Abstract
The HeartMate 3 left ventricular assist device (LVAD) is now the only centrifugal pump intended for durable support being actively manufactured and implanted for adults in the United States. The changes in preload and afterload that accompany common clinical scenarios experienced by patients with an LVAD will cause specific changes to the LVAD pump parameters, namely, the pump power, pulsatility index, and flow. Appropriate care of this unique, and growing, population requires a full understanding of these variables as well as the underlying physiologic principles governing their derivation. The aim of this review is to focus on the updated functionality of the HeartMate 3, specifically in comparison to the HeartMate II, as well as the application of pump parameter interpretation to common clinical scenarios.
Keywords: Left ventricular assist device, HeartMate, pump parameters, pulsatility index
Graphical Abstract

There are an estimated 300,000 patients in the United States living with heart failure (HF) who may be eligible for advanced HF therapies, specifically left ventricular assist device (LVAD) or cardiac transplantation.1–3 Owing to limited organ availability and strict patient selection, only about 3000 patients underwent cardiac transplantation in 2019.4,5 Meanwhile, LVAD technology continues to improve significantly. According to the 2019 Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) report, the 1-year survival was 82%, but recent advances in pump technology with the HeartMate 3 (HM3; Abbott, Abbott Park, IL) have improved quantity, and quality of life for these patients.6 In the MOMENTUM 3 study, a randomized controlled trial comparing the HM3 with the HeartMate II (HMII; Abbott), the HM3 had significantly better survival free from disabling stroke or need for re-operation for pump removal at 1 (84.0% and 74.7%, respectively) and 2 years (74.8% and 60.6%, respectively), compared with the HMII.7
Although the HM3 has become the preferred Abbott pump, many patients with previously implanted HMII pumps continue to be cared for across LVAD centers. Additionally, the HeartWare HVAD (Medtronic, Minneapolis, MN) devices were commonly implanted, until the recently announced cessation in production.8 Each pump has its own specific design and operation characteristics. LVAD function is directly correlated with cardiac hemodynamics. The pump flows respond to hemodynamic changes throughout each individual cardiac cycle, and these outputs vary in response to any changes in these parameters. Changes in preload and afterload that accompany common clinical scenarios experienced by patients with an LVAD will cause specific changes to pump parameters, namely, pump power, pulsatility index (PI), and flow. Appropriate care of this unique, and growing, population requires a full understanding of these variables and their downstream effects.
The aim of this review was to focus on the updated functionality of the HM3, specifically in comparison with the HMII, as well as the application of pump parameter interpretation to common clinical scenarios. Care for patients implanted with the HVAD pump continues despite the cessation of new implants, but will not be a focus in this review as it has been previously addressed by Rich and Burkhoff.9
HM3 Pump Design
The HM3 is a fully magnetically levitated LVAD and has 4 unique features: (1) a fully magnetically levitated rotor, (2) large blood flow pathways, (3) intrinsic pulsatility, and (4) an intradevice operating system.10
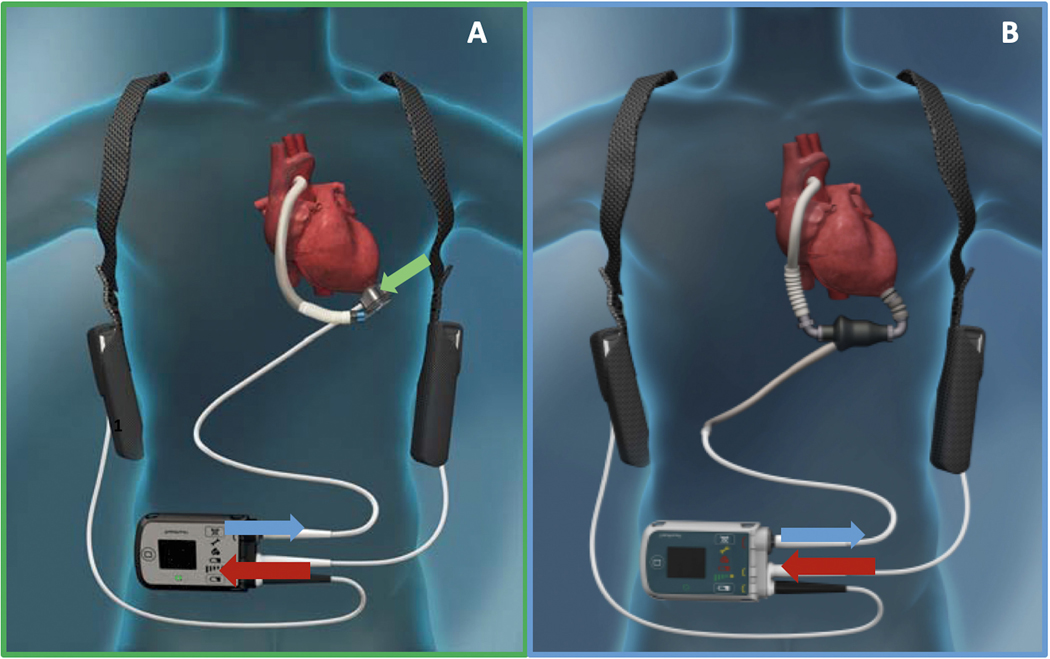
The rotor is fully levitated and self-centered, without the need for hydrodynamic or mechanical bearings. This Full MagLev technology (Abbott) decreases the shear stress and compressive forces seen with hydrodynamic bearings.10 Additionally, the HM3 has large, consistent blood flow pathways owing to the rotor and inlet design. Although hydrodynamic bearing rotors and inlets have narrow blood flow pathways, the HM3 inlet pathways are 10 20 times larger. These larger pathways minimize shear stress, avoid stasis, and decrease activation of thrombogenic blood components.10 The intrinsic pulsatility mechanism, often referred to as the artificial pulse, is preprogrammed within the device, and is automatically active at a set rotor speed above 4000 RPM. The software is set to cycle 30 times every minute (i.e., every 2 seconds), but does not have an effect on total net flow. Every 2 seconds, the rotor will decrease by 2000 RPM, from the set speed, for 0.15 seconds, then increase by 4000 RPMs for 0.20 seconds, and finally return to the set speed. This allows for routine washing of the pump, which further decreases stasis.10 Finally, the HM3 has an on-board computer inside the pump itself that allows for isolation of the rotor drive power. The rotor drive power is the component of the total pump power that is directly attributable to generating flow through the LVAD. This feature results in a more accurate estimation of flow, compared with the competing signals interpreted by the HMII, which has the operating system within the controller Figure 1A–B. The HMII is unable to isolate rotor drive power from the total pump power and, thus, the power flow relationship for the HMII is less well-defined at low flow states. The complex interplay of power, pulsatility, and flow in the HM3 and HMII is described in detail later in this article.
