Abstract
Objective
The purpose of this study was to describe the clinical presentation and physiologic profile of individuals with varying degrees of severity of multisystem inflammatory syndrome in children (MIS-C).
Methods
We performed a retrospective study of children diagnosed with MIS-C admitted to a single quaternary children’s hospital from May 2020 to April 2021. We created an MIS-C severity score using the following parameters: hospital admission status (e.g., floor vs intensive care unit), need for inotropic or vasoactive medications, and need for mechanical ventilation. Univariate and multivariate analyses were performed to associate risk factors corresponding to the MIS-C severity score.
Results
The study included 152 children who were followed for 14 days post hospital admission. A stepwise forward selection process identified seven physiologic variables associated with “severe” MIS-C according to a logistic regression. Specifically, a combination of elevated creatinine (p = 0.013), international normalized ratio (p = 0.002), brain natriuretic peptide (p = 0.001), white blood cell count (p = 0.009), ferritin (p = 0.041), respiratory rate (p = 0.047), and decreased albumin (p = 0.047) led to an excellent discrimination between mild versus severe MIS-C (AUC = 0.915).
Conclusion
This study derived a physiologic profile associated with the stratification of MIS-C severity.
Impact
Based on a cohort of 152 individuals diagnosed with MIS-C, this study derived a nomenclature that stratifies the severity of MIS-C.
Investigated demographic, presentational vital signs, and blood analytes associated with severity of illness.
Identification of a multivariate physiologic profile that strongly associates with MIS-C severity.
This model allows the care team to recognize patients likely to require a higher level of intensive care.
Introduction
Multisystem inflammatory syndrome in children (MIS-C) is a serious hyperinflammatory condition following an acute infection with the SARS-CoV-2 virus in children and adolescents and characterized by fever, rash, conjunctival injection, and inflammation of the heart, lungs, kidneys, brain, or gastrointestinal organs.1,2 MIS-C has varying degrees of clinical presentation and in the more severe forms, it affects multiple body systems.3–5 Given the novelty of MIS-C, previous literature has mainly focused on describing the typical stigmata of the disease, the similarities to and differences from Kawasaki disease, and hospital course after administration of intravenous immune globulin (IVIG) and/or corticosteroids.6–15 More recently, studies describe the clinical spectrum of MIS-C and the correlation of certain factors (e.g., demographics, laboratory markers) with disease severity.3,16,17
Despite increasing knowledge regarding MIS-C, questions still remain. For instance, which children with coronavirus disease 2019 (COVID-19) will develop MIS-C? In addition, which children with MIS-C exhibit a “severe” form of the disease? While some children recover with supportive therapy, others require aggressive treatment before the clinical response. Thus, our goal was to design a novel MIS-C severity score (MSS) quantifying the level of critical care for hospitalized MIS-C patients and to depict the physiologic profile of patients who experience a severe course of illness. Using granular data, usually not possible to accrue in a multi-center study, we created a model, consisting of demographic characteristics, early laboratory results, and first-registered vital signs, to identify patients who develop severe MIS-C.
Methods
Study population and physiologic data collection
This retrospective study evaluates children admitted to Texas Children’s Hospital, USA, with a diagnosis of MIS-C, between May 2020 and April 2021. The Institutional Review Board of Baylor College of Medicine approved this study with a waiver of consent under protocol H-49737. Subjects were identified from our institutional database that includes all patients admitted with MIS-C according to the US Centers for Disease Control and Prevention (CDC) case definition: age <21 years presenting with fever; laboratory evidence of inflammation; severe illness with multisystem involvement (≥2 organs); no alternative plausible diagnosis; and positive for current or recent SARS-CoV-2 infection by reverse transcription-polymerase chain reaction (RT-PCR) test, serology or antigen test, or COVID-19 exposure within the preceding 4 weeks.1 The timing of the diagnosis was typically within 12 h of presentation to our hospital depending on the availability of the RT-PCR and/or serology testing. The patient database was adjudicated by an internal group of experts including clinicians from the critical care, cardiology, rheumatology, and infectious disease divisions of the hospital.
Data collected from the electronic medical record includes demographic characteristics (age, sex, race, and ethnicity) and clinical characteristics (weight, height, body mass index [BMI], date of hospital admission and discharge, date of intensive care unit admission and discharge, and dates of intubation and extubation).
All medication records, laboratory results, and monitored vital signs were retrieved using the Sickbay data platform (Medical Informatics Corp, Houston, TX). Medications missing dosages or with no reported date/time of administration were excluded from the analysis. Laboratory data obtained included the first recorded results of the following:
Immune activation/infection/inflammation—white blood cells (WBC), neutrophils, lymphocytes, procalcitonin, ferritin, fibrinogen, C-reactive protein (CRP).
Cardiovascular—brain natriuretic peptide (BNP), troponin I, lactate.
Renal—creatinine, blood urea nitrogen (BUN), electrolytes.
Gastrointestinal/liver—albumin, aspartate transaminase (AST), alanine transaminase (ALT), gamma-glutamyl transferase (GGT), alkaline phosphatase.
Coagulation—international normalized ratio (INR), partial thromboplastin time (PTT), prothrombin time (PT), thrombin time, fibrinogen, D dimer.
Hematologic—hemoglobin (HGB), hematocrit (HCT), platelets, mean corpuscular hemoglobin (MCH).
Recorded vital sign data included heart rate (HR), peripheral oxygen saturation (SpO2), invasive and noninvasive mean (mBP), systolic (sBP), and diastolic (dBP) blood pressure, and respiratory rate (RR). These signals are synchronized and captured at a sampling frequency of 0.5 Hz. We retrieved the first 3 h of the vital signs for each patient and computed an average as the representative value for each patient. The results of the first recorded echocardiographic measurement were also retrieved to assess the left-ventricular ejection fraction (LVEF) and changes in coronary vessel diameter. A minimal portion of the laboratory tests returned as “failed” or “insufficient sample” for a valid result. These missing data were imputed using the median from the cohort for each lab type.
