Abstract
Effector Th17 cells, including IFN-γ-IL-17+ (eTh17) and IFN-γ+IL-17+ (eTh17/1) subsets, play critical pathogenic functions in the induction of autoimmunity. As acute inflammation subsides, a small proportion of the effectors survive and convert to memory Th17 cells (mTh17), which sustain chronic inflammation in autoimmune diseases. Herein, we investigated the differential contributions of eTh17 versus eTh17/1 to the memory pool using an experimental model of ocular autoimmune disease. Our results show that adoptive transfer of Tbx21−/− CD4+ T cells or conditional deletion of Tbx21 in Th17 cells leads to diminished eTh17/1 in acute phase and functionally compromised mTh17 in chronic phase. Further, adoptive transfer of disease-specific eTh17/1, but not eTh17, leads to generation of mTh17 and sustained ocular inflammation. Collectively, our data demonstrate that T-bet-dependent eTh17/1 cells generated during the acute inflammation are the principle effector precursors of pathogenic mTh17 cells that sustain the chronicity of autoimmune inflammation.
Keywords: memory Th17, effector Th17/1, T-bet
1. INTRODUCTION
Long-lived memory T cells pose a unique capacity of mounting a faster and augmented immune response upon re-exposure to the same antigen [1,2]. This memory response can effectively protect the host from reinfections, and is the basis of vaccination [1,2]. On the other hand, accumulating evidence suggests that memory T cell-mediated response can also be harmful by driving chronic inflammation and autoimmune disease [2–5]. In the past decade, much advance has been achieved in understanding the epigenetic regulation, heterogeneity, homing properties, and function of memory T cells; however, the process of their generation largely remains a puzzle, particularly for the CD4+ T cell compartment, which is of paramount importance for design of vaccines in infections and effective immunotherapies in autoimmune disease.
CD4+ T helper (Th) cells are highly heterogeneous, and various subsets including Th1, Th2, Th9, Th17, Th22 and T follicular helper cells, have been characterized by their respective expression of signature cytokines and master transcription factors [6–8]. Among them, Th17 cells, which express the signature cytokine IL-17 regulated by the transcription factor retinoic acid receptor-related orphan receptor-γt (RORγt), have been recently demonstrated to play principal pathogenic function in autoimmune diseases, evidenced by the critical roles of memory Th17 (mTh17) cells in maintaining the chronic inflammation in multiple sclerosis, rheumatic arthritis, psoriasis and ocular surface autoimmune disease [2–4,9]. We have recently revealed that the long-term survival of these autoreactive mTh17 cells requires environmental IL-7 and IL-15 signaling both at target tissue and in local draining lymphoid compartment, and their generation follows the linear effector-to-memory pathway [2,10,11], a process that is reciprocally regulated by environmental IL-2 and IL-23 signaling during the contraction phase of the primary response [12]. However, it has remained unclear whether there is any specific subset of effector cells poised to enter the memory pool while the others are destined to apoptosis during the contraction phase; or whether simply a small portion of effector cells randomly differentiate into memory cells [13]. Interestingly, the preponderance of evidence has demonstrated high plasticity of effector Th17 cells. For example, Th17 cells can express the phenotypes of other Th subsets under specific conditions, such as the IFN-γ+ Th1-like phenotype termed effector Th17/1 (eTh17/1) cells. In autoimmune ocular surface disease, we have shown two effector Th17 subsets – CD4+IFN-γ-IL-17+ (“single-positive” eTh17) and CD4+IFN-γ+IL-17+ (“double-positive” eTh17/1) cells critically participating in the initial acute inflammation and subsequent flares in chronic phase [14]. Given that IFN-γ expression in T cells is principally driven by the transcription factor T-bet, which is implicated in the development of memory B cells, memory CD8+ T, and memory Th1 cells [15–17], along with that T-bet-expressing cells have better survival ability than RORγt-expressing cells during the contraction phase [13,18], we hypothesized that eTh17 and eTh17/1 subsets that expand in acute inflammation differentially contribute to the subsequent formation of mTh17 pool in autoimmunity.
In this study, we demonstrate that mTh17 cells express T-bet, at a lower level, as eTh17/1 do, and naïve CD4+ T cells lacking T-bet or IL17+CD4+ T cells with conditional deletion of T-bet fail to develop into eTh17/1 cells and pathogenic mTh17 cells, along with the loss of the ability to induce chronic inflammation. Furthermore, eTh17/1 cells show significantly superior abilities over eTh17 to form memory Th17 both in vitro and in vivo. Thus, our findings expand our current understanding of Th17-mediated chronic autoimmune inflammation by depicting the specific effector subset that is preferably programmed to become long-lived memory Th17 cells.
2. MATERIALS AND METHODS
2.1. Animals
Female 6- to 8-wk-old wild-type (WT) C57BL/6 mice (Charles River Laboratories), B6.Rag1−/− mice, and B6.Tbx21−/− mice (Jackson Laboratory) were used for this study. B6.IL-17Acre mice and B6.Tbx21F/F mice (Jackson Laboratory) were crossbred in house to generate T-bet conditional knockout IL-17AcreTbx21F/F (IL-17AΔTbx21) mice. All transgenic mice were maintained in specific pathogen-free conditions. Mutant genes were confirmed using PCR conducted by Transnetyx, and the same littermates which possess loxP sites flanking of Tbx21 were used as control (Tbx21F/F). All animal experiments were approved by the Schepens Eye Research Institute Animal Care and Use Committee and adhered to the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research.
2.2. Murine model of autoimmune dry eye disease (DED)
Mice were placed in a controlled-environment chamber without scopolamine injection with a relative humidity<15%, airflow of 15 L/min and a constant temperature of 21–23 °C for 14 consecutive days (acute DED). Thereafter, mice were transferred to a standard non-desiccated vivarium for an extended period up to 5–6 weeks (chronic DED). For rechallenge experiments, chronic DED mice were placed back into the controlled-environment chamber for additional 6 days [12,19]. Disease severity was observed by an investigator who was masked to the grouping, and evaluated using fluorescein (Sigma-Aldrich) staining and scored using the National Eye Institute (Bethesda, MD) grading system, giving a score from 0 to 3 for each of the five areas of the cornea [20].
2.3. In vitro generation of memory Th17 cells from effector Th17 and Th17/1 cells
Effector Th17 (CD4+IL-17+IFN-γ-) and Th17/1 (CD4+IL-17+IFN-γ+) cells were sorted from acute DED animals, as we have previously described [14]. In brief, CD4+ T cells were first enriched using a CD4+ T cell isolation kit (Miltenyi Biotec), and then Th17 and Th17/1 cells were stained and sorted using IL-17 and IFN-γ cytokine secretion assay kits (Miltenyi Biotec) and a BD FACSAria sorter (BD Bioscience). Each subset of cells were cultured with IL-23 (1μg/ml) for 48 hours [12]. The generation of mTh17 cells were examined using flow cytometry.
2.4. In vitro antigen-specific re-activation of memory T cells
CD44hi and CD44low subsets of CD4+ T cells were sorted from the draining lymph nodes (DLN) of chronic DED, and in vitro stimulated with the cognate corneal antigens (100 μg/ml) for 18 hours. The corneal antigens were tissue homogenates prepared from the corneal tissues that were harvested from animals housed in a sterile cage and fed with sterile water and food, and the tissue collection and homogenization was performed using sterile techniques to avoid induction of any foreign antigens. The activation profiles were evaluated on T cells using flow cytometry-based CD154 activation assay [21,22], as well as on the culture supernatants for secreted IL-17 and IFN-γ levels using commercially available ELISA kits (eBioscience).
