Abstract
Background
Gestational diabetes mellitus is one of the most frequent pregnancy complications with a global prevalence of 13.4% in 2021. Pregnant women with COVID-19 and gestational diabetes mellitus are 3.3 times more likely to be admitted to an intensive care unit than women without gestational diabetes mellitus. Data on the association of gestational diabetes mellitus with maternal and neonatal pregnancy outcomes in pregnant women with SARS-CoV-2 infection are lacking.
Objective
This study aimed to investigate whether gestational diabetes mellitus is an independent risk factor for adverse maternal and fetal and neonatal outcomes in pregnant women with COVID-19.
Study Design
The COVID-19-Related Obstetric and Neonatal Outcome Study is a registry-based multicentric prospective observational study from Germany and Linz, Austria. Pregnant women with clinically confirmed COVID-19 were enrolled between April 3, 2020, and August 24, 2021, at any stage of pregnancy. Obstetricians and neonatologists of 115 hospitals actively provided data to the COVID-19-Related Obstetric and Neonatal Outcome Study. For collecting data, a cloud-based electronic data platform was developed. Women and neonates were observed until hospital discharge. Information on demographic characteristics, comorbidities, medical history, COVID-19–associated symptoms and treatments, pregnancy, and birth outcomes were entered by the local sites. Information on the periconceptional body mass index was collected. A primary combined maternal endpoint was defined as (1) admission to an intensive care unit (including maternal mortality), (2) viral pneumonia, and/or (3) oxygen supplementation. A primary combined fetal and neonatal endpoint was defined as (1) stillbirth at ≥24 0/7 weeks of gestation, (2) neonatal death ≤7 days after delivery, and/or (3) transfer to a neonatal intensive care unit. Multivariable logistic regression analysis was performed to evaluate the modulating effect of gestational diabetes mellitus on the defined endpoints.
Results
Of the 1490 women with COVID-19 (mean age, 31.0±5.2 years; 40.7% nulliparous), 140 (9.4%) were diagnosed with gestational diabetes mellitus; of these, 42.9% were treated with insulin. Overall, gestational diabetes mellitus was not associated with an adverse maternal outcome (odds ratio, 1.50; 95% confidence interval, 0.88–2.57). However, in women who were overweight or obese, gestational diabetes mellitus was independently associated with the primary maternal outcome (adjusted odds ratio, 2.69; 95% confidence interval, 1.43–5.07). Women who were overweight or obese with gestational diabetes mellitus requiring insulin treatment were found to have an increased risk of a severe course of COVID-19 (adjusted odds ratio, 3.05; 95% confidence interval, 1.38–6.73). Adverse maternal outcomes were more common when COVID-19 was diagnosed with or shortly after gestational diabetes mellitus diagnosis than COVID-19 diagnosis before gestational diabetes mellitus diagnosis (19.6% vs 5.6%; P<.05). Maternal gestational diabetes mellitus and maternal preconception body mass index of ≥25 kg/m2 increased the risk of adverse fetal and neonatal outcomes (adjusted odds ratio, 1.83; 95% confidence interval, 1.05–3.18). Furthermore, overweight and obesity (irrespective of gestational diabetes mellitus status) were influential factors for the maternal (adjusted odds ratio, 1.87; 95% confidence interval, 1.26–2.75) and neonatal (adjusted odds ratio, 1.81; 95% confidence interval, 1.32–2.48) primary endpoints compared with underweight or normal weight.
Conclusion
Gestational diabetes mellitus, combined with periconceptional overweight or obesity, was independently associated with a severe maternal course of COVID-19, especially when the mother required insulin and COVID-19 was diagnosed with or after gestational diabetes mellitus diagnosis. These combined factors exhibited a moderate effect on neonatal outcomes. Women with gestational diabetes mellitus and a body mass index of ≥25 kg/m2 were a particularly vulnerable group in the case of COVID-19.
Key words: diabetes mellitus, invasive ventilation, large for gestational age, maternal pregnancy outcomes, morbidity, obesity, pregnancy, SARS-CoV-2
AJOG at a Glance.
Why was this study conducted?
This study aimed to quantify independent associations between gestational diabetes mellitus (GDM) and severe COVID-19 in pregnancy.
Key findings
After adjusting for confounders, GDM was independently associated with a more severe course of COVID-19 in women who were overweight (adjusted odds ratio [aOR], 2.90; 95% confidence interval [CI], 1.21–6.95) or obese (aOR, 2.54; 95% CI, 1.13–5.67), but not in women with normal weight. Women who were overweight or obese with insulin-treated GDM experienced a further increased risk of adverse outcomes (aOR, 3.05; 95% CI, 1.38–6.73). Adverse maternal outcomes were more common when COVID-19 was diagnosed with or shortly after GDM diagnosis than COVID-19 diagnosis before GDM diagnosis (19.6% vs 5.6%; P<.05). Neonatal adverse outcomes were associated with GDM and body mass index of ≥25 kg/m2 in their mothers (aOR, 1.83; 95% CI, 1.05–3.18).
What does this add to what is known?
Pregnant women who are overweight or obese before or at the beginning of pregnancy with combined GDM and COVID-19 represented a vulnerable group.
Introduction
Pregnancy is considered an independent risk factor for severe COVID-19,1 and pregnancy outcomes are negatively influenced by preexisting comorbidities, such as cardiovascular disease, obesity, and diabetes mellitus (DM).2 Obesity is associated with an unfavorable outcome in pregnant women, and its combination with abnormal glucose metabolism further enhances this effect.3 Hyperglycemia in pregnancy is a continuum. Preexisting DM is at 1 end of the spectrum as a chronic disease, whereas gestational DM (GDM) represents the low end, first detected in pregnancy and usually resolving after delivery. GDM requiring insulin is regarded as a more severe form of the disorder.
Entry of SARS-CoV-2 into the body is mediated by its binding to the angiotensin-converting enzyme 2 (ACE2) receptor.4 In DM, the entry of the virus may be facilitated by an increase of ACE2 receptor expression by hyperinsulinemia, which is further enhanced by the surface glycoprotein dipeptidyl peptidase-4.5 DM may be associated with complement defects, immunodeficiency, and increased inflammatory activity. In nonpregnant individuals with COVID-19 inhospital hyperglycemia and inflammation are independently associated with a severe course.6 In addition, it has been shown that SARS-CoV-2 may damage pancreatic beta cells, resulting in an increased incidence of newly diagnosed DM.7 , 8
Little is known about the modulatory role of GDM in pregnant women with COVID-19. GDM is one of the most frequent pregnancy complications with a global prevalence of 13.4% and a Europe prevalence of 7.8%.9 , 10 Although GDM is a milder form of hyperglycemia, pregnant women with COVID-19 and GDM are 3.3 times more likely to be admitted to an intensive care unit (ICU) than women without DM; however, here, severe disease and invasive ventilation in women with GDM were not increased.2 Analysis of data from registries can be skewed, either lacking specific information about GDM and its differentiation from DM or the data extraction is solely based on International Classification of Diseases codes.11, 12, 13
For the current study, data from the “COVID-19–Related Obstetric and Neonatal Outcome Study” (CRONOS), a hospital-based registry study in Germany and Linz, Austria, were used to evaluate data on the diagnosis and treatment of GDM. The aim of this subgroup analysis of the CRONOS registry was to investigate whether GDM represents an independent risk factor for adverse maternal and neonatal outcomes in pregnant women with COVID-19.
Material and Methods
CRONOS is a multicentric prospective observational study established by the German Society of Perinatal Medicine in April 2020 to offer a timely and fact-based extension of the counseling of pregnant women. Parts of the study results have been published.14, 15, 16 Ethics approval was obtained (University Hospital Schleswig-Holstein, Kiel, Germany; file number D 451/20). Inpatient women with confirmed COVID-19 were included. No woman in our cohort was vaccinated against COVID-19 before their SARS-CoV-2 infection.
Data management
For collecting data, a reporting form was developed using the cloud-based electronic data capture platform of the service provider castoredc.com (Amsterdam, Netherlands). According to the study protocol, all women were prospectively enrolled in the study at first presentation in the maternity hospitals. Informed consent was either obtained in the antepartum period or waived in the postpartum period if presented with former COVID-19 in the current pregnancy. Information on demographic characteristics, comorbidities, previous and current pregnancy characteristics, COVID-19–associated symptoms and treatments, pregnancy and birth-specific events, and neonatal outcomes were entered for each pregnant woman.
Study cohort
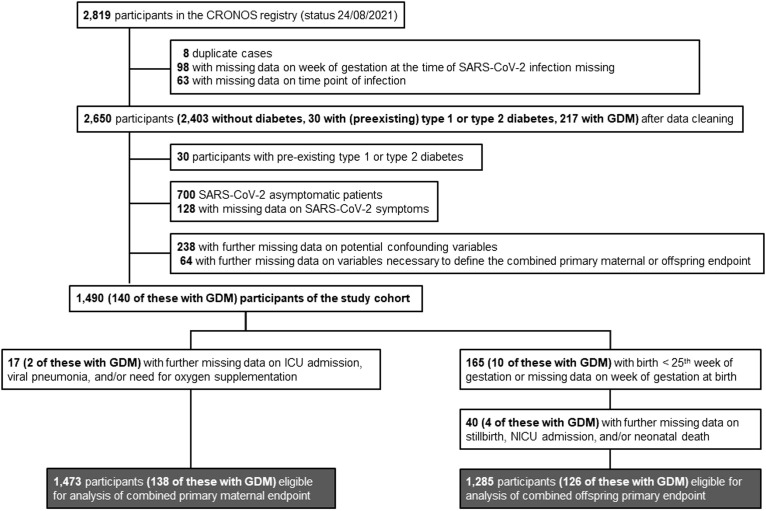
At the time point of data extraction, obstetricians and neonatologists from 115 maternity hospitals had actively provided data to CRONOS. A total of 2819 extracted cases from April 3, 2020, to August 24, 2021, underwent review and plausibility check (Figure 1 ). Patients who tested positive for SARS-CoV-2 but did not have COVID-19–associated symptoms were excluded from the final analysis. The final study cohort was composed of 1490 women. Of these, the diagnosis of COVID-19 was confirmed by a real-time polymerase chain reaction (PCR) test in 1319 (88.5%), by a positive antigen test result in 40 (2.7%), and by positive SARS-CoV-2 antibodies in 37 (2.5%). In 92 cases (6.2%), the type of positive test result was unknown, and in 2 cases (0.1%), the diagnosis was clinically based on the presence of COVID-19 symptoms. The data extraction was performed 2 months after the Delta variant became dominant in Germany.