Definition of the MIS-C severity score and outcome
To quantify the disease severity, we defined an MSS as a 5-point ordinal scale from 0 to 4 as follows: MSS = 4 was assigned to a patient being in the hospital ICU on mechanical ventilation with or without vasoactive-inotropic support; MSS = 3 was assigned to a patient in the hospital ICU with vasoactive-inotropic support but not on mechanical ventilation; MSS = 2 corresponded to a patient being in the hospital ICU but without vasoactive-inotropic support or mechanical ventilation; MSS = 1 was assigned to a patient who was in the hospital but not in the ICU nor on vasoactive-inotropic support or mechanical ventilation; MSS = 0 described a patient who was discharged. This scale quantified an accumulation of critical care for the MIS-C patients. The MSS was assessed on a daily basis, and it is important to note that a patient’s MSS could change from day to day as the level of care escalated or deescalated during hospitalization. We also defined peak-MSS as the greatest MSS experienced by a patient over their hospitalization.
The primary outcome of the study was a binary variable defined as “severe MIS-C” for those patients whose peak-MSS was greater than or equal to 3, and “mild MIS-C” for those patients whose peak-MSS was less than 3. Using this binary outcome, we performed analyses summarizing the admission physiologic profile of patients based on demographics, early laboratory results, and first-registered vital signs.
Statistical analysis
Analysis of vital signs was preceded by z score transformation based on the age- and sex-adjusted mean and standard deviation for each of the vital signs from the healthy population as published by Baker-Smith et al.18 The BMI was also transformed to age- and sex-adjusted z scores using growth charts published by the US CDC.19 Comparison of any two groups occurred using non-parametric two-sided Wilcoxon rank-sum test for continuous variables (laboratory results, vital signs, age, BMI) and Fisher’s exact test to assess the frequency of categorical variables. The association between physiologic data and the binary labeling of mild vs severe MIS-C was first tested using univariate logistic regression analyses. We also examined associations between the physiologic variables and the primary outcome via a multivariate logistic regression model. We employed a stepwise forward selection process based on the Akaike information criterion (AIC) and statistical significance of the variables. At each step, the selection process examined all remaining variables to add the variable that decreased the AIC the most while maintaining significant p values for included variables. The AIC penalizes models with too many variables that do not add sufficient information. Thus, this selection process excludes the least important variables to arrive at a model with a reduced number of significant variables.20 To test against overfitting, the reduced multivariate logistic model was analyzed using the TRIPOD guidelines for a Type 1b test using re-sampling of the data.21 The level of collinearity among covariates was quantified using the variance of inflation (VIF) coefficient. For both the univariate and multivariate, the continuous variables were normalized by subtracting the median and dividing by the interquartile range. All analyses were performed using the “Statsmodels v0.12.2” library available in the Python environment. For all tests, statistical significance was concluded at a p value <0.05.
Results
Demographics and clinical data for the entire cohort
Table 1 demonstrates the details of patient demographics. The cohort included 152 patients of age <6 years (30%), in between 6 and 12 years (42%), and >12 years (28%). There was a predominance of male children (58%). The cohort was predominantly Hispanic (56%). Based on age- and sex-adjusted percentiles for the general population, the BMI for the cohort was significantly skewed toward overweight. Thirty-six percent of the cohort presented with reduced LVEF (<55%). The median length of hospital stay was 6.8 days (IQR = 4.8–9.0 days). One hundred and six (70%) patients were admitted to an ICU at some point during their hospitalization. For these patients, the length of stay in the ICU had a median of 3.8 days (IQR = 2.6–6.4 days).
Table 1.
Demographic and clinical characteristics of the cohort.
| Variables | Entire cohort, n (%) | Mild MIS-C, n (%) | Severe MIS-C, n (%) | p value |
|---|---|---|---|---|
| Overall frequencies | 152 (100) | 87 (57) | 65 (43) | n/a |
| Age | ||||
| <6 years | 45 (30) | 35 (40) | 10 (15) | <0.001 |
| 6–12 years | 64 (42) | 34 (39) | 30 (46) | 0.239 |
| >12 years | 43 (28) | 18 (21) | 25 (38) | 0.013 |
| Sex | ||||
| Female | 64 (42) | 37 (43) | 27 (42) | 0.518 |
| Male | 88 (58) | 50 (57) | 38 (58) | 0.518 |
| Race/ethnicity | ||||
| Hispanic/Latino | 85 (56) | 51 (59) | 34 (52) | 0.271 |
| Non-Hispanic black | 31 (20) | 13 (15) | 18 (28) | 0.043 |
| Non-Hispanic white | 25 (16) | 16 (18) | 9 (14) | 0.301 |
| Non-Hispanic Asian | 6 (4) | 3 (3) | 3 (5) | 0.514 |
| Non-Hispanic other | 5 (3) | 4 (5) | 1 (2) | 0.287 |
| BMI | ||||
| Underweight (BMI < 5th) | 10 (7) | 7 (8) | 3 (5) | 0.309 |
| Healthy weight (5th ≤ BMI < 85th) | 73 (48) | 50 (57) | 23 (35) | 0.006 |
| Overweight (85th ≤ BMI) | 69 (45) | 30 (34) | 39 (60) | 0.001 |
| Echocardiography | ||||
| LVEF < 55% | 54 (36) | 18 (21) | 36 (55) | <0.001 |
| Coronary dilation | 40 (26) | 24 (28) | 16 (25) | 0.413 |
| Medications | ||||
| Immunomodulators (IVIG or Anakinra) | 123 (81) | 60 (69) | 63 (97) | <0.001 |
| Steroids | 146 (96) | 81 (93) | 65 (100) | 0.033 |
| Steroids alone | 28 (18) | 26 (30) | 2 (3) | <0.001 |
| Steroids + IVIG, but not Anakinra | 31 (20) | 29 (33) | 2 (3) | <0.001 |
| Steroids + Anakinra, but not IVIG | 50 (33) | 12 (14) | 38 (58) | <0.001 |
| Steroids + IVIG + Anakinra | 37 (24) | 14 (16) | 23 (35) | 0.005 |
| Volume expansion | 140 (92) | 80 (92) | 60 (92) | 0.593 |
| Calcium chloride bolus | 28 (18) | 2 (2) | 26 (40) | <0.001 |
| Anticoagulant | 99 (65) | 40 (46) | 59 (91) | <0.001 |
| Antiplatelet | 93 (61) | 52 (60) | 41 (63) | 0.404 |
| Therapy initiation | ||||
| Immunomodulator initiation within 24 h | 92 (61) | 37 (43) | 55 (85) | <0.001 |
| Immunomodulator initiation within 48 h | 110 (72) | 51 (59) | 59 (91) | <0.001 |
| Steroid initiation within 24 h | 116 (76) | 59 (68) | 57 (88) | 0.003 |
| Steroid initiation within 48 h | 137 (90) | 75 (86) | 62 (95) | 0.051 |
| Hospitalization | ||||
| Prolonged hospital stay (>7 days) | 73 (48) | 22 (25) | 51 (78) | <0.001 |
| Prolonged ICU stay (>4 days) | 52 (34) | 10 (11) | 42 (65) | <0.001 |
Body mass index (BMI) was categorized using age- and sex-adjusted growth percentile curves published by the CDC. The p values were computed using Fisher’s method to test differences in frequencies between mild MIS-C and severe MIS-C groups for each row.