Fig. 1.
A – HeartMate 3, B – HeartMate II, Green arrow Rotor drive power needed to rotate the rotor and generate blood flow, Blue arrows – Pump power going down Driveline to the pump, Red arrows – System Power going into the Controller from batteries.
Pump Power and PI
Pump Power
The power associated with the left ventricular assist system can be broken down into the system power, the power going into the controller from the batteries or grounded power source, and the pump power. The pump power includes the driveline power, motor operating power, bearing power, and rotor drive power. The driveline power refers to the power that is needed to overcome the resistance of the driveline, the motor operating power is the power that is used to operate the electronics of the motor, and the bearing power is the power needed to overcome energy losses from friction of the bearings, in the case of the HMII, and operating of the magnetic levitation for the HM3. None of these powers are directly involved in the operation of the rotor to generate blood flow. The rotor drive power is the only component of power that is involved directly in generating the rotational spin of the rotor and thus generating blood flow. Notably, owing to the on-board computer located within the intrapericardial device of the HM3, the device is able to measure rotor drive power in isolation. This feature allows for a more direct measure of flow than in the HMII, which instead uses the total pump power to derive flow (Fig. 1A, B).
The pump power is a direct measure of the pump motor voltage and current, which can change in response to shifting hemodynamics or pathophysiologic insults. For instance, pump power increases in the setting of rotor thrombus, although this change can be gradual or abrupt, or changes in loading conditions, such as with aortic insufficiency (AI). The relationship of power to specific clinical scenarios is discussed elsewhere in this review.
Power–Flow Curves
The power flow curves represent the direct relationship between power and flow. The lower the power, the lower the flow calculated by the pump; the higher the power, the higher the flow calculated by the pump. This relationship adjusts based on the pump set speed and varies between the HMII and HM3. As described later in this article, the HM3 uses the rotor drive power as its source of power to estimate flow, whereas the HMII uses the total pump power to estimate flow. Within the normal, expected operating flows of the 2 devices, power and flow are linearly correlated. However, at lower flow rates, generally less than 2.5 L/min, the relationship becomes curvilinear for the HMII. For this reason, the HMII will not estimate flows of less than 2.5 L/min and thus has a tendency to overestimate diastolic flows. Conversely, the HM3 has a curvilinear relation of power to flow at high flows and can thus underestimate peak systolic flows (Fig. 2 A, B).
Fig. 2.
A – HeartMate II; B – HeartMate 3; Ovals – region of imprecise flow calculations.
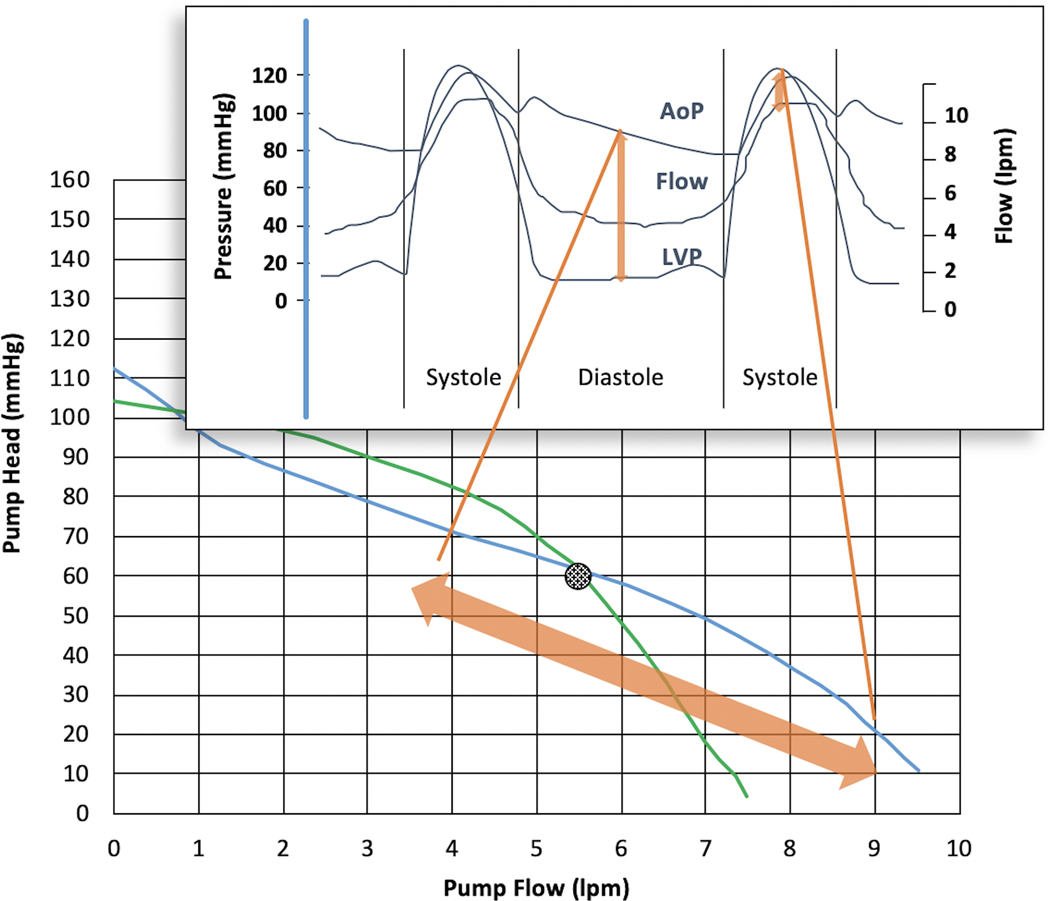
The power and flow change throughout each cardiac cycle as the pressure across the pump changes with normal cardiac function (Fig. 3). When considering the variations in power throughout each cardiac cycle, the imprecision of flow estimation at the extremes of power means that there is a tendency for diastolic flow (i.e., minimum flow) to be overestimated in certain clinical scenarios with the HMII and for systolic flow (i.e., maximum flow) to be underestimated in certain scenarios with the HM3 (Fig. 4).
Fig. 3.
Variation in pump power throughout cardiac cycle in relation to LVAD flow and aortic and left ventricular pressures.
Fig. 4.
Estimated Flow throughout Cardiac Cycle based on Power.
