Abstract
CD4+ follicular helper T (Tfh) cells play a vital role in providing help for B cells undergoing selection and differentiation into activated antibody-secreting cells in mammalian germinal centers (GCs). Increasing evidence suggests that Tfh cells are a heterogeneous population that generates cytokine-skewed immune responses – a reflection of the microenvironment during differentiation. This has important ramifications for Tfh-mediated B cell help. Because Tfh subsets can have opposing effects on GC B cell responses, we discuss current findings regarding the differentiation and functions of cytokine-skewed Tfh cells in modulating GC B cell differentiation. Antibodies are important weapons against infectious diseases but can also be pathogenic mediators in some autoimmune conditions. Since cytokine-skewed Tfh cells can influence the magnitude and quality of the humoral response, we address the roles of cytokine-skewed Tfh cells in disease.
Tfh cells are key players in the mammalian humoral immune response
Plasma cells that secrete class-switched (see Glossary) high-affinity antibodies and long-lived memory B cells are the main cellular components conferring efficacious and long-lasting humoral responses against invading pathogens [1]. The ability of B cells to undergo selection and differentiation into long-lived plasma cells and memory B cells is dependent on signals from T follicular helper (Tfh) cells within GCs [2,3] (Figure 1). Tfh cells are a distinct T cell subset that can be distinguished from other CD4+ T cell subsets by their surface expression of C-X-C chemokine receptor 5 (CXCR5) [4,5], programmed cell death protein 1 (PD-1), and inducible T cell co-stimulator (ICOS), and low expression of chemokine (C-C motif) receptor 7 (CCR7) [4,5] – the latter enabling Tfh cell migration to and retention in the GC [6]. Tfh cells are also characterized by the expression of the transcription factor (TF) B cell lymphoma 6 protein (Bcl-6) [7–9] and the production of interleukin (IL)-21 in mice and humans [10].
Figure 1. T follicular helper (Tfh) cell roles in mammalian B cell differentiation.
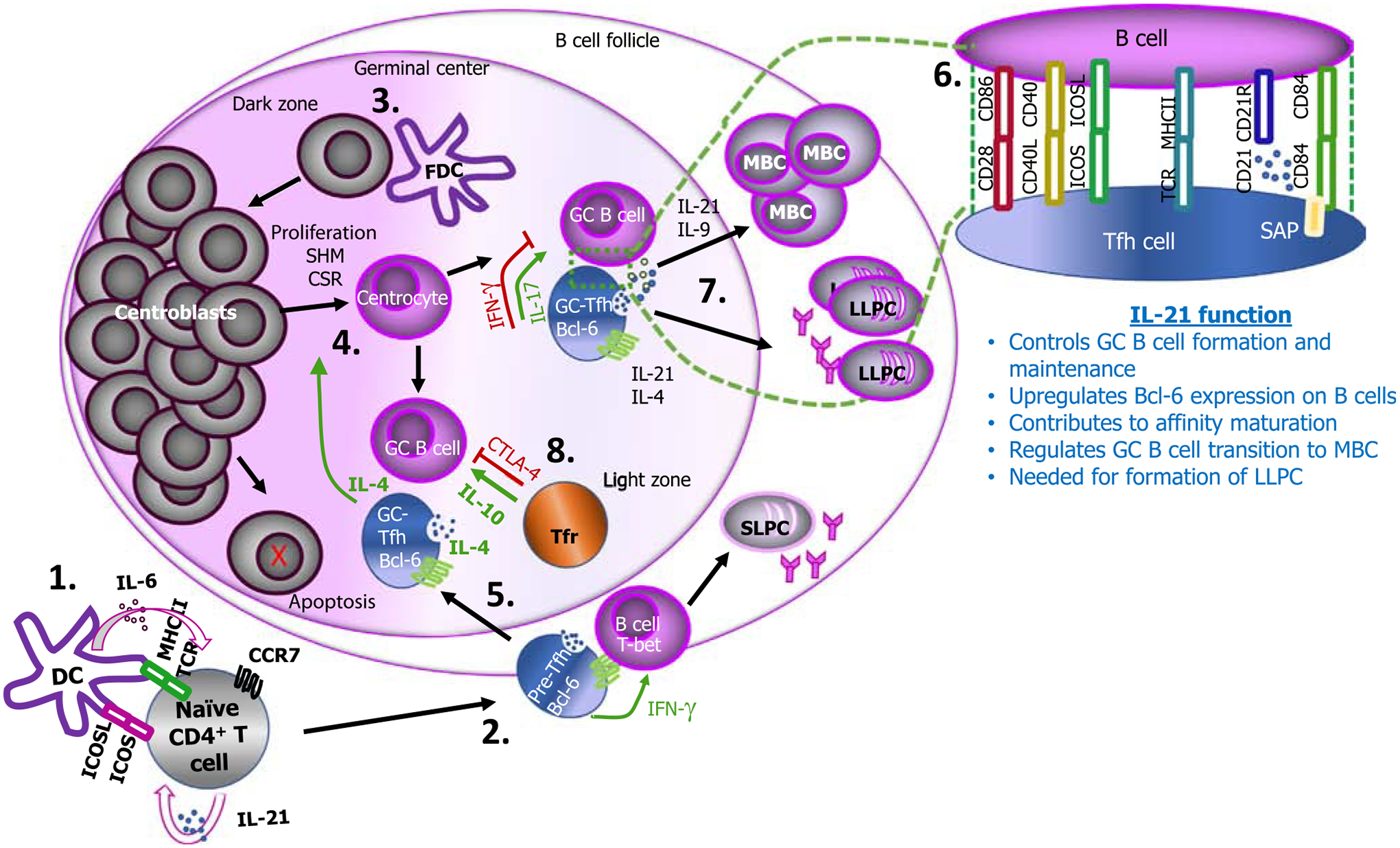
Dendritic cells (DCs) induce initial priming of naïve CD4+ T cells in the presence of interleukin (IL)-6 and through inducible T cell co-stimulator (ICOS)/ICOS-L (ligand) interactions. This process is accompanied by antigen recognition through peptide-loaded MHC-II on DCs and differentiation of T cells into pre-Tfh cells, characterized by upregulation of B cell lymphoma 6 protein (Bcl-6) and C-X-C chemokine receptor 5 (CXCR5), while downregulating CCR7 (1). Autocrine signaling of IL-21 on Tfh cells enhances their development and maintenance. Expression of CXCR5 enables Tfh cells to migrate to B cell follicles where they interact with B cells, leading to B cell differentiation into short-lived extrafollicular plasma cells (SLPCs) (2). Follicular dendritic cells (FDCs) interact with follicular B cells, and this allows B cells to migrate to the dark zone where they differentiate into centroblasts (3). Centroblasts undergo somatic hypermutation (SHM), class-switch recombination (CSR), and proliferation, and subsequently migrate to the light zone to form centrocytes (4). In addition, IL-4 produced by Tfh cells may mediate centrocyte differentiation. B cell clones in the dark zone with low affinity or that are autoreactive can undergo additional SHM or die because of apoptosis. The cognate interaction between pre-Tfh cell and B cells allows full differentiation of Tfh cells, and these migrate to the GC (5) where they provide help to B cells activated by FDCs via cytokines that in some cases may include IL-17. CD28/CD86, CD40L/CD40, ICOS/ICOS-L, TCR/MHCII, CD21/CD21R, and SAP-associated receptor (CD84)/CD84, mediate cognate interactions between T and B cells (6). This GC reaction between Tfh and B cells results in memory B cell (MBC) and long-lived plasma cell (LLPC) formation supported by IL21, IL-9, and IL-4 (7). Regulatory T follicular (Tfr) cells can suppress T–B cell interactions through cytotoxic T lymphocyte-associated protein 4 (CTLA-4) but enhance B cell maturation via IL-10 (8). Arrows in green signify a positive signal for B cell maturation; arrows in red signify a negative signal.
Tfh-derived IL-21 [3,11] plays several crucial roles, including the formation and maintenance of GCs and the development of plasma cells and memory B cells [1]. Recent studies of responses during infection in mice and humans reveal that Tfh cells can produce a more varied repertoire of cytokines than the canonical IL-21. Indeed, Tfh cells are a heterogeneous population of cells that are capable of coexpressing low, but biologically meaningful, amounts of TFs that are more typical of other T cells subsets, such as T-box expressed in T cells [T-bet; type 1 T helper (Th1) cells], GATA-binding protein 3 (GATA-3; Th2 cells), retinoic acid receptor-related orphan receptor γ (ROR-γt; Th17 cells) and Forkhead box protein 3 [FoxP3; T regulatory (Treg) cells] (Figure 2 and Box 1), as well as cytokines associated with these TFs [12–15]. Although the roles of these cytokines are well defined in classical helper T cell responses, the functions of Tfh-derived canonical T helper cell cytokines within the GC are poorly understood, but can have consequences for the resulting humoral response.
Figure 2. Proposed models for the differentiation of cytokine-skewed T follicular helper (Tfh) cell subsets in mice and humans.
