Abstract
High concentrations of acetylene (10 to 50% [vol/vol] gas phase) were required to inhibit the growth of Burkholderia cepacia G4 on toluene, while 1% (vol/vol) (gas phase) propyne or 1-butyne completely inhibited growth. Low concentrations of longer-chain alkynes (C5 to C10) were also effective inhibitors of toluene-dependent growth, and 2- and 3-alkynes were more potent inhibitors than their 1-alkyne counterparts. Exposure of toluene-grown B. cepacia G4 to alkynes resulted in the irreversible loss of toluene- and o-cresol-dependent O2 uptake activities, while acetate- and 3-methylcatechol-dependent O2 uptake activities were unaffected. Toluene-dependent O2 uptake decreased upon the addition of 1-butyne in a concentration- and time-dependent manner. The loss of activity followed first-order kinetics, with apparent rate constants ranging from 0.25 min−1 to 2.45 min−1. Increasing concentrations of toluene afforded protection from the inhibitory effects of 1-butyne. Furthermore, oxygen, supplied as H2O2, was required for inhibition by 1-butyne. These results suggest that alkynes are specific, mechanism-based inactivators of toluene 2-monooxygenase in B. cepacia G4, although the simplest alkyne, acetylene, was relatively ineffective compared to longer alkynes. Alkene analogs of acetylene and propyne—ethylene and propylene—were not inactivators of toluene 2-monooxygenase activity in B. cepacia G4 but were oxidized to their respective epoxides, with apparent Ks and Vmax values of 39.7 μM and 112.3 nmol min−1 mg of protein−1 for ethylene and 32.3 μM and 89.2 nmol min−1 mg of protein−1 for propylene.
Molecules that inactivate an enzyme after undergoing a catalytic transition in the active site are known as mechanism-based inactivators or suicide substrates (4, 29, 33). Posing as a substrate, the inactivator binds to the target enzyme and is catalytically converted into a reactive species that can covalently bind to an active-site amino acid or prosthetic group, causing a concurrent loss of enzyme activity. Due to the specificity and irreversible nature of this interaction, mechanism-based inactivators are versatile tools that have been used as probes for enzyme mechanisms, as therapeutic agents, and as inhibitors of microbial processes (4, 24, 29, 33).
Alkynes are a well-known class of mechanism-based inactivators of a number of oxygenase enzymes, including several bacterial monooxygenases (15, 28). For example, the simplest alkyne, acetylene, is a potent inactivator of ammonia monooxygenase (AMO) from the nitrifying bacterium Nitrosomonas europaea. Inhibition of AMO activity in N. europaea by acetylene has been shown to be a saturable, time- and O2-dependent reaction, and incubation of cells with 14C2H2 results in the specific, covalent radiolabeling of a membrane polypeptide which is thought to contain the active site of the enzyme (17). Similar kinetic and radiolabeling results obtained from studies with methanotrophic bacteria indicate that acetylene is a mechanism-based inactivator of both the particulate and soluble (sMMO) forms of methane monooxygenase (MMO) (7, 25, 30). In spite of the differences among these three enzymes, they are all inactivated by low concentrations of acetylene (0.01 to 0.03%) (7, 18), and are all capable of oxidizing the chlorinated solvent trichloroethylene (TCE) (2, 8, 23, 31).
A variety of other microorganisms are also known to oxidize TCE through the activity of nonspecific oxygenase enzymes. Among these, most attention has been given to the toluene-oxidizing organism Burkholderia cepacia G4. This organism initiates the metabolism of toluene via successive hydroxylations at the ortho and then the adjacent meta position of the aromatic ring, immediately followed by meta cleavage of the catechol intermediate (21, 27). Genetic and biochemical studies strongly suggest that the enzyme toluene 2-monooxygenase is singularly responsible for both of the hydroxylation reactions required to initiate toluene catabolism and for the cometabolic oxidation of TCE by B. cepacia G4 (21, 22, 26). Furthermore, biochemical analysis of the purified enzyme and sequence comparisons indicate that toluene 2-monooxygenase is part of a family of binuclear-iron enzymes that contains several other hydrocarbon- and TCE-oxidizing oxygenases, including the well-characterized sMMO (11, 21, 35).
Despite the strong catalytic and structural similarities between toluene 2-monooxygenase and sMMO, these two enzymes appear to differ considerably in their sensitivity to acetylene. While sMMO-catalyzed reactions such as TCE oxidation are known to be readily inactivated by acetylene (1, 25), a recent study suggested that this compound is a weak inhibitor of the TCE-degrading activity of B. cepacia G4 (20). These observations suggested two possibilities to us. First, it is possible that acetylene exerts its inhibitory effects on toluene oxidation through a mechanism different from the inactivation-based mechanisms observed for several other bacterial oxygenases. Second, it is possible that acetylene acts as a conventional, albeit unusually weak, mechanism-based inactivator of toluene-oxidizing activity. The aim of the present study was to resolve these questions by examining the effects of acetylene and other alkynes on the toluene-oxidizing activity of B. cepacia G4.
MATERIALS AND METHODS
Chemicals and reagents.
Acetylene was generated from calcium carbide (technical grade; Aldrich, Milwaukee, Wis.). Propyne (97%), 1-hexyne, phenylacetylene, 3-phenyl-propyne, 1-ethynylcyclohexylamine, toluene, o-cresol, 3-methylcatechol, propylene, and propylene oxide were obtained from Aldrich. 1- and 2-Butyne, 1- and 2-pentyne, 2- and 3-hexyne, and 1-decyne were obtained from Farchan Laboratories, Inc. (Gainesville, Fla.). Other reagents and their sources included N,N-dimethylformamide (Sigma, St. Louis, Mo.), ethylene (Airco, Murray Hill, N.J.), and ethylene oxide (MG Industries, Malvern, Pa.). All other chemicals were of reagent grade or better.
Bacterial strain and culture conditions.
B. cepacia G4 was kindly provided by Malcolm Shields (University of West Florida, Pensacola) and was maintained on minimal medium agar plates containing 20 mM lactate. The minimal medium contained (per liter) 0.5 g of NH4NO3, 0.2 g of MgSO4 · 7H2O, 0.05 g of CaCl2 · 2H2O, 0.01 g of disodium EDTA, 0.005 g of FeCl3, 50 ml of 1 M KH2PO4-K2HPO4 (pH 7.0), and 10 ml of trace elements solution (0.143 g of H3BO3, 0.102 g of MgSO4 · 7H2O, 0.032 g of ZnSO4 · 7H2O, 0.01 g of CoCl2 · 4H2O, 0.008 g of CuSO4 · 5H2O, and 0.005 g of Na2MoO4 · 2H2O per liter). Liquid cultures were grown overnight with shaking (200 rpm) at 30°C in glass serum vials (160 ml) containing minimal medium (60 ml) and either lactate (20 mM) or toluene (94 μmol, 1 mM aqueous phase; added neat). The vials were sealed with butyl rubber stoppers. At 4 h before harvest, additional toluene (94 μmol) was added to toluene-grown bacteria. Lactate-grown cells were not amended before harvest. Cells were pelleted by centrifugation (6000 × g, 10 min) and resuspended in 30 ml of phosphate buffer (50 mM KH2PO4-K2HPO4 [pH 7.0]). Cells were then recentrifuged, resuspended in 30 ml of phosphate buffer, and pelleted again. Finally, the washed cells were resuspended in phosphate buffer (1 ml), yielding a final concentration of 2 to 4 mg of protein per ml. The concentrated cell suspension was stored at room temperature for ≤3 h before use. Toluene-oxidizing activities remained constant over this storage period.