Figure 1.
Flowchart showing the CRONOS participants being eligible for analysis
CRONOS, COVID-19–Related Obstetric and Neonatal Outcome Study; GDM, gestational diabetes mellitus; ICU, intensive care unit; NICU, neonatal intensive care unit.
Kleinwechter. Gestational diabetes mellitus and COVID-19: COVID-19–Related Obstetric and Neonatal Outcome Study registry results. Am J Obstet Gynecol 2022.
Definition of gestational diabetes mellitus, gestational diabetes mellitus therapy, data acquisition
GDM was defined according to the International Diabetes in Pregnancy Study Groups criteria17 In Germany, a 2-step approach is required.18 First, a 50-g nonfasting 1-hour screen is performed between 24 and 28 weeks of gestation. Women with a test result of ≥135 mg/dL complete a 75-g oral glucose tolerance test (OGTT). GDM is confirmed if any of the following venous plasma glucose values are met or exceeded: fasting, 92 mg/dL; 1 hour, 180 mg/dL; and 2 hours, 153 mg/dL. The diagnosis can be made without OGTT if the screening plasma glucose value exceeds 200 mg/dL. Furthermore, GDM was assumed to be confirmed in 7 women who were classified as GDM by their resident gynecologist according to OGTT results, but the quantitative plasma glucose results were not documented in their maternal health records. GDM was treated according to the German guidelines.18 Specific data regarding GDM were entered online by each hospital on site. Quality and completeness checks were performed by the central study organization. GDM data, OGTT values, and treatment of GDM were validated.
Definition of body mass index categories
Body mass index (BMI) was classified according to the World Health Organization: underweight, <18.5 kg/m2; normal weight, 18.5 to 24.9 kg/m2; overweight, ≥25 kg/m2; and obesity, ≥30 kg/m2. For clarity, overweight or obesity in this article refers to a BMI of ≥25 kg/m2.
Outcomes and endpoint definition
We conducted a subgroup analysis on pregnant women with COVID-19, defining GDM as cases (exposure) and pregnant women with COVID-19 without GDM or DM as controls. We defined the combined primary maternal endpoint as (1) admission to an ICU (including maternal mortality), (2) diagnosis of viral pneumonia, and/or (3) need for oxygen supplementation. The combined primary fetal and neonatal endpoint was defined as the presence of (1) stillbirth at ≥24 0/7 weeks of gestation, (2) neonatal death ≤7 days after delivery, and/or (3) neonatal admission to the neonatal ICU (NICU). We prespecified secondary endpoints: maternal admission to an ICU (including maternal mortality), viral pneumonia, need for oxygen supplementation, neonatal transfer to the NICU, hypertensive disorders in pregnancy (gestational hypertension, preeclampsia, and preexisting hypertensive diseases), cesarean delivery, small and large for gestational age (SGA of <10th percentile and LGA of >90th percentile for gestational age and sex). Power calculation was done on preliminary data through November 2020, estimating that 119 cases, 1190 controls, and 110 cases, 1100 controls (1:10), would be sufficient to detect a difference of at least 10% with an alpha level of.05 and power of 80% in the maternal and fetal and neonatal combined primary endpoints, respectively.
Statistical methodology
Model 1
We examined the association of GDM (as exposure, reference group: women without any type of DM) with the defined primary maternal endpoint and with the primary fetal and neonatal endpoint, respectively, using logistic regression models adjusted for BMI (modeled as a 2- or 3-level variable) only. The multivariable-adjusted model included BMI, week of gestation at the onset of COVID-19 symptoms (or at PCR-positive test result), multiples (yes or no), maternal age at the time of positive COVID-19 test result, language competence (communication possible with or without problems), nicotine or smoking during pregnancy (yes or no), parity (0 or ≥1), and hypertensive disorders in pregnancy (yes or no).
Model 2
We examined the association of GDM and BMI category in 4 subgroups of women, that is, (1) no GDM or DM, BMI of <25 kg/m2 (reference group); (2) no GDM or DM, BMI of ≥25 kg/m2; (3) GDM, BMI of <25 kg/m2; and (4) GDM, BMI of ≥25 kg/m2 with the primary maternal and fetal and neonatal endpoints using logistic regression models. In a sensitivity analysis, a 3-level stratification of BMI was performed, thus comparing 6 subgroups. For the multivariable-adjusted model, the aforementioned covariates were considered.
Model 3
In addition, individuals were stratified by the requirement for insulin treatment, thus comparing 6 subgroups: (1) no GDM or DM, BMI of <25 kg/m2 (reference group); (2) no GDM of DM, BMI of ≥25 kg/m2; (3) GDM without insulin, BMI of <25 kg/m2; (4) GDM without insulin, BMI of ≥25 kg/m2; (5) GDM with insulin, BMI of <25 kg/m2; and (6) GDM with insulin, BMI of ≥25 kg/m2.
In a subgroup analysis, we included only women with GDM and complete documentation of 75-g OGTT values at the time of GDM diagnosis and information on GDM treatment. The association between venous plasma glucose concentrations from OGTT (continuous variables, all included in the same model) and the combined primary endpoints (dependent variable) was assessed using logistic regression analysis, adjusted for potential confounders as defined above, including GDM treatment (basic management or insulin). The adjusted odds ratios (ORs) and 95% confidence intervals (CIs) to develop the combined primary endpoint per 1 mg/dL increase in plasma glucose concentrations are presented.
Statistical analyses were performed using SAS software (version 9.4; SAS Institute, Cary, NC). P values of <.05 were considered statistically significant.
Results
Among the 1490 symptomatic women in the study cohort, 140 (9.4%) had GDM (Figure 1). Moreover, 74% of women with COVID-19 were diagnosed simultaneously with or shortly after GDM diagnosis. Most women with GDM showed abnormal fasting glucose concentrations during OGTT, either isolated or in combination with 1 pathologic postprandial glucose concentration value. GDM was treated with insulin in 43% of women (Table 1 ). Overall, 41% of women in the study cohort were nulliparous. Low language competency, a surrogate parameter in our cohort for low socioeconomic status, was found in 25% of women. Women with GDM were older and more likely to be overweight or obese than women without GDM or DM (Table 1).
Table 1.
General characteristics of the total study cohort and according to DM status
| Characteristic | n (no GDM or DM/GDM) | Total | No GDM or preexisting DM | GDM |
|---|---|---|---|---|
| 5 most commonly reported COVID-19–related symptoms in symptomatic cases | 1260/131 | Malaise (60%), cough (60%), fatigue (53%), loss of sense of smell or taste (51%), nasal obstructions (41%) | Malaise (60%), cough (59%), fatigue (53%), loss of sense of smell or taste (51%), nasal obstructions (41%) | Cough (66%), malaise (57%), fatigue (53%), loss of sense of smell or taste (47%), nasal obstructions (46%) |
| Week of gestation at the onset of COVID-19 symptoms (OR at PCR-positive test result) | 1350/140 | 28.2±9.8 | 28.1±9.9 | 28.9±8.9 |
| Week of gestation of gestational diabetes mellitus diagnosis | 0/140 | — | — | 25.3±4.3 |
| Glucose-lowering therapy Basic management Conventional insulin therapy Intensified insulin therapy |
0/140 | — | — | 80 (57.1) 43 (30.7) 17 (12.1) |
| Fasting venous plasma glucose concentration (mg/dL)a | 0/130 | — | — | 96.6±9.7 |
| Venous plasma glucose concentration after 1 h during OGTT (mg/dL)a | 0/124 | — | — | 175.7±31.7 |
| Venous plasma glucose concentration after 2 h during OGTT (mg/dL)a | 0/121 | — | — | 135.1±29.3 |
| Pathologic venous plasma glucose concentration during OGTT Only at fasting Only after 1 h Only after 2 h At fasting and after 1 or 2 h After 1 and 2 h At all 3 time points |
0/120 | — | — | 44 (36.7) 13 (10.8) 10 (8.3) 33 (27.5) 6 (5.0) 14 (11.7) |
| Maternal age at positive COVID-19 test result (y) | 1350/140 | 31.0±5.2 | 30.9±5.2b | 32.0±5.0b |
| Maternal BMI before or at the beginning of pregnancy (kg/m2) Underweight or normal weight Overweight Obese |
1350/140 | 803 (53.9) 387 (26.0) 300 (20.1) |
768 (56.9)c 340 (25.2)c 242 (17.9)c |
35 (25.0)c 47 (33.6)c 58 (41.4)c |
| Language competence (communication possible without any problems) | 1350/140 | 1269 (85.2) | 1153 (85.4) | 116 (82.9) |
| Continent of birth Europe North and South America Africa Asia Australia |
1334/139 | 1015 (68.9) 13 (0.9) 50 (3.4) 394 (26.8) 1 (0.07) |
936 (70.2) 12 (0.9) 43 (3.2) 342 (25.6) 1 (0.07) |
79 (56.8)b 1 (0.7) 7 (5.0) 52 (37.4) 0 (0.0) |
| Nicotine or smoking during pregnancy (yes) | 1350/140 | 47 (3.2) | 39 (2.9) | 8 (5.7) |
| Nulliparous (yes) | 1350/140 | 606 (40.7) | 557 (41.3) | 49 (35.0) |
| Hypertensive disorders in pregnancy (yes) | 1350/140 | 94 (6.3) | 76 (5.6)c | 18 (12.9)c |
| Mother in ICU (yes) | 1334/137 | 68 (4.6) | 54 (4.1)d | 14 (10.2)d |
| Maternal intubation (yes) | 1334/137 | 29 (2.0) | 24 (1.8) | 5 (3.7) |
| Maternal oxygen supplementation (yes) | 1335/138 | 119 (8.1) | 100 (7.5)b | 19 (13.8)b |
| Maternal viral pneumonia (yes) | 1323/136 | 91 (6.2) | 76 (5.7)b | 15 (11.0)b |
| Mother deceased (yes) | 1333/137 | 4 (0.3) | 4 (0.3) | 0 (0.0) |
| Combined primary maternal endpoint (yes) | 1335/138 | 139 (9.4) | 117 (8.8)d | 22 (15.9)d |
| Multiples (yes) | 1350/140 | 44 (3.0) | 37 (2.7) | 7 (5.0) |
| Premature rupture of membranes or spontaneous preterm delivery (yes) | 1159/126 | 187 (14.6) | 168 (14.5) | 19 (15.1) |
| Stillbirth (yes) | 1159/126 | 13 (1.01) | 11 (0.95) | 2 (1.59) |
| Umbilical cord arterial pH<7.1 (yes) | 1126/123 | 46 (3.7) | 40 (3.6) | 6 (4.9) |
| Apgar score of <7 at 5 min (yes) | 1126/123 | 29 (2.3) | 24 (2.1) | 5 (4.1) |
| Child in neonatal ICU (yes) | 1148/124 | 203 (16.0) | 177 (15.4) | 26 (21.0) |
| Child deceased after delivery (yes) | 1147/126 | 4 (0.3) | 3 (0.3) | 1 (0.8) |
| Combined primary offspring endpoint (yes) | 1159/126 | 217 (16.9) | 189 (16.3) | 28 (22.2) |
| Mode of delivery (cesarean delivery) | 1192/130 | 495 (37.4) | 438 (36.7) | 57 (43.9) |
| Small for gestational age (yes) | 1021/113 | 79 (7.0) | 70 (6.9) | 9 (8.0) |
| Large for gestational age (yes) | 1021/113 | 103 (9.1) | 77 (7.5)c | 26 (23.0)c |
Data are presented as number/total number (percentage) or mean±standard deviation, unless otherwise specified. P<.05, P<.001, and P<.01 are for comparison between no GDM or preexisting DM and GDM based on the chi-squared test or Fisher exact test (categorical variables) or Student t test (continuous normally distributed variables).
