and alcohol Stroop. The 6.5-minute task consisted of sixteen counterbalanced 10-trial blocks (4 of each block type). Each trial consisted of a word presented in capital letters, in red, yellow, green, or blue font (1800 ms) on a black background followed by a fixation cross (200 ms). The four block types were: Congruent (e.g., “GREEN” in green font); Incongruent (e.g., “GREEN” in blue font); Neutral (e.g., “YEAR” in red font); and Alcohol (e.g., “BEER” in blue font) (Figure 1). 50% of the incongruent and congruent block trials were neutral words to prevent reliance on word-reading strategies. Responses were recorded if they occurred within the presentation window. Fixation blocks (4 total, 20 sec each) were included after every four Stroop blocks to reduce participant fatigue. Three participants were excluded for poor Stroop performance (i.e., <70% accuracy on any condition).
Figure 1. Stroop Task Behavioral Performance.
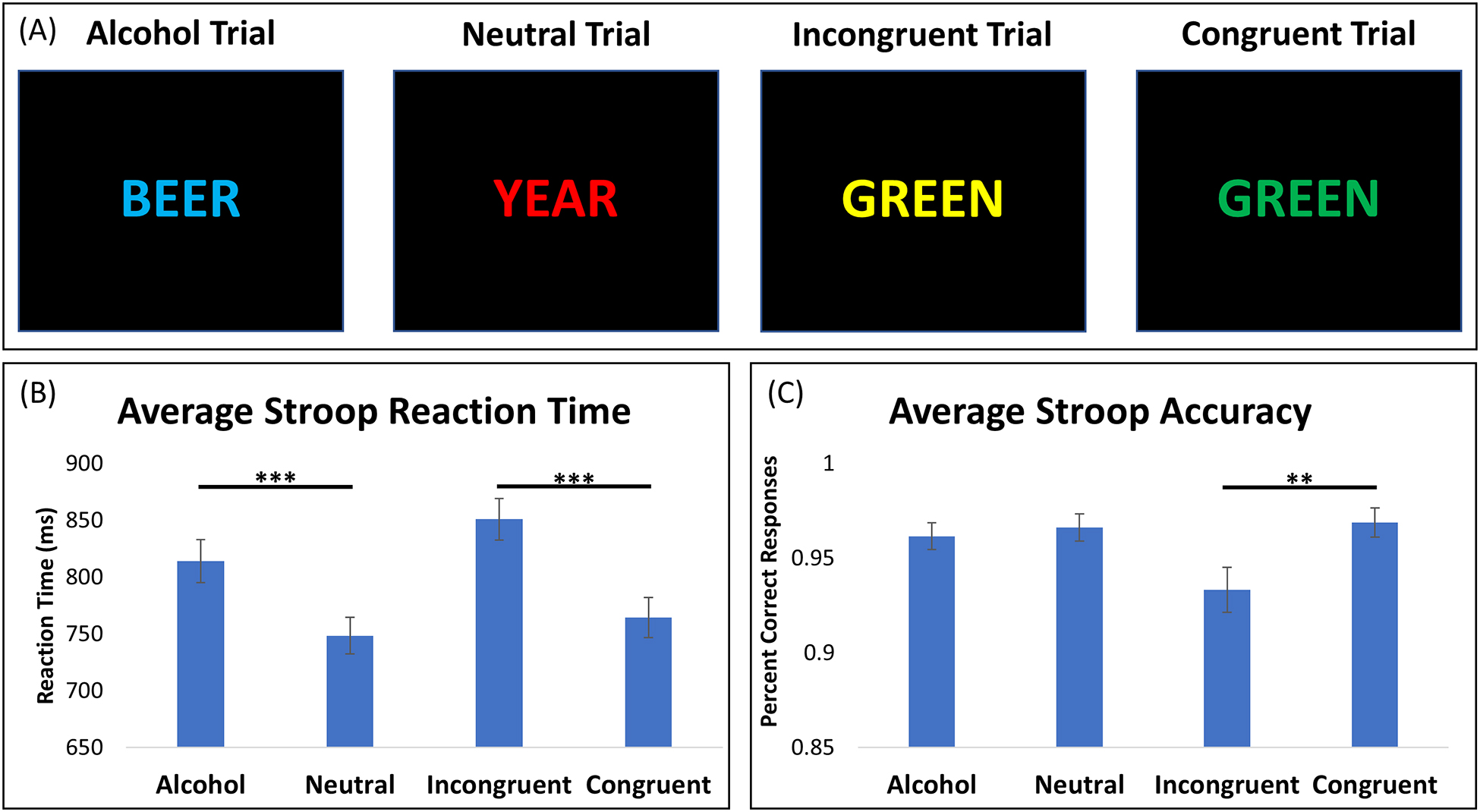
(A) Example trials of the 6.5-minute fMRI Stroop task, consisting of sixteen 10-trial blocks. Each block was counterbalanced for each condition: alcohol, neutral, incongruent, and congruent words. (B) Average reaction times for each Stroop condition. Participants responded significantly slower for alcohol versus neutral trials (t = 7.85, p < 0.001) and incongruent versus congruent trials (t = 8.78, p < 0.001). (C) Average accuracy for each condition. Participant accuracy did not significantly differ between alcohol and neutral conditions (t = 0.83, p = 0.41), but was significantly lower for incongruent trials compared to congruent trials (t = 3.67, p = 0.01). ** = p < 0.01; *** = p < 0.001
fMRI Processing
Scanning was performed on a Siemens Prisma 3T scanner with a 64-channel head coil. Data were acquired with a repetition time (TR) of 720 ms, echo time (TE) of 30 ms, 66 slices, posterior to anterior phase encoding direction, flip angle = 66, voxel size 2.5 × 2.5 × 2.5, GRAPPA factor of 2, and multiband acceleration factor of 6. Multiecho multiplanar rapidly acquired gradient echo-structural images were acquired (TR=2530 ms, TE1=3.3 ms; TE2=6.98; TE3=8.79 ms; TE4=10.65; flip angle =7; resolution = 1.33 × 1 × 1 mm).
Data analyses were conducted in the FMRIB Software Library (FSL; Jenkinson et al., 2012). Pre-processing included MCFLIRT motion correction, brain extraction using BET, slice timing correction, smoothing with a 6-mm Gaussian kernel of full width half maximum, and high-pass filtering at 0.01 Hz. Data were cleaned using Spikefix (https://github.com/bbfrederick/spikefix) to remove intensity spikes and include single-point confound regressors for noise/motion-related time points in participants’ general linear models. Confound regressors in subject-level models included motion time courses (translation and rotation in the x, y, and z planes) and the Spikefix noise/motion artifacts. Data were denoised using independent component analysis with FSL MELODIC for each participant, in which noise components are individually characterized and removed from the analysis (Smith et al., 2004). Spatial and temporal features of each component were visually inspected to identify and remove noise-related components (Griffanti et al., 2017). 