Abstract
In order to study the influence of compressed carbon dioxide, over a range of pressures (1.5 to 5.5 MPa) and exposure times (up to 7 h), on the survival of Escherichia coli, Saccharomyces cerevisiae, and Enterococcus faecalis, a new pressurizable reactor system was conceived. Microbial cells were inoculated onto a solid hydrophilic medium and treated at room temperature; their sensitivities to inactivation varied greatly. The CO2 treatment had an enhanced efficiency in cell destruction when the pressure and the duration of exposure were increased. The effects of these parameters on the loss of viability was also studied by response-surface methodology. This study showed that a linear correlation exists between microbial inactivation and CO2 pressure and exposure time, and in it models were proposed which were adequate to predict the experimental values. The end point acidity was measured for all the samples in order to understand the mechanism of microbial inactivation. The pHs of the treated samples did not vary, regardless of the experimental conditions. Other parameters, such as water content and pressure release time, were also investigated.
Carbon dioxide has a dual physiological role in microorganisms since it can both stimulate and inhibit cell development. The inhibitory action has been increasingly exploited to improve the hygiene of both liquid and solid foodstuffs by protecting them from bacterial spoilage (6, 7, 9, 20). Blickstad et al. (3) reported the effect of carbon dioxide on the microflora of pork and found that increasing the partial pressure of carbon dioxide added to the packaging atmosphere prolonged the shelf life of the meat.
The effect of compressed carbon dioxide on different types of microorganisms has been studied by several researchers (1, 4, 5, 8–11, 15–17). Microbial inactivation by CO2 is dependent on many parameters. Some of the experimental conditions, such as temperature, pressure, and moisture, contribute to a more effective treatment by increasing the diffusivity of CO2 (10, 11, 16, 19). Within certain limits, a longer duration of exposure to carbon dioxide permits better sterilization; exposure time can be decreased by increasing the temperature (1). Microbial resistance to CO2 also depends on the type of microorganism, the phase of growth, and the suspension medium, the last of which can inhibit the bactericidal effect of compressed CO2, especially in some food systems rich in proteins (20).
Various hypotheses have been proposed to explain the microbicidal activity of carbon dioxide. Lin et al. (13, 14) have observed that, at high pressure, supercritical carbon dioxide penetrates cells and ruptures them when it is released suddenly. They were even able to improve the rate of disruption by repeatedly releasing the CO2 pressure. Fraser (8) has also evoked this subject; he observed that the best microbial destruction results were obtained when the CO2 pressure was released as rapidly as possible. In food with a high water content, CO2 dissolves in the water to form carbonic acid, according to the following equilibrium reactions:
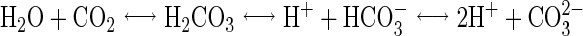 |
Thus, dissolved CO2 acts by lowering the pH of the medium, and the resulting acidity leads to a disturbance of some biological systems within the cells. It was therefore suggested that microbial inhibition was due to an alteration in the properties of the cell (membrane, cytoplasm, enzymes, etc.) (6). However, a reduction in the pH of the medium is not sufficient to account for the antimicrobial action of CO2, since it shows a specific inhibitory effect which is greater than that of the other acids used to lower medium acidity (hydrochloric acid, phosphoric acid, etc.) (2, 9, 12, 16). These acids do not penetrate the microbial cells as easily as carbon dioxide.
This paper describes the feasibility of using a new pressurizable reactor system, developed by our laboratory, to destroy microorganisms. Our work focused on the study of the inactivation by compressed carbon dioxide of three microorganism strains inoculated onto filter paper disks. The parameters explored were mainly exposure time and pressure at room temperature. The effect of each parameter was investigated in a systematic study and followed up by response-surface methodology. The end point acidity of the samples was determined by measuring the pH of the suspensions in which the filter paper disks were disintegrated. Moreover, we observed the effect of water content on cell destruction and compared the results obtained with sudden and slow decompression rates.
MATERIALS AND METHODS
Growth of cells.