Analytical and other methods.
Hydrocarbons were analyzed with a Shimadzu (Kyoto, Japan) GC-8A chromatograph equipped with a flame ionization detector and a stainless steel column (0.3 by 122 cm) packed with Porapak Q 80-100 mesh (Alltech, Deerfield, Ill.). The column temperature was 135°C for the experiment described in the legend to Fig. 6. Propylene and propylene oxide were determined at a column temperature of 125°C, whereas ethylene and ethylene oxide were determined at a column temperature of 100°C. The injector and detector temperatures were set at 200°C for all analyses. Substrate consumption was determined by peak area quantification with an HP3395 integrator (Hewlett Packard, Palo Alto, Calif.). Data for determination of stoichiometry and kinetic constants were obtained by comparison of propylene and epoxide peak heights to standard curves constructed from known amounts of the authentic compounds. The concentration ranges for primary substrates used to construct standard curves were as follows: 0.1 to 5.0 μmol for propylene oxide, 0.2 to 10.0 μmol for ethylene oxide, and 0.45 to 8.9 μmol for propylene. All standard curves were linear over these substrate ranges, with an r2 value of ≥0.998.
FIG. 6.
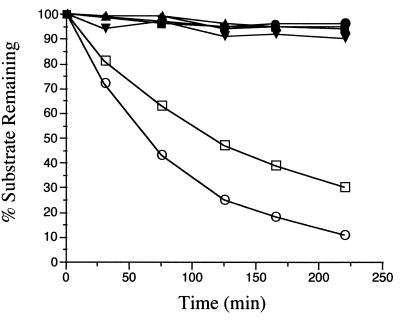
Time course of alkene and alkyne depletion catalyzed by B. cepacia G4. Cells were incubated with 0.45 μmol of each compound as described in Materials and Methods. 1-Butyne (4.5 μmol) was added to inactivate toluene 2-monooxygenase activity in selected samples. Symbols: □, ethylene; ○, propylene; ■, ethylene plus 1-butyne; •, propylene plus 1-butyne; ▾, ethylene (heat-killed cells); ⧫, acetylene; ▴, propyne.
The aqueous concentration of toluene in two-phase systems was calculated with a dimensionless Henry’s constant that was determined empirically to be 0.343 at 30°C (13). The concentrations of ethylene and propylene in the aqueous phase were calculated with Henry’s constants derived from the data of Wilhelm et al. (34). The presence of B. cepacia G4 cells at the concentrations used in our experiments did not affect the partitioning of toluene, ethylene, or propylene between the liquid (1.5 ml of phosphate buffer) and gas (8.5 ml) phases compared to rubber cell-free controls. Protein concentrations were determined with the biuret assay (12) following cell solubilization in 3 N NaOH for 30 min at 65°C. Bovine serum albumin was used as the standard.
Growth inhibition by alkynes.
Toluene- or lactate-grown cells were cultivated in glass serum vials (160 ml) sealed with Teflon-lined butyl rubber stoppers (Supelco, Bellefonte, Pa.). Prior to inoculation, the required concentrations of toluene, lactate, and alkynes were added to the vials through the stoppers by use of sterile syringes. The vials were then incubated under experimental conditions (30°C with shaking [200 rpm]) for at least 2 h to allow equilibration of the hydrocarbons between the gas and liquid phases. The incubations were initiated by the addition of concentrated, toluene-grown B. cepacia G4. The cells were injected into the sealed vials through the stoppers to give an initial optical density at 600 nm of 0.05. Samples (1 ml) were aseptically withdrawn from sealed vials throughout the experiment to monitor cell growth, as determined by measurements of optical density at 600 nm. In all experiments, acetylene, propyne, and 1-butyne were added as gases. Other alkynes and aromatics were added from stock solutions prepared in N,N-dimethylformamide. Abiotic control incubations were also established for each experimental condition to determine the effect of repeated puncturing of the stoppers on the concentrations of each substrate and each inhibitor. Headspace analysis by gas chromatography revealed that the abiotic losses of all substrates were less than 5% of the initial values.
Inhibition of O2 uptake by alkynes.
O2 uptake measurements were determined with a Clark (Yellow Springs, Ohio)-style O2 electrode mounted in a glass water-jacketed reaction vessel (1.6 ml) maintained at 30°C. In all assays, the reaction chamber was filled with phosphate buffer before the addition of cells or other reactants. A basal rate of cellular respiration was established by measuring O2 uptake in the absence of substrate, and this value was subtracted from all substrate-induced rates to yield the substrate-dependent O2 uptake rate. To determine rates of alkyne-dependent inactivation of toluene-dependent O2 uptake, tangents were drawn to the nonlinear O2 electrode traces at selected times following the addition of each inactivator. The rates were plotted against time and were fitted to a first-order exponential decay curve to determine the apparent rate constant for activity loss (kobs) at each inactivator concentration.
The specificity of alkyne inactivation in B. cepacia G4 was determined by measuring the effects of alkyne exposure on various substrate-specific O2 uptake rates. Concentrated toluene-grown cells (130 μg of protein) were added to phosphate buffer (final reaction volume, 1 ml) in glass serum vials (10 ml) sealed with Teflon-lined butyl rubber stoppers. Specific alkynes (0.45 μmol) were added separately to individual vials with a gas-tight syringe, and the vials were incubated for 30 min in a shaking water bath (30°C, 150 rpm). A control vial to which no alkyne was added was included. After the reaction period, the cells were sedimented in a microcentrifuge (30 s at 14,000 × g), washed twice with phosphate buffer, and resuspended in phosphate buffer (150 μl). Samples of the washed cell suspension were then examined for residual O2 uptake activity in the presence of toluene (200 μM), o-cresol (200 μM), 3-methylcatechol (400 μM), or acetate (1 mM). Residual activity was determined by comparing the substrate-dependent O2 uptake rate obtained from alkyne-treated cells with the corresponding rate determined from cells recovered from the control vial.
Requirement of O2 for inactivation.