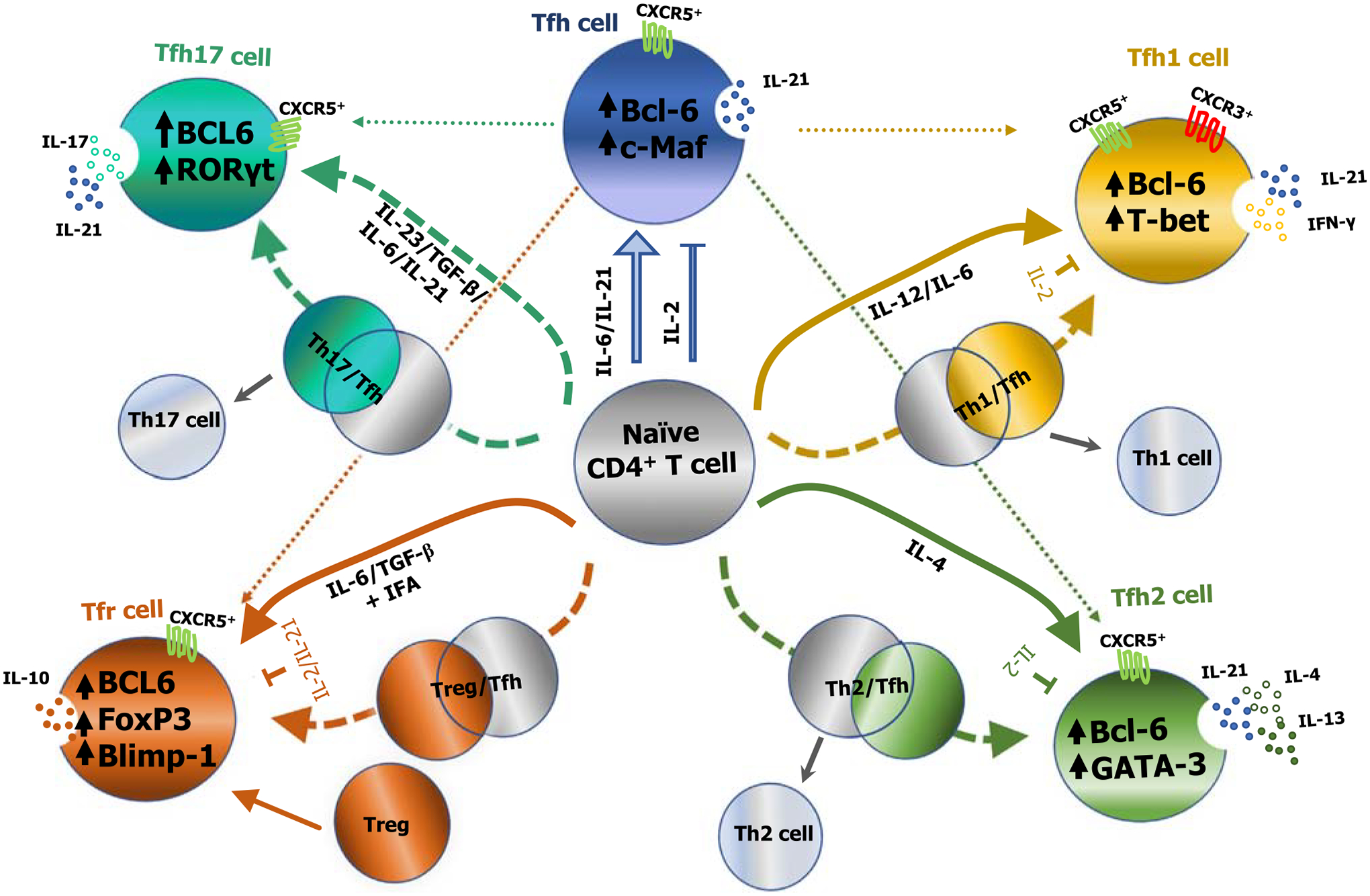
A schematic representation of the differentiation of cytokine-skewed Tfh cells from naïve CD4+ T cells is shown. External stimuli including the cytokine milieu, as well as intrinsic factors such as lineage-specific transcription factors (TFs), induce the differentiation of naïve CD4+ T cells into subsets of cytokine-skewed Tfh cell subsets. Polarizing cytokines can induce Tfh cells to coexpress transcriptional programming that is typical of other CD4+ helper T cells, in addition to Bcl-6 that is typically expressed by Tfh cells. It is possible that a partial ‘hybrid’ [T helper (Th)1/Tfh, Th2/Tfh, Th17/Tfh, and T regulatory cell (Treg)/Tfh] state occurs before maturing into different cytokine-skewed germinal center (GC)-Tfh cells. In view of this, Tfh cells can produce limited quantities of canonical cytokines that have been used to identify other T helper subsets, in addition to interleukin (IL)-21, typically associated with classical Tfh cells. The majority of T follicular regulatory (Tfr) cells arise via differentiation from infiltrating Tregs, although some also differentiate from naïve CD4+ T cells. Broken lines indicate hypothesized differentiation pathways for cytokine-skewed Tfh subsets [12]. Abbreviations: Bcl-6, B cell lymphoma-6; Blimp-1, B lymphocyte-induced maturation protein-1; c-Maf, c-musculoaponeurotic fibrosarcoma oncogene homolog; CXCR5, C-X-C chemokine receptor 5; FoxP3, forkhead box protein 3; GATA-3, GATA-binding protein 3; IFN-γ, interferon γ; RORγt, RAR-related orphan receptor γ; T-bet, T-box expressed in T cells; Tfh1, Th1-like Tfh cells; Tfh2, Th2-like Tfh cells; Tfh17, Th17-like Tfh cells.
Box 1. Priming of cytokine-skewed Tfh cells.
Conventional Tfh cells
IL-6 and IL-21 are essential for Tfh cell differentiation. Engagement of IL-21R through IL-21 autocrine signaling leads to the activation of STAT3, upregulation of Bcl-6, and CD4+ T cell differentiation into Tfh cells. Bcl-6 expression enables down-stream expression of E proteins which drive the expression of CXCR5, the follicle homing chemokine receptor that identifies Tfh cells [31]. Differentiated Tfh cells produced copious amounts of IL-2 but remain unresponsive to this cytokine through intrinsic IL-6 signaling [102] that prevents STAT5 from accessing the Il2rb gene locus, in turn preventing upregulation of IL-2Rβ and activation of the T cell receptor/IL-2/STAT5 positive feedback loop [102].
Tfh1 cells
Adoption of a Th1 phenotype leads to the development of a Th1 and Tfh ‘hybrid’ cell type referred to as Th1-like Tfh (Tfh1) cells. This population is characterized by a phenotypic profile of CXCR3+CXCR5+CCR6− and expression of IFN-γ in addition to IL-21 [103]. In vitro differentiation of mouse [44] and human [45] naïve CD4+ T cells into Tfh1 cells appears to require IL-12R signaling, but not IL-6 or IFN-γ receptor signaling. Ligation of the IL-12 receptor activates STAT4 and STAT3 in mouse [44] and human [45] CD4+ T cells, leading to the expression of a Th1 and Tfh transcriptional profile in this hybrid population, driven by T-bet and Bcl-6, respectively [44]. IL-27 signaling also drives a Tfh1 phenotype [46] whereas IL-2 signaling suppresses this differentiation [44].
Tfh2 cells
These can be identified based on their production of IL-4 and IL-21 [36,104] and a lack of expression of the two chemokine receptors CXCR3 and CCR6 [28]. The differentiation of Tfh2 cells is dependent on IL-4 but is suppressed by IL-6 via STAT3 signaling [105]. Tfh2 cells express Bcl-6 as well as GATA-3. Given that IL-4 is pivotal to B cell survival and affinity maturation [69,106,107], the importance of Tfh2 cells extends beyond the induction of class switching of B cells to IgE [108,109]. IL-4 secreted by Tfh2 cells supports humoral immunity in situations where IgE is not made, such as in IgG4-mediated diseases [89–91].
Tfh17 cells
Characterized by IL-21 and IL-17 production, the differentiation of Tfh17 cells is dependent on IL-23, ICOS, and RORγt [24]. Tfh17-associated IL-17 may enhance cognate T–B cell interactions, leading to spontaneous GC development [71]. The IL-17 produced from Tfh17 cells also induces switching to IgG2a and IgG3 [77].
Tfr cells
Regulation of follicular responses is mediated by T follicular regulatory (Tfr) cells. Tfr cells express Treg signature molecules such as the TF forkhead box (Fox) p3, CTLA-4, and GITR [15,52], but are deficient in the expression of IL-4, IL-21, and CD40L – molecules that provide help signals to B cells [15]. Tfr cells can differentiate from naïve CD4 T helper cells originating from conventional FoxP3+ Treg precursor cells as well as after immunization with foreign protein emulsified in incomplete Freunds adjuvant (IFA) in a PD1-ligand dependent manner [15,55,110,111]. Like conventional Tfh cells, IL-6 plays a key role in the differentiation of Tfr cells, and STAT3 deficiency abrogating signaling from IL-6R leads to a decrease in Tfr cells in both the spleen and Peyer’s patches following antigen stimulation [112]. IL-21, the major cytokine produced by Tfh cells, suppresses Tfr cell differentiation [113].
The GC response is crucial in the generation of efficacious humoral immunity. An understanding of the conditions that regulate cytokine production by Tfh cells could be harnessed to enhance humoral immunity to pathogens. The limited quantities of canonical T helper lineage cytokines produced by Tfh cells make these subsets difficult to identify through commonly used assays that measure cellular cytokine production following antigen restimulation. To more clearly identify antigen-specific Tfh cells, approaches such as activation-induced marker assays were recently developed to examine the upregulation of generic activation markers such as CD25 and OX40 following antigen restimulation, instead of cytokines [16,17]. This has enabled an advance in our understanding of Tfh biology. We discuss here the differentiation and functional consequences of heterogeneous Tfh cells that we refer to as ‘cytokine-skewed’, and discuss the roles of cytokine-skewed Tfh cells during B cell help for different inflammatory environments.
Development of cytokine-skewed Tfh cells
Tfh cells were first described in human tonsils [18–20], although analyses of human Tfh cells have thus far mostly focused on circulating Tfh (cTfh) cells with the phenotypic profile of PD-1+CXCR5+ surface expression. The use of mouse genetic models has defined crucial TFs that regulate early Tfh cell differentiation and commitment (reviewed in [21]). The dominant TF that is required for and controls the programming of Tfh cells is Bcl-6 [7–9,21].
Bcl-6 is a key TF in Tfh cell differentiation
Early Tfh cell differentiation is positively influenced by additional TFs including cellular musculoaponeurotic fibrosarcoma oncogene homolog (c-Maf) [22–24], lymphoid enhancer-binding factor 1 (LEF-1), and T cell factor 1 (TCF-1) [25,26]. Multipotent in all aspects of the formation and maintenance of the GC reaction, the cytokine IL-21 is crucial for the development of plasma cells (Figure 1) [3,10,11]. Studies in mice [27] and humans [23] have shown that c-Maf is a key inducer of IL-21 production and is therefore a key molecule conferring Tfh function. However, simultaneous expression of Bcl-6 is necessary to repress transcriptional programming that instructs other T helper cell lineage subsets.