2.5. T cell Adoptive transfer
DLN cells from naive Tbx21−/− or wild-type C57BL/6 mice were harvested and naïve CD4+ T cells were isolated via negative selection with a naïve CD4+ T cell isolation kit (Miltenyi Biotec). 1 × 106 naïve CD4+ T cells were injected i.v. into naïve B6.Rag1−/− mice, which were then immediately placed in the controlled-environment chamber for 14 d. In addition, 2 × 104 mTh17 cells isolated from DLN of IL-17AΔTbx21 chronic DED mice (using the similar sorting methods described in 2.3) were injected i.v. into B6. Rag1−/− mice which were subsequently exposed to desiccating stress for 7 days. Furthermore, 2 × 104 eTh17 or eTh17/1 cells isolated from DLN of wild-type acute DED mice (as described in 2.3) were injected i.v. into B6.Rag1−/− mice which had been exposed to desiccating stress for 2 weeks. After adoptive transfer, Rag1−/− mice were placed in normal vivarium and were injected i.p. with 100 of anti-IL-23 Ab (R&D System) or 100 of isotype IgG (R&D System) immediately and 2 d after cell transfer.
2.6. Flow cytometry analysis
Eye-draining lymph nodes and conjunctival tissues were collected and single-cell suspensions were prepared. Conjunctival tissues were first digested in RPMI 1640 (Invitrogen) with 2 mg/ml DNase I and 2 mg/ml collagenase type IV (Roche) at 37 °C. The following antibodies were used: FITC-conjugated or Brilliant Violet 421-conjugated anti-CD4 (Biolegend), APC-conjugated anti-IL-17, FITC- conjugated anti-IFN-γ, PE-conjugated anti-RORγt, PE-conjugated anti-T-bet, PE-conjugated anti-CD154 (all from eBioscience), and PE-conjugated anti-IL-23R (R&D System). For intracellular cytokine staining, cells were first stimulated with PMA plus inomycin (Sigma-Aldrich) for 5 h at 37°C and 5% CO2 in the presence of GolgiStop (BD Biosciences). All the Abs with their matched isotype control and fix-perm buffer were purchased from Thermo Fisher Scientific. Stained cells were examined with an LSR II flow cytometer (BD Biosciences), and the results were analyzed using FlowJo software (Tree Star).
2.7. Statistical analysis
Statistical analysis was performed using the Statistical Package of Social Sciences software (IBM SPSS, version 22). For comparison of multiple groups, ANOVA followed by Bonferroni’s multiple comparisons post hoc test was used to analyze the statistical significance. For comparison of two groups, an unpaired, two-tailed Student’s t test was used for statistical analysis. Results are presented as mean ± SEM. P < 0.05 was considered significant.
3. RESULTS
3.1. Memory Th17 cells express transcriptional factor T-bet
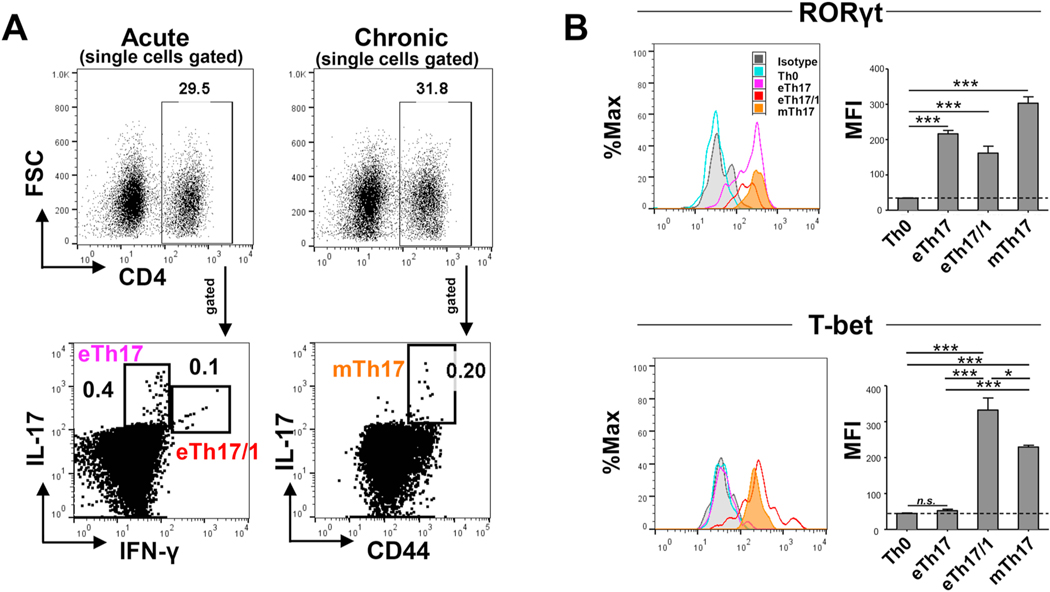
The master transcription factors determining T helper cell lineage fate critically regulate CD4+ T cell differentiation and function, including RORγt critical for promoting Th17 differentiation, and T-bet for promoting Th1 differentiation [6]. Our recent work has demonstrated effector Th17 (eTh17, IFN-γ-IL-17+) and Th17/1 (eTh17/1, IFN-γ+IL-17+) in inducing acute ocular surface inflammation [14], and subsequently a small portion of these effectors (without differentiation of eTh17 vs. eTh17/1) enter memory pool and become mTh17 that maintain the chronic ocular surface inflammation [12]. Thus, using the same murine model of DED, we first delineated the expression patterns of RORγt and T-bet in eTh17, eTh17/1 and mTh17 cells. As expected, eTh17 and eTh17/1 from acute DED, and mTh17 cells from chronic DED all expressed high levels of RORγt, and eTh17/1 cells additionally express high levels of T-bet while eTh17 cells do not. Interestingly, although mTh17 cells from chronic DED primarily express IL-17 but not IFN-γ [4], they do express T-bet, at a lower level than eTh17/1 (Fig. 1). These data demonstrate the closer similarity in expression of T cell lineage transcription factors between eTh17/1 and mTh17 cells.
Figure 1. Expression of transcription factors by effector Th17 (including eTh17 and eTh17/1, CD44lowIL-17+CD4+) and memory Th17 (mTh17, CD44hiIL-17+CD4+) cells in autoimmune inflammation.
(A) Mice were exposed to desiccating stress for 14 d to induce acute dry eye disease (DED), and eye-draining lymph nodes (DLNs) were collected and analyzed for eTh17 (IFN-γ-IL-17+) and eTh17/1 (IFN-γ+IL-17+) cells. In addition, another group of mice subjected to 14 d desiccating stress were returned to normal vivarium for additional 14 d to induce chronic DED, in which DLNs were collected and analyzed for mTh17 (CD44hiIL-17+). (B) Further analysis of DED-derived eTh17, eTh17/1 and mTh17 cells in (A) for their expression levels of Th17 and Th1 master transcription factors RORγt and T-bet, respectively. Representative histograms shown on the left and summary graphs of mean fluorescein intensities (MFI) are exhibited on the right. Naïve Th0 cells serve as the baseline control. Data shown are mean ± SEM from one representative experiment (n = 4–5/group) out of two performed. *, p<0.05; ***, p<0.001. n.s., not significant.