PI and PI Events
The PI represents the relationship of the maximum-to-minimum differential with the average power. This ratio is represented as:
The power is measured over 15-second intervals, and then averaged to generate the PI. Therefore, the PI reflects the variability of pump power, which is directly related to pump flow. Under most clinical scenarios, the PI can then reflect the native heart contractility as flow variation and thus power variation between peak systole and diastole will be higher as underlying left ventricular contractility increases. As mentioned elsewhere in this article, the HM3 uses the rotor drive power to calculate the PI, whereas the HMII uses total pump power. Consequently, the HM3 PI has a more complete appreciation for diastolic oscillations in flow and power and, thus, has a better representation of flow and power variability through the pump during conditions that predispose to under filling of the left ventricle.
A PI event is triggered if there is a greater than 45% difference noted between the per-second PI and the average PI. This difference can be driven by either the Powermax or Powermin. PI events are often misassociated with suction events, but notably, most PI events are not related to ventricular suction. Other examples of PI event triggers include acute changes in the left ventricular preload or afterload and beat-to-beat changes in left ventricular volumes, including arrhythmias, coughing or sneezing, sudden changes in power or pump speed, or concurrent use of an intra-aortic balloon pump (IABP) (Table 1). In response to a PI event, the pump speed will automatically drop to its low speed limit and then re-advance to the set speed in 50- or 100-RPM increments for the HM3 and HMII, respectively. If another PI event is triggered before returning to the set speed the cycle will repeat, and it will continue to do so until the cause of the PI event is addressed. To that end, frequent PI events and large changes in speed may indicate a more pathologic underlying condition that should be addressed. Finally, it is important to note that there are no audible alarms associated with a PI event.
Table 1.
Examples of PI Event Triggers
| Sudden changes in patient volume status |
| Sudden changes in power or pump speed |
| Arrhythmias (eg, atrial fibrillation, premature ventricular contractions) |
| Ventricular suction |
| Coughing |
| Sneezing |
| Intra-aortic balloon pump use |
Determinants of Pump Flow
Pump flow (Q) is equal to the ratio between rotor speed and the difference in pressures from the inflow to the outflow cannula, also known as the pump head pressure (H).
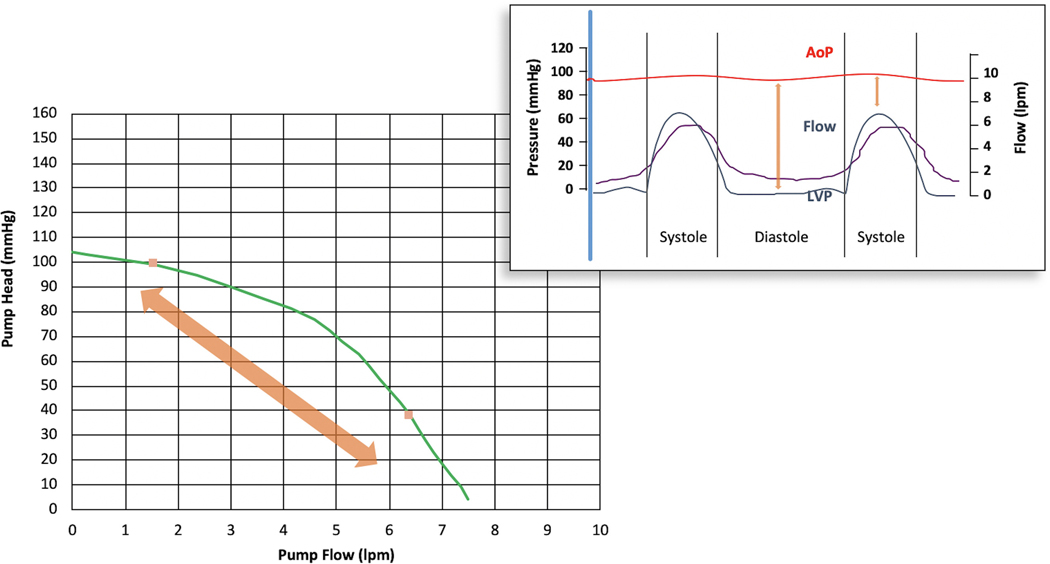
The pressure difference can be approximated by the difference between left ventricular pressure (LVP) (i.e., Pinflow) and aortic pressure (AoP) (i.e., Poutflow). Therefore, the flow through the LVAD is inversely proportional to the pressure difference. This feature manifests every beat of the cardiac cycle; as the pressure difference increases during diastole, pump flow decreases, and as the difference decreases during systole, the pump flow increases. On a beat-to-beat basis, the aortic pressure changes minimally when compared with the dynamic changes in LVP (Fig. 5).
Fig. 5.
Variation in pump flow throughout cardiac cycle in relation to LVAD flow and aortic and left ventricular pressures.
Even during diastolic filling, the LVP can be more dynamic, depending on the preload. Previous work analyzing HVAD waveforms have shown steeper increases in the LVP when the left atrial pressure is higher, which translates to a steeper rate of change in flow. Conversely, lower left atrial pressure results in a blunted LVP, and subsequently more stable flows through the LVAD.11,12
Although the beat-to-beat oscillation of the cardiac cycle accounts for the pulsatility seen in the pump flow, more macro hemodynamic changes will result in larger scale shifts in pump flow. Clinical changes that increase the pressure difference, such as a decrease in preload (i.e., Pinflow) or an increase in afterload (i.e., Poutflow), will decrease the flow through the device. The relationship between the pressure difference and flow is thus illustrated through HQ curves.
HQ Curves
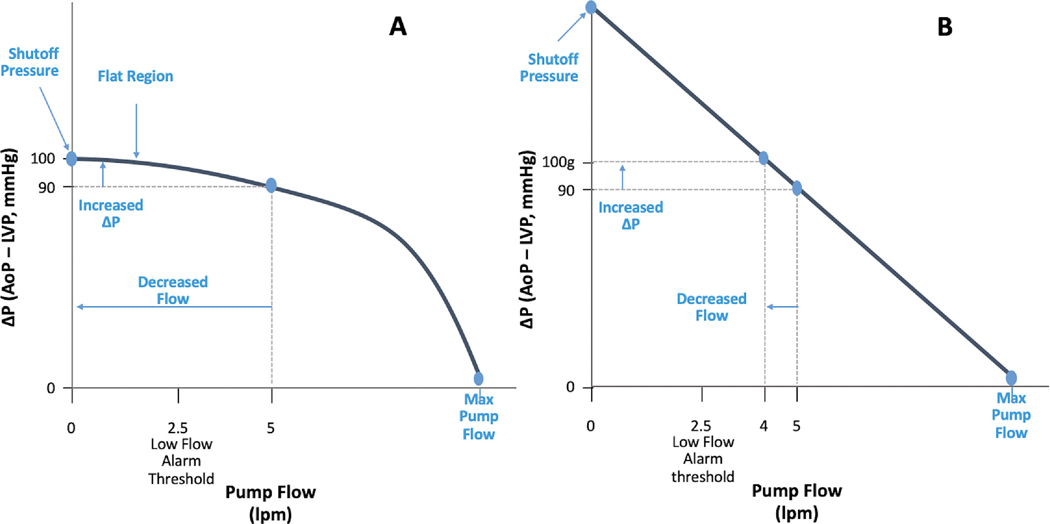
The relationship that determines pump flow is represented graphically with HQ curves. Each LVAD has its own unique HQ curve, meaning that the HQ relationship varies between the HM3, HMII, and HVAD. Additionally, for a given pump, there is a series of HQ curves for all of the various operating speeds. For example, as the speed is increased, the corresponding HQ curve shifts up and to the right. The shutoff pressure is the pressure gradient across the LVAD for a given set speed that leads to net zero flow. Any pressure gradient in excess to the shutoff pressure will lead to retrograde flow down the outflow cannula.