Many TFs serve as negative regulators of Tfh cell differentiation. As shown in mice, the repressive activity of Bcl-6 is antagonized by B lymphocyte-induced maturation protein-1 (Blimp-1) [7]. Other TFs, including Foxo1 [28,29] and ID2 [30], act to antagonize Bcl-6 or other pro-Tfh programs and promote the differentiation of other T helper lineage cells. These findings suggest that, beyond direct Blimp1-mediated Bcl-6 repression, Bcl-6 indirectly and positively instructs other genes involved in Tfh cell differentiation via a ‘repressor of repressor mechanism’ [21,31].
Models of differentiation
Beyond the differentiation of CD4+ T cells into the Tfh lineage, the expression of cytokines more traditionally associated with T helper cells has been described. The influential role of signature cytokines from T helper cell subsets with respect to humoral responses, combined with the key role of Tfh cells in providing help to B cells for activation, indicates a potentially important role for cytokine-skewed Tfh cells in shaping humoral responses. The developmental pathways leading to cytokine-skewed Tfh cells are incompletely understood. Proposed pathways include a linear route for naïve CD4+ T cells to become cytokine-skewed Tfh, as well as a pathway that incorporates a transitional hybrid state between Tfh cells and other CD4+ helper T subsets [12] (Figure 2). Identification of a pathway that involves a transient differentiation state could be important in maintaining flexibility for gene expression profiles of Tfh cells, with ramifications in terms of Tfh cell plasticity, as the immune response progresses. Other studies have shown that classical T helper cells, such as Th2 cells, can further differentiate into Tfh cells [14]. Regardless of the developmental pathways, once induced, Bcl-6 promotes Tfh cell differentiation by repressing other helper T cell lineage-associated signature genes [32]. Bcl-6 accomplishes this repressive function by binding to the promoters of Th1/Th2/Th17 TF genes (e.g., encoding T-bet, GATA-3, and RORγt), leading to suppression of interferon γ (IFN-γ) and IL-17 production in human tonsillar cells and mice [8]. The transitional model that incorporates a hybrid cell type can explain the bifurcation of Tfh and classical T helper cells, as well as the identification of cells displaying characteristics of both.
Differentiation of Tfh1 cells
Tfh1 cells are the best-studied subset of Tfh cells that have partial qualities of polarization towards other T helper lineages, and these are generated during type 1 inflammatory responses. A transient increase in ICOS+PD-1+CXCR5+CXCR3+ circulating Tfh1 (cTfh1) cells was observed in humans following vaccination with influenza virus trivalent inactivated vaccine relative to controls; this increase correlated positively with high-affinity antibody production [33], suggesting that there might be a functional correlation between the number of cTfh1 cells and the generation of high-avidity antibodies. Moreover, the priming of naïve CD4+ T cells in the presence of the polarizing cytokine IL-12 or infection of mice with Toxoplasma gondii (a strong inducer of Th1 cell responses) can increase the expression of Tbx21, Bcl6, and Il21 genes via signal transducer and activator of transcription 4 (STAT4), and transiently generate cells that exhibit features of both Th1 and Tfh cells [12]. This supports the idea that cytokine-skewed Tfh cells may develop through a transitional hybrid state (Figure 2).
In vitro polarization of CD4+ T cells sorted from naïve wild-type (WT) and Tbx21−/− mice showed that Th1 differentiation proceeds with an increase in T-bet expression which represses Tfh cell differentiation and promotes full differentiation of Th1 cells [12]. However, T-bet can also recruit Bcl-6 to the Ifng locus to curtail excessive IFN-γ production [34]. Thus, the development of Tfh, Th1, and Tfh1 cells may depend on the ratio of T-bet and Bcl-6 in the cells, thus explaining, at least in part, observations that all three subsets of T cells can coexist in type 1 inflammatory conditions [12,13]. Indeed, this hypothesis is supported by a second study where the expression of both T-bet and Bcl-6 was monitored in CD4+ T cells cultured under Th1-polarizing conditions, and in the presence of varying concentrations of IL-2 [13]. Although a higher concentration of IL-2 inhibits Bcl-6 expression in Th1 cells, it does not have an impact on T-bet expression, indicating that the T-bet:Bcl-6 ratio, which is modulated in part by IL-2, can dictate the Bcl-6–Blimp1 axis balance, and may be important in maintaining the flexibility between the Th1/Tfh gene profiles [13]. Furthermore, CXCR5+ Tfh cells circulating in human blood, referred to as cTfh cells, express low amounts of Bcl-6 [35,36]; thus, the lack of Bcl-6-mediated transcriptional repressor activity in cTfh cells in humans might help to explain a higher frequency of cytokine-skewed Tfh cells in which T helper cell-associated transcripts and cytokines are not suppressed once they enter the circulation [37]. Of note, the epigenetic modifications that differentiate cTfh cells from their tissue-resident counterparts in humans are currently unknown, although cTfh cells may functionally resemble GC Tfh cells in lymphoid organs [36–38].
Control of loci by epigenetic modifications that include dynamic DNA methylation, chromatin remodeling through histone modification, and noncoding RNAs seems to play an essential role in the development of cytokine-skewed Tfh cells. For instance, STAT TFs that transduce signals from cytokine receptors can control transcriptional programming by promoting the deposition of repressive or permissive epigenetic marks at specific loci [42,43]. STAT4 transduces signals from the IL-12 receptor and has been found to control permissive H3K36me3 and H3K4me3 modifications that regulate gene loci such as Bcl6, Pdcd1, and Il21 that are important in Th1 and Tfh cell differentiation [12]. Moreover, IL-12-driven differentiation of Tfh1 cells occurs through phosphorylation of STAT1 and STAT4 [44,45] which act by suppressing the histone repressive mark H3K27me3 on the Tbx21 and Bcl6 gene loci [45]. This results in IL-12-driven expression of Tfh cell-associated genes such as those encoding ICOS and Bcl-6 in CD4+ T cells [44]. In addition, STAT3, which transduces signals from the IL-6 receptor, also regulates the commitment of CD4+ T cells to either a Tfh1 or Th1 phenotype in a Plasmodium sp. mouse infection model by regulating T-bet expression to avoid potential immunopathology [46].
Differentiation of Tfh2 cells
Tfh cells can produce the classical Th2 cytokine IL-4 [14]. This can occur after the production of IL-21 has been initiated following immunization of mice with nitrophenyl-haptenated ovalbumin (NP-OVA) [39], but this is not a strict requirement given that IL-4-producing Th2 cells, when adoptively transferred into naïve mice and challenged with antigen (schistosome egg antigen), can differentiate into IL-4-producing Tfh2 cells [14]. IL-4 does not appear to play a role in the formation of mouse GCs until pre-T follicular helper (pre-Tfh) cells are in the follicle (Figure 1), thus influencing centrocyte formation in the follicular B cell population [39]. Tfh2 cells can be distinguished from other subsets of Tfh cells by the quantity of IL-4 produced. Although all Tfh cells open the Il4 locus to facilitate centrocyte formation, not all continue to produce IL-4 [39,40]. Of note, most virus-specific Tfh1 cells do not express IL-4, but the GC Tfh cell subset does in the context of acute lymphocytic choriomeningitis virus (LCMV; Armstrong strain) acute infection in mice [41]. Thus, sustained IL-4 production appears to be the hallmark of Tfh2 cells, and Tfh cells skewed towards other subsets such as Tfh1 cells do not produce IL-4 to any appreciable concentration in mice immunized with unadjuvanted soluble antigen from T. gondii [40].
Differentiation of Tfh17 cells
Tfh cells coproducing IL-17 in addition to IL-21 [24], termed Tfh17 cells, have also been described in mice. Although comparatively little is known about these cells, they appear to share many characteristics of Th17 cells in that their differentiation is dependent on the TF RORγt [47], as well as on receptor ligation via IL-6, IL-21, and transforming growth factor (TGF)-β [48–50]. In vitro stimulation experiments of mouse naïve T cells suggest that the expansion and maintenance of Tfh17 cells is mediated by IL-23 [24,51], but the interplay of Bcl-6 and RORγt in the regulation of the coexpression of IL-21 and IL-17 has not been studied in great detail. However, there appears to be a key role for c-Maf, which is important in IL-21 expression, both in the induction of ICOS expression [24] and presumably also in the coinduction of Bcl-6 [22–24], leading to coexpression of IL-17 and IL-21. Thus, these key molecules described in Tfh cells play an important role in the differentiation of Tfh17 cells, but are likely not factors that might directly lead to IL-17 expression in this subset.
Regulation of GC responses by T follicular regulatory cells
Once formed, GC responses are known to be tempered by T follicular regulatory (Tfr) cells in murine studies. Similarly to Tregs, Tfr cells express Treg signature molecules such as the TFs Foxp3, cytotoxic T lymphocyte-associated protein 4 (CTLA-4), and GITR [15,52], but are deficient in the expression of IL-4, IL-21, and CD40L – molecules that are crucial for B cell help [15]. In Tfr-diphtheria toxin receptor (DTR) (Foxp3creCxcr5DTR/WT) mice in which Tfr cells were deleted at days 5–9 after immunization with NP-OVA, Tfr cells exerted an early effect on antigen-specific B cells before the formation of GCs, as demonstrated by an increased frequency of GC B cells compared with control mice (Foxp3crecxcr5WT/WT) [53]. This finding suggested that Tfr cells might modulate extrafollicular B cell responses. However, FoxP3-expressing Tfr cells also display restrained GC B cell proliferation and promote the generation of high-affinity antibodies; although the vast majority of Tfr cells are generated from infiltrating Tregs (e.g., [54]), they also develop directly in the GC from naïve T cells, but in smaller numbers [55] (Figure 2). In addition, circulating Tfr (cTfr) cells are generated in the peripheral lymphoid tissue before T and B cell interactions [56], and studies in mice in which cTfr cells were sorted from immunized mice, and cocultured with B cells in a suppression assay, suggested that cTfr cells may have a limited capacity to suppress Tfh cells and IgG1 in B cells compared with lymph node-resident Tfr cells [57]. This suppression might be acquired concomitantly with GC infiltration because cTfr cells are able to migrate to secondary lymphoid organs and partake in GC reactions in mice [57]. Once in the GC, Tfr cells can maintain their suppressive function via the expression of polycomb protein Ezh2 – a chromatin-modifying enzyme that is necessary for the development of T cell homeostasis and cytokine production [42,58,59], and is crucial in maintaining Tfr cell transcriptional programming [60] via direct binding to Foxp3 [59–61].
