Abstract
In a two-part process, we assessed elements of the principal hormonal pathway regulating iron homeostasis in human neonates. Part 1: Quantifying erythropoietin (Epo), erythroferrone (ERFE), hepcidin, and relevant serum and erythrocytic iron-related metrics in umbilical cord blood from term (n = 13) and preterm (n = 10) neonates, and from neonates born to mothers with diabetes and obesity (n = 13); Part 2: Quantifying serum Epo, ERFE, and hepcidin before and following darbepoetin administration. Part 1: We measured Epo, ERFE and hepcidin in all cord blood samples. Epo and ERFE levels did not differ between the three groups. Preterm neonates had the lowest hepcidin levels, while neonates born to diabetic women with a very high BMI had the lowest ferritin and RET-He levels. Part 2: Following darbepoetin dosing, ERFE levels generally increased (p < 0.05) and hepcidin levels generally fell (p < 0.05). Our observations suggest that the Epo/ERFE/hepcidin axis is intact in the newborn period.
Keywords: Iron, Supplementation, RET-He, Erythropoietin, Erythroferrone, Hepcidin
1. Introduction
Iron deficiency in newborn infants can result in substantial and persistent neurocognitive dysfunction [1–3]. Consequently, efforts are needed to prevent, or to promptly and adequately treat, neonatal iron deficiency [4,5]. Enteral iron supplementation will not always prevent or treat neonatal iron deficiency: the success of enteral iron dosing depends, in part, on the integrity of the patient’s iron homeostatic mechanisms. Those mechanisms are well described in adults, but little is known about them in neonates [6–9].
The iron-regulatory hormonal axis includes erythropoietin (Epo), erythroferrone (ERFE), hepcidin, and ferroportin. In brief (Fig. 1) when Epo binds to cognate receptors on the surface of erythroid progenitors, erythrocytic clonal proliferation occurs. The Epo-stimulated erythroid progenitors rapidly produce ERFE and ERFE blood levels consequently rise [10]. High circulating levels of ERFE suppress hepcidin production by the liver [11]. Elevated hepcidin levels trigger degradation of ferroportin, the iron exporter, thereby inhibiting absorption of enteral iron and preventing mobilization of iron from storage [12,13]. In contrast, low hepcidin levels (seen with high ERFE levels) foster absorption of enteral iron through ferroportin-mediated iron transport [12–14].
Fig. 1.
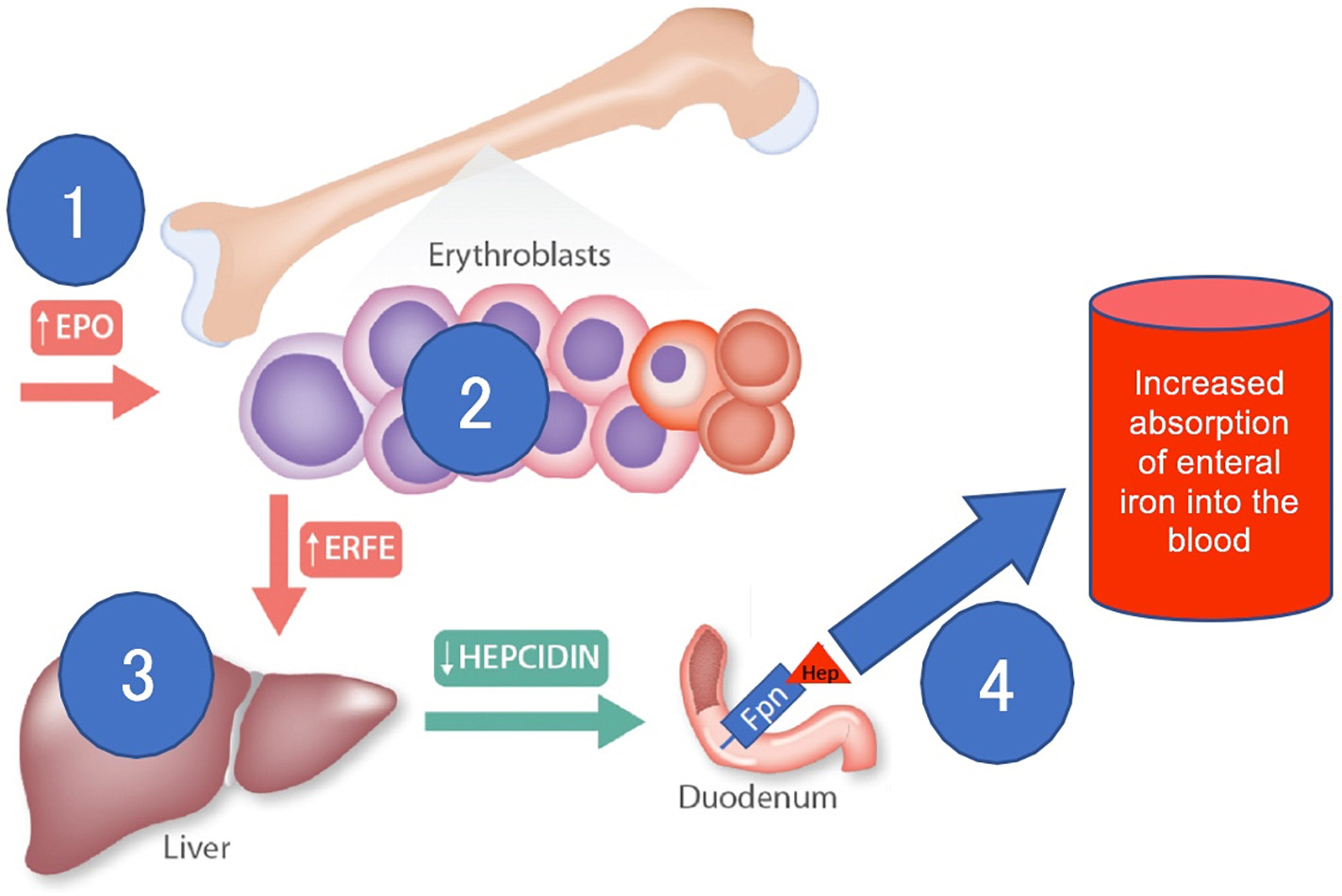
Schematic representation of the erythropoietin (EPO), erythroferrone (ERFE), hepcidin, ferroportin (Fpn) axis, as it pertains to the regulation of intestinal iron absorption. High EPO levels (1) stimulate erythroblasts to produce ERFE (2). High ERFE levels reduce hepcidin production in the liver (3). Low hepcidin levels facilitate absorption of enteral iron through ferroportin (4). Modified from Ganz et al. [23].
