Abstract
BACKGROUND
Bronchopulmonary dysplasia is a prevalent complication after extremely preterm birth. Inflammation with mechanical ventilation may contribute to its development. Whether hydrocortisone treatment after the second postnatal week can improve survival without bronchopulmonary dysplasia and without adverse neurodevelopmental effects is unknown.
METHODS
We conducted a trial involving infants who had a gestational age of less than 30 weeks and who had been intubated for at least 7 days at 14 to 28 days. Infants were randomly assigned to receive either hydrocortisone (4 mg per kilogram of body weight per day tapered over a period of 10 days) or placebo. Mandatory extubation thresholds were specified. The primary efficacy outcome was survival without moderate or severe bronchopulmonary dysplasia at 36 weeks of postmenstrual age, and the primary safety outcome was survival without moderate or severe neurodevelopmental impairment at 22 to 26 months of corrected age.
RESULTS
We enrolled 800 infants (mean [±SD] birth weight, 715±167 g; mean gestational age, 24.9±1.5 weeks). Survival without moderate or severe bronchopulmonary dysplasia at 36 weeks occurred in 66 of 398 infants (16.6%) in the hydrocortisone group and in 53 of 402 (13.2%) in the placebo group (adjusted rate ratio, 1.27; 95% confidence interval [CI], 0.93 to 1.74). Two-year outcomes were known for 91.0% of the infants. Survival without moderate or severe neurodevelopmental impairment occurred in 132 of 358 infants (36.9%) in the hydrocortisone group and in 134 of 359 (37.3%) in the placebo group (adjusted rate ratio, 0.98; 95% CI, 0.81 to 1.18). Hypertension that was treated with medication occurred more frequently with hydrocortisone than with placebo (4.3% vs. 1.0%). Other adverse events were similar in the two groups.
CONCLUSIONS
In this trial involving preterm infants, hydrocortisone treatment starting on postnatal day 14 to 28 did not result in substantially higher survival without moderate or severe bronchopulmonary dysplasia than placebo. Survival without moderate or severe neurodevelopmental impairment did not differ substantially between the two groups. (Funded by the National Institutes of Health; ClinicalTrials.gov number, NCT01353313.)
Bronchopulmonary dysplasia remains prevalent after extremely preterm birth, affecting about half of survivors, and has not decreased over time.1–5 Its cause is multifactorial; prematurity, mechanical ventilation, oxygen exposure, and inflammation are postulated to contribute to its development.3 A diagnosis of bronchopulmonary dysplasia is associated with subsequent abnormal growth, neurodevelopment, and respiratory function.5–7 Dexamethasone, a powerful glucocorticoid, became widely used in the 1990s after reports of shortterm respiratory benefit.8,9 Use declined when adverse effects emerged, particularly neurodevelopmental effects such as cognitive impairment and cerebral palsy4,10–12; however, many extremely preterm infants continue to be exposed.4,12
Dexamethasone is not an ideal therapeutic agent owing to its long biologic half-life, suppression of cortisol production, and disruption of the balance between mineralocorticoid and glucocorticoid signaling in the brain.13 Hydrocortisone has several potentially advantageous qualities. Like cortisol, it binds to both mineralocorticoid and glucocorticoid receptors in the brain. In animal models, hydrocortisone has neither the apoptotic effects of dexamethasone in the hippocampus nor the growth-restricting effects on brain and body weight.13–15 Randomized trials of hydrocortisone involving human infants are limited; however, in a recent randomized trial of hydrocortisone initiated between postnatal days 7 and 14 with a starting dose of 5 mg per kilogram of body weight per day tapered over a period of 3 weeks, there was no evidence of adverse neurodevelopmental effects at 2 years.16
Lower-dose hydrocortisone (1 to 2 mg per kilogram per day, estimated physiologic replacement17), given in the first postnatal week as prophylaxis for relative adrenal insufficiency, decreased the incidence of death or bronchopulmonary dysplasia.18 Whether a higher, antiinflammatory dose of hydrocortisone given later can improve respiratory outcomes was not known when this trial started in 2011. This trial was undertaken to determine both the efficacy of hydrocortisone in increasing survival without bronchopulmonary dysplasia and its longer-term safety, as assessed by survival without moderate or severe neurodevelopmental impairment at 22 to 26 months of age, adjusted for prematurity.
METHODS
TRIAL DESIGN AND OVERSIGHT
This double-masked, placebo-controlled, randomized trial was designed by investigators of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Neonatal Research Network (NRN) and was conducted in its 19 U.S. academic centers (including 50 neonatal intensive care units). Authors from RTI International had full access to the data and take responsibility for its integrity and the accuracy of analysis. The first author drafted the manuscript. Other author contributions are described in the Supplementary Appendix (available with the full text of this article at NEJM.org); all the authors vouch for the accuracy and completeness of the data and for the fidelity of the trial to the protocol (available at NEJM.org). The trial was approved by institutional review boards at each center; after written consent was obtained from a parent or guardian, infants were enrolled from August 22, 2011, to February 4, 2018. Follow-up visits occurred from August 18, 2013, to March 29, 2020.