Although IL-10 was previously thought to play a significant role in the suppression capacity of Tfr cells, B cell-intrinsic IL-10 signaling promoted GC responses and anti-parasite immunity in a mouse model of malaria [62,63]. It is reasonable to speculate that suppression is instead mediated by other molecules on the Tfr surface, such as CTLA-4; indeed, specific deletion of CTLA-4 from Tregs does not restrain the expansion of GC and Tfh cells, despite an increase in the frequency of both Treg and Tfr cells in these mutant mice relative to controls [64]. However, these possibilities remain to be investigated under a more robust condition in which CTLA-4 is specifically knocked out from Tfr cells. Thus, the mechanisms by which Tfr cells function remain speculative and require further investigation.
Plasticity of cytokine-skewed Tfh cells
Although there is evidence that Tfh cells can maintain Tfh cell commitment [65], it is unclear whether Tfh subsets are plastic with respect to differential skewing of cytokines. However, epigenetic control of specific gene loci in Tfh cells can be altered to facilitate the transition of Tfh-like to conventional T helper cell subsets, as demonstrated in a study that utilized in vitro and ex vivo repolarized murine Tfh cells [66,67]. Analysis of adoptively transferred CXCR5+ memory Tfh cells in the acute LCMV infection mouse model suggests that, although the majority of secondary effector cells can recall a Tfh response upon rechallenge with LCMV, those that exhibit some plasticity by downregulating CXCR5 also maintain low expression of Th1 effector molecules, including IFN-γ and granzyme B [65]. Of note, DNA methylation at the Gzmb promoter is a hallmark of memory Tfh cells compared with memory Th1 cells in mice [65]. Together, these data suggest that, although Tfh cells may exhibit some plasticity, there may still be some epigenetic restrictions on the extent of such plasticity.
Regarding Tfh2 cells in the house dust mite mouse allergy model, those cells that were reactive to the antigen, and which secreted IL-4 on initial sensitization, could also transition to become conventional IL-4 and IL-13 double-producing Th2 cells upon restimulation, suggesting that Tfh2 might be precursors to Th2 cells [66]. However, Th2 cells can also differentiate into Tfh2 cells [14]. These data support the idea that Tfh cells can express permissive histone modifications such as H3K4me at specific gene loci (e.g., Tbx21, Gata3, and Rorc) that are associated with classical Th cell subsets, thereby enabling Tfh cells to express effector molecules and perform effector functions [67]. The plasticity of Tfh cells is vital to the generation of short-term effector Th cells, as well as long-term humoral immunity.
The role of cytokine-skewed Tfh cells in B cell differentiation
In humans, B cells in the GC interact with Tfh cells through CD40/CD40L, CD28/CD86, and ICOS/ICOS-L interactions [23], constituting the GC reaction in which B cells undergo multiple rounds of differentiation and proliferation in the GC dark zone. This involves a series of maturation steps, including somatic hypermutation, class switching, and affinity maturation, eventually resulting in a population of differentiated plasma cells and memory B cells (Figure 1). Tfh cells provide a plethora of signals to differentiating B cells, including IL-21 which is absolutely required for plasma cell formation and somatic hypermutation, as well as producing IL-9, which is essential for the formation of memory B cells in the GC [68] via stimulation of memory precursor cells in both mice and humans. The role of classical T helper cytokines in the GC with respect to the B cell maturation process is incompletely understood but is not inconsequential. Temporal and spatial effects influence the contributions of these cytokines to GC formation and the B cell differentiation process, and some studies demonstrate that cytokine production is asynchronous [69].
The role of pre-Tfh cells compared with GC Tfh cells in class-switch recombination (CSR) is difficult to dissect. However, recent studies have demonstrated that CSR is initiated before GC formation [70]. Therefore, cytokine production by pre-Tfh cells may play a role in class switching before the formation of the GC. There is evidence that IL-17 associated with Tfh17 cells might enhance cognate T–B cell interactions, leading to spontaneous GC development, as shown in autoimmune BXD2 mice [71]. This might potentially occur via suppressed mobility of B cells within the B cell follicle, thereby fostering GC reactions, or by stabilizing Tfh cells in the light zone [71]; indeed, interruption of IL-17 signaling abrogates autoreactive B cell responses [72]. However, the mechanism underlying this observation remains unclear. There is stronger evidence for the role of cytokine-skewed Tfh cells in isotype class switching. Specifically, both Tfh1 [44,73] and Tfh2 [74] cells are instrumental in isotype switching: on the one hand, in the absence of Tfh1 cells, there is a lack of isotype switching to IgG2c and IgG2a [73,75] and of enhanced IgG1 [76] isotype switching in response to viral infection in mice (Figure 3), with consequences for the maintenance of long-term neutralizing antibodies [73]. On the other hand, sustained IL-4 production by murine Tfh2 cells, along with IL-13, can drive switching to IgE [74], and in vitro stimulation of murine B cells in the presence of exogenous IL-17 appears to induce switching to IgG2a and IgG3 [77]. Given the proximity of Tfh cells to the GC where B cells undergo phenotypic and functional development, the production of cytokines associated with other T helper cell lineages by cytokine-skewed Tfh cells is paramount for efficacious antibody responses.
Figure 3. Cytokine-skewed T follicular helper (Tfh) cell subsets may play varied roles in modulating antibody isotype class switching and affinity maturation in both mice and humans.
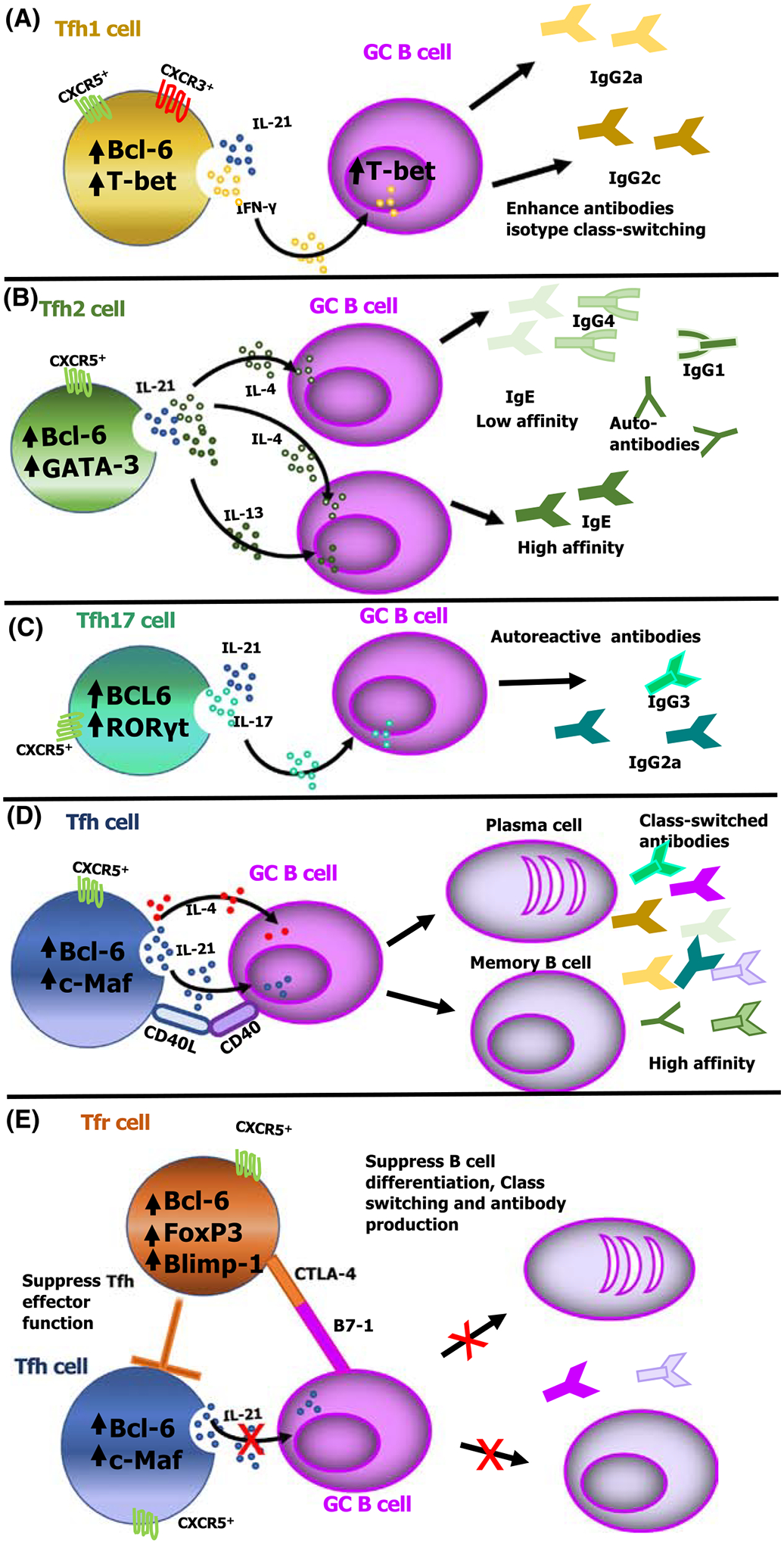
(A) Interferon (IFN)-γ produced by Th1-like Tfh (Tfh1) cells induces the expression of T-box expressed in T cells (T-bet) on B cells that promotes IgG2a isotype class switching in response to lymphocytic choriomeningitis virus (LCMV) or Zika viral infection and IgG2c isotype class switching in response to Plasmodium parasites, albeit by limiting the overall germinal center (GC) B cell response [73,75,78]. (B) Cytokines secreted from Th2-like Tfh (Tfh2) cells influence the affinity of the antibody produced, where IL-4 from Tfh2 cells promotes low-affinity IgG1 and IgE, whereas coproduction of interleukin (IL)-4 and IL-13 induces high-affinity IgE. (C) IL-17 is produced by Th17-like Tfh (Tfh17) cells that induce antibody isotype class switching to IgG2a and IgG3, and induction of autoreactive antibodies. (D) Conventional Tfh cells produce effector molecules such as IL-21 and IL-4 that induce B cell differentiation into plasma and memory B cells. (E) T follicular regulatory (Tfr) cells mediate regulatory functions by expressing cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), a coinhibitory receptor that interacts with B7–1/B7–2 on B cells, resulting in direct suppression of IL-21 production by Tfh cells, and disrupting cognate interactions between Tfh and B cells. This suppressive function of Tfr cells on either B or Tfh cells subsequently limits B cell differentiation into memory or plasma cells [57,124]. Abbreviations: Bcl-6, B cell lymphoma 6 protein; c-Maf, cellular musculoaponeurotic fibrosarcoma oncogene homolog; CXCR, C-X-C chemokine receptor; GATA-3, GATA-binding protein 3; RORγt, RAR-related orphan receptor γ.