DM, diabetes mellitus; GDM, gestational diabetes mellitus; ICU, intensive care unit; OGTT, oral glucose tolerance test; OR, odds ratio; PCR, polymerase chain reaction.
Kleinwechter. Gestational diabetes mellitus and COVID-19: COVID-19–Related Obstetric and Neonatal Outcome Study registry results. Am J Obstet Gynecol 2022.
To convert to mmol/L multiply mg/dL with 0.055
P<.05
P<.001
P<.01.
Associations of diabetes status with combined primary maternal endpoint
In the overall sample, the odds to develop the combined primary maternal endpoint were comparable between women with GDM and those without any type of DM (model 1) (Table 2 ). Women with a BMI above the normal range (≥25 kg/m2, including further stratification in overweight and obesity) had significantly higher odds for the maternal endpoint than women who were underweight or who had normal weight (models 1) (Table 2 and Supplemental Table 1). After stratifying the sample according to BMI categories, we observed that GDM was associated with 2.69 times higher odds for the combined maternal endpoint after multivariable adjustment in women who were overweight and obese (reference group: no GDM or DM, BMI of <25 kg/m2) (model 2) (Table 2). The highest odds associated with GDM was observed in women who were overweight or obese requiring insulin therapy (model 3) (Table 2). Women with COVID-19 diagnosis with or after GDM diagnosis had a statistically significant higher frequency of the combined maternal endpoint than women with COVID-19 diagnosis before GDM diagnosis (19.6% vs 5.6%; P<.05) (Supplemental Table 2). In women who were underweight or had normal weight, the risk to develop the combined maternal endpoint was not different between women with GDM, irrespective of insulin use, and women without any type of DM (models 2 and 3) (Table 2).
Table 2.
Associations among diabetes mellitus status (gestational diabetes mellitus vs no diabetes mellitus), body mass index (underweight or normal weight vs overweight or obese), and combined primary maternal endpoint
| Variable | Combined primary maternal endpoint |
||||
|---|---|---|---|---|---|
| n (column %) | Yes (n [row %]) | No (n [row %]) | Crude or only BMI (aOR [95% CI])a | Multivariable (aOR [95% CI])b | |
| Model 1 | |||||
| GDM | |||||
| No GDM or preexisting DM | 1335 (90.6) | 117 (8.8) | 1218 (91.2) | Ref | Ref |
| GDM, no DM | 138 (9.4) | 22 (15.9) | 116 (84.1) | 1.56 (0.94–2.59) | 1.50 (0.88–2.57) |
| BMIc | |||||
| Underweight or normal weight (<25 kg/m2) | 796 (54.0) | 48 (6.0) | 748 (94.0) | Ref | Ref |
| Overweight or obese (≥25 kg/m2) | 677 (46.0) | 91 (13.4) | 586 (86.6) | 2.29 (1.58–3.33)d | 1.87 (1.26–2.75)d |
| Model 2 | |||||
| No GDM or DM, underweight or normal weight | 761 (51.7) | 44 (5.9) | 717 (94.2) | Ref | Ref |
| No GDM or DM, overweight or obese | 574 (39.0) | 73 (12.7) | 501 (87.3) | 2.37 (1.61–3.51)d | 1.93 (1.28–2.90)d |
| GDM, underweight or normal weight | 35 (2.4) | 4 (11.4) | 31 (88.6) | 2.10 (0.71–6.22) | 2.01 (0.65–6.25) |
| GDM, overweight or obese | 103 (7.0) | 18 (17.5) | 85 (82.5) | 3.45 (1.91–6.24)d | 2.69 (1.43–5.07)d |
| Model 3 | |||||
| No GDM or DM, underweight or normal weight | 761 (51.7) | 44 (5.8) | 717 (94.2) | Ref | Ref |
| No GDM or DM, overweight or obese | 574 (39.0) | 73 (12.7) | 501 (37.6) | 2.37 (1.61–3.51)d | 1.93 (1.28–2.90)d |
| GDM without insulin, underweight or normal weight | 26 (1.8) | 3 (11.5) | 23 (88.5) | 2.13 (0.61–7.35) | 1.98 (0.53–7.34) |
| GDM without insulin, overweight or obese | 54 (3.7) | 7 (13.0) | 47 (87.0) | 2.43 (1.04–5.68)d | 2.30 (0.94–5.63) |
| GDM with insulin, underweight or normal weight | 9 (0.6) | 1 (11.1) | 8 (88.9) | 2.04 (0.25–16.65) | 2.09 (0.24–18.01) |
| GDM with insulin, overweight or obese | 49 (3.3) | 11 (22.4) | 38 (77.6) | 4.72 (2.26–9.86)d | 3.05 (1.38–6.73)d |
Data are presented as aOR (95% CI) using logistic regression analyses with combined primary maternal endpoint (yes or no) as the dependent variable.
aOR, odds ratio; BMI, body mass index; CI, confidence interval; DM, diabetes mellitus; GDM, gestational diabetes mellitus; Ref, reference interval.
Kleinwechter. Gestational diabetes mellitus and COVID-19: COVID-19–Related Obstetric and Neonatal Outcome Study registry results. Am J Obstet Gynecol 2022.
Model 1 adjusted for BMI only and models 2 and 3 unadjusted
Additionally adjusted for week of gestation at the onset of COVID-19 symptoms (or at a positive polymerase chain reaction test result), multiples (yes or no), maternal age at positive COVID-19 test result, language competence (communication possible with or without problems), nicotine or smoking during pregnancy (yes or no), parity (0 or ≥1), and hypertensive disorders in pregnancy (yes or no)
Maternal BMI before or at the beginning of pregnancy (kg/m2)
P<.05.
Associations of diabetes status with combined primary fetal and neonatal endpoint
In the multivariable-adjusted model, the odds of developing the combined primary fetal and neonatal endpoint were not different among the offspring of women with GDM and those without any DM in the overall sample (model 1) (Table 3 ). Contrastingly, increased maternal BMI was associated with an approximately 80% higher risk to develop the combined endpoint (models 1) (Table 3 and Supplemental Table 3). In stratified analyses, we observed a risk increment of 83% and 91% for offspring of mothers who were overweight or obese with and without GDM, respectively, for the combined fetal and neonatal endpoint, compared with offspring of mothers of the reference group (model 2) (Table 3). There was no statistically significant difference in the primary fetal and neonatal endpoint depending on the time of COVID-19 diagnosis before or with or after GDM diagnosis (24.2% vs 21.5%) (Supplemental Table 2). Unlike the findings for the combined maternal endpoint, offspring of mothers using insulin seemed to already have an increased risk of developing the combined fetal and neonatal endpoint with a maternal BMI of <25 kg/m2 (model 3) (Table 3).
Table 3.