46 participants had usable neuroimaging data.
Statistical Analyses
To assess behavioral interference, we used t-tests to compare alcohol versus neutral word reaction time and accuracy and incongruent versus congruent reaction time and accuracy. Mean reaction times for each condition were calculated for each participant before subtracting to create Stroop effect reaction times (i.e., alcohol RT – neutral RT; incongruent RT – congruent RT). Accuracy for each condition was calculated for each participant and subtracted to create Stroop effect accuracy (i.e., alcohol accuracy – neutral accuracy; incongruent accuracy – congruent accuracy).
We defined two a priori regions of interest (ROI) in the dACC and rACC (Figure 2, Figure 3). The dACC ROI was comprised of two bilateral spheres 5-voxels in diameter around the coordinates x = ± 6, y = 18, z = 40 that spatially overlap with dACC activation from prior classic Stroop research (Banich et al., 2000; Laird et al., 2005). The rACC was constructed by creating a sphere 5-voxels in diameter around the coordinates x = 0, y = 38, z = −4 that has been used previously by our group (Janes et al., 2018) and spatially overlaps with prior rACC activation during a drug Stroop task (Froeliger et al., 2012), and was related to cue-induced craving and relapse in treatment-seeking adults with AUD (Seo et al., 2013). To compare ACC activity during the two Stroop tasks, beta-weights from individual first-level models for the alcohol > neutral and incongruent > congruent contrasts were extracted and compared using paired-samples t-tests. ADS scores were correlated with the beta-weights for the rACC and dACC ROIs for each contrast to test whether Stroop task activation varied by AUD severity. Finally, exploratory whole-brain analyses were conducted for the alcohol and classic Stroop contrasts (i.e., alcohol > neutral and incongruent > congruent) using FSL Randomise with cluster-based thresholding (Z = 2.3; 5000 permutations) to control for familywise error at p < 0.05. The combination of Randomise and MELODIC denoising is an ideal method for reducing familywise error, especially in block-design task fMRI data (Eklund et al., 2019; Eklund et al., 2016). Supplementary analyses investigated ACC activity to each Stroop condition separately and associations between ACC activity and ADS subscales.
Figure 2: Differences in Activity in Anterior Cingulate Regions of Interest During Alcohol and Classic Stroop.
