Version Changes
Revised. Amendments from Version 1
Changes to the manuscript include: Indicated the name of species as Fusarium concentricum Sated the photos with high resolution MIC values and graph have been added in results section Uses and active constituents of the selected plants have been added in introduction section Antifungal activities of the selected plants have been included in the discussion section.
Abstract
Background: Fusarium concentricum is one of the most devastating fungi responsible for fruit and vegetable crops rot worldwide. The present study was designed to find an ecofriendly control measure for pathogenic Fusarium concentricum, using suitable bioagents.
Methods: Medicinal plant extracts were evaluated or their antifungal activities against Fusarium concentricum using the poisoned food and serial dilution methods. Antagonistic potency of some nonpathogenic microbes was also assessed on Fusarium concentricum using the dual culture method.
Results: Highest inhibition of growth of Fusarium concentricum was observed with 68.1% (0.389 mg per 90 mm Petri plate) of mycelia on Coccinia grandis plant leaf extract, in comparison to the control grown with 100.0% (1.22 mg/dish). The tested plants extract showed MIC values rages of 80-150 µg/ml on the isolated fungus. The highest inhibition of radial growth was observed using Trichoderma viride on Fusarium concentricum (46.01% inhibition).
Conclusions: The findings of present study would be benevolent for antifungal drug development to control Fusarium concentricum causing fruit and vegetable rot.
Keywords: Fusarium sp., Plants extract, Non-pathogenic microbes, Antagonisms, Biocontrol
Introduction
Fusarium species are a large genus of hyaline filamentous mold fungi, responsible for fruit and vegetable crop rot ( Al-Najada & Gherbawy, 2015; Ziedan et al., 2018). Fusarium species are deeply invasive and can cause hematogenously disseminated infections with high mortality in neutropenic patients ( Dignani & Anaissie, 2004). Numerous species of Fusarium contribute to yield loss and reduced quality to varying degrees by infection with some mycotoxins ( O’Donnell et al., 2009). They also cause decay of various fruit in storage and postharvest conditions ( Whiteside et al., 1988). The fruit rot caused by Fusarium incurs enormous yield losses and is often observed in fields and markets ( Baria et al., 2015). The application of antagonistic agents and plant extracts in agriculture are becoming a major focus of plant protection research. Different preservatives or fungicide treatments are frequently applied to manage fruits diseases and decay, which is an alarming health concern ( Munhuweyi et al., 2020). Since most chemical fungicides are highly toxic to humans and animals and they frequently cause water and soil pollution ( Al-Najada & Gherbawy, 2015). Moreover, continuous and indiscriminate use is leading to the development of fungicide resistant strains of pathogens ( Tafinta et al., 2013). There are some previous reports on plants extract based controlling techniques in Fusarium fungi causing diseases for some crops and plants ( Baria et al., 2015; Ziedan et al., 2018).
In Bangladesh, fruit rot is a destructive disease caused by Fusarium species on pre-harvest and postharvest fruits. To the best of our knowledge, there is no previous research on biological control of this pathogenic fungus. Therefore, the main objective of the study was to find an ecofriendly control system of Fusarium concentricum to decrease fruit and vegetable rot in Bangladesh.
Methods
Collection of Fusarium concentricum
A pure culture of Fusarium sp. was previously isolated and identified (Accession No. MT856371) from postharvest Citrus reticulata fruit rot ( Hasan et al., 2020a). The culture was preserved at the Department of Genetic Engineering and Biotechnology, University of Rajshahi (Rajshahi, Bangladesh).
In vitro assessment of antifungal potential of selected plants
For antifungal activities screening, six healthy, mature medicinal plants, Allium sativum, Zingiber officinale, Coccinia grandis, Brassica juncea, Ocimum tenuiflorum and Hibiscus rosa-sinensis were collected from Mirzapur, Binodpur and Kajla village, Motihar, Rajshahi, Bangladesh.