Associations among diabetes mellitus status (gestational diabetes mellitus vs no diabetes mellitus), body mass index (underweight or normal weight vs overweight or obese), and combined primary fetal and neonatal endpoint
| Variable | Combined primary fetal and neonatal endpoint |
||||
|---|---|---|---|---|---|
| n (column %) | Yes (n [row %]) | No (n [row %]) | Crude or only BMI (aOR [95% CI])a | Multivariable (aOR [95% CI])b | |
| Model 1 | |||||
| GDM | |||||
| No GDM or preexisting DM | 126 (9.8) | 189 (16.3) | 970 (83.7) | Ref | Ref |
| GDM, no DM | 1159 (90.2) | 28 (2.2) | 98 (77.8) | 1.20 (0.76–1.90) | 1.11 (0.69–1.79) |
| BMIc | |||||
| Underweight or normal weight (<25 kg/m2) | 690 (53.7) | 87 (12.6) | 603 (87.4) | Ref | Ref |
| Overweight or obese (≥25 kg/m2) | 595 (46.3) | 120 (21.9) | 465 (78.2) | 1.89 (1.40–2.56)d | 1.81 (1.32–2.48)d |
| Model 2 | |||||
| No GDM or DM, underweight or normal weight | 660 (51.4) | 81 (12.3) | 579 (87.7) | Ref | Ref |
| No GDM or DM, overweight or obese | 499 (38.8) | 108 (21.6) | 391 (78.4) | 1.97 (1.44–2.71)d | 1.91 (1.37–2.65)d |
| GDM, underweight or normal weight | 30 (2.3) | 6 (20.0) | 24 (80.0) | 1.79 (0.71–4.50) | 1.90 (0.75–4.82) |
| GDM, overweight or obese | 96 (7.5) | 22 (22.9) | 74 (77.1) | 2.13 (1.25–3.61)d | 1.83 (1.05–3.18)d |
| Model 3 | |||||
| No GDM or DM, underweight or normal weight | 660 (51.4) | 81 (12.3) | 579 (87.7) | Ref | Ref |
| No GDM or DM, overweight or obese | 499 (38.8) | 108 (21.6) | 391 (78.4) | 1.97 (1.44–2.71)d | 1.91 (1.37–2.65)d |
| GDM without insulin, underweight or normal weight | 22 (1.7) | 3 (13.6) | 19 (86.4) | 1.13 (0.33–3.90) | 1.17 (0.33–4.09) |
| GDM without insulin, overweight or obese | 52 (4.1) | 11 (21.2) | 41 (78.9) | 1.92 (0.95–3.88) | 1.65 (0.79–3.46) |
| GDM with insulin, underweight or normal weight | 8 (0.6) | 3 (37.5) | 5 (62.5) | 4.29 (1.01–18.29)d | 4.76 (1.11–20.41)d |
| GDM with insulin, overweight or obese | 44 (3.4) | 11 (25.0) | 33 (75.0) | 2.38 (1.16–4.90)d | 2.03 (0.96–4.32) |
Data are presented as aOR (95% CI) using logistic regression analyses with combined primary offspring endpoint (yes or no) as the dependent variable.
aOR, odds ratio; BMI, body mass index; CI, confidence interval; DM, diabetes mellitus; GDM, gestational diabetes mellitus; Ref, reference interval.
Kleinwechter. Gestational diabetes mellitus and COVID-19: COVID-19–Related Obstetric and Neonatal Outcome Study registry results. Am J Obstet Gynecol 2022.
Model 1 adjusted for BMI only and models 2 and 3 unadjusted
Additionally adjusted for week of gestation at the onset of COVID-19 symptoms (or at a positive polymerase chain reaction test result), multiples (yes or no), maternal age at positive COVID-19 test result, language competence (communication possible with or without problems), nicotine or smoking during pregnancy (yes or no), parity (0 or ≥1), and hypertensive disorders in pregnancy (yes or no)
Maternal BMI before or at the beginning of pregnancy (kg/m2)
P<.05.
Associations of diabetes status with secondary endpoints
Analysis of maternal admission to an ICU (including maternal mortality), oxygen supplementation, viral pneumonia, NICU admission (as single endpoints), and cesarean delivery confirmed that the vulnerable subgroups at increased risk of adverse outcomes were women who were overweight or obese and their offspring. Excluding cesarean delivery, the risk further increased if the mothers additionally had GDM (models 2) (Supplemental Table 4). The risk for hypertensive disorders in pregnancy was increased in women with GDM compared with their counterparts without DM (model 1) (Supplemental Table 4). Although the offspring of women with GDM did not differ in their risk to be born SGA compared with the offspring of women without any type of DM, the offspring of women with GDM were approximately 3 times more likely to be born LGA (models 1). After additional stratification, the offspring of women with GDM and who were overweight or obese had increased odds for LGA compared with the offspring of women without any type of DM and who were underweight or had normal weight (models 2 and 3) (Supplemental Table 4).
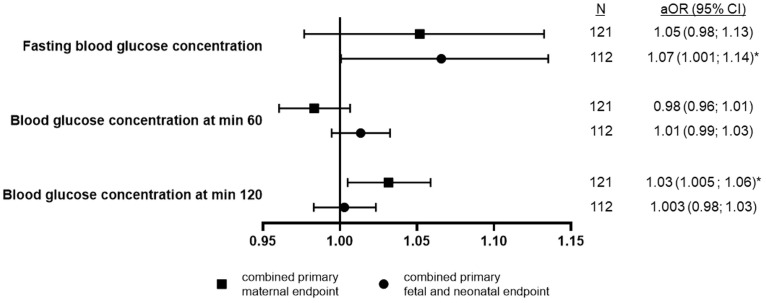
Associations of plasma glucose concentrations with combined primary endpoints
We performed a subgroup analysis of women with GDM to assess the association of venous plasma glucose concentrations from OGTT at the time of GDM diagnosis with the combined primary endpoints. Higher glucose concentrations after 2 hours were independently associated with higher odds to develop the combined primary maternal endpoint. Fasting glucose concentrations were independently and directly associated with the combined primary fetal and neonatal endpoint (Figure 2 ).
Figure 2.
Associations between plasma glucose concentrations from OGTT and primary endpoints
Data are presented as aOR (95% CI) using logistic regression analyses for combined primary maternal and fetal and neonatal endpoints (yes or no) as the dependent variable (separate model for each) per 1 mg/dL increase in venous plasma glucose concentrations from OGTT (continuous variables, all included in the same model). Adjusted for week of gestation at the onset of COVID-19 symptoms (or at PCR-positive test result), multiples (yes or no), maternal age at positive COVID-19 test result, language competence (communication possible with or without problems), parity (0 or ≥1), maternal BMI category before or at the beginning of pregnancy (BMI, <25 or ≥25 kg/m2), hypertensive disorders in pregnancy (yes or no), and type of GDM therapy (diet or insulin). The model with combined primary fetal and neonatal endpoint was additionally adjusted for nicotine or smoking during pregnancy (yes or no) as a quasi-complete separation of data points was observed with this potential confounding variable. The asterisk represents P<.05.
aOR, adjusted odds ratio; BMI, body mass index; CI, confidence interval; OGTT, oral glucose tolerance test; PCR, polymerase chain reaction.
Kleinwechter. Gestational diabetes mellitus and COVID-19: COVID-19–Related Obstetric and Neonatal Outcome Study registry results. Am J Obstet Gynecol 2022.
Comment
Principal findings
Pregnant women with GDM and COVID-19 undergo a more severe course of the infection if they are overweight or obese periconceptionally or require insulin in association with a periconceptional BMI of ≥25 kg/m2. Adverse maternal outcomes were more common when COVID-19 was diagnosed with or shortly after GDM diagnosis than COVID-19 diagnosis before GDM diagnosis. These combined factors had a moderate influence on adverse neonatal outcomes. In women with a BMI of <25 kg/m2 and COVID-19, GDM (with or without insulin) had no adverse effect on the severity of the maternal course.
Hypothesized mechanism of action and further research
COVID-19 may interact with the course of GDM19 and vice versa. Here, 74% of women with COVID-19 that occurred simultaneously with or shortly after GDM diagnosis experienced a more severe course than women with COVID-19 that occurred before GDM diagnosis. Of note, 43% of women were treated with insulin, supporting previous studies that inflammation may worsen hyperglycemia. In large randomized trials, the insulin rates were 7% and 20%, respectively.20 , 21 The INTERCOVID study reported an increased risk ratio for COVID-19 in women with insulin-treated GDM regardless of their BMI.22
On their first consultation with their obstetrician, pregnant women should be informed of the measures to reduce the risk of GDM, for example, increasing exercise, cessation of smoking, switching to a healthy diet, and weight gain control according to the US National Academy of Medicine guidelines, which are the same in Germany. For example, women with obesity should gain weight not more than 9 kg until delivery. All women with GDM diagnosis should receive individual counseling on diet, undergo education, and start self-measurement of capillary blood glucose. The aforementioned measures for prevention are recommended to be intensified after GDM diagnosis to reduce the frequency of insulin use; monitoring visits to discuss the progress should be more frequent. In addition, fetal growth should be monitored regularly by ultrasound examination to detect fetal macrosomia, which is following the German national guidelines on the management of GDM.18
Here, the severe course of COVID-19 only affected women who were overweight or obese (with or without GDM), allowing us to conclude that BMI is a relevant factor in the course of the disease. Obesity is associated with an unfavorable outcome in pregnant women, and its combination with abnormal glucose metabolism further enhances this effect.3 Although we had no information on the quality of metabolic control after GDM diagnosis, the 3-fold increased rate of LGA in women with GDM compared with women without any type of DM (23% vs 7.5%) was indicative of the challenge in achieving normoglycemia in COVID-19. In the trials mentioned above, the LGA rate was 13% at most; moreover, in randomized screening trials, the LGA rate was even <10%.23 , 24 Our data did not confirm an increased rate of stillbirth in GDM as reported in the analysis from the Premier Healthcare Database.13 Independent of GDM status, the US Centers for Disease Control and Prevention reported a 47% overall increased risk of stillbirth with COVID-19, increasing further when the Delta virus variant was dominant.12
The coexistence of GDM and overweight or obesity is common and has been seen in 75% of pregnancies in our GDM subgroup vs 43.1% of pregnancies without DM. Typical for insulin resistance in the cases of GDM, we saw an isolated elevated fasting plasma glucose, the only abnormal result in 36.7% of pregnancies and an additional 39.2% of pregnancies in combination with abnormal results of the 1-hour or 2-hour OGTT value.25 All 3 OGTT values were elevated in 11.7% of pregnancies. According to the literature and based on these results, we can presume that women with GDM and COVID-19 in our cohort are at high risk of developing type 2 DM during the next 5 to 10 years.26
GDM prevalence seems to be increasing during the pandemic.13 , 27, 28, 29 The 30% increased risk of GDM observed in the United States may be explained by a change in women’s lifestyles under the conditions of the pandemic.29 Unfavorable lifestyle modification is known to increase the risk of developing impaired glucose tolerance during pregnancy. Our data have shown that only 1 of 3 values of OGTT was associated with a severe course in the mother and offspring, respectively. Thus, OGTT results seem to have a limited predictive value on adverse pregnancy outcomes in COVID-19. Further research is needed to evaluate the relationship between the quality of GDM therapy and the course and outcome of COVID-19. Because pregnant women may have a higher proportion of asymptomatic infections,30 further research is required on asymptomatic pregnant women with SARS-CoV-2 infection and GDM.