A culture of toluene-grown B. cepacia G4 (500 ml) was harvested and resuspended in phosphate buffer (8 ml). A portion of this concentrated cell suspension was used to completely fill an O2 electrode chamber, which was quickly (<1 min) driven anaerobic by endogenous respiration. A 5.5-cm capillary inlet filled with the same concentrated cell suspension prevented atmospheric O2 from entering the electrode chamber during the experiment. At various times after the chamber became anaerobic, cell samples (20 μl) were removed and added to another O2 electrode chamber containing phosphate buffer and toluene (400 μM) to measure toluene-dependent O2 uptake rates. At 30 min after the initiation of the experiment, 1-butyne (120 μM, final concentration) was added to the anaerobic cell suspension, and at 54 and 77 min, H2O2 (400 nmol) was introduced into the chamber as a source of O2. In a control experiment, the procedure was repeated without the addition of 1-butyne. In all cases, the additions of substrates and inhibitors to the electrode chambers involved dilution of the reaction mixture by 2% or less. Both electrode chambers were maintained at 30°C with a circulating water bath.
Alkyne and alkene oxidation.
Consumption of short-chain alkenes and alkynes by B. cepacia G4 in resting-cell assays was examined by gas chromatography. Concentrated toluene-grown B. cepacia G4 cells (300 μg of protein) were added to phosphate buffer (final reaction volume, 1 ml) in glass serum vials (10 ml) sealed with Teflon-lined butyl rubber stoppers. Heat-inactivated cells (95°C, 15 min) were added to one vial as a control. In two other vials, cells were preincubated (30 min, 30°C) with 1-butyne (4.5 μmol) to inactivate toluene 2-monooxygenase activity prior to the addition of alkenes. Reactions were initiated by the addition of 10 μl (0.45 μmol) of either ethylene, propylene, acetylene, or propyne with a gas-tight microsyringe. Reaction mixtures were incubated at 30°C with shaking (150 rpm); periodically, headspace samples (20 μl) were analyzed by gas chromatography as described above. Liquid samples (4 μl) were removed at selected times for comparative analysis of soluble products against epoxide standards (see above).
The epoxide nature of the products obtained from the transformation of ethylene and propylene by B. cepacia G4 was confirmed by acid hydrolysis. Epoxides undergo C—O bond cleavage under acidic conditions to yield 1,2-diols, which are not detectable by gas chromatographic analysis of headspace samples (100 μl) under the conditions described above (epoxides are detectable). Therefore, headspace samples from reaction vials in which ethylene or propylene had been consumed were analyzed by gas chromatography before and after the addition of 9 N H2SO4 (50 μl).
For examination of the stoichiometry of propylene oxidation to propylene oxide, cells (300 μg of protein) and propylene (5.5 μmol) were incubated at 30°C with shaking (150 rpm) for 4 h. Headspace (20 μl) and liquid (4 μl) samples were analyzed by gas chromatography (see above) at selected times.
Kinetic analysis of propylene and ethylene oxidation.
Various amounts of ethylene or propylene (0.45 to 25 μmol) were added to sealed glass serum vials (10 ml) containing phosphate buffer (final reaction volume, 1 ml). The alkenes were allowed to equilibrate between the gas and liquid phases by shaking (150 rpm) for 2 h at 30°C. Concentrated toluene-grown B. cepacia G4 cells (400 to 600 μg of protein) were added to initiate the reaction. After 20 min, liquid samples (4 μl) were removed for analysis of epoxide formation by gas chromatography. The rates of epoxide formation (v) were plotted against the corresponding initial propylene or ethylene concentrations (S) and fit by least-squares regression analysis to the Michaelis-Menten equation [v = (Vmax × S)/(Km + S)] to determine the apparent Ks and Vmax.
RESULTS
Inhibition of growth by alkynes.
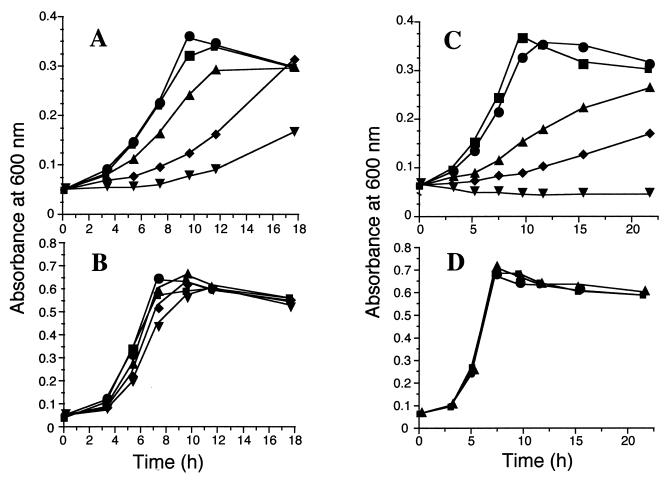
In the presence of 1% (vol/vol) acetylene (gas phase), the growth curve of B. cepacia G4 was similar to that of a control containing no acetylene. However, the presence of 10, 25, and 50% acetylene progressively inhibited growth (Fig. 1A). Acetylene concentrations remained constant throughout the experiments, as determined by gas chromatography (data not shown). The growth of B. cepacia G4 on lactate was largely unaffected by the presence of acetylene at all concentrations tested (Fig. 1B). In contrast to acetylene, considerably lower concentrations of 1-butyne were required to inhibit the growth of B. cepacia G4 on toluene (Fig. 1C). 1-Butyne exhibited inhibitory effects at levels as low as 0.05%, while 1.0% completely halted growth over the full time course of the experiment. Similar results were obtained with equivalent concentrations of propyne (data not shown). The growth of B. cepacia G4 on lactate was unaffected by the addition of 1% propyne or 1% 1-butyne (Fig. 1D). Additionally, B. cepacia G4 grew in the presence of toluene and 1% 1-butyne upon the addition of 20 mM lactate (data not shown).
FIG. 1.
Growth of B. cepacia G4 on 1 mM toluene (A and C) and 20 mM lactate (B and D) in the presence of various alkynes. Symbols in panels A and B represent initial amounts (volume/volume) (gas phase) of acetylene added to growth vials: ■, 0%; •, 1%; ▴, 10%; ⧫, 25%; and ▾, 50%. Symbols in panel C represent initial amounts (volume/volume) (gas phase) of 1-butyne added to growth vials: ■, 0%; •, 0.01%; ▴, 0.05%; ⧫, 0.1%; and ▾, 1.0%. Symbols in panel D: ■, no alkyne; •, 1% propyne; ▴, 1% 1-butyne.