Collected plants were washed with water to remove dust from the plants’ surface and dried in room temperature. Plant extract preparation and fractionation was performed according to the method by Kader et al. (2018). Different parts of selected plants- bulb of Allium sativum; rhizome of Zingiber officinale; leaves of Coccinia grandis, Brassica juncea, Ocimum tenuiflorum; and flowers of Hibiscus rosa-sinensis plants were cut into small species and ground by blender to form fine powder. The dried powder of the plants (100gm of each plant) were rinsed in methanol (500ml) using a conical flask, and were incubated in a shaking incubator with occasional shaking for fourteen days. The liquid contents were pressed through Markin cloth followed by filtration using Whatman no. 1 filter paper. Obtained filtered liquids were dehydrated in vacuo to leave a blackish and sticky mass. The extracts were collected in vials and preserved in a refrigerator at 4°C.
The inhibitory effect of different plant extracts was measured by following the poisoned food technique ( Balamurugan, 2014). For this, 20µg of each plant extract was added to 20ml of potato dextrose agar (PDA) to fill a 90mm size Petri plate and mixed well. After solidification, seven day old 6 mm size fungal plug was placed in the center of the Petri plates. The Petri plates were incubated at 35°C for seven days in static condition.
Determination of minimum inhibitory concentration (MIC)
The required MIC values of selected plants extracts were measured through the serial dilution technique ( Hussain et al., 2010). The extract of 1 gm of plant-based methanol leaves was dissolved into 1 ml methanol to obtain this same stock solution. Fungal suspension was taken into each test tube and added 50-150µg of plants extracts. The tested tubes were then incubated for 3 days. The progression of the isolated fungus was detected in tubes in which the strength of the substances was just below the inhibition level.
In vitro antagonistic test
For evaluation of antagonistic effects, six non-pathogenic pure microbe cultures, Escherichia coli (pathogenic), Rhizobium phaseoli, Rhizobium leguminosarum, Neofusicoccum mangifera, Trichoderma viride and Pestalotiopsis sp. were used against Fusarium concentricum. The pure microbe cultures were kindly provided by Dr. Md. Salah Uddin, Associate Professor and Director, Microbiology Lab., Department of Genetic Engineering and Biotechnology, University of Rajshahi, as the part of collaboration.
To assess the antagonistic effects, the dual culture technique was used, as previously described ( Vethavalli & Sudha, 2012). A mycelial disc of 6 mm diameter was cut from the periphery of both antagonist cultures and the test pathogen and placed on a Petri plate with PDA media. For the control, only the test pathogen was placed in the centre of a Petri plate. The Petri plates were incubated at 35°C in darkness.
Data analysis
The inhibition percentage of mycelial growth= [(Gc-Gt)/Gc] × 100; Where, Gc = Mycelial growth in terms of colony diameter in control set, Gt= Mycelial growth in terms of colony diameter in treatment set. The inhibition percentages of Fusarium species growth were calculated using the following formula: Inhibition percentage (%) =100× (dc– dt)/dc; Where, dc = radial growth of pathogen in control, dt = radial growth of pathogen in dual culture. Mean values were compared through least significant different test using SAS software, version 9.4M5 (SAS Inc., Cary, NC, USA). All the experiment and test were replicated thrice.
Results
In vitro screening of extracts presenting antifungal activity
All plant extracts showed a degree of growth inhibition of the tested fungus at the same concentrations. The highest inhibition of growth of the isolates was observed at 68.1% of mycelium on Coccinia grandis, which was followed by 64.1% on Allium sativum, in comparison to the control culture (100.0%). Hibiscus rosa-sinensis showed the lowest inhibition of mycelium with 29.6% against the fungal isolate in comparison to the control. The results are presented in Figure 1 and Figure 2.
Figure 1. Effect of different methanol plant extracts on percentage inhibition of mycelial growth of Fusarium concentricum.