Three microbial strains were used as test organisms: Escherichia coli IP 6532, Enterococcus faecalis ATCC 29212, and Saccharomyces cerevisiae CBS 400. Before each test, microorganisms from cultures stored at 4°C were grown overnight in recovery medium containing (per liter) 5 g of peptone, 2 g of yeast extract, 5 g of NaCl, and 5 g of glucose. The bacteria were incubated at 37°C; the yeast was incubated at 30°C. The cell concentration in the resulting suspensions was generally about 108 CFU/ml. The absence of contamination in the suspensions was confirmed by Gram staining.
Sample preparation.
Hydrophilic filter paper disks (50-mm diameter, Whatman no. 1) were autoclaved at 120°C for 20 min. Just before the tests were conducted, each microbial suspension was aseptically dispersed onto a filter paper disk, which will retain all the deposited suspension volume (100 μl). The inoculated disk was placed vertically inside a sterile flask (50 ml) containing a small Teflon-covered magnetic stirring bar and five sterile glass beads (5-mm diameter). Flasks were kept closed until it was time to perform the experiment.
Apparatus.
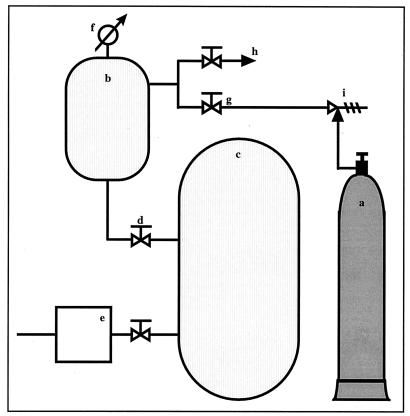
The processing reactor used in this work was made entirely of stainless steel (Fig. 1). The reactor consisted of a pressure vessel with an internal volume of 5 liters (Fig. 1b) connected to a 1,600-liter vacuum tank (Fig. 1c); the two tanks were separated by a large-diameter valve (Fig. 1d). Compressed carbon dioxide was supplied from a gas cylinder (Fig. 1a) which was linked, by a valve (Fig. 1g), to the pressure vessel. A precision manometer (Fig. 1f), CITEC (AISI 316 TI/L), was installed in the vessel to measure the pressure. Except where mentioned, experiments were carried out with a sudden release of pressure, which was obtained by opening the decompression valve (Fig. 1d) between the two tanks. The pressure in the vacuum tank could be adjusted between 5 kPa and atmospheric pressure, and the pressure at the end of the experiment could be controlled. On the other hand, the vacuum was maintained in the vessel before application of CO2 pressure, and this avoided any possibility of having a CO2-air mixture at the beginning of the experiment.
FIG. 1.
Schematic representation of the pressurizable reactor configuration. a, compressed CO2; b, pressure vessel; c, vacuum tank; d, decompression valve; e, vacuum pump; f, precision manometer; g, compression valve; h, outlet; i, pressure regulator.
Process.
Open flasks containing filter paper disks were placed in the pressure vessel at the beginning of each experiment. The vessel was immediately sealed, and when all the tubing connections were secured, the vacuum was established by connecting the vessel to the vacuum tank. After the decompression valve was closed, carbon dioxide (Air Liquide, 99.7% pure) was injected into the vessel at the selected pressure. Microbial cells were treated at pressures ranging from 1.5 to 5.5 MPa and for various exposure times. The apparatus allows carbon dioxide to be injected very quickly (within 10 s). The holding time began as soon as the vessel reached the experimental pressure and ended with a sudden (0.4-s) release of the CO2 pressure. All of the experiments were carried out at room temperature. The flasks in the vessel were closed and removed, and the surviving cells were immediately counted. Before each test, the inside surfaces of the vessel were wiped clean with cotton moistened with a 70% alcohol solution. The reported results are the average of at least duplicate tests.
Enumeration of living microorganisms.