Although the HQ is a dependent relationship, it is not always linear. To illustrate this, 2 sample HQ curves are shown in Figs. 6 A and B. Fig. 6A shows an HQ curve in which the device is flowing on the flat portion of the curve. Here, a change of only ten mmHg will decrease the flow from 5 liters per minute (lpm) to 0 lpm. On the other hand, in Fig. 6B the HQ curve is steeper, and thus a 10 mm Hg pressure decrease only decreases flow from 5 lpm to 4 lpm.
Fig. 6.
A – LVAD operating on the flat part of the HQ curve B – LVAD operating on the steep part of the HQ curve; DeltaP flow. AoP, aortic pressure; LVP, left ventricular pressure.
Clinically, this scenario can be seen when comparing the HMII and the HVAD. The HVAD operates on a much flatter HQ curve, and is thus very afterload sensitive (i.e., a small increase in systemic blood pressure can have an exaggerated effect on the flow). Conversely, the HMII operates on a relatively steep HQ curve and is thus less sensitive to changes in pressure differences (Fig. 7A). The HM3 is unique in that it has a flatter sloped HQ curve when operating at lower flows (i.e., <4 lpm), but steeps when at higher flows (i.e., >4 lpm) (Fig. 7B).
Fig. 7.
A – HeartMate II HQ Curve, B - HeartMate 3 HQ Curve.
Power, Pulsatility, and Flow–Putting It All Together
Although power is the input to the PI equation, power is related to pump flow as defined elsewhere in this article by the power–flow curves. Therefore, PI behavior can be thought of in terms of pump flow (Fig. 8). Any condition that leads to a heightened variation in flow will thus also lead to a heightened variation in power given that directly proportional relationship. As described previously in this article, the lack of precision of flow and power discrimination for the HMII at flows of less than 2.5 L/min means that low flows may be falsely overestimated with this pump leading to a reduction in both estimated flow pulsatility and power pulsatility during clinical scenarios resulting in low flow through the LVAD.
Fig. 8.
Pump flow–pulsatility vs time. PI, pulsatility index.
Variations in Pump Parameters in Clinical Practice
The information on the HMII and HM3 interrogation screens can provide practical information to inform care in a variety of clinical scenarios. The following are common situations seen in patients with an LVAD, and their effects on pump function are described.
Hypovolemia
LVAD function can be complicated by an abnormally low, or high, preload. Hypovolemic states are commonly owing to dehydration, overdiuresis, inappropriately high speeds, or gastrointestinal bleeding. Right ventricular (RV) failure can also cause similar effects as hypovolemia, owing to decreased ability to fill the left side of the heart, and is discussed in greater detail in a later section of this review. When hypovolemic, the lower LVP results in a higher pressure gradient across the pump (i.e., between the left ventricle and the aorta), which results in a decreased average flow. Flow pulsatility will also change, because the diastolic flow and systolic flow are not affected equally by the volume change. Lower filling pressures decrease LVP augmentation owing to the Frank Starling mechanism, leading to a decrease in the systolic flow. Low filling pressures also increase the diastolic pressure differential, which will decrease the diastolic flow. Depending on the LVAD speed and where a given patient is on the HQ curve during diastole (i.e., whether they are operating on the flat part of the HQ curve or steep part of the HQ curve), flow pulsatility can either increase or decrease. The flow and pulsatility differences between normovolemia and hypovolemia are illustrated in Figs. 9 and 10.
Fig. 9.
Normovolemia HQ Curve with Associated Flows throughout Cardiac Cycle.
Fig. 10.
Hypovolemia HQ Curve with Associated Flows throughout Cardiac Cycle.
The interpretation of these flow changes is not universal across all pumps. As discussed elsewhere in this article, the HMII is inaccurate at calculating flow at lower speeds, whereas the HM3 remains accurate. Therefore, the decrease in the average flow and diastolic flow seen with hypovolemia results in a lower PI in HMII, but a comparatively greater PI in with HM3 (Fig. 11).
Fig. 11.
Estimated Flow vs Time.
Hypervolemia
Despite the improved cardiac support from the LVAD, these patients can still become volume overloaded. This condition can be due to inadequate diuresis, inappropriately low speeds, or dietary indiscretion. AI can also cause hypervolemic-type physiology and will be discussed in greater detail. In hypervolemic states, the higher LVP results in a lower pressure gradient across the pump, which results in an increased average flow. Flow pulsatility also changes, because higher filling pressures decrease the diastolic pressure differential and systolic gradient disproportionately. Depending on the LVAD speed and where a given patient is operating on the HQ curve, this can either increase or decrease flow pulsatility. Given the imprecision of flow estimation at high flows, the HM3 may underestimate the peak flow, instead interpreting this as less flow and power variation and thus a decrease in the PI. Conversely, the HMII fully appreciates all of the flow under these settings, and the PI will be comparatively higher. The flow and pulsatility differences between normovolemia and hypervolemia are illustrated in Figs. 9 and 12.
Fig. 12.
Hypervolemia HQ Curve with Associated Flows throughout Cardiac Cycle.
Hypertension
Blood pressure is most often measured manually with the use of a sphygmomanometer and a Doppler signal to assess for the Doppler opening pressure, which is the best noninvasive surrogate for the mean arterial pressure. Owing to the artificial pulse in the HM3, a standard blood pressure may be able to be measured, although the mean arterial pressure seemingly remains more accurate, even in the HM3.13 Regardless of method of blood pressure measurement, patients with an LVAD require a lower mean arterial pressure goal than would normally be considered in a patient with non-LVAD HF owing to the association with adverse outcomes, specifically strokes.14–16 The significant changes in flows can be appreciated by again noting the flattening of the HQ curve at these higher pressures. As described elsewhere in this article, a smaller increase in pressure here significantly affects flow.