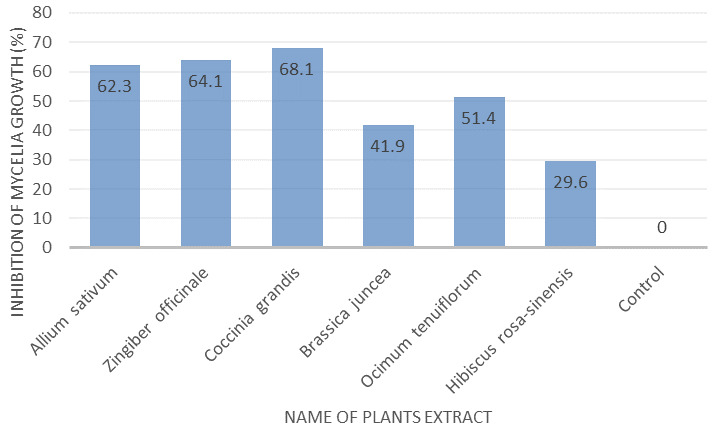
Mycelia were collected after seven days of incubation on potato dextrose agar at 35°C in darkness. 90 mm Petri plates were used to culture the tested fungus.
Figure 2. Petri dish images showing the effect of plant extracts on inhibition of mycelial growth of Fusarium concentricum.
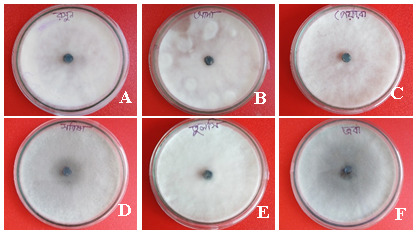
( A) Allium sativum, ( B) Zingiber officinale, ( C) Coccinia grandis, ( D) Brassica juncea, ( E) Ocimum tenuiflorum, and ( F) Hibiscus rosa-sinensis. Mycelia were collected after seven days of incubation on potato dextrose agar at 35°C in darkness.
Determination of minimum inhibitory concentration (MIC)
The tested plants extracts showed the MIC values ranges from 80-150µg/ml against the fungus Fusarium concentricum growth. Highest MIC value of isolated fungus was 80µg/ml on Coccinia grandis plant extract and lowest MIC value of isolated fungus was 150 µg/ml on Hibiscus rosa-sinensis plant extract ( Figure 3).
Figure 3. Minimum inhibitory concentration (MIC) of methanol extract showing the effect of plant extracts on inhibition of mycelial growth of Fusarium concentricum.
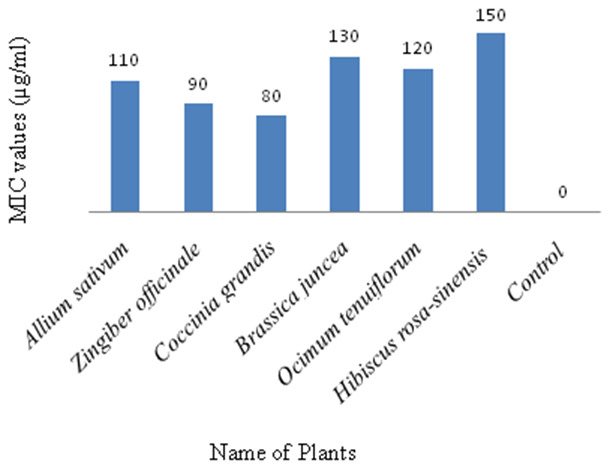
( A) Allium sativum, ( B) Zingiber officinale, ( C) Coccinia grandis, ( D) Brassica juncea, ( E) Ocimum tenuiflorum, and ( F) Hibiscus rosa-sinensis. Mycelia were collected after seven days of incubation on potato dextrose at 35°C in darkness.
In vitro antagonistic assay
The highest percentage inhibition of radial growth was observed with Trichoderma viride (46.01%) against Fusarium concentricum which was followed by 43.33% and 32.05% on Escherichia coli and Rhizobium phaseoli, respectively ( Figure 4). The antagonistic agent Rhizobium leguminosarum did not show any inhibitory activity against the isolated fungus ( Figure 4). The control group also did not show any inhibition of radial growth of Fusarium concentricum.
Figure 4. Growth inhibition effects of microbial agents on Fusarium concentricum.
