Abstract
Background
Inefficiency of in vitro fertilization (IVF) programs can be caused by implantation failures. The uterine microbiota can influence the implantation process. However, it still remains unclear whether opportunistic microorganisms detected in the endometrium have a negative impact on the implantation success. The aim of our study was to evaluate the influence of the uterine microbiota on the embryo implantation success in patients undergoing assisted reproductive technologies.
Methods
The study included 130 women diagnosed with infertility. The patients were divided into three groups: group I included women with the first IVF attempt (n = 39); group II included patients with recurrent implantation failure following embryo transfer with ovarian stimulation (n = 27); group III consisted of women with recurrent implantation failure following frozen-thawed embryo transfer (n = 64). We performed microbiological examination of the embryo transfer catheter which was removed from the uterine cavity after embryo transfer; cervical discharge of all the patients was studied as well. Thirty patients were selected for metagenomic sequencing.
Results
The study showed that the uterine cavity is not free of microorganisms. A total of 44 species of microorganisms were detected: 26 species of opportunistic organisms and 18 species of commensals (14 species of lactobacilli and 4 species of bifidobacteria). Obligate anaerobic microorganisms and Gardnerella vaginalis were detected more frequently in group I compared to group III (strict anaerobes—15.4 and 1.6%; G. vaginalis—12.8 and 1.6%, respectively) (p < 0.05). However, this fact did not have a negative influence on the pregnancy rate: it was 51.3% in group I, it was 29.6% and 35.9% in women with recurrent implantation failures, respectively.
Conclusion
Opportunistic microorganisms which were revealed in low or moderate titers (103–105 CFU/ml) in the uterine cavity and cervical canal did not affect the pregnancy rate in the women in the study groups. The microflora of the uterine cavity and cervical canal differed in qualitative composition in 87.9% of patients, therefore, we can suggest that the uterine cavity may form its own microbiota. The microbiota of the uterine cavity is characterized by fewer species diversity compared to the microbiota of the cervical canal.
Keywords: Infertility, Assisted reproductive technologies, Uterine microbiota, Metagenomic sequencing, Embryo implantation
Background
Overcoming infertility in patients with recurrent implantation failures (RIF) is a highly topical issue in reproductive medicine. RIF—failure to achieve pregnancy after multiple IVF attempts [1]. A favorable outcome of assisted reproductive technologies (ART) programs depends on a coordinated dialogue between the receptive endometrium and a high-quality embryo [1, 2].
Uterine microbiota and its influence on implantation success have been the subject of recent research. Microbiota are a group of microorganisms which are present in a human biotope and coexist symbiotically with the host organism. Due to the intensive study of the human microbiome, the role of the uterine microbiota during embryo transfer has been much emphasized [3–9].
To date, it still remains unclear whether opportunistic microorganisms detected in the endometrium have a negative impact on implantation, what composition of the uterine microbiota is considered to be normal and which one is associated with dysbiosis having an adverse effect on implantation, and where is the line between the norm and pathology in quantitative and qualitative ratio [10–13].
Therefore, the aim of the study was to evaluate the effect of the uterine microbiota on the success of implantation, the course of early pregnancy, and rate of live births in women undergoing IVF.
Methods
The study included 130 reproductive-aged patients who underwent treatment at the National Medical Research Center for Obstetrics, Gynecology and Perinatology, Moscow, Russia. All of them complained of infertility.
The participants of the study met the following inclusion criteria: age from 23 to 37 years, regular menstrual cycle, absence of endometrial pathology confirmed by the ultrasound assessment, transfer of good quality embryos.
The exclusion criteria were the presence of interstitial and/or subserous uterine fibroids measuring more than 4 cm which could deform the uterine cavity, stage III–IV endometriosis, intrauterine pathology (intrauterine septum, endometrial polyp, chronic endometritis), severe pathozoospermia of a partner.
The patients were divided into three groups: group I consisted of women with the first IVF attempt (n = 39); group II included patients with recurrent implantation failure (RIF) following embryo transfer with ovarian stimulation (n = 27); group III consisted of women with RIF following frozen-thawed embryo transfer (n = 64).
Microbiological examination of the cervical microbiota of all women was performed before embryo transfer to the uterine cavity. The vaginal part of the cervix was cleansed with a sterile cotton swab to exclude contamination of the cervical canal by the vaginal microflora. The material was collected from the cervical canal using Dacron swab and placed into test tubes with Ames transport medium (Medical Wire, Great Britain). The composition of the microbiota was studied using culturomics and with a wide variety of selective and non-selective culture media. Cervical canal discharge was inoculated for selective and non-selective agar media. After embryo transfer into the uterine cavity, the distal fragment of the embryo catheter was cut off with sterile scissors and placed in a test tube with culture media (1 ml) used for hemocultures (Oxoid, Great Britain). After 48 h of cultivation in an anaerobic box (Jouan, France) filled with a three-component gas mixture (N2-80%; CO2-10%; H2-10%), the composition of the microbiota was identified by culturomics.
Isolated microorganisms were identified using time-of-flight mass spectrometry (MALDI-TOF MS) with an Autoflex II mass spectrometer and Maldi BioTyper software, version 3.0 (Bruker Daltoniks, Germany).
All women had a selective single embryo transfer. Patients of groups I and II underwent ovarian hyperstimulation with gonadotropin-releasing hormone (GnRH) antagonists from days 2–3 of the menstrual cycle. Standard dose of human chorionic gonadotropin (10,000 IU) was used as an ovulation trigger.