PARTICIPANTS
Infants were eligible from 14 to 28 postnatal days if they had an estimated gestational age at birth of less than 30 weeks, were born at or admitted to an NRN site at no more than 72 hours of postnatal age, had received mechanical ventilation through an endotracheal tube for at least 7 days, and were receiving mechanical ventilation through an endotracheal tube at trial entry. Among the exclusions were major congenital anomalies, a decision to limit intensive life support, indomethacin or ibuprofen treatment within 48 hours before trial entry, and previous systemic glucocorticoid treatment for bronchopulmonary dysplasia. Infants were also excluded if they had received hydrocortisone or other systemic glucocorticoids for at least 14 cumulative days or within 7 days before trial entry.
RANDOMIZATION
Randomization was performed individually in a 1:1 ratio by the NRN Data Coordinating Center at RTI International with the use of permuted-block randomization (in blocks of two and four), with stratification performed within center according to gestational age (<27 weeks vs. 27 to <30 weeks). Pharmacy personnel prepared masked hydrocortisone or placebo; all the other personnel remained unaware of the trial-group assignments.
INTERVENTION
Infants received saline placebo or hydrocortisone sodium succinate (Solu-Cortef plain, Pfizer), administered intravenously or orally (if no intravenous line was available) and tapered over a period of 10 days (4 mg per kilogram per day for 2 days, 2 mg per kilogram per day for 3 days, 1 mg per kilogram per day for 3 days, and 0.5 mg per kilogram per day for 2 days, on the basis of a review of antiinflammatory dosing schedules17). Mandatory extubation thresholds were specified. Although extubation could be elected at the discretion of the attending physician, an extubation attempt was required within 72 hours after starting hydrocortisone or placebo and within 24 hours after the following criteria had been met: a fraction of inspired oxygen (Fio2) of less than 0.40 to maintain a saturation of at least 88%, a mean airway pressure of less than 8 cm of water, and a hemodynamically stable condition in the opinion of the clinical team. Trial personnel reviewed settings daily to monitor adherence. Successful extubation was defined as remaining extubated for at least 1 week, including at least 3 days after the last dose of hydrocortisone or placebo.
PRIMARY OUTCOME
The primary outcome included both efficacy and safety measures. The efficacy outcome was improvement in survival without physiologically defined moderate or severe bronchopulmonary dysplasia at 36 weeks of postmenstrual age, defined as the use of supplemental oxygen, positive-pressure ventilation, or both to maintain an oxygen saturation of more than 90%. An ambient-air challenge was performed for infants estimated to be receiving an Fio2 of less than 0.30 by nasal cannula.6,19 The safety outcome was survival without moderate or severe neurodevelopmental impairment at 22 to 26 months of corrected age, determined by an in-person trial visit and defined as any of the following: a Bayley Scales of Infant and Toddler Development–III (Bayley-III) cognitive composite score of less than 85 (standardized mean [±SD], 100±15; range, 55 to 145) or motor composite score of less than 85 (standardized mean, 100; range, 45 to 155), with lower scores indicating greater impairment20; a Gross Motor Function Classification System (GMFCS) level of at least II (on a scale from level 0 to V, with 0 indicating normal gross motor function and higher levels indicating greater impairment)21; severe vision impairment in both eyes (consistent with a visual acuity of <20/200); or bilateral hearing impairment with or without amplification (on the basis of observation during the trial visit; report by the parent, guardian, or primary caregiver; or chart review). The Bayley-III, neurosensory, and neurologic examinations were administered by annually certified examiners who were unaware of the trial-group assignments.22
SECONDARY OUTCOMES
The secondary outcomes were standard complications and growth measures after extremely preterm birth at 36 weeks of postmenstrual age and at 22 to 26 months of corrected age; successful extubation during the intervention period, defined as remaining extubated for at least 1 week, including at least 3 days after the last dose of hydrocortisone or placebo; use of open-label dexamethasone; and respiratory status at 40 weeks of postmenstrual age, according to the same measures used at 36 weeks. According to the protocol, dexamethasone was restricted to infants remaining on mechanical ventilation. Use of hydrocortisone after the treatment period was a protocol violation. Specific adverse events of interest included spontaneous gastrointestinal perforation, culture-proven sepsis, hyperglycemia resulting in insulin treatment, and hypertension treated with antihypertensive agents.
STATISTICAL ANALYSIS
A sample size of 800 infants was chosen for 80% power to detect an increase in survival without moderate or severe bronchopulmonary dysplasia of 10 percentage points from a baseline survival of 35% or less (estimated from NRN data); a two-sided type I error of 0.05 was used. Simulations were used to ensure a probability of at least 80% of a successful safety evaluation, defined as follows. On the basis of previous data, we anticipated that hydrocortisone treatment would be associated with improved survival without moderate or severe neurodevelopmental impairment.23,24 We did not anticipate finding a significant benefit with this sample size; therefore, the safety evaluation was considered to be successful if either the risk of death or neurodevelopmental impairment was lower in the hydrocortisone group than in the placebo group or there was a higher risk of death or neurodevelopmental impairment in the hydrocortisone group than in the placebo group but the lower limit of a one-sided 95% confidence interval for the ratio of increased benefit for bronchopulmonary dysplasia to increased risk of neurodevelopmental impairment was equal to or greater than 4.