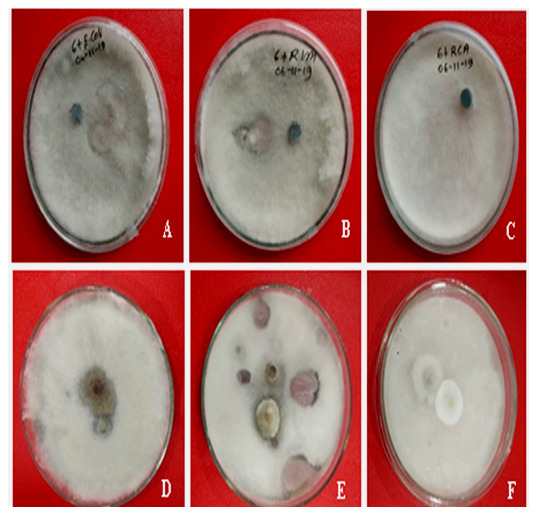
( A) Escherichia coli, ( B) Rhizobium phaseoli, ( C) Rhizobium leguminosarum, ( D) Neofusicoccum mangifera, and ( E) Trichoderma viride and ( F) Pestalotiopsis sp., Mycelia were collected after seven days of incubation on potato dextrose agar at 35°C in darkness.
Discussion
Fruit rot caused by Fusarium species is very common in Bangladesh. The main objective of the present study was to study biological control measures for this fungus. Plant extracts are now a superior choice to control different plant pathogens, as reported by several previous studies ( Hasan et al., 2020b; Kareem & Al-Araji, 2017; Parveen et al., 2014). In our study, we found that the plant extracts Allium sativum, Zingiber officinale, and Coccinia grandis have significant inhibitory effects on mycelial growth of Fusarium concentricum. Hosen & Shamsi (2019) also found significant antifungal activity using Allium sativum (53.85%), Ocimum sanctum (48.72%) and Zingiber officinale (49.35%) against Fusarium solani and F. oxysporum. Our current results were also supported by data from Khatun et al. (2020) and Kareem & Al-Araji (2017). In our present study, Coccinia grandis plant leave extract showed a promising MIC capability to control Fusarium concentricum fungus. Piper betle plant extracts showed a significant antifungal potency on Fusarium oxysporum ( Gnanasekaran et al., 2015). For the confirmation of antagonist effects on the radial growth of the pathogen in dual culture have been previously reported by Akhtar et al. (2010). By contrast, Nasrin et al. (2018) reported 87% inhibition potency on mycelium growth of Fusarium oxysporum f. sp. lycopersici by Calotropis proceraon plant extract. In our study, Trichoderma viride, Escherichia coli, Rhizobium phaseoli and Alternaria sp. showed significant antagonistic activity against Fusarium concentricum. Trichoderma viride showed 45.88% growth inhibition on Fusarium merismoides fungi in a study by Hosen & Shamsi (2019), which support our present findings. Nasrin et al. (2018) also reported 82% inhibition radial growth by Trichoderma sp. against Fusarium oxysporum f. sp. lycopersici. In contrast, Bashar & Chakma (2014) reported that volatile substances produced by T. viride, A. niger, A. flavus and A. fumigatus showed 29.75, 20.15, 15.78 and 12.25% growth inhibition, respectively, on F. oxysporum.
In the current investigation, there are some limitations. Although this study showed that some plant extracts and nonpathogenic microbes could control the fungal stain, the number of plant extracts and microbes was limited. In addition, we used only methanol solvent for extraction and did not use other extractions. Moreover, we performed only in vitro techniques for antifungal potency screening and did not use any in vivo techniques. Therefore, we need to perform further studies to detect ecofriendly control this devastating fungal stain in the future.
Conclusions
We evaluated different biological control measures for the devastating Fusarium fungi. Various medicinal plants extracts and non-pathogenic microbes showed promising inhibitory activities on Fusarium concentricum in vitro. These identified control measures of Fusarium concentricum shows the importance of further research on Fusarium taxonomy to decline the risk of Fusarium concentricum caused fruit rot in Bangladesh.
Data availability
Underlying data
Figshare: Effect of plant extracts on inhibition of mycelial growth of the Fusarium species. https://doi.org/10.6084/m9.figshare.13096262 ( Hasan, 2020a). This project contains the images of Petri plates for each treatment condition.
Figshare: Effect of antagonistic agents on inhibition of mycelial growth of the Fusarium species. https://doi.org/10.6084/m9.figshare.13096325 ( Hasan, 2020b). This project contains the images of Petri plates for each treatment condition.