Beyond isotype switching, a wider role for Tfh1 cells in the GC reaction has been shown in the induction of T-bet in B cells via IFN-γ signaling [78]. This has been linked to retention of GC B cells in the dark zone and affinity maturation during Plasmodium sp. infection in mice [79] leading to an enhanced anti-malarial response relative to controls (as evidenced from specific deficiency of T-bet in B cells). However, this finding was not reproduced in another study reporting that T-bet expression in B cells suppressed anti-Plasmodium humoral immunity [62]. The discrepancy between these two reports may be due to differences in the models used – the latter study used a model of Plasmodium infection that did not require chemotherapeutic intervention to keep the mice alive [79]. Nonetheless, the latter study also determined that intrinsic IL-10 signaling in B cells is necessary to limit the suppressive effects of IFN-γ within the GC, presumably supporting a suppressive IL-10 effect from Tfh1 cells on the generation of humoral immunity [62]. This notion is also supported by studies of LCMV infection in mice where IL-10+IL-21+ double-producing Tfh cells present within the spleen during chronic (clone 13 strain) but not acute (Armstrong) LCMV infection enhanced humoral immunity and viral control [80]. This role of IL-10-producing Tfr cells might also extend to enhancing type 2 helper responses, as suggested by a study in a mouse model of induced peanut allergy in which Tfr-produced IL-10 enhanced GC responses and increased antigen-specific serum IgE in control mice relative to Tfr-deficient (Bcl6fl/flFoxp3cre) mice [81]. In humans, by contrast, a subset of tonsillar follicular CD25+ FoxP3−IL-10+ T cells – that otherwise functionally and phenotypically resembled mouse Tfr cells – prevented isotype class switching to IgE without having any suppressive role on plasma cell differentiation [82]; this suggested that suppression was restricted to specific aspects of the humoral response. This difference between species highlights one of the challenges in assessing the role of cytokine-skewed Tfh cells where data from mouse models may not always translate to humans.
The role of cytokine-skewed Tfh cells in immunity to infection
Cytokine-skewed Tfh cells arise in different infectious disease settings, concomitantly with a diverse suite of effector cytokine-driven immune effector mechanisms that are required for the control of different pathogens, not least of which are those mediated by different antibody isotypes. In line with the role of cytokine-skewed Tfh cells in antibody class switching in GC B cells, there is mounting evidence for their importance in the generation of neutralizing antibodies, particularly in human viral infections such as hepatitis C virus (HCV) [83] and HIV-1 [84,85], in which the frequency of cTfh1 cells correlated with a broadly reactive antiviral humoral response. Similarly, IgE is a key mediator of type 2 inflammation that contributes to anti-helminth immunity in a variety of helminth infections in both mice and humans, and its production is thought to be mediated by IL-4 produced by CD4+ T cells [86].
Tfh2 cells are now emerging as the most crucial regulatory component of IgE secretion during intestinal helminth infection via the production of IL-4. Depletion of Tfh2 cells, but not of Th2 cells, in the lymph node microenvironment impaired IgE+ plasma cell differentiation and antibody production during Heligmosomoides polygyrus infection in mice [87,88]. Moreover, Tfh2 cells can induce IgG1-producing as well as IgE-producing plasma cells in response to helminth infection, and the production of both isotypes is compromised in the absence of IL-4 signaling [14,88]. These data underscore the importance of Tfh2 cell-derived IL-4 as the crucial cytokine source in directing humoral responses to helminth infections. Although IL-4 might play a role in the formation of centrocytes in the GC, it does not appear to play a role in affinity maturation. IL-4-producing Tfh2 can induce low-affinity IgE or IgG4 responses [89–91], and low-affinity IgE-producing plasma cells develop when there is a direct class switch from IgM to IgE. This is illustrated by the inability of IgG1-deficient mice to produce high-affinity IgE, even after repeated immunization with NP19KLH [92]. By contrast, high-affinity antibodies can develop upon sequential class switching from IgM to IgG1 to IgE [92–94], as observed in Nippostrongylus brasillensis infection in mice. Noteworthy, low-affinity/directly switched IgE was found in GC B cells, while high-affinity/sequentially switched IgE was found in plasma cells [92–94].
Although IL-4 remains the key defining cytokine of type 2 immunity, Th2 cells can produce other type 2 cytokines, including IL-5 and IL-13 [95]. A study showed that plasma cells producing higher-affinity IgE during anaphylaxis can be driven by multipotent Tfh2 cells coexpressing IL-4 and IL-13 (termed Tfh13 cells) [74], suggesting that Tfh2 cells might have similar characteristics to Th2 cells in this regard (Figure 3). This observation suggests that cytokines produced by Tfh2/Tfh13 cells might contribute to defining the affinity of the antibodies produced. Although the putative mechanism behind this observation is not fully understood, we posit that the kinetics of antibody isotype class switching driven by different subsets of Tfh2 in the GC might be a key factor in the affinity of the antibody produced.
Cytokine-skewed Tfh cells do not always appear to be beneficial (Box 2). During Plasmodium sp. infections, cTfh1 cells isolated from children infected with Plasmodium falciparum have been shown to be inferior at providing B cell help compared with conventional cTfh cells, as evidenced by the decrease in their ability to induce isotype class switching in naïve B cells cocultured with superantigen SEB (staphylococcal enterotoxin B) [96]. Specifically, this impaired function of Tfh1 cells has been attributed to IFN-γ production by both Th1 and Tfh1 cells during Plasmodium infection in both mice and humans; indeed, blockade of IFN-γ or deletion of T-bet enhanced Tfh cell differentiation relative to controls [96,97]. This is surprising given the importance of Th1 responses [98,99] and antibodies [100,101] in malaria, as well as the preferential activation of cTfh1 over conventional cTfh cells. However, the observation that IFN-γ suppresses GC responses was supported by at least one other study in rodent Plasmodium infection, attributing it to the induction of T-bet in GC B cells [62] – which led to the differentiation of atypical memory B cells and antibody skewing towards an IgG3 isotype [78]. Since cTfh1 cells upregulate CXCR3, a chemokine receptor that allows lymphocyte migration, it is also possible that, in this setting, CXCR3 expressed on cTfh1 cells enhances Tfh1 cell trafficking from the GCs towards the T–B cell border [97], leading to expedited and inefficient cognate interaction between Tfh1 cells and B cells, and inferior antibody production. Regardless of the mechanism, it is not clear why these potential explanations might not also apply to the generation of antibodies in viral infection (Figure 3). The function of cTfh1 cells in humoral immunity is thus context-dependent and pathogen-specific because cTfh1 cells can be beneficial in viral infections, but detrimental in some parasitic infections.
Box 2. Contribution of cytokine-skewed Tfh cells to autoimmune and allergic disease.
Many autoimmune diseases are known to be driven in part by autoantibodies. The importance of Tfh cells as driving factors in the development of robust class-switched and high-affinity auto-antibody responses places Tfh cells as a key cell subset in the development of a range of autoimmune and allergic diseases such as systemic lupus erythematosus (SLE) and anaphylaxis. IgE has long been identified as the perpetuator of allergic responses leading to asthma [86] and is the main source of IL-4 that drives class switching to IgE; this can be driven by Tfh2 cells rather than by conventional CD4+ Th2 cells in mice [74,87]. In peripheral blood, IL-21/IL-4 double-producing Tfh2 cells have been reported to be increased in allergic asthma patients in peripheral blood, and correlate positively with total serum IgE [114,115]. Similarly, a heightened number of Tfh2 cells in the spleen correlated with disease incidence in mice with specific deletion of Ets1 in CD4+ T cells – a mouse model of SLE-like disease – concomitant with modulation of IL-4 signaling and increasing IgE titers [116].
Not all autoimmune conditions are characterized by the development of Tfh2 cells alone. Juvenile dermatomyositis (JDM) is a systemic autoimmune disease that alters the frequency of different subsets of cytokine-skewed human cTfh cells, and a mixed population of cTfh2 and cTfh17 cells in the periphery correlates with disease activity [36]. The significance of this finding is unclear, but it may be related to findings in BXD2 mice, a recombinant inbred mouse strain that develops autoimmunity including spontaneous arthritis [71] – when derived from CXCR5+ICOS+IL-21R+IL-17R+ Tfh (Tfh17) cells in these mice, IL-17 has been implicated in mediating spontaneous GC development [72]. In the absence of a functional IL-17 receptor in BXD2 Il17ra−/− mice, Tfh cells were defective in their ability to localize to the light zone of the GC, although in vitro cocultured B cell responses remained unaffected. These data suggest that IL-17 signaling can influence Tfh cell positioning, perhaps via upregulation of G protein signaling (RGS) and chemokine-mediated positioning within the GC to interact with B cells [72]. This suggests that Tfh17 cells might be relevant targets for therapeutic interventions in some autoimmune diseases, and they therefore merit further investigation.