3.2. T-bet is required for naïve CD4+ T cells to differentiate into eTh17/1 in autoimmune DED
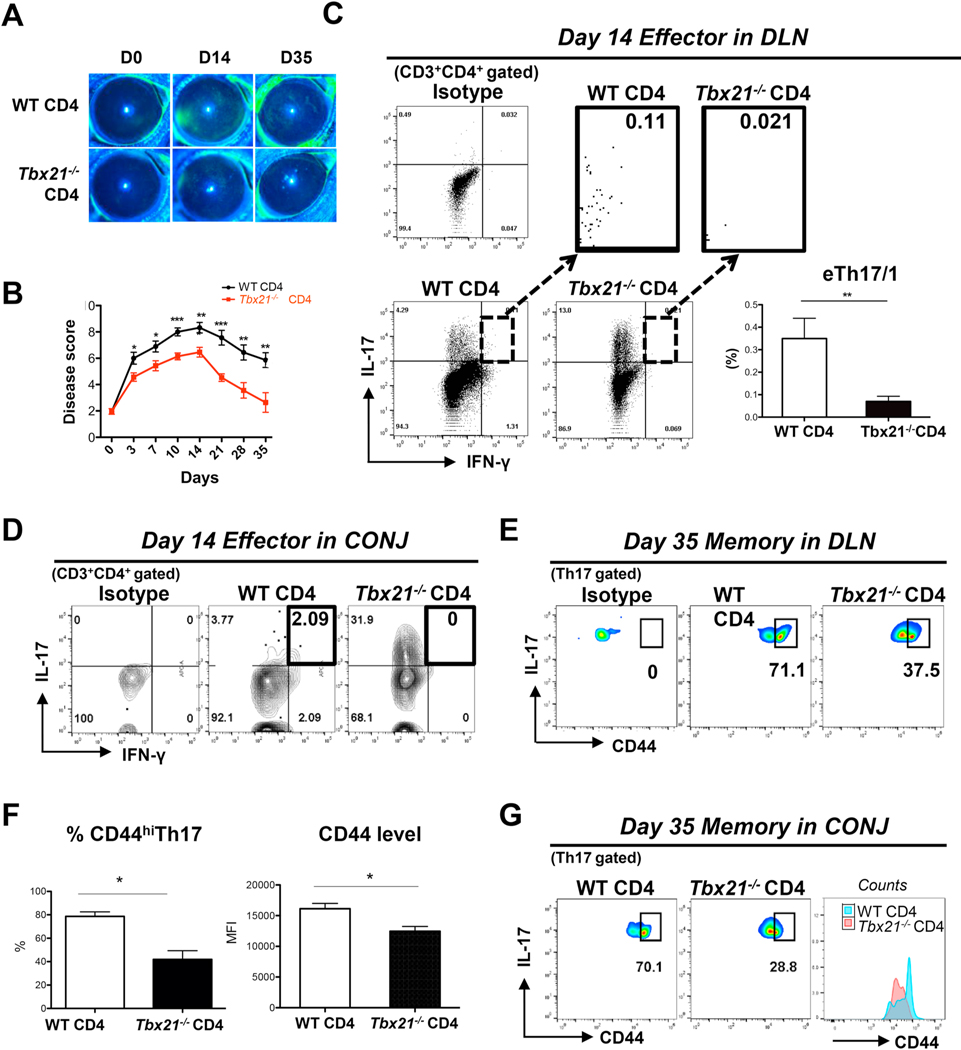
Similar to our findings, IFN-γ-expressing eTh17/1 cells in other autoimmune diseases have been demonstrated to express the transcription factor T-bet, however, there has been a debate on the indispensable role of T-bet in the development of eTh17/1, which seems to be disease- or tissue-dependent [23–26]. To determine whether T-bet is critical to the generation of eTh17/1 in ocular surface autoimmunity, naïve CD4+ T cells were collected from the DLN of Tbx21−/− mice and were adoptively transferred to Rag1−/− mice. The recipient mice were subsequently exposed to desiccating stress to induce acute DED. Compared to recipients of wild-type (WT) naïve CD4+ T cells, those receiving Tbx21−/− naïve CD4+ T cells showed comparable CD4+ T cell migration to ocular tissue (Supplementary Fig. 1), but milder acute disease by day 14 (Fig. 2A and 2B), along with the lack of generation of IFN-γ+IL-17+ eTh17/1 cells in both DLN and ocular surface (Fig. 2C and 2D), consistent with the our previous findings of eTh17/1 in inducing exacerbated ocular surface inflammation [14].
Figure 2. Adoptive transfer of naïve CD4+ T cells isolated from Tbx21−/− mice to Rag1−/− mice fails to induce chronic DED with impaired generation of mTh17 cells.
Naïve CD4+ T cells were isolated from wild-type (WT) or Tbx21−/− mice and then i.v. injected into Rag1−/− mice. After adoptive transfer, these Rag1−/− recipients were immediately subjected to desiccating stress for 14 d and then relocated to normal non-desiccating environment where they were maintained until day 35. (A) Clinical disease severity was evaluated by corneal fluorescein staining (CFS) and representative images show baseline, acute (day 14) and chronic (day 35) stage of DED. (B) Kinetic DED scores in each group are summarized as mean ± SEM in linear graphs (n = 28 eyes per group at acute stage, 16 eyes per group at chronic stage, pooled from two independent experiments). (C) Representative flow cytometry plots show frequencies of eTh17/1 cells in draining lymph nodes (DLN) of Rag1−/− recipients in acute DED (day 14). The frequencies of eTh17/1 cells (mean ± SEM) are summarized in the bar graphs (n = 6 per group). (D) Flow cytometry plots show frequencies of eTh17/1 cells in conjunctiva (CONJ) of Rag1−/− recipients in acute DED (day 14). Ocular tissues were pooled from 6 animals in each group for the analysis, and data shown are one representative out of two performed. (E) Representative flow cytometry plots show frequencies of CD44hi mTh17 cells in DLNs of Rag1−/− recipients in chronic DED (day 35). (F) The proportion of CD44hi mTh17 cells among Th17 population and the expression intensity of CD44 (MFI) by Th17 cells in DLNs of chronic DED from one representative experiment out of two independent experiments performed are summarized as mean ± SEM in the bar graphs (n = 3–6 per group). (G) Flow cytometry plots show frequencies of CD44hi mTh17 in conjunctiva of Rag1−/− recipients in chronic DED (day 35). Ocular tissues were pooled from 4 animals in each group for the analysis, and data shown are one representative out of two performed.*, p<0.05; **, p<0.01; ***, p<0.001.
3.3. Absence of eTh17/1 is associated with failed development of chronic ocular surface inflammation and substantial shrink of memory Th17 pool
As the disease progressed to a chronic stage by day 35 in WT CD4+ T cell recipients, those receiving Tbx21−/− CD4+ T cells did not develop persistent ocular surface inflammation (Fig. 2A and 2B). Furthermore, along with the failed generation of pathogenic eTh17/1 cells at day 14, the subsequent generation of CD44hi mTh17 cell population at day 35 in Tbx21−/− CD4+ T cell recipients was also significantly impaired (Fig. 2E and 2G). These data suggest that T-bet-dependent generation of pathogenic eTh17/1 in acute inflammation is required for efficient formation of pathogenic memory Th17 pool that drives sustained inflammation post-acute insult.