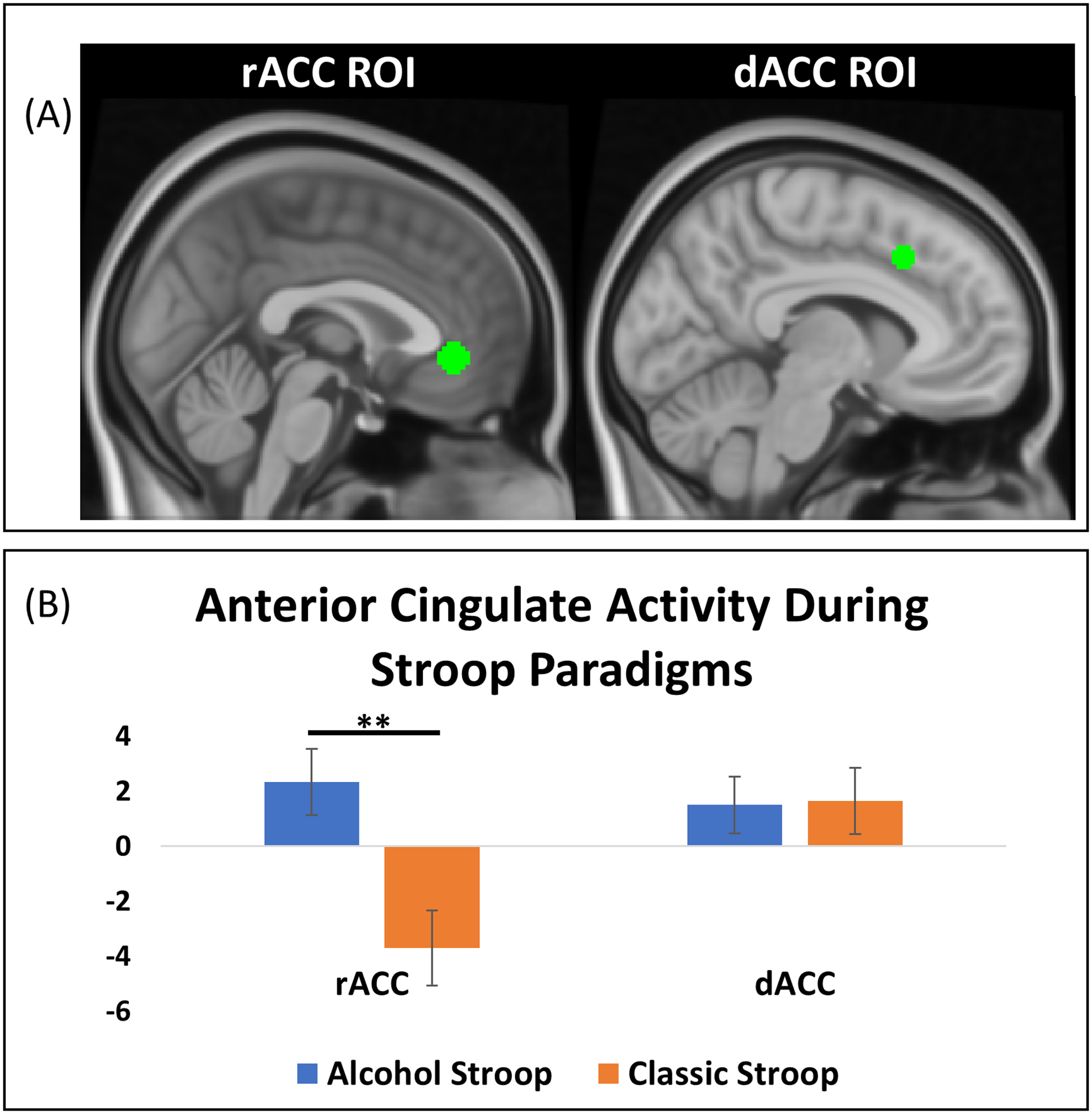
(A) The rACC region of interest (left) was constructed by creating a sphere 5-voxels in diameter around the coordinates x = 0, y = 38, z = −4. The dACC region of interest (right) was constructed by creating two bilateral spheres 5-voxels in diameter around the coordinates x = ± 6, y = 18, z = 40 (B) Activity in the rACC ROI significantly differed between the alcohol and classic Stroop, with the alcohol Stroop showing relatively increased activity (t = 2.80, p = 0.01). Activity in the dACC ROI did not significantly differ between the alcohol and classic Stroop (t = −0.08, p = 0.94). * = p <0.05, ** = p < 0.01.