Frozen-thawed embryo transfer was carried out in a natural ovulatory cycle without hormone replacement therapy. After embryo transfer, dydrogesterone (30 mg/day) was administered orally from the day of transvaginal puncture of the ovaries in hyperstimulation cycle. There was a comparative analysis of the results obtained in women both in the ovarian hyperstimulation cycles and in frozen-thawed oocyte cycles, depending on pregnancy or its absence.
At the second stage of the study, 30 samples of biomaterial from the uterine cavity (a fragment of an embryo transfer catheter in the culture medium) were selected for next-generation sequencing with a subsequent comparison with microbiological data.
Variable regions V3-V4 were chosen to determine the species composition of the uterine microbiota using next-generation sequencing. Amplification of the selected region was performed with primers 357F and 806R; DNA extraction from samples was performed using PREP-RAPID DNA Extraction Kit, and amplification of the 16S rRNA gene sequence was carried out using the DTprime detecting thermocycler (“DNA-Technology, Research & Production”, LLC, Russia). The quality of the obtained amplicons was evaluated in a 2% agarose gel. The quality and concentration of DNA libraries for next-generation sequencing were checked using a bioanalyzer device. Sequencing was performed using the Illumina MiSeq system (USA) v2 kits according to the manufacturer’s protocol. Data analysis was conducted using the QIMME software package.
Results
We performed a quantitative and qualitative analysis of the composition of the cervical canal microflora in women of the study groups.
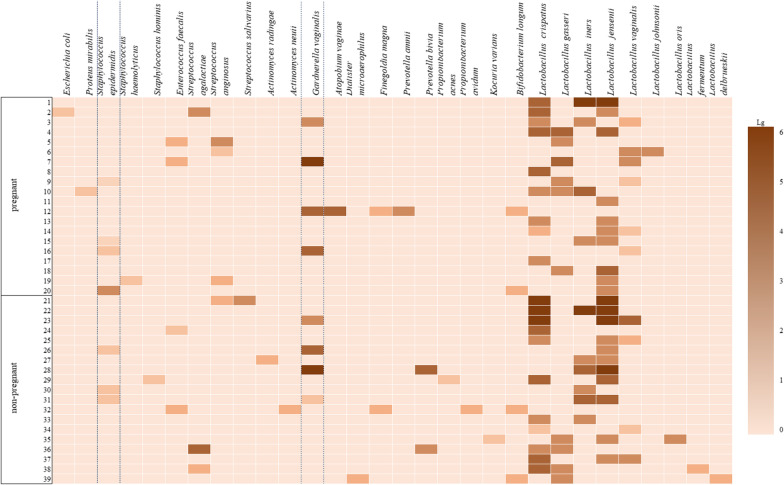
We revealed 30 species of opportunistic microorganisms in patients with the first IVF attempt, namely, 20 species of opportunistic microorganisms and 10 species of commensals. Lactobacilli were most often detected in moderate (104–105 CFU/ml) or large (106 CFU/ml or more) amounts more often in women who became pregnant than in those who did not conceive (90.0 and 73.7%, respectively), but there was no statistically significant difference (p > 0.05). Opportunistic microorganisms were identified mainly in low titers (103 CFU/ml or less). The exceptions were Gardnerella and obligate anaerobic microorganisms. For example, moderate or high titers of G. vaginalis were mainly detected in pregnant women (moderate titers—15%, high titers—5%) and in women who did not become pregnant (high titers—5.3%, moderate titers—10.6%). On the contrary, obligate anaerobic microorganisms were detected more frequently in low (15.8%) or moderate titers (10.5%) in women who did not conceive; pregnant women showed the same level of low and moderate titers (5% each), but there was no statistically significant difference either. Other opportunistic microorganisms, such as enterococci, enterobacteria and actinomycetes, colonized the cervical canal only in low concentrations. The level of microbial colonization taking into account the frequency of occurrence and quantitative assessment of microorganisms in women of group I is shown in Fig. 1.
Fig. 1.
Quantitative evaluation of microorganisms in patients with the first IVF attempt
The patients of group I who were pregnant or did not become pregnant showed no statistically significant difference in the frequency of detection of lactobacilli and opportunistic microorganisms in low, moderate or high concentrations in the cervical canal. Frequent colonization with lactobacilli in moderate or high concentrations, as well as frequent detection of opportunistic microorganisms (G. vaginalis) in moderate or high titers in patients of group I who became pregnant, did not significantly affect the implantation of the embryo.