3.4. Conditional deletion of Tbx21 in IL-17+CD4+ T cells prevents the generation of pathogenic mTh17 cells
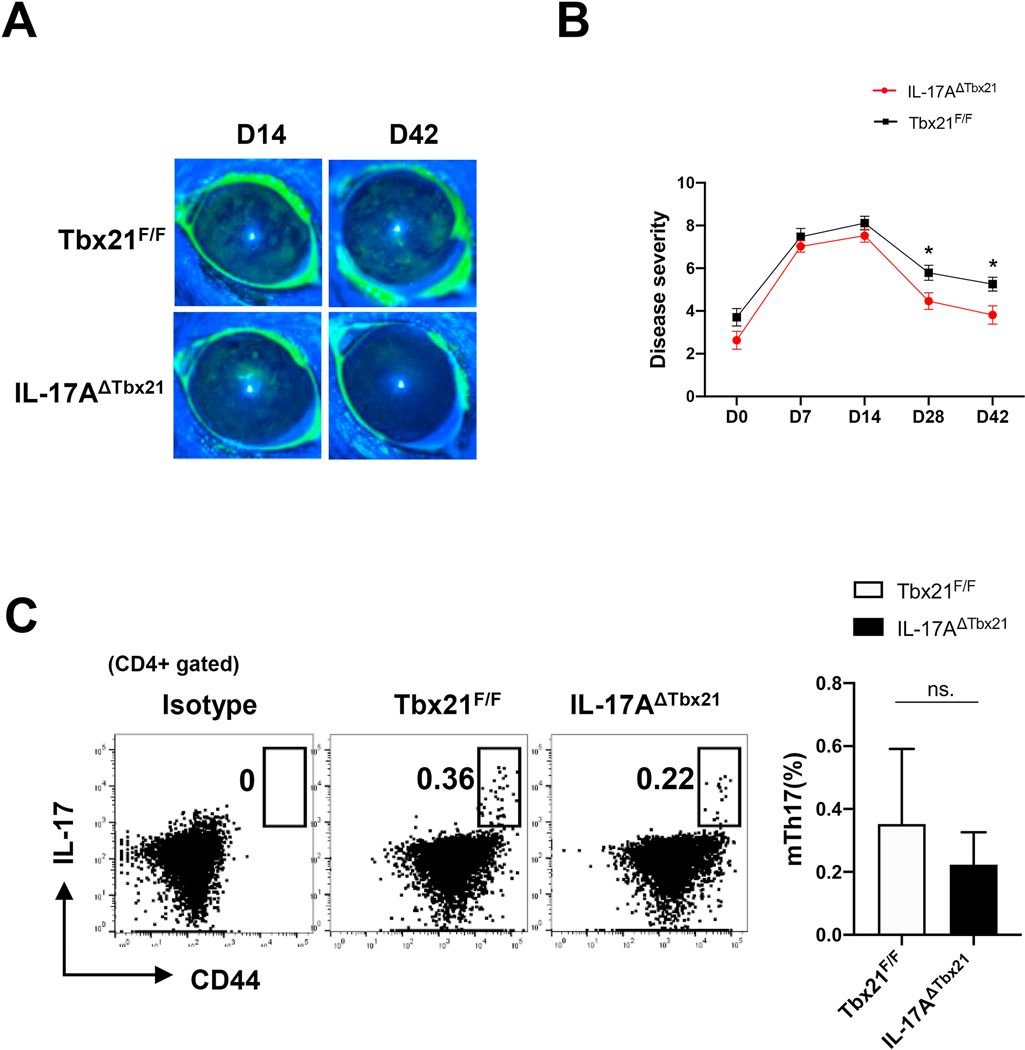
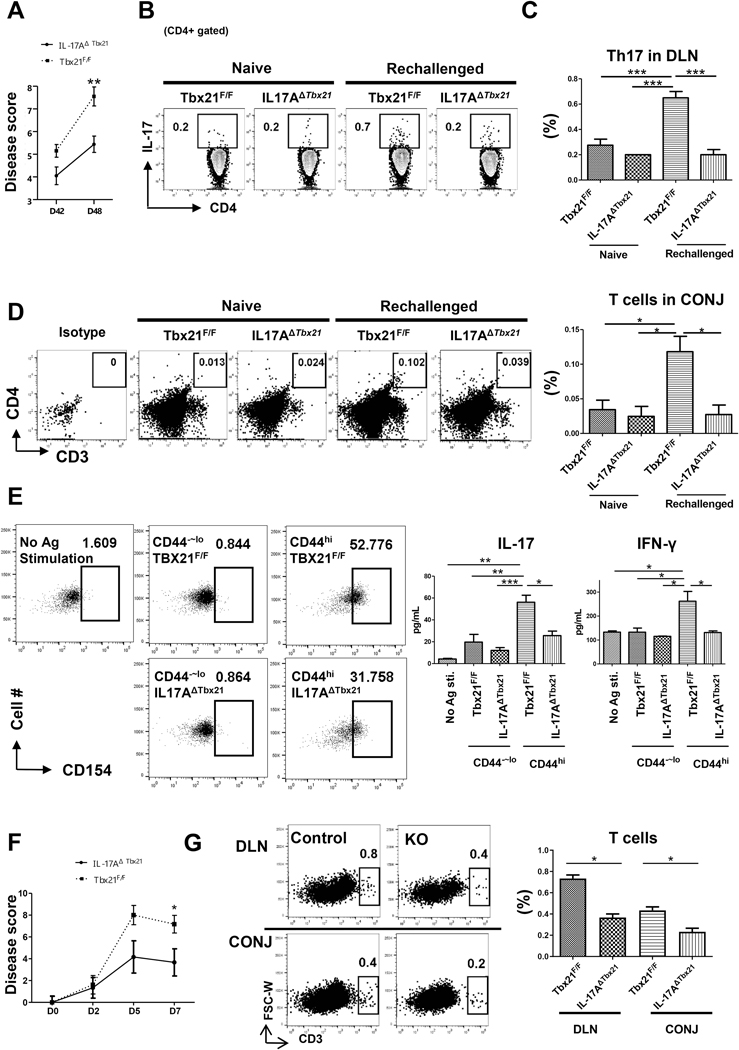
To determine a potentially causal relationship between the absence of eTh17/1 cells and impaired mTh17 generation, we used mice with conditional knockout of T-bet on IL-17+CD4+ T cells (IL-17AΔTbx21) to evaluate the generation of memory Th17 pool. Lack of T-bet in Th17 cells did not significantly reduce the acute disease severity, but significantly suppressed the development of chronic disease (Fig. 3A and 3B). Interestingly, although IL-17AΔTbx21 animals failed to generate eTh17/1 cells at day 14 (Supplementary Fig. 2), there was no significant difference in the frequencies of CD44hi mTh17 cells at day 42 between IL-17AΔTbx21 and control (Tbx21F/F) mice (Fig. 3C), suggesting that in IL-17AΔTbx21 mice, lack of eTh17/1 cells does not affect the frequencies of “memory-phenotype” Th17 cells, but primarily compromises the pro-inflammatory function of mTh17 cells. To precisely examine the functionality of mTh17 cells, we performed in vivo recall response, in vitro antigen re-stimulation, and ex vivo adoptive transfer experiments. Chronic DED at day 42 were re-challenged with a short-term desiccating stress, IL-17AΔTbx21 group showed significantly less disease exacerbation than control group (Fig. 4A), along with significantly impaired re-conversion of memory cells to effector cells, including eTh17 and eTh17/1 cells (Fig. 4B, 4C and Supplementary Fig. 3), and their failed infiltration in ocular surface (Fig. 4D). In addition, CD44hi memory-phenotype T cells isolated from chronic DED (day 42) were in vitro re-stimulated with corneal tissue extracted proteins, and those from IL-17AΔTbx21 group exhibited lower up-regulation of the activation marker CD154 [21,22], and significantly less expression of both IL-17 and IFN-γ (Fig. 4E). Further, the specific subset of IL-17-producing memory-phenotype T cells (mTh17) were isolated from chronic DED that was induced in either IL-17AΔTbx21 or littermates control animals, and then adoptively transferred to normal Rag1−/− mice. The ‘control mTh17’ recipients showed significantly more severe disease than the ‘IL-17AΔTbx21 mTh17’ recipients (Fig. 4F), along with significantly higher amounts of T cells recovered in both conjunctiva and draining lymph nodes (Fig. 4G). These data have thus demonstrated that conditional T-bet deficiency in Th17 cells leads to failed generation of eTh17/1 cells and subsequently functionally impaired mTh17 cells that are unable to sustain chronic ocular surface inflammation.
Figure 3. Conditional deletion of Tbx21 in Th17 cells suppresses development of chronic ocular inflammation.
(A) IL-17AΔTbx21 and control (Tbx21F/F) mice were exposed to desiccating stress for 14 d and then transfer to normal non-desiccating vivarium. Clinical disease severity was evaluated by corneal fluorescein staining (CFS) scores and representative images at day14 (acute disease) and day 42 (chronic disease) are shown. (B) Kinetic disease scores in each group are summarized as mean ± SEM in linear graphs (n = 44 eyes in IL-17AΔTbx21, 36 eyes in Tbx21F/F group at acute stage, 28 eyes per group at chronic stage, pooled from three independent experiments). (C) Representative flow cytometry plots show frequencies of CD44hi mTh17 cells in DLNs at day 42 (chronic disease), and summarized as mean ± SEM in the bar graph (n = 14 per group, pooled from 3 independent experiments). *, p<0.05; ***, p<0.001; n.s., not significant.
Figure 4. Conditional deletion of Tbx21 in Th17 cells inhibits the generation of functional mTh17.