The viability of S. cerevisiae, E. coli, and E. faecalis was determined by counting the number of CFU per milliliter. Each processed disk was diluted with 30 ml of an aqueous TS solution (1 g of tryptone per liter, 8.5 g of NaCl per liter). The contents of the flask were then mixed well by magnetic stirring for 30 min. This mixing led to a total disintegration of the filter paper, and the microorganisms were subsequently dispersed in the solution. The dispersion obtained was then diluted, and 1 ml of the appropriate dilution was plated on duplicate petri dishes containing a suitable growth medium. Yeast was grown on yeast glucose agar (Biokar Diagnostics, BK 053) (per liter: yeast extract, 5 g; glucose, 20 g; bacteriological agar, 15 g). The bacteria were grown on nutrient agar (Biokar Diagnostics, BK 021) (per liter: meat extract, 5 g; tryptone, 10 g; NaCl, 5 g; bacteriological agar, 15 g). Colonies were counted after 24 h of incubation at 37°C (bacteria) and at 30°C (yeast). Microbial cells in the control samples were counted by the same procedures described for treated samples. The acidity of each inoculated disk was expressed by measuring the pH of the TS solution in which the filter paper was suspended after its disintegration. Measurements were realized with an electrode connected to a pH meter (pH 539, Wissenschaftlich-Technische-Werkstätten). Whenever necessary, the water content of the inoculated disks was determined at 100°C with an infrared balance (Mettler LP16).
Design of experiments.
Response surface is a statistical methodology which consists of two distinct parts, the design of the experiment and the analysis of the data (18). As for the experimental design, two factors, CO2 pressure (P) and exposure time (t), were studied at five levels. The response for each treatment level is the response variable. It was assumed that the main response variables were the rate of inactivation and the acidity. Response-surface methodology is used to study the relationship between the response variables and the factors; it also allows a mathematical model to be developed that can predict the value of the response variable for given levels of pressure and exposure time. A central composite design with two factors was used (Table 1). This set of designs consists of a full or fractional two-level experiment (coded as ±1), designed to value all of the linear and interaction terms, and of center points and star points (coded as 0 and ±α, respectively) used to estimate the quadratic terms (18). This randomized design yielded 10 experiments with 4 (22) factorial points, 4 (22) star points with α = 1.68, and 2 replications of the center point. The levels of the two factors were chosen on the basis of preliminary trials. Response-surface contours and other statistical analyses were obtained by the appropriate procedures described in STATGRAPHICS Plus 1.4 (Manugistics, Inc., Rockville, Md.).
TABLE 1.
Coded levels for independent variables used in developing the experimental data
| Parameter | Actual values (in original units) at a variable coded level of:
|
||||
|---|---|---|---|---|---|
| −α | −1 | 0 | +1 | +α | |
| P (MPa) | 2.32 | 3 | 4 | 5 | 5.68 |
| t (min) | 18.6 | 90 | 195 | 300 | 371.4 |
The response variable Y was experimentally measured. In our case, a second-degree polynomial equation was assumed to approach the true function, suggesting an interaction between the two factors, Y = β0 + β1x1 + β2x2 + β11x12 + β22x22 + β12x1x2, where β0, β1, β2, β11, β22, and β12 are coefficients for the coded model and x1 and x2 are the coded factors related to P and t, respectively. The linear relationship is expressed by the equations x1 = 2(P − P*)/d1 and x2 = 2(t − t*)/d2, where * is the mean of the factorial levels and d1 and d2 are the differences between the low and high levels, respectively, of the factor; x1, x2 ∈ [−1;+1], P ∈ [30;50], and t ∈ [90;300].
RESULTS AND DISCUSSION
Systematic study of pressure and exposure time.