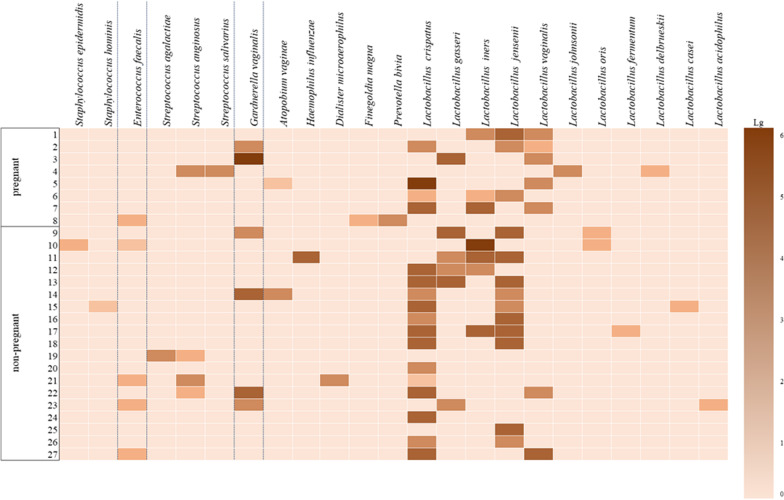
Among 23 species of microorganisms revealed in women with RIF following embryo transfer with ovarian stimulation (group II), there were 12 species of opportunistic microorganisms and 11 species of commensals, lactobacilli. Lactobacilli in moderate or large amounts were more often detected in women who did not become pregnant than in pregnant women (63.2 and 50.0%, respectively) in patients of group II compared to patients of group I, but the results were not statistically significant (p > 0.05). Opportunistic microorganisms were cultured in low, moderate or high concentrations. Therefore, enterococci and coagulase-negative staphylococci were detected only in low concentrations; streptococci in both subgroups were cultured mainly in moderate concentrations and dominated in the group of pregnant women (12.5 and 5.3%, respectively). Gardnerella was found in both subgroups in moderate concentrations more often in women who were not pregnant (21.1%), and in high concentration in one pregnant woman (12.5%); strict anaerobes in low and moderate titers were also isolated more frequently in pregnant women (12.5 and 10.5%, respectively). The level of microbial colonization taking into account the frequency of occurrence and quantitative evaluation of microorganisms in women of group II is shown in Fig. 2.
Fig. 2.
Quantitative evaluation of microorganisms in patients with RIF following embryo transfer and ovarian stimulation
The patients of group II who had RIF following embryo transfer with ovarian stimulation showed a lower frequency of detected lactobacilli, a lower degree of their contamination of the cervical canal and more frequent colonization of the cervical canal by opportunistic microorganisms (obligate anaerobes) in low and moderate concentration. However, there was no negative influence on the implantation and we may suggest that the presence of microorganisms in the cervical canal, which is anatomically as close as possible to the uterine cavity, in low and moderate concentrations does not affect the implantation and the beginning of pregnancy.
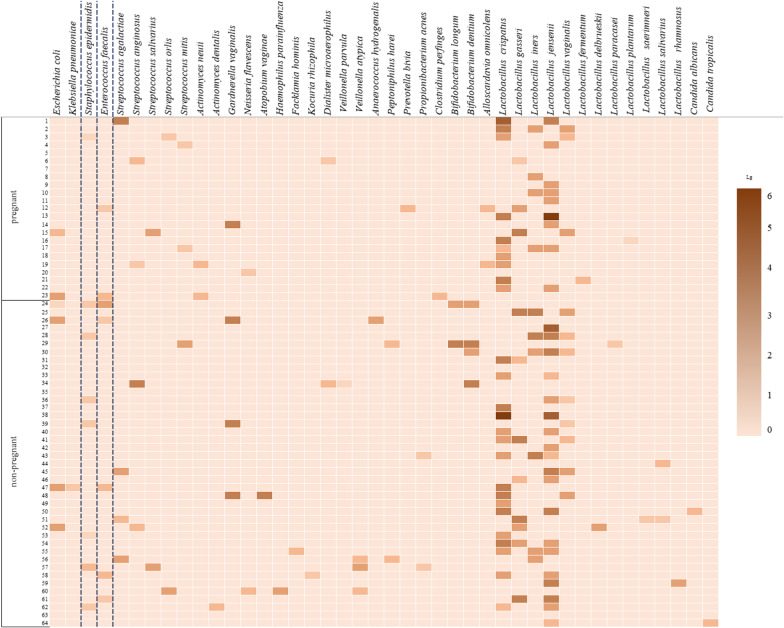
A total of 42 species were identified in patients with RIF following frozen-thawed embryo transfer (group III), namely, 25 species of opportunistic microorganisms and 15 species of commensals, 2 species of yeast fungi. Lactobacilli were most often revealed in moderate or large concentrations in women of group III, more frequently in women who had pregnancy than in women who did not become pregnant (60.9% and 51.2%, respectively), but no statistically significant difference was found (p > 0.05). Opportunistic microorganisms were detected mainly in low concentrations, except Gardnerella which was found in both subgroups in moderate concentrations (4.3% and 7.3%, respectively). Strict anaerobes were detected in pregnant women only in low concentrations (13.0%), women who did not conceive showed the same frequency in low and moderate titers (9.75%). Coagulase-negative staphylococci and enterococci were almost always present in low concentrations.
There was no statistically significant difference in the frequency of colonization of the cervical canal by the microorganisms in low and, what is more important, in moderate concentrations between pregnant and non-pregnant women (p > 0.05). The level of microbial colonization taking into account the frequency of occurrence and quantitative evaluation of microorganisms in women of group III is shown in Fig. 3.
Fig. 3.
Quantitative evaluation of microorganisms in patients with RIF following frozen-thawed embryo transfer
The patients with RIF following frozen-thawed embryo transfer who did not conceive were less likely to have colonization of the cervical canal with lactobacilli in high or moderate concentrations than those who became pregnant. On the contrary, opportunistic microorganisms (streptococci, enterobacteria, and especially strict anaerobes and Gardnerella) were revealed in moderate concentrations. However, due to the absence of a statistically significant difference in these indicators, we cannot consider the decrease in the titer of lactobacilli, as well as the increase in the number of opportunistic microorganisms to moderate values as a risk factor for implantation failure.