(A) Chronic DED mice (desiccating stress from day 1 to 14, followed by normal non-desiccating environment until day 42) were re-challenged with another short-term desiccating stress (6 days in duration until day 48). Kinetic scores summarized the disease scores immediately before re-challenge and after 6-day re-challenge. (n=20 per group, pooled from three independent experiments). (B) Representative flow cytometry plots show frequencies of Th17 effector cells (CD4+IL17+) in draining lymph nodes (DLN) after re-challenge. (C) The frequencies of effector cells (mean ± SEM) from one representative experiment out of two performed are summarized in the bar graphs (n = 4 per group). (D) Representative flow cytometry plots showing CD4+ T cells infiltration in conjunctiva (CONJ) upon re-challenge. Six eye tissues were pooled together. The frequencies of CD4+ T cells are summarized in the bar graphs (n = 3 per group). (E) CD4+CD44hi T cells were isolated from chronic DED mice (day 42) and in vitro stimulated with corneal tissue homogenates. Cells were then evaluated for CD154 expression by flow cytometry, and the culture supernatants were assayed for IL-17 and IFN-γ by ELISA (n = 3–4 per group). (F) mTh17 cells isolated from chronic DED mice were adoptively transferred to normal Rag1−/− mice which were subsequently exposed to desiccating stress, and the kinetic disease scores are summarized (n = 6 per group). (G) Representative flow cytometric plots showing CD4+ T cells infiltration in DLN and the CONJ, and the frequencies of T cells are summarized in the bar graph (n = 3 per group). *, p< 0.05; **, p<0.01, ***, p<0.001.
3.5. The eTh17/1 cells, but not eTh17 cells, exhibit substantial ability to convert to mTh17 cells in vitro
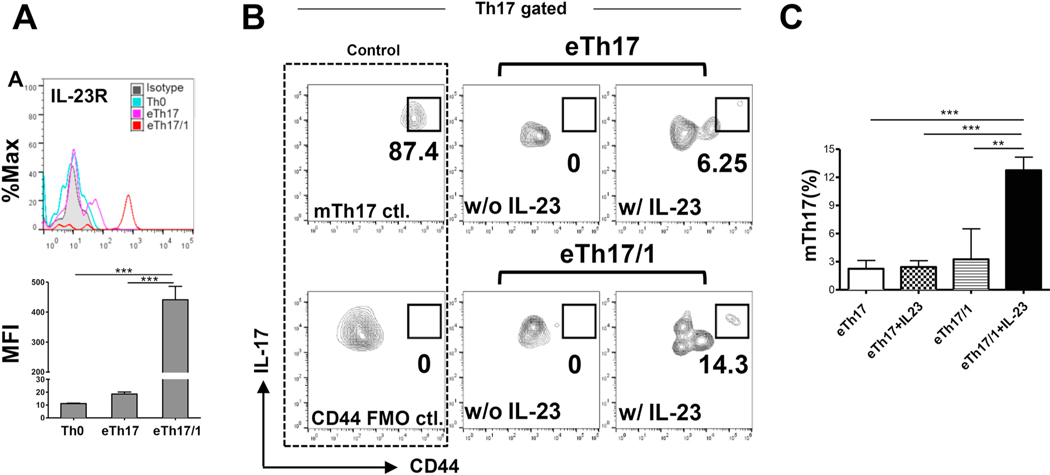
We have recently shown that IL-23 is the key cytokine that promotes effector Th17 cells to enter the memory pool [12], so we first compared the IL-23 receptor (IL-23R) expression in the two DED effector Th17 subsets: eTh17 and eTh17/1. Flow cytometric analysis showed that although both subsets expressed IL-23R, eTh17/1 cells expressed significantly higher levels than eTh17 cells (Fig. 5A), suggesting that eTh17/1 cells can more efficiently convert to mTh17 cells in response to IL-23 stimulation. We next isolated eTh17/1 and eTh17 cells from DLN of acute DED mice and cultured them with IL-23 for 48 hours. By the end of the culturing, a substantial mTh17 population characterized as CD44hiIL-17+CD4+ cells was only recovered from eTh17/1 culture, but not eTh17 culture (Fig. 5B and 5C). Consistent with our previous findings on the key role of IL-23 in mTh17 generation, without the support of IL-23, eTh17/1 was not able to convert to mTh17 cells (Fig. 5B and 5C). There was also a noted CD44int population in both eTh17 and eTh17/1 cultures stimulated with the IL-23, suggesting effector-to-memory conversion a continuous and dynamic process. These data demonstrate that eTh17/1 cells are the predominant effector precursors of mTh17 cells and their conversion requires the support of IL-23 in in vitro condition.
Figure 5. IL-23 promotes the conversion of effector Th17/1 cells to memory Th17 cells ex vivo.
(A) Representative flow cytometry histograms show the expression of IL-23R in naive T cells (Th0), and eTh17 and eTh17/1 cells from autoimmune DED mice (same gating strategy as Fig. 1A). The expression levels of IL-23R by each T cell subset are summarized as mean ± SEM of mean fluorescein intensities (MFIs) in the bar graph. (B) Enriched fresh eTh17 and eTh17/1 cells from DLNs of acute DED mice were cultured in the presence or absence of IL-23 for 48 h. Representative flow cytometry plots from four independent experiments show the population of CD44hi mTh17 cells at the end of culture. The mTh17 cells analyzed in wild-type chronic DED mice serve as a positive control for the staining (mTh17 ctl.). (C) The frequencies of mTh17 cells in each group are summarized as mean ± SEM. Bar graphs summarize data from 4 independent experiments (n = 4–6 in eTh17/1 groups, n = 12–13 in eTh17 groups). **, p<0.01; ***, p<0.001
3.6. The eTh17/1 cells, but not eTh17 cells, are able to effectively induce persisting ocular surface inflammation with generation of mTh17 in vivo
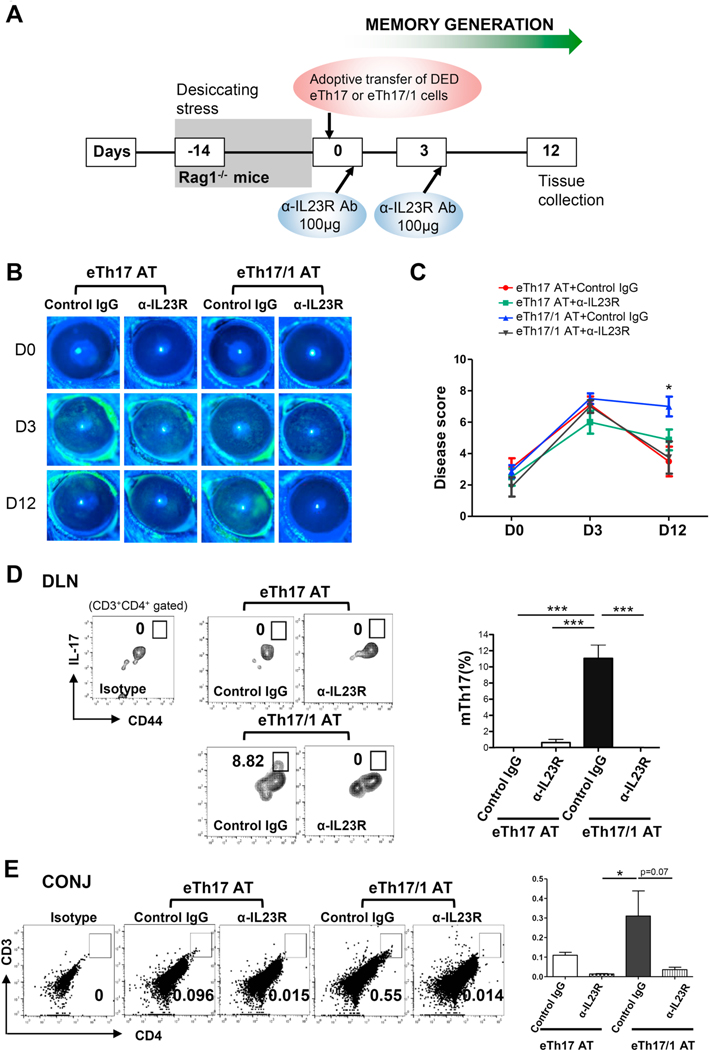
Next, we assessed whether eTh17/1 cells are similarly effective in in vivo conversion to mTh17 as observed in vitro. We exposed Rag1−/− mice to desiccating stress for 14 days to induce a natural, IL-23-enriched microenvironment [12], followed by adoptive transfer of wild-type acute DED mice-derived eTh17/1 or eTh17 cells. Immediately after adoptive transfer, the recipient mice were relocated to normal non-desiccating vivarium to induce the contraction of effector T cells and simultaneous generation of memory T cells [12] (Fig. 6A). After 14 days desiccating stress, the Rag1−/− mice did not develop DED (indicated as day 0 in Fig. 6B and 6C), consistent with our previous observation [14], due to the lack of T cells. Upon receiving the DED effector T cells, both eTh17 and eTh17/1 recipients rapidly developed significant clinical disease with similar severity by day 3. However, by day 12, only eTh17/1 recipients showed persistent severe disease, while eTh17 recipients showed dramatic amelioration to nearly normal scores (Fig. 6B and 6C), indicating that the transferred eTh17/1 has superior ability over eTh17 to maintain the disease for a longer period. Indeed, further analysis revealed a substantial CD44hi mTh17 population without detectable effector Th17 cells at day 12 in eTh17/1 recipients; in contrast, there was no significant effector Th17 or memory Th17 cells were recovered from eTh17 recipients at day 12 (Fig. 6D), demonstrating that eTh17/1 is the predominant subset of effector precursors of mTh17 in vivo. Consistent with the persisting ocular surface disease, significant CD4+ T cell infiltration were detected in the conjunctiva at the ocular surface in the eTh17/1 recipients (Fig. 6E). Similar to our in vitro findings, an intermittent status between established memory and effector Th17 cells, characterized as CD44hiIL17low cells was identified in the eTh17/1 recipients (Fig. 6D), and they may represent those still undergoing memory conversion process. Furthermore, given the critical role of IL-23 in generating mTh17 cells [12], and especially, in driving eTh17/1 to mTh17 pool in vitro (Fig. 5B and 5C), we determined the effect of blocking IL-23 signaling on disease induction and progression in eTh17 and eTh17/1 recipients by injection of an anti-IL-23R Ab at the same time of T cell adoptive transfer (Fig. 6A). We found that blockade of IL-23R abolished the ability of DED-derived eTh17/1 cells in inducing persisting disease in Rag1−/− recipients (day 12, Fig. 6B and 6C), along with diminished CD4+ T cell infiltration in conjunctiva and impaired generation of mTh17 (Fig. 6D and 6E).
