Abstract
Capillaries are equipped to sense neurovascular coupling agents released onto the outer wall of a capillary, translating these external signals into electrical/Ca2+ changes that play a crucial role in blood flow regulation and ensuring that neuronal demands are met. However, control mechanisms attributable to forces imposed onto the lumen are less clear. Here, we show that Piezo1 channels act as mechanosensors in central nervous system (CNS) capillaries. Electrophysiological analyses confirmed expression and function of Piezo1 channels in brain cortical and retinal capillaries. Activation of Piezo1 channels evoked currents that were sensitive to endothelial cell-specific Piezo1 deletion. Using genetically encoded Ca2+ indicator mice and an ex vivo pressurized retina preparation, we found that activation of Piezo1 channels by mechanical forces triggered Ca2+ signals in capillary endothelial cells. Collectively, these findings indicate that Piezo1 channels are capillary mechanosensors that initiate crucial Ca2+ signals and could therefore have a profound impact on CNS blood flow control.
Graphical Abstract
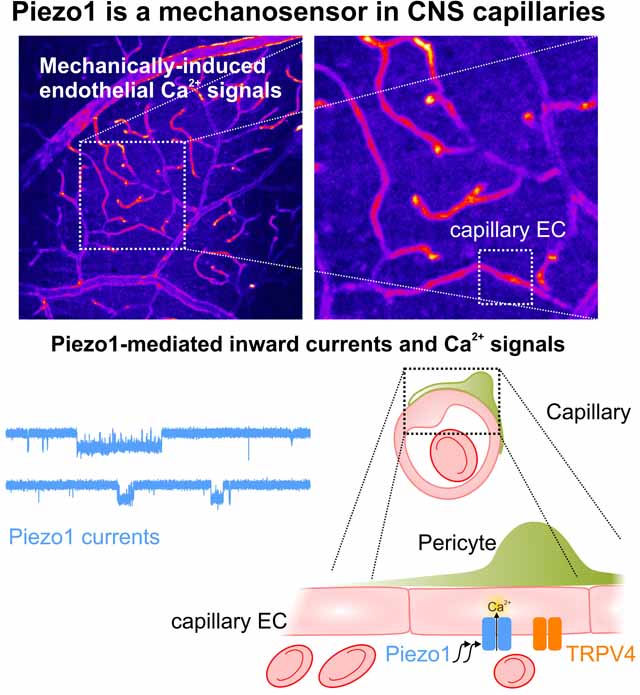
Capillaries are equipped to sense neurovascular coupling agents that play a crucial role in blood flow regulation and ensuring that neuronal demands are met. However, control mechanisms that regulate the forces imposed onto the lumen have been unclear. This study provides evidence that Piezo1 channel is expressed and functional in CNS capillaries. The activation of Piezo1 channel by mechanical forces in the vascular lumen triggers endothelial calcium signals.
INTRODUCTION
The continuous pumping of blood by the heart creates a range of life-long mechanical forces on the vascular system, from pulsatile oscillations to changes in flow and pressure. Vascular cells experience oscillatory and laminar shear stress (due to blood flow) and circumferential stretch (due to intravascular pressure)1,2. Shear stress—the frictional force generated by fluid flow over a surface—represents a major form of mechanical force experienced by endothelial cells (ECs)3,4. The ability of ECs to sense (mechanosensation) and respond to (mechanotransduction) mechanical forces is in fact indispensable for vascular maturation and is also thought to be crucial for blood pressure regulation5–7.
Mechanotransduction is mediated by several mechanisms, including ionotropic channel gating and metabotropic receptor activation8–11. Vascular responses to mechanical stimuli require the concerted action of different proteins. Slow metabotropic mechanisms, such as those mediated by Gq protein-coupled receptors (GqPCRs), involve mechanical activation independent of ligand binding11,12. On the other hand, faster-acting ion channels in ECs, including the Piezo1 channel and osmosensitive TRPV4 channel, have been implicated in mechanosensation6,7,13–15. Both of these channels are cation permeable, but only Piezo1, discovered about a decade ago, is a bona fide mechanosensor14,16. Activation of Piezo1 by a mechanical stimulus leads to Na+/Ca2+ influx, which triggers electrical signals and initiates second messenger signaling pathways16. Global or EC-specific genetic deletion of the Piezo1 channel in mice is embryonically lethal, reflecting the crucial role of endothelial Piezo1 in sensing shear stress and transducing Ca2+-dependent EC migration and alignment during development6,7. Piezo1 in ECs is also critical through adulthood, controlling arterial tone under certain circumstances (e.g., during exercise)17 and regulating blood pressure through activation of nitric oxide synthase (NOS) and increased release of the vasodilator, NO5. While several studies have reported Piezo1 activity in peripheral arterial ECs, whether Piezo1 is expressed and functional in the central nervous system (CNS) vasculature generally, or in capillaries in particular, is not known.
The cerebral circulation is composed of a network of interconnected surface vessels with considerable capacity for redirecting blood flow18. Arterioles penetrate orthogonally into the brain, and branch into smaller arterioles, transitioning to capillaries that dramatically extend the area of perfusion. Capillaries, the smallest of all blood vessels, are narrow (~5 μm diameter) tubes consisting of a single layer of capillary ECs (cECs). We previously showed that inwardly rectifying Kir2.1 channels in cECs are activated by K+ released from active neurons, driving a propagating hyperpolarization that travels upstream to dilate arterioles19. This mechanism is a major contributor to the neurovascular coupling (NVC) process that mediates an increase in local blood flow to active neurons (functional hyperemia)19–21. Because the diameter of red blood cells (RBCs) is slightly larger than the capillary diameter, blood cells are ‘squeezed’ through single file, imparting unique mechanical forces on the capillary endothelium. However, whether brain capillaries sense the forces associated with blood movement and whether such mechanosensation contributes to NVC are currently unknown.
Here, we demonstrate that Piezo1 channels are present and functional in CNS cECs. Specifically, we found that Piezo1 channels in cECs in the brain cortex and retina activate in response to mechanical and/or pharmacological stimuli. We further show that changes in intravascular pressure/flow trigger endothelial Ca2+ transients in retinal cECs, and that these transients are augmented by pharmacological activation of Piezo1 channels. Collectively, our findings establish that the Piezo1 channel is a mechanosensor in CNS capillaries that is capable of mediating mechanically induced endothelial Ca2+ transients, highlighting the potential of this channel to play a role in cerebral blood flow (CBF) regulation.
MATERIALS AND METHODS
For detailed methodological information, please refer to the Data Supplement. For research materials, please see the Major Resources Table.
Data Availability:
All study data are included in the article and supporting information.
RESULTS
Changes in pressure and flow evoke endothelial Ca2+ transients in the retinal vasculature
We have previously shown that NVC agents, such as K+ and receptor agonists, derived from neurons and/or astrocytes act on cECs to trigger downstream effects that alter CBF19,20,22. However, less is known about luminal forces that act on the vascular EC layer and whether these forces affect capillary signaling. Imparting a controlled intraluminal mechanical stimulus onto brain cECs in vivo is a challenging, and likely unfeasible, undertaking. As an alternative approach, we exploited the retinal circulation, which provides experimental advantages compared with the brain, such as its relatively two-dimensional angioarchitecture and the fact that it is supplied by a single ophthalmic artery. In this ex vivo pressurized retina preparation, the ophthalmic artery of a flattened retina, pinned down with the vitreal surface facing up (en face preparation), is cannulated, and calibrated changes in intravascular pressure are generated by varying the pressure at the ophthalmic artery, thereby altering flow and shear stress throughout the retinal vasculature (Fig. 1a)23,24. While the downstream pressure change is not precisely known—a major limitation of all ex vivo and in vivo investigations of vascular networks—changes applied at the ophthalmic artery are expected to translate into changes in pressure/flow at the capillary level that are within the physiological range. Importantly, the retina is developmentally and anatomically part of the brain, and similar to the brain cortex, it possesses various elements of the neurovascular unit (NVU), which typically comprises neurons, astrocytes, ECs, smooth muscle cells (SMCs) and pericytes25–27. For these experiments, we used retinal preparations from an EC-specific, genetically encoded Ca2+-indicator (Cdh5-GCaMP8) mouse (Fig. 1a, 1b) in which the indicator, GCaMP8, is expressed under the control of the endothelium-specific cadherin 5 (Cdh5) promoter, allowing changes in Ca2+ levels to be monitored in the endothelium of all vascular components (arterioles, capillaries, and venules)22. Elevating pressure at the ophthalmic artery from 20 to 80 mmHg, a stimulus that increases perfusion pressure, flow and shear forces in all vascular segments, triggered robust EC Ca2+ signals throughout the retinal vasculature that varied in fluorescence magnitude from 3.0% to 40.5% (Fig. 1b and movie S1-left). These mechanically driven Ca2+ signals were evident throughout the retinal vasculature, including in capillaries, venules and arterioles (Fig. 1c), reaching maximum Ca2+ fluorescence changes (ΔF/F0) of 0.19 ± 0.02, 0.15 ± 0.03 and 0.12 ± 0.01, respectively (Fig. 1c, 1d). These Ca2+ elevations in response to changes in pressure were sustained, as depicted in Figure 1c. Focusing on capillary regions of interest (ROIs), we found that gradually elevating the intravascular pressure from 20 to 80 mmHg (20-mmHg increments) evoked a pressure-dependent rise in cEC [Ca2+]i that returned to baseline upon lowering pressure back to 20 mmHg (Fig. 1e and Suppl. Fig. 1). Little or no change in fluorescence was observed in response to pressure in non-vascular background areas. These observations collectively indicate that mechanical forces trigger reversible and graded Ca2+ signals in the retinal endothelium, particularly in capillary ECs.