LVEF left-ventricular ejection fraction.
Table 2 summarizes the vital signs as z scores (referent to age- and sex-adjusted mean and standard deviation for healthy population). Of note, patients typically presented with tachycardia, tachypnea, and hypotension. Blood oxygen saturation levels were consistently normal in the cohort, but these measurements did not account for the level of respiratory support that the patients may have received upon admission.
Table 2.
Continuous variables.
| Variable name | Units | Entire cohort median (IQR) | Mild MIS-C median (IQR) | Severe MIS-C median (IQR) | p value |
|---|---|---|---|---|---|
| Cardiac markers | |||||
| Lactate | mmol/L | 1.60 (1.50–1.62) | 1.60 (1.60–1.60) | 1.80 (1.30–2.90) | 0.016 |
| BNP | pg/mL | 132.70 (42.08–400.05) | 67.80 (28.35–232.60) | 349.00 (115.90–851.50) | <0.001 |
| Troponin | ng/mL | 0.02 (0.01–0.12) | 0.01 (0.01–0.05) | 0.09 (0.01–0.42) | <0.001 |
| LVEF | % | 59.00 (50.75–64.06) | 62.09 (56.00–65.04) | 53.00 (44.00–60.95) | <0.001 |
| Inflammatory/infection markers | |||||
| C-reactive protein | mg/dL | 18.05 (11.25–24.20) | 15.90 (7.80–21.15) | 22.70 (15.50–29.10) | <0.001 |
| Ferritin | ng/mL | 303.00 (176.50–606.25) | 207.00 (125.50–399.50) | 457.00 (271.00–782.00) | <0.001 |
| Fibrinogen | mg/dL | 559.50 (504.75–672.75) | 551.00 (506.00–623.00) | 583.00 (501.00–719.00) | 0.120 |
| Lymph | 103/µL | 1.02 (0.69–1.65) | 1.02 (0.73–1.75) | 0.95 (0.67–1.47) | 0.390 |
| Procalcitonin | ng/mL | 3.94 (1.55–11.36) | 2.25 (1.13–6.99) | 8.07 (3.29–26.45) | <0.001 |
| Segmented neut | % | 81.40 (74.28–86.15) | 80.40 (69.20–83.30) | 84.70 (79.30–88.30) | <0.001 |
| WBC | 103/µL | 9.49 (7.21–12.43) | 8.08 (6.09–11.14) | 11.41 (9.13–13.84) | <0.001 |
| Coagulation markers | |||||
| Platelets count | 103/µL | 158.00 (113.75–208.25) | 173.00 (132.50–230.50) | 140.00 (102.00–191.00) | 0.009 |
| D dimer | µg/mL | 3.21 (2.12–4.81) | 2.84 (1.92–4.14) | 3.63 (2.68–5.66) | 0.004 |
| PTT | s | 33.10 (31.07–36.65) | 33.10 (31.50–34.35) | 33.30 (29.40–39.00) | 0.296 |
| INR | 1.20 (1.20–1.30) | 1.20 (1.10–1.20) | 1.30 (1.20–1.40) | <0.001 | |
| Protime | s | 15.60 (14.97–16.50) | 15.60 (14.35–15.60) | 16.00 (15.30–17.20) | <0.001 |
| Thrombin time | s | 15.50 (14.95–15.90) | 15.50 (15.40–16.20) | 15.30 (14.50–15.90) | 0.021 |
| Renal markers | |||||
| Sodium | mmol/L | 133.00 (130.00–135.00) | 133.00 (131.00–135.00) | 132.00 (129.00–134.00) | 0.055 |
| Potassium | mmol/L | 3.90 (3.60–4.22) | 4.00 (3.70–4.30) | 3.70 (3.40–4.10) | <0.001 |
| Magnesium | mg/dL | 2.10 (2.00–2.30) | 2.10 (2.00–2.30) | 2.10 (2.00–2.40) | 0.736 |
| Phosphorus | mg/dL | 3.60 (3.20–4.10) | 3.70 (3.35–4.10) | 3.50 (2.90–4.00) | 0.077 |
| Ionized calcium | mmol/L | 1.16 (1.15–1.17) | 1.16 (1.15–1.17) | 1.16 (1.10–1.20) | 0.407 |
| BUN | mg/dL | 13.00 (10.00–20.00) | 12.00 (9.50–13.50) | 18.00 (13.00–28.00) | <0.001 |
| Creatinine | mg/dL | 0.47 (0.34–0.79) | 0.38 (0.31–0.54) | 0.73 (0.46–1.18) | <0.001 |
| GI/liver markers | |||||
| AST | U/L | 56.00 (41.00–77.25) | 52.00 (40.00–71.50) | 59.00 (44.00–84.00) | 0.091 |
| ALT | U/L | 37.00 (24.00–60.25) | 30.00 (22.00–50.00) | 43.00 (26.00–67.00) | 0.018 |
| GGT | U/L | 42.00 (31.00–62.75) | 42.00 (25.00–42.00) | 51.00 (37.00–89.00) | <0.001 |
| Alkaline phosphatase | U/L | 138.00 (107.75–162.25) | 138.00 (108.50–151.00) | 139.00 (104.00–166.00) | 0.485 |
| Albumin | g/dL | 3.50 (3.10–3.82) | 3.60 (3.30–3.90) | 3.20 (2.90–3.70) | <0.001 |
| Hematological markers | |||||
| HGB | g/dL | 11.20 (10.38–12.30) | 11.20 (10.30–12.10) | 11.30 (10.50–12.30) | 0.718 |
| HCT | % | 33.15 (31.00–35.88) | 33.10 (31.20–35.75) | 33.20 (30.50–36.50) | 0.933 |
| RBC | 106/µL | 4.13 (3.83–4.49) | 4.14 (3.85–4.50) | 4.09 (3.78–4.49) | 0.685 |
| MCH | pg | 27.40 (26.27–28.60) | 27.30 (26.05–28.45) | 27.50 (26.70–28.70) | 0.186 |
| MCV | fL | 80.80 (78.50–82.95) | 80.70 (78.20–82.90) | 80.90 (79.00–83.10) | 0.630 |
| MCHC | g/dL | 33.90 (33.10–34.70) | 33.90 (33.00–34.60) | 34.00 (33.30–35.10) | 0.160 |
| Vital signs | |||||
| HR | z score | 1.88 (1.37–2.71) | 1.67 (1.19–2.28) | 2.18 (1.68–2.81) | 0.016 |