Figshare: Effects of different plants extract by methanol on inhibition percentages of mycelial growth of the Fusarium species, https://doi.org/10.6084/m9.figshare.13134953 ( Hasan, 2020c).
Figshare: Effect of antagonistic agents on inhibition of mycelial growth of the Fusarium species, https://doi.org/10.6084/m9.figshare.13134962 ( Hasan, 2020d).
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
Acknowledgements
A previous version of this article is available on Figshare: https://doi.org/10.6084/m9.figshare.13012517 ( Hasan, 2020e).
Funding Statement
This research work was supported by the Ministry of Education, Government of the People's Republic of Bangladesh (2019-2021) [No.3900.0000.09.06.217/2BS 91/170].
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 2; peer review: 1 approved
References
- Akhtar J, Kumar V, Tiu KR, et al. : Integrated management of banded leaf and sheath blight disease of maize. Plant Disease Research. 2010;25(1):35–38. Reference Source [Google Scholar]
- Al-Najada AR, Gherbawy YA: Molecular Identification of Spoilage Fungi Isolated from Fruit and Vegetables and Their Control with Chitosan. Food Biotechnol. 2015;29(2):166–184. 10.1080/08905436.2015.1027222 [DOI] [Google Scholar]
- Balamurugan S: In vitro antifungal activity of Citrus aurantifolia Linn plant extracts against phytopathogenic fungi Macrophomina phaseolina. International Letters of Natural Sciences. 2014;13:70–74. 10.18052/www.scipress.com/ILNS.13.70 [DOI] [Google Scholar]
- Baria TT, Patil RK, Patel JK: Ecofriendly Management of Fusarium Fruit Rot of Citrus. The Bioscan. 2015;10(4):1807–1811. Reference Source [Google Scholar]
- Bashar MA, Chakma M: In vitro control of Fusarium solani and F. oxysporum the causative agent of brinjal wilt. Dhaka Univ J Biol Sci. 2014;23(1):53–60. 10.3329/dujbs.v23i1.19826 [DOI] [Google Scholar]
- Dignani MC, Anaissie E: Human fusariosis. Clin Microbiol Infect. 2004;10 Suppl 1:67–75. 10.1111/j.1470-9465.2004.00845.x [DOI] [PubMed] [Google Scholar]
- Gnanasekaran P, Salique SM, Panneerselvam A, et al. : In vitro Biological Control of Fusarium oxysporum f. sp. Cubense by using Some Indian Medicinal Plants. Int J Current Res and Review. 2015;3(11):107–116. Reference Source [Google Scholar]
- Hasan M: Effect of plant extracts on inhibition of mycelial growth of the Fusarium species. Figshare. Figure.2020a. 10.6084/m9.figshare.13096262 [DOI] [Google Scholar]
- Hasan M: Effect of antagonistic agents on inhibition of mycelial growth of the Fusarium species. Figshare. Figure.2020b. 10.6084/m9.figshare.13096325 [DOI] [Google Scholar]
- Hasan M: Effects of different plants extract by methanol on inhibition percentages of mycelial growth of the Fusarium species. figshare. Dataset.2020c. 10.6084/m9.figshare.13134953.v1 [DOI] [Google Scholar]
- Hasan M: Effect of antagonistic agents on inhibition of mycelial growth of the Fusarium species. figshare. Dataset.2020d. 10.6084/m9.figshare.13134962.v1 [DOI] [Google Scholar]
- Hasan M: Evaluation of Possible Biological Control of Fusarium sp. by Plants Extract and Antagonistic Species In Vitro. figshare. Preprint.2020e. 10.6084/m9.figshare.13012517 [DOI] [Google Scholar]