Eight protocol-specified interim analyses were conducted after every 100 infants reached status (death, discharge, transfer, or 120 postnatal days) to compare the incidence of serious adverse events (nosocomial sepsis, intestinal perforation, respiratory deterioration or pneumonia, and death) between the two trial groups. A Pocock boundary of ±2.555, with a corresponding significance level of 0.011, was used for the interim safety evaluation.25 Two futility analyses were conducted to evaluate the possibility of stopping the trial when approximately 400 and 600 infants had been evaluated for the primary efficacy outcome. Both design-based conditional power and the conditional power under current trend were computed.26 Because we did not have hypothesized effect sizes for the secondary outcomes, only the conditional power under current trend was computed for these outcomes. If these analyses showed a design-based conditional power for the primary outcome of 0.1 or less and provided the data and safety monitoring committee with little evidence of a benefit for any of the secondary outcomes, the data and safety monitoring committee was to consider stopping enrollment owing to futility.
All analyses were conducted according to the intention-to-treat principle with the use of SAS software, version 14.3 (SAS Institute).27 Robust Poisson regression with control for center and gestational-age strata as fixed effects was used to obtain adjusted relative risk (rate ratio) estimates and 95% confidence intervals for the primary efficacy and safety outcomes and binary secondary outcomes.28 There was no prespecified plan to adjust for multiplicity of testing for the secondary outcomes, and a P value is reported only for the primary outcome (with a two-sided P value of <0.05 considered to indicate statistical significance); all other analyses are reported as point estimates and 95% confidence intervals and are considered to be exploratory. Missing data regarding the primary safety outcome were assumed to be missing completely at random; sensitivity analyses were conducted by assuming all missing outcomes were either events or nonevents.
When the model failed to converge owing to sparse outcome data at some centers, Mantel–Haenszel estimates for stratified data were obtained.29 Because the robust Poisson regression models did not converge, the Gail–Simon test for interactions between treatment effects and infant subgroups was used to test for the heterogeneity of the treatment effect on the risk of the primary efficacy outcome according to center, gestational-age strata, and sex.30 In addition, Fenton growth curve z scores for weight, length, and head circumference were calculated and analyzed with the use of linear regression.31 Adverse events were analyzed with the use of Poisson regression, and duration of respiratory support and length of hospital stay were analyzed with the use of median regression. All models were adjusted for center and gestational-age strata. The Cox proportional-hazards model was used to compare timing of successful extubation, and Kaplan–Meier estimates of the probability of remaining intubated within 14 days were computed.32
RESULTS
TRIAL POPULATION
Details on screening, randomization, and follow-up are shown in Figure 1. The mean (±SD) birth weight was 715±167 g, and the mean gestational age was 24.9±1.5 weeks. The outcome of survival without moderate or severe bronchopulmonary dysplasia was known for all enrolled infants, and the outcome of survival without moderate or severe neurodevelopmental impairment was known for 91.0%. Maternal and infant demographic characteristics are shown in Table 1. Baseline characteristics did not differ substantially between the two groups except for sex, with more boys assigned to placebo than to hydrocortisone. Characteristics of enrolled infants were similar to those eligible but not enrolled (Table S5 in the Supplementary Appendix) and were representative of the wider population affected by bronchopulmonary dysplasia (Table S6).