| RR | z score | 1.89 (0.83–3.91) | 1.34 (0.36–2.09) | 3.18 (1.30–5.04) | <0.001 |
| mBP | z score | –0.56 (–1.25 to 0.12) | –0.11 (–0.65 to 0.98) | –0.98 (–1.57 to –0.37) | <0.001 |
| sBP | z score | –0.81 (–1.48 to 0.17) | –0.24 (–0.97 to 0.88) | –1.32 (–1.94 to –0.64) | <0.001 |
| dBP | z score | –0.74 (–1.17 to 0.14) | –0.18 (–0.73 to 0.91) | –1.01 (–1.47 to –0.60) | <0.001 |
| Continuous demographics | |||||
| Age | years | 9.17 (4.89–13.09) | 7.74 (3.67–11.28) | 10.27 (8.32–14.40) | <0.001 |
| BMI | z score | 0.87 (–0.03 to 1.71) | 0.58 (–0.18 to 1.42) | 1.44 (0.44–2.13) | 0.002 |
First laboratory results and first-registered vital signs upon admission for the entire cohort, and for the mild and severe MIS-C groups. Vital signs and BMI were transformed to z scores using age- and sex-adjusted mean and standard deviation from the general population. The p values were computed using the non-parametric two-sided Wilcoxon rank-sum method to compare the mild MIS-C and severe MIS-C groups.
Steroids and immunomodulators (IVIG or Anakinra) were the mainstay therapies. About 81% and 96% of the cohort received immunomodulators and steroids, respectively, with a greater proportion among the severe patients. By definition, all severe patients received inotropic/vasoactive support. Volume expansion and calcium chloride were also used to counter cardiovascular compromise. Anticoagulant (65%) and antiplatelet (61%) therapies were also administered to the majority of the patients. Table 1 also shows that most patients who received immunomodulators and steroids had their initial dose within 48 h.
Demographics and clinical data associated with mild vs severe MIS-C
The mild and severe MIS-C groups contained 87 (57%) and 65 (43%) patients, respectively. Table 1 highlights differences between the two groups pertaining to demographic information, medications, and duration of hospitalization. There is a significant association between MIS-C severity and age, with younger patients (age <6 years) being more likely to develop mild MIS-C (p < 0.001) and older patients (age >12 years) having severe MIS-C (p = 0.013). Healthy weight BMI (p = 0.006) and overweight BMI (p = 0.001) are associated with mild and severe MIS-C, respectively. Non-Hispanic black group (p = 0.043) experience more severe MIS-C despite no inter-racial difference in age (p = 0.489) or BMI (p = 0.744). A greater fraction of the severe MIS-C group had a lower initial LVEF (p < 0.001). Coronary dilation did not show a significantly different incidence between the mild and severe MIS-C groups. Prolonged length of stay (more than 7 days) occurred in 25% of the mild MIS-C group and 78% of the severe MIS-C group (p < 0.001). Similarly, a significantly (p < 0.001) higher proportion of patients required a prolonged length of stay in the ICU (more than 4 days) in the severe MIS-C group (65%) than in the mild MIS-C group (11%). Table 1 also shows that a greater proportion in the severe MIS-C group, in comparison to the mild MIS-C group, received immunomodulators (p < 0.001) and steroids (p < 0.033), and that the severe MIS-C group received their initial dose of therapies earlier than the mild MIS-C group.
There was also a significant difference in laboratory markers of systemic inflammatory/dysfunction between the mild and severe groups of MIS-C. In addition, tachycardia (p = 0.016), tachypnea (p < 0.001), and hypotension (p < 0.001) are more aberrant in the severe MIS-C group (refer to Table 2).
Trajectory of the MSS
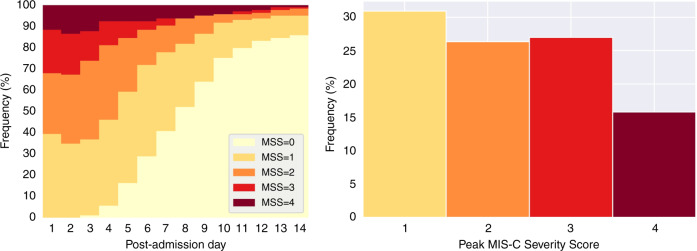
The day-to-day moving distribution of the MSS is graphically displayed in Fig. 1 (left) as daily stacked bar plots. On the first day, 40% of the cohort was categorized to an MSS = 1, 28% experienced MSS = 2, 20% experienced MSS = 3, and 12% experienced MSS = 4. On days 2–3, we observed a shift toward higher MSS values, before some patients started to show resolution of the illness around days 4–5. By 1 week, half of the cohort was discharged from the hospital. One can also observe that 15% of the cohort required a prolonged hospital stay of at least 14 days.