Elevated blood pressure will, of course, increase the pressure differential across the pump and the decrease flow, but the diastolic pressure gradient is affected more than the systolic gradient, and pulsatility subsequently increases. As can be visualized in Figs. 13 and 14, while all flows decrease owing to the increased pressure differential, the average and minimum flow decrease out of proportion to the decrease in the maximum flow. Again, there are differences between the HMII and HM3 owing to the inaccurate lower flow estimation. This difference will result in a PI decrease in HMII patients with hypertension, but a PI increase in HM3 patients with hypertension (Fig. 11).
Fig. 13.
Normotensive HQ Curve with Associated Flows throughout Cardiac Cycle.
Fig. 14.
Hypertensive HQ Curve with Associated Flows throughout Cardiac Cycle.
RV Failure
Although the implantation of the HM3 can address the LV dysfunction in patients with end-stage HF, RV failure remains a common complication, occurring in 9%–40% of all patients with an LVAD, and 34% of patients at the 2-year follow-up in the MOMENTUM 3 study.7,17 RV failure is affected early on by a sudden increase in preload from the improved left ventricular output with the LVAD implanted. This leads to increased RV wall stretch, wall tension, and myocardial demand. Additionally, changes in the position of the interventricular septum with LVAD unloading and subsequent reverse remodeling can also decrease RV contractility. RV failure results in a decreased ability to fill the LV, thus decreasing the LV preload. Because the LVAD can only sense what is occurring in the LV, the decreased LV pressure seen in RV failure will resemble the pump parameters of hypovolemia, namely, decreased preload leads to a greater pressure gradient across the pump, thus decreasing flow and increasing the PI (see Fig. 15). The PI will be higher in the HM3 compared with the HMII owing to improved sensitivity of detecting decreased flows (Fig. 11).
Fig. 15.
Right Ventricular Failure HQ Curve with Associated Flows throughout Cardiac Cycle.
Aortic Insufficiency
AI is a relatively common phenomenon in continuous flow patients with an LVAD, noted in 6%–32% of patients with a continuous flow LVAD at 1 year, and up to 24%–33% of patients at 3 years.18–22 AI occurs owing to a combination of decreased aortic valve opening, causing leaflet fusion and deterioration, as well as the continuous, reverse pressure gradient elicited by the pump.19,23–26 It is important to diagnose AI because it associated with increased hospitalizations and mortality,22 and treatment is possible, including surgical or transcatheter valve replacement, percutaneous occlusion devices, or orthotopic heart transplantation.27–29 The diagnosis of AI severity can be difficult on an echocardiogram and has shown to be more accurate with measurement of the systolic-to-diastolic peak velocity ratio and diastolic slope of the outflow canula, although this has not yet been validated in the HM3.30
HM3 pump parameters can help to alert the clinician to the development of AI. Owing to the regurgitant flow, the left ventricular end-diastolic pressure will increase. Simultaneously, enhanced aortic runoff will decrease the aortic pressure, leading to a narrowing of the pressure gradient. This change will result in increased overall mean flow along with a decrease in the PI (Figs. 16 and 17).
Fig. 16.
No Aortic Insufficiency HQ Curve with Associated Flows throughout Cardiac Cycle.
Fig. 17.
Aortic Insufficiency HQ Curve with Associated Flows throughout Cardiac Cycle.
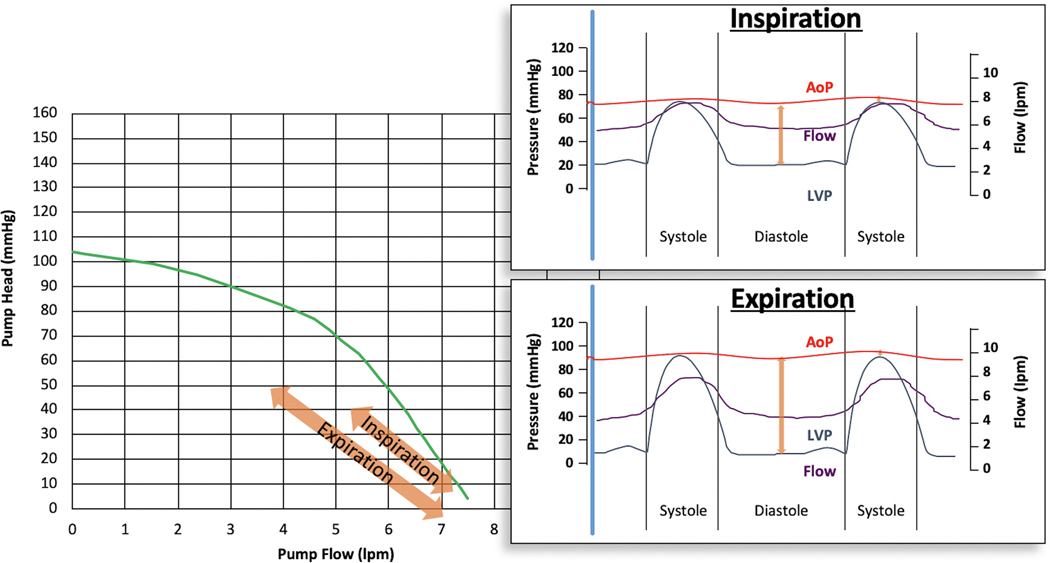
Cardiac Tamponade
The occurrence of cardiac tamponade is not uncommon after LVAD implantation, occurring in approximately 3.2%–7.2% of patients, likely owing to both the operation itself and early use of anticoagulation.31 In cardiac tamponade, the restrictive physiology of early tamponade will cause an initial increase, and equalization, of intracardiac pressures with respiratory variation. During inspiration, preload will increase, which will decrease the diastolic pressure gradient across the LVAD, effectively increasing flow but, decreasing the PI. During expiration, the preload decreases, which increases the diastolic pressure gradient and leads to decreased total flow with an increase in the PI (Fig. 18). Because HMII pumps detect higher flows more accurately than the HM3, the PI will be comparatively higher in the HMII than the HM3 during both inspiration and expiration.
Fig. 18.
Cardiac Tamponade HQ Curves during Inspiration and Expiration with Associated Flows throughout Cardiac Cycle.