Clinical implications
Large studies of hospitalized pregnant women with COVID-19 have reported increased maternal morbidity and mortality compared with nonpregnant women.1 , 2 , 31 Physiological associations of GDM (ie, hyperinsulinemia) may enhance ACE2 receptor expression followed by facilitated SARS-CoV-2 entry. In addition, GDM may affect the damage of pancreatic beta cells in more severe cases on insulin therapy, followed by an excess incidence of type 2 DM. Therefore, measures are needed to detect GDM as early as possible, recommending lifestyle changes (eg, a healthy diet or regular exercise) and encouraging women to take part in regular follow-up examinations, for example, OGTT, fasting plasma glucose, and Hba1c testing for diabetes diagnosis. Vaccination with an mRNA vaccine has the potential to substantially reduce COVID-19–associated inflammation, which may further decrease hyperglycemia in pregnancy.
Our data have provided additional information for caregivers of pregnant women. GDM is a common complication in pregnancy. Women with GDM, combined with periconceptional overweight or obesity, should be considered a vulnerable group in COVID-19, especially if they require insulin. The analysis of the CRONOS subgroup of patients with COVID-19 with admission to the ICU showed that GDM was the most common comorbidity.15 Despite high LGA frequency, GDM seemed to have little effect on neonatal outcomes. The decision between conservative management in pregnant patients with COVID-19 and the risk of iatrogenic preterm delivery requires interdisciplinary monitoring and consultation. Vaccination with an mRNA vaccine combined with hygiene protection measures is an important option for preventing or mitigating the severe course of COVID-19 in pregnancy.32 , 33 The pandemic should not discourage women from undergoing GDM screening or planning their birth in a hospital to receive the full benefits of adequate care.34
Strengths and limitations of the study
Here, we used data from a homogeneous cohort and rigorous data monitoring concerning the confirmation of COVID-19, GDM, and its therapy and adverse maternal and offspring outcomes. Diligent data monitoring and recalls and plausibility tests of reported cases and laboratory test results allowed us to detect and eliminate discrepancies.35
Some limitations should be mentioned. First, the hospitals reporting data only represent 30% of all births in Germany. Second, we cannot exclude that residual confounders remained unconsidered in the analysis; for example, it is unclear whether or not all hospitals reported all their pregnant women with COVID-19 during the study period. Small numbers in model 3 led to limited interpretation because of large CI intervals. Here, we used LGA as a surrogate for blood glucose control and quality of DM management but with limited validity as exact data are not available. Moreover, our study did not include women with asymptomatic SARS-CoV-2 infection or a cohort without COVID-19. This limited the generalization of data to those women with COVID-19 during their pregnancy. Third, we had no information on monogenic DM, but we presumed from our data that the incidence is low. Lastly, some diagnostic and treatment decisions may have been influenced by uncertainties during the pandemic and high workloads.
Conclusions
GDM, combined with periconceptional overweight or obesity, was independently associated with a severe maternal course of COVID-19, especially when insulin was required for treatment. Adverse maternal outcomes were more common when COVID-19 was diagnosed with or shortly after GDM diagnosis than COVID-19 diagnosis before GDM diagnosis. These combined factors had a moderate adverse effect on neonatal outcomes. Caregivers for women with GDM and COVID-19 should be aware of the additional risks that GDM poses.
Acknowledgments
We are very grateful to the participating pregnant women, the contributing institutions, and the local researchers involved in the study. The Appendix contains the details of the institutions. We especially thank Prof Werner A. Scherbaum, MD, Düsseldorf, Germany, and Prof Wolfgang Lieb, MD, MSc, Kiel, Germany, for reviewing the manuscript and for their helpful comments and Dr Katrina Kraft, MD, Starnberg, Germany, for language editing. All of them have no conflict of interest.
Footnotes
The authors report no conflict of interest.
The study has received funding from the German Diabetes Foundation (FP-0439-2021) and the Krumme Foundation. The funding organizations have no involvement in the design and conduct of the study; collection, management, analysis, interpretation of the data, preparation, review, and approval of the manuscript; or decision to submit the manuscript for publication.
This study is registered in the German Clinical Trial Registry (identification number DRKS00021208; https://www.drks.de/drks_web/navigate.do?navigationId=trial.HTML&TRIAL_ID=DRKS00021208)
Cite this article as: Kleinwechter HJ, Weber KS, Mingers N, et al. Gestational diabetes mellitus and COVID-19: results from the COVID-19–Related Obstetric and Neonatal Outcome Study. Am J Obstet Gynecol 2022;227:631.e1-19.
Appendix
Members (registered maternity units) of the “COVID-19-Related Obstetric and Neonatal Outcome Study”
Participating institutions and principal investigators
-
•
University Hospital TU, Dresden, Germany: M.R.
-
•
University Hospital, Erlangen, Germany: Alexander Hein
-
•
Kepler University Hospital, Linz, Austria: Peter Oppelt
-
•
University Hospital, Munich, Germany: T.K.
-
•
Vivantes Hospital Neukoelln, Berlin, Germany: B.R.
-
•
Hospital Links der Weser, Bremen, Germany: Bastian Riebe
-
•
St. Josef Hospital, Berlin, Germany: U.M.S.G.
-
•
Charité University Hospital, Berlin, Germany: C.E.
-
•
Vivantes Hospital Friedrichshain, Berlin, Germany: A.J.
-
•
University Hospital, Essen, Germany: A.I.
-
•
Sana Hospital Lichtenberg, Berlin, Germany: I.D.S.
-
•
City Hospital, Munich, Germany: C.B.
-
•
City Hospital, Dortmund, Germany: Charlotte Rohlwink
-
•
University Hospital, Freiburg, Germany: M.K.
-
•
City Hospital, Darmstadt, Germany: Jula Manz
-
•
Protestant Hospital Spandau, Berlin, Germany: Nina Axnick
-
•
City Hospital, Stuttgart, Germany: Angela Lihs
-
•
City Hospital, Loerrach, Germany: Michael Bohlmann
-
•
Hospital Hallerwiese, Nuremberg, Germany: E. H.G.
-
•
University Hospital, Jena, Germany: T.G.
-
•
University Hospital, Dresden, Germany: M.R.
-
•
Albertinen Hospital, Hamburg, Germany: Katharina Lang
-
•
Diakovere Hospital, Hannover, Germany: C.M.
-
•
University Hospital, Hannover, Germany: C.V.K.
-
•
University Hospital, Witten-Herdecke, Germany: Marketa Vasku
-
•
Technical University Hospital, Munich, Germany: B.K.
-
•
University Hospital, Giessen, Germany: Gentiana Ibrahimi
-
•
Mathias Hospital, Rheine, Germany: Christina Froehlich
-
•
University Hospital, Mainz, Germany: Parnian Parvanta
-
•
Hospital Sachsenhausen, Frankfurt, Germany: Norman Doehring
-
•
St. Hedwig Hospital, Regensburg, Germany: Sebastian Haeussler
-
•
Florence Nightingale Hospital, Duesseldorf, Germany: M.B.
-
•
Diakonissen Foundation Hospital, Speyer, Germany: Verena Haeffner
-
•
St. Johannes Hospital, Dortmund, Germany: Monika Palz-Fleige
-
•
Dritter Orden Hospital, Munich, Germany: Franz Edler von Koch
-
•
St. Franziskus Hospital, Muenster, Germany: Franziska Pugge
-
•
University Hospital, Muenster, Germany: M.S.
-
•
University Hospital, Aachen, Germany: Cordula Franz
-
•
City Hospital, Hanau, Germany: Georgi Popivanov
-
•
City Hospital, Starnberg, Germany: V.H.
-
•
St. Elisabeth Hospital, Halle, Germany: Sven Seeger
-
•
Vivantes Hospital Schoeneberg, Berlin, Germany: Timo Hentrich
-
•
City Hospital, Kiel, Germany: Marek Struck
-
•
University Hospital, Marburg, Germany: C.K.
-
•
Bayreuth Hospital, Bayreuth, Germany: C.K.
-
•
City Hospital, Datteln, Germany: Miriam Morgen
-
•
General Hospital Barmbek, Hamburg, Germany: Marcel Malan
-
•
District Hospital, Weiden, Germany: Ines Ehrhardt
-
•
General Hospital Wandsbek, Hamburg, Germany: Tamina Rawnaq
-
•
City Hospital, Mönchengladbach, Germany: Gunnar Schwennicke
-
•
Red Cross Hospital, Munich, Germany: Nadine Brunner
-
•
City Hospital, Bayreuth, Germany: Anja Leonhardt
-
•
University Hospital, Kiel, Germany: U.P.
-
•
Red Cross Hospital, Ludwigsburg, Germany: Nadine Petitjean
-
•
Ortenau Hospital, Offenburg, Germany: L.P.
-
•
Waldshut-Tiengen Hospital, Waldshut, Germany: Jeanette Teeuwen-Mutter
-
•
University Hospital, Ulm, Germany: Frank Reister
-
•
University Hospital, Halle, Germany: K.R.
-
•
St. Johannes Hospital, Duisburg, Germany: Asimina Katsiouni
-
•
Traunstein Hospital, Traunstein, Germany: Christian Schindlbeck
-
•
Helios Hospital, Sangerhausen, Germany: O.P.
-
•
University Hospital, Magdeburg, Germany: Franziska Fettig
-
•
Sana Hospital, Duisburg, Germany: M.S.
-
•
University Hospital of Saarland, Bad Homburg, Germany: Zoltan Takacs
-
•
Dr Geisenhofer Clinic, Munich, Germany: L.K.
-
•
University Hospital, Rostock, Germany: J.S.
-
•
St. Juergen Hospital, Bremen, Germany: K.L.