Fig. 1. Trajectory of the MSS.
Daily stacked bar plot of the MSS (left) and frequency histogram of the peak-MSS (right).
The frequency histogram of the peak-MSS is shown in Fig. 1 (right). We observe that 31% of the cohort reached a peak-MSS = 1, 26% attained peak-MSS = 2, 27% attained peak-MSS = 3, and 16% reach the highest peak-MSS = 4. Consequently, the cohort was divided into 57% mild MIS-C and 43% severe MIS-C.
Physiologic profile of mild vs severe MIS-C
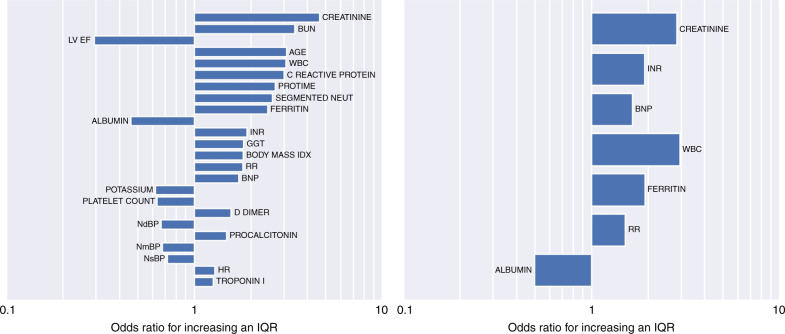
Univariate logistic regression analyses are shown in Table 3. The variables (including demographics, labs, and vitals) are organized according to the increasing AIC. Figure 2 shows odds ratios associated with an interquartile range increase in the respective clinical variables. Among demographics, age and BMI had a significant association with the primary outcome. Laboratory results that serve as surrogates for renal compromise (creatinine, BUN, potassium), immune activation and infection (WBC, neutrophils, procalcitonin, CRP, ferritin), coagulation (protime, INR, platelets, D dimer), cardiac involvement (LVEF, BNP, troponin), and gastrointestinal involvement (albumin, GGT) reached significant associations.
Table 3.
Univariate models.
| Lab name | AIC | AUC | IQR | OR | p value |
|---|---|---|---|---|---|
| Creatinine | 167.75 | 0.78 | 0.45 | 4.63 | <0.001 |
| BUN | 175.22 | 0.75 | 10.00 | 3.42 | <0.001 |
| WBC | 185.64 | 0.72 | 5.22 | 3.07 | <0.001 |
| INR | 186.71 | 0.71 | 0.10 | 1.91 | <0.001 |
| LVEF | 187.02 | 0.71 | 13.31 | 0.29 | <0.001 |
| Protime | 187.81 | 0.70 | 1.53 | 2.70 | <0.001 |
| Lactate | 188.53 | 0.61 | 0.13 | 1.14 | <0.001 |
| Troponin I | 190.63 | 0.71 | 0.11 | 1.26 | 0.003 |
| RR | 191.06 | 0.65 | 1.43 | 1.81 | <0.001 |
| Ferritin | 191.33 | 0.74 | 429.75 | 2.46 | <0.001 |
| CRP | 191.97 | 0.70 | 12.95 | 3.00 | <0.001 |
| Segmented neut | 192.13 | 0.71 | 11.87 | 2.61 | <0.001 |
| BNP | 192.13 | 0.76 | 357.98 | 1.73 | <0.001 |
| dBP | 193.37 | 0.72 | 0.42 | 0.67 | <0.001 |
| mBP | 193.64 | 0.70 | 0.45 | 0.68 | <0.001 |
| GGT | 194.03 | 0.69 | 31.75 | 1.83 | <0.001 |
| Procalcitonin | 194.73 | 0.73 | 9.80 | 1.49 | 0.001 |
| Age | 196.60 | 0.68 | 8.20 | 3.09 | <0.001 |
| Albumin | 199.32 | 0.66 | 0.72 | 0.46 | <0.001 |
| sBP | 199.70 | 0.68 | 0.61 | 0.72 | 0.003 |
| D dimer | 203.10 | 0.64 | 2.69 | 1.57 | 0.010 |
| Potassium | 204.33 | 0.66 | 0.63 | 0.62 | 0.013 |
| HR | 205.94 | 0.62 | 0.57 | 1.29 | 0.026 |
| Platelet | 206.23 | 0.62 | 94.50 | 0.63 | 0.028 |
| BMI | 206.91 | 0.64 | 1.75 | 1.82 | 0.014 |
| Fibrinogen | 208.08 | 0.57 | 168.00 | 1.41 | 0.069 |
| MCHC | 208.17 | 0.57 | 1.60 | 1.50 | 0.077 |
| Sodium | 208.98 | 0.59 | 5.00 | 0.70 | 0.117 |
| ALT | 209.32 | 0.61 | 36.25 | 1.20 | 0.161 |
| AST | 209.38 | 0.58 | 36.25 | 1.20 | 0.158 |
| MCH | 209.74 | 0.56 | 2.32 | 1.29 | 0.194 |
| Lymph | 210.33 | 0.54 | 0.96 | 0.84 | 0.287 |
| PTT | 210.49 | 0.55 | 5.58 | 1.19 | 0.311 |
| SpO2 | 210.75 | 0.50 | 0.88 | 0.87 | 0.381 |
| Thrombin time | 210.92 | 0.39 | 0.95 | 1.02 | 0.494 |
| HGB | 210.97 | 0.52 | 1.93 | 1.17 | 0.460 |
| Phosphorus | 211.06 | 0.58 | 0.90 | 0.90 | 0.502 |
| MCV | 211.07 | 0.52 | 4.45 | 1.11 | 0.503 |
| HCT | 211.42 | 0.50 | 4.88 | 1.07 | 0.747 |
| Ionized calcium | 211.46 | 0.54 | 0.02 | 0.67 | 0.799 |
| Alkaline phosphatase | 211.48 | 0.47 | 54.50 | 0.97 | 0.842 |
| Magnesium | 211.50 | 0.52 | 0.30 | 1.03 | 0.896 |
| RBC | 211.52 | 0.48 | 0.67 | 1.01 | 0.957 |
Results from univariate logistic regression models for the classification of mild vs severe MIS-C. Variables are listed according to the increasing AIC. The area under the receiving operating characteristic curve (AUC) is listed as well. Odds ratios (OR) are associated with an interquartile range (IQR) increase in the respective variables.