Fig. 1: Changes in pressure/flow evoke endothelial Ca2+ transients in the retinal vasculature.

(a) Schematic illustration of the pressurized retina vasculature preparation. Top: Diagram showing the eyes and their circuit connection to the visual cortex of the mouse. Bottom: After first isolating eyes from euthanized mice, the intact retina is obtained without damaging the ophthalmic artery, then the ophthalmic artery is cannulated and the retinal cup is cut open into four retinal petals and pinned to the recording stage. Right: A gravity column is used to control pressure at the ophthalmic artery, and a transducer is used to monitor pressure changes. (b) Representative images of the entire en face ex vivo retinal preparation from a Cdh5-GCaMP8 mouse. Pressure at the ophthalmic artery was elevated from 20 to 80 to 20 mmHg, and Ca2+ transients were monitored. Inset: Venules (V), capillaries (C), and arterioles (A) could be characterized based on their angioarchitecture and vessel diameter. Representative images (each from a 30-s recording) depict the median response during a pressure step. (c) Traces of line scans showing Ca2+ transients in arterioles, capillaries, and venules in response to elevating intravascular pressure at the cannulated ophthalmic artery from 20 to 80 mmHg (n = 6 retina preparations from 6 mice). (d) Scatter plots of changes in fluorescence in arterioles, capillaries, and venules in response to pressure elevation. Data are presented as means (bold dotted horizontal lines) ± SEM (error bars) (*P < 0.05, ns indicates not significant, Kruskal-Wallis test followed by Dunn’s multiple comparisons test; arterioles, n = 17 ROIs/6 retinas/6 mice; capillaries, n = 20 ROIs/6 retinas/6 mice; venules, n = 19 ROIs/6 retinas/6 mice). (e) Left: Averaged fluorescence from capillary ROIs (yellow; n = 3 ROIs) and non-vascular background (gray; n = 3 ROIs) from the same retina preparation in response to the depicted pressure changes (20→40→60→80→20 mmHg). Solid lines are means and dotted lines are SEM. Right: Fluorescence changes in capillary segments (yellow) and neighboring non-capillary background ROIs (gray). Data were obtained from 3 retina preparations from 3 Cdh5-GCaMP8 mice.
Mechanical stimulation induces single-channel currents in CNS cECs
As a first step in determining the molecular basis for pressure-evoked Ca2+ signals in retinal cECs, we investigated these cells for the presence of a mechanosensitive Ca2+ conductance using patch-clamp electrophysiology. To this end, we isolated cECs from whole retinas by mechanical homogenization and enzymatic digestion (see Methods, Fig. 2a) and applied the cell-attached configuration of the patch-clamp technique. Notably, the morphology of isolated cECs is different from that of arterial ECs. Individual cECs appear as collapsed forms of the small tubes that exist in vivo, whereas arterial ECs, which have a characteristic cobblestone appearance in vivo, appear as patches of cells with a roundish shape upon isolation19,28,29. Freshly isolated retinal cECs were bathed in a 140-mM K+ solution (similar to intracellular [K+]i), yielding a zero membrane potential, and patch pipettes were filled with a 3-mM K+ solution (similar to extracellular [K+] in the cerebrospinal fluid and the vitreous fluid). Patches were held at −50 mV after achieving a giga-ohm seal (Fig. 2a). Application of gradually increasing negative pressure (from 0 to −40 mmHg) onto the patch evoked single-channel–like openings whose open times became more prolonged as the negative pressure increased, consistent with a graded mechanosensitive current (Fig. 2b). Application of a constant negative pressure (−10 mmHg) also increased the open probability in a reversible manner (Fig. 2c). Fitting the amplitude distribution of the mechanosensitive current to a Gaussian histogram yielded an estimated unitary current of 0.99 ± 0.04 pA; at −50 mV, this unitary current corresponded to a single-channel conductance of ~20 pS. In contrast, the single-channel conductance of the TRPV4 channel, which is also Ca2+-permeable and expressed in cECs28, is more than double this value (~45–60 pS)13,30. Moreover, mechanically evoked currents persisted under conditions in which TRPV4 was blocked (Fig. 2c). Taken together, these findings demonstrate the presence of a mechanosensitive cationic channel in retinal capillaries that is distinct from TRPV4.
Fig. 2: Mechanical stimulation evokes a current with an intermediate conductance in CNS capillary ECs.

(a) Experimental protocol for the isolation of retinal cECs. Retinal cups were dissected from isolated eyeballs, homogenized, and then filtered. Retained capillaries were eluted into enzymatic solution for digestion, then washed and triturated (see Methods). Right box: Cell-attached approach for electrophysiology in cECs. A micropipette with slight positive pressure was advanced until it touched a cEC, after which suction was applied to attain a giga-ohm seal. Mechanical stimulation of the attached patch was achieved by delivering calibrated negative pressure. (b) Representative traces measured from a retinal cEC bathed in a 140-mM K+ solution using the cell-attached mode and a pipette filled with a 3-mM K+ solution. The patch was voltage-clamped at −50 mV, and negative pressure (0 to −40 mmHg) was gradually applied to the patch pipette. (c) Representative traces and amplitude histograms (right) from a retinal cEC without negative pressure (0 mmHg, top), followed by application of negative pressure (−10 mmHg, middle), and then no pressure (bottom). The pipette solution was supplemented with 1 μM RR and 100 μM Ba2+. Amplitude histograms and open probabilities (NPO) reflect pressure-evoked changes. C: closed, O: open. Representative traces (from ≥5-min recordings) depict median responses to pressure.
Mechanosensitive single-channel currents in CNS cECs are mediated by Piezo1 channels
Next, we sought to identify the ion channel underlying the observed mechanically driven currents (Fig. 2) and possibly Ca2+ signals (Fig. 1). Because our findings suggested that these currents are mediated by a channel type other than TRPV4, we next turned to literature reports on peripheral ECs5,17,31 and recent RNA sequencing databases of CNS ECs32,33, which led us to focus on the bona fide mechanosensitive channel, Piezo1. To this end, we patch-clamped retinal cECs in the cell-attached mode in the presence of the Kir2.1 channel blocker Ba2+ (100 μM) and the TRPV4 inhibitor GSK2193874 (hereafter, GSK219 [1 μM]). No or very few single-channel openings were evident in control recordings performed at −50 mV in the absence of a negative-pressure stimulus. However, inclusion of the Piezo1 activator Yoda1 (5 μM 34) in the pipette lead to increased single-channel activity with a unitary current of −1.01 ± 0.01 pA (Fig. 3a), a value similar to that observed in response to negative pressure (Fig. 2). This Yoda1-induced current was suppressed by the trivalent cation, Gd3+ (30 μM; Fig. 3a, b), a nonspecific inhibitor of mechanically activated cation currents35–37. Single-channel currents increased with membrane potential hyperpolarization with a slope conductance of 22.7 pS (Fig. 3c), consistent with the previously reported conductance of the Piezo1 channel9,17. These results collectively suggest that the Piezo1 channel is present and functional in retinal cECs and could underlie mechanically driven responses in these cells.
Fig. 3: Retinal cECs express functional Piezo1 channels.

(a, b) Representative traces and averaged NPO values for single-channel openings obtained in the cell-attached mode from retinal cECs in the absence (control) or presence of Yoda1 (5 μM) in the 3 mM K+ pipette solution. GSK219 (1 μM; TRPV4 blocker) and Ba2+ (100 μM) were included in the pipette solution. Amplitude histogram demonstrating the unitary Yoda1-evoked current. In a third group, 30 μM Gd3+ was added to the pipette and bath solutions. Each point in the scatter plot was obtained from a single cEC (number of cECs is indicated in the figure). (c) Unitary current-voltage relationship plotted using averaged unitary currents measured at the following voltages (mV): −30 (n = 6 cECs), −50 (n = 7), −70 (n = 7), −90 (n = 7), and −110 (n = 6). Conductance obtained from the slope was 22.7 pS.