- Hasan MF, Islam MA, Sikdar B: First report on molecular identification of Fusarium species causing fruit rot of mandarin ( Citrus reticulata) in Bangladesh [version 1; peer review: 1 approved]. F1000Res. 2020a;9:1212. 10.12688/f1000research.26464.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan MF, Islam MA, Sikdar B: PCR and Sequencing Base Detection of Gummosis Disease on Citrus aurantifolia Caused by Lasiodiplodia theobromae and Evaluation of Its Antagonisms. J Adv Microbiol. 2020b;20(3):77–90. 10.9734/jamb/2020/v20i330230 [DOI] [Google Scholar]
- Hosen MD, Shamsi S: In vitro antagonism of Trichoderma verede and Aspergillus spp. against a pathogenic seed borne fungus of sesame. J Bangladesh Acad Sci. 2019;43(1):17–23. 10.3329/jbas.v43i1.42229 [DOI] [Google Scholar]
- Hussain A, Wahab S, Zarin I, et al. : Antibacterial Activity of the Leaves of Coccinia indica (W. and A) Wof India. Advances in Biological Research. 2010;4(5):241–248. Reference Source [Google Scholar]
- Kader SMA, Hasan M, Ahmed S, et al. : Antioxidant, Antibacterial and Cytotoxic activities of Ethanol extract and its different fractions of Sterculia cordata leaves. Discovery Phytomedicine. 2018;5(3):26–33. 10.15562/phytomedicine.2018.64 [DOI] [Google Scholar]
- Kareem HJ, Al-Araji AM: Evaluation of Trichoderma Harzianum Biological Control Against Fusarium Oxysporum F. Sp. Melongenae. Journal of Science. 2017;58(4B):2051–2060. Reference Source [Google Scholar]
- Khatun MJ, Khalequzzaman KM, Naher MS, et al. : Management of Fusarium Wilt of Tomato by Botanicals and Biocontrol Agents and Their Effect on Yield. Bangladesh J Bot. 2020;49(1):71–74. 10.3329/bjb.v49i1.49095 [DOI] [Google Scholar]
- Munhuweyi K, Mpai S, Sivakumar D: Extension of Avocado Fruit Postharvest Quality Using Non-Chemical Treatments. Agronomy. 2020;10(2):212. 10.3390/agronomy10020212 [DOI] [Google Scholar]
- Nasrin L, Podder S, Mahmud MR: Investigation of Potential Biological Control of Fusarium Oxysporum f.sp. Lycopersici by Plant Extracts, Antagonistic sp. and Chemical Elicitors In Vitro. Fungal Genom Biol. 2018;8(1):155. 10.4172/2165-8056.1000155 [DOI] [Google Scholar]
- O’Donnell K, Sutton DA, Rinaldi MG, et al. : Novel multilocus sequence typing scheme reveals high genetic diversity of human pathogenic members of the Fusarium incarnatum-F. equiseti and F. chlamydosporum species complexes within the United States. J Clin Microbiol. 2009;47(12):3851–3861. 10.1128/JCM.01616-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parveen S, Wani AH, Ganie AA, et al. : Antifungal activity of some plant extracts on some pathogenic fungi. Archives of Phytopathology and Plant Protection. 2014;47(3):279–284. 10.1080/03235408.2013.808857 [DOI] [Google Scholar]
- Tafinta IY, Shehu K, Abdulganiyyu H, et al. : Isolation and Identification of Fungi Associated with the Spoilage of Sweet Orange ( Citrus Sinensis) Fruits In Sokoto State. Nigerian Journal of Basic and Applied Science. 2013;21(3):193–6. 10.4314/njbas.v21i3.4 [DOI] [Google Scholar]
- Vethavalli S, Sudha SS: In vitro and in silico studies on biocontrol agent of bacterial strains against Fusarium oxysporum f. sp. lycopersici. Research in Biotechnology. 2012;3(2):22–31. Reference Source [Google Scholar]
- Whiteside J, Bennett J, Holtzblatt K: Usability engineering: our experience and evolution. In: M. Helander (Ed.), Handbook of human-computer interaction. New York, North Holland,1988;791–817. 10.1016/B978-0-444-70536-5.50041-5 [DOI] [Google Scholar]
- Ziedan ESH, Khattab AA, Sahab AF: New fungi causing postharvest spoilage of cucumber fruits and their molecular characterization in Egypt. J Plant Prot Res. 2018;58(4):362–371. 10.24425/jppr.2018.124650 [DOI] [Google Scholar]