Fig. 2. Univariate and multivariate analyses.
Odds ratios for the univariate (left) and multivariate (right) logistic regression analyses for the classification of mild vs severe MIS-C. Odds ratios are associated with an interquartile range increase in the respective variables. The interquartile ranges are listed in Tables 3 and 4.
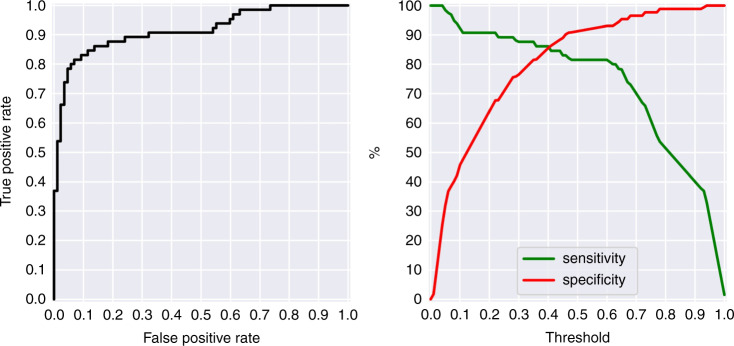
The results of the stepwise forward selection multivariate logistic regression are shown in Table 4. This selection process identified creatinine (p = 0.013), INR (p = 0.002), BNP (p = 0.001), WBC (p = 0.009), ferritin (p = 0.041), RR (p = 0.047), and albumin (p = 0.047) as the variables that associated most with severe MIS-C. The odds ratios for each of these explanatory factors are displayed in Fig. 2. The model performed well (area under the curve, AUC = 0.915) at discriminating mild versus severe MIS-C (see receiver operating characteristic curve (ROC) in Fig. 3 (left)). Figure 3 (right) depicts the sensitivity–specificity curve according to classification threshold.
Table 4.
Multivariate model.
| Variables | Coef. | Std error | OR | p value |
|---|---|---|---|---|
| Intercept | –1.7327 | 0.343 | n/a | <0.001 |
| Creatinine | 1.0444 | 0.421 | 2.84 | 0.013 |
| INR | 0.6509 | 0.208 | 1.92 | 0.002 |
| BNP | 0.5013 | 0.155 | 1.65 | 0.001 |
| WBC | 1.0833 | 0.417 | 2.95 | 0.009 |
| Ferritin | 0.6568 | 0.322 | 1.93 | 0.041 |
| Respiratory rate | 0.4136 | 0.208 | 1.51 | 0.047 |
| Albumin | –0.6962 | 0.351 | 0.50 | 0.047 |
Results from multivariate logistic regression for the classification of mild vs severe MIS-C. Odds ratios (OR) are associated with an interquartile range increase in the respective variables. The median and interquartile range for each variable are displayed in Table 2. The pseudo R-square value = 0.4844, the LLR p value = 8.39E–19, and the performance ROC AUC = 0.915.
Fig. 3. Classification performance.
Receiver operating characteristic curve (left), and sensitivity and specificity curves (right) for the multivariate logistic model to classify mild vs severe MIS-C.
To test the model against overfitting, a TRIPOD Type 1b testing analysis using re-sampling was employed to generate 100 repetitions of a 50/50 random split of the data to train and test the model. The average ROC AUC over the training subset was 92.0% and the average AUC over the testing subsets was 90.4%. To evaluate the collinearity of the model features, the VIF was computed and found to be 1.35 which is well accepted (VIF < 10) as an indication that the level of collinearity is low.
Discussion
Our single-center study of 152 individuals describes the clinical spectrum of MIS-C. While the majority of children with MIS-C require ICU admission, little information exists illustrating the presentation or clinical course between children with varying degrees of the condition. Therefore, we created an MSS based on hospital or ICU admission and the need for inotropic/vasoactive support with/without mechanical ventilation. The importance of this MSS lies in its strong associations with demographics, first-registered vital signs, and early laboratory characteristics (see Tables 1 and 2). These associations allow the care team to recognize patients likely to require a higher level of intensive care. The results of this study should not be interpreted as a predictive model of severity, but rather as an associative profile linked to the severity of illness. The results of this study may in the future be used as a stepping stone to develop a predictive tool that would have to be validated on a separate or multi-institutional cohort.
To find the most informative (according to the AIC) physiologic risk profile associated with severe MIS-C, we created a stepwise-selected logistic model to associate demographics, vital signs, and laboratory markers with the mild vs severe classification of MIS-C. The following parameters: creatinine, INR, BNP, WBC, ferritin, albumin, and RR z score were selected as the most informative risk factors, leading to a classification performance quantified by an AUC = 0.915 for differentiating between mild vs severe MIS-C patients (see Table 4, Figs. 2 and 3).
Previous studies have shown the effectiveness of ICU scoring systems in identifying patient outcomes. The acute physiology and chronic health evaluation, simplified acute physiology score, and pediatric risk of mortality score collect data on the first day of ICU admission to prognosticate mortality.22,23 Although validated, these scoring systems cannot be readily applied to MIS-C for several reasons. First, most children survive hospitalization with MIS-C. Second, the number of variables that are needed to calculate the score are numerous and they do not include inflammatory parameters that are typical of MIS-C. Lastly, these severity scores were developed for general illnesses that led to ICU admission. Thus, we sought to develop an MSS that led to the development of a logistic regression model to identify patients likely to require a higher level of intensive care.
Similar to previous reports,3,16,17 age and race strongly associate with severe MIS-C. Although children between the ages of 6–12 were more likely to develop MIS-C, the frequency of intensive care unit admission, inotropic support, and mechanical ventilation was higher in older children. The disproportional representation of MIS-C in black and Hispanic children is well characterized in the literature. In this study, 76.3% (n = 116 of 152) of children diagnosed with MIS-C were either Hispanic or non-Hispanic black. Of note, non-Hispanic black children had a higher predisposition to the severe form of MIS-C (Table 1).