To investigate whether cECs in other CNS areas also express functional Piezo1 channels, we applied a recording protocol similar to that in Figure 3 to cECs isolated from the brain cortex. Yoda1 triggered a number of channel openings in each cell, with an average open probability (NP0) of 0.49 (Fig. 4a–4d), reflecting the number of channels in the patch (N) and their open probability (P0). Yoda1-evoked currents were blocked by Gd3+ (30 μM) and GsMTx-4 (2.5 μM)—both of which inhibit Piezo1 as well as a number of other types of ion channels35,36,38–40 (Fig. 4c, d). The unitary conductance of Yoda1-induced currents in brain cortical cECs was ~20 pS (Fig. 4e). Consistent with the mechanosensitive properties of these currents, application of negative pressure (−10 mmHg) to the attached patch in the presence of Yoda1 further and profoundly increased the open probability of Piezo1 currents, an increase that was reversed upon cessation of the mechanical force (Fig. 4f). Taken together, these observations support the idea that the Piezo1 channel is functional in brain cortical cECs and is activated by mechanical and pharmacological stimuli.
Fig. 4: Brain cortical cECs express functional Piezo1 channels.

(a) Cell-attached recordings at a holding membrane potential of −50 mV. Brain cECs were bathed in a 140-mM K+ solution, and the pipette was filled with a 6-mM K+ solution containing RR (1 μM) and Ba2+ (100 μM). (b) Currents with Yoda1 (5 μM) included in the pipette. (c, d) Traces and summary data showing the open probability (NP0) of Yoda1-induced currents. Yoda1 (5 μM) was included in the pipette solution in the absence (control; n = 9) or presence of Gd3+ (30 μM; n = 6) or GsMTx-4 (2.5 μM; n = 9). *P ≤ 0.05 and **P ≤ 0.01 (Kruskal-Wallis test followed by Dunn’s multiple comparisons test). (e) Traces and plot of unitary current-voltage relationship, used to estimate the single-channel conductance. Currents were recorded at −30 mV (n = 6 cECs), −50 (n = 10), −70 (n = 9), −90 (n = 7), and −110 (n = 4). (f) Representative current traces from a patch exposed to Yoda1 and held at −50 mV. Pressure was stepped from 0 mmHg to −10 mmHg by applying suction via the patch pipette and held for 30 seconds before returning to 0 mmHg.
To specifically attribute the mechanically evoked currents to Piezo1 and confirm the specificity of Yoda1 actions, we generated inducible EC-specific Piezo1-knockout (Piezo1-EC-KO) mice by crossing mice harboring loxP sites flanking exons 20–23 of the Piezo1 gene (Piezo1fl/fl mice) with mice expressing inducible Cre-recombinase under the control of the vascular endothelial Cdh5 promoter (Cdh5-Cre/ERT2). Cre-recombinase expression was induced in Cre-recombinase–expressing homozygous Piezo1fl/fl offspring by treating with tamoxifen, yielding Piezo1-EC-KO mice. Piezo1fl/fl mice that lacked expression of Cre-recombinase similarly received tamoxifen and were used as controls (Piezo1-EC-Ctrl). Using brain cortical cECs, we found that application of negative pressure through the patch pipette (−10 mmHg) failed to trigger Piezo1 currents in Piezo1-EC-KO cECs (Fig. 5a) but successfully evoked currents in all cECs from Piezo1-EC-Ctrl mice (Fig. 5b, c). Furthermore, inclusion of Yoda1 (5 μM) in the pipette solution increased the open probability of Piezo1-like currents in Piezo1-EC-Ctrl cECs but not in Piezo1-EC-KO cECs (Fig. 5d). Yoda1 similarly evoked an increase in Piezo1 open probability in retinal cECs from control mice, but not KO mice (Suppl.Fig. 2), which previous reports have shown have no major differences in retinal vascular networks compared with wild-type mice17. Under our experimental settings, we did not detect mechanically induced unitary currents with an amplitude consistent with TRPV4 channel openings in KO or control cECs. Collectively, these findings support the selectivity of Yoda1 and the expression of functional Piezo1 channels in native cortical brain cECs from wild-type (Figs. 3 and 4) and Piezo1fl/fl mice (Fig. 5).
Fig. 5: Genetic deletion and RR abolish Piezo1 activity.

(a, b) Representative traces from a cEC from an EC-specific Piezo1-knockout (Piezo1EC-KO) mouse (a) and a cEC from a control mouse (b). Recordings were obtained in the cell-attached mode at a holding potential of −50 mV before (0 mmHg) and after the application of negative pressure (−10 mmHg) onto the patch. (c) Scatter plots of the open probability (NPO) of pressure-induced Piezo1-like single-channel openings in Piezo1EC-KO and control cECs (**P < 0.01, Wilcoxon test; Piezo1EC-KO, n = 6 cECs/4 mice; Control, n = 9 cECs/3 mice). (d) Scatter plots of the open probability of Yoda1-induced channel openings in Piezo1EC-KO and control cECs (**P < 0.01, Mann-Whitney test; Piezo1EC-KO, n = 12 cECs/4 mice; Control, n = 12 cECs/4 mice). ns indicates not significant. Horizontal black lines depict means and error bars are SEM. (e) Representative traces of cell-attached, Yoda1-induced, single-channel inward currents in the presence of 1, 10 or 30 μM RR, recorded at a holding potential of −50 mV. Right insets: Magnified channel openings of the marked segments. Traces are representative of 7 cECs (1 μM RR), 6 cECs (10 μM), and 4 cECs (30 μM). (f) Open-time histograms in the presence of 1 or 5 μM RR in the pipette solution. Lines show a single exponential fit to a Marquardt algorithm, yielding mean open times of 8.9 ms (1 μM) and 3.3 ms (5 μM). (g) Concentration-dependent decrease in open time and estimated IC50 (n = 4, 10, 12, 3, 1 cECs at 0, 1, 5, 10, 30 μM RR, respectively). (h) Reciprocal of mean open time (1/ƬO) plotted against the external [RR].
Ruthenium red blocks Piezo1 activity in brain cECs
Ruthenium red (RR) is a polycationic, non-selective blocker that inhibits several types of ion channels, including those involved in Ca2+ flux such as TRP channels, ryanodine receptors, voltage-gated Ca2+ channels, and mitochondrial Ca2+ uniporters41–44. Nanomolar RR blocks the vanilloid TRP channels, TRPV1 and TRPV4, in a voltage-dependent manner45–47. In contrast, RR blocks other ion channels with half-inhibitory concentrations well into the micromolar range. Interestingly, it has been shown that RR can block the mouse Piezo1 channel at distinctively higher concentrations (IC50 ≈ 5.4 μM at −80 mV48) than those required to block TRPV4 channel (IC50 ≈ 5 nM for inhibition of TRPV4-mediated Ca2+ signals at physiological membrane potentials46). Therefore, to test the sensitivity of Yoda1-induced currents in capillaries to RR, we assessed the open probability of Yoda1-evoked currents in brain cortical cECs under conditions in which the pipette solution, which is extracellular to the attached patch, was supplemented with 0, 1, 5, 10 or 30 μM RR. As depicted in Figure 5e, Piezo1 currents recorded at −50 mV were inhibited by RR in a concentration-dependent manner with a calculated half-inhibitory concentration (IC50) at this voltage of 2.9 μM, a value comparable to that reported by others (5.4 μM at −80 mV,48). RR shortened the mean channel open time by blocking the channel pore. The RR-concentration dependence of the shortening of Piezo1 channel openings, determined from histograms at different external RR levels (Fig. 5f), is shown in Figure 5g. These analyses further showed that the reciprocal of open time was a linear function of RR concentration, with a blocking rate constant (slope, kb) of 0.015 μM−1 ms−1 (Fig. 5h). The closed times within opening bursts observed at [RR] ≥ 10 μM were dominated by blocking events; thus, the reciprocal of their mean provided an estimated unblocking rate constant (k-b) of 0.13 ms−1. Therefore, we estimated the dissociation constant, KD (k-b/kb), at −50 mV to be ~8.7 μM (Fig. 5h).