After the comparative analysis of the cervical canal microorganisms in women of the study groups, we found that the most common microorganisms of the cervical canal in all groups of women were lactobacilli, which were slightly more frequent in women with embryo transfer with ovarian stimulation (groups I and II) compared to women with frozen-thawed embryo transfer (group III), but statistically significant difference was not noted. Two species of lactobacilli, L. jensenii and L. crispatus, prevailed in the frequency of colonization in all groups, but women of groups I and II had a certain predominance (p > 0.05). The cervical canal was colonized only by lactobacilli in almost 40.0% of women of all groups (33.3%; 44.4% and 36.0%, respectively), mainly by associations of two or three species. Opportunistic microorganisms were found in every second woman in all groups (with a slight predominance in group I). In all groups, there was no statistically significant difference in the colonization of the cervical canal with facultative and obligate anaerobes. G. vaginalis was revealed more frequently in women of groups I (20.5%) and II (22.2%) compared to group III (6.3%), but the difference was statistically insignificant (p > 0.05).
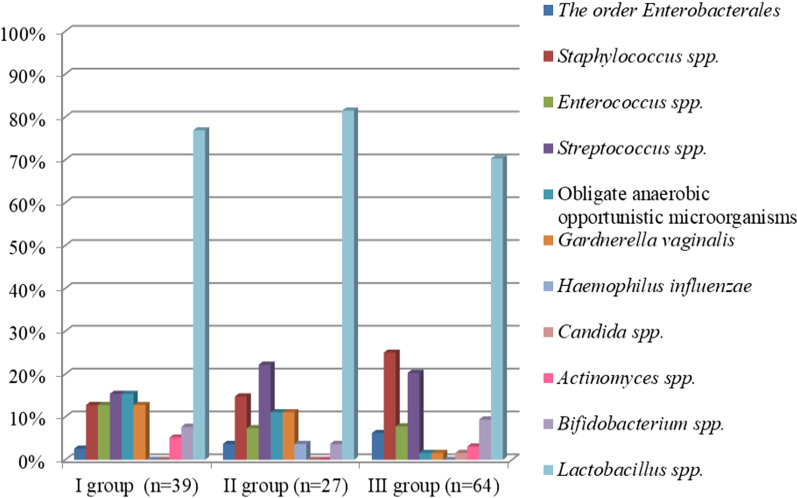
Uterine cavity aspirate of the patients was analyzed as well. The results of a comparative analysis of the uterine microbiota in the groups of women are shown in Fig. 4.
Fig. 4.
Comparative analysis of the frequency of detecting microorganisms in the uterine cavity in study groups. Note I–III G. vaginalis (p = 0.05); I–III obligate anaerobic opportunistic microorganisms (p = 0.02)
Culture-based analysis of the content of the uterine cavity in patients of all three groups showed the presence of microflora in 89.2% of cases. The uterine cavity in women of groups I, II and III was found to be sterile only in 10.3%, 7.4% and 12.5% of cases, respectively. The species of microorganisms isolated from the uterine cavity were diverse. Lactobacilli (14 species) were dominant in all groups, three of them were more frequent, namely L. jensenii, L. crispatus and L. vaginalis. The frequency of isolation of L. jensenii in the study groups was 48.7%, 51.9% and 40.6%, respectively. The isolation rate of L. crispatus was 35.9%, 25.9% and 31.3%, respectively. The frequency of occurrence of L. vaginalis in women of the study groups was 2.6%, 11.1% and 9.4%. There was no statistically significant difference in the frequency of colonization of the uterine cavity by lactobacilli and in isolation rate of individual types among patients of the study groups (p > 0.05). Isolation rate of opportunistic microorganisms was the same in patients of groups I, II, and III (46.1, 44.4 and 48.4%, respectively (p > 0.05)).
Enterobacteria were detected more often in pregnant women with RIF following frozen-thawed embryo transfer (group III) (17.4%) compared to the women who became pregnant after the first IVF attempt (group I) (5%) and women with RIF following embryo transfer with ovarian stimulation (group II) (12.5%). Staphylococci were detected more frequently in patients of group III (25.0%) in comparison with patients of group I (12.9%) and group II (14.8%). Streptococci were revealed more frequently in patients with RIF in groups II (22.2%) and III (20.3%) compared to women of group I with IVF attempt (15.4%). It is worth noting that isolation rate of obligate anaerobic microorganisms in women with the first IVF attempt was 15.4% compared to patients with RIF following frozen-thawed embryo transfer whose rate was 1.6%. The frequency of detection of G. vaginalis in women with the first IVF attempt was 12.8% and it was 1.6% in women with RIF following frozen-thawed embryo transfer. Thus, isolation rate of obligate anaerobic microorganisms and G. vaginalis was statistically significantly higher in group I compared to group III (p = 0.02, p = 0.05, respectively).
Metagenomic study of 30 samples of uterine cavity aspirate was carried out. The most common bacterial genera (the occurrence is higher than 5.0%) are presented according to the sequencing of a fragment of the 16S ribosomal RNA gene.
An extremely low concentration of bacterial DNA was found in all samples of the sterile uterine cavity compared to the concentration of DNA in negative control samples. Microorganisms of the genera Lactobacillus, Tepidimonas, Streptococcus, Proteus, Acinetobacter, Enterococcus, Pseudomonas, Veillonella were detected in these samples; such genera were also detected in negative control samples.
In most cases, the data on representation of microorganisms in the uterine cavity obtained after microbiological studies were consistent with the data obtained after sequencing. In some cases, microbiological studies failed to detect some bacterial genera that were revealed by sequencing. These results can be explained by the presence of microorganisms which did not grow on the culture medium for various reasons, however their DNA was present in the sample.