Figure 3: AUD Severity and dACC reactivity during Classic Stroop Interference.

AUD severity measured using the Alcohol Dependence Scale (ADS) was associated with increased reactivity in the dACC region of interest during classic Stroop interference (r = 0.31, p = 0.03).
Results
Behavioral Results
Reaction times were significantly slower for alcohol trials versus neutral trials (t=7.85, p<0.001) and for incongruent trials versus congruent trials (t=8.78, p<0.001) (Figure 1B). Accuracy did not significantly differ between alcohol and neutral conditions (t=0.83, p=0.41), but was significantly lower for incongruent trials versus congruent trials (t=3.67, p=0.01) (Figure 1C). Importantly, the average accuracy for incongruent blocks was 93% indicating high accuracy, despite performance being significantly higher for the congruent blocks.
Anterior Cingulate Response During the Alcohol and Classic Stroop
Comparing the Stroop contrasts (i.e., alcohol > neutral contrast versus incongruent > congruent contrast), rACC activation significantly differed between the two tasks (t=2.80, p=0.01). Specifically, rACC activity was higher during the alcohol > neutral contrast relative to the incongruent > congruent contrast. In contrast, dACC activity did not significantly differ between the alcohol > neutral and incongruent > congruent contrasts (t=−0.08, p=0.94).
AUD Severity and Stroop Reactivity
Self-reported ADS scores positively correlated with dACC activity to the incongruent > congruent contrast (r=0.31, p=0.03), but not during the alcohol > neutral contrast (r=−0.05, p=0.75), and a post-hoc analyses indicated that these associations were significantly different (Z=1.72, p=0.04). Supplementary analyses revealed that the association between ADS and dACC activity was driven by ADS items relating to loss of control (Mejldal et al., 2020). AUD severity was not associated with rACC activity (p’s > 0.46).
Whole Brain Analyses
During the alcohol Stroop, participants displayed increased activity in the dACC/supplementary motor area, pre-and post-central gyrus, and occipital lobe (Table 1, Figure 4). During the classic Stroop, participants displayed increased activity in the bilateral cerebellum, occipital lobe, and inferior parietal lobe. These findings were not influenced by including Stroop accuracy or reaction time in the model.
Table 1. Whole-brain activation for alcohol and classic Stroop paradigms.
Analyses were conducted using FSL Randomise with cluster-based thresholding (Z = 2.3; 5000 permutations) to control for familywise error at p < 0.05. Brain regions were identified using the AAL atlas (Tzourio-Mazoyer et al., 2002) labels for the MNI coordinates of the cluster peaks.
| Contrast | Region | Z-Score | Cluster Size | |||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Alcohol > Neutral | cerebellum crus 1, lingual gyrus, fusiform gyrus, middle occipital lobe | 3.54 | 2166 | −26 | −92 | −4 |
| lingual gyrus, inferior occipital lobe, calcarine fissure | 3.54 | 1406 | 26 | −91 | −1 | |
| pre- and postcentral gyri | 3.54 | 931 | 42 | −16 | 58 | |
| superior frontal gyrus/SMA/midcingulate | 3.54 | 751 | −9 | 2 | 55 | |
| Incongruent > Congruent | cerebellum VI, middle and inferior occipital lobe | 3.54 | 1872 | −25 | −91 | −7 |
| inferior parietal lobe, pre- and post-central gyri | 3.54 | 1847 | −32 | −40 | 54 | |
| inferior and middle occipital lobe, lingual gyrus | 3.54 | 1195 | 25 | −93 | 1 |
Figure 4: Whole Brain Activation for Alcohol and Classic Stroop Paradigms.

The alcohol Stroop (alcohol > neutral contrast) was associated with increased reactivity in the cerebellum, occipital lobe, pre and postcentral gyri, and a cluster encompassing the dACC/supplementary motor area/superior frontal cortex (left). Image is displayed at the dACC cluster maxima (MNI coordinates: x = −4 y = 20 z = 42). The classic Stroop (incongruent > congruent contrast) was associated with increased reactivity in the cerebellum, occipital lobe, and inferior parietal lobe (right). Image is displayed at the third local maxima in the inferior parietal lobe cluster, third local maxima (MNI coordinates: x = −32 y = −52 z = 50). Analyses were conducted using FSL Randomise with cluster-based thresholding (Z = 2.3; 5000 permutations)
Discussion
In treatment-seeking adults with AUD, we identified behavioral interference effects and different patterns of brain reactivity to the alcohol and classic Stroop paradigms. Participants responded slower to alcohol trials versus neutral trials and slower to incongruent trials versus congruent trials. This finding validates our task by replicating previous work, showing slower reaction times to interfering non-drug stimuli (i.e., interference; Stroop, 1935) and drug-related stimuli in individuals with AUD/SUD (Lusher et al., 2004).