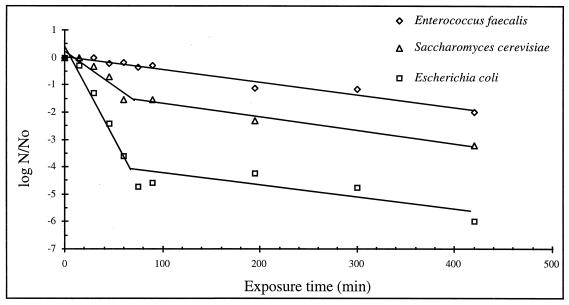
The rate of inactivation of the three microorganisms as a function of exposure time was expressed as the logarithm of the ratio of surviving cells after treatment with CO2 (N) to the cell count in the control culture (N0) (Fig. 2). The error of the initial countable population (N0) of an inoculated filter paper disk was about 25% (12 replicates). The treatment pressure chosen was 5 MPa. Zero time of treatment represents the control samples. The inactivation of the microbial cells was enhanced by increasing the exposure time. The curve for each strain has a characteristic shape that defines its own resistance. E. coli was destroyed the most quickly. The survival curves showed that E. coli was inactivated by about 3.6 log cycles after 60 min of treatment, whereas only 1.5-log and 0.2-log inactivation occurred for S. cerevisiae and E. faecalis, respectively. Experimental plots corresponding to the inactivation of E. faecalis could be placed on a straight line, showing a linear regression with a 158.7-min D value (decimal reduction time). The mechanism of destruction, ruled by a first-order kinetics, agreed with results obtained by Cuq et al. (5) at constant pressure (0.6 MPa) and temperature (55°C). Carbon dioxide under pressure exhibited a different effect against S. cerevisiae and E. coli. The results presented survival curves of two linear stages. The rate of inactivation is rapid at first, with a significantly slower rate at the later stage. The two biphasic curves showed an inflexion point which occurred after about 90 min of exposure time. These results suggest that the microorganisms were destroyed more easily at the first straight portion; the second one involved an increase in the destruction resistance. At higher pressure and temperature, Lin et al. (15) and Ballestra et al. (1) have reported the presence of the first linear regression in the kinetics of the inactivation of S. cerevisiae and E. coli, respectively. But in their studies, this stage was preceded by an earlier one with a slower rate of inactivation; it corresponded to little values of exposure time (<30 min) not exploited in this paper.
FIG. 2.
Rate of destruction of different strains of microorganisms as a function of exposure time at a CO2 pressure of 5 MPa.
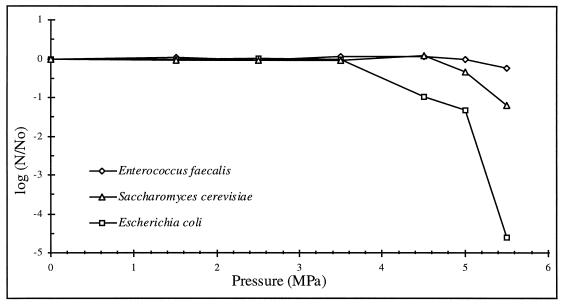
Figure 3 illustrates the numbers of surviving cells as a function of CO2 pressure. Experiments showed that the inactivation rates of the three microbial strains appeared insensitive to pressures below 3.5 MPa. The beginning of the inactivation did not occur at the same pressure; it was strain dependent. E. coli had, once more, the least pressure resistance. Only a slight effect on E. faecalis cells was noted. The drop in viability may have occurred, for each strain, when the minimal required pressure allowed carbon dioxide to penetrate into the cells during the 30-min incubation period.
FIG. 3.
Rate of destruction of different strains of microorganisms as a function of CO2 pressure with 30 min of exposure time.
Methodology of response surfaces.
The effects of the two factors (P and t) were studied only with E. coli and S. cerevisiae; E. faecalis was much more resistant than these two strains, and a very slow rate of destruction occurred at the explored levels. Analysis of variance showed that the destruction rate, expressed as log N/N0, was dependent on these two factors, which confirms our results obtained previously. The regression coefficients are shown in Table 2, where the P values (probability) should be less than 0.05 to indicate whether a correlation exists between the two factors and the response variable.
TABLE 2.
Regression coefficients and P values (probabilities) for the response surface models
| Parameter |
E. coli
|
S. cerevisiae
|
||
|---|---|---|---|---|
| Coefficient | P value | Coefficient | P value | |
| Constant | 10.66 | −6.66 | ||
| P | −3.84 | 0.005 | 3.96 | 0 |
| t | −0.023 | 0.07 | 0.016 | 0.01 |
| P2 | 0.28 | 0.48 | −0.6 | 0.02 |
| t2 | 0.00005 | 0.18 | −0.00001 | 0.57 |
| Pt | −0.001 | 0.84 | −0.0046 | 0.09 |
The equations describing the response surfaces are as follows: for E. coli, log N/N0 = 10.66 − 3.84 P − 0.023 t + 0.28 P2 + 0.00005 t2 − 0.001 Pt, with R2 = 0.91; for S. cerevisiae, log N/N0 = −6.66 + 3.96 P + 0.016 t − 0.6 P2 − 0.00001 t2 − 0.0046 Pt, with R2 = 0.98.