The marked inhibitory effects of propyne and 1-butyne on the toluene-dependent growth of B. cepacia G4 led us to examine the response of the organism to other alkynes (Table 1). All linear alkynes tested, except acetylene, were potent inhibitors of toluene-dependent growth. Interestingly, linear alkynes with interior triple bonds were more potent inhibitors of growth than their counterparts with terminal bonds. The aromatic alkynes phenylacetylene and 3-phenylpropyne, suppressed growth at the higher concentrations tested but were less effective at lower concentrations. A yellow product was visible in vials containing ≤4.5 μmol of phenylacetylene or 3-phenylpropyne following the incubation period, suggesting that a meta-ring cleavage product had accumulated. It is possible that ring cleavage of the aromatic alkynes resulted in a yellow product or that these aromatic alkynes inhibited the metabolism of 2-hydroxy-6-oxohepta-2,4-dienoic acid (the product of ring cleavage in toluene metabolism by B. cepacia G4). The cyclic alkyne ethynylcyclohexylamine was an extremely weak inhibitor of growth.
TABLE 1.
Inhibition of toluene-dependent growth of B. cepacia G4 by alkynes
| Inhibitor |
A600 of culture grown in the presence of an inhibitor ata:
|
||
|---|---|---|---|
| 45 μmolb | 4.5 μmol | 0.45 μmol | |
| Acetylene | 0.35 ± 0.03 (−) | 0.35 ± 0.02 (−) | 0.35 ± 0.02 (−) |
| Propyne | 0.04 ± 0.00 (++) | 0.10 ± 0.01 (+) | 0.30 ± 0.09 (+) |
| 1-Butyne | 0.04 ± 0.00 (++) | 0.06 ± 0.02 (++) | 0.27 ± 0.05 (+) |
| 2-Butyne | 0.03 ± 0.00 (++) | 0.03 ± 0.00 (++) | 0.04 ± 0.00 (++) |
| 1-Pentyne | 0.04 ± 0.01 (++) | 0.04 ± 0.01 (++) | 0.19 ± 0.02 (+) |
| 2-Pentyne | 0.04 ± 0.00 (++) | 0.04 ± 0.00 (++) | 0.04 ± 0.01 (++) |
| 1-Hexyne | 0.05 ± 0.01 (++) | 0.05 ± 0.01 (++) | 0.18 ± 0.04 (+) |
| 2-Hexyne | 0.04 ± 0.00 (++) | 0.04 ± 0.00 (++) | 0.05 ± 0.01 (++) |
| 3-Hexyne | 0.04 ± 0.00 (++) | 0.04 ± 0.01 (++) | 0.08 ± 0.01 (+) |
| 1-Decyne | 0.05 ± 0.01 (++) | 0.07 ± 0.03 (++) | 0.23 ± 0.10 (+) |
| Phenylacetylene | 0.03 ± 0.00 (++) | 0.18 ± 0.02 (+) | 0.29 ± 0.01 (+) |
| 3-Phenylpropyne | 0.05 ± 0.01 (++) | 0.23 ± 0.03 (+) | 0.37 ± 0.06 (−) |
| 1-Ethynylcyclohexylamine | 0.29 ± 0.06 (+) | 0.38 ± 0.02 (−) | 0.39 ± 0.04 (−) |
B. cepacia G4 was grown as a batch culture with 94 μmol of toluene in the presence of the indicated amount of inhibitor for 9.5 h. The A600 at time zero was ca. 0.05. Values are the mean ± standard deviation for triplicate experiments. Symbols in parentheses indicate levels of inhibition. Complete inhibition (++) is defined as a final A600 of ≤0.075, partial inhibition (+) is defined as a final A600 of between 0.075 and 0.30, and no inhibition (−) is defined as a final A600 of ≥0.30. The final A600 of cells grown in the absence of an alkyne was 0.341 ± 0.014.
Gaseous alkynes (acetylene, propyne, and 1-butyne) were assumed to behave as ideal gases, i.e., 45 μmol = 1 ml of gaseous alkyne = 1% (vol/vol) gas phase in experimental vials.
Inactivation of toluene-dependent O2 uptake by alkynes.
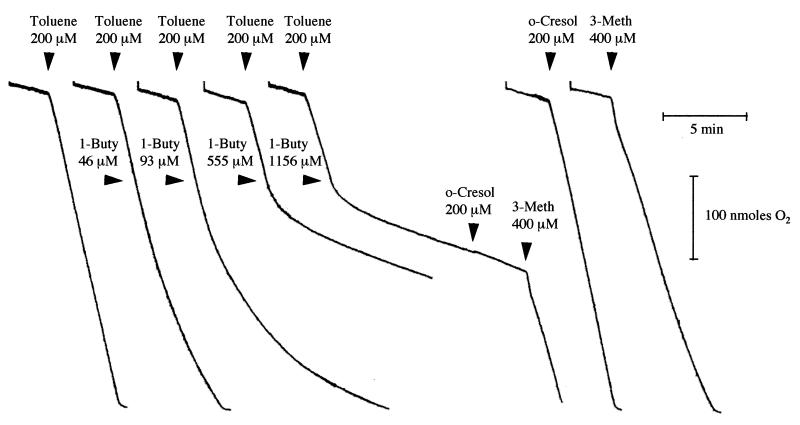
To further investigate the effect of alkynes on toluene-grown B. cepacia G4, we examined toluene-dependent O2 uptake by cells (from a concentrated stock) in the presence of specific concentrations of 1-butyne. After the addition of toluene (200 μM), a constant rate of O2 uptake was established. The addition of 1-butyne resulted in a time-dependent loss of O2 uptake activity, and the rate of inactivation depended on the concentration of 1-butyne (Fig. 2). After the addition of 1-butyne, the rates of O2 uptake eventually stabilized (in reactions containing sufficient O2) at levels comparable to the basal rate of O2 uptake obtained prior to the addition of toluene. The addition of o-cresol to cells inactivated with 1-butyne did not stimulate O2 uptake; however, the addition of 3-methylcatechol resulted in an O2 uptake rate that was within 10% of that determined when 3-methylcatechol was added to untreated cells (Fig. 2). Acetylene also inhibited toluene-dependent O2 uptake by B. cepacia G4 in a time- and concentration-dependent manner, although much higher concentrations were required to produce an observable effect (data not shown).
FIG. 2.
Effects of 1-butyne on substrate-dependent O2 uptake by B. cepacia G4. The time at which a given substrate or inactivator was added to the O2 electrode chamber containing cells (130 μg of protein) is depicted next to each trace by arrowheads. Abbreviations: 3-Meth, 3-methylcatechol; 1-Buty, 1-butyne.