Figure 1 (facing page). Recruitment, Randomization, and Follow-up.
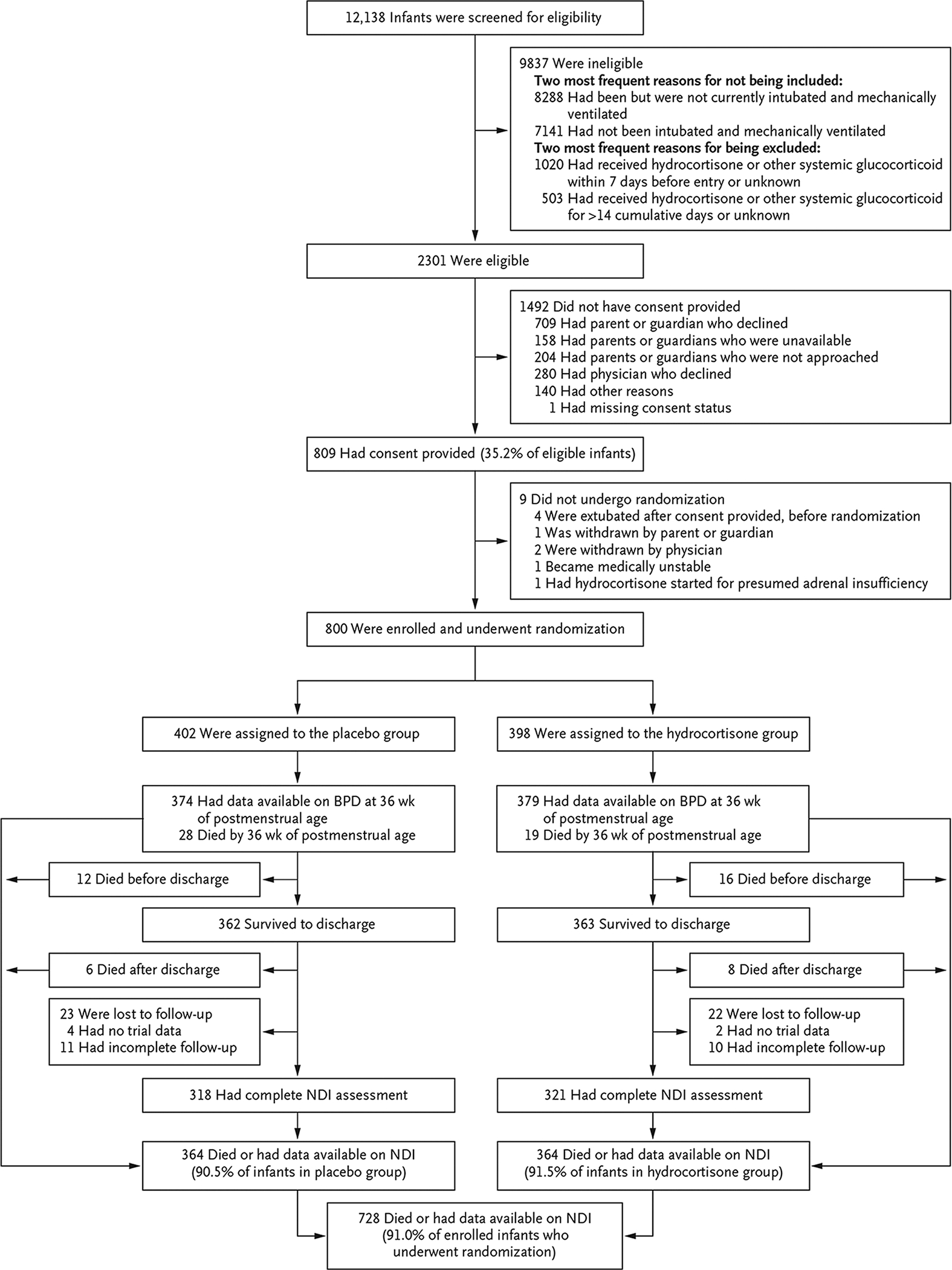
Infants were enrolled at 50 hospitals; however, the centers participating in the Neonatal Research Network changed over time, and the timing of approval by institutional review boards varied. Therefore, the number of hospitals enrolling at any given time was lower than 50. Although 1523 infants were excluded for previous receipt of glucocorticoids, only 339 of these infants had received glucocorticoid therapy for bronchopulmonary dysplasia (BPD). Thus, 3.4% of the 9837 ineligible infants were excluded for this reason. The majority (1184) had received early therapy for hypotension. There were 11 infants for whom the severity of neurodevelopmental impairment (NDI) could not be determined. According to the protocol for the follow-up study, from which we obtained the NDI data in our trial, NDI could be determined if a component of a binary indicator in the NDI definition was known as “Yes,” but the severity level could be determined only when other components of level of severity were not missing.
Table 1.
Characteristics of the Infants at Baseline.*
| Characteristic | Hydrocortisone (N = 398) | Placebo (N = 402) |
|---|---|---|
| Maternal age — yr | 28.5±6.2 | 28.2±6.4 |
| Race — no./total no. (%)† | ||
| Black | 139/388 (35.8) | 168/391 (43.0) |
| White | 231/388 (59.5) | 206/391 (52.7) |
| Other | 18/388 (4.6) | 17/391 (4.3) |
| Hispanic or Latino ethnic group — no./total no. (%)† | 66/392 (16.8) | 60/397 (15.1) |
| Mother’s education — no./total no. (%) | ||
| Less than high school diploma | 68/396 (17.2) | 59/397 (14.9) |
| High school diploma | 101/396 (25.5) | 108/397 (27.2) |
| Partial college | 78/396 (19.7) | 85/397 (21.4) |
| College degree or more | 66/396 (16.7) | 69/397 (17.4) |
| Unknown | 83/396 (21.0) | 76/397 (19.1) |
| Received any antenatal glucocorticoids — no./total no. (%) | 343/395 (86.8) | 357/402 (88.8) |
| Birth weight — g | 710.3±162.7 | 720.4±171.8 |
| Gestational age — wk | 24.8±1.5 | 24.9±1.5 |
| Male sex — no (%) | 186 (46.7) | 235 (58.5) |
| Median 5-min Apgar score (5th to 95th percentile) | 6 (1–9) | 6 (2–9) |
| Small for gestational age — no. (%) | 46 (11.6) | 44 (10.9) |
| Multiple birth — no. (%) | 95 (23.9) | 102 (25.4) |
| 5-min Apgar score ≤5 — no./total no. (%) | 162/396 (40.9) | 155/397 (39.0) |
| Median no. of postnatal days at randomization (5th to 95th percentile) | 21 (14–28) | 21 (15–28) |
| Highest mode of support at trial entry — no./total no. (%) | ||
| High-frequency ventilator | 131/398 (32.9) | 119/401 (29.7) |
| Conventional ventilator | 265/398 (66.6) | 282/401 (70.3) |
| Nasal IPPV | 2/398 (0.5)‡ | 0/401 |
| Fio2 at trial entry | 0.52±0.21 | 0.50±0.20 |
| Respiratory index at trial entry§ | 596±350 | 569±319 |
Plus–minus values are means ±SD. Percentages may not total 100 because of rounding. Fio2 denotes fraction of inspired oxygen, and IPPV intermittent positive-pressure ventilation.
Race and ethnic group were reported by the parent or guardian.
Infants were intubated at randomization but extubated before administration of hydrocortisone or placebo.
The respiratory index was calculated as mean airway pressure × Fio2 × 100.