Other purported mechanosensitive membrane proteins are dispensable for Piezo1 channel activation in brain cECs
We previously characterized TRPV4 channel activity in brain cECs (Fig. 6a)28. The single-channel conductance of TRPV4 at physiological membrane potentials (45–60 pS13,30) is quite distinct from that of the Piezo1 channel reported by us (Figs. 3 and 4) and others9,17,48. Here, using the cell-attached mode and a bath solution containing a 140-mM K+ solution, we recorded single TRPV4 and Piezo1 channels in the same patch of a brain cEC following activation with Yoda1 and the TRPV4 activator GSK1016790A (hereafter, GSK101), respectively, delivered via the patch pipette. Currents at a holding potential of −50 mV exhibited two distinct opening levels: one similar to that observed in Figure 4, with a unitary current of −0.96 pA, and the other with a unitary current of −2.32 pA (Fig. 6b). The estimated conductances based on these unitary currents were 20 and 47 pS, respectively, values consistent with Piezo1 and TRPV4 conductances at this recording voltage30,48. Note that TRPV4 openings were strikingly less frequent, presumably owing to endogenous tonic inhibition of TRPV4 channels by PIP228 and possibly due to lower channel density. These different-level openings were simultaneously detected under conditions in which both Piezo1 and TRPV4 channels were pharmacologically activated (Fig. 6c), highlighting the distinct nature of TRPV4 and Piezo1 channel openings.
Fig. 6: The purported mechanosensors, TRPV4 and GqPCR, are dispensable for Piezo1 channel activation in brain cECs.

(a) Brain cECs express the Piezo1 channel, TRPV4 channel, and different GqPCRs. (b) Representative traces illustrating TRPV4 and Piezo1 currents in a brain cEC bathed in a 140-mM K+ solution, recorded in cell-attached mode with a pipette filled with a 3-mM K+ solution supplemented with Yoda1 (5 μM), GSK101 (3 μM, TRPV4 activator), and Ba2+ (100 μM). (c) Representative trace obtained using similar experimental conditions as in b in a different cEC showing overlapping openings of different unitary currents. Blue: Piezo1 channel openings and levels of openings. Orange: a TRPV4 channel opening on top of an open Piezo1 channel. (d) Scatter plots and averaged open probability (NP0) of Piezo1 channel openings in brain cECs from a) a C57BL/6J mouse in the absence (n = 8) and presence (n = 9) of Yoda1 in the pipette; b) a TRPV4-KO mouse in the presence of Yoda1 alone (n = 7) or together with GSK101 (n = 7); and c) a brain endothelial Gαq/11-KO (be-Gαq/11-KO) mouse in the presence of Yoda1 (n = 10 cECs). (e) Unitary currents (scattered data points) and calculated single-channel conductances (text next to data points; means ± SEM) under different conditions. GSK101-induced currents were observed only in C57BL/6J cECs treated with GSK101.
TRPV4 channels are sensitive to changes in osmotic pressure49 and some studies have suggested that they activate in response to shear stress15,50. However, whether TRPV4 is truly mechanosensitive is debatable, with recent evidence from heterologous expression systems arguing against a role for TRPV4 in mechanosensation51. To determine whether the mechanosensitive activation of Piezo1 is at all dependent on TRPV4 channel activity, we measured Piezo1 currents in brain cortical cECs isolated from TRPV4-knockout (TRPV4-KO) mice (Fig. 6d). Yoda1 induced Piezo1 currents in TRPV4-KO cECs in the presence or absence of GSK101 (Fig. 6d) with measured unitary currents and estimated conductance comparable to those observed in wild-type mice (Fig. 6e), indicating that TRPV4 activity is unnecessary for Piezo1 activation. Notably, GSK101 evoked TRPV4 openings in cECs from wild-type C57BL/6J mice, but not those from TRPV4-KO mice (Fig. 6e).
It has also been reported that several GqPCRs are sensitive to mechanical stimuli11,12 and thus could conceivably be involved in Piezo1 activation. To confirm that Piezo1 channel activation is independent of GqPCR signaling, we measured Yoda1-induced currents in cECs isolated from mice with tamoxifen-inducible, brain EC-specific knockout of Gαq/11 (be-Gαq/11-KO mice)52. Yoda1 evoked single-channel openings similar to those in C57BL/6J cECs, with a unitary current of −1.04 ± 0.01 pA and an estimated conductance of ~21 pS (Fig. 6e). The open probability of Yoda1-evoked currents was similar to that observed under other conditions (Fig. 6e), suggesting that functional expression of Gαq/11 is not necessary for Piezo1 activation.
Collectively, these observations indicate that TRPV4 channels and GqPCRs—both purported mechanosensors—are dispensable for Piezo1 activation in CNS capillaries.
Piezo1 channels mediate mechanically driven endothelial Ca2+ transients
Having identified functional, mechanosensitive Piezo1 currents in retinal and cortical cECs (Figs. 3 and 4), we circled back around to the original observation that motivated our investigation of mechanosensitive ion channels in CNS capillaries. We asked whether mechanically driven EC Ca2+ transients induced by changes in intravascular pressure—and thus flow and shear stress—are mediated by Piezo1 channels. To this end, we tested the effects of the Piezo1 activator Yoda1 on pressure-induced Ca2+ transients in whole Cdh5-GCaMP8 retina preparations following application of the pressure-step protocol shown in Figure 1.
Wide-field imaging of whole Cdh5-GCaMP8 retina preparations showed that cEC Ca2+ responses induced by elevating pressure at the ophthalmic artery from 20 mmHg to 80 mmHg for 60 seconds (maximum fluorescence increase: 13.8%) were profoundly enhanced by supplementing the perfusion solution with 30 μM Yoda1 (maximum fluorescence increase: 27.6%; Fig. 7a–d and movie S1-middle). Moreover, this Yoda1-induced increase in Ca2+ fluorescence persisted, consistent with the mode of action of Yoda1, which sustains open Piezo1 channels34. Yoda1 had no effect on steady-state EC Ca2+ fluorescence at 20 mmHg compared with baseline controls. Because Yoda1 was bath applied and thus acts globally (Fig. 7a–f and movie S1), the observed effects could be attributable to direct or indirect endothelial mechanisms. To distinguish between these two possibilities, we directly applied 30 μM Yoda1 focally onto a capillary of interest and imaged Ca2+ using spinning-disk confocal microscopy. In capillaries of retinas pressurized to 40 mmHg (at the ophthalmic artery), application of Yoda1 evoked increases in Ca2+ fluorescence ranging from 3.4- to 17.3-fold (mean: 9.3-fold; Fig. 7g, Suppl. Fig. 3, and movie S2). In control experiments, application of Yoda1 onto capillaries of unpressurized retinas (Fig. 7h and movie S3) or application of aCSF onto capillaries of a pressurized retina (Fig. 7i, j) caused lesser Ca2+ responses in cECs (Yoda1/0 mmHg: 3.5-fold; aCSF/40 mmHg: 2.2-fold), compared with the 9.3-fold increase observed under Yoda1/40 mmHg conditions (Fig. 7j). Along the same lines, a change in pressure from 40 to 80 mmHg evoked Ca2+ transients in cECs (movie S4) that were substantially boosted by focal application of Yoda1 onto the capillary of interest simultaneously with the pressure change (Suppl. Fig. 4 and movie S5).
Figure 7. Mechanically induced endothelial Ca2+ transients are driven by Piezo1.

(a, b) Representative micrographs of a retinal preparation from a Cdh5-GCaMP8 mouse, perfused with a solution supplemented with 30 μM Yoda1, showing Ca2+ transients at 20 mmHg (left) and after elevating intravascular pressure to 80 mmHg. (a) Whole retina preparation; (b) magnification of boxed region in a. (c) Representative micrographs of endothelial Ca2+ responses to a pressure change sequence (20→80→20 mmHg) in capillaries under control conditions and in the presence of Yoda1 (30 μM) or Yoda1+RR (10 μM). (d) Individual capillary line scans from ROIs of the micrographs shown in c. Up and down-arrows represent pressure elevation (20→80 mmHg) and reduction (80→20 mmHg), respectively. Bold lines are averaged scans from the experiment in panel c. (e) Averaged GCaMP8 fluorescence under control conditions (black) and in the presence of Yoda1 alone (blue) or Yoda1+RR (10 μM, red) (n = 3 retinal preparations from 3 Cdh5-GCaMP8 mice). (f) Scatter plots and averaged peak changes in GCaMP8 fluorescence (ΔF/F0) in response to pressure elevation from 20 to 80 mmHg under the three conditions in c (Friedman test followed by Dunn’s multiple comparisons test; n = 10 ROIs/3 retinas/3 mice). (g, h) Focal application of Yoda1 (30 μM; 3 s, 1 psi) onto retinal capillaries in a pressurized (40 mmHg; g) or unpressurized (0 mmHg; h) ex vivo retina preparation. TRITC-dextran was added to the Yoda1 solution for visualization and confirmation of ejection. (i) Ca2+ transients in different capillaries segments under different conditions. Left: Application of Yoda1 onto capillaries of a pressurized retina (baseline: 10 s; ejection: 3 s; post-ejection: 27 s). Right top: Application of Yoda1 onto unpressurized retinal capillaries. Right bottom: Application of aCSF onto pressurized retinal capillaries. Each color represents an independent Cdh5-GCaMP8 preparation. (j) Averaged scatter plots of fold-change in fluorescence under the conditions shown in i. (k) Capillary EC Ca2+ responses to a pressure-change sequence (20→80→20 mmHg) under control conditions and with application of GSK219 (1 μM) alone or together with 1 or 10 μM RR. (l) Averaged GCaMP8 fluorescence under control conditions (black) and in the presence of GSK219 (1 μM) alone (yellow) or GSK219+10 μM RR (red) (n = 3 retina preparations from 3 Cdh5-GCaMP8 mice). (m) Scatter plots and averaged peak changes in GCaMP8 fluorescence in response to pressure elevation from 20 to 80 mmHg under the three conditions shown in l (Friedman test followed by Dunn’s multiple comparisons test; n = 9 ROIs/3 retinas/3 mice).