The quantitative ratio of microorganisms was determined by sequencing, but these data should be considered carefully, since we studied the samples of the culture medium, but not the sample itself, and the ratio may depend on the growth rate of various microorganisms.
We conducted a comparative analysis of the microbiota of the uterine cavity and the cervical canal of 130 women. The analysis showed that the microbiota of the uterine cavity and the cervical canal was identical only in 14 (12.1%) of 116 women, and it was different in qualitative composition in 102 (87.9%) patients. When comparing the isolation rate of various components of the microbiota of the uterine cavity and the cervical canal, it was noted that a statistically significant difference was observed in the isolation rate of G. vaginalis (p = 0.01) and the most common lactobacillus species: L. crispatus (p = 0.01), L. gasseri (p = 0.01), L. vaginalis (p = 0.01) and L. iners (p = 0.01). As for the other species, the difference was not statistically significant (p > 0.05).
We analyzed the reproductive outcomes in women of the study groups (Table 1).
Table 1.
Characteristics of pregnancy outcomes in patients of the study groups
| Parameter | Group I (n = 39) | Group II (n = 27) | Group III (n = 64) |
|---|---|---|---|
| Pregnancy rate | 51.3% (n = 20) | 29.6% (n = 8) | 35.9% (n = 23) |
| Biochemical pregnancy | 5.0% (n = 1) | 0% (n = 0) | 4.3% (n = 1) |
| Missed abortion | 0% (n = 0) | 12.5% (n = 1) | 17.4% (n = 4) |
| Spontaneous miscarriage | 10.0% (n = 2) | 0% (n = 0) | 4.3% (n = 1) |
| Ectopic pregnancy | 5.0% (n = 1) | 0% (n = 0) | 0% (n = 0) |
| Preterm birth | 5.0% (n = 1) | 12.5% (n = 1) | 0% (n = 0) |
| Full-term delivery | 75.0% (n = 15) | 75.0% (n = 6) | 73.9% (n = 17) |
Group I included patients with the first IVF attempt (n = 39); group II consisted of women with recurrent implantation failure following embryo transfer with ovarian stimulation (n = 27); group III included patients with recurrent implantation failure following frozen-thawed embryo transfer (n = 64)
According to the data presented in Table 1, the ratio of pregnancy rate to embryo transfer in patients of group I was 51.3% and it was 1.7 times higher than in women of group II (29.6%) and 1.4 times higher than in women of group III (35.9%), but the difference was not statistically significant (p > 0.05). The analysis of pregnancy outcomes in the study groups showed the absence of missed abortions in patients with the first IVF attempt (0%), but this indicator was 12.5% and 17.4% in patients with RIF, groups II and III, respectively. However, statistically significant difference was not found (p > 0.05). The ratio of full–time deliveries to the number of pregnancies was 75%, 75% and 73.9% in the groups, respectively.
Discussion
The importance of uterine microbiota in embryo transfer has recently received a lot of attention in reproductive medicine. There is no consensus regarding the sterility of the uterine cavity. And there is still no clear understanding of the influence of the endometrial microflora on the implantation processes. Moreover, it is not obvious what refers to eubiotic state of microbial ecology and what is dysbiotic disorder, as there is no clear line between these two concepts. The detection of opportunistic microorganisms in the reproductive tract and antibacterial therapy frequently fail to result in success [14–16].
The results of our study demonstrated that 87.7% of women had uterine microflora. Microbial communities can be found in the uterine cavity and the results of our study are consistent with the results of other authors [17–24].
The research conducted by Moreno et al. [17] demonstrated the influence of the uterine microbiota on implantation. It was revealed that the group of patients with a decreased percentage of Lactobacilli (< 90%) and prevalent opportunistic microorganisms (> 10%) in the uterine cavity in comparison with the group with prevalent Lactobacilli (> 90%) had a significantly lower implantation rate (60.7% vs. 23.1%, p = 0.02), pregnancy rate (70.6% vs. 33.3%, p = 0.03), progressing pregnancy (58.8% vs. 13.3%, p = 0.02) and birth rate (58.8% vs. 6.7%, p = 0.02).
The study of Kyono [25, 26] presented the analysis of the pregnancy rate in patients with prevalent Lactobacilli (Lactobacilli > 80%) and in patients without dominance of Lactobacilli (Lactobacilli < 80% and opportunistic microorganisms > 20%) in the uterine cavity. Pregnancy rate was higher in women with prevalent Lactobacilli (> 80%) compared to patients without dominant Lactobacilli (< 80%), 61.3% vs. 40%, respectively.
The opposite evidence is provided by the studies of other authors. Kotaro et al. [27] showed that the predominance of Lactobacilli in the uterine cavity (> 90%) was observed more often in women with RIF—64.3% (18/28) than in the control group—38.9% (7/18). The analysis of the vaginal samples showed similar results: Lactobacilli dominated in women with RIF—67.9% (19/28), unlike patients with the first IVF attempt—44.4% (8/18). Isolation rate of Gardnerella was 39.3% (11/28) in women with RIF and 27.7% (5/18) in the control group.
Thus, the researchers tend to focus on pathogenetically significant interaction between the endometrial microbiota and its immunity, and not just confirmation of the presence of microorganisms in the endometrium.
The results of our study are consistent with the results of other researchers [23–26]. There is no clear evidence that the presence of dominant Lactobacilli in the uterine cavity are beneficial in terms of pregnancy outcomes. But the restoration of the uterine microbiota with the aim of the predominance of Lactobacilli can have a positive effect on embryo implantation. The presence of opportunistic microorganisms in the uterine cavity did not affect the pregnancy rate.