Our study found that almost half of the children diagnosed with MIS-C had a healthy weight BMI. In the majority of cases having a healthy BMI was protective, while an overweight BMI was associated with a higher MSS. These findings parallel those described by Abrams et al. in which obesity was a factor associated with worse outcomes in children with MIS-C.3 Theories behind this link are supported by studies that have shown that adipose cells have more SARS-CoV-2 receptors. As in this study, a J-shaped relationship between BMI and poor outcome was described in a meta-analysis focused on COVID-19 in adults.24
Our stratification of MIS-C is clinically and physiologically relevant because of its association with cardiac echo assessment, vital signs, medications received, and blood analytes (Tables 1 and 2). The degree of cardiac involvement in the more severe group is evidenced by the left-ventricular function. Approximately half of the patients with severe MIS-C had an initial echocardiogram that showed reduced LVEF. In contrast, only one-fifth of MIS-C cases in the mild group had left-ventricular dysfunction.
The seven variables associated with severe MIS-C can be crudely distributed to six distinct organ systems. The systems included renal (creatinine), hematologic (INR, ferritin), cardiac (BNP), immunologic (WBC), pulmonary (RR), and the gastrointestinal system (albumin). A way to view our findings is that the number of organ systems involved increases the odds of developing severe MIS-C. As the pathophysiology of MIS-C is still under investigation, it is difficult to extrapolate how these associations are linked at the molecular level for each organ system. Current hypotheses explaining which children will develop MIS-C include: (i) hyper-activation of neutrophils and monocytes, (ii) reduced number of T and B cells, (iii) altered response of T and B cells, (iv) auto-antibodies, and (v) formation of superantigens.25–29 It is possible to hypothesize that children with severe MIS-C have a greater dysregulation in the aforementioned processes. These relationships will need to be tested in future studies.
Patients with severe MIS-C had higher utilization of biologic agents (e.g., anakinra). In this study, 94% (61 of 65) of children with severe MIS-C received anakinra. Son et al. evaluated initial MIS-C therapy and found that 20.6% of children received the combination of IVIG, glucocorticoid, and a biologic agent.13 Similarly, we found that the extent of clinical severity (e.g., ICU admission, vasoactive-inotropic support, mechanical ventilation) correlated with the more frequent use of a biologic agent. Furthermore, their study also showed that the length of hospital stay was longer in their more “severe” cohort.
Dysregulation of hematologic markers was higher in the severe cohort of MIS-C (see Table 2). Of note, BNP, troponin, procalcitonin, creatinine, and ferritin were at least twice as elevated in the children with severe MIS-C in comparison with mild MIS-C. Another point of interest is that patients having the severe form of MIS-C are associated with a longer hospital and ICU stay.
Limitations
Our study has limitations. Our data were derived from one center which may decrease the potential generalizability of our findings. However, given the rarity with which MIS-C occurs, the sample size and granularity of information from this single-site institution is a strength. For instance, our MSS is based on the precise day-to-day documentation of ICU stay, start, and discontinuation of inotropic/vasoactive medications, mechanical ventilation, and daily records of laboratories and vital signs. Second, the selection of therapies was institutionally dependent since there were no well-established national guidelines. Given the novelty and lack of evidence for treating MIS-C, clinicians treated according to similar protocols for Kawasaki disease, shock syndrome, or sepsis, and in conjunction with pediatric infectious disease and rheumatology specialists. Those patients transferred from an outside hospital typically received treatments limited to stabilization. But the precise knowledge of outside treatments is lacking in this retrospective study. Next, symptoms and their onset were not reported; however, most MIS-C studies have not shown a difference in these characteristics according to ICU admission, vasopressor use, or intubation. This may be due to the fact that documentation of disease course prior to presentation at the hospital is inaccurate and subjective as it is self-reported. It is also important to stress that while we refer to “mild” and “severe” MIS-C phenotypes, all of these children were sick enough to be hospitalized, and 70% were admitted to ICU during their hospitalization. Finally, our findings are preliminary and would warrant further validation in a larger or separate cohort with access to similar clinical and physiologic data.
Conclusion
Herein, we developed a severity score to stratify individuals diagnosed with MIS-C. The more severe form of MIS-C was defined based on ICU admission, inotropic/vasoactive support, or the need for mechanical ventilation. The most informative physiologic profile associated with the severe form of MIS-C included elevated creatinine, INR, BNP, WBC, ferritin and RR, and reduced albumin. These variables, incorporated into a logistic regression, led to an excellent classification performance (ROC AUC = 0.915) for differentiating between mild vs severe MIS-C patients in our institution and warrants validation in separate cohorts.
Author contributions
F.S., S.A., and Al.M. conceptualized and designed the study, performed data collection and analyses, drafted, and revised the manuscript, and approved the final version. Ax.M. helped with data collection and revised the manuscript. A.A., S.K.S.T., C.G.R., L.S., and S.F. critically revised the manuscript.
Funding
This project was funded in part by a grant from the National Institutes of Health: R61HD105593.
Data availability
The datasets generated during and/or analyzed during the current study are not publicly available due to protected health information restrictions but are available from the corresponding author on reasonable request and in compliance with the institutional review board and required inter-institutional data-sharing agreements.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.CDC. Information for Healthcare Providers About Multisystem Inflammatory Syndrome in Children (Mis-C)https://www.cdc.gov/mis-c/hcp/ (2021).