Concluding remarks
The identification of different subsets of cytokine-skewed Tfh cells that develop under different polarizing conditions, mostly investigated in mice, underscores the complexity of the transcriptional programming and inflammatory environment that regulates Tfh cell biology. There is still much to be discovered about the functions of these different Tfh subsets (see Outstanding questions). Furthermore, the varying capacity of different Tfh cell subsets to provide B cell help raises a relevant question about how the functionalities of these cells might be harnessed to modulate the number and type of Tfh cell subsets. Interrogating this aspect of Tfh cells heterogeneity will be important for improving strategies to design efficacious candidate vaccines against infectious diseases (Box 3) in which key effector mechanisms may develop under polarized immune conditions, and in which skewing humoral immunity might generate an optimal protective response.
Outstanding questions.
Given that Tfh cells do not differentiate in the absence of directional cytokine-skewing, what are the major functions that non-skewed, IL-6-driven, conventional Tfh cells play in the activation of B cells, isotype class switching, and the humoral immune response in different infection models?
In vitro studies have clearly demonstrated that cytokine-skewed Tfh cells can differentiate from naïve CD4+ T cells in the presence of various cytokines. To what extent could these polarizing cytokines drive conventional Tfh cells to become cytokine-skewed?
What are the epigenetic modifications that distinguish conventional Tfh cells from their cytokine-skewed counterparts?
What are the intrinsic mechanisms, such as post-translational regulation or epigenetic modifications, that could regulate the stringency of cytokine production by these cells over and above regulation by Bcl-6?
Does skewing occur before or after T cell follicular commitment?
Is cytokine-skewing reversible?
How heterogeneous are Tfr cells, and what are the mechanisms of GC suppression?
How do different pathogens skew Tfh cell polarization to avoid protective immune responses?
Tfh1 cells can enhance or impair antibody responses depending on the infection model. How could the molecular mechanisms that govern this cell differentiation be targeted in the generation of appropriate immune responses and efficacious antibody responses?
Studies in mice have shown that direct class switching (from IgM to IgE) and sequential class switching (from IgM to IgG1 to IgE) are mediated by cytokines and affect the affinity of IgE in Tfh2 cell responses. How do the kinetics of antibody isotype class switching driven by different subsets of cytokine-skewed Tfh cells dictate the affinity of the antibody produced?
Are cytokine-skewed Tfh cell subsets able to differentiate into a phenotype that can recall the appropriate effector function upon reinfection? If so, what is the longevity of these memory cells?
Box 3. Cytokine-skewed Tfh cell development towards a memory phenotype.
One of the hallmarks of CD4+ T cells is their central function in immunological memory. Following pathogen clearance, effector CD4+ T cells undergo a contraction phase, leaving behind only a subset of this cell population with a differentiated memory phenotype [117]. Memory Tfh cells are no exception and have been shown to be present in both mice [65] and humans [37]. They are able to recall Tfh cell–B cell helper function following secondary challenge [65,116,118–120]. Two main subsets of memory Tfh cells have been identified – circulatory Bcl-6loCXCR5loICOS+PD-1+CD69− memory Tfh cells [37,121] and draining lymph node-resident Bcl-6+CXCR5+ICOShiPD-1+CD69+ antigen-specific memory Tfh cells may [121,122] – both of which are functional in providing B cell help. Many questions remain regarding the plasticity of memory Tfh cell reactivation initiated by antigen-specific memory B cells [123]. However, the epigenetic programming that retains the pattern of gene expression and transcription profiles in Tfh cells may extend to cytokine secretion [65].
Highlights.
The inflammatory environment and the cytokine milieu induce T follicular helper (Tfh) cells to differentiate into different subsets that pertain to type 1 inflammatory responses (Tfh1), type 2 inflammatory responses (Tfh2), and T regulatory responses (Tfr), as well as the production of interleukin (IL)-17 (Tfh17) cells with varying capacity to provide help to B cells in mammals.
Cytokine-skewed Tfh cells contribute to class-switch recombination before germinal center (GC) formation and modify B cell maturation within GCs.
The accessibility in conventional Tfh cells of specific gene loci typical of CD4+ T helper cells through epigenetic programming might facilitate their transition into Tfh1, Tfh2, Tfh17, or Tfr phenotypes.
The heterogeneity of Tfh cells underscores their ability to modulate different humoral immune responses to different pathogens, all with varying consequences. For instance, production of interferon (IFN)-γ by Tfh1 cells can impair B cell function during Plasmodium sp. infection but enhances B cell function in some viral infections.
Cytokines produced by Tfh2/Tfh13 cells can mediate not only antibody class switching but also the affinity of the IgE produced.
The suppressive activity of Tfr cells depends on their origin, as well as on their location.
Acknowledgments
This work was supported by a grant from the National Institute for Allergy and Infectious Diseases to T.J.L. (R01AI123425).
Glossary
- Activation-induced marker assay
utilizes activation markers such as CD25 and OX40 to identify antigen-specific T cells following restimulation with antigen without the need for intracellular cytokine staining
- Affinity maturation
a change taking place in B cells that allows improvement of their antigen-binding affinity and the generation of antibodies with higher affinity for a particular antigen
- B cell lymphoma 6 (Bcl-6)
a TF that identifies Tfh cells
- B lymphocyte-induced maturation protein 1 (Blimp-1)
a TF that represses the expression of Bcl6, or of other genes involved in Tfh cell development, thereby serving as a potent inhibitor of Tfh cell differentiation
- BXD2 mice
a recombinant inbred mouse strain generated by crossing offspring from C57BL/6J and DBA/2J mice for >20 generations. These mice spontaneously develop autoimmune disease
- Centrocytes
nondividing B cells in the light zone of the GC that can undergo selection and terminally differentiate into either memory B cells or antibody-producing plasma cells
- Class switching
rearrangement of the heavy chain locus of an immunoglobulin in mature B cells, leading to a different immunoglobulin isotype
- Cytotoxic T lymphocyte-associated protein 4 (CTLA-4)
a negative regulator of immune responses
- C-X-C chemokine receptor 5 (CXCR5)
a member of the CXC chemokine receptor family that responds to chemokine CXCL13
- Forkhead box protein 3 (FoxP3)
a TF that identifies regulatory Tregs
- GATA-binding protein 3 (GATA-3)
a TF involved in the development and function of Th2 cells
- Inducible T cell co-stimulator (ICOS)
a T cell co-stimulatory molecule
- Interferon γ (IFN-γ)
a key cytokine typically found in type 1-skewed immune responses
- Lymphoid enhancer-binding factor 1 (LEF-1)
a TF that promotes early differentiation of Tfh cells
- Programmed cell death protein 1 (PD1)
a molecule normally associated with downregulation of T cell effector responses
- Pre-T follicular helper (pre-Tfh) cells
these are formed immediately after T cell priming, before GC formation. They are important in extrafollicular antibody responses and GC initiation
- Retinoic acid receptor-related orphan receptor γ (ROR-γt)
a TF mediating the development, maintenance, and function of Th17 cells
- Somatic hypermutation
point mutations that occur in the variable regions of both the heavy and light chains of immunoglobulins, leading to the generation of high-affinity antibodies
- Signal transducer and activator of transcription (STAT)
a family of TFs activated by Janus kinases to mediate intracellular signaling
- T-box expressed in T cells (T-bet/TBX21)
a TF associated with IFN-γ transcription
- T cell factor 1 (TCF-1)
a TF important for T cell development and helper T cell lineage commitment
- Tfh1 cells
Th1-like Tfh cells that express stereotypical molecules of Tfh cells as well as the canonical Th1 cytokine IFN-γ
- Tfh2 cells
Th2-like Tfh cells that express stereotypical molecules of Tfh cells as well as the canonical Th2 cytokine IL-4
- Tfh17 cells
Th17-like Tfh cells that express stereotypical molecules of Tfh cells as well as the canonical Th17 cytokine IL-17
- T follicular helper (Tfh) cells
a subset of CD4+ T helper cells whose key function is to provide help for B cells to become antibody-secreting cells
- T follicular regulatory (Tfr) cells
express stereotypical molecules of Tfh cells as well as FoxP3, the canonical TF of Tregs
Footnotes
Declaration of interests
The authors declare no conflicts of interest.