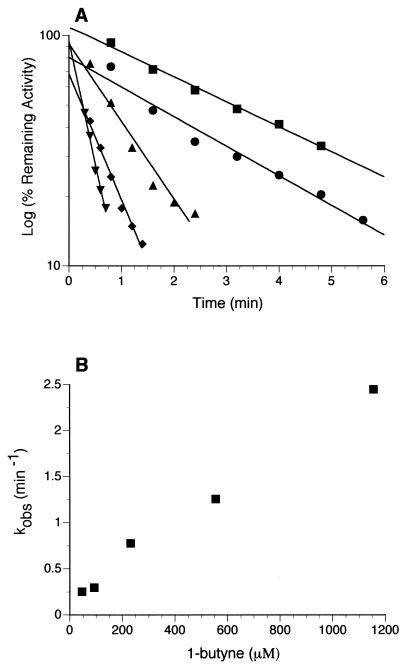
The time-dependent loss of toluene-dependent O2 uptake activity in B. cepacia G4 during exposure to 1-butyne was slowed by increasing concentrations of toluene. Cells incubated in the presence of 70 μM 1-butyne and 150, 250, 400, and 750 μM toluene lost 71, 56, 39, and 24% of their respective toluene-dependent O2 uptake activities over a 4-min period (data not shown). In the absence of 1-butyne, O2 uptake rates were constant (until O2 became limiting, at ≥5 min) and similar (within 3% of each other) over the range of toluene concentrations examined in this experiment (150 to 750 μM). The time- and concentration-dependent inactivation of toluene-dependent O2 uptake by 1-butyne could also be described as a pseudo-first-order reaction (Fig. 3A). The observed rate constant for inactivation, kobs, was determined at each concentration of 1-butyne tested as described in Materials and Methods. The kobs values ranged from 0.25 min to 2.45 min−1, and although inactivation rates were dependent upon inactivator concentration, saturation was not observed (Fig. 3B). Even at the highest concentration (1.1 mM 1-butyne) at which reliable rates of inactivation could still be determined, kobs was still proportional to the concentration of the inactivator.
FIG. 3.
Kinetics of inactivation of toluene-dependent O2 uptake in B. cepacia G4 by 1-butyne. (A) Time course of inactivation of toluene-dependent O2 uptake in the presence of various micromolar concentrations of 1-butyne: ■, 46; •, 93; ▴, 231; ⧫, 555; and ▾, 1,156. Cells (130 μg of protein) were mixed with phosphate buffer and toluene (200 μM) in an O2 electrode chamber (1.6 ml) prior to 1-butyne addition. O2 uptake rates were determined at the indicated times following 1-butyne addition as described in Materials and Methods and plotted as the log of the percentage of remaining activity. (B) Rate of inactivation (kobs) versus 1-butyne concentration. Values for kobs were determined as described in Materials and Methods.
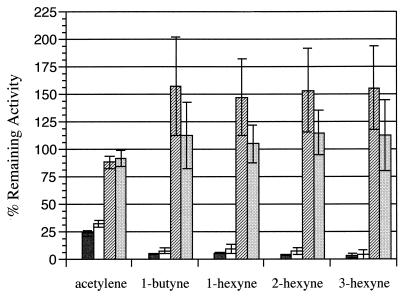
The effects of 1-, 2-, and 3-hexyne on toluene-dependent O2 uptake were also investigated. The addition of 0.45 μmol (281 μM) of each of these alkynes to a reaction mixture of cells and toluene (200 μM) resulted in a rapid-time-dependent loss of activity (data not shown). Following complete inactivation of toluene-dependent O2 uptake activity by each hexyne isomer, 3-methylcatechol (400 μM) stimulated O2 uptake in treated cells to within 10% of the 3-methylcatechol-dependent rate determined in the absence of alkynes (as was demonstrated for cells inactivated with 1-butyne [Fig. 2]). The kobs values for 2- and 3-hexyne were approximately twice that of 1-hexyne, consistent with the increased effectiveness of 2- and 3-hexyne relative to 1-hexyne as inhibitors of growth (Table 1). These results suggest that subterminal alkynes are more effective inactivators of toluene 2-monooxygenase activity in B. cepacia G4 than their N-terminal counterparts.
The specificity of alkyne-based inactivation was further examined by comparing toluene-, o-cresol-, 3-methylcatechol-, and acetate-dependent O2 uptake rates after exposure of toluene-grown cells of B. cepacia G4 to acetylene, 1-butyne, or 1-, 2-, or 3-hexyne. While toluene- and o-cresol-dependent O2 uptake activities were irreversibly inactivated after exposure of the cells to each alkyne, 3-methylcatechol- and acetate-dependent O2 uptake activities were uninhibited (Fig. 4). Surprisingly, cells incubated in the presence of alkynes (with the exception of acetylene) exhibited higher levels of 3-methylcatechol-dependent O2 uptake activity than control cells. Organic solvents, such as ethanol or acetone, are known to protect catechol 2,3-dioxygenases from O2-dependent autoinactivation (5). Perhaps linear alkynes other than acetylene acted in a similar manner in these experiments.
FIG. 4.
Irreversible effects of alkyne exposure on substrate-dependent O2 uptake by B. cepacia G4. Cells (130 μg of protein) were incubated in the presence of an alkyne (indicated on the x axis) for 30 min and washed twice in phosphate buffer, and the remaining O2 uptake activities stimulated by 200 μM toluene (black bars), 200 μM o-cresol (white bars), 400 μM 3-methylcatechol (hatched bars), or 1 mM acetate (stippled bars) were calculated relative to that of a control (see Materials and Methods). Cells were exposed to 45 μmol of acetylene and 0.45 μmol of the other alkynes. Values are the mean ± standard deviation for three trials (acetylene treated cells, n = 2).
Requirement of O2 for inactivation.
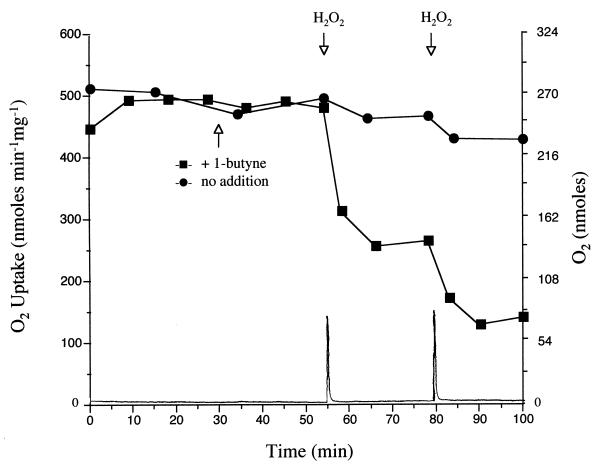
Since O2 is a required cosubstrate in monooxygenase-catalyzed reactions, the inactivation potential of 1-butyne was examined under anaerobic conditions. Toluene-grown cells of B. cepacia G4 retained full toluene-dependent O2 uptake activity when incubated anaerobically for 24 min in the presence of 1-butyne (120 μM) (Fig. 5). When H2O2 (400 nmol) was added to the anaerobic cell suspension, a measurable amount of O2 was produced and rapidly consumed. Immediately following the addition of H2O2, cellular toluene-dependent O2 uptake activity rapidly decreased to a lower constant level, which was approximately 50% the activity before the addition of H2O2. A further addition of H2O2 (400 nmol) resulted in the loss of an additional 25% of the original toluene-dependent O2 uptake activity. In contrast, cells incubated under anaerobic conditions and exposed to 800 nmol of H2O2 in the absence of 1-butyne retained 85% of their toluene-dependent O2 uptake activity, demonstrating that the addition of H2O2 to B. cepacia G4 cells, which could result in the production of potentially damaging oxygen species, does not cause a substantial loss of toluene-dependent O2 uptake activity. These results indicate that the cosubstrate O2 is required for the loss of toluene 2-monooxygenase activity in the presence of 1-butyne.