Figure 6. Adoptive transfer of eTh17/1, but not eTh17, induces and maintains DED, along with the generation of mTh17 cells in vivo.
(A) Experimental design. Rag1−/− mice was exposed to desiccating stress for 14 d, followed by adoptive transfer of wild-type DED mice-derived eTh17 or eTh17/1 cells via i.v. injection (2×104). Immediately after adoptive transfer (day 0), the Rag1−/− recipients were transferred to normal vivarium for additional 12 d. Some of the recipients also received anti-IL23 receptor (IL-23R) antibody treatment intraperitoneally at day 0 and 3. (B) Clinical disease severity was evaluated by corneal fluorescein staining scores and representative images show baseline (day 0, the day of adoptive transfer) and day 3, 12 post-adoptive transfer. (C) Clinical disease scores in each group at day 12, pooled from three independent experiments, are summarized as mean ± SEM (n = 6–8 eyes per group). *, p<0.05 for eTh17/1 AT vs eTh17 AT, eTh17/1 AT vs eTh17/1 AT+anti-IL23R, and eTh17/1 AT vs eTh17 AT+anti-IL23R. (D) Representative flow cytometry plots from three independent experiments show generation of CD44hi mTh17 cells in eye-draining lymph nodes (DLN) of mice with adoptive transfer of eTh17 or eTh17/1 cells at day 12 post-transfer. The frequencies of mTh17 cells (mean ± SEM) are summarized in the bar graphs (n = 3–4 per group). (E) Conjunctival tissues were pooled from 3 animals in each group for flow cytometric analysis of infiltration of CD4+ T cells at day 12 post-transfer, and data shown are one representative out of two performed. The frequencies of T cells (mean ± SEM) are summarized in the bar graphs (n = 3 per group). *p<0.05; ***, p<0.001
4. DISCUSSION
Memory Th17 cells (mTh17) not only provide protective immunity against repeated infections, but also play pathogenic functions in driving chronic autoimmune diseases [2–4,9]. Unlike its CD8+ counterpart, CD4+ T cell memory is less well understood due to the complex heterogeneity of CD4+ T cell subsets. Our recent efforts on investigating the precise roles of immunological memory in sustaining chronic ocular surface inflammation have revealed that mTh17 cells are generated from their lineage-committed effector precursors under IL-23 stimulation during the contraction phase of primary response, and maintained under both IL-7 and IL-15 stimulation for long term [12]. A subsequent critical question is whether there is any inherent determinant(s) in the effector cells deciding their destinies of either converting to long-lived memory cells or undergoing apoptosis. In the context of autoimmunity and chronic inflammation, it is well documented that two effector Th17 subsets – IFN-γ-IL-17+ eTh17 and IFN-γ+IL-17+ eTh17/1 – play critical pathogenic roles in inducing full-blown disease [2,25,27,28], including autoimmune dry eye disease [14]. Herein, we demonstrate for the first time that T-bet is required for the generation of eTh17/1 cells, which serve as the principal effector precursors entering Th17 memory pool in chronic autoimmune inflammation.
Our findings reveal that mTh17 express the Th1-lineage transcription factor T-bet, which is also highly expressed by eTh17/1 but not eTh17 cells. Interestingly, although all eTh17, eTh17/1, and mTh17 express Th17-lineage transcription factor RORγt, as expected, the expression level of RORγt is the lowest in eTh17/1, which is related to the reciprocal suppression on RORγt by T-bet [13,29]. We further demonstrate that T-bet is indispensable for eTh17/1 differentiation in our ocular autoimmunity model, consistent with the report in experimental autoimmune encephalomyelitis [25], but contradictory to the findings in colitis models where T-bet is dispensable for eTh17/1 generation [23,24]. Therefore, the regulation of eTh17/1 development by T-bet is disease- or tissue-dependent.
T-bet has been implicated to play a critical role in immunological memory formation. Compared to RORγt-expressing cells, T-bet-expressing cells convert to memory cells more efficiently by gaining survival benefit from regulating CD27, BCL-2, CD122 expression and IL-15 signaling, which are essential for the survival and proliferation of memory T cells [15,18,19]. Further, the T-bet expressing levels by effector Th1 cells are implicated in regulating their subsequent differentiation to either “central memory (Tcm)” or “effector memory (Tem)” subtypes, with T-betlow effectors preferentially giving rise to Tcm while T-bethigh effectors substantially giving rise to Tem [2,18]. As Tem is characterized by distinct up-regulation of homing receptors, which facilitate their migration to non-lymphoid sites of inflammation, as well as profound production of inflammatory cytokines upon re-stimulation, Tem is believed to the dominant subtype of CD4+ memory T cells playing pathogenic roles in various autoimmune diseases [2,4,13,18,30]. Consistently, we have previously demonstrated that mTh17 is indeed characterized as Tem that are capable of maintaining chronic ocular inflammation and mounting a heightened recall response [4]. Indeed, T-bet-deficient CD4+ T cells were capable of efficiently migrating to ocular tissues after being adoptively transferred to Rag1−/− mice (Supplementary Fig. 1), but were defective in developing into eTh17/1 and mTh17 cells (Fig. 2), indicating that the reduced disease in T-bet-deficient CD4+ T cell recipients is not due to inferior migration ability resulting from T-bet deficiency as previously reported [31]. In addition, as mTh17 cells maintain chronic inflammation by primarily producing IL-17 but not IFN-γ [4], the reduced chronic inflammation is unlikely due to defective IFN-γ production by T-bet-deficient CD4+ T cells. Furthermore, selective deficiency of eTh17/1 (with other T-bet expressing cells intact, such as classic Th1 and NK cells) led to failed generation of pathogenic mTh17 cells and development of chronic disease (Fig. 3 and 4). It is noted that the suppression of disease severity in the mice with conditional Tbx21 deletion in Th17 cells (Fig. 3B) is lower than that in the Rag1−/− mice receiving Tbx21-depleted CD4+ T cells (Fig. 2B), especially in the acute stage, suggesting additional contribution to disease by other immune cells that are present in the IL-17AΔTbx21 model but not in the Rag1−/− mice, such as B lymphocytes that have been shown to promote acute disease via activation by effector Th17 cells [32,33]. Compared to the Rag1−/− model, the IL-17AΔTbx21 model also harbors abilities of mounting conventional Th1 and CD8+ T cell responses, but neither of them is critically involved in DED pathogenesis [14,34]. Taken together, our current study demonstrates T-bet is an essential inherent factor regulating eTh17/1 generation and their subsequent conversion to long-lived effector memory cells.