-
•
District Hospital, Ebersberg, Germany: Stephan Hasmueller
-
•
City Hospital, Zittau, Germany: Bettina Hollenbach
-
•
Saalfeld Hospital, Saalfeld, Germany: Dietrich Hager
-
•
University Hospital, Lübeck, Germany: Julia Rothe
-
•
Diakonissen Hospital, Flensburg, Germany: Barabara Fleig
-
•
Marien Hospital, Bottrop, Germany: Katharina Freienstein
-
•
Fürstenfeldbruck Hospital, Fürstenfeldbruck, Germany: Kerstin Brettschneider
-
•
Eggenfelden Hospital, Rottal-Inn, Germany: Irmgard Drost
-
•
Sophien and Hufeland Hospital, Weimar, Germany: Cathleen Heinemann
-
•
District Hospital, Freiberg, Germany: Martina Sperling
-
•
Hospital Bad Salzungen, Bad Salzungen, Germany: Ines Tonndorf
-
•
University Hospital, Duesseldorf, Germany: Melva Baum
-
•
Neuwerk Hospital, Mönchengladbach, Germany: Vanessa Nikolai
-
•
Heart-Jesu-Hospital, Muenster, Germany: Joachim Zucker-Reimann
-
•
Neuwied Hospital, Neuwied, Germany: Richard Berger
-
•
University Hospital, Tuebingen, Germany: Harald Abele
-
•
Heidenheim Hospital, Heidenheim, Germany: Annika Knesebeck
-
•
Caritas Maria Heimsuchung Hospital Pankow, Berlin, Germany: Jens Rohne
-
•
Dietrich-Bonhoeffer Hospital, Neubrandenburg, Germany: Konstanze Kissing-Pahl
-
•
Imland Hospital, Rendsburg, Germany: Annika Ertel
-
•
Itzehoe Hospital, Itzehoe, Germany: Laura Pohlmann
-
•
Ubbo-Emmius Hospital, Aurich, Germany: Kathrin Meyer-Eckle
-
•
Protestant Hospital of Bethel Foundation, Bielefeld, Germany: C.B.J.
-
•
Kalk Hospital, Koeln, Germany: Martin Dambowy
-
•
LAKUMED Hospital, Landsberg, Germany: Mirjam Ulrich
-
•
District Hospital, Erding, Germany: I.B.
-
•
St. Theresien Hospital, Nuremberg, Germany: Ulf Dammer
-
•
Caritas Hospital, Bad Mergentheim, Germany: Sönke Ebert
-
•
KMG Hospital, Sömmerda, Germany: Katharina Feistner
-
•
University Hospital, Leipzig, Germany: Holger Stepan
-
•
Sana Hanse Hospital, Wismar, Germany: J.S.
-
•
City Hospital, Solingen, Germany: Elina Voigthaus
-
•
EUREGIO Hospital, Nordhorn, Germany: Susanne Beckmann
-
•
Christophorus Hospital, Coesfeld, Germany: Elisabeth Edeler
-
•
Medius Hospital, Nürtingen, Germany: Tanja Scheufele-Klein
-
•
University Hospital, Wuerzburg, Germany: Catharina Bartmann
-
•
Diakonissen Hospital, Dresden, Germany: A.T.
-
•
Westcoast Hospital, Heide, Germany: S.E.H.
-
•
City Hospital, Karlsruhe, Germany: Magdalena Tackenberg
Supplemental Table 1.
Associations among diabetes mellitus status (gestational diabetes mellitus vs no diabetes mellitus), body mass index (underweight or normal weight vs overweight vs obese), and combined primary maternal endpoint
| Variable | Combined primary maternal endpoint |
||
|---|---|---|---|
| n (column %) | Crude or only BMI (aOR [95% CI])a | Multivariable (aOR [95% CI])b | |
| Model 1 | |||
| GDM | |||
| No GDM or preexisting DM | 1335 (90.6) | Ref | Ref |
| GDM, no DM | 138 (9.4) | 1.56 (0.94–2.59) | 1.50 (0.87–2.56) |
| BMIc | |||
| Underweight or normal weight (<25 kg/m2) | 796 (54.0) | Ref | Ref |
| Overweight (≥25 kg/m2) | 380 (25.8) | 2.28 (1.50–3.46)d | 1.84 (1.19–2.85)d |
| Obese (≥30 kg/m2) | 297 (20.2) | 2.32 (1.48–3.64)d | 1.90 (1.17–3.07)d |
| Model 2 | |||
| No GDM or DM, underweight or normal weight | 761 (51.7) | Ref | Ref |
| No GDM or DM, overweight | 335 (22.7) | 2.34 (1.5–3.64)d | 1.87 (1.18–2.97)d |
| No GDM or DM, obese | 239 (16.2) | 2.43 (1.5–3.94)d | 2.01 (1.21–3.37)d |
| GDM, underweight or normal weight | 35 (2.4) | 2.10 (0.71–6.22) | 2.01 (0.65–6.25) |
| GDM, overweight | 45 (3.1) | 3.52 (1.55–8.02)d | 2.90 (1.21–6.95)d |
| GDM, obese | 58 (3.9) | 3.40 (1.61–7.16)d | 2.54 (1.13–5.67)d |
Data are presented as aOR (CI) using logistic regression analyses with combined primary maternal endpoint (yes or no) as the dependent variable.
aOR, odds ratio; BMI, body mass index; CI, confidence interval; DM, diabetes mellitus; GDM, gestational diabetes mellitus; Ref, reference interval.
Kleinwechter. Gestational diabetes mellitus and COVID-19: COVID-19–Related Obstetric and Neonatal Outcome Study registry results. Am J Obstet Gynecol 2022.
Model 1 adjusted for BMI only and model 2 unadjusted
Additionally adjusted for week of gestation at the onset of COVID-19 symptoms (or at a positive polymerase chain reaction test result), multiples (yes or no), maternal age at positive COVID-19 test result, language competence (communication possible with or without problems), nicotine or smoking during pregnancy (yes or no), parity (0 or ≥1), and hypertensive disorders in pregnancy (yes or no)
Maternal BMI before or at the beginning of pregnancy (kg/m2)
P<.05.
Supplemental Table 2.
Comparison of timing of COVID-19 with prevalence of primary and secondary endpoints among the subgroup of women with gestational diabetes mellitus
| Variable | n (GDM) | Diagnosis of COVID-19 before the diagnosis of GDM | Diagnosis of COVID-19 with or after the diagnosis of GDM | |
|---|---|---|---|---|
| Combined primary maternal endpoint (yes) | 138 | 2 (5.6) | 20 (19.6) | a |
| Combined primary fetal and neonatal endpoint (yes) | 126 | 8 (24.2) | 20 (21.5) | |
| Mother in ICU (yes) | 137 | 1 (2.8) | 13 (12.9) | |
| Maternal oxygen supplementation (yes) | 138 | 2 (5.6) | 17 (16.7) | |
| Maternal viral pneumonia (yes) | 136 | 2 (5.6) | 13 (13.0) | |
| Cesarean delivery (yes) | 130 | 17 (50.0) | 40 (41.7) | |
| Child in neonatal ICU (yes) | 124 | 25 (78.1) | 73 (79.4) | |
| Small for gestational age (yes) | 113 | 3 (9.7) | 6 (7.3) | |
| Large for gestational age (yes) | 113 | 7 (22.6) | 19 (23.2) |
Data are presented as number (percentage).
GDM, gestational diabetes mellitus; ICU, intensive care unit.
Kleinwechter. Gestational diabetes mellitus and COVID-19: COVID-19–Related Obstetric and Neonatal Outcome Study registry results. Am J Obstet Gynecol 2022.
P<.05 for comparison between the diagnosis of COVID-19 before the diagnosis of GDM and the diagnosis of COVID-19 with or after the diagnosis of GDM based on the chi-squared test or Fisher exact test.
Supplemental Table 3.
Associations between diabetes mellitus status (gestational diabetes mellitus vs no diabetes mellitus), body mass index (underweight or normal weight vs overweight vs obese), and combined primary fetal and neonatal endpoint
| Variable | Combined primary fetal and neonatal endpoint |
||
|---|---|---|---|
| n (column %) | Crude or only BMI (aOR [95% CI])a | Multivariable (aOR [95% CI])b | |
| Model 1 | |||
| GDM) | |||
| No GDM or preexisting DM | 126 (9.8) | Ref | Ref |
| GDM, no DM | 1159 (90.2) | 1.21 (0.76–1.92) | 1.12 (0.70–1.81) |
| BMIc | |||
| Underweight or normal weight (<25 kg/m2) | 690 (53.7) | Ref | Ref |
| Overweight (≥25 kg/m2) | 336 (26.2) | 1.96 (1.39–2.76)d | 1.87 (1.31–2.67)d |
| Obese (≥30 kg/m2) | 259 (20.2) | 1.81 (1.24–2.65)d | 1.72 (1.15–2.56)d |
| Model 2 | |||
| No GDM or DM, underweight or normal weight | 660 (51.4) | Ref | Ref |
| No GDM or DM, overweight | 292 (22.7) | 2.01 (1.40–2.88)d | 1.92 (1.32–2.80)d |
| No GDM or DM, obese | 207 (16.1) | 1.93 (1.29–2.90)d | 1.88 (1.23–2.87)d |
| GDM, underweight or normal weight | 30 (2.3) | 1.79 (0.71–4.50) | 1.90 (0.75–4.83) |
| GDM, overweight | 44 (3.4) | 2.38 (1.16–4.90)d | 2.16 (1.01–4.59)d |
| GDM, obese | 52 (4.1) | 1.92 (0.95–3.88) | 1.57 (0.75–3.30) |
Data are presented as aOR (95% CI) using logistic regression analyses with combined primary offspring endpoint (yes or no) as the dependent variable.
aOR, odds ratio; BMI, body mass index; CI, confidence interval; DM, diabetes mellitus; GDM, gestational diabetes mellitus; Ref, reference interval.
Kleinwechter. Gestational diabetes mellitus and COVID-19: COVID-19–Related Obstetric and Neonatal Outcome Study registry results. Am J Obstet Gynecol 2022.
Model 1 adjusted for BMI only and model 2 unadjusted
Additionally adjusted for week of gestation at the onset of COVID-19 symptoms (or at a positive polymerase chain reaction test result), multiples (yes or no), maternal age at positive COVID-19 test result, language competence (communication possible with or without problems), nicotine or smoking during pregnancy (yes or no), parity (0 or ≥1), and hypertensive disorders in pregnancy (yes or no)
Maternal BMI before or at the beginning of pregnancy (kg/m2)
P<.05.