Probably, uterine microbiota is a set of functionally associated microorganisms [24]. Certain microorganisms of the uterine cavity obviously support its homeostasis in every healthy individual. Biofilms, which are microbial communities, play an important role. Bacteria in biofilms have certain physiological properties. Normal biofilms in the human body are represented by microbial communities that form the physiological microflora of the skin, oral cavity, vagina, intestines, etc. But there are also pathological biofilms, which are often associated with chronic inflammatory processes.
The qualitative composition of microbiota of the uterine cavity and cervical canal was different in 87.9% of patients, therefore, one cannot exclude the formation of an independent microbiota in the uterine cavity, which is characterized by less species diversity compared to the cervical canal.
There is still no clear opinion about the composition of the microbiota of the upper and lower genital tracts. Moreno et al. found that microbial composition of the vagina and endometrium was different in 20% of the examined samples [17, 20]. Differences between these biotopes were found by Wee et al., who compared the uterine microbiota with vaginal and cervical samples in fertile and infertile women [28]. These results indicate that the microbiota of the upper and lower parts of the reproductive tract may be similar, but not always identical.
A separate stage of the study was the metagenomic sequencing of the uterine microbiota (the distal part of the embryo transfer catheter in the culture medium). This method of studying microbiota is described in the studies of some researchers [21, 22]. In most cases, the microorganism diversity in the uterine cavity was the same according to microbiological studies and metagenomic analysis. In some cases, there were bacterial genera that were not identified with microbiological studies. The results can be explained by the presence of microorganisms in the sample; these microorganisms did not grow in the culture media for various reasons, but their DNA was present in the sample. The obtained data may be due to the presence of trace amounts of bacterial DNA in the reagents and indicate that sequencing method is not sufficiently specific for analyzing samples with an extremely low DNA concentration. It can be assumed that besides the presence of microorganisms in the uterine cavity, a quantitative assessment of the composition of the microflora is important; however, it can be problematic to carry out the assessment at the time of embryo transfer due to insufficient amount of material obtained with an embryo transfer catheter.
Therefore, the use of this method for assessing the microflora of the uterine cavity at the time of embryo transfer remains questionable. The use of sequencing may be appropriate for a comprehensive assessment of the endometrial microbiota and its receptivity when obtaining biological material using a pipelle biopsy, as the material has a larger biomass than in case of embryo transfer catheter.
The condition of the whole organism is also essential since the endometrium can be protected against the destructive effect of the microbial factor. Microorganisms are known to be inactivated by cells involved in innate and adaptive immunity responses under physiological conditions [29]. In the early stages, this process is carried out by Toll-, NOD-, and RIG-like receptors which are located on the surface of cells of the immune system, epithelial cells, endothelial cells, and fibroblasts [29]. Microbial colonization of the endometrium is also restrained by Toll-like receptors, which stimulate the mechanisms of innate antimicrobial resistance by interacting with the structures of the microbial cell [30].
We analyzed the reproductive outcomes in women included in the study. The ratio of pregnancy rate to embryo transfer in patients of group I was maximal (51.3%), compared to the patients with RIF (29.6% and 35.9%, respectively). In spite of the fact that isolation rate of obligate anaerobic microorganisms and G. vaginalis was statistically significantly higher in group I compared to group III (strict anaerobes—15.4 and 1.6%; G. vaginalis—12.8 and 1.6%, respectively) (p = 0.02; p = 0.05), it did not negatively influence the implantation process in this group of women. The rate of missed abortions was 12.5% and 17.4% in patients with RIF (groups II and III, respectively), but there were no cases of missed abortions among women with the first IVF attempt. The ratio of full–term deliveries to the number of pregnancies was 75%, 75% and 73.9% in the groups, respectively.
Conclusion
The most common microorganisms in the composition of the microbiota of the uterine cavity were lactobacilli in women who became pregnant and who were not pregnant: lactobacilli were 80.0% and 73.7% in group I, respectively; 90.2% and 79.0% in group II, and 60.9% and 75.6% in group III. The results of the study do not support the hypothesis about the role of lactobacilli and non-lactobacilli microbiota in the genesis of embryo implantation failures.
The comparative data on the composition of the microbiota of the uterine cavity and the cervical canal confirm the concept of non-sterility of the uterus and the existence of microbiota which is different from the microbiota of the lower parts of the reproductive tract. The qualitative composition and isolation rate of various types of microorganisms found in the uterus and cervical canal in the general cohort of women were identical only in 12.1% of women, and they differed in 87.9% of women.
The isolation rate of opportunistic microorganisms from the uterine cavity and the cervical canal, as well as the quantitative composition of their detection in the cervical canal in moderate concentrations (up to 105 CFU/ml) among pregnant and non-pregnant women did not have statistically significant differences and did not affect the outcomes of ART in the study groups.
Therefore, taking into account the lack of statistical data confirming the influence of certain uterine microorganisms on the reproductive outcome, it is necessary to continue studying uterine microbiota. It is important to consider not only the present microorganisms, but also the individual characteristics of the whole organism, as well as the interaction of microorganisms with the organism. A clear understanding of the mechanisms of dysbiotic disorders leading to chronic inflammatory processes in the uterine cavity, as well as its timely diagnosis and treatment, is one of the fundamental issues in the treatment of patients with a history of implantation failures.
Acknowledgements
Not applicable.