PRIMARY EFFICACY OUTCOME
At 36 weeks of postmenstrual age, 66 of 398 infants (16.6%) in the hydrocortisone group were alive without moderate or severe bronchopulmonary dysplasia, as compared with 53 of 402 infants (13.2%) in the placebo group (adjusted rate ratio, 1.27; 95% confidence interval [CI], 0.93 to 1.74) (Table 2). Results for components of the primary outcome were similar in the two groups (Table 2), as was the severity of bronchopulmonary dysplasia in survivors33 (Table 3). The rate ratios and 95% confidence intervals in subgroups defined by sex, gestational-age strata, and center are shown in Figure S1. The Gail–Simon test did not reveal evidence of differing effect according to center, gestational-age stratum, sex, or birth year; however, an interaction cannot be ruled out, because the trial was not powered for subgroup effects.
Table 2.
Primary Outcomes and Their Components.*
| Outcome | Hydrocortisone (N = 398) | Placebo (N = 402) | Rate Ratio (95% CI)† |
|---|---|---|---|
| Efficacy | |||
| Survival without moderate or severe BPD at 36 wk of postmenstrual age — no. (%) | 66 (16.6) | 53 (13.2) | 1.27 (0.93–1.74) |
| Death by 36 wk of postmenstrual age — no. (%) | 19 (4.8) | 28 (7.0) | 0.66 (0.38–1.16)‡ |
| Moderate or severe BPD at 36 wk of postmenstrual age — no./total no. (%) | 313/379 (82.6) | 321/374 (85.8) | 0.96 (0.91–1.02) |
| Safety § ¶ | |||
| Survival without moderate or severe NDI — no./total no. (%) | 132/358 (36.9) | 134/359 (37.3) | 0.98 (0.81–1.18) |
| Survival without severe NDI — no./total no. (%) | 230/358 (64.2) | 231/359 (64.3) | 1.01 (0.90–1.12) |
| Known to have died by follow-up — no. (%) | 43 (10.8) | 46 (11.4) | 0.91 (0.62–1.34)‡ |
| Moderate or severe NDI in survivors — no./total no. (%) | 183/315 (58.1) | 179/313 (57.2) | 1.03 (0.90–1.17) |
BPD denotes bronchopulmonary dysplasia, and CI confidence interval.
Rate ratios were adjusted for center and gestational-age strata.
Shown is the Mantel–Haenszel estimate.
Neurodevelopmental impairment (NDI) was defined as one or more of the following: a Bayley Scales of Infant and Toddler Development–III (Bayley-III) cognitive score of less than 85 (standardized mean [±SD], 100±15; range, 55 to 145) or a Bayley-III motor score of less than 85 (standardized mean, 100; range, 45 to 155), with lower scores indicating greater impairment; a Gross Motor Function Classification System (GMFCS) level of at least II (on a scale from level I to V, with I indicating normal and higher levels indicating greater impairment); severe vision impairment in both eyes (consistent with a visual acuity of <20/200); or bilateral hearing impairment with or without amplification (on the basis of observation during the trial visit; report by the parent, guardian, or primary caregiver; or chart review).
NDI severity could not be determined for 11 infants. According to the protocol for the follow-up study, from which we obtained the NDI data in our trial, NDI could be determined if a component of a binary indicator in the NDI definition was known as “Yes,” but the severity level could be determined only when all components of level of severity were documented.
Table 3.
Other In-hospital Outcomes and Other Outcomes at 22 to 26 Months.*
| Outcome | Hydrocortisone | Placebo | Rate Ratio (95% CI)† |
|---|---|---|---|
| Other in-hospital outcomes | |||
| Death before discharge — no./total no. (%) | 35/398 (8.8) | 40/402 (10.0) | 0.86 (0.56 to 1.31) |
| BPD grade at 36 wk of postmenstrual age — no./total no. (%)‡ | |||
| No support, ambient air | 40/375 (10.7) | 33/367 (9.0) | 1.18 (0.78 to 1.80) |
| Supplemental oxygen through nasal cannula at flow rate of ≤2 liters/min | 120/375 (32.0) | 124/367 (33.8) | 0.94 (0.77 to 1.15) |
| Supplemental oxygen through nasal cannula at flow rate of >2 liters/min, CPAP, or nasal IPPV | 152/375 (40.5) | 159/367 (43.3) | 0.94 (0.80 to 1.10) |
| Invasive positive-pressure ventilation | 63/375 (16.8) | 51/367 (13.9) | 1.21 (0.87 to 1.69) |
| Median no. of days of mechanical ventilation to 36 wk of postmenstrual age among survivors to 36 wk (5th to 95th percentile)§ | 37 (18 to 80) | 40 (19 to 78) | −3 (−5 to −1)¶ |
| Median duration of oxygen supplementation up to status among survivors to discharge (5th to 95th percentile) — days | 105 (51 to 120) | 104 (55 to 120) | 0 (−3 to 3)¶ |
| Median length of hospital stay among survivors to discharge (5th to 95th percentile) — days | 125 (71 to 297) | 122 (40 to 250) | 3 (−2 to 8)¶ |
| Dexamethasone given before 36 wk of postmenstrual age — no./total no. (%) | 150/378 (39.7) | 157/373 (42.1) | 0.95 (0.80 to 1.12)‖ |