Supplemental Table 4.
Associations among diabetes mellitus status (gestational diabetes mellitus vs no diabetes mellitus), body mass index (underweight or normal weight vs overweight vs obese), and secondary endpoints
| Variable | n (column %) | Crude or only BMI (aOR [95% CI])a | Multivariable (aOR [95% CI])b |
|---|---|---|---|
| Mother in ICU | |||
| Model 1 | |||
| GDM | |||
| No GDM or preexisting DM | 1334 (90.7) | Ref | Ref |
| GDM, no DM | 137 (9.3) | 2.00 (1.07–3.76)c | 1.84 (0.95–3.56) |
| BMId | |||
| Underweight or normal weight (<25 kg/m2) | 795 (54.0) | Ref | Ref |
| Overweight or obese (≥25 kg/m2) | 676 (46.0) | 3.15 (1.80–5.50)c | 2.41 (1.36–4.30)c |
| Model 2 | |||
| No GDM or DM, underweight or normal weight | 760 (51.7) | Ref | Ref |
| No GDM or DM, overweight or obese | 575 (39.0) | 3.30 (1.82–5.97)c | 2.54 (1.37–4.68)c |
| GDM, underweight or normal weight | 25 (2.4) | 2.82 (0.62–12.77) | 2.64 (0.56–12.53) |
| GDM, overweight or obese | 102 (6.9) | 6.20 (2.84–13.52)c | 4.34 (1.91–9.87)c |
| Oxygen supplementation (mother) | |||
| Model 1 | |||
| GDM | |||
| No GDM or preexisting DM | 1335 (90.6) | Ref | Ref |
| GDM, no DM | 138 (9.4) | 1.58 (0.92–2.70) | 1.49 (0.85–2.62) |
| BMId | |||
| Underweight or normal weight (<25 kg/m2) | 796 (54.0) | Ref | Ref |
| Overweight or obese (≥25 kg/m2) | 677 (46.0) | 2.18 (1.46–3.24)c | 1.74 (1.15–2.64)c |
| Model 2 | |||
| No GDM or DM, underweight or normal weight | 761 (51.7) | Ref | Ref |
| No GDM or DM, overweight or obese | 574 (39.0) | 2.30 (1.51–3.50)c | 1.85 (1.20–2.87)c |
| GDM, underweight or normal weight | 35 (2.4) | 2.45 (0.82–7.31) | 2.42 (0.77–7.57) |
| GDM, overweight or obese | 103 (7.0) | 3.24 (1.71–6.13)c | 2.42 (1.23–4.76)c |
| Viral pneumonia (mother) | |||
| Model 1 | |||
| GDM | |||
| No GDM or preexisting DM | 1323 (90.7) | Ref | Ref |
| GDM, no DM | 136 (9.3) | 1.63 (0.89–2.95) | 1.54 (0.82–2.88) |
| BMId | |||
| Underweight or normal weight (<25 kg/m2) | 792 (54.3) | Ref | Ref |
| Overweight or obese (≥25 kg/m2) | 667 (45.7) | 2.17 (1.38–3.40)c | 1.68 (1.05–2.69)c |
| Model 2 | |||
| No GDM or DM, underweight or normal weight | 758 (52.0) | Ref | Ref |
| No GDM or DM, overweight or obese | 565 (38.7) | 2.28 (1.42–3.67)c | 1.77 (1.08–2.90)c |
| GDM, underweight or normal weight | 34 (2.3) | 2.43 (0.70–8.42) | 2.31 (0.63–8.41) |
| GDM, overweight or obese | 102 (7.0) | 3.35 (1.65–6.8)c | 2.46 (1.17–5.18)c |
| Hypertensive disorders in pregnancy | |||
| Model 1 | |||
| GDM | |||
| No GDM or preexisting DM | 1335 (90.6) | Ref | Ref |
| GDM, no DM | 138 (9.4) | 1.85 (1.06–3.24)c | 1.78 (1.01–3.15)c |
| BMId | |||
| Underweight or normal weight (<25 kg/m2) | 796 (54.0) | Ref | Ref |
| Overweight or obese (≥25 kg/m2) | 677 (46.0) | 3.06 (1.91–4.9)c | 3.14 (1.94–5.08)c |
| Model 2 | |||
| No GDM or DM, underweight or normal weight | 761 (51.7) | Ref | Ref |
| No GDM or DM, overweight or obese | 574 (39.0) | 2.87 (1.76–4.69)c | 2.97 (1.8–4.89)c |
| GDM, underweight or normal weight | 35 (2.4) | 0.87 (0.11–6.58) | 0.89 (0.12–6.81) |
| GDM, overweight or obese | 103 (7.0) | 5.82 (3.02–11.21)c | 5.74 (2.92–11.29)c |
| Cesarean delivery | |||
| Model 1 | |||
| GDM | |||
| No GDM or preexisting DM | 1192 (90.2) | Ref | Ref |
| GDM, no DM | 130 (9.8) | 1.13 (0.78–1.65) | 1.06 (0.73–1.56) |
| BMId | |||
| Underweight or normal weight (<25 kg/m2) | 709 (53.6) | Ref | Ref |
| Overweight or obese (≥25 kg/m2) | 613 (46.4) | 1.71 (1.36–2.15)c | 1.68 (1.32–2.13)c |
| Model 2 | |||
| No GDM or DM, underweight or normal weight | 678 (51.3) | Ref | Ref |
| No GDM or DM, overweight or obese | 514 (38.9) | 1.75 (1.38–2.22)c | 1.73 (1.35–2.21)c |
| GDM, underweight or normal weight | 31 (2.3) | 1.40 (0.67–2.93) | 1.43 (0.68–3.03) |
| GDM, overweight or obese | 99 (7.5) | 1.84 (1.20–2.83)c | 1.67 (1.07–2.59)c |
| Neonatal ICU admission | |||
| Model 1 | |||
| GDM | |||
| No GDM or preexisting DM | 1148 (90.3) | Ref | Ref |
| GDM, no DM | 124 (9.8) | 1.19 (0.74–1.90) | 1.12 (0.68–1.82) |
| BMId | |||
| Underweight or normal weight (<25 kg/m2) | 683 (53.7) | Ref | Ref |
| Overweight or obese (≥25 kg/m2) | 589 (46.3) | 1.95 (1.43–2.66)c | 1.86 (1.34–2.58)c |
| Model 2 | |||
| No GDM or DM, underweight or normal weight | 653 (51.3) | Ref | Ref |
| No GDM or DM, overweight or obese | 495 (38.9) | 2.06 (1.49–2.85)c | 1.99 (1.42–2.80)c |
| GDM, underweight or normal weight | 30 (2.4) | 1.96 (0.77–4.94) | 2.12 (0.83–5.41) |
| GDM, overweight or obese | 94 (7.4) | 2.11 (1.22–3.67)c | 1.85 (1.04–3.29)c |
| Small for gestational age | |||
| Model 1 | |||
| GDM | |||
| No GDM or preexisting DM | 1021 (90.0) | Ref | Ref |
| GDM, no DM | 113 (10.0) | 1.54 (0.73–3.25) | 1.50 (0.70–3.19) |
| BMId | |||
| Underweight or normal weight (<25 kg/m2) | 619 (54.6) | Ref | Ref |
| Overweight or obese (≥25 kg/m2) | 515 (45.4) | 0.44 (0.26–0.74)c | 0.45 (0.27–0.77)c |
| Model 2 | |||
| No GDM or DM, underweight or normal weight | 590 (52.0) | Ref | Ref |
| No GDM or DM, overweight or obese | 431 (38.0) | 0.45 (0.26–0.78)c | 0.47 (0.27–0.82)c |
| GDM, underweight or normal weight | 29 (2.6) | 1.66 (0.56–4.94) | 1.68 (0.55–5.07) |
| GDM, overweight or obese | 84 (7.4) | 0.65 (0.25–1.69) | 0.64 (0.24–1.68) |
| Large for gestational age | |||
| Model 1 | |||
| GDM | |||
| No GDM or preexisting DM | 1021 (90.0) | Ref | Ref |
| GDM, no DM | 113 (10.0) | 3.10 (1.86–5.16)c | 3.13 (1.87–5.26)c |
| BMId | |||
| Underweight or normal weight (<25 kg/m2) | 619 (54.6) | Ref | Ref |
| Overweight/obese (≥25 kg/m2) | 515 (45.4) | 1.78 (1.16–2.73)c | 1.71 (1.10–2.66)c |
| Model 2 | |||
| No GDM or DM, underweight or normal weight | 590 (52.0) | Ref | Ref |
| No GDM or DM, overweight or obese | 431 (38.0) | 1.71 (1.07–2.73)c | 1.66 (1.03–2.67)c |
| GDM, underweight or normal weight | 29 (2.6) | 2.54 (0.84–7.69)c | 2.66 (0.87–8.13) |
| GDM, overweight or obese | 84 (7.4) | 5.63 (3.11–10.19)c | 5.46 (2.96–10.07)c |
| Model 3 | |||
| No GDM or DM, underweight or normal weight | 590 (52.0) | Ref | Ref |
| No GDM or DM, overweight or obese | 431 (38.0) | 1.71 (1.07–2.73)c | 1.66 (1.03–2.66)c |
| GDM without insulin, underweight or normal weight | 21 (1.9) | 2.64 (0.74–9.40) | 2.84 (0.79–10.28) |
| GDM without insulin, overweight or obese | 40 (3.5) | 6.01 (2.78–13.04)c | 6.16 (2.79–13.59)c |
| GDM with insulin, underweight or normal weight | 8 (0.7) | 2.27 (0.27–18.93) | 2.20 (0.26–18.76) |
| GDM with insulin, overweight or obese | 44 (3.9) | 5.29 (2.46–11.34)c | 4.89 (2.23–10.72)c |
Data are presented as aOR (95% CI) using logistic regression analyses with secondary endpoints (yes or no) as the dependent variable (separate model for each).
aOR, odds ratio; BMI, body mass index; CI, confidence interval; DM, diabetes mellitus; GDM, gestational diabetes mellitus; ICU, intensive care unit; Ref, reference interval.