We found increased rACC activity to the alcohol > neutral contrast relative to the incongruent > congruent contrast, but no significant differences in dACC activity. The increased rACC response during the alcohol versus classic Stroop is consistent with prior research indicating the rACC’s role in resolving conflict during emotional interference (Egner et al., 2008; Etkin et al., 2006; Mohanty et al., 2007), and with research in individuals with stimulant use disorders who display increased activity in the rACC and dACC to drug > neutral (in addition to the posterior cingulate and cerebellum) but not incongruent > neutral words (Ersche et al., 2010). Studies of drug cue-reactivity show increased (or reduced suppression of) rACC activity in individuals with SUD (Goudriaan et al., 2010; Janes et al., 2016; Zhang and Volkow, 2019). One interpretation of this pattern of activation is that the personal relevance of alcohol cues engages the rACC, which has been previously implicated in both internally-oriented thinking and emotional processing (Qin and Northoff, 2011; Whalen et al., 1998). The rACC is generally suppressed during tasks requiring attention and cognitive control with affectively neutral material, and is activated during self-referential thinking (Buckner et al., 2008). This interpretation is consistent with our supplementary analyses comparing ACC activity for each Stroop condition. We found that the rACC was generally suppressed during all Stroop conditions, however there was reduced suppression for congruent versus incongruent and a trend towards reduced suppression for alcohol relative versus neutral. Reduced rACC suppression to congruent versus incongruent conditions and a trend for alcohol versus neutral conditions may reflect two different processes: increased suppression for the cognitively demanding incongruent trials and reduced suppression for the self-relevant, distracting alcohol trials respectively.
Whole-brain analysis also revealed distinct patterns of brain activation during the alcohol and classic Stroop. Participants displayed increased activity in regions linked to attention, cognitive control, and motor preparation, including the dACC and pre-and post-central gyrus to alcohol > neutral. During incongruent > congruent, participants displayed greater activity in parietal lobe regions that spatially overlap with the dorsal attention network (Figure 4; Vossel et al., 2014; Yeo et al., 2011). The dorsal attention network is involved in directing top-down controlled attention selection (Vossel et al., 2014), such as the top-down orienting necessary for successful Stroop performance. Whole brain analyses showed significant dACC activation during alcohol > neutral but not during incongruent > congruent. However, when directly comparing activation in the dACC ROI, there were no differences between the alcohol > neutral and incongruent > congruent contrasts. This suggests that task-related dACC activity did not differ between the alcohol and classic Stroop, despite only the dACC activity to alcohol > neutral surviving stringent whole-brain correction. Finally, we did not see significant activation in prefrontal regions typically recruited during Stroop paradigms (Xu et al., 2016). Future work that uses an ROI approach or compares groups (i.e., AUD versus controls) may be better positioned to probe differences in prefrontal reactivity during Stroop paradigms.
AUD severity was linked to dACC activity to incongruent >congruent but not alcohol>neutral and was unrelated to rACC activity. To better understand clinical features that might be driving this finding, supplementary analyses of ADS subscales were conducted (Mejldal et al., 2020). Findings indicated that the correlation between ADS scores and dACC activity was driven by ADS items relating to loss of control over drinking. The dACC is involved in orienting attention and deploying necessary top-down cognitive control to execute task demands (Shenhav et al., 2016). Consistent with our findings, prior research has found that to maintain accuracy on the Stroop task, heavy binge drinking young adults had greater activation in cognitive control brain regions than light drinkers (Molnar et al., 2018). Problematic alcohol use may contribute to more severe impairments in brain regions related to cognitive control, thus requiring even greater activity to meet the cognitive demands of the Stroop. Moreover, the incongruent condition had the slowest reaction times and lowest accuracy compared to the three other Stroop conditions (all p’s < 0.01), suggesting that the semantically similar distractor stimuli are the most taxing on cognitive control resources, especially for individuals with more severe AUD. Thus, it is plausible that the association between AUD and dACC function is most evident during highly challenging cognitive tasks.
It was somewhat surprising that AUD severity was not related to brain or behavioral responses to the alcohol Stroop. Prior research indicates that drug Stroop brain activity was related to cocaine craving (Goldstein et al., 2009) and post-treatment cocaine use (Marhe et al., 2013). However, several behavioral studies report no association between AUD severity and alcohol Stroop performance (Field et al., 2013; Luehring-Jones et al., 2017; Suffoletto et al., 2020). It may be that cue-related attentional bias is more closely tied to addiction severity for some SUDs than others. It is also possible that alcohol-related interference occurs quite early in the addiction process, and does not vary by severity of AUD, whereas deficits in general cognitive control deficits are either a risk factor for or consequence of more severe problems with alcohol.