Abbreviations
- IVF
In vitro fertilization
- RIF
Recurrent implantation failure
- ART
Assisted reproductive technologies
- CFU
Colony forming units
- GnRH
Gonadotropin-releasing hormone
Author contributions
The authors developed the design of the study, collected the material, analyzed, interpreted, processed the data, and wrote the text of the manuscript. All authors read and approved the final manuscript.
Funding
The study has been performed within the experimental scientific research “Development of an integrated approach to the prediction, prevention and correction of dysbiotic disorders of the intestinal and vaginal microbiota and pathologies provoked by them in women and newborns” AAAA-A20-120022790038-4.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Declarations
Ethics approval and consent to participate
The study was approved by the Ethical Review Board of National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov, Moscow, Russia (Protocol No.9—16/09/2017). The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. Informed consent was obtained from all participants.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
The original online version of this article was revised: the reference no. 23 has been updated.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
5/15/2023
A Correction to this paper has been published: 10.1186/s12905-023-02302-6
References
- 1.Savelyeva GM, Sukhikh GT, Serov VN, Radzinsky VE, Manukhin IB. Gynecology. National guidelines (Rus). 2nd ed. Moscow: Geotar-Media, 2019.
- 2.Unanyan AL, Sidorova IS, Kogan EA, Belogubova SYu, Demura TA, Elisavetskaya AM, Sizova NM. Endometriosis, adenomyosis, chronic endometritis: clinical and pathogenetic relationships and reproductive failures. Obstet Gynecol (Rus). 2018;(10):136–40.
- 3.Perez-Muñoz ME, Arrieta MC, Ramer-Tait AE, et al. A critical assessment of the “sterile womb” and “in utero colonization” hypotheses: implications for research on the pioneer infant microbiome. Microbiome. 2017;5:48. doi: 10.1186/s40168-017-0268-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.D’Ippolito S, Di Nicuolo F, Pontecorvi A, Gratta M, Scambia G, Di Simone N. Endometrial microbes and microbiome: recent insights on the inflammatory and immune players” of the human endometrium. Am J Reprod Immunol. 2018;80(6):e13065. doi: 10.1111/aji.13065. [DOI] [PubMed] [Google Scholar]
- 5.Benner M, Ferwerda G, Joosten I, van der Molen RG. How uterine microbiota might be responsible for a receptive, fertile endometrium. Hum Reprod Update. 2018;24(4):393–415. [DOI] [PubMed]
- 6.Walther-Antonio MR, Chen J, Multinu F, Hokenstad A, Distad TJ, Cheek EH, Keeney GL, Creedon DJ, Nelson H, Mariani A, et al. Potential contribution of the uterine microbiome in the development of endometrial cancer. Genome Med. 2016;8:122. doi: 10.1186/s13073-016-0368-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Franasiak JM, Alecsandru D, Forman EJ, Gemmell LC, Goldberg JM, Llarena N, Margolis C, Laven J, Schoenmakers S, Seli E. A review of the pathophysiology of recurrent implantation failure. Fertil Steril. 2021;116(6):1436–1448. doi: 10.1016/j.fertnstert.2021.09.014. [DOI] [PubMed] [Google Scholar]
- 8.Mlodzik N, Lukaszuk K, Sieg W, Jakiel G, Smolarczyk R. Endometrial microbiota—Do they mean more than we have expected? Ginekol Pol. 2020;91(1):45–48. doi: 10.5603/GP.2020.0010. [DOI] [PubMed] [Google Scholar]
- 9.Salliss ME, Farland LV, Mahnert ND, Herbst-Kralovetz MM. The role of gut and genital microbiota and the estrobolome in endometriosis, infertility and chronic pelvic pain. Hum Reprod Update. 2021;28(1):92–131. doi: 10.1093/humupd/dmab035. [DOI] [PubMed] [Google Scholar]
- 10.Chen P, Chen P, Guo Y, Fang C, Li T. Interaction between chronic endometritis caused endometrial microbiota disorder and endometrial immune environment change in recurrent implantation failure. Front Immunol. 2021;4(12):748447. doi: 10.3389/fimmu.2021.748447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sezer O, Soyer Çalışkan C, Celik S, Kilic SS, Kuruoglu T, Unluguzel Ustun G, Yurtcu N. Assessment of vaginal and endometrial microbiota by real-time PCR in women with unexplained infertility. J Obstet Gynaecol Res. 2022;48(1):129–139. doi: 10.1111/jog.15060. [DOI] [PubMed] [Google Scholar]
- 12.Diaz-Martínez MDC, Bernabeu A, Lledó B, Carratalá-Munuera C, Quesada JA, Lozano FM, Ruiz V, Morales R, Llácer J, Ten J, Castillo JC, Rodríguez A, Nouni-García R, López-Pineda A, Moliner B, Bernabeu R. Impact of the vaginal and endometrial microbiome pattern on assisted reproduction outcomes. J Clin Med. 2021;10(18):4063. doi: 10.3390/jcm10184063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Toson B, Simon C, Moreno I. The endometrial microbiome and its impact on human conception. Int J Mol Sci. 2022;23(1):485. doi: 10.3390/ijms23010485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miles SM, Hardy BL, Merrell DS. Investigation of the microbiota of the reproductive tract in women undergoing a total hysterectomy and bilateral salpingo-oopherectomy. Fertil Steril. 2017;107:813–820.e81. doi: 10.1016/j.fertnstert.2016.11.028. [DOI] [PubMed] [Google Scholar]