Inflow or Outflow Obstruction
Inflow obstructions are most often owing to thrombus. This entity narrows the space in which blood can enter the motor, but as long as thrombus does not get pulled into the rotor itself, it will not cause a rotor thrombosis and the associated power spikes. Outflow graft obstruction is a rare complication, but has been reported, specifically in the HM3. Outflow obstructions can be due to thrombus or fibrin material within the graft, or due to a twisting or kinking of the graft externally. These obstructions often present first as low-flow alarms without evident reason. They can be difficult to diagnose, but a computed tomography scan, echocardiography, and invasive hemodynamics have been shown to be helpful.32–35 Regardless of the etiology, an outflow graft obstruction will narrow the path within which blood is flowing, and significantly increase the outflow pressure and associated gradient throughout the cardiac cycle.
With either an inflow or an outflow cannula obstruction, flow pulsatility, and thus the PI, will decrease owing a lack of harmonic amplification. In health, blood flow in large diameter central vessels is initially amplified owing to harmonic amplification before it is later dampened into a non-pulsatile flow by the arterioles and capillaries in an attempt to act as an elastic buffer to minimize trauma from high-pressure flow at the tissue level.36 In the case of either an inflow or outflow cannula obstruction, impedance at the level of the obstruction leads to a more central damping of the pressure and flow oscillation, thus decreasing the pulsatility and PI. In cases of a complete obstruction, flow will become zero and there will be no pulsatility (Fig. 19)
Fig. 19.
Degree of Inflow or Outflow Obstruction and Associated Harmonic Oscillation.
The occlusions present in both inflow and outflow obstructions are constant throughout the cardiac cycle. This results in decreased maximum and minimum flows, leading to lower total flows, frequently triggering persistent low-flow alarms, and an associated lower PI. It is important to note that the manufacturer’s HQ curves do not apply in this setting; these HQ curves assume that the pump and all of the cannulae are anatomically intact. In the setting of inflow or outflow cannula malfunction, the pressure and flow relationships are less relevant owing to the fixed obstruction. The HMII will display a lower PI than the HM3 owing to the improved sensitivity of lower flows seen with the HM3 (Fig. 11).
Ventricular Arrhythmias
Prolonged ventricular arrhythmias, whether tachycardia or fibrillation, often result in RV failure. Subsequently, pump parameters will most often resemble that of RV failure. Although the LV benefits from continuous LVAD support, the RV does not. As ventricular tachycardia or fibrillation continues, the RV will not contract, thus LV preload will decrease, the pump pressure gradient will increase, and flows will decrease. Similarly, the LV also will lose native contractility during active ventricular arrhythmias, and there will be minimal changes in pressure during the cardiac cycle, resulting in decreased pulsatility. In contrast, a premature ventricular contraction, which by definition is nonsustained, will only cause a brief decrease in preload followed by a compensatory pause that allows for more time for filling of the left ventricle. Thus, the oscillation between normal and decreased flows will manifest as an increase in pulsatility.
Intra-aortic Balloon Pump
In the context of LVADs, IABPs are usually only seen in the acute postoperative setting, often as a remnant of pre-LVAD cardiac support. Although this is support most often temporary, it is important to be able to interpret the pump parameters in the setting of LVAD use. IABPs augment systolic flow by the creation of a vacuum effect during systolic deflation, and augment coronary perfusion pressure by diastolic inflation. Additionally, energy is stored in the walls of the compliant aorta during balloon inflation and the elastic recoil after balloon deflation further promotes enhanced diastolic runoff and a decrease in late diastolic pressures. In the setting of a continuous flow LVAD, the early diastolic augmentation will increase the pressure gradient across the pump leading to a decrease in the diastolic LVAD flow, whereas balloon deflation during systole will decrease the pressure gradient across the LVAD and augment systolic flows. Whether or not this increases or decreases the mean flow for the patient will depend on their loading conditions and hemodynamics with the IABP off. In the example (Fig. 20), the patient is hypervolemic and, thus, has higher flows and a lower PI. Regardless of the patients baseline conditions, the hemodynamic effects of the IABP will increase the PI.
Fig. 20.
Intra-aortic Balloon Pump HQ Curves during “On” and “Off” Modes with Associated Flows throughout Cardiac Cycle.
Changes in RPM
Each pump has its own unique set of HQ curves. As discussed throughout this review, on a beat-to-beat basis, flow varies along the continuum of the specific HQ curve the patient is on, which depends on the type of pump and the patient’s set speed. If the patient’s set speed is adjusted, their flow and pulsatility will change as well. Decreasing a patient’s RPM will decrease their mean flow and increase pulsatility, whereas increasing their RPMs will increase the mean flow and comparatively decrease pulsatility (Fig. 21).
Fig. 21.
High and Low RPM HQ Curves with Associated Flows throughout Cardiac Cycle.
Recovery
Most patients with an LVAD remain on durable mechanical circulatory support until cardiac transplantation or death. However, INTERMACS registry data indicate that approximately 1% of patients with an LVAD will be explanted per year, and over a 3-year period 9% fit clinical criteria to be explanted.37 A great deal of research is ongoing in the field of cardiac recovery in patients with an LVAD with the aim of increasing this percentage. Recently, a prospective, multicenter trial of an optimization protocol including medications and frequent echocardiography resulted in a 40% explant rate among 36 patients with an HMII.38 It is, therefore, important to recognize evidence of underlying cardiac recovery in a patient with a HMII or HM3 LVAD.
As the heart recovers, it will generate increasingly more pressure in systole, to the point of overcoming the aortic pressure and opening the aortic valve on every beat. Specifically, if the systolic pressure generated is great enough to overcome the aortic pressure and open the aortic valve, then the pump will be at maximum flow, because the difference in the pressure head reaches zero. A patient with an LVAD with cardiac recovery would have an increase in peak and mean flows, and therefore have high flows with high pulsatility. However, in recovery, often there is enhanced unloading of the left ventricle leading to higher diastolic pressure gradients with associated decreases in diastolic flow. If this diastolic decrease in flow outpaces the systolic augmentation, there may be a decrease in the total mean flow. In either situation, pulsatility will remain high (Fig. 22).
Fig. 22.
Cardiac Recovery HQ Curve with Associated Flows throughout Cardiac Cycle.
Summary or Approach
LVAD pump parameters should be used in the context of an individual patient’s history and clinical examination, with serum laboratory markers and transthoracic echocardiography as indicated. Within that context, flow, which is derived from power, should be assessed first (Fig. 23). In the HM3 high flow is concerning for AI, hypervolemia, or cardiac recovery, whereas in the HMII rotor thrombus should be considered as well. Next, the PI should be evaluated. In the HM3, a high PI should bring severe hypovolemia, hypertension, cardiac tamponade, and cardiac recovery to the top of the differential, along with presence of an IABP. RV failure, hypervolemia, and some arrhythmias may present similarly, although the PI in these syndromes can be variable. A low PI in a HM3 should prompt consideration of inflow or outflow obstruction and AI (Table 2). Conversely, in the HMII, the PI will be increased with hypervolemia or in the presence of an IABP, but decreased in all of the other clinical syndromes discussed in this review (Table 3). Finally, PI events will be noted in all of these situations, except for AI, if the pump parameter change is clinically meaningful. To this end, the pump should be interrogated as part of routine evaluation.