Finally, we tested whether the increase in Ca2+ signals induced by Yoda1 was sensitive to the pore blocker, RR. In the presence of bath-applied Yoda1 (30 μM), application of RR at a concentration of 10 μM, chosen based on the RR concentration-dependent inhibition of Piezo1 currents (see Fig. 5), reduced baseline Ca2+ fluorescence at 20 mmHg (Fig. 7c–e) and significantly attenuated cEC Ca2+ responses to pressure elevation (66.8% ± 3.5% inhibition; Fig. 7c–f and movie S1-right). In the absence of Yoda1, changing pressure from 20 to 80 mmHg at the upstream ophthalmic artery triggered an increase in capillary Ca2+ transients that was profoundly inhibited by application of 10 μM RR (Fig. 7l). These transients were not altered by the selective TRPV4 inhibitor GSK219 (1 μM) or 1 μM RR—a concentration sufficient to block TRPV4 (Fig. 7k and28) but not Piezo1 (see Fig. 5 and48). Taken together, these observations point to the involvement of Piezo1 channels in sensing mechanical forces and driving Ca2+ influx and argue against the direct involvement of TRPV4 channels in pressure/flow-induced increases in EC [Ca2+].
DISCUSSION
Effective NVC evokes vascular responses that match or exceed neural demands. This process is driven by agents released from neurons and/or astrocytes onto the outer vascular wall, and ultimately leads to changes in the hemodynamic profile53. These latter intravascular changes manifest as arterial/arteriolar dilation and increases in RBC flux. While these hemodynamic changes are typically viewed as the end result of NVC, accumulating evidence has shown that changes in mechanical forces can themselves act as stimuli capable of triggering responses in sensitive biological systems—including the vasculature3,54,55. In particular, research over the past decade has demonstrated a crucial role for the Piezo1 channel in mediating such physiological functions16,56. In this study, we provide the first functional evidence for Piezo1 channel expression in the CNS capillary endothelium and further demonstrate that this channel is the crucial molecular mediator of mechanically evoked Ca2+ signals.
The first report of Piezo1 channels in the cardiovascular system revealed an indispensable role for endothelial Piezo1 channels in vascular development in utero6,7. In fact, genetic ablation of Piezo1 in mice is lethal, reflecting the crucial role of EC Piezo1 channels in sensing blood flow once the heart starts beating6,7. Subsequent studies have also demonstrated a role for the Piezo1 channel in regulating vascular tone in adulthood5,17. Different groups have studied the impact of shear stress on EC Piezo1 channels in the mesenteric circulation, reporting that shear stress activates Piezo1 and leads to the production of the potent vasodilator NO and/or changes in the membrane potential of ECs5,17. While the effect of laminar and disturbed blood flow on arterial Piezo1 channels in the peripheral circulation has been explored, little had been known about functional expression of Piezo1 in the CNS vasculature in general and in capillaries in particular. An intriguing aspect of capillaries is that their diameter is similar to the size of an RBC57,58, producing a distinctive hemodynamic profile. RBCs pass single file through capillary segments such that their transit is thought to impose frictional forces on cECs of the capillary lumen that could conceivably activate a mechanosensor such as Piezo1. In this study, we showed that a change in the forces experienced by CNS capillaries triggers cEC Ca2+ transients and an inward cationic current. However, it should be noted that changing pressure at the ophthalmic artery of the retina preparation changes pressure, flow and shear stress at the capillary level; thus, further investigations are needed to determine whether strain is also a significant activator of Piezo1 channels. Multiple lines of evidence further revealed that the Ca2+-permeable Piezo1 channel activates in response to pharmacological or mechanical stimuli, and that these effects are abolished by genetic ablation of Piezo1.
Piezo1 is a Ca2+-permeable channel that has been shown to mediate Ca2+ transients in response to physiologically relevant stimuli in arterial ECs5, vascular tip cells59, pancreatic acinar cells60, RBCs61,62, and chondrocytes14. We recently demonstrated a hierarchy of Ca2+ events in brain capillaries driven by the activity of nearby neurons22. Neuronal activity generates chemical signals in the form of receptor agonists that engage GqPCR signaling, leading to inositol 1,4,5-trisphosphate (IP3) receptor–mediated Ca2+-release events22. These latter events are enhanced by Ca2+ influx through TRPV4 channels, presumably via disinhibition of TRPV4 through GqPCR signaling-mediated depletion of PIP228. One downstream consequence of brain cEC Ca2+ signaling is Ca2+-dependent synthesis of the vasodilator NO, which relaxes contractile pericytes in initial segments of the capillary bed and selectively increases local blood flow22. Whereas GqPCR/IP3R- and TRPV4-mediated Ca2+ signals in cECs are triggered by neuronal activity, we reasoned that changes in the hemodynamic profile, such as during hyperemia, trigger Piezo1 activation and subsequent Ca2+ transients. We show in the present study that altering intravascular hemodynamics by changing pressure/flow throughout the retina vasculature leads to mechanically driven Ca2+ signals through activation of the Piezo1 channel. The impact of Piezo1-mediated Ca2+ signaling on blood flow regulation remains to be tested, but likely involves NO generation22 and local fine tuning of blood flow.
There are two foreseeable scenarios in which the potential role of capillary Piezo1 channels might come into play in the regulation of CBF. First, mechanical forces lead to Piezo1-mediated Ca2+ signals that could locally affect CBF through the generation of NO and subsequent mural cell (pericyte, SMC) relaxation. Second, as a non-selective cation channel, Piezo1 could mediate a depolarizing conductance in cECs. Conceptually, hyperemia-evoked Piezo1 activation could depolarize cECs, an action that we predict would represent a “built-in brake system” that allows membrane-potential resetting, and thus would support the repeated operation of our previously reported hyperpolarization-dependent NVC mechanism19. The two scenarios outlined here are not necessarily mutually exclusive; they could exist side by side and regulate CBF through spatially and temporally distinct mechanisms. In support of this proposition, the Ca2+/Na+-permeable TRPV4 channel seems to serve dual purposes in brain capillaries: regulating membrane potential28 and mediating Ca2+ signals22. Whether and how Piezo1 channels regulate CBF remains unclear and will require experimental manipulation of Piezo1 with concurrent monitoring of CBF, a focus of ongoing investigations.
cECs play a crucial role in NVC19–22,28,63, and the evidence presented here suggests that Piezo1-mediated mechanosensation in capillaries likely contributes to this process. Recent single-cell RNA sequencing analyses indicate that Piezo1 is highly expressed in ECs of the CNS32,33. In comparison, mural cells (SMCs and pericytes), glia (astrocytes), and neurons show little or no Piezo1 mRNA expression9,32,33,64. It is likely that ECs within the neurovascular unit (NVU), which also consists of neurons, astrocytes, SMCs and pericytes53, are the cell type that experiences dynamic mechanical forces. A limited number of studies, however, have suggested a functional role for Piezo1 in SMCs in certain vascular beds65,66, astrocytes67, and specific subsets of neurons68,69. Although Piezo1 channels are functional and crucial in the endothelium, including in CNS capillaries, further work is needed to fully understand whether other cell types in the NVU express Piezo1, and if so, whether they contribute to mechanosensation and the regulation of CBF.
The demonstration that brain capillaries sense different stimuli—not only electrical19 and chemical20,22,28, but also mechanical—is likely to have profound implications for cerebrovascular function and CBF control. Our study specifically suggests that this vascular ‘response’ can itself be a ‘trigger’, initiating a secondary cascade of events attributable to the mechanosensitivity of capillaries to changes in blood flow. The present study also raises many new questions about Piezo1 in health and disease. A growing number of human PIEZO1 mutations—both loss and gain of function—have been discovered70–72. These mutations have been linked to several pathologies73, but it remains unclear whether altered channel function affects vascular function and CBF control. Additionally, it is likely that the ability of vascular Piezo1 to modulate blood flow is altered in cardiovascular diseases where vascular hemodynamic forces are disrupted (e.g., hypertension and atherosclerosis)3,54,74,75. Whether vascular mechanosensation is compromised in diseases with a cardiovascular component, such as Alzheimer’s disease, hypertension, dementia and stroke, is similarly uncertain.