- 15.Chen C, Song X, Wei W, Zhong H, Dai J, Lan Z, Li F, Yu X, Feng Q, Wang Z, et al. The microbiota continuum along the female reproductive tract and its relation to uterine-related diseases. Nat Commun. 2017;8:875. doi: 10.1038/s41467-017-00901-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fang R-L, Chen L-X, Shu W-S, Yao S-Z, Wang S-W, Chen Y-Q. Barcoded sequencing reveals diverse intrauterine microbiomes in patients suffering with endometrial polyps. Am J Transl Res. 2016;8:1581–1592. [PMC free article] [PubMed] [Google Scholar]
- 17.Moreno I, Franasiak JM. Endometrial microbiota—new player in town. Fertil Steril. 2017;108(1):32–39. doi: 10.1016/j.fertnstert.2017.05.034. [DOI] [PubMed] [Google Scholar]
- 18.Franasiak JM, Scott RT. Endometrial microbiome. Curr Opin Obstet Gynecol 2017; 29:46–152. [DOI] [PubMed]
- 19.Mitchell CM, Haick A, Nkwopara E, Garcia R, Rendi M, Agnew K, Fredricks DN, Eschenbach D. Colonization of the upper genital tract by vaginal bacterial species in nonpregnant women. Am J Obstet Gynecol 2015;212(5):611-e1. [DOI] [PMC free article] [PubMed]
- 20.Moreno I, Codoñer FM, Vilella F, Valbuena D, Martinez-Blanch JF, Jimenez-Almazán J, Alonso R, Alamá P, Remohí J, Pellicer A, Ramon D, Simon C. Evidence that the endometrial microbiota has an effect on implantation success or failure. Am J Obstet Gynecol. 2016;215(6):684–703. doi: 10.1016/j.ajog.2016.09.075. [DOI] [PubMed] [Google Scholar]
- 21.Tao X, Franasiak JM, Zhan Y, Scott RT, Rajchel J, Bedard J, Newby R, Scott RT, Treff NR, Chu T. Characterizing the endometrial microbiome by analyzing the ultra-low bacteria from embryo transfer catheter tips in IVF cycles: next generation sequencing (NGS) analysis of the 16 S ribosomal gene. Hum Microbiome J. 2017;3:15–21. doi: 10.1016/j.humic.2017.01.004. [DOI] [Google Scholar]
- 22.Franasiak JM, Werner MD, Juneau CR, Tao X, Landis J, Zhan Y, Treff NR, Scott RT. Endometrial microbiome at the time of embryo transfer: next-generation sequencing of the 16 S ribosomal subunit. J Assist Reprod Genet 2016;33:129–136). [DOI] [PMC free article] [PubMed]
- 23.Einenkel R, Zygmunt M, Muzzio DO. Microorganisms in the healthy upper reproductive tract: from denial to beneficial assignments for reproductive biology. Reprod Biol 2019;19(2):113–118. 10.1016/j.repbio.2019.04.001. Epub 2019 Apr 22. PMID: 31023521. [DOI] [PubMed]
- 24.Verstraelen H, Vilchez-Vargas R, Desimpel F, Jauregui R, Vankeirsbilck N, Weyers S, Verhelst R, De Sutter P, Pieper DH, Van De Wiele T. Characterisation of the human uterine microbiome in non-pregnant women through deep sequencing of the V1–2 region of the 16 S rRNA gene. PeerJ 2016;4:e1602. [DOI] [PMC free article] [PubMed]
- 25.Kyono K, Hashimoto T, Kikuchi S, Nagai Y, Sakuraba Y. A pilot study and case reports on endometrial microbiota and pregnancy outcome: an analysis using 16S rRNA gene sequencing among IVF patients, and trial therapeutic intervention for dysbiotic endometrium. Reprod Med Biol. 2018;18(1):72–82. [DOI] [PMC free article] [PubMed]
- 26.Hashimoto T, Kyono K. Does dysbiotic endometrium affect blastocyst implantation in IVF patients? J Assist Reprod Genet. 2019;36:2471–2479. doi: 10.1007/s10815-019-01630-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kitaya K, Nagai Y, Arai W, Sakuraba Y, Ishikawa T. Characterization of Microbiota in Endometrial Fluid and Vaginal Secretions in Infertile Women with Repeated Implantation Failure. Hindawi Mediat Inflam. 2019;21(2019):4893437. doi: 10.1155/2019/4893437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wee BA, Thomas M, Sweeney EL, Frentiu FD, Samios M, Ravel J, et al. A retrospective pilot study to determine whether the reproductive tract microbiota differs between women with a history of infertility and fertile women. Aust N Z J Obstet Gynaecol. 2018;58:341. doi: 10.1111/ajo.12754. [DOI] [PubMed] [Google Scholar]
- 29.Benjelloun F, Quillay H, Cannou C, Marlin R, Madec Y, Fernandez H, Chrétien F, Le Grand R, Barré-Sinoussi F, Nugeyre MT, Menu E. Activation of toll-like receptors differentially modulates inflammation in the human reproductive tract: preliminary findings. Front Immunol. 2020;4(11):1655. doi: 10.3389/fimmu.2020.01655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kharayat NS, Sharma GC, Kumar GR, Bisht D, Chaudhary G, Singh SK, Das GK, Garg AK, Kumar H, Krishnaswamy N. Differential expression of endometrial toll-like receptors (TLRs) and antimicrobial peptides (AMPs) in the buffalo (Bubalus bubalis) with endometritis. Vet Res Commun. 2019;43(4):261–269. doi: 10.1007/s11259-019-09761-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.