Fig. 23.
Summary of Differential Diagnosis Based on Pump Flow and Pulsatility.
Table 2.
Summary of HeartMate 3 Pump Parameter Changes
| Condition | Flow Estimate | Power | PI | PI Event |
|---|---|---|---|---|
|
| ||||
| Typical range | 3–6 Ipm | 3–6 w | 2–6 | None |
| When to call | Drop of ≥1 L from | ±2 from | ±2 from baseline | |
| baseline | baseline | OR < 2 | ||
| Severe hypovolemia | ↓ | ↓ | ↑ | Yes |
| Hypertension | ↓ | ↓ | ↑ | Yes |
| Tamponade | ↓ | ↓ | ↑ | Yes |
| Severe RV failure | ↓ | ↓ | Variable | Yes |
| Arrhythmias | ↓ | ↓ | Variable | Yes |
| Inflow obstruction | ↓ | ↓ | ↓ | Yes |
| Outflow obstruction | ↓ | ↓ | ↓ | Yes |
| Aortic insufficiency | ↑ | ↑ | ↓ | Less frequent |
| Cardiac recovery | Variable | Variable | ↑ | Variable |
| IABP | ↓ | ↓ | ↑ | More frequent |
| Rotor thrombus | ↑ | ↑ | ↓ | Less frequent |
IABP, intra-aortic balloon pump; OR, odds ratio; PI, pulsatility index, RV, right ventricular.
Table 3.
Summary of HeartMate II Pump Parameter Changes
| Condition | Flow Estimate | Power | PI | PI Event |
|---|---|---|---|---|
|
| ||||
| Typical range | 3–6 Ipm | 3–6 w | 2–6 | None |
| When to call | Drop of ≥ 1 L from baseline | ±2 from baseline | ±2 from baseline OR < 2 | |
| Severe hypovolemia | ↓ | ↓ | ↓ | Yes |
| Hypertension | ↓ | ↓ | ↓ | Yes |
| Tamponade | ↓ | ↓ | ↓ | Yes |
| Severe RV failure | ↓ | ↓ | ↓ | Yes |
| Arrhythmias | ↓ | ↓ | ↓ | Yes |
| Inflow obstruction | ↓ | ↓ | ↓ | Yes |
| Outflow obstruction | ↓ | ↓ | ↓ | Yes |
| Aortic insufficiency | ↑ | ↑ | ↓ | Less frequent |
| Cardiac recovery | Variable | Variable | ↑ | Variable |
| IABP | No change | No change | ↑ | Yes |
| Rotor thrombus | ↑ | ↑ | ↓ | Less frequent |
Abbreviations as in Table 2.
Conclusions
The accurate interpretation of LVAD pump parameters is a key aspect of clinical care for this patient population. The ability to incorporate these variables into standard practice is imperative to the accurate evaluation, diagnosis, and treatment of patients with LVADs. Finally, we anticipate an improved understanding of patient hemodynamics and their associated pump parameters will benefit patient care and further research in the field.
Supplementary Material
Lay Summary.
Left ventricular assist devices are implanted for durable, long-term therapy in patients with advanced heart failure. These heart pumps provide continuous flow to the body, but the rate and pattern of flow changes will change in response to clinical conditions.
This review covers the underlying mechanisms related to these changes in pump flow, so as to improve clinician understanding and interpretation.
The accurate interpretation of left ventricular assist device pump parameters is a key aspect of clinical care for this patient population. The ability to incorporate these variables into standard practice is imperative to the accurate evaluation, diagnosis, and treatment of patients with LVADs
Acknowledgments
The authors thank Abbott Laboratories (Abbott Park, IL) for providing copyright authorization to include and publish select figures in this manuscript.
Footnotes
Supplementary materials
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.cardfail.2021.11.016.
References
- 1.Miller LW. Left ventricular assist devices are underutilized. Circulation 2011;123:1552–8. [DOI] [PubMed] [Google Scholar]
- 2.Kittleson MM, Shah P, Lala A, et al. INTERMACS profiles and outcomes of ambulatory advanced heart failure patients: a report from the REVIVAL Registry. J Heart Lung Transplant 2020;39:16–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ambardekar AV, Kittleson MM, Palardy M, et al. Outcomes with ambulatory advanced heart failure from the Medical Arm of Mechanically Assisted Circulatory Support (MedaMACS) Registry. J Heart Lung Transplant 2019;38:408–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Truby LK, Rogers JG. Advanced heart failure: epidemiology, diagnosis, and therapeutic approaches. JACC Heart Fail 2020;8:523–36. [DOI] [PubMed] [Google Scholar]
- 5.Khush KK, Cherikh WS, Chambers DC, et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Thirty-sixth adult heart transplantation report - 2019; focus theme: donor and recipient size match. J Heart Lung Transplant 2019;38:1056–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Teuteberg JJ, Cleveland JC Jr, Cowger J, et al. The Society of Thoracic Surgeons INTERMACS 2019 annual report: the changing landscape of devices and indications. Ann Thorac Surg 2020;109:649–60. [DOI] [PubMed] [Google Scholar]
- 7.Mehra MR, Goldstein DJ, Uriel N, et al. Two-year outcomes with a magnetically levitated cardiac pump in heart failure. N Engl J Med 2018;378:1386–95. [DOI] [PubMed] [Google Scholar]
- 8.Njoku N. Urgent medical device communication notification letter Medtronic HVAD System. Dublin: Medtronic; 2021. [Google Scholar]
- 9.Rich JD, Burkhoff D. HVAD flow waveform morphologies: theoretical foundation and implications for clinical practice. ASAIO J 2017;63:526–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bourque K, Cotter C, Dague C, et al. Design rationale and preclinical evaluation of the HeartMate 3 left ventricular assist system for hemocompatibility. ASAIO J 2016;62:375–83. [DOI] [PubMed] [Google Scholar]
- 11.Grinstein J, Rodgers D, Kalantari S, et al. HVAD waveform analysis as a noninvasive marker of pulmonary capillary wedge pressure: a first step toward the development of a smart left ventricular assist device pump. ASAIO J 2018;64:10–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grinstein J, Imamura T, Kruse E, et al. Echocardiographic predictors of hemodynamics in patients supported with left ventricular assist devices. J Card Fail 2018;24:561–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li S, Beckman JA, Welch NG, et al. Accuracy of Doppler blood pressure measurement in continuous-flow left ventricular assist device patients. ESC Heart Fail 2019;6:793–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Teuteberg JJ, Slaughter MS, Rogers JG, et al. The HVAD left ventricular assist device: risk factors for neurological events and risk mitigation strategies. JACC Heart Fail 2015;3:818–28. [DOI] [PubMed] [Google Scholar]