It is well known that IL-23 is required for initially differentiated Th17 cells to acquire full function [28]. Our recent work has observed a persisting high IL-23 level in the microenvironment during expansion phase throughout to the contraction phase of the primary response, and demonstrated that IL-23 signaling during the contraction phase is essential to dictate effector Th17 to enter memory pool [12], thus having expanded our knowledge on the role of IL-23 in Th17 immunity. Mechanistically, IL-23 drives memory T cell generation through exerting anti-apoptotic function and promoting IL-15R and IL-7R expression [12]. In addition, IL-23R signaling also promotes T-bet expression in differentiated Th17 cells and their plastic differentiation to eTh17/1 cells [14]. On the other hand, it remains to be determined whether T-bet reciprocally regulates IL-23R expression in Th17 immunity. Considering the critical role of IL-23 signaling in Th17 memory formation, we examined the IL-23R expression by eTh17 and eTh17/1 cells, and found that eTh17/1 cells express much higher level of IL-23R than eTh17. Deficiency of IL-23R has been reported to cause effector Th17 cells susceptible to cell death [35]. Thus, such discrepancy in IL-23R expression level implicates that eTh17/1 are more responsive to IL-23 stimulation which may prevent cellular apoptosis during contraction phase and promote them more efficiently to enter memory pool than eTh17 effectors.
We and others have previously shown that both disease-specific eTh17 and eTh17/1 are able to adoptively transfer the same disease to naive recipients [14,36,37], and our present study has confirmed these findings, and beyond this, further demonstrated that once the acute disease is induced, eTh17 cells are unable to effectively maintain the disease demonstrated by rapid resolution of the disease, while eTh17/1 cells are capable of maintaining the persistent corneal epitheliopathy, along with their conversion to mTh17 cells. Although it is possible for eTh17 to further differentiate to eTh17/1, and subsequently convert to mTh17 in vivo [6,38], such conversion usually happens in a highly inflammatory environment during the expansion phase of immune response [14,38], and thus in our intentionally created “memory formation” environment that recapitulates the contraction phase (mice returned to a normal vivarium at the time of adoptive transfer) of immune response, the microenvironment is probably unfavorable for efficient plastic conversion of eTh17 to eTh17/1 cells. Moreover, our data from Tbx21−/− CD4+ T cell adoptive transfer and IL-17AΔTbx21 animal studies exhibit while eTh17/1 cells are absent in acute inflammation due to lack of T-bet, memory-phenotype Th17 cells are still detected later after the resolution of the acute disease, suggesting that eTh17 cells may contribute to memory pool, at least phenotypically, in a smaller degree.
5. CONCLUSIONS
In conclusion, our present work has untangled eTh17/1, whose development is dependent on T-bet expression, as the principal effector precursors differentiating to mTh17 cells during the transition of acute to chronic inflammation, further highlighting the critical role of eTh17/1 cells not only as potent pathogenic effectors in acute inflammation, but also as essential memory precursors leading to chronic inflammation in autoimmunity. These findings provide novel avenues to design effective immunotherapy for autoimmune diseases.
Supplementary Material
Highlights.
T-bet is required for generation of autoreactive IFN-γ+IL-17+ Th17/1 effectors.
Conditional deletion of T-bet abolishes the pathogenic memory Th17 cells.
Th17/1 effectors substantially convert to memory Th17 cells in vitro and in vivo.
ACKNOWLEDGEMENTS
Dr. Nai-Wen Fan would like to thank the Yin Shu-Tien Foundation for their support through the Taipei Veterans General Hospital-National Yang-Ming University Excellent Physician Scientists Cultivation Program (No. 106-V-A-002).
This work was supported by National Institutes of Health grants EY20889 (R.D.) and P30EY003790 (Massachusetts Eye and Ear).
Footnotes
Nai-Wen Fan: Methodology, Investigation, Validation, Formal analysis, Writing - Original Draft, Writing - Review & Editing, Visualization
Shudan Wang: Investigation, Validation, Formal analysis, Writing - Review & Editing
Gustavo Ortiz: Investigation
Sunil K. Chauhan: Conceptualization, Methodology
Yihe Chen: Conceptualization, Methodology, Formal analysis, Writing - Original Draft, Writing - Review & Editing, Visualization, Supervision
Reza Dana: Conceptualization, Methodology, Writing - Review & Editing, Supervision, Funding acquisition
Conflict of interest: R.D. and S.K.C. hold equity in Aramis Biosciences which owns intellectual property related to targeting IL-17 in ocular surface diseases. R.D., Y.C. and S.K.C. are inventors of a patent related to targeting memory Th17 cells in ocular immunoinflammatory diseases (owned by Massachusetts Eye and Ear).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Ahmed R, Bevan MJ, Reiner SL, Fearon DT. The precursors of memory: models and controversies. Nat Rev Immunol. 9 (2009) 662–668. 10.1038/nri2619. [DOI] [PubMed] [Google Scholar]
- [2].Raphael I, Joern RR, Forsthuber TG. Memory CD4(+) T Cells in Immunity and Autoimmune Diseases. Cells. 9 (2020). 10.3390/cells9030531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Yang J, Sundrud MS, Skepner J, Yamagata T. Targeting Th17 cells in autoimmune diseases. Trends Pharmacol Sci. 35 (2014) 493–500. 10.1016/j.tips.2014.07.006. [DOI] [PubMed] [Google Scholar]
- [4].Chen Y, Chauhan SK, Lee HS, Saban DR, Dana R. Chronic dry eye disease is principally mediated by effector memory Th17 cells. Mucosal Immunol. 7 (2014) 38–45. 10.1038/mi.2013.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Deng Q, Luo Y, Chang C, Wu H, Ding Y, Xiao R. The Emerging Epigenetic Role of CD8+T Cells in Autoimmune Diseases: A Systematic Review. Front Immunol. 10 (2019) 856. 10.3389/fimmu.2019.00856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].DuPage M, Bluestone JA. Harnessing the plasticity of CD4(+) T cells to treat immune-mediated disease. Nat Rev Immunol. 16 (2016) 149–163. 10.1038/nri.2015.18. [DOI] [PubMed] [Google Scholar]
- [7].Cosmi L, Maggi L, Santarlasci V, Liotta F, Annunziato F. T helper cells plasticity in inflammation. Cytometry A. 85 (2014) 36–42. 10.1002/cyto.a.22348. [DOI] [PubMed] [Google Scholar]
- [8].Kamali AN, Noorbakhsh SM, Hamedifar H, Jadidi-Niaragh F, Yazdani R, Bautista JM et al. A role for Th1-like Th17 cells in the pathogenesis of inflammatory and autoimmune disorders. Mol Immunol. 105 (2019) 107–115. 10.1016/j.molimm.2018.11.015. [DOI] [PubMed] [Google Scholar]
- [9].McGeachy MJ. Th17 memory cells: live long and proliferate. J Leukoc Biol. 94 (2013) 921–926. 10.1189/jlb.0313113. [DOI] [PubMed] [Google Scholar]