Assessing the integrity of this axis in neonates could fill a knowledge gap important to understanding perinatal iron biology. Immaturity or disruption in this axis with different disease states (such as preterm birth or maternal diabetes/obesity) might be mechanistically involved in iron deficiency in some neonates, and might be responsible for failure of enteral iron supplementation to adequately prevent or treat iron deficiency in others.
As a step toward filling this knowledge gap we performed a pilot study to quantify the serum levels of these regulatory molecules, along with iron, ferritin, and CBC parameters that reflect iron-limited erythropoiesis. We also assessed whether, after administering recombinant erythropoietin (darbepoetin) to neonates, their blood levels of the iron regulatory molecules change in the pattern seen in adults, thereby fostering an increase in absorption of enteral iron.
2. Materials and methods
The protocol was approved by the Institutional Review Board of Intermountain Healthcare and the study was performed in accordance with the Declaration of Helsinki. For the first part of the study, umbilical cord blood was drawn at birth using a deidentified approach, recording only whether the sample was from a healthy term delivery (≥37 weeks), a healthy preterm delivery (28–32 weeks), or from a pregnancy complicated by both obesity (BMI > 30) and diabetes (type 1, type 2, or gestational). “Healthy” was defined as the absence of maternal diabetes and maternal obesity in a normally grown neonate (birth weight 10 – 90th percentile [15]).
For the second part of the study, parents of NICU patients provided written informed consent for their child’s participation. Neonates were eligible for the study if they had an order from their clinical provider to receive darbepoetin at a dose of 10 micrograms/kg body weight. After obtaining parental consent, and within 24 h before the subcutaneous darbepoetin was given, blood was obtained to measure ERFE and hepcidin levels. Then, within a 24 to 72 h “window” following the darbepoetin dose, ERFE and hepcidin levels were repeated. Research nurses attempted to time this phlebotomy for when a clinically-ordered phlebotomy was needed, so as to reduce venipuncture for study only. Patient serum was transferred to a cryotube, labeled, and stored at −80 °C until the collection of all specimens was completed for batch-analysis.
Hepcidin and ERFE levels in serum were determined using Intrinsic Lifesciences Hepcidin IDx™ or Erythroferrone IE™ ELISA kits according to the manufacturer’s directions (Intrinsic Lifesciences, La Jolla, CA) [16]. Erythropoietin levels in cord blood serum were determined using the Human EPO/Erythropoietin ELISA kit according to the manufacturer’s directions (Sigma-Aldrich, St. Louis, MO). The CBCs were performed on Sysmex analyzers (Sysmex Americas, Inc., Lincolnshire, IL) in the Intermountain Healthcare clinical laboratory. Sysmex quality control procedures were performed daily as recommended by the manufacturer. Serum iron, serum ferritin, and total iron binding capacity were also performed by the Intermountain Healthcare clinical laboratory using standard operating procedures.
Study data were entered into REDCap for workflow and analysis. The gestational age field was populated by information from the infant’s medical record. All statistical analyses were done using the R statistical language and environment (R Foundation, Vienna, Austria).
3. Results
3.1. Umbilical cord blood
Features of the three groups of neonates are summarized in Table 1. The healthy term neonates and those born to obese/diabetic mothers were of similar gestational age and birth weight. Preterm infants were (by design) of earlier gestational age and consequently of lower birth weight than the other two groups. Mother’s BMIs were similar in the healthy term and the healthy preterm groups, but higher in those born to obese/diabetic mothers.
Table 1.