Kleinwechter. Gestational diabetes mellitus and COVID-19: COVID-19–Related Obstetric and Neonatal Outcome Study registry results. Am J Obstet Gynecol 2022.
Model 1 adjusted for BMI only and models 2 and 3 unadjusted
Additionally adjusted for week of gestation at the onset of COVID-19 symptoms (or at a positive polymerase chain reaction test result), multiples (yes or no), maternal age at positive COVID-19 test result, language competence (communication possible with or without problems), nicotine or smoking during pregnancy (yes or no), parity (0 or ≥1), and hypertensive disorders in pregnancy (yes or no). Models with viral pneumonia as the dependent variable were not adjusted for nicotine or smoking during pregnancy as a quasi-complete separation of data points was observed
P<.05
Maternal BMI before or at the beginning of pregnancy (kg/m2).
Supplementary Data
Kleinwechter. Gestational diabetes mellitus and COVID-19: COVID-19–Related Obstetric and Neonatal Outcome Study registry results. Am J Obstet Gynecol 2022.
References
- 1.Zambrano L.D., Ellington S., Strid P., et al. Update: characteristics of symptomatic women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status - United States, January 22-October 3, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1641–1647. doi: 10.15585/mmwr.mm6944e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allotey J., Stallings E., Bonet M., et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ. 2020;370:m3320. doi: 10.1136/bmj.m3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li M.F., Ke J.F., Ma L., et al. Maternal pre-pregnancy obesity combined with abnormal glucose metabolism further increases adverse pregnancy outcomes in Chinese pregnant women. Front Endocrinol (Lausanne) 2021;12:754406. doi: 10.3389/fendo.2021.754406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lan J., Ge J., Yu J., et al. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature. 2020;581:215–220. doi: 10.1038/s41586-020-2180-5. [DOI] [PubMed] [Google Scholar]
- 5.Shekhar S., Wurth R., Kamilaris C.D.C., et al. Endocrine conditions and COVID-19. Horm Metab Res. 2020;52:471–484. doi: 10.1055/a-1172-1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vasbinder A., Anderson E., Shadid H., et al. Inflammation, hyperglycemia, and adverse outcomes in individuals with diabetes mellitus hospitalized for COVID-19. Diabetes Care. 2022;45:692–700. doi: 10.2337/dc21-2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sathish T., Kapoor N., Cao Y., Tapp R.J., Zimmet P. Proportion of newly diagnosed diabetes in COVID-19 patients: a systematic review and meta-analysis. Diabetes Obes Metab. 2021;23:870–874. doi: 10.1111/dom.14269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barrett C.E., Koyama A.K., Alvarez P., et al. Risk for newly diagnosed diabetes >30 days after SARS-CoV-2 infection among persons aged <18 years - United States, March 1, 2020-June 28, 2021. MMWR Morb Mortal Wkly Rep. 2022;71:59–65. doi: 10.15585/mmwr.mm7102e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.IDF Diabetes Atlas Diabetes around the world in 2021. 2021. https://www.diabetesatlas.org Available at:
- 10.Wang H., Li N., Chivese T., et al. IDF Diabetes Atlas: estimation of global and regional gestational diabetes mellitus prevalence for 2021 by International Association of Diabetes in Pregnancy Study Group’s Criteria. Diabetes Res Clin Pract. 2021;183:109050. doi: 10.1016/j.diabres.2021.109050. [DOI] [PubMed] [Google Scholar]
- 11.Metz T.D., Clifton R.G., Hughes B.L., et al. Disease severity and perinatal outcomes of pregnant patients with coronavirus disease 2019 (COVID-19) Obstet Gynecol. 2021;137:571–580. doi: 10.1097/AOG.0000000000004339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DeSisto C.L., Wallace B., Simeone R.M., et al. Risk for stillbirth among women with and without COVID-19 at delivery hospitalization - United States, March 2020-September 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1640–1645. doi: 10.15585/mmwr.mm7047e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jering K.S., Claggett B.L., Cunningham J.W., et al. Clinical characteristics and outcomes of hospitalized women giving birth with and without COVID-19. JAMA Intern Med. 2021;181:714–717. doi: 10.1001/jamainternmed.2020.9241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pecks U., Kuschel B., Mense L., Oppelt P., Rüdiger M., CRONOS Network Pregnancy and SARS-CoV-2 infection in Germany-the CRONOS registry. Dtsch Arztebl Int. 2020;117:841–842. doi: 10.3238/arztebl.2020.0841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sitter M., Pecks U., Rüdiger M., et al. Pregnant and postpartum women requiring intensive care treatment for COVID-19-first data from the CRONOS-registry. J Clin Med. 2022;11:701. doi: 10.3390/jcm11030701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mand N., Iannaccone A., Longardt A.C., et al. Neonatal outcome following maternal infection with SARS-CoV-2 in Germany: COVID-19-related Obstetric and Neonatal Outcome Study (CRONOS) Arch Dis Child Fetal Neonatal Ed. 2021 doi: 10.1136/archdischild-2021-322100. [Epub ahead or print] [DOI] [PubMed] [Google Scholar]
- 17.International Association of Diabetes and Pregnancy Study Groups Consensus Panel. Metzger B.E., Gabbe S.G., et al. International Association of Diabetes and Pregnancy Study Groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care. 2010;33:676–682. doi: 10.2337/dc09-1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schäfer-Graf U., Laubner K., Hummel S., et al. Gestational diabetes mellitus (GDM), diagnostics, therapy and follow-up care. Exp Clin Endocrinol Diabetes. 2021;129:S9–S19. doi: 10.1055/a-1284-6011. [DOI] [PubMed] [Google Scholar]
- 19.Wei S.Q., Bilodeau-Bertrand M., Liu S., Auger N. The impact of COVID-19 on pregnancy outcomes: a systematic review and meta-analysis. CMAJ. 2021;193:E540–E548. doi: 10.1503/cmaj.202604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crowther C.A., Hiller J.E., Moss J.R., et al. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. N Engl J Med. 2005;352:2477–2486. doi: 10.1056/NEJMoa042973. [DOI] [PubMed] [Google Scholar]
- 21.Landon M.B., Spong C.Y., Thom E., et al. A multicenter, randomized trial of treatment for mild gestational diabetes. N Engl J Med. 2009;361:1339–1348. doi: 10.1056/NEJMoa0902430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eskenazi B., Rauch S., Iurlaro E., et al. Diabetes mellitus, maternal adiposity, and insulin-dependent gestational diabetes are associated with COVID-19 in pregnancy: the INTERCOVID study. Am J Obstet Gynecol. 2021 doi: 10.1016/j.ajog.2021.12.032. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hillier T.A., Pedula K.L., Ogasawara K.K., et al. A pragmatic, randomized clinical trial of gestational diabetes screening. N Engl J Med. 2021;384:895–904. doi: 10.1056/NEJMoa2026028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davis E.M., Abebe K.Z., Simhan H.N., et al. Perinatal outcomes of two screening strategies for gestational diabetes mellitus: a randomized controlled trial. Obstet Gynecol. 2021;138:6–15. doi: 10.1097/AOG.0000000000004431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Powe C.E., Allard C., Battista M.C., et al. Heterogeneous contribution of insulin sensitivity and secretion defects to gestational diabetes mellitus. Diabetes Care. 2016;39:1052–1055. doi: 10.2337/dc15-2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hiersch L., Shah B.R., Berger H., et al. Oral glucose tolerance test results in pregnancy can be used to individualize the risk of future maternal type 2 diabetes mellitus in women with gestational diabetes mellitus. Diabetes Care. 2021;44:1860–1867. doi: 10.2337/dc21-0659. [DOI] [PubMed] [Google Scholar]
- 27.Sun S., Savitz D.A., Wellenius G.A. Changes in adverse pregnancy outcomes associated with the COVID-19 pandemic in the United States. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.29560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zanardo V., Tortora D., Sandri A., Severino L., Mesirca P., Straface G. COVID-19 pandemic: impact on gestational diabetes mellitus prevalence. Diabetes Res Clin Pract. 2022;183:109149. doi: 10.1016/j.diabres.2021.109149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu Y., Dai M., Tang S. Effect of initial COVID-19 outbreak during first trimester on pregnancy outcome in Wuxi, China. BMC Pregnancy Childbirth. 2022;22:54. doi: 10.1186/s12884-022-04395-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ma Q., Liu J., Liu Q., et al. Global percentage of asymptomatic SARS-CoV-2 infections among the tested population and individuals with confirmed COVID-19 diagnosis: a systematic review and meta-analysis. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.37257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ackerman C.M., Nguyen J.L., Ambati S., et al. Clinical and pregnancy outcomes of coronavirus disease 2019 among hospitalized pregnant women in the United States. Open Forum Infect Dis. 2021;9:ofab429. doi: 10.1093/ofid/ofab429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dagan N., Barda N., Biron-Shental T., et al. Effectiveness of the BNT162b2 mRNA COVID-19 vaccine in pregnancy. Nat Med. 2021;27:1693–1695. doi: 10.1038/s41591-021-01490-8. [DOI] [PubMed] [Google Scholar]
- 33.Barda N., Dagan N., Cohen C., et al. Effectiveness of a third dose of the BNT162b2 mRNA COVID-19 vaccine for preventing severe outcomes in Israel: an observational study. Lancet. 2021;398:2093–2100. doi: 10.1016/S0140-6736(21)02249-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grünebaum A., Bornstein E., Katz A., Chervenak F.A. Worsening risk profiles of out-of-hospital births during the COVID-19 pandemic. Am J Obstet Gynecol. 2022;226:137–138. doi: 10.1016/j.ajog.2021.11.1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Regan A.K., Arah O.A., Sullivan S.G. Performance of diagnostic coding and laboratory testing results to measure COVID-19 during pregnancy and associations with pregnancy outcomes. Paediatr Perinat Epidemiol. 2022 doi: 10.1111/ppe.12863. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Kleinwechter. Gestational diabetes mellitus and COVID-19: COVID-19–Related Obstetric and Neonatal Outcome Study registry results. Am J Obstet Gynecol 2022.