The study has several limitations. The study leveraged within-person comparisons to characterize alcohol- and non-alcohol-related interference in AUD. However, the study lacked a control group, which would allow for between-group comparisons. Also, the ADS is an older measure of AUD problems and may not fully encompass DSM-5 conceptualizations of AUD (e.g., craving) (Mejldal et al., 2020), yet, we are encouraged that our findings were driven by ADS items probing loss of control, which might be most conceptually linked to Stroop interference. The sample had few non-white participants; thus, results require replication in more diverse samples. Also, the association between AUD severity and dACC reactivity during the classic Stroop suggests a small-to-medium effect size. Replication in a larger sample would provide additional support for the relationship between dACC activity and AUD severity.
Conclusion
Using a unique Stroop paradigm, we examined the behavioral and brain responses to alcohol and non-alcohol-related Stroop interference in adults with AUD. Although both the alcohol and the classic Stroop resulted in behavioral interference (i.e., slowed reaction times), the pattern of cingulate activity differed between the alcohol and classic Stroop. The rACC displayed increased activity to the alcohol Stroop compared to the classic Stroop, potentially suggesting an interfering effect of self-relevant alcohol cues on non-alcohol-related task goals. Although dACC activity did not differ between the two Stroop tasks, dACC activity during the classic Stroop was related to AUD severity, suggesting that participants with more severe AUD required greater recruitment of the dACC to meet the task demands of the classic Stroop. Our findings have implications for treatment development. Specifically, while treatments that reduce the impact of alcohol cues may benefit individuals across a wide range of AUD severity, treatments that also improve executive function may be particularly beneficial for individuals with more severe AUD.
Supplementary Material
Author Disclosures
Role of Funding Source
Supported by National Institute on Alcohol Abuse and Alcoholism, R01 AA015923 (TJF), National Institute on Drug Abuse, K02 DA042987 (ACJ), DA015036 (PI: Lukas), National Institute of Mental Health, R56 MH117131 and R01 MH117131. This work was supported by the intramural research program of the National Institute on Drug Abuse, National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Banich MT, Milham MP, Atchley R, Cohen NJ, Webb A, Wszalek T, Kramer AF, Liang Z-P, Wright A, Shenker J, 2000. fMRI studies of Stroop tasks reveal unique roles of anterior and posterior brain systems in attentional selection. Journal of cognitive neuroscience 12(6), 988–1000. [DOI] [PubMed] [Google Scholar]
- Brewer JA, Worhunsky PD, Carroll KM, Rounsaville BJ, Potenza MN, 2008. Pretreatment brain activation during stroop task is associated with outcomes in cocaine-dependent patients. Biological psychiatry 64(11), 998–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TA, Barlow DH, 2014. Anxiety and related disorders interview schedule for DSM-5 (ADIS-5L).: Lifetime version. Client interview schedule. Oxford University Press. [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL, 2008. The brain’s default network: anatomy, function, and relevance to disease. Annals of the New York Academy of Sciences 1124, 1–38. [DOI] [PubMed] [Google Scholar]
- Czapla M, Baeuchl C, Simon JJ, Richter B, Kluge M, Friederich H-C, Mann K, Herpertz SC, Loeber S, 2017. Do alcohol-dependent patients show different neural activation during response inhibition than healthy controls in an alcohol-related fMRI go/no-go-task? Psychopharmacology 234(6), 1001–1015. [DOI] [PubMed] [Google Scholar]
- Egner T, Etkin A, Gale S, Hirsch J, 2008. Dissociable neural systems resolve conflict from emotional versus nonemotional distracters. Cerebral cortex 18(6), 1475–1484. [DOI] [PubMed] [Google Scholar]
- Eklund A, Knutsson H, Nichols TE, 2019. Cluster failure revisited: Impact of first level design and physiological noise on cluster false positive rates. Human Brain Mapping 40(7), 2017–2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eklund A, Nichols TE, Knutsson H, 2016. Cluster failure: why fMRI inferences for spatial extent have inflated false-positive rates. Proceedings of the National Academy of Sciences 113(28), 7900–7905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ersche KD, Bullmore ET, Craig KJ, Shabbir SS, Abbott S, Müller U, Ooi C, Suckling J, Barnes A, Sahakian BJ, Merlo-Pich EV, Robbins TW, 2010. Influence of Compulsivity of Drug Abuse on Dopaminergic Modulation of Attentional Bias in Stimulant Dependence. Archives of General Psychiatry 67(6), 632–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Egner T, Peraza DM, Kandel ER, Hirsch J, 2006. Resolving emotional conflict: a role for the rostral anterior cingulate cortex in modulating activity in the amygdala. Neuron 51(6), 871–882. [DOI] [PubMed] [Google Scholar]