- 2.Rowley AH, Shulman ST, Arditi M. Immune pathogenesis of COVID-19-related multisystem inflammatory syndrome in children. J. Clin. Investig. 2020;130:5619–5621. doi: 10.1172/JCI143840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abrams JY, et al. Factors linked to severe outcomes in multisystem inflammatory syndrome in children (MIS-C) in the USA: a retrospective surveillance study. Lancet Child Adolesc. Health. 2021;5:323–331. doi: 10.1016/S2352-4642(21)00050-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jiang L, et al. COVID-19 and multisystem inflammatory syndrome in children and adolescents. Lancet Infect. Dis. 2020;20:e276–e288. doi: 10.1016/S1473-3099(20)30651-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Villacis-Nunez DS, et al. Giant coronary aneurysms in multisystem inflammatory syndrome in children associated with SARS-CoV-2 infection. JACC Case Rep. 2021;3:1499–1508. doi: 10.1016/j.jaccas.2021.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carrasco-Sanchez FJ, et al. Admission hyperglycaemia as a predictor of mortality in patients hospitalized with COVID-19 regardless of diabetes status: data from the Spanish Semi-COVID-19 Registry. Ann. Med. 2021;53:103–116. doi: 10.1080/07853890.2020.1836566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davies, P., du Pre, P., Lillie, J. & Kanthimathinathan, H. K. One-year outcomes of critical care patients post-COVID-19 multisystem inflammatory syndrome in children. JAMA Pediatr.175, 1281–1283 (2021). [DOI] [PMC free article] [PubMed]
- 8.Feldstein LR, et al. Characteristics and outcomes of US children and adolescents with multisystem inflammatory syndrome in children (MIS-C) compared with severe acute Covid-19. JAMA. 2021;325:1074–1087. doi: 10.1001/jama.2021.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hsu TY, et al. Laboratory trends, hyperinflammation, and clinical outcomes for patients with a systemic rheumatic disease admitted to hospital for COVID-19: a retrospective, comparative cohort study. Lancet Rheumatol. 2021;3:e638–e647. doi: 10.1016/S2665-9913(21)00140-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Payne AB, et al. Incidence of multisystem inflammatory syndrome in children among US persons infected with SARS-CoV-2. JAMA Netw. Open. 2021;4:e2116420. doi: 10.1001/jamanetworkopen.2021.16420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pignatelli R, et al. Pediatric multisystem SARS CoV2 with versus without cardiac involvement: a multicenter study from Latin America. Eur. J. Pediatr. 2021;180:2879–2888. doi: 10.1007/s00431-021-04052-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rodriguez-Smith JJ, et al. Inflammatory biomarkers in COVID-19-associated multisystem inflammatory syndrome in children, Kawasaki disease, and macrophage activation syndrome: a cohort study. Lancet Rheumatol. 2021;3:e574–e584. doi: 10.1016/S2665-9913(21)00139-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Son MBF, et al. Multisystem inflammatory syndrome in children – initial therapy and outcomes. N. Engl. J. Med. 2021;385:23–34. doi: 10.1056/NEJMoa2102605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Whittaker E, et al. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA. 2020;324:259–269. doi: 10.1001/jama.2020.10369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nile SH, et al. COVID-19: pathogenesis, cytokine storm and therapeutic potential of interferons. Cytokine Growth Factor Rev. 2020;53:66–70. doi: 10.1016/j.cytogfr.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fernandes DM, et al. Severe acute respiratory syndrome Coronavirus 2 clinical syndromes and predictors of disease severity in hospitalized children and youth. J. Pediatr. 2021;230:23–31. doi: 10.1016/j.jpeds.2020.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nino-Taravilla C, Otaola-Arca H, Lara-Aguilera N, Zuleta-Morales Y, Ortiz-Fritz P. Multisystem inflammatory syndrome in children, Chile, May-August 2020. Emerg. Infect. Dis. 2021;27:1457–1461. doi: 10.3201/eid2705.204591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baker-Smith, C. M. et al. Diagnosis, evaluation, and management of high blood pressure in children and adolescents. Pediatrics142, e20182096 (2018). [DOI] [PubMed]
- 19.CDC. Percentile Data Files with Lms Valueshttps://www.cdc.gov/growthcharts/percentile_data_files.htm (2021).
- 20.Hastie, T., Tibshirani, R. & Friedman, J. The Elements of Statistical Learning 2nd edn (Springer, 2009).
- 21.Collins GS, Reitsma JB, Altman DG, Moons KG, Group T. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (Tripod): The Tripod Statement. The Tripod Group. Circulation. 2015;131:211–219. doi: 10.1161/CIRCULATIONAHA.114.014508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pollack MM, Patel KM, Ruttimann UE. PRISM III: an updated pediatric risk of mortality score. Crit. Care Med. 1996;24:743–752. doi: 10.1097/00003246-199605000-00004. [DOI] [PubMed] [Google Scholar]
- 23.Pollack MM, Yeh TS, Ruttiman UE, Holbrook PR, Fields AI. Evaluation of pediatric intensive care. Crit. Care Med. 1984;12:376–383. doi: 10.1097/00003246-198404000-00008. [DOI] [PubMed] [Google Scholar]
- 24.Huang HK, et al. The J-shaped relationship between body mass index and mortality in patients with COVID-19: a dose-response meta-analysis. Diabetes Obes. Metab. 2021;23:1701–1709. doi: 10.1111/dom.14382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carter MJ, et al. Peripheral immunophenotypes in children with multisystem inflammatory syndrome associated with SARS-CoV-2 infection. Nat. Med. 2020;26:1701–1707. doi: 10.1038/s41591-020-1054-6. [DOI] [PubMed] [Google Scholar]
- 26.Cheng MH, et al. Superantigenic character of an insert unique to SARS-CoV-2 spike supported by skewed TCR repertoire in patients with hyperinflammation. Proc. Natl Acad. Sci. USA. 2020;117:25254–25262. doi: 10.1073/pnas.2010722117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vella, L. A. et al. Deep immune profiling of MIS-C demonstrates marked but transient immune activation compared to adult and pediatric COVID-19. Sci. Immunol.6, eabf7570 (2021). [DOI] [PMC free article] [PubMed]
- 28.Vella, L. A. & Rowley, A. H. Current insights into the pathophysiology of multisystem inflammatory syndrome in children. Curr. Pediatr. Rep. 1–10 (2021). [DOI] [PMC free article] [PubMed]
- 29.Porritt, R. A. et al. The autoimmune signature of hyperinflammatory multisystem inflammatory syndrome in children. J. Clin. Invest.131, e151520 (2021). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are not publicly available due to protected health information restrictions but are available from the corresponding author on reasonable request and in compliance with the institutional review board and required inter-institutional data-sharing agreements.