Umbilical cord blood findings. Features including iron-related results and CBC findings (mean ± SD, 95% CI, 2-sided t-test in black, non-parametric Wilcoxon rank sum test in bold).
| Healthy term (n = 13) | Obese/diabetic (n = 13) | Healthy preterm (n = 10) |
P values |
|||
|---|---|---|---|---|---|---|
| Term vs. O/D | Term vs. preterm | O/D vs. preterm | ||||
|
| ||||||
| Gestational age (wk/days) | 38 2/7 ± 1 2/7 | 38 1/7 ± 1 1/7 | 31 2/7 ± 1 6/7 | 0.578 | <0.001 | <0.001 |
| 37 5/7–39 0/7 | 37 3/7–38 5/7 | 30 1/7–32 2/7 | ||||
| Birth weight (gm) | 3236 ± 456 | 3837 ± 123 | 1695 ± 473 | 0.494 | <0.001 | <0.001 |
| 2988–3484 | 3771–3903 | 1402–1988 | ||||
| Mother’s BMI | 29.6 ± 7.0 | 42.1 ± 13.2 | 26.6 ± 4.2 | 0.010 | 0.167 | <0.001 |
| 25.5–33.7 | 35.0–49.2 | 23.5–29.7 | ||||
| Serum iron (μg/dL) | 219 ± 201 | 124 ± 63 | 101 ± 67 | 0.049 | 0.039 | 0.617 |
| 110–329 | 87–161 | 63–156 | ||||
| TIBC (μg/dL) | 216 ± 39 | 230 ± 68 | 191 ± 22 | 0.531 | 0.158 | 0.155 |
| 195–237 | 190–269 | 168–214 | ||||
| Serum ferritin (ng/mL) | 136 ± 49 | 152 ± 181 | 70 ± 69 | 0.368 | 0.030 | 0.246 |
| 110–162 | 11–258 | 22–118 | ||||
| Hgb (g/dL) | 15.6 ± 1.5 | 15.3 ± 1.2 | 17.7 ± 1.7 | 0.590 | 0.012 | 0.002 |
| 14.6–17.4 | 14.6–16.0 | 16.7–19.7 | ||||
| HCT (%) | 47.0 ± 5.1 | 46.1 ± 3.5 | 50.3 ± 5.1 | 0.637 | 0.177 | 0.043 |
| 44.0–50.7 | 44.1–48.1 | 47.2–53.4 | ||||
| MCV (fL) | 105.9 ± 2.9 | 107.8 ± 4.8 | 111.9 ± 8.3 | 0.295 | 0.045 | 0.180 |
| 104.1–107.7 | 105.0–110.6 | 106.8–117.0 | ||||
| MCH (pg) | 34.9 ± 1.5 | 35.7 ± 2.2 | 39.1 ± 3.9 | 0.335 | 0.003 | 0.023 |
| 34.0–35.8 | 34.4–37.0 | 36.7–41.5 | ||||
| Retics (%) | 4.3 ± 0.8 | 4.3 ± 0.9 | 6.8 ± 1.3 | 0.720 | <0.001 | <0.001 |
| 3.8–4.8 | 3.9–10.3 | 5.6–8.0 | ||||
| RET-He (pg) | 34.5 ± 2.2 | 33.2 ± 3.8 | 36.6 ± 9.6 | 0.406 | 0.016 | 0.038 |
| 33.0–35.8 | 31.0–35.4 | 35.9–38.0 | ||||
| Erythropoietin (mU/mL) | 4.5 ± 6.0 | 18.5 ± 44.7 | 4.7 ± 6.1 | 0.298 | 0.826 | 0.749 |
| 1.3–7.7 | 0.0–53.0 | 1.0–7.4 | ||||
| Erythroferrone (ng/mL) | 1.6 ± 1.3 | 2.8 ± 4.1 | 1.0 ± 0.8 | 0.952 | 0.337 | 0.646 |
| 0.9–2.3 | 0.4–5.2 | 0.5–1.5 | ||||
| Hepcidin (ng/mL) | 70.8 ± 37.0 | 34.2 ± 21.3 | 30.5 ± 22.8 | 0.043 | 0.004 | 0.069 |
| 52.1–89.5 | 19.4–49.0 | 15.6–45.4 | ||||
Wk, week; gm, gram; TIBC, total iron binding capacity; Hgb, hemoglobin; HCT, hematocrit; MCV mean corpuscular volume; MCH, mean corpuscular hemoglobin; Retics, reticulocytes; RET—He, reticulocyte hemoglobin content; O/D, obese and diabetic.
Compared with the healthy term neonates, the groups born to obese/diabetic mothers or born prematurely had low serum iron levels. Ferritin levels were lower in the preterm than the term group. Among those born to obese/diabetic mothers, ferritin levels varied widely and were not parametrically distributed. Two neonates had levels less <10 ng/mL, had the lowest RET-He values, and were born to the mothers with the highest BMI values. The preterm group had higher MCV, MCH, reticulocyte count, and RET-He values than did the other groups.
All three iron-regulatory hormones were detected in umbilical cord blood samples. Hepcidin levels were lower in the preterm group than the term group. Fig. 2 compares umbilical cord blood Epo, ERFE, and Hepcidin values in the three groups. Serum hepcidin levels had a moderately strong correlation with serum ferritin levels (Pearson correlation r = 0.760; 95% C.I. 0.545–0.881; Fig. 2 panel D).
Fig. 2.
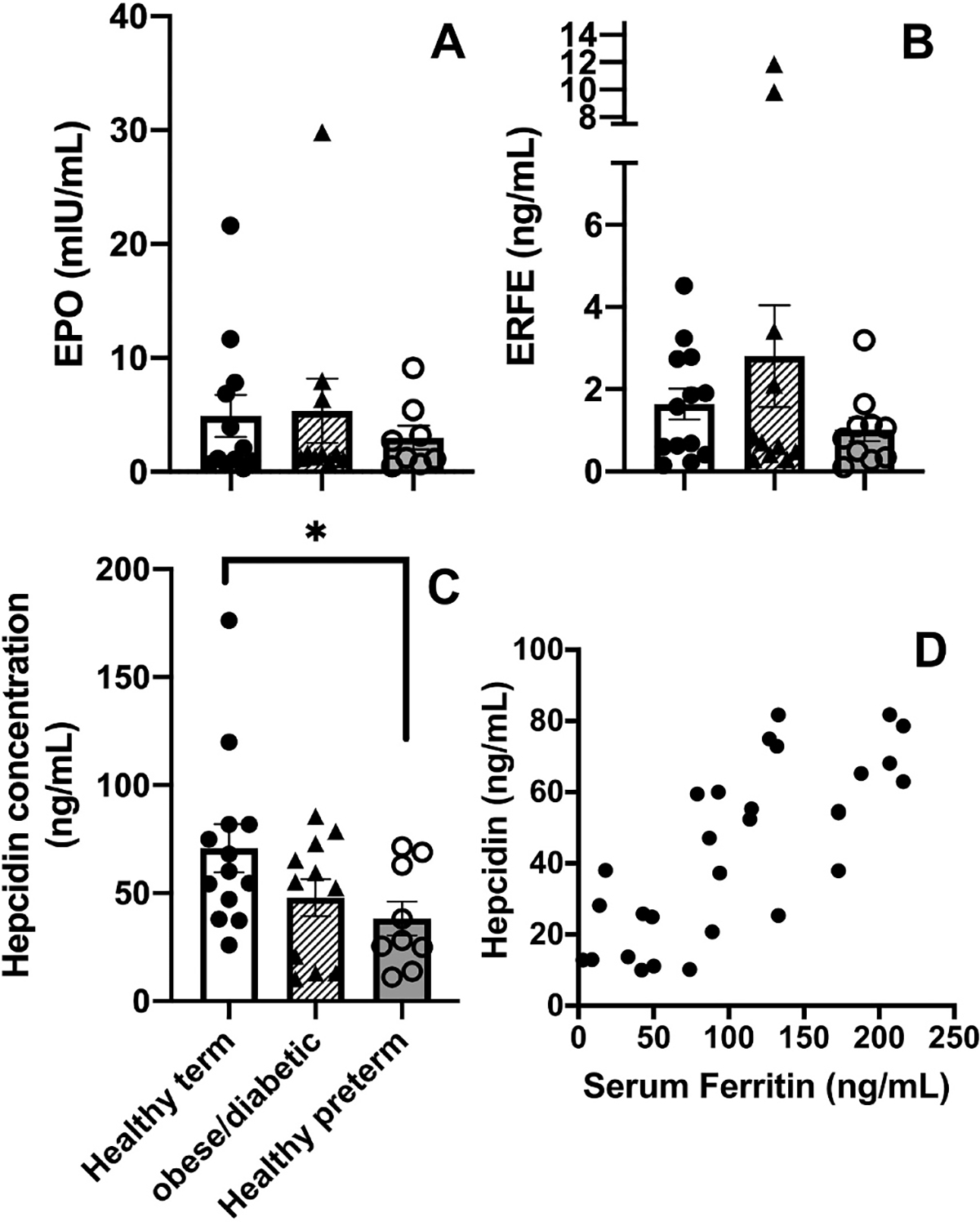
Erythropoietin (EPO), Erythroferrone (ERFE), and Hepcidin concentrations in umbilical cord blood of healthy term neonates, neonates born to obese/diabetic mothers, and healthy preterm neonates (panels A-C). Scatter plot of paired serum Ferritin and Hepcidin concentrations (panel D).
3.2. Before and after darbepoetin administration
Features of the darbepoetin recipients are shown in Table 2. All ten were very low birth weight (605 to 1460 g) and all had been born prematurely (23 6/7 to 30 3/7 weeks gestation). In eight of the ten, the darbepoetin dose was followed by an increase in serum ERFE level (as typically occurs in adult subjects). However, in the two with the highest ERFE levels before the darbepoetin dose (numbers 1 and 3 in Table 2), the ERFE level did not increase (also see Fig. 3 panel A). Patient number 1, who had the longest interval between darbepoetin dose and phlebotomy (141 h), was one of these two (Fig. 3).
Table 2.
Features and findings among study-neonates treated with darbepoetin.
| No. | Birth wt (g) | GA @ Birth (w/d) | Sex | Mother BMI | Postnatal age at Darbe dose (d) | Iron dosing before Darbe? | Darbe dose given (date/time) | Post-Darbe blood draw (date/time) | Interval between Darbe and blood draw (h) | Pre-Darbe Hgb (g/dL) | Pre-Darbe RET-He (pg) | Pre-Darbe ERFE (ng/mL) | Post-Darbe ERFE (ng/mL) | Pre-Darbe Hepcidin (ng/mL) | Post-Darbe Hepcidin (ng/mL) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||||
| 1 | 995 | 27 0 | F | 25.3 | 53 | Yes | 1/28/20 15:06 |
2/3/20 12:10 |
141 | 1/21/20 12.6 |
1/21/20 29.1 |
0.9 | 0.4 | 6.6 | 8.2 |
| 2 | 930 | 25 1 | M | 35.5 | 14 | Yes | 1/28/20 16:29 |
1/31/20 04:20 |
60 | 1/28/20 13.3 |
1/27/20 25.0 |
0.4 | 1.5 | 69.8 | 48.8 |
| 3 | 1050 | 30 3 | F | 57.0 | 1 | No | 1/28/20 16:29 |
1/31/20 04:20 |
60 | 1/27/20 16.5 |
– | 3.4 | 2.1 | 6.6 | 12.6 |
| 4 | 945 | 25 3 | F | 34.4 | 21 | Yes | 2/4/20 13:08 |
2/6/20 04:00 |
39 | 2/6/20 13.1 |
2/3/20 25.6 |
0.3 | 0.4 | 212.5 | 167.1 |
| 5 | 1460 | 29 5 | F | 22.6 | 0 | No | 2/6/20 15:07 |
2/9/20 05:00 |
62 | 2/6/20 15.7 |
– | 0.2 | 0.3 | 125.0 | 47.4 |
| 6 | 1335 | 28 5 | F | 34.8 | 26 | Yes | 2/11/20 14:20 |
2/14/20 08:55 |
66 | – | 2/10/20 24.3 |
0.4 | 1.5 | 7.3 | 7.2 |
| 7 | 605 | 23 6 | F | 26.8 | 47 | Yes | 2/17/20 14:51 |
2/18/20 19:11 |
29 | 2/14/20 11.6 |
– | 0.2 | 0.3 | 29.9 | 14.1 |
| 8 | 690 | 23 6 | M | 26.8 | 47 | Yes | 2/17/20 15:33 |
2/18/20 07:16 |
16 | 2/18/20 10.3 |
– | 0.2 | 0.4 | 17.9 | 52.7a |
| 9 | 610 | 23 6 | F | 26.8 | 47 | Yes | 2/17/20 16:02 |
2/19/20 04:00 |
36 | 2/19/20 11.1 |
– | 0.1 | 0.2 | 26.0 | 19.8 |
| 10 | 945 | 25 3 | F | 34.4 | 42 | Yes | 2/25/20 14:00 |
2/26/20 05:20 |
15 | 2/24/20 12.8 |
2/10/20 31.7 |
0.3 | 0.5 | 178.7 | 88.6 |
No., number; Wt, weight; g, grams; GA, gestational age; w, weeks; d, days; h, hours; Hgb, hemoglobin.
Pneumonia developed between pre- and post-darbe phlebotomy (FiO2 increased to 100%, ventilator settings increased, x-ray consistent with pneumonia).
Fig. 3.
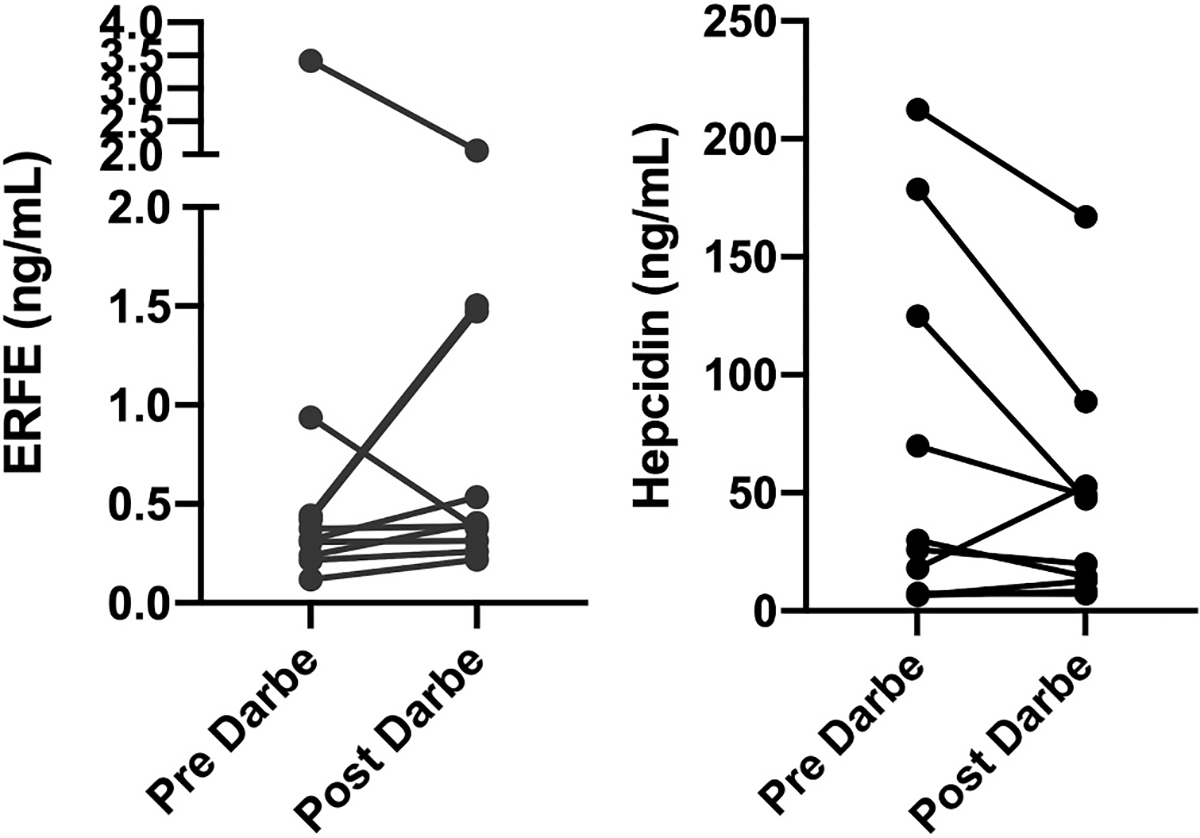
Erythroferrone (ERFE) and hepcidin serum levels before and following darbepoetin (Darbe) dosing (10 μg/kg subcutaneous). Individual values are shown by dots. A solid line connects the paired before vs. after darbepoetin administration.
In a similar manner, in seven of the ten, the darbepoetin dose was followed by a fall in serum hepcidin level. However, in the two with the highest ERFE levels and also the lowest hepcidin levels before the darbepoetin (numbers 1 and 3) the hepcidin level did not fall (Fig. 3 panel B). The hepcidin level also did not fall after darbepoetin dosing in patient eight. That patient developed pneumonia about the time of the darbepoetin dosing.
Three of the ten (numbers 4, 5, and 10) had pre-darbepoetin hepcidin levels much higher than the other seven. These three all had evidence of a respiratory infection and were being treated with intravenous antibiotics at the time of the darbepoetin dosing. Patient 4 (hepcidin 212.5 ng/mL) required mechanical ventilation and was treated with cephalexin for alpha hemolytic streptococci from a tracheal aspirate accompanying respiratory deterioration. Patient 5 (hepcidin 125.0 ng/mL) required mechanical ventilation and was being treated with ampicillin and gentamicin for presumed infection and an elevated CRP (1.4 mg/dL). Patient ten (hepcidin 178.7 ng/mL) was on mechanical ventilation treated with cephazolin for methicillin-sensitive Staphylococcus aureus respiratory infection.
4. Discussion
Iron sufficiency during the neonatal period is important for erythropoiesis, mitochondrial respiration, nucleic acid replication, immune function, and brain development [17]. Iron sufficiency is particularly crucial for neonates receiving treatment with recombinant Epo, because inadequate iron availability during accelerated erythropoiesis can deplete iron stores and precipitate multi-organ iron deficiency [18]. Moreover, due to the prioritization of iron stores to support erythropoiesis over the iron needs of other organs, it is possible that even moderate iron limitation could result in deficient brain iron [19]. Neonatal animal models suggest that deficient brain iron can cause neurological damage that persists even after the iron deficiency is corrected [20]. Consequently, avoiding iron deficiency in neonates who are receiving erythropoietin treatment is an important facet of assuring their optimal neurodevelopment.
In general, iron supplementation is provided to neonates in the form of enteral ferrous sulfate, or as part of a preparation of enteral multivitamins plus iron [21]. For enteral iron dosing to be effective, molecular iron in the bowel lumen must enter the circulation through ferroportin-mediated iron export from the basal surface of enterocytes [22]. Hepcidin is a key determinant of enteral iron absorption. Specifically, high levels of hepcidin bind to and degrade ferroportin, thereby impeding enteral iron absorption, while low levels of hepcidin have the opposite effect, promoting absorption of enteral iron. Consequently, appropriate regulation of the hepcidin level is critical to the success of enteral iron therapy in maintaining iron sufficiency.
The hormonal pathway regulating the hepcidin level after recombinant Epo dosing (or increased endogenous Epo production) is sometimes termed the erythropoietin-erythroferrone- hepcidin axis. The pilot data in our present study suggest that this axis is intact in most neonates. Specifically, the relevant hormones are present in cord blood in concentrations consistent with iron status, and after Epo dosing their serum levels change in the pattern described in adults [23]. Namely, cord blood hepcidin is lower in infants with evidence of low iron status (low ferritin and low iron). In most of the infants, after administering darbepoetin (a long-acting erythropoietin), ERFE increased and hepcidin decreased. This information generates some level of assurance that following recombinant Epo administration to neonates, their absorption of enterally administered iron should increase, thereby increasing their circulating iron levels.
We caution that our pilot data does not guarantee that enteral iron will be absorbed sufficiently well in all treated neonates so as to prevent iron deficiency. In fact, three recent reports indicate that iron deficiency can indeed develop in neonates during treatment with Epo despite enteral iron administration. Siddappa et al. followed a cohort of 116 extremely low birth weight (<1000 g) neonates who were receiving Epo three times per week. Additional iron was given if serum ferritin levels were low, yet 60% of the neonates had ferritin levels that fell to ≤75 mg/mL [18]. A low serum ferritin (<50 ng/mL) was present in 38% of Epo and Darbe recipients compared to 6% of placebo recipients in our previous randomized placebo controlled trial [24]. Despite the increased numbers of infants with low ferritin in the Epo and Darbe group, cognitive outcomes were significantly better in the Epo/Darbe group than the placebo group, underscoring the need to identify optimal iron dosing with Epo or Darbe therapy [25]. Recently, our group reported that iron deficiency, defined as a RET-He <25 pg, is present in 25% of neonates treated with darbepoetin and iron [26–28]. Similarly, in the PENUT trial, Epo treatment of neonates <28 weeks gestation significantly reduced red cell transfusions, however 38% of the Epo recipients dropped their ferritin level below 75 ng/mL [29,30]. Thus, clearly some neonates treated with Epo and enteral iron do not absorb sufficient iron to prevent iron deficiency. Perhaps in some of the Epo-treated neonates with impending iron deficiency, further increasing the enteral iron dose would be sufficient. However, if any have a high hepcidin level (due either to inflammation or disrupted regulatory mechanisms) increasing their enteral iron dose could fail because iron absorption is inhibited.
Murine experiments by Sangkhae et al. showed that high maternal hepcidin levels caused fetal iron deficiency anemia and severe growth restriction of the placenta and fetus. Moreover, the brains of fetuses with iron deficiency due to high hepcidin contained less iron and were significantly smaller than normal, suggesting that the fetal murine brain is particularly sensitive to decreases in the iron supply [8,9]. We previously reported higher than expected serum hepcidin levels in iron deficient mothers who delivered iron deficient neonates [31]. Similarly, Fisher et al. found elevated cord blood hepcidin levels, and low cord blood iron, when mothers had intraamniotic infections [32].
We recognize that the preliminary nature and small sample sizes of our pilot data require confirmation before conclusions are certain. However, our findings suggest an intact iron regulatory axis in the majority of neonates. There are clinical scenarios in neonates where this regulatory axis could inhibit adequate iron absorption. For example, hepcidin levels can increase during infection and inflammation, thus iron deficient infants with infections might not absorb enteral iron well. Indeed, we observed high hepcidin levels in three subjects with respiratory infection. There appears to be a strong effect of infection and/or inflammation on hepcidin regulation in neonates. We wonder whether it would be informative to measure hepcidin levels in neonates when increasing doses of enteral iron appear to be ineffective in correcting their iron deficiency. Perhaps intravenous iron dosing should be considered for neonates who have a high hepcidin level but require supplemental iron. We are currently conducting studies to evaluate that approach, with the goal of achieving iron sufficiency in all NICU patients.
Acknowledgements
The authors thank the Intermountain Healthcare Neonatal Research Nurses; Kimberlee Weaver Lewis, RN, Trisha A. Marchant, RN, Melody Parry, RN, Jennifer O. Elmont, RN, and Susan Christensen, RN. We also thank Molly Adams for assistance with the Institutional Review Board application and communications, Vickie L. Baer, RN for REDcap development.
Funding statement
The study was supported in part by grant U54DK110858 from the US Public Health Service, and by funds from the Department of Pediatrics, University of Utah Health, Salt Lake City, UT.
Footnotes
CRediT authorship contribution statement
Timothy M. Bahr: Conceptualization, Data curation, Formal analysis, Writing – review and edition. Diane M. Ward: Conceptualization, Methodology, Validation, Data curation, Supervision, Formal analysis, Writing – review and edition, Project administration, Funding acquisition. Xuan Jia: Data curation, review and edition. Robin K. Ohls: Formal analysis, Writing – review and edition. Kendell R. German: Formal analysis, Writing – review and edition. Robert D. Christensen: Conceptualization, Data curation, Supervision, Formal analysis, Writing original draft, Review and edition, Project administration, Funding acquisition.
Declaration of competing interest
The authors have no conflicts of interest to disclose relevant to this study.
References
- [1].Georgieff MK, The role of iron in neurodevelopment: fetal iron deficiency and the developing hippocampus, Biochem. Soc. Trans. 36 (2008) 1267–1271, 10.1042/BST0361267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Armony-Sivan R, Zhu B, Clark KM, et al. , Iron deficiency at both birth and 9 months predicts right frontal EEG asymmetry in infancy, Dev. Psychobiol. 58 (2016) 462–470, 10.1002/dev.21388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Georgieff MK, Iron assessment to protect the developing brain, Am. J. Clin. Nutr. 106 (2017) 1588S–1593S, 10.3945/ajcn.117.155846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Cusick SE, Georgieff MK, Rao R, Approaches for reducing the risk of early-life iron deficiency-induced brain dysfunction in children, Nutrients 17 (2018) 227, 10.3390/nu10020227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Geng F, Mai X, Zhan J, et al. , Timing of iron deficiency and recognition memory in infancy, Nutr. Neurosci. 7 (2020) 1–10, 10.1080/1028415X.2019.1704991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Cao C, Fleming MD, The placenta: the forgotten essential organ of iron transport, Nutr. Rev. 74 (2016) 421–431, 10.1093/nutrit/nuw009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Delaney KM, Guillet R, Fleming RE, et al. , Umbilical cord serum ferritin concentration is inversely associated with umbilical cord hemoglobin in neonates born to adolescents carrying singletons and women carrying multiples, J. Nutr. 149 (2019) 406–415, 10.1093/jn/nxy286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Sangkhae V, Nemeth E, Placental iron transport: the mechanism and regulatory circuits, Free Radic. Biol. Med. 133 (2019) 254–261, 10.1016/j.freeradbiomed.2018.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Sangkhae V, Fisher AL, Chua KJ, Ruchala P, Ganz T, Nemeth E, Maternal hepcidin determines embryo iron homeostasis, Blood 136 (2020) 2206–2216, 10.1182/blood.2020005745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Kautz L, Jung G, Valore EV, Rivella S, Nemeth E, Ganz T, Identification of erythroferrone as an erythroid regulator of iron metabolism, Nat. Genet. 46 (2014) 678–84 oi: 10.1038/ng.2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Coffey R, Ganz T, Erythroferrone: an erythroid regulator of hepcidin and iron metabolism, Hemasphere 28 (2018), e35, 10.1097/HS9.0000000000000035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Nemeth E, Tuttle MS, Powelson J, et al. , Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization, Science 306 (2004) 2090–2093, 10.1126/science.1104742. [DOI] [PubMed] [Google Scholar]
- [13].Ward De Domenico DM, Langelier C, et al. , The molecular mechanism of hepcidin-mediated ferroportin down-regulation, Mol. Biol. Cell. 18 (2007) 2569–2578, 10.1091/mbc.e07-01-0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Ward DM, Kaplan J, Ferroportin-mediated iron transport: expression and regulation, Biochim. Biophys. Acta 1823 (2012) 1426–1433, 10.1016/j.bbamcr.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Christensen RD, Henry E, Kiehn TI, Street JL, Pattern of daily weights among low birth weight neonates in the neonatal intensive care unit: data from a multihospital health-care system, J. Perinatol. 26 (2006) 37–43, 10.1038/sj.jp.7211431. [DOI] [PubMed] [Google Scholar]
- [16].Ganz T, Jung G, Naeim A, et al. , Immunoassay for human serum erythroferrone, Blood 130 (2017) 1243–1246, 10.1182/blood-2017-04-777987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Cao C, O’Brien KO, Pregnancy and iron homeostasis: an update, Nut. Rev. 71 (2013) 35–51, 10.1111/j.1753-4887.2012.00550.x. [DOI] [PubMed] [Google Scholar]
- [18].Siddapa AM, Olson RM, Spector M, E, et al. , High prevalence of iron deficiency despite standardized high-dose iron supplementation during recombinant erythropoietin therapy in extremely low gestational age newborns, J. Pediatr. 111 (2020) 98–105, 10.1016/j.jpeds.2020.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Zamora TG, Guiang SF 3rd, Widness JA, Georgieff MK, Iron is prioritized to red blood cells over the brain in phlebotomized anemic newborn lambs, Pediatr. Res. 79 (2016) 922–928, 10.1038/pr.2016.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Georgieff MK, Iron deficiency in pregnancy, Am. J. Obstet. Gynecol. 223 (2020) 516–524, 10.1016/j.ajog.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].MacQueen BC, Baer VL, Scott DM, et al. , Iron supplements for infants at risk for iron deficiency, Glob. Pediatr. Health. 25 (2017), 10.1177/2333794X17703836, 4:2333794X17703836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Yang Q, Liu W, Zhang S, Liu S, The cardinal roles of ferroportin and its partners in controlling cellular iron in and out, Life Sci. 258 (2020) 118135, 10.1016/j.lfs.2020.118135. [DOI] [PubMed] [Google Scholar]
- [23].Ganz T, The discovery of the iron-regulatory hormone hepcidin, Clin. Chem. 65 (2019) 1330–1331, 10.1373/clinchem.2019.306407. [DOI] [PubMed] [Google Scholar]
- [24].Ohls RK, Christensen RD, Kamath-Rayne BD, et al. , A randomized, masked, placebo-controlled study of darbepoetin alfa in preterm infants, Pediatrics. 132 (2013) e119–e127, 10.1542/peds.2013-0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Ohls RK, Kamath-Rayne BD, Christensen RD, et al. , Cognitive outcomes of preterm infants randomized to darbepoetin, erythropoietin, or placebo, Pediatrics. 133 (2014) 1023–1030, 10.1542/peds.2013-4307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Bahr TM, Baer VL, Christensen TR, et al. , Reconciling discordant values for serum ferritin vs. reticulocyte hemoglobin content, J. Perinatol. Oct 5 (2020), 10.1038/s41372-292-00845-2. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- [27].Gerday E, Brereton JB, Bahr TM, et al. , Urinary ferritin; a potential noninvasive way to screen NICU patients for iron deficiency, J. Perinatol. Jul 24 (2020), 10.1038/s41372-020-0746-6. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- [28].Bahr TM, Carr NR NR, Christensen TR, et al. , Quality improvement initiative to increase the consistency and effectiveness of iron supplementation for NICU patients.
- [29].Juul SE, Comstock BA, Wadhawan R, et al. , PENUT trial consortium. A randomized trial of erythropoietin for neuroprotection in preterm infants, N. Engl. J. Med. 382 (2020) 233–243, 10.1056/NEJMoa1907423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Juul SE, Vu PT, Comstock BA, et al. , PENUT trial consortium, Effect of high-dose erythropoietin on blood transfusions in extremely low gestational age neonates: Post hoc analysis of a randomized clinical trial, JAMA Pediatr. 174 (2020) 933–943, 10.1001/jamapediatrics.2020.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].MacQueen BC, Christensen RD, Baer VL, Ward DM, Snow GL, Screening umbilical cord blood for congenital iron deficiency, Blood Cells Mol. Dis. 77 (2019) 95–100, 10.1016/j.bcmd.2019.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Fisher AL, Sangkhae V, Presicce P, et al. , Fetal and amniotic fluid iron homeostasis in healthy and complicated murine, macaque, and human pregnancy, JCI Insight 27 (2020), e135321, 10.1172/jci.insight.135321. [DOI] [PMC free article] [PubMed] [Google Scholar]