Table 2 showed that the rate of destruction of the two microbial strains could have a linear correlation with CO2 pressure and exposure time. These data were reflected by the P values. Moreover, in the case of S. cerevisiae, CO2 pressure had a quadratic effect on the response variable, as well as a little two-factor interaction. Exposure time had no significant quadratic relationship with the rate of destruction. The coefficients of determination (R2) revealed that the models were adequate and that they predicted the experimental values.
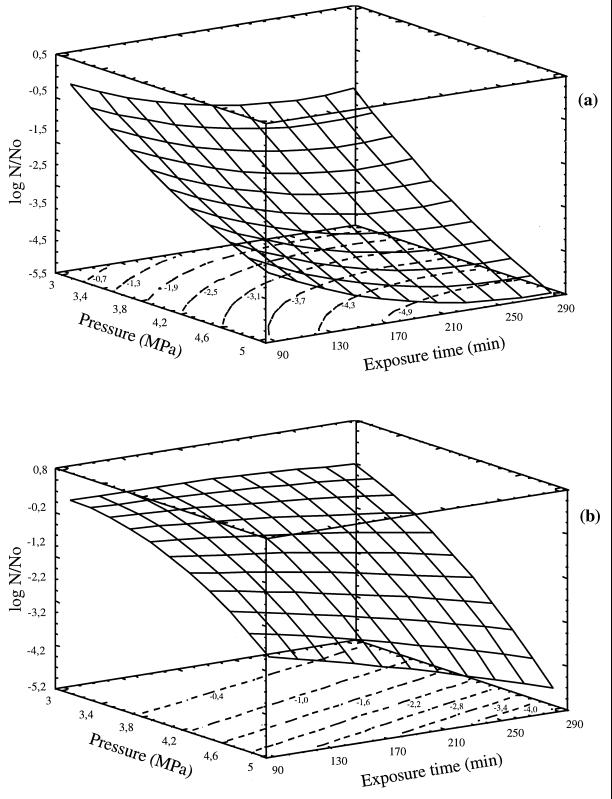
Response surfaces and contour plots of the rate of destruction are presented in Fig. 4. The numbers on the contour plots showed log N/N0 values.
FIG. 4.
Response surfaces and contour plots for the rate of destruction of E. coli (a) and S. cerevisiae (b) at different levels of independent variables.
Compressed CO2 was highly toxic to both microbes. The microbial inactivation was affected and improved more by increasing CO2 pressure than by prolonging exposure time. The exposure time had little effect, particularly at the lowest pressure. Its effect was enhanced by increasing CO2 pressure; this observation is more clear in the case of S. cerevisiae, for which the inactivation was negligible at 3 MPa. The effect of CO2 pressure was more marked even at the shortest exposure times. For example, E. coli populations were reduced 2.5 and 4 logs by increasing the CO2 pressure from 3 to 5 MPa after 90 and 330 min of exposure time, respectively.
The experimental data demonstrated that increasing the CO2 pressure and exposure time reduced microbial cell numbers (9), even if the microbes were inoculated on hydrophilic disks. The three microorganisms differed in their resistances to the inhibitory effect of carbon dioxide (16). This difference in sensitivity could be related to their cell envelope and its carbon dioxide permeability. Higher pressures and longer exposure times might favor the solubility of CO2, which acts by increasing the acidity of the suspension medium (17). Thus, the observed microbicidal effect is due not only to the experimental conditions but also to the specificity of the gas used (20). Pressurization with a mixture of N2 and O2 (80%–20%) at 8.5 MPa for 4 h had no antimicrobial effect and did not reduce the counts of any of the three strains.
The efficiency of the newly developed reactor system in microbial inactivation by compressed CO2 has been demonstrated. The reactor concept offers the advantage of being both a simple and a safe process, with controlled rates of compression and decompression. The results obtained are of particular interest for industrial applications. The filter paper disk is a solid medium, and the feasibility of inactivation in microbial suspension has also been demonstrated. These results have encouraged us to pursue this study with different types of food or other products with a high value added tax (VAT) value. Some products demand a nonthermal treatment for sterilization, since heat may cause undesirable alterations in their quality.
End-point acidity.
The second response variable investigated with the same experimental design was the variation in acidity of the samples before and after treatment. Building on the previous work, the effect of acidity and its correlation with the rate of destruction were investigated. The results of these measurements were practically the same for both strains, and so the average values were reported in Table 3. The pHs of the treated samples were the same, regardless of the treatment pressure and exposure time.