- Field M, Christiansen P, Cole J, Goudie A, 2007. Delay discounting and the alcohol Stroop in heavy drinking adolescents. Addiction 102(4), 579–586. [DOI] [PubMed] [Google Scholar]
- Field M, Cox WM, 2008. Attentional bias in addictive behaviors: A review of its development, causes, and consequences. Drug and Alcohol Dependence 97(1), 1–20. [DOI] [PubMed] [Google Scholar]
- Field M, Marhe R, Franken IH, 2014. The clinical relevance of attentional bias in substance use disorders. CNS spectrums 19(3), 225–230. [DOI] [PubMed] [Google Scholar]
- Field M, Mogg K, Mann B, Bennett GA, Bradley BP, 2013. Attentional biases in abstinent alcoholics and their association with craving. Psychology of Addictive Behaviors 27(1), 71–80. [DOI] [PubMed] [Google Scholar]
- Froeliger B, Modlin L, Wang L, Kozink RV, McClernon FJ, 2012. Nicotine withdrawal modulates frontal brain function during an affective Stroop task. Psychopharmacology 220(4), 707–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Alia-Klein N, Tomasi D, Carrillo JH, Maloney T, Woicik PA, Wang R, Telang F, Volkow ND, 2009. Anterior cingulate cortex hypoactivations to an emotionally salient task in cocaine addiction. Proceedings of the National Academy of Sciences 106(23), 9453–9458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goudriaan AE, De Ruiter MB, Van Den Brink W, Oosterlaan J, Veltman DJ, 2010. Brain activation patterns associated with cue reactivity and craving in abstinent problem gamblers, heavy smokers and healthy controls: an fMRI study. Addiction biology 15(4), 491–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffanti L, Douaud G, Bijsterbosch J, Evangelisti S, Alfaro-Almagro F, Glasser MF, Duff EP, Fitzgibbon S, Westphal R, Carone D, 2017. Hand classification of fMRI ICA noise components. Neuroimage 154, 188–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janes AC, Betts J, Jensen JE, Lukas SE, 2016. Dorsal anterior cingulate glutamate is associated with engagement of the default mode network during exposure to smoking cues. Drug and alcohol dependence 167, 75–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janes AC, Zegel M, Ohashi K, Betts J, Molokotos E, Olson D, Moran L, Pizzagalli DA, 2018. Nicotine normalizes cortico-striatal connectivity in non-smoking individuals with major depressive disorder. Neuropsychopharmacology 43(12), 2445–2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM, 2012. Fsl. Neuroimage 62(2), 782–790. [DOI] [PubMed] [Google Scholar]
- Jones A, Rose AK, Cole J, Field M, 2013. Effects of Alcohol Cues on Craving and Ad Libitum Alcohol Consumption in Social Drinkers: The Role of Disinhibition. Journal of Experimental Psychopathology 4(3), 239–249. [Google Scholar]
- Laird AR, McMillan KM, Lancaster JL, Kochunov P, Turkeltaub PE, Pardo JV, Fox PT, 2005. A comparison of label-based review and ALE meta-analysis in the Stroop task. Human brain mapping 25(1), 6–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luehring-Jones P, Louis C, Dennis-Tiwary TA, Erblich J, 2017. A Single Session of Attentional Bias Modification Reduces Alcohol Craving and Implicit Measures of Alcohol Bias in Young Adult Drinkers. Alcoholism: Clinical and Experimental Research 41(12), 2207–2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lusher J, Chandler C, Ball D, 2004. Alcohol dependence and the alcohol Stroop paradigm: evidence and issues. Drug and Alcohol Dependence 75(3), 225–231. [DOI] [PubMed] [Google Scholar]
- Marhe R, Luijten M, van de Wetering BJM, Smits M, Franken IHA, 2013. Individual Differences in Anterior Cingulate Activation Associated with Attentional Bias Predict Cocaine Use After Treatment. Neuropsychopharmacology 38(6), 1085–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mejldal A, Andersen K, Bilberg R, Braun B, Bogenschutz M, Bühringer G, Nielsen AS, Silke B, 2020. The Alcohol Dependence Scale and DSM-5 alcohol use disorder: Severity ratings correspond insufficiently in older patients. International Journal of Methods in Psychiatric Research 29(1), e1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohanty A, Engels AS, Herrington JD, Heller W, Ringo Ho MH, Banich MT, Webb AG, Warren SL, Miller GA, 2007. Differential engagement of anterior cingulate cortex subdivisions for cognitive and emotional function. Psychophysiology 44(3), 343–351. [DOI] [PubMed] [Google Scholar]