| z Score for weight for age at 36 wk | −1.68±1.01 | −1.65±0.98 | −0.04 (−0.17 to 0.09)** |
| z Score for height for age at 36 wk | −2.33±1.16 | −2.21±1.14 | −0.13 (−0.29 to 0.04)** |
| z Score for head circumference for age at 36 wk | −1.68±1.34 | −1.74±1.17 | 0.06 (−0.11 to 0.23)** |
| Other 2-yr follow-up outcomes | |||
| Severity of NDI on Bayley-III — no./total no. (%)†† | |||
| Normal or mild | 132/315 (41.9) | 134/313 (42.8) | 0.97 (0.81 to 1.15) |
| Moderate | 98/315 (31.1) | 97/313 (31.0) | 1.02 (0.81 to 1.27) |
| Severe or profound | 85/315 (27.0) | 82/313 (26.2) | 1.01 (0.78 to 1.31)‖ |
| GMFCS level of II or higher — no./total no. (%) | 48/331 (14.5) | 41/329 (12.5) | 1.14 (0.77 to 1.69) |
| Moderate or severe cerebral palsy — no./total no. (%)‡‡ | 41/330 (12.4) | 33/329 (10.0) | 1.20 (0.78 to 1.83)‖ |
| Severe hearing impairment — no./total no. (%) | 9/328 (2.7) | 14/326 (4.3) | 0.64 (0.28 to 1.48)‖ |
| No or some functional vision — no./total no. (%) | 5/330 (1.5) | 9/329 (2.7) | 0.53 (0.17 to 1.63)‖ |
| z Score for follow-up weight | |||
| Infants with available data | 316 | 319 | |
| Mean | −0.51±1.04 | −0.44±1.15 | −0.08 (−0.25 to 0.09)** |
| z Score for follow-up length | |||
| Infants with available data | 314 | 316 | |
| Mean | −0.93±1.17 | −0.94±1.22 | −0.01 (−0.19 to 0.17)** |
| z Score for follow-up head circumference | |||
| Infants with available data | 301 | 314 | |
| Mean | −0.45±1.41 | −0.35±1.42 | −0.12 (−0.34 to 0.10)** |
Plus–minus values are means ±SD. CPAP denotes continuous positive airway pressure.
Widths of 95% confidence intervals were not adjusted for multiplicity and are provided for descriptive purposes only.
BPD was graded according to the grades developed by Jensen et al.33 An ambient-air challenge was performed for infants estimated to be receiving an Fio2 of less than 0.30 by nasal cannula6,19; therefore, some infants receiving oxygen by nasal cannula were determined not to have physiologically defined BPD.
Mechanical ventilation was defined as a high-frequency ventilator or a conventional ventilator.
Shown is the median difference (95% CI).
Shown is the Mantel–Haenszel estimate.
Shown is the mean difference (95% CI).
NDI severity could not be determined for 11 infants. According to the protocol of the follow-up study, NDI could be determined if a component of a binary indicator in the NDI definition was known as “Yes,” but the severity level could be determined only when all components of level of severity were known.
Cerebral palsy was diagnosed when there were definite abnormalities observed in the neuromotor examination, and functional challenges were classified as moderate if the GMFCS level was II or III and severe if the GMFCS level was IV or V.34
PRIMARY SAFETY OUTCOME
At 22 to 26 months of corrected age, survival without moderate or severe neurodevelopmental impairment occurred in 132 of 358 infants (36.9%) in the hydrocortisone group and in 134 of 359 (37.3%) in the placebo group (adjusted rate ratio, 0.98; 95% CI, 0.81 to 1.18) (Table 2). In sensitivity analyses that accounted for missing data regarding the primary safety outcome, the results were materially unchanged, regardless of whether missing outcomes were assumed to be events (adjusted rate ratio, 0.99; 95% CI, 0.82 to 1.20) or nonevents (adjusted rate ratio, 0.98; 95% CI, 0.84 to 1.14). Other neurodevelopmental and growth outcomes did not differ substantially between the two groups (Tables 3 and S1).
SECONDARY EFFICACY OUTCOMES
During the treatment period, more infants in the hydrocortisone group were successfully extubated than in the placebo group (178 of 398 [44.7%] vs. 135 of 402 [33.6%]) (Fig. 2). The rate ratio for extubation was 1.54 (95% CI, 1.23 to 1.93). The hydrocortisone group had fewer days of mechanical ventilation than the placebo group before 36 weeks of postmenstrual age (median, 37 days vs. 40 days; median difference, −3 days; 95% CI, −5 to −1) and before status (120 postnatal days; median, 37 days vs. 41 days; median difference, −4 days; 95% CI, −7 to −1). However, similar numbers in each group had been extubated by 36 weeks of postmenstrual age, and total duration of supplemental oxygen therapy and length of hospital stay did not differ substantially between the two groups (Table 3). Open-label dexamethasone was administered to 39.7% of the infants in the hydrocortisone group and 42.1% of those in the placebo group (Table 3). Other secondary outcomes were similar between the two groups (Tables 3 and S1).
Figure 2. Infants Extubated According to Day of Treatment.