References
- 1.Oracki SA et al. (2010) Plasma cell development and survival. Immunol. Rev 237, 140–159 [DOI] [PubMed] [Google Scholar]
- 2.Lee SK et al. (2011) B cell priming for extrafollicular antibody responses requires Bcl-6 expression by T cells. J. Exp. Med 208, 1377–1388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zotos D et al. (2010) IL-21 regulates germinal center B cell differentiation and proliferation through a B cell-intrinsic mechanism. J. Exp. Med 207, 365–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim CH et al. (2001) Subspecialization of CXCR5+ T cells: B helper activity is focused in a germinal center–localized subset of CXCR5+ T cells. J. Exp. Med 193, 1373–1382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haynes NM et al. (2007) Role of CXCR5 and CCR7 in follicular Th cell positioning and appearance of a programmed cell death gene-1high germinal center-associated subpopulation. J. Immunol 179, 5099–5108 [DOI] [PubMed] [Google Scholar]
- 6.Hardtke S et al. (2005) Balanced expression of CXCR5 and CCR7 on follicular T helper cells determines their transient positioning to lymph node follicles and is essential for efficient B-cell help. Blood 106, 1924–1931 [DOI] [PubMed] [Google Scholar]
- 7.Johnston RJ et al. (2009) Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science 325, 1006–1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu D et al. (2009) The transcriptional repressor Bcl-6 directs T follicular helper cell lineage commitment. Immunity 31, 457–468 [DOI] [PubMed] [Google Scholar]
- 9.Nurieva RI et al. (2009) Bcl6 mediates the development of T follicular helper cells. Science 325, 1001–1005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bryant VL et al. (2007) Cytokine-mediated regulation of human B cell differentiation into Ig-secreting cells: predominant role of IL-21 produced by CXCR5+ T follicular helper cells. J. Immunol 79, 8180–8190 [DOI] [PubMed] [Google Scholar]
- 11.Linterman MA et al. (2010) IL-21 acts directly on B cells to regulate Bcl-6 expression and germinal center responses. J. Exp. Med 207, 353–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakayamada S et al. (2011) Early Th1 cell differentiation is marked by a Tfh cell-like transition. Immunity 35, 919–931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oestreich KJ et al. (2012) Molecular mechanisms that control the expression and activity of Bcl-6 in TH1 cells to regulate flexibility with a TFH-like gene profile. Nat. Immunol 13, 405–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zaretsky AG et al. (2009) T follicular helper cells differentiate from Th2 cells in response to helminth antigens. J. Exp. Med 206, 991–999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Linterman M et al. (2011) Foxp3+ follicular regulatory T cells control T follicular helper cells and the germinal centre response. Nat. Med 17, 975–982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dan JM et al. (2016) A cytokine-independent approach to identify antigen-specific human germinal center T follicular helper cells and rare antigen-specific CD4+ T cells in blood. J. Immunol 197, 983–993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Havenar-Daughton C et al. (2016) Cytokine-independent detection of antigen-specific germinal center T follicular helper cells in immunized nonhuman primates using a live cell activation-induced marker technique. J. Immunol 197, 994–1002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schaerli P et al. (2000) CXC chemokine receptor 5 expression defines follicular homing T cells with B cell helper function. J. Exp. Med 192, 1553–1562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Breitfeld D et al. (2000) Follicular B helper T cells express CXC chemokine receptor 5, localize to B cell follicles, and support immunoglobulin production. J. Exp. Med 192, 1545–1552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim CH et al. (2001) Subspecialization of CXCR5+ T cells: B helper activity is focused in a germinal center-localized subset of CXCR5+ T cells. J. Exp. Med 193, 1373–1381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choi J and Crotty S (2021) Bcl6-Mediated transcriptional regulation of follicular helper T cells (TFH). Trends Immunol 42, 336–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Andris F et al. (2017) The transcription factor c-Maf promotes the differentiation of follicular helper T cells. Front. Immunol 8, 480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kroenke MA et al. (2012) Bcl6 and Maf cooperate to instruct human follicular helper CD4 T cell differentiation. J. Immunol 188, 3734–3744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bauquet AT et al. (2009) The costimulatory molecule ICOS regulates the expression of c-Maf and IL-21 in the development of follicular T helper cells and TH-17 cells. Nat. Immunol 10, 167–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Choi YS et al. (2015) LEF-1 and TCF-1 orchestrate T FH differentiation by regulating differentiation circuits upstream of the transcriptional repressor Bcl6. Nat. Immunol 16, 980–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu L et al. (2015) The transcription factor TCF-1 initiates the differentiation of T FH cells during acute viral infection. Nat. Immunol 16, 991–999 [DOI] [PubMed] [Google Scholar]
- 27.Hiramatsu Y et al. (2010) c-Maf activates the promoter and enhancer of the IL-21 gene, and TGF-β inhibits c-Maf-induced IL-21 production in CD4+ T cells. J. Leukoc. Biol 87, 703–712 [DOI] [PubMed] [Google Scholar]
- 28.Xiao N et al. (2014) The E3 ubiquitin ligase Itch is required for the differentiation of follicular helper T cells. Nat. Immunol 15, 657–666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stone EL et al. (2015) ICOS coreceptor signaling inactivates the transcription factor FOXO1 to promote Tfh cell differentiation. Immunity 42, 239–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shaw LA et al. (2016) Id2 reinforces TH 1 differentiation and inhibits E2A to repress T FH differentiation. Nat. Immunol 17, 834–843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Choi J et al. (2020) Bcl-6 is the nexus transcription factor of T follicular helper cells via repressor-of-repressor circuits. Nat. Immunol 21, 777–789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hatzi K et al. (2015) BCL6 orchestrates Tfh cell differentiation via multiple distinct mechanisms. J. Exp. Med 212, 539–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bentebibel S-E et al. (2016) ICOS+ PD-1+ CXCR3+ T follicular helper cells contribute to the generation of high-avidity antibodies following influenza vaccination. Sci. Rep 6, 26494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oestreich KJ et al. (2011) The lineage-defining factors T-bet and Bcl-6 collaborate to regulate Th1 gene expression patterns. J. Exp. Med 208, 1001–1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Simpson N et al. (2010) Expansion of circulating T cells resembling follicular helper T cells is a fixed phenotype that identifies a subset of severe systemic lupus erythematosus. Arthritis Rheum 62, 234–244 [DOI] [PubMed] [Google Scholar]
- 36.Morita R et al. (2011) Human blood CXCR5+ CD4+ T cells are counterparts of T follicular cells and contain specific subsets that differentially support antibody secretion. Immunity 34, 108–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Locci M et al. (2013) Human circulating PD-1+ CXCR3− CXCR5+ memory Tfh cells are highly functional and correlate with broadly neutralizing HIV antibody responses. Immunity 39, 758–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brenna E et al. (2020) CD4+ T follicular helper cells in human tonsils and blood are clonally convergent but divergent from non-Tfh CD4+ cells. Cell Rep 30, 137–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gonzalez DG et al. (2018) Nonredundant roles of IL-21 and IL-4 in the phased initiation of germinal center B cells and subsequent self-renewal transitions. J. Immunol 201, 3569–3579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fairfax KC et al. (2015) IL-4-secreting secondary T follicular helper (Tfh) cells arise from memory T cells, not persisting Tfh cells, through a B cell-dependent mechanism. J. Immunol 194, 2999–3010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yusuf I et al. (2010) Germinal center T follicular helper cell IL-4 production is dependent on signaling lymphocytic activation molecule receptor (CD150). J. Immunol 185, 190–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mandal M et al. (2011) Epigenetic repression of the Igk locus by STAT5-mediated recruitment of the histone methyltransferase Ezh2. Nat. Immunol 12, 1212–1220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Villarino AV et al. (2017) Mechanisms and consequences of Jak–STAT signaling in the immune system. Nat. Immunol 18, 374–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Powell MD et al. (2019) IL-12 signaling drives the differentiation and function of a TH 1-derived T FH1-like cell population. Sci. Rep 9, 13991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ma X et al. (2018) Expansion of T follicular helper-T helper 1 like cells through epigenetic regulation by signal transducer and activator of transcription factors. Ann. Rheum. Dis 77, 1354–1361 [DOI] [PubMed] [Google Scholar]
- 46.Carpio VH et al. (2020) T helper plasticity is orchestrated by STAT3, Bcl6 and Blimp-1 balancing pathology and protection in malaria. iScience, 101310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ivanov II et al. (2006) The orphan nuclear receptor RORγt directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell 126, 1121–1133 [DOI] [PubMed] [Google Scholar]
- 48.Mangan PR et al. (2006) Transforming growth factor-β induces development of the TH 17 lineage. Nature 441, 231–234 [DOI] [PubMed] [Google Scholar]
- 49.Veldhoen M et al. (2006) TGFβ in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity 24, 179–189 [DOI] [PubMed] [Google Scholar]
- 50.Zhou L et al. (2007) IL-6 programs TH-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat. Immunol 8, 967–974 [DOI] [PubMed] [Google Scholar]
- 51.Langrish CL et al. (2005) IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J. Exp. Med 201, 233–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sage PT et al. (2014) The coinhibitory receptor CTLA-4 controls B cell responses by modulating T follicular helper, T follicular regulatory, and T regulatory cells. Immunity 41, 1026–1039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Clement RL et al. (2019) Follicular regulatory T cells control humoral and allergic immunity by restraining early B cell responses. Nat. Immunol 20, 1360–1371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Maceiras AR et al. (2017) T follicular helper and T follicular regulatory cells have different TCR specificity. Nat. Commun 8, 15067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Aloulou M et al. (2016) Follicular regulatory T cells can be specific for the immunizing antigen and derive from naive T cells. Nat. Commun 7, 10579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fonseca VR et al. (2017) Human blood Tfr cells are indicators of ongoing humoral activity not fully licensed with suppressive function. Sci. Immunol 2, eaan1487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sage PT et al. (2014) Circulating T follicular regulatory and helper cells have memory-like properties. J. Clin. Invest 124, 5191–5204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tumes DJ et al. (2013) The polycomb protein Ezh2 regulates differentiation and plasticity of CD4+ T helper type 1 and type 2 cells. Immunity 39, 819–832 [DOI] [PubMed] [Google Scholar]