- [10].Lohning M, Hegazy AN, Pinschewer DD, Busse D, Lang KS, Hofer T. et al. Long-lived virus-reactive memory T cells generated from purified cytokine-secreting T helper type 1 and type 2 effectors. J Exp Med. 205 (2008) 53–61. 10.1084/jem.20071855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Harrington LE, Janowski KM, Oliver JR, Zajac AJ, Weaver CT. Memory CD4 T cells emerge from effector T-cell progenitors. Nature. 452 (2008) 356–60. 10.1038/nature06672. [DOI] [PubMed] [Google Scholar]
- [12].Chen Y, Shao C, Fan NW, Nakao T, Amouzegar A, Chauhan SK et al. The functions of IL-23 and IL-2 on driving autoimmune effector T-helper 17 cells into the memory pool in dry eye disease. Mucosal Immunol. 14 (2021) 177–186. 10.1038/s41385-020-0289-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Pepper M, Jenkins MK. Origins of CD4(+) effector and central memory T cells. Nat Immunol. 12 (2011) 467–471. 10.1038/ni.2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Chen Y, Chauhan SK, Shao C, Omoto M, Inomata T, Dana R. IFN-gamma-Expressing Th17 Cells Are Required for Development of Severe Ocular Surface Autoimmunity. J Immunol. 199 (2017) 1163–1169. 10.4049/jimmunol.1602144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Intlekofer AM, Takemoto N, Wherry EJ, Longworth SA, Northrup JT, Palanivel VR et al. Effector and memory CD8+ T cell fate coupled by T-bet and eomesodermin. Nat Immunol. 6 (2005) 1236–1244. 10.1038/ni1268. [DOI] [PubMed] [Google Scholar]
- [16].Knox JJ, Myles A, Cancro MP. T-bet(+) memory B cells: Generation, function, and fate. Immunol Rev. 288 (2019) 149–160. 10.1111/imr.12736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Yu SF, Zhang YN, Yang BY, Wu CY. Human memory, but not naive, CD4+ T cells expressing transcription factor T-bet might drive rapid cytokine production. J Biol Chem. 289 (2014) 35561–35569. 10.1074/jbc.M114.608745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Pepper M, Linehan JL, Pagan AJ, Zell T, Dileepan T, Cleary PP et al. Different routes of bacterial infection induce long-lived TH1 memory cells and short-lived TH17 cells. Nat Immunol. 11 (2010) 83–89. 10.1038/ni.1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Chen Y, Chauhan SK, Tan X, Dana R. Interleukin-7 and −15 maintain pathogenic memory Th17 cells in autoimmunity. J Autoimmun. 77 (2017) 96–103. 10.1016/j.jaut.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Lemp MA. Report of the National Eye Institute/Industry workshop on Clinical Trials in Dry Eyes. CLAO J. 21 (1995) 221–232. [PubMed] [Google Scholar]
- [21].Frentsch M, Arbach O, Kirchhoff D, Moewes B, Worm M, Rothe M. et al. Direct access to CD4+ T cells specific for defined antigens according to CD154 expression. Nat Med. 11 (2005) 1118–1124. 10.1038/nm1292. [DOI] [PubMed] [Google Scholar]
- [22].Kirchhoff D, Frentsch M, Leclerk P, Bumann D, Rausch S, Hartmann S. et al. Identification and isolation of murine antigen-reactive T cells according to CD154 expression. Eur J Immunol. 37 (2007) 2370–2377. 10.1002/eji.200737322. [DOI] [PubMed] [Google Scholar]
- [23].Harbour SN, Maynard CL, Zindl CL, Schoeb TR, Weaver CT. Th17 cells give rise to Th1 cells that are required for the pathogenesis of colitis. Proc Natl Acad Sci U S A. 112 (2015) 7061–7066. 10.1073/pnas.1415675112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Krausgruber T, Schiering C, Adelmann K, Harrison OJ, Chomka A, Pearson C et al. T-bet is a key modulator of IL-23-driven pathogenic CD4(+) T cell responses in the intestine. Nat Commun. 7 (2016) 11627. 10.1038/ncomms11627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Wang Y, Godec J, Ben-Aissa K, Cui K, Zhao K, Pucsek AB et al. The transcription factors T-bet and Runx are required for the ontogeny of pathogenic interferon-gamma-producing T helper 17 cells. Immunity. 40 (2014) 355–366. 10.1016/j.immuni.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Duhen R, Glatigny S, Arbelaez CA, Blair TC, Oukka M, Bettelli E. Cutting edge: the pathogenicity of IFN-gamma-producing Th17 cells is independent of T-bet. J Immunol. 190 (2013) 4478–4482. 10.4049/jimmunol.1203172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Bazzazi H, Aghaei M, Memarian A, Asgarian-Omran H, Behnampour N, Yazdani Y. Th1-Th17 Ratio as a New Insight in Rheumatoid Arthritis Disease. Iran J Allergy Asthma Immunol. 17 (2018) 68–77. [PubMed] [Google Scholar]
- [28].Annunziato F, Cosmi L, Santarlasci V, Maggi L, Liotta F, Mazzinghi B. et al. Phenotypic and functional features of human Th17 cells. J Exp Med. 204 (2007) 1849–1861. 10.1084/jem.20070663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Lazarevic V, Chen X, Shim JH, Hwang ES, Jang E, Bolm AN et al. T-bet represses T(H)17 differentiation by preventing Runx1-mediated activation of the gene encoding RORgammat. Nat Immunol. 12 (2011) 96–104. 10.1038/ni.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Fritsch RD, Shen X, Illei GG, Yarboro CH, Prussin C, Hathcock KS et al. Abnormal differentiation of memory T cells in systemic lupus erythematosus. Arthritis Rheum. 54 (2006) 2184–2197. 10.1002/art.21943. [DOI] [PubMed] [Google Scholar]
- [31].Lord GM, Rao RM, Choe H, Sullivan BM, Lichtman AH, Luscinskas FW et al. T-bet is required for optimal proinflammatory CD4+ T-cell trafficking. Blood. 106 (2005) 3432–3439. 10.1182/blood-2005-04-1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Subbarayal B, Chauhan SK, Di Zazzo A, Dana R. IL-17 Augments B Cell Activation in Ocular Surface Autoimmunity. J Immunol. 197 (2016) 3464–3470. 10.4049/jimmunol.1502641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Stern ME, Schaumburg CS, Siemasko KF, Gao J, Wheeler LA, Grupe DA et al. Autoantibodies contribute to the immunopathogenesis of experimental dry eye disease. Invest Ophthalmol Vis Sci. 53 (2012) 2062–2075. 10.1167/iovs.11-9299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].El Annan J, Chauhan SK, Ecoiffier T, Zhang Q, Saban DR, Dana R. Characterization of effector T cells in dry eye disease. Invest Ophthalmol Vis Sci. 50 (2009) 3802–3807. 10.1167/iovs.08-2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].McGeachy MJ, Chen Y, Tato CM, Laurence A, Joyce-Shaikh B, Blumenschein WM et al. The interleukin 23 receptor is essential for the terminal differentiation of interleukin 17-producing effector T helper cells in vivo. Nat Immunol. 10 (2009) 314–324. 10.1038/ni.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Hirota K, Duarte JH, Veldhoen M, Hornsby E, Li Y, Cua DJ et al. Fate mapping of IL-17-producing T cells in inflammatory responses. Nat Immunol. 12 (2011) 255–263. 10.1038/ni.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Choi G, Park YJ, Cho M, Moon H, Kim D, Kang CY et al. A critical role for Th17 cell-derived TGF-beta1 in regulating the stability and pathogenicity of autoimmune Th17 cells. Exp Mol Med (2021). 10.1038/s12276-021-00632-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Cosmi L, Santarlasci V, Maggi L, Liotta F, Annunziato F. Th17 plasticity: pathophysiology and treatment of chronic inflammatory disorders. Curr Opin Pharmacol. 17 (2014) 12–16. 10.1016/j.coph.2014.06.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.