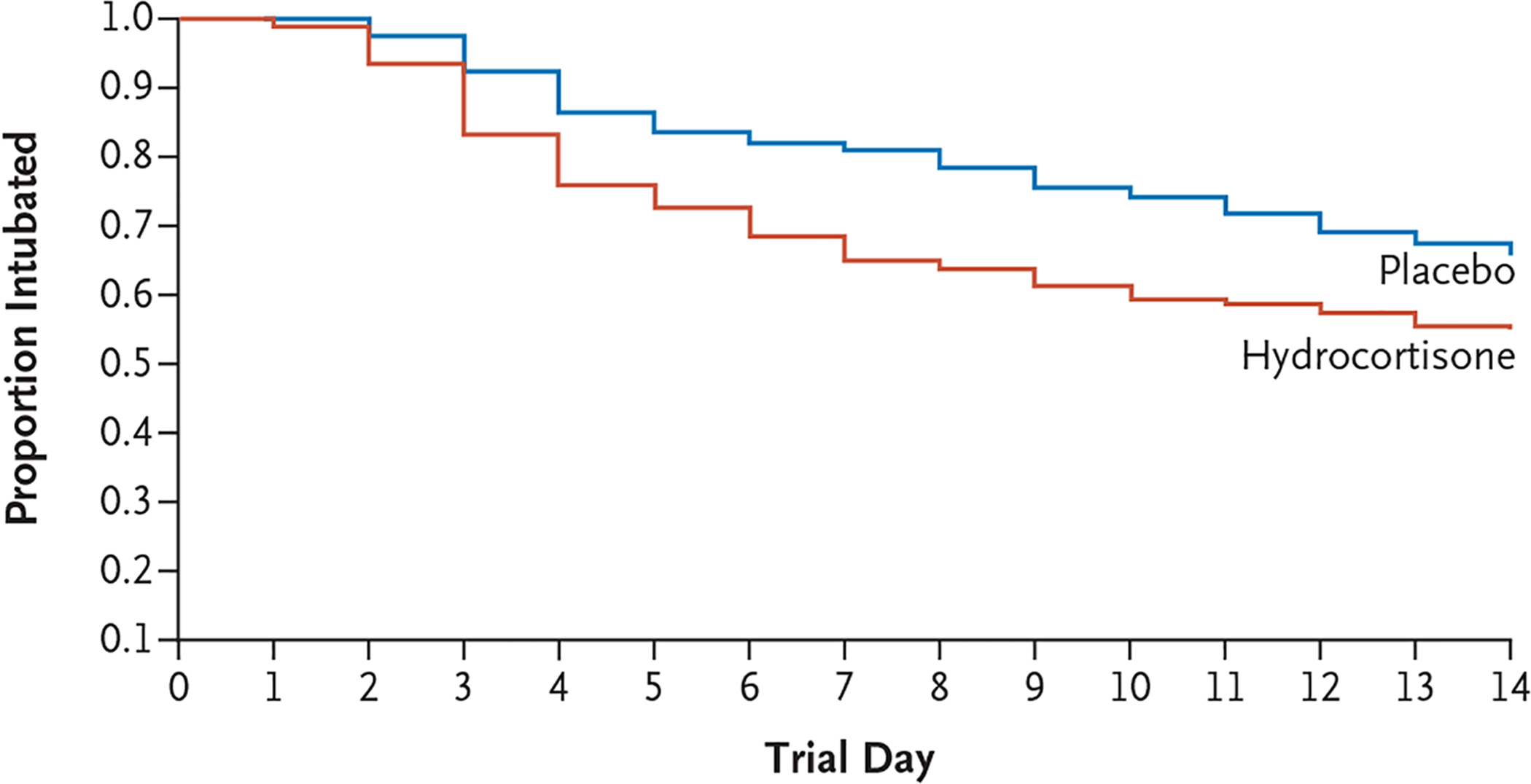
Shown are Kaplan–Meier estimates of the proportion of infants remaining intubated within the first 14 days of the trial. The probability of being extubated by the end of the treatment period was 44.7% in the hydrocortisone group and 33.6% in the placebo group. The rate ratio for extubation estimated from the proportional-hazards model was 1.54 (95% CI, 1.23 to 1.93).
ADVERSE EVENTS AND PROTOCOL VIOLATIONS
Hypertension was more common in the hydrocortisone group; 4.3% were treated with antihypertensive medication, as compared with 1.0% in the placebo group (adjusted rate ratio, 4.27; 95% CI, 1.45 to 12.55) (Table S2). No infant was discharged while receiving antihypertensive medication. The frequency of other adverse events and causes of death were similar in the two groups, as shown in Tables S2 (adverse events) and S3 (causes of death). Protocol violations were also similar in the two groups (Table S4); the most frequent violations were a missed dose (7.0% in the hydrocortisone group and 8.0% in the placebo group) and dexamethasone treatment within the 14-day trial period (6.8% and 5.7%, respectively).
DISCUSSION
In this multicenter, randomized, placebo-controlled trial involving infants at high risk for bronchopulmonary dysplasia, hydrocortisone treatment did not lead to substantially higher survival without moderate or severe bronchopulmonary dysplasia at 36 weeks of postmenstrual age than placebo, nor did hydrocortisone treatment alter the severity of bronchopulmonary dysplasia assessed at that time. The great majority of infants (634 of 753, 84.2%) had received a diagnosis of moderate or severe bronchopulmonary dysplasia at 36 weeks, a finding similar to that of previous reports of infants mechanically ventilated for more than 36 days.35 Thus, our hypothesis that earlier extubation would result in a decreased incidence of moderate or severe bronchopulmonary dysplasia or death was not supported by these results. Survival without moderate or severe neurodevelopmental impairment and other growth and neurodevelopmental outcomes assessed at 2-year follow-up were similar in the two groups.