FIG. 5.
Toluene-dependent O2 uptake by anaerobically incubated B. cepacia G4 exposed (■) or not exposed (•) to 1-butyne. A dense suspension of cells was kept anaerobic by mixing in an O2 electrode chamber throughout the course of the experiment. 1-Butyne (120 μM) was added at 30 min. H2O2 (400 nmol) was added at 54 and 77 min. Toluene-dependent O2 uptake rates were measured after transfer of 20-μl samples of the dense cell suspension into another O2 electrode chamber as described in Materials and Methods. The trace at the bottom represents the amount of O2 (nanomoles) in the dense cell suspension over the course of the 1-butyne amendment experiment.
Alkene oxidation by B. cepacia G4.
Since alkenes are effective mechanism-based inactivators of certain monooxygenases, such as cytochrome P-450 (25, 30), we examined the possibility that alkene analogs of acetylene and propyne—ethylene and propylene—could inactivate monooxygenase activity in B. cepacia G4. Initial experiments indicated that ethylene and propylene were not inactivators of toluene 2-monooxygenase activity in B. cepacia G4 but were rapidly consumed by toluene-grown cells (data not shown). To further examine the substrate efficacy of alkenes versus alkynes, we monitored the consumption of acetylene, propyne, ethylene, and propylene by toluene-grown B. cepacia G4 with resting-cell assays. Both ethylene and propylene were consumed by B. cepacia G4, whereas acetylene and propyne remained at constant levels (Fig. 6). Additionally, the presence of 1-butyne completely inhibited the consumption of ethylene and propylene (Fig. 6). The consumption of both ethylene and propylene was accompanied by the accumulation of a single reaction product for each, as determined by gas-phase sampling. The retention times of these products matched those of ethylene oxide and propylene oxide, respectively, and the epoxide nature of these products was confirmed by their disappearance from the gas phase after the addition of H2SO4 (see Materials and Methods). A ratio of 0.96 for propylene consumed to propylene oxide produced was observed over a 4-h time course in a separate resting-cell assay (data not shown).
Kinetics of ethylene and propylene oxidation.
The kinetics of ethylene and propylene oxidation by toluene-grown B. cepacia G4 were further investigated with short-term, whole-cell assays. The amounts of propylene oxide and ethylene oxide produced from a range of initial propylene and ethylene concentrations were determined following 20-min incubations. The rates of propylene oxide and ethylene oxide production were constant over this time course for all concentrations tested (data not shown). Ks and Vmax values were 39.7 μM and 112.3 nmol min−1 mg of protein−1 for ethylene and 32.3 μM and 89.2 nmol min−1 mg of protein−1 for propylene (values are averages from duplicate experiments [≤20% variation]).
DISCUSSION
In this study, we have demonstrated that a variety of alkynes act as potent inhibitors of the toluene-dependent growth of B. cepacia G4. Using representative alkynes, we have also demonstrated that toluene 2-monooxygenase activity is specifically inactivated by these compounds. Based on both our kinetic and growth studies, we conclude that alkynes represent a general class of mechanism-based inactivators for toluene 2-monooxygenase activity in B. cepacia G4.
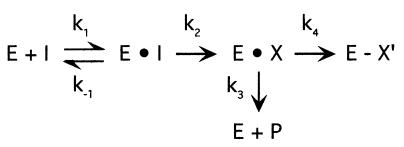
The simplest kinetic model for a mechanism-based inactivator is presented in Fig. 7 (29). According to this model, a number of kinetic criteria should be met to describe a molecule as a mechanism-based inactivator (4, 29, 33), and in this study we have satisfied many of these requirements. First, the inactivation of toluene 2-monooxygenase activity in B. cepacia G4 was time dependent, and the loss of enzyme activity followed pseudo-first-order kinetics (Fig. 3A). Second, a substrate for the target enzyme, toluene, protected against inactivation by 1-butyne, suggesting that there is a competitive interaction between these two compounds for the active site in toluene 2-monooxygenase. Third, the inactivation of toluene 2-monooxygenase activity in B. cepacia G4 by alkynes was irreversible (Fig. 4). Fourth, a catalytically favorable environment for toluene 2-monooxygenase was required for alkyne-dependent inactivation. This result addresses the most characteristic feature of mechanism-based inactivators, which is that the target enzyme is required to catalytically activate the inactivator. Like all other monooxygenases, toluene 2-monooxygenase has a requirement for O2 and for a source of a reductant to effect catalysis. Since it is not possible to limit the availability of a reductant to the enzyme in whole-cell experiments, we demonstrated that O2 is a necessary cosubstrate for 1-butyne-dependent inactivation of toluene 2-monooxygenase activity in B. cepacia G4 (Fig. 5). Although the H2O2 used in our experiment may directly provide both O2 and reductant to the monooxgenase via a “peroxide shunt” (21), this possibility supports rather than detracts from our conclusion that the alkyne-dependent inactivation of toluene 2-monooxygenase activity requires a catalytically functional form of the enzyme.
FIG. 7.
Kinetic model for a mechanism-based inactivator. I, inactivator; E, enzyme; P, product (any transformed species of I that diffuses away from the active site); E · I, reversible enzyme-inactivator complex; E · X, reactive intermediate; E − X′, inactivated enzyme (29). k represents the rate constant for each reaction step.
Although we have satisfied many of the important criteria of mechanism-based inactivation with regard to the effects of alkynes on toluene 2-monooxygenase activity in B. cepacia G4, other aspects of this type of inhibition were not demonstrated in this study. For instance, an additional kinetic criterion is that the inactivation reaction should exhibit saturation kinetics. This criterion requires that the rate of enzyme inactivation is proportional to the inactivator concentration at low inactivator concentrations and that it approaches a constant, maximal value at higher concentrations. Although we demonstrated a strong concentration dependence for the inactivation of toluene-dependent O2 uptake in B. cepacia G4 by 1-butyne, the reaction did not appear to be saturable under the conditions tested (Fig. 3B). This apparent lack of saturation could have been due to intrinsic features of toluene 2-monooxygenase. For example, the maximal rate of inactivation could be fast relative to the formation of the enzyme-inactivator complex; alternatively, inactivator transformation by the target enzyme might not be preceded by a conventional enzyme-inactivator binding step. It is also possible that the rate of inactivation of toluene 2-monooxygenase activity in B. cepacia G4 by alkynes could be saturated at higher concentrations. However, at the highest alkyne concentrations tested in our experiments, the observed rates of inactivation were the highest rates that we could determine given the finite response time of the O2 electrode.