TABLE 3.
pHs of the diluted inoculated disks after treatment with CO2 under various experimental conditions
| Pressure (MPa) | Exposure time (min) | End-point pH after treatment |
|---|---|---|
| 2.32 | 195 | 5.2 |
| 3 | 90 | 5.4 |
| 3 | 300 | 5.3 |
| 4 | 18.6 | 5.2 |
| 4 | 195 | 5.2 |
| 4 | 195 | 5.3 |
| 4 | 371.4 | 5.4 |
| 5 | 90 | 5.2 |
| 5 | 300 | 5.3 |
| 5.68 | 195 | 5.3 |
The same drop in pH, compared with the control sample (pH 6.8), was observed in all of the experiments. Since CO2 dissolves in aqueous solution to form an acid, pH was lower after each treatment, but with further increases in experimental condition values, the measured pHs did not change significantly. All the P values calculated by the experimental design were greater than 0.1, confirming that the end-point acidity was not a function of the pressure or the exposure time. The lowered acidity may help increase cell permeability to ease cellular penetration of the fluid (16). This drop in pH will irreversibly inhibit essential metabolic systems, as suggested by Haas et al. (9).
Influence of moisture.
We conducted further experiments with E. coli and S. cerevisiae to investigate the influence of water content of the samples on microbial inactivation. Six filter papers were, as usual, inoculated with the microbial suspension, but the water contents of five of them were increased by addition of different volumes of sterile distilled water. The water contents of the six filter papers were 37, 52, 62, 67, 72, and 75%. All of these samples were treated under the same experimental conditions (5 MPa over 300 min). After treatment, it appeared that the rate of destruction and the drop in pH were similar for all the samples and for the two microbial strains (data not shown). A minimum of water content seems to allow the mechanism of inactivation. Thus, to clearly show the effect of a small water content, two filter papers were inoculated with an E. coli suspension and treated at 5 MPa for 300 min. Before treatment, one of the samples was dried at 30°C for 7 h; the second kept its initial water content.
The results in Table 4 indicated a remarkable decrease in the rate of destruction for the dried filter paper and showed that the treatment was much less effective, whereas the acidity had a similar decrease in both cases. Microbial inactivation strongly depends on the water content. Some researchers (9, 11, 19) demonstrated that moisture was essential to the antimicrobial action of carbon dioxide under pressure. Lin et al. (16, 17) suggested that swollen cell walls, due to the presence of water, become more CO2 permeable.
TABLE 4.
Effect of water content on microbial inactivation
| Substrate | Water content (%) | End-point pH after treatment | Log (N/N0) (E. coli) | Log (N/N0) (S. cerevisiae) |
|---|---|---|---|---|
| Filter paper without drying | 37 | 5.3 | −4.8 | −2.3 |
| Filter paper with drying | 6 | 5.6 | −0.09 | −0.57 |
Influence of the rate of decompression.
We have also looked at the effect of the rate of decompression. All of the experiments in this work were undertaken with as rapid a release of pressure as possible: only 0.4 s was necessary to obtain a vacuum in the processing reactor. In order to evaluate the influence of this factor, comparative pressurization tests were performed with three different decompression times: 0.4 s, 15 min, and 50 min. The experiments were conducted for 195 min at 4 MPa for all microbial strains studied. The microorganism counts after treatment showed that there was no significant difference, with similar rates of destruction in the three experiments. Although the rate of decompression had no effect in our study, some researchers (8, 13, 14) have insisted that rapid decompression is a parameter that enhances the inactivation process. The explanation that we suggest is that these authors worked under CO2-supercritical conditions (pressure and temperature), in which the fluid penetrates more easily into the cells. Rapid decompression may provoke cell rupture as a result of the expansion of CO2 within the cells. This hypothesis will require further investigation.
ACKNOWLEDGMENT
We are grateful to the regional council of Poitou-Charentes, France, for their financial support.