- 59.Yang X-P et al. (2015) EZH2 is crucial for both differentiation of regulatory T cells and T effector cell expansion. Sci. Rep 5, 10643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hou S et al. (2019) FoxP3 and Ezh2 regulate Tfr cell suppressive function and transcriptional program. J. Exp. Med 216, 605–620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kwon H-K et al. (2017) Different molecular complexes that mediate transcriptional induction and repression by FoxP3. Nat. Immunol 18, 1238–1248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Guthmiller JJ et al. (2017) Cutting Edge: IL-10 is essential for the generation of germinal center B cell responses and anti-plasmodium humoral immunity. J. Immunol 198, 617–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Laidlaw BJ et al. (2017) Interleukin-10 from CD4+ follicular regulatory T cells promotes the germinal center response. Sci. Immunol 2, eaan4767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wing JB et al. (2014) Regulatory T cells control antigen-specific expansion of Tfh cell number and humoral immune responses via the coreceptor CTLA-4. Immunity 41, 1013–1025 [DOI] [PubMed] [Google Scholar]
- 65.Hale JS et al. (2013) Distinct memory CD4+ T cells with commitment to T follicular helper-and T helper 1-cell lineages are generated after acute viral infection. Immunity 38, 805–817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ballesteros-Tato A et al. (2016) T follicular helper cell plasticity shapes pathogenic T helper 2 cell-mediated immunity to inhaled house dust mite. Immunity 44, 259–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lu KT et al. (2011) Functional and epigenetic studies reveal multistep differentiation and plasticity of in vitro-generated and in vivo-derived follicular T helper cells. Immunity 35, 622–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang Y et al. (2017) Germinal-center development of memory B cells driven by IL-9 from follicular helper T cells. Nat. Immunol 18, 921–930 [DOI] [PubMed] [Google Scholar]
- 69.Weinstein JS et al. (2016) TFH cells progressively differentiate to regulate the germinal center response. Nat. Immunol 17, 1197–1205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Roco JA et al. (2019) Class-switch recombination occurs infrequently in germinal centers. Immunity 51, 337–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hsu H-C et al. (2008) Interleukin 17-producing T helper cells and interleukin 17 orchestrate autoreactive germinal center development in autoimmune BXD2 mice. Nat. Immunol 9, 166–175 [DOI] [PubMed] [Google Scholar]
- 72.Ding Y et al. (2013) IL-17RA is essential for optimal localization of follicular Th cells in the germinal center light zone to promote autoantibody-producing B cells. J. Immunol 191, 1614–1624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liang H et al. (2019) ZIKV infection induces robust Th1-like Tfh cell and long-term protective antibody responses in immunocompetent mice. Nat. Commun 10, 3859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gowthaman U et al. (2019) Identification of a T follicular helper cell subset that drives anaphylactic IgE. Science 365, eaaw6433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Barnett BE et al. (2016) B cell-intrinsic T-bet expression is required to control chronic viral infection. J. Immunol 197, 1017–1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sheikh AA et al. (2019) Context-dependent role for T-bet in T follicular helper differentiation and germinal center function following viral infection. Cell Rep 28, 1758–1772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mitsdoerffer M et al. (2010) Proinflammatory T helper type 17 cells are effective B-cell helpers. Proc. Natl. Acad. Sci 107, 14292–14297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Obeng-Adjei N et al. (2017) Malaria-induced interferon-γ drives the expansion of Tbethi atypical memory B cells. PLoS Pathog 13, e1006576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ly A et al. (2019) Transcription factor T-bet in B cells modulates germinal center polarization and antibody affinity maturation in response to malaria. Cell Rep 29, 2257–2269 [DOI] [PubMed] [Google Scholar]
- 80.Xin G et al. (2018) Single-cell RNA sequencing unveils an IL-10-producing helper subset that sustains humoral immunity during persistent infection. Nat. Commun 9, 5037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Xie MM et al. (2020) T follicular regulatory cells and IL-10 promote food antigen-specific IgE. J. Clin. Invest 130, 3820–3832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cañete PF et al. (2019) Regulatory roles of IL-10–producing human follicular T cells. J. Exp. Med 216, 1843–1856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhang J et al. (2019) Circulating CXCR3+ Tfh cells positively correlate with neutralizing antibody responses in HCV-infected patients. Sci. Rep 9, 10090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Martin-Gayo E et al. (2017) Circulating CXCR5CXCR3PD-1lo Tfh-like cells in HIV-1 controllers with neutralizing antibody breadth. JCI Insight 2, e89574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Baiyegunhi O et al. (2018) Frequencies of circulating Th1-biased T follicular helper cells in acute HIV-1 infection correlate with the development of HIV-specific antibody responses and lower set point viral load. J. Virol 92, e00659–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Stone KD et al. (2010) IgE, mast cells, basophils, and eosinophils. J. Allergy Clin. Immunol 125, S73–S80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Meli AP et al. (2017) T follicular helper cell-derived IL-4 is required for IgE production during intestinal helminth infection. J. Immunol 199, 244–252 [DOI] [PubMed] [Google Scholar]
- 88.King IL and Mohrs M (2009) IL-4–producing CD4+ T cells in reactive lymph nodes during helminth infection are T follicular helper cells. J. Exp. Med 206, 1001–1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Akiyama M et al. (2016) Enhanced IgG4 production by follicular helper 2 T cells and the involvement of follicular helper 1 T cells in the pathogenesis of IgG4-related disease. Arthritis Res. Ther 18, 167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Akiyama M et al. (2018) Interleukin-4 contributes to the shift of balance of IgG subclasses toward IgG4 in IgG4-related disease. Cytokine 110, 416–419 [DOI] [PubMed] [Google Scholar]
- 91.Cargill T et al. (2019) Activated T-follicular helper 2 cells are associated with disease activity in IgG4-related sclerosing cholangitis and pancreatitis. Clin. Trans. Gastroenterol 10, e00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Xiong H et al. (2012) Sequential class switching is required for the generation of high affinity IgE antibodies. J. Exp. Med 209, 353–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.He J-S et al. (2013) The distinctive germinal center phase of IgE+ B lymphocytes limits their contribution to the classical memory response. J. Exp. Med 210, 2755–2771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.He J-S et al. (2017) IgG1 memory B cells keep the memory of IgE responses. Nat. Commun 8, 641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Liang H-E et al. (2012) Divergent expression patterns of IL-4 and IL-13 define unique functions in allergic immunity. Nat. Immunol 13, 58–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Obeng-Adjei N et al. (2015) Circulating Th1-cell-type Tfh cells that exhibit impaired B cell help are preferentially activated during acute malaria in children. Cell Rep 13, 425–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ryg-Cornejo V et al. (2016) Severe malaria infections impair germinal center responses by inhibiting T follicular helper cell differentiation 14(1) pp. 68–81 [DOI] [PubMed] [Google Scholar]
- 98.King T and Lamb T (2015) Interferon-gamma: the Jekyll and Hyde of malaria. PLoS Pathog 11, e1005118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wikenheiser DJ et al. (2016) The costimulatory molecule ICOS regulates host Th1 and follicular Th cell differentiation in response to Plasmodium chabaudi AS infection. J. Immunol 196, 778–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cohen S et al. (1961) Gamma-globulin and acquired immunity to human malaria. Nature 192, 733–737 [DOI] [PubMed] [Google Scholar]
- 101.Sabchareon A et al. (1991) Parasitologic and clinical human response to immunoglobulin administration in falciparum malaria. Am. J. Trop. Med. Hyg 45, 297–308 [DOI] [PubMed] [Google Scholar]
- 102.Papillion A et al. (2019) Inhibition of IL-2 responsiveness by IL-6 is required for the generation of GC-TFH cells. Sci. Immunol 4, eaaw7636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yu S et al. (2015) IL-12 induced the generation of IL-21-and IFN-γ-co-expressing poly-functional CD4+ T cells from human naive CD4+ T cells. Cell Cycle 14, 3362–3372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Schmitt N et al. (2014) Phenotype and functions of memory Tfh cells in human blood. Trends Immunol 35, 436–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hercor M et al. (2017) Antigen-presenting cell-derived IL-6 restricts the expression of GATA3 and IL-4 by follicular helper T cells. J. Leukoc. Biol 101, 5–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kobayashi T et al. (2017) Follicular helper T cells mediate IgE antibody response to airborne allergens. J. Allergy Clin. Immunol 139, 300–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Reinhardt RL et al. (2009) Cytokine-secreting follicular T cells shape the antibody repertoire. Nat. Immunol 10, 385–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhu J et al. (2004) Conditional deletion of Gata3 shows its essential function in TH1-TH2 responses. Nat. Immunol 5, 1157–1165 [DOI] [PubMed] [Google Scholar]
- 109.Takeda K et al. (1996) Essential role of Stat6 in IL-4 signalling. Nature 380, 627–630 [DOI] [PubMed] [Google Scholar]
- 110.Wollenberg I et al. (2011) Regulation of the germinal center reaction by Foxp3+ follicular regulatory T cells. J. Immunol 187, 4553–4560 [DOI] [PubMed] [Google Scholar]
- 111.Chung Y et al. (2011) Follicular regulatory T cells expressing Foxp3 and Bcl-6 suppress germinal center reactions. Nat. Med 17, 983–988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wu H et al. (2016) Stat3 is important for follicular regulatory T cell differentiation. PLoS One 11, e0155040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Jandl C et al. (2017) IL-21 restricts T follicular regulatory T cell proliferation through Bcl-6 mediated inhibition of responsiveness to IL-2. Nat. Commun 8, 14647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gong F et al. (2018) Circulating CXCR5+ CD4+ T cells participate in the IgE accumulation in allergic asthma. Immunol. Lett 197, 9–14 [DOI] [PubMed] [Google Scholar]
- 115.Yao Y et al. (2018) Correlation of allergen-specific T follicular helper cell counts with specific IgE levels and efficacy of allergen immunotherapy. J. Allergy Clin. Immunol 142, 321–324 [DOI] [PubMed] [Google Scholar]
- 116.Kim CJ et al. (2018) The transcription factor Ets1 suppresses T follicular helper type 2 cell differentiation to halt the onset of systemic lupus erythematosus. Immunity 49, 1034–1048 [DOI] [PubMed] [Google Scholar]
- 117.Nguyen QP et al. (2019) Origins of CD4+ circulating and tissue-resident memory T-cells. Immunology 157, 3–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Weber JP et al. (2012) T-follicular helper cells survive as long-term memory cells. Eur. J. Immunol 42, 1981–1988 [DOI] [PubMed] [Google Scholar]
- 119.MacLeod MK et al. (2011) Memory CD4 T cells that express CXCR5 provide accelerated help to B cells. J. Immunol 186, 2889–2896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Liu X et al. (2012) Bcl6 expression specifies the T follicular helper cell program in vivo. J. Exp. Med 209, 1841–1852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Asrir A et al. (2017) Interconnected subsets of memory follicular helper T cells have different effector functions. Nat. Commun 8, 847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Fazilleau N et al. (2007) Lymphoid reservoirs of antigen-specific memory T helper cells. Nat. Immunol 8, 753–761 [DOI] [PubMed] [Google Scholar]
- 123.Ise W et al. (2014) Memory B cells contribute to rapid Bcl6 expression by memory follicular helper T cells. Proc. Natl. Acad. Sci 111, 11792–11797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sage PT et al. (2016) Suppression by T FR cells leads to durable and selective inhibition of B cell effector function. Nat. Immunol 17, 1436–1446 [DOI] [PMC free article] [PubMed] [Google Scholar]