Collectively, this study provides compelling evidence that CNS capillaries express Piezo1 channels and further shows that the activity of these channels is triggered by physiologically relevant forces. Capillary Piezo1 channels can thus be envisioned as vascular mechanosensors that control signaling modalities in the brain microvasculature and thereby play an important role in CBF regulation.
Supplementary Material
NOVELTY and SIGNIFICANCE.
What Is Known?
Central nervous system (CNS) capillaries play an important role in blood flow regulation.
Brain capillaries translate external signals, released by active neurons onto the outer capillary wall, to increase blood flow.
Changes in blood flow in a narrow capillary applies mechanical forces on the vessel wall, but it is unknown whether CNS capillaries sense mechanical stimuli.
What New Information Does This Article Contribute?
CNS capillaries sense mechanical stimuli and initiate a calcium signal and an inward current.
Piezo1 channel is a mechanosensitive channel that is expressed and functional in retina and brain cortex capillaries.
Genetic or pharmacologic inhibition of endothelial Piezo1 channel suppress the ability of capillaries to sense mechanical forces.
Capillary endothelial Piezo1 activity is crucial for the translation of mechanical forces into calcium signals that could regulate blood flow.
ACKNOWLEDGMENTS
We thank D. Enders and T. Wellman for technical assistance and J. Wenzel and M. Schwaninger (University of Lübeck) for providing brain endothelial Gαq/11-knockout mice.
Sources of Funding
This study was supported by the American Heart Association (17POST33650030 and 20CDA35310097 to OFH), the Totman Medical Research Trust (to OFH and MTN); the Fondation Leducq Transatlantic Network of Excellence on the Pathogenesis of Small Vessel Disease of the Brain (to MTN); the European Union Horizon 2020 Research and Innovation Program SVDs@target under the grant agreement n° 666881 (to MTN); and grants from the National Institute of General Medical Sciences (P20-GM-135007, Vermont Center for Cardiovascular and Brain Health [to OFH and MTN]), National Institute of Neurological Disorders and Stroke and National Institute of Aging (R01-NS-110656 to MTN), National Institute of Diabetes and Digestive and Kidney Diseases (R37-DK-053832 to MTN), and the National Heart, Lung, and Blood Institute (F32-HL-152576 to NRK; R01-HL-121706, 7UM- HL-1207704, R01-HL-131181, and R35-HL-140027 [to MTN]). The authors acknowledge the support and guidance of the staff and investigators of the Vermont Center for Cardiovascular and Brain Health.
Nonstandard Abbreviations and Acronyms
- aCSF
artificial cerebrospinal fluid
- be-Gαq/11-KO
brain endothelial specific Gαq/11 knockout mouse
- Cdh5
cadherin 5
- Cdh5-GCaMP8
green calcium fluorescence indicator under control of Cadherin 5 promoter
- cECs
Capillary endothelial cells
- CNS
Central nervous system
- ECs
Endothelial cells
- GqPCRs
Gq protein-coupled receptors
- mmHg
millimeter mercury
- NO
Nitric oxide
- NOS
Nitric oxide synthase
- NPO
open probability
- NVC
neurovascular coupling
- NVU
neurovascular unit
- Piezo1-EC-Ctrl
endothelial cell specific Piezo1 control mouse
- Piezo1-EC-KO
endothelial cell specific Piezo1 knockout mouse
- PIP2
phosphatidylinositol 4,5-bisphosphate
- RBC
red blood cell
- CBF
cerebral blood flow
- ROI
region of interest
- RR
ruthenium red
- SMC
smooth muscle cell
- TRPV4
transient receptor potential vanilloid 4 channels
Footnotes
CONFLICT OF INTEREST
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
REFERENCES
- 1.Dessalles C, Leclech C, Castagnino A, Barakat A. Integration of substrate- and flow-derived stresses in endothelial cell mechanobiology. Commun Biol 2021;4. doi: 10.1038/S42003-021-02285-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hahn C, Schwartz MA. Mechanotransduction in vascular physiology and atherogenesis. Nat Rev Mol Cell Biol 2009;10:53–62. doi: 10.1038/nrm2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Resnick N, Gimbrone MA. Hemodynamic forces are complex regulators of endothelial gene expression. FASEB J 1995;9:874–882. doi: 10.1096/fasebj.9.10.7615157. [DOI] [PubMed] [Google Scholar]
- 4.Reneman RS, Hoeks APG. Wall shear stress as measured in vivo: Consequences for the design of the arterial system. Med Biol Eng Comput 2008;46:499–507. doi: 10.1007/s11517-008-0330-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang S, Chennupati R, Kaur H, Iring A, Wettschureck N, Offermanns S. Endothelial cation channel PIEZO1 controls blood pressure by mediating flow-induced ATP release. J Clin Invest 2016;126:4527–4536. doi: 10.1172/JCI87343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ranade S, Qiu Z, Woo S-H, Hur S, Murthy S, Cahalan S, Xu J, Mathur J, Bandell M, Coste B, et al. Piezo1, a mechanically activated ion channel, is required for vascular development in mice. Proc Natl Acad Sci 2014;111:10347–10352. doi: 10.1073/pnas.1409233111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li J, Hou B, Tumova S, Muraki K, Bruns A, Ludlow M, Sedo A, Hyman AJ, McKeown L, Young RS, et al. Piezo1 integration of vascular architecture with physiological force. Nature 2014;515:279–282. doi: 10.1038/nature13701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ranade SS, Syeda R, Patapoutian A. Mechanically Activated Ion Channels. Neuron 2015;87:1162–1179. doi: 10.1016/J.NEURON.2015.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coste B, Mathur J, Schmidt M, Earley TJ, Ranade S, Petrus MJ, Dubin AE, Patapoutian A. Piezo1 and Piezo2 Are Essential Components of Distinct Mechanically Activated Cation Channels. Science (80- ) 2010;330:55–60. doi: 10.1126/science.1193270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zou Y, Akazawa H, Qin Y, Sano M, Takano H, Minamino T, Makita N, Iwanaga K, Zhu W, Kudoh S, et al. Mechanical stress activates angiotensin II type 1 receptor without the involvement of angiotensin II. Nat Cell Biol 2004;6:499–506. doi: 10.1038/ncb1137. [DOI] [PubMed] [Google Scholar]
- 11.y Schnitzler MM, Storch U, Meibers S, Nurwakagari P, Breit A, Essin K, Gollasch M, Gudermann T. Gq-coupled receptors as mechanosensors mediating myogenic vasoconstriction. EMBO J 2008;27:3092–3103. doi: 10.1038/emboj.2008.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu J, Mathur J, Vessières E, Hammack S, Nonomura K, Favre J, Grimaud L, Petrus M, Francisco A, Li J, et al. GPR68 Senses Flow and Is Essential for Vascular Physiology. Cell 2018;173:762–775.e16. doi: 10.1016/j.cell.2018.03.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Loukin S, Zhou X, Su Z, Saimi Y, Kung C. Wild-type and Brachyolmia-causing Mutant TRPV4 Channels Respond Directly to Stretch Force. J Biol Chem 2010;285:27176–27181. doi: 10.1074/jbc.M110.143370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rocio Servin-Vences M, Moroni M, Lewin GR, Poole K. Direct measurement of TRPV4 and PIEZO1 activity reveals multiple mechanotransduction pathways in chondrocytes. Elife 2017;6. doi: 10.7554/eLife.21074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hartmannsgruber V, Heyken W-T, Kacik M, Kaistha A, Grgic I, Harteneck C, Liedtke W, Hoyer J, Köhler R. Arterial response to shear stress critically depends on endothelial TRPV4 expression. PLoS One 2007;2:e827. doi: 10.1371/journal.pone.0000827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murthy SE, Dubin AE, Patapoutian A. Piezos thrive under pressure: mechanically activated ion channels in health and disease. Nat Rev Mol Cell Biol 2017;18:771–783. doi: 10.1038/nrm.2017.92. [DOI] [PubMed] [Google Scholar]