Another feature of mechanism-based inactivation that we did not demonstrate in this study is a 1:1 ratio of covalent attachment of inactivator to target enzyme. This criterion is infrequently demonstrated for mechanism-based inactivators because the necessary experimental approaches usually require sources of enzyme with defined specific activities and an identifiable (usually radiolabeled) inactivator. Our attempts with 14C2H2 labeling in B. cepacia G4 resulted in an inefficient, nonspecific labeling of proteins and other cellular constituents (data not shown). Unfortunately, a 14C-labeled alkyne other than acetylene is not commercially available, so the covalent modification of toluene 2-monooxygenase was not investigated with longer alkynes. The unresolved kinetic considerations discussed above would benefit from additional studies with purified toluene 2-monooxygenase.
It is of interest that acetylene appears to be an ineffective mechanism-based inactivator of toluene 2-monooxygenase activity in B. cepacia G4 relative to other alkynes. Two possible explanations for the poor inactivation capacity of acetylene are that the partition ratio for acetylene is large and that the dissociation constant for acetylene is very high. The partition ratio represents the number of times that the activated inactivator is released as a product per inactivation event (29, 32), or k3/k4, as depicted in Fig. 7. Therefore, a large partition ratio describes an inefficient inactivator. In this study, there was no evidence of acetylene transformation by B. cepacia G4 during growth experiments or resting-cell assays (Fig. 6), suggesting that acetylene does not act as a mechanism-based inactivator with a large partition ratio. If the dissociation constant for acetylene is high relative to that of propyne, it seems reasonable that a similar pattern could be demonstrated for the alkene analogs of these compounds—ethylene and propylene. Our results indicated that these alkenes were not inactivators of toluene 2-monooxygenase activity in B. cepacia G4, but they were oxidized by this enzyme to their respective epoxides, with no evidence of further breakdown. Surprisingly, the apparent Ks values for ethylene and propylene (39.7 and 32.3 μM, respectively) were quite similar. In comparison, two previous studies reported apparent Ks values for TCE of 3 and 6 μM in B. cepacia G4 (10, 19), while the Km value for TCE with purified toluene 2-monooxygenase from this organism was reported to be 12 μM (22). Further analysis indicated that acetylene was a poor inhibitor of ethylene and propylene oxidation (data not shown). These results suggest that acetylene binds poorly to toluene 2-monooxygenase, whereas C2 alkenes, such as ethylene and TCE (22), exhibit a much higher affinity for the enzyme.
Much of the current interest in B. cepacia G4 results from the rapid rate of TCE degradation that is supported by toluene-grown cells of this microorganism. While our results are consistent with earlier observations that acetylene is a relatively weak inhibitor of TCE degradation by B. cepacia G4 (20), they also extend these earlier studies and provide two important observations that could be of use in future studies of TCE biodegradation. First, as noted earlier, several TCE-oxidizing monooxygenase systems are inactivated by low concentrations of acetylene (7, 18). Since toluene 2-monooxygenase activity is relatively immune to inactivation by acetylene, this effect could potentially be exploited to determine which type of enzyme activity is responsible for TCE oxidation in undefined microbial mixtures. A similar approach could also be used with other compounds containing internal triple bonds. For example, we have demonstrated that 2-alkynes are potent inactivators of toluene-oxidizing activity in B. cepacia G4, whereas these compounds are known to be largely ineffective inactivators of both sMMO and AMO (3, 7, 30). Second, our observation that B. cepacia G4 can readily oxidize both ethylene and propylene supports the general observation that this activity is common to TCE-degrading strains. Methane (6)-, propane (14)-, and ammonia (16)-oxidizing bacteria are all capable of cometabolic alkene epoxidation, while other TCE-degrading bacteria, such as Xanthobacter Py2 (9), can grow on simple alkenes. Although these coincident catalytic activities are perhaps unsurprising given the structural similarities between TCE and simple n-alkenes, our confirmation that this theme extends to toluene-oxidizing bacteria suggests that alkene-oxidizing activity might represent the basis for a nontoxic assay for determining the TCE-degrading “potential” of an undefined microbial population.
ACKNOWLEDGMENTS
We thank Evan deSzoeke for performing preliminary growth experiments and Malcolm Shields for providing B. cepacia G4.
Funding of this study was provided by the Office of Research and Development, U.S. Environmental Protection Agency, under agreement R-815738 through the Western Region Hazardous Substance Research Center.
REFERENCES
- 1.Alvarez-Cohen L, McCarty P L. Effects of toxicity, aeration, and reductant supply on trichloroethylene transformation by a mixed methanotrophic culture. Appl Environ Microbiol. 1991;57:228–235. doi: 10.1128/aem.57.1.228-235.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arciero D, Vannelli T, Logan M, Hooper A B. Degradation of trichloroethylene by the ammonia-oxidizing bacterium Nitrosomonas europaea. Biochem Biophys Res Commun. 1989;159:640–643. doi: 10.1016/0006-291x(89)90042-9. [DOI] [PubMed] [Google Scholar]
- 3.Arp D J, Hommes N G, Hyman M R, Juliette L Y, Keener W K, Russell S A, Sayavedra-Soto L A. Ammonia monooxygenase from Nitrosomonas europaea. In: Lidstrom M E, Tabita F R, editors. Microbial growth on C1 compounds. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1996. pp. 159–166. [Google Scholar]
- 4.Ator M A, Montellano P R O D. Mechanism-based (suicide) enzyme inactivation. In: Sigman D S, Boyer P E, editors. The enzymes. XIX. San Diego, Calif: Academic Press, Inc.; 1990. pp. 214–282. [Google Scholar]
- 5.Buswell J A. Metabolism of phenol and cresols by Bacillus stearothermophilus. J Bacteriol. 1975;124:1077–1083. doi: 10.1128/jb.124.3.1077-1083.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Colby J, Stirling D I, Dalton H. The soluble methane mono-oxygenase of Methylococcus capsulatus (Bath). Its ability to oxygenate n-alkanes, n-alkenes, ethers, and alicyclic, aromatic and heterocyclic compounds. Biochem J. 1977;165:395–402. doi: 10.1042/bj1650395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dalton H, Whittenbury R. The acetylene reduction technique as an assay for the nitrogenase activity in the methane oxidising bacterium Methylococcus capsulatus strain. Bath Arch Microbiol. 1976;109:147–151. [Google Scholar]