- 15.Najjar SS, Slaughter MS, Pagani FD, et al. An analysis of pump thrombus events in patients in the Heart-Ware ADVANCE bridge to transplant and continued access protocol trial. J Heart Lung Transplant 2014;33:23–34. [DOI] [PubMed] [Google Scholar]
- 16.Feldman D, Pamboukian SV, Teuteberg JJ, et al. The 2013 International Society for Heart and Lung Transplantation Guidelines for mechanical circulatory support: executive summary. J Heart Lung Transplant 2013;32:157–87. [DOI] [PubMed] [Google Scholar]
- 17.Cogswell R, John R, Shaffer A. Right ventricular failure after left ventricular assist device. Cardiol Clin 2020;38: 219–25. [DOI] [PubMed] [Google Scholar]
- 18.Kirklin JK, Pagani FD, Kormos RL, et al. Eighth annual INTERMACS report: special focus on framing the impact of adverse events. J Heart Lung Transplant 2017;36:1080–6. [DOI] [PubMed] [Google Scholar]
- 19.Jorde UP, Uriel N, Nahumi N, et al. Prevalence, significance, and management of aortic insufficiency in continuous flow left ventricular assist device recipients. Circ Heart Fail 2014;7:310–9. [DOI] [PubMed] [Google Scholar]
- 20.Soleimani B, Haouzi A, Manoskey A, Stephenson ER, El-Banayosy A, Pae WE. Development of aortic insufficiency in patients supported with continuous flow left ventricular assist devices. ASAIO J 2012;58:326–9. [DOI] [PubMed] [Google Scholar]
- 21.Holley CT, Fitzpatrick M, Roy SS, et al. Aortic insufficiency in continuous-flow left ventricular assist device support patients is common but does not impact long-term mortality. J Heart Lung Transplant 2017;36:91–6. [DOI] [PubMed] [Google Scholar]
- 22.Truby LK, Garan AR, Givens RC, et al. Aortic insufficiency during contemporary left ventricular assist device support: analysis of the INTERMACS registry. JACC Heart Fail 2018;6:951–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pak SW, Uriel N, Takayama H, et al. Prevalence of de novo aortic insufficiency during long-term support with left ventricular assist devices. J Heart Lung Transplant 2010;29:1172–6. [DOI] [PubMed] [Google Scholar]
- 24.Mudd JO, Cuda JD, Halushka M, Soderlund KA, Conte JV, Russell SD. Fusion of aortic valve commissures in patients supported by a continuous axial flow left ventricular assist device. J Heart Lung Transplant 2008; 27:1269–74. [DOI] [PubMed] [Google Scholar]
- 25.Holtz J, Teuteberg J. Management of aortic insufficiency in the continuous flow left ventricular assist device population. Curr Heart Fail Rep 2014;11:103–10. [DOI] [PubMed] [Google Scholar]
- 26.Sayer G, Sarswat N, Kim GH, et al. The hemodynamic effects of aortic insufficiency in patients supported with continuous-flow left ventricular assist devices. J Card Fail 2017;23:545–51. [DOI] [PubMed] [Google Scholar]
- 27.Yehya A, Rajagopal V, Meduri C, et al. Short-term results with transcatheter aortic valve replacement for treatment of left ventricular assist device patients with symptomatic aortic insufficiency. J Heart Lung Transplant 2019;38:920–6. [DOI] [PubMed] [Google Scholar]
- 28.Phan K, Haswell JM, Xu J, et al. Percutaneous transcatheter interventions for aortic insufficiency in continuous-flow left ventricular assist device patients: a systematic review and meta-analysis. ASAIO J 2017;63: 117–22. [DOI] [PubMed] [Google Scholar]
- 29.Belkin MN IT, Fujino T, Kanelidis AJ, et al. Transcatheter aortic valve replacement in left ventricular assist device patients with aortic regurgitation. Structural Heart 2020;4:107–12. [Google Scholar]
- 30.Grinstein J, Kruse E, Sayer G, et al. Accurate quantification methods for aortic insufficiency severity in patients with LVAD: role of diastolic flow acceleration and systolic-to-diastolic peak velocity ratio of outflow cannula. JACC Cardiovasc Imaging 2016;9:641–51. [DOI] [PubMed] [Google Scholar]
- 31.Briasoulis A, Inampudi C, Akintoye E, Adegbala O, Alvarez P, Bhama J. Trends in utilization, mortality, major complications, and cost after left ventricular assist device implantation in the United States (2009 to 2014). Am J Cardiol 2018;121:1214–8. [DOI] [PubMed] [Google Scholar]
- 32.Belkin MN, Venturini J, Nathan S, Grinstein J. Left ventricular assist device performance under pressure: troubleshooting outflow graft dysfunction. Circ Heart Fail 2020;13:e007098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaku Y, Naka Y, Witer L, et al. Late inflow or outflow obstruction requiring surgical intervention after HeartMate 3 left ventricular assist device insertion. Interact Cardiovasc Thorac Surg 2020;31:626–8. [DOI] [PubMed] [Google Scholar]
- 34.Aghayev A, Mehra MR. Cardiac CT to diagnose left ventricular assist device outflow graft twist. Radiology 2019;292:537. [DOI] [PubMed] [Google Scholar]
- 35.Su L, Hironaka CE, Chen FY, Couper GS, Kiernan MS, Kawabori M. Untwist the twist: instant hemodynamic improvement in known HeartMate 3 complication. J Artif Organs 2020. [DOI] [PubMed] [Google Scholar]
- 36.Ooi H, Chung W, Biolo A. Arterial stiffness and vascular load in heart failure. Congest Heart Fail 2008;14:31–6. [DOI] [PubMed] [Google Scholar]
- 37.Topkara VK, Garan AR, Fine B, et al. Myocardial recovery in patients receiving contemporary left ventricular assist devices: results from the Interagency Registry for Mechanically Assisted Circulatory Support (INTER-MACS). Circ Heart Fail 2016;9:e002897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Birks EJ, Drakos SG, Patel SR, et al. Prospective Multicenter Study of Myocardial Recovery Using Left Ventricular Assist Devices (RESTAGE-HF [Remission from Stage D Heart Failure]): medium-term and primary end point results. Circulation 2020;142:2016–28. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.