- 17.Rode B, Shi J, Endesh N, Drinkhill MJ, Webster PJ, Lotteau SJ, Bailey MA, Yuldasheva NY, Ludlow MJ, Cubbon RM, et al. Piezo1 channels sense whole body physical activity to reset cardiovascular homeostasis and enhance performance. Nat Commun 2017;8:350. doi: 10.1038/s41467-017-00429-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blinder P, Tsai PS, Kaufhold JP, Knutsen PM, Suhl H, Kleinfeld D. The cortical angiome: an interconnected vascular network with noncolumnar patterns of blood flow. Nat Neurosci 2013;16:889–897. doi: 10.1038/nn.3426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Longden TA, Dabertrand F, Koide M, Gonzales AL, Tykocki NR, Brayden JE, Hill-Eubanks D, Nelson MT. Capillary K+-sensing initiates retrograde hyperpolarization to increase local cerebral blood flow. Nat Neurosci 2017;20:717–726. doi: 10.1038/nn.4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harraz OF, Longden TA, Dabertrand F, Hill-Eubanks D, Nelson MT. Endothelial GqPCR activity controls capillary electrical signaling and brain blood flow through PIP2 depletion. Proc Natl Acad Sci U S A 2018;115:E3569–E3577. doi: 10.1073/pnas.1800201115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dabertrand F, Harraz OF, Koide M, Longden TA, Rosehart AC, Hill-Eubanks DC, Joutel A, Nelson MT. PIP2 corrects cerebral blood flow deficits in small vessel disease by rescuing capillary Kir2.1 activity. Proc Natl Acad Sci U S A 2021;118. doi: 10.1073/PNAS.2025998118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Longden TA, Mughal A, Hennig GW, Harraz OF, Shui B, Lee FK, Lee JC, Reining S, Kotlikoff MI, König GM, et al. Local IP3 receptor-mediated Ca2+ signals compound to direct blood flow in brain capillaries. Sci Adv 2021;7. doi: 10.1126/SCIADV.ABH0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ratelade J, Klug NR, Lombardi D, Angelim MKSC, Dabertrand F, Domenga-Denier V, Al-Shahi Salman R, Smith C, Gerbeau JF, Nelson MT, et al. Reducing Hypermuscularization of the Transitional Segment between Arterioles and Capillaries Protects against Spontaneous Intracerebral Hemorrhage. Circulation 2020:2078–2094. doi: 10.1161/CIRCULATIONAHA.119.040963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gonzales AL, Klug NR, Moshkforoush A, Lee JC, Lee FK, Shui B, Tsoukias NM, Kotlikoff MI, Hill-Eubanks D, Nelson MT. Contractile pericytes determine the direction of blood flow at capillary junctions. Proc Natl Acad Sci U S A 2020;117:27022–27033. doi: 10.1073/PNAS.1922755117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.London A, Benhar I, Schwartz M. The retina as a window to the brain-from eye research to CNS disorders. Nat Rev Neurol 2013;9:44–53. doi: 10.1038/NRNEUROL.2012.227. [DOI] [PubMed] [Google Scholar]
- 26.Ratelade J, Mezouar N, Domenga-Denier V, Rochey A, Plaisier E, Joutel A. Severity of arterial defects in the retina correlates with the burden of intracerebral haemorrhage in COL4A1-related stroke. J Pathol 2018;244:408–420. doi: 10.1002/PATH.5023. [DOI] [PubMed] [Google Scholar]
- 27.Metea M, Newman E. Signalling within the neurovascular unit in the mammalian retina. Exp Physiol 2007;92:635–640. doi: 10.1113/EXPPHYSIOL.2006.036376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harraz OF, Longden TA, Hill-Eubanks D, Nelson MT. PIP2 depletion promotes TRPV4 channel activity in mouse brain capillary endothelial cells. Elife 2018;7. doi: 10.7554/eLife.38689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sonkusare SK, Bonev AD, Ledoux J, Liedtke W, Kotlikoff MI, Heppner TJ, Hill-Eubanks DC, Nelson MT. Elementary Ca2+ signals through endothelial TRPV4 channels regulate vascular function. Science 2012;336:597–601. doi: 10.1126/science.1216283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Watanabe H, Vriens J, Suh SH, Benham CD, Droogmans G, Nilius B. Heat-evoked Activation of TRPV4 Channels in a HEK293 Cell Expression System and in Native Mouse Aorta Endothelial Cells. J Biol Chem 2002;277:47044–47051. doi: 10.1074/jbc.M208277200. [DOI] [PubMed] [Google Scholar]
- 31.John L, Ko NL, Gokin A, Gokina N, Mandalà M, Osol G. The Piezo1 cation channel mediates uterine artery shear stress mechanotransduction and vasodilation during rat pregnancy. Am J Physiol Heart Circ Physiol 2018;315:H1019–H1026. doi: 10.1152/ajpheart.00103.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vanlandewijck M, He L, Mäe MA, Andrae J, Ando K, Del Gaudio F, Nahar K, Lebouvier T, Laviña B, Gouveia L, et al. A molecular atlas of cell types and zonation in the brain vasculature. Nature 2018;554:475–480. doi: 10.1038/nature25739. [DOI] [PubMed] [Google Scholar]
- 33.Saunders A, Macosko EZ, Wysoker A, Goldman M, Krienen FM, de Rivera H, Bien E, Baum M, Bortolin L, Wang S, et al. Molecular Diversity and Specializations among the Cells of the Adult Mouse Brain. Cell 2018;174:1015–1030.e16. doi: 10.1016/j.cell.2018.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Syeda R, Xu J, Dubin AE, Coste B, Mathur J, Huynh T, Matzen J, Lao J, Tully DC, Engels IH, et al. Chemical activation of the mechanotransduction channel Piezo1. Elife 2015;4. doi: 10.7554/eLife.07369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ermakov YA, Kamaraju K, Sengupta K, Sukharev S. Gadolinium Ions Block Mechanosensitive Channels by Altering the Packing and Lateral Pressure of Anionic Lipids. Biophys J 2010;98:1018. doi: 10.1016/J.BPJ.2009.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang X-C, Sachs F Block of Stretch-Activated Ion Channels in Xenopus Oocytes by Gadolinium and Calcium Ions. Science (80- ) 1989;243:1068–1071. doi: 10.1126/SCIENCE.2466333. [DOI] [PubMed] [Google Scholar]
- 37.Caldwell RA, Clemo HF, Baumgarten CM. Using gadolinium to identify stretch-activated channels: technical considerations. Https://DoiOrg/101152/Ajpcell19982752C619 1998;275. doi: 10.1152/AJPCELL.1998.275.2.C619. [DOI] [PubMed] [Google Scholar]
- 38.Bae C, Sachs F, Gottlieb P. The mechanosensitive ion channel Piezo1 is inhibited by the peptide GsMTx4. Biochemistry 2011;50:6295–6300. doi: 10.1021/BI200770Q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li H, Xu J, Shen Z-S, Wang G-M, Tang M, Du X-R, Lv Y-T, Wang J-J, Zhang F-F, Qi Z, et al. The neuropeptide GsMTx4 inhibits a mechanosensitive BK channel through the voltage-dependent modification specific to mechano-gating. J Biol Chem 2019;294:11892–11909. doi: 10.1074/JBC.RA118.005511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gnanasambandam R, Ghatak C, Yasmann A, Nishizawa K, Sachs F, Ladokhin AS, Sukharev SI, Suchyna TM. GsMTx4: Mechanism of Inhibiting Mechanosensitive Ion Channels. Biophys J 2017;112:31. doi: 10.1016/J.BPJ.2016.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Broekemeier K, Krebsbach R, Pfeiffer D. Inhibition of the mitochondrial Ca2+ uniporter by pure and impure ruthenium red. Mol Cell Biochem 1994;139:33–40. doi: 10.1007/BF00944201. [DOI] [PubMed] [Google Scholar]
- 42.Cibulsky SM, Sather WA. Block by ruthenium red of cloned neuronal voltage-gated calcium channels. J Pharmacol Exp Ther 1999;289:1447–1453. [PubMed] [Google Scholar]
- 43.Hamilton M, Lundy P. Effect of ruthenium red on voltage-sensitive Ca++ channels. J Pharmacol Exp Ther 1995;273:940–947. [PubMed] [Google Scholar]
- 44.Xu L, Tripathy A, Pasek D, Meissner G. Ruthenium red modifies the cardiac and skeletal muscle Ca(2+) release channels (ryanodine receptors) by multiple mechanisms. J Biol Chem 1999;274:32680–32691. doi: 10.1074/JBC.274.46.32680. [DOI] [PubMed] [Google Scholar]
- 45.Güler AD, Lee H, Iida T, Shimizu I, Tominaga M, Caterina M. Heat-evoked activation of the ion channel, TRPV4. J Neurosci 2002;22:6408–6414. doi: 10.1523/jneurosci.22-15-06408.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Phan MN, Leddy HA, Votta BJ, Kumar S, Levy DS, Lipshutz DB, Lee SH, Liedtke W, Guilak F. Functional characterization of TRPV4 as an osmotically sensitive ion channel in porcine articular chondrocytes. Arthritis Rheum 2009;60:3028–3037. doi: 10.1002/ART.24799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ding XL, Wang YH, Ning LP, Zhang Y, Ge HY, Jiang H, Wang R, Yue S-W. Involvement of TRPV4-NO-cGMP-PKG pathways in the development of thermal hyperalgesia following chronic compression of the dorsal root ganglion in rats. Behav Brain Res 2010;208:194–201. doi: 10.1016/J.BBR.2009.11.034. [DOI] [PubMed] [Google Scholar]