- 8.DiSpirito A A, Gulledge J, Shiemke A K, Murrel J C, Lidstrom M E, Krema C L. Trichloroethylene oxidation by the membrane-associated methane monooxygenase in type I, type II and type X methanotrophs. Biodegradation. 1992;2:151–164. [Google Scholar]
- 9.Ensign S A, Hyman M R, Arp D J. Cometabolic degradation of chlorinated alkenes by alkene monooxygenase in a propylene-grown Xanthobacter strain. Appl Environ Microbiol. 1992;58:3038–3046. doi: 10.1128/aem.58.9.3038-3046.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Folsom B R, Chapman P J, Pritchard P H. Phenol and trichloroethylene degradation by Pseudomonas cepacia G4: kinetics and interactions between substrates. Appl Environ Microbiol. 1990;56:1279–1285. doi: 10.1128/aem.56.5.1279-1285.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fox B G, Shanklin J, Ai J, Loehr T M, Sanders-Loehr J. Resonance raman evidence for an Fe-O-Fe center in stearoyl-ACP desaturase. Primary sequence identity with other diiron-oxo proteins. Biochemistry. 1994;33:12776–12786. doi: 10.1021/bi00209a008. [DOI] [PubMed] [Google Scholar]
- 12.Gornall A G, Bardawill C J, David M M. Determination of serum proteins by means of the Biuret reaction. J Biol Chem. 1949;177:751–766. [PubMed] [Google Scholar]
- 13.Gossett J M. Measurement of Henry’s law constants for C1 and C2 chlorinated hydrocarbons. Environ Sci Technol. 1987;21:202–208. [Google Scholar]
- 14.Hou C T, Patel R, Laskin A I, Barnabe N, Barist I. Epoxidation of short alkenes by resting-cell suspensions of propane-grown bacteria. Appl Environ Microbiol. 1983;46:171–177. doi: 10.1128/aem.46.1.171-177.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hyman M R, Arp D J. Acetylene inhibition of metalloenzymes. Anal Biochem. 1988;173:207–220. doi: 10.1016/0003-2697(88)90181-9. [DOI] [PubMed] [Google Scholar]
- 16.Hyman M R, Murton I B, Arp D J. Interaction of ammonia monooxygenase from Nitrosomonas europaea with alkanes, alkenes, and alkynes. Appl Environ Microbiol. 1988;54:3187–3190. doi: 10.1128/aem.54.12.3187-3190.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hyman M R, Wood P M. Suicidal inactivation and labeling of ammonia monooxygenase by acetylene. Biochem J. 1985;227:719–725. doi: 10.1042/bj2270719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hynes R K, Knowles R. Inhibition by acetylene of ammonia oxidation in Nitrosomonas europaea. FEMS Microbiol Lett. 1978;4:319–321. doi: 10.1093/femsle/fnx068. [DOI] [PubMed] [Google Scholar]
- 19.Landa A S, Sipkema E M, Weijma J, Beenackers A A C M, Dolfing J, Janssen D B. Cometabolic degradation of trichloroethylene by Pseudomonas cepacia G4 in a chemostat with toluene as the primary substrate. Appl Environ Microbiol. 1994;60:3368–3374. doi: 10.1128/aem.60.9.3368-3374.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McClay K, Fox B G, Steffan R J. Chloroform mineralization by toluene-oxidizing bacteria. Appl Environ Microbiol. 1996;62:2716–2722. doi: 10.1128/aem.62.8.2716-2722.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Newman L M, Wackett L P. Purification and characterization of toluene 2-monooxygenase from Burkholderia cepacia G4. Biochemistry. 1995;34:14066–14076. doi: 10.1021/bi00043a012. [DOI] [PubMed] [Google Scholar]
- 22.Newman L M, Wackett L P. Trichloroethylene oxidation by purified toluene 2-monooxygenase: products, kinetics, and turnover-dependent inactivation. J Bacteriol. 1997;179:90–96. doi: 10.1128/jb.179.1.90-96.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oldenhuis R, Vink R L J M, Janssen D B, Witholt B. Degradation of chlorinated aliphatic hydrocarbons by Methylosinus trichosporium OB3b expressing soluble methane monooxygenase. Appl Environ Microbiol. 1989;55:2819–2826. doi: 10.1128/aem.55.11.2819-2826.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ortiz de Montellano P R, Almira Correia M. Inhibition of cytochrome P450 enzymes. In: Ortiz de Montellano P R, editor. Cytochrome P450. 2nd ed. New York, N.Y: Plenum Press; 1991. pp. 305–364. [Google Scholar]
- 25.Prior S D, Dalton H. Acetylene as a suicide substrate and active site probe for methane monooxygenase from Methylococcus capsulatus (Bath) FEMS Microbiol Lett. 1985;29:105–109. [Google Scholar]
- 26.Shields M S, Montgomery S O, Chapman P J, Cuskey S M, Pritchard P H. Mutants of Pseudomonas cepacia G4 defective in catabolism of aromatic compounds and trichloroethylene. Appl Environ Microbiol. 1991;57:1935–1941. doi: 10.1128/aem.57.7.1935-1941.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shields M S, Montgomery S O, Chapman P J, Cuskey S M, Pritchard P H. Novel pathway of toluene catabolism in the trichloroethylene-degrading bacterium G4. Appl Environ Microbiol. 1989;55:1624–1629. doi: 10.1128/aem.55.6.1624-1629.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Silverman R B. Mechanism-based enzyme inactivation: chemistry and enzymology. Vol. 2. Boca Raton, Fla: CRC Press, Inc.; 1988. Oxygenation reactions; pp. 191–252. [Google Scholar]
- 29.Silverman R B. Mechanism-based enzyme inactivation: chemistry and enzymology. Vol. 1. Boca Raton, Fla: CRC Press, Inc.; 1988. The birth of mechanism-based enzyme inactivation; pp. 3–33. [Google Scholar]
- 30.Stirling D I, Dalton H. Effect of metal-binding agents and other compounds on methane oxidation by two strains of Methylococcus capsulatus. Arch Microbiol. 1977;114:71–76. doi: 10.1007/BF00429633. [DOI] [PubMed] [Google Scholar]
- 31.Tsein H, Brusseau G A, Hanson R S, Wackett L P. Biodegradation of trichloroethylene by Methylosinus trichosporium OB3b. Appl Environ Microbiol. 1989;55:3155–3161. doi: 10.1128/aem.55.12.3155-3161.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Waley S G. Kinetics of suicide substrates. Practical procedures for determining parameters. Biochem J. 1985;227:843–849. doi: 10.1042/bj2270843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walsh C. Suicide substrates: mechanism-based enzyme inactivators. Tetrahedron. 1982;38:871–909. [Google Scholar]
- 34.Wilhelm E, Battino R, Wilcock R J. Low-pressure solubility of gases in liquid water. Chem Rev. 1977;77:219–262. [Google Scholar]
- 35.Wilkins R G. Binuclear iron centres in proteins. Chem Soc Rev. 1992;21:171–178. [Google Scholar]