- Molnar SM, Beaton LE, Happer JP, Holcomb LA, Huang S, Arienzo D, Marinkovic K, 2018. Behavioral and brain activity indices of cognitive control deficits in binge drinkers. Brain sciences 8(1), 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motzkin JC, Baskin-Sommers A, Newman JP, Kiehl KA, Koenigs M, 2014. Neural correlates of substance abuse: Reduced functional connectivity between areas underlying reward and cognitive control. Human Brain Mapping 35(9), 4282–4292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller-Oehring EM, Le Berre A-P, Serventi M, Kalon E, Haas AL, Padula CB, Schulte T, 2019. Brain activation to cannabis-and alcohol-related words in alcohol use disorder. Psychiatry Research: Neuroimaging 294, 111005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Ray RD, Cooper JC, Robertson ER, Chopra S, Gabrieli JDE, Gross JJ, 2004. For better or for worse: neural systems supporting the cognitive down- and up-regulation of negative emotion. NeuroImage 23(2), 483–499. [DOI] [PubMed] [Google Scholar]
- Papachristou H, Nederkoorn C, Havermans R, van der Horst M, Jansen A, 2012. Can’t stop the craving: The effect of impulsivity on cue-elicited craving for alcohol in heavy and light social drinkers. Psychopharmacology 219(2), 511–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin P, Northoff G, 2011. How is our self related to midline regions and the default-mode network? Neuroimage 57(3), 1221–1233. [DOI] [PubMed] [Google Scholar]
- Seo D, Lacadie CM, Tuit K, Hong K-I, Constable RT, Sinha R, 2013. Disrupted ventromedial prefrontal function, alcohol craving, and subsequent relapse risk. JAMA psychiatry 70(7), 727–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenhav A, Cohen JD, Botvinick MM, 2016. Dorsal anterior cingulate cortex and the value of control. Nature Neuroscience 19(10), 1286–1291. [DOI] [PubMed] [Google Scholar]
- Skinner HA, Horn JL, 1984. Alcohol dependence scale (ADS): User’s guide. Addiction Research Foundation. [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, 2004. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23, S208–S219. [DOI] [PubMed] [Google Scholar]
- Stroop JR, 1935. Studies of interference in serial verbal reactions. Journal of experimental psychology 18(6), 643. [Google Scholar]
- Suffoletto B, Field M, Chung T, 2020. Attentional and approach biases to alcohol cues among young adult drinkers: An ecological momentary assessment study. Experimental and Clinical Psychopharmacology 28(6), 649–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M, 2002. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 15(1), 273–289. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Koob GF, McLellan AT, 2016. Neurobiologic Advances from the Brain Disease Model of Addiction. New England Journal of Medicine 374(4), 363–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vossel S, Geng JJ, Fink GR, 2014. Dorsal and ventral attention systems: distinct neural circuits but collaborative roles. The Neuroscientist 20(2), 150–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalen PJ, Bush G, McNally RJ, Wilhelm S, McInerney SC, Jenike MA, Rauch SL, 1998. The emotional counting Stroop paradigm: a functional magnetic resonance imaging probe of the anterior cingulate affective division. Biological psychiatry 44(12), 1219–1228. [DOI] [PubMed] [Google Scholar]
- Wilcox CE, Clifford J, Ling J, Mayer AR, Bigelow R, Bogenschutz MP, Tonigan JS, 2020. Stroop-related cerebellar and temporal activation is correlated with negative affect and alcohol use disorder severity. Brain Imaging Behav 14(2), 586–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JMG, Mathews A, MacLeod C, 1996. The emotional Stroop task and psychopathology. Psychological bulletin 120(1), 3. [DOI] [PubMed] [Google Scholar]
- Xu M, Xu G, Yang Y, 2016. Neural systems underlying emotional and non-emotional interference processing: An ALE meta-analysis of functional neuroimaging studies. Frontiers in behavioral neuroscience 10, 220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo BT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, Roffman JL, Smoller JW, Zöllei L, Polimeni JR, 2011. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. Journal of neurophysiology 106, 1125–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Volkow ND, 2019. Brain default-mode network dysfunction in addiction. Neuroimage 200, 313–331. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