- 48.Coste B, Xiao B, Santos J, Syeda R, Grandl J, Spencer K, Kim SE, Schmidt M, Mathur J, Dubin AE, et al. Piezo proteins are pore-forming subunits of mechanically activated channels. Nature 2012;483:176–181. doi: 10.1038/NATURE10812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liedtke W, Choe Y, Martí-Renom MA, Bell AM, Denis CS, Sali A, Hudspeth AJ, Friedman JM, Heller S. Vanilloid receptor-related osmotically activated channel (VR-OAC), a candidate vertebrate osmoreceptor. Cell 2000;103:525–535. doi: 10.1016/s0092-8674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mendoza SA, Fang J, Gutterman DD, Wilcox DA, Bubolz AH, Li R, Suzuki M, Zhang DX. TRPV4-mediated endothelial Ca2+ influx and vasodilation in response to shear stress. Https://DoiOrg/101152/Ajpheart008542009 2010;298:466–476. doi: 10.1152/AJPHEART.00854.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nikolaev YA, Cox CD, Ridone P, Rohde PR, Cordero-Morales JF, Vásquez V, Laver DR, Martinac B. Mammalian TRP ion channels are insensitive to membrane stretch. J Cell Sci 2019;132. doi: 10.1242/JCS.238360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wenzel J, Hansen CE, Bettoni C, Vogt MA, Lembrich B, Natsagdorj R, Huber G, Brands J, Schmidt K, Assmann JC, et al. Impaired endothelium-mediated cerebrovascular reactivity promotes anxiety and respiration disorders in mice. Proc Natl Acad Sci 2020;117:1753–1761. doi: 10.1073/PNAS.1907467117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Iadecola C The Neurovascular Unit Coming of Age: A Journey through Neurovascular Coupling in Health and Disease. Neuron 2017;96:17–42. doi: 10.1016/j.neuron.2017.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gimbrone MA. Vascular Endothelium, Hemodynamic Forces, and Atherogenesis. Am J Pathol 1999;155:1–5. doi: 10.1016/S0002-9440(10)65090-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hamill OP, Martinac B. Molecular Basis of Mechanotransduction in Living Cells. Https://DoiOrg/101152/Physrev2001812685 2001;81:685–740. doi: 10.1152/PHYSREV.2001.81.2.685. [DOI] [PubMed] [Google Scholar]
- 56.Beech DJ, Kalli AC. Force Sensing by Piezo Channels in Cardiovascular Health and Disease. Arterioscler Thromb Vasc Biol 2019:ATVBAHA119313348. doi: 10.1161/ATVBAHA.119.313348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Snyder G, Sheafor B. Red Blood Cells: Centerpiece in the Evolution of the Vertebrate Circulatory System(1). Am Zool 1999;39:189–189. [Google Scholar]
- 58.Krogh A The supply of oxygen to the tissues and the regulation of the capillary circulation. J Physiol 1919;52:457–474. doi: 10.1113/JPHYSIOL.1919.SP001844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu T ting, Du X fei, Zhang B bing, Zi H xing, Yan Y, Yin J an, Hou H, Gu S-Y, Chen Q, Du J-L. Piezo1-Mediated Ca2+ Activities Regulate Brain Vascular Pathfinding during Development. Neuron 2020;108:180–192.e5. doi: 10.1016/J.NEURON.2020.07.025. [DOI] [PubMed] [Google Scholar]
- 60.Swain SM, Romac JM-J, Shahid RA, Pandol SJ, Liedtke W, Vigna SR, Liddle RA. TRPV4 channel opening mediates pressure-induced pancreatitis initiated by Piezo1 activation. J Clin Invest 2020;130:2527–2541. doi: 10.1172/JCI134111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Danielczok JG, Terriac E, Hertz L, Petkova-Kirova P, Lautenschläger F, Laschke MW, Kaestner L. Red Blood Cell Passage of Small Capillaries Is Associated with Transient Ca2+-mediated Adaptations. Front Physiol 2017;0:979. doi: 10.3389/FPHYS.2017.00979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cahalan SM, Lukacs V, Ranade SS, Chien S, Bandell M, Patapoutian A. Piezo1 links mechanical forces to red blood cell volume. Elife 2015;4. doi: 10.7554/eLife.07370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Thakore P, Alvarado MG, Ali S, Mughal A, Pires PW, Yamasaki E, Pritchard HAT, Isakson BE, Tran CHT, Earley S. Brain endothelial cell trpa1 channels initiate neurovascular coupling. Elife 2021;10:1–84. doi: 10.7554/ELIFE.63040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang Y, Chen K, Sloan SA, Bennett ML, Scholze AR, O’Keeffe S, Phatnani HP, Guarnieri P, Caneda C, Ruderisch N, et al. An RNA-Sequencing Transcriptome and Splicing Database of Glia, Neurons, and Vascular Cells of the Cerebral Cortex. J Neurosci 2014;34:11929. doi: 10.1523/JNEUROSCI.1860-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liao J, Lu W, Chen Y, Duan X, Zhang C, Luo X, Lin Z, Chen J, Liu S, Yan H, et al. Upregulation of Piezo1 (Piezo Type Mechanosensitive Ion Channel Component 1) Enhances the Intracellular Free Calcium in Pulmonary Arterial Smooth Muscle Cells From Idiopathic Pulmonary Arterial Hypertension Patients. Hypertension 2021;77:1974–1989. doi: 10.1161/HYPERTENSIONAHA.120.16629. [DOI] [PubMed] [Google Scholar]
- 66.Retailleau K, Duprat F, Arhatte M, Ranade SS, Peyronnet R, Martins JR, Jodar M, Moro C, Offermanns S, Feng Y, et al. Piezo1 in Smooth Muscle Cells Is Involved in Hypertension-Dependent Arterial Remodeling. Cell Rep 2015;13:1161–1171. doi: 10.1016/J.CELREP.2015.09.072. [DOI] [PubMed] [Google Scholar]
- 67.Velasco-Estevez M, Rolle SO, Mampay M, Dev KK, Sheridan GK. Piezo1 regulates calcium oscillations and cytokine release from astrocytes. Glia 2020;68:145–160. doi: 10.1002/GLIA.23709. [DOI] [PubMed] [Google Scholar]
- 68.Wang J, La J-H, Hamill OP. PIEZO1 Is Selectively Expressed in Small Diameter Mouse DRG Neurons Distinct From Neurons Strongly Expressing TRPV1. Front Mol Neurosci 2019;0:178. doi: 10.3389/FNMOL.2019.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zeng W-Z, Marshall KL, Min S, Daou I, Chapleau MW, Abboud FM, Liberles SD, Patapoutian A. PIEZOs mediate neuronal sensing of blood pressure and the baroreceptor reflex. Science (80- ) 2018;362:464–467. doi: 10.1126/SCIENCE.AAU6324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Albuisson J, Murthy SE, Bandell M, Coste B, Louis-dit-Picard H, Mathur J, Fénéant-Thibault M, Tertian G, de Jaureguiberry J-P, Syfuss P-Y, et al. Dehydrated hereditary stomatocytosis linked to gain-of-function mutations in mechanically activated PIEZO1 ion channels. Nat Commun 2013 41 2013;4:1–9. doi: 10.1038/ncomms2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ma S, Cahalan S, LaMonte G, Grubaugh ND, Zeng W, Murthy SE, Paytas E, Gamini R, Lukacs V, Whitwam T, et al. Common PIEZO1 Allele in African Populations Causes RBC Dehydration and Attenuates Plasmodium Infection. Cell 2018;173:443–455.e12. doi: 10.1016/j.cell.2018.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lukacs V, Mathur J, Mao R, Bayrak-Toydemir P, Procter M, Cahalan SM, Kim HJ, Bandell M, Longo N, Day RW, et al. Impaired PIEZO1 function in patients with a novel autosomal recessive congenital lymphatic dysplasia. Nat Commun 2015 61 2015;6:1–7. doi: 10.1038/ncomms9329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Alper SL. Genetic Diseases of PIEZO1 and PIEZO2 Dysfunction. Curr Top Membr 2017;79:97–134. doi: 10.1016/BS.CTM.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 74.Malek AM, Alper SL, Izumo S. Hemodynamic Shear Stress and Its Role in Atherosclerosis. JAMA 1999;282:2035–2042. doi: 10.1001/JAMA.282.21.2035. [DOI] [PubMed] [Google Scholar]
- 75.Lund-Johansen P Haemodynamics in Essential Hypertension. Clin Sci 1980;59:343s–354s. doi: 10.1042/CS059343S. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and supporting information.