REFERENCES
- 1.Ballestra P, Abreu Da Silva A, Cuq J-L. Inactivation of Escherichia coli by carbon dioxide under pressure. J Food Sci. 1996;61:829–831. , 836. [Google Scholar]
- 2.Becker Z E. A comparison between the action of carbonic acid and other acids upon the living cell. Protoplasma. 1933;25:161–175. [Google Scholar]
- 3.Blickstad E, Enfors S-O, Molin G. Effect of hyperbaric carbon dioxide pressure on the microbial flora of pork stored at 4 or 14°C. J Appl Bacteriol. 1981;50:493–504. [Google Scholar]
- 4.Cuq J L, Ballestra P. Neuvièmes rencontres scientifiques et technologiques des industries alimentaires. Nancy, Paris, France: Lavoisier; 1997. Effets antimicrobiens de l’anhydride carbonique sous pression; pp. 393–398. [Google Scholar]
- 5.Cuq J L, Roussel H, Vivier D, Caron J-P. Etude des effets des gaz sous pression. 2. Influence sur l’inactivation thermique des micro-organismes. Sci Aliments. 1993;13:677–698. [Google Scholar]
- 6.Dixon N M, Kell D B. The inhibition by CO2 of the growth and metabolism of micro-organisms. J Appl Bacteriol. 1989;67:109–136. doi: 10.1111/j.1365-2672.1989.tb03387.x. [DOI] [PubMed] [Google Scholar]
- 7.Donald J R, Jones C L, MacLean A R M. The effect of carbonation on bacteria in beverages. Am J Public Health. 1924;14:122–128. doi: 10.2105/ajph.14.2.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fraser D. Bursting bacteria by release of gas pressure. Nature. 1951;167:33–34. doi: 10.1038/167033b0. [DOI] [PubMed] [Google Scholar]
- 9.Haas G J, Prescott J R, Dudley E, Dik R, Hintlian C, Keane L. Inactivation of microorganisms by carbon dioxide under pressure. J Food Safety. 1989;9:253–265. [Google Scholar]
- 10.Isenschmid A, Marison L W, von Stockar U. The influence of pressure and temperature of compressed CO2 on the survival of yeast cells. J Biotechnol. 1995;39:229–237. doi: 10.1016/0168-1656(95)00018-l. [DOI] [PubMed] [Google Scholar]
- 11.Kamihira M, Taniguchi M, Kobayashi T. Sterilization of microorganisms with supercritical carbon dioxide. Agric Biol Chem. 1987;51:407–412. [Google Scholar]
- 12.King J S, Mabitt L A. Preservation of raw milk by the addition of carbon dioxide. J Dairy Res. 1982;49:439–447. [Google Scholar]
- 13.Lin H-M, Chan E-C, Chen C, Chen L-F. Disintegration of yeast cells by pressurized carbon dioxide. Biotechnol Prog. 1991;7:201–204. [Google Scholar]
- 14.Lin H-M, Yang Z, Chen L-F. An improved method for disruption of microbial cells with pressurized carbon dioxide. Biotechnol Prog. 1992;8:165–166. doi: 10.1021/bp00014a012. [DOI] [PubMed] [Google Scholar]
- 15.Lin H-M, Yang Z, Chen L-F. Inactivation of Saccharomyces cerevisiae by supercritical and subcritical carbon dioxide. Biotechnol Prog. 1992;8:458–461. [Google Scholar]
- 16.Lin H-M, Yang Z, Chen L-F. Inactivation of Leuconostoc dextranicum with carbon dioxide under pressure. Chem Eng J. 1993;52:B29–B34. [Google Scholar]
- 17.Lin H-M, Cao N, Chen L-F. Antimicrobial effect of pressurized carbon dioxide on Listeria monocytogenes. J Food Sci. 1994;59:657–659. [Google Scholar]
- 18.Lorenzen T J, Anderson V L. Design of experiments. A no-name approach. New York, N.Y: Marcel Dekker Inc.; 1993. [Google Scholar]
- 19.Taniguchi M, Suzuki H, Sato M, Kobayashi T. Sterilization of plasma powder by treatment with supercritical carbon dioxide. Agric Biol Chem. 1987;51:3425–3426. [Google Scholar]
- 20.Wei C I, Balaban M O, Fernando S Y, Peplow A J. Bacterial effect of high pressure CO2 treatment on foods spiked with Listeria or Salmonella. J Food Prot. 1991;54:189–193. doi: 10.4315/0362-028X-54.3.189. [DOI] [PubMed] [Google Scholar]