These results are consistent with those of the only known previous multicenter, randomized, controlled trial of hydrocortisone treatment begun after the first postnatal week, which enrolled 372 intubated infants between postnatal day 7 and day 14 and provided treatment for 22 days, starting with 5 mg per kilogram per day.36 That trial also showed that hydrocortisone increased the incidence of extubation during the treatment period but did not result in a significant improvement in survival without moderate or severe bronchopulmonary dysplasia. Outcomes at 2 years showed no significant difference in death or neurodevelopmental impairment between the trial groups.16 In contrast, randomized, controlled trials involving the use of lower-dose hydrocortisone in the first postnatal week as prophylaxis for possible relative adrenal insufficiency (1 to 2 mg per kilogram per day, estimated physiologic replacement dose17) showed a significant improvement in survival without moderate or severe bronchopulmonary dysplasia, as assessed in an individual patient data meta-analysis.18 Because early lung inflammation has been linked to bronchopulmonary dysplasia, earlier therapy could be more effective by ameliorating early adrenal insufficiency and thereby decreasing early lung inflammation,37,38 whereas later therapy may be ineffective owing to the presence of established inflammatory injury.
In reviews of glucocorticoid therapy, the authors concluded that although dexamethasone given after the first week probably decreases the risk of death or bronchopulmonary dysplasia, no recommendation can be made regarding the appropriate dose or timing; in addition, long-term follow up has been inadequate to evaluate neurodevelopmental effects.39,40 Thus, a new multicenter trial is warranted to address these questions.
The only monitored adverse event that was more frequent in the hydrocortisone group than in the placebo group was hypertension (4.3% vs. 1.0% of infants treated with an antihypertensive agent). The incidences of culture-proven sepsis and of necrotizing enterocolitis were similar in the two groups (Table S1). Use of systemic glucocorticoids within the 14-day trial period or later was also similar in the two groups (Tables 3 and S1).
Strengths of this trial include a multicenter design, a larger sample than previous studies, a well-established multicenter clinical trials network, and certification of all the examiners performing the neurologic examinations and Bayley-III assessments at 2 years.22 Limitations include the facts that only 35% of the eligible patients were enrolled and that there was a high incidence of subsequent treatment with open-label dexamethasone. Although this incidence is similar to that in many previous studies,40 it may limit interpretation of results. Only infants surviving to 14 days were eligible, which may limit generalizability. In addition, outcomes were limited to 2 years of follow-up. Currently, these children are being evaluated at 5 to 6 years to assess pulmonary and developmental outcomes.
We found that a 10-day tapering course of hydrocortisone for extremely preterm infants who remained intubated at 14 to 28 days did not result in substantially higher survival without moderate or severe bronchopulmonary dysplasia at 36 weeks of postmenstrual age than placebo. We found no adverse effects on growth or neurodevelopment at 2 years of corrected age, confirming the safety of this therapy to 2 years, as used in the trial.
Supplementary Material
Acknowledgments
The National Institutes of Health, including the Eunice Kennedy Shriver National Institute of Child Health and Human Development (U10 HD21373, UG1 HD21364, UG1 HD21385, UG1 HD27851, UG1 HD27853, UG1 HD27856, UG1 HD27880, UG1 HD27904, UG1 HD34216, UG1 HD36790, UG1 HD40492, UG1 HD40689, UG1 HD53089, UG1 HD53109, UG1 HD68244, UG1 HD68270, UG1 HD68278, UG1 HD68263, UG1 HD68284; UG1 HD87226, and UG1 HD87229) and the National Center for Advancing Translational Sciences (UL1 TR6, UL1 TR41, UL1 TR42, UL1 TR77, UL1 TR93, UL1 TR105, UL1 TR442, UL1 TR454, and UL1 TR1117), provided grant support for the Neonatal Research Network, including support for this trial.
APPENDIX
The authors’ full names and academic degrees are as follows: Kristi L. Watterberg, M.D., Michele C. Walsh, M.D., Lei Li, Ph.D., Sanjay Chawla, M.D., Carl T. D’Angio, M.D., Ronald N. Goldberg, M.D., Susan R. Hintz, M.D., M.S.Epi., Matthew M. Laughon, M.D., M.P.H., Bradley A. Yoder, M.D., Kathleen A. Kennedy, M.D., M.P.H., Georgia E. McDavid, R.N., Conra Backstrom-Lacy, R.N., Abhik Das, Ph.D., Margaret M. Crawford, B.S., C.C.R.P., Martin Keszler, M.D., Gregory M. Sokol, M.D., Brenda B. Poindexter, M.D., Namasivayam Ambalavanan, M.D., Anna Maria Hibbs, M.D., M.S.C.E., William E. Truog, M.D., Barbara Schmidt, M.D., Myra H. Wyckoff, M.D., Amir M. Khan, M.D., Meena Garg, M.D., Patricia R. Chess, M.D., Anne M. Reynolds, M.D., Mohannad Moallem, M.D., Edward F. Bell, M.D., Lauritz R. Meyer, M.D., Ravi M. Patel, M.D., Krisa P. Van Meurs, M.D., C. Michael Cotten, M.D., M.H.S., Elisabeth C. McGowan, M.D., Abbey C. Hines, Psy.D., H.S.P.P., Stephanie Merhar, M.D., Myriam Peralta-Carcelen, M.D., M.P.H., Deanne E. Wilson-Costello, M.D., Howard W. Kilbride, M.D., Sara B. DeMauro, M.D., M.S.C.E., Roy J. Heyne, M.D., Ricardo A. Mosquera, M.D., