To the editor:
With the dominance of the most recent severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variant of concern Omicron (B.1.1.529), the question arises, what is the reason for its high contagiousness in coronavirus disease 2019 (COVID-19)–vaccinated dialysis patients compared with the previous SARS-CoV-2 variants Delta and wild type?
The objective of this study was, therefore, to analyze humoral and cellular immunity directed against the Omicron variant of concern compared with the wild-type or Delta variant of concern in hemodialysis patients (n = 42; Supplementary Table S1) immunized with 4 doses of mRNA COVID-19 vaccine. Titers of neutralizing antibodies (NAbs) against wild type, Delta, and Omicron were estimated by SARS-CoV-2 spike-protein (S-protein) pseudovirus assays. T-cell immunity reactive against wild-type, Delta-derived, and Omicron-derived S-protein was analyzed by multiparameter flow cytometry (Supplementary Figure S1). The analyses were performed 8 to 9 weeks following the fourth doses.
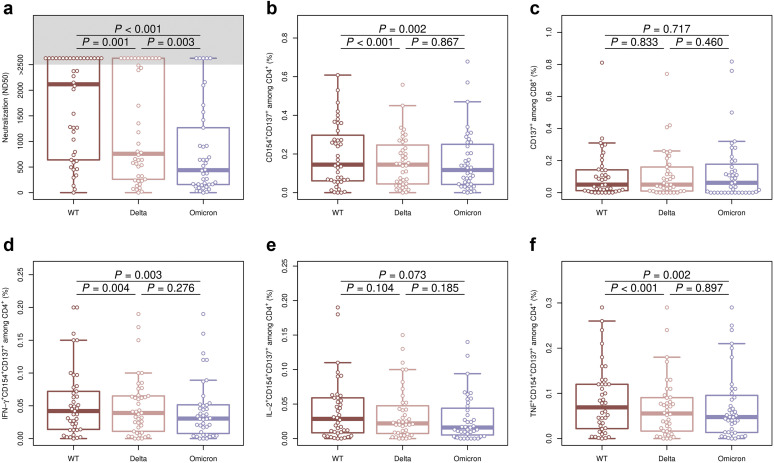
The hemodialysis patients had a clear seroconversion after 4 doses of SARS-CoV-2 vaccination, with a significantly higher titer of NAb against wild type compared with Delta or Omicron S-protein (median [interquartile range]-50% Neutralizing Dose [ND50] = 2117 [663–2560], 759 [276–2560], and 439 [160–1180], respectively). Interestingly, the NAb titer against Delta was significantly higher compared with Omicron-specific NAb (Figure 1 a). Although the number of patients with a detectable S-protein–reactive CD4 T-cell response was nearly identical (40–41 of 42 patients; Supplementary Table S2), the magnitude of response was significantly lower against Omicron- and Delta-derived S-protein compared with wild type (Figure 1b). In contrast, significantly fewer patients showed a detectable S-protein–reactive CD8 T-cell response against Omicron but not Delta compared with wild type (P = 0.0304, Fisher exact test; Supplementary Table S2), although the magnitude of response was not different between all 3 SARS-CoV-2 variants (Figure 1c).
Figure 1.
Comparison of humoral and cellular immunity directed against spike protein (S-protein) derived from Omicron, Delta, and wild-type (WT) variants of concern in hemodialysis patients vaccinated with 4 mRNA coronavirus disease 2019 (COVID-19) vaccine doses. (a) Isolated serum from hemodialysis patients was analyzed for Omicron-, Delta-, and WT-specific neutralizing antibodies (50% Neutralizing Dose [ND50]). (b–f) Isolated peripheral blood mononuclear cells from hemodialysis patients were stimulated for 16 hours with 1 μg/ml severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) S-protein overlapping peptide pools from WT (dark red box plots), Delta (light red box plots), or Omicron (blue box plots). S-protein–reactive T helper cells were identified as life/dead-marker–CD3+CD4+CD137+CD154+ (b), and S-protein–reactive cytotoxic T cells were identified as life/dead-marker–CD3+CD8+CD137+ (c). Within the S-protein–reactive CD4 T-cell population, antibodies against interferon-γ (IFN-γ) (d), interleukin-2 (IL-2) (e), and tumor necrosis factor (TNF) (f) were used to detect T helper cell 1 cytokine-producing T helper cells. Groups were compared using 2-sided, unpaired Mann-Whitney U test; P ≤ 0.050 was defined as significant.
With regard to the functionality, the frequency of Omicron and Delta S-protein–reactive CD4+ T cells producing T helper cell 1 cytokines interferon-γ and tumor necrosis factor was significantly lower compared with wild-type S-protein–reactive CD4+ T cells (Figure 1d and f).
In line with the results from the general populations,1 , 2 hemodialysis patients show a clearly decreased humoral and cellular immune response against Omicron compared with SARS-CoV-2 wild type after 4 doses of vaccination. Of interest, Omicron-specific NAb titer was significantly lower compared with Delta-specific NAb, explaining the more efficient evasion of Omicron from neutralizing antibodies3 and its efficient spread in vaccinated individuals.4 Because the humoral immune response of dialysis patients strongly benefits from a fourth compared with a third vaccination,5 an adjustment of the vaccination procedure should be recommended.
Data Statement
The data will be available on request.
Acknowledgments
We feel deep gratitude to the patients who donated their blood samples for this project. We would like to acknowledge the excellent assistance of dialysis staff of Dialyse-Bochum as well as the expertise and technical assistance of immune diagnostic laboratory (Sviatlana Kaliszczyk, Patrizia Wehler, Sarah Skrzypczyk, Eva Kohut, Julia Kurek, and Jan Zapka) of Center for Translational Medicine at Marien Hospital Herne. This work was supported by grants of Mercator Foundation, COVIDDataNet.NRW, and the EpiCov project from Arbeitsgemeinschaft industrieller Forschungsvereinigungen (AiF)/Zentrale Innovationsprogramm Mittelstand (ZIM).
Footnotes
Supplementary Methods.
Table S1. Cohort characteristics.
Table S2. Number of patients with CD4 or CD8 T-cell response.
Figure S1. Gating strategy to identify spike-protein (S-protein)–reactive T cells among CD4+ T and CD8+ T cells.
Supplementary Material
References
- 1.GeurtsvanKessel C.H., Geers D., Schmitz K.S., et al. Divergent SARS-CoV-2 Omicron–reactive T and B cell responses in COVID-19 vaccine recipients. Sci Immunol. 2022;7:1–15. doi: 10.1126/sciimmunol.abo2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ariën K.K., Heydrickx L., Michiels J., et al. Three doses of BNT162b2 vaccine confer neutralising antibody capacity against the SARS-CoV-2 Omicron variant. NPJ Vaccines. 2022;7:4–6. doi: 10.1038/s41541-022-00459-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoffmann M., Krüger N., Schulz S., et al. The Omicron variant is highly resistant against antibody-mediated neutralization: implications for control of the COVID-19 pandemic. Cell. 2022;185:447–456. doi: 10.1016/j.cell.2021.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arora P., Zhang L., Rocha C., et al. Comparable neutralisation evasion of SARS-CoV-2 omicron subvariants BA.1, BA.2, and BA.3. Lancet Infect Dis. 2022;22:766–767. doi: 10.1016/S1473-3099(22)00224-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cinkilic O., Anft M., Blazquez-Navarro A., et al. Inferior humoral and sustained cellular immunity against wild-type and omicron variant of concern in hemodialysis patients immunized with 3 SARS-CoV-2 vaccine doses compared with 4 doses. Kidney Int. 2022;101:1287–1289. doi: 10.1016/j.kint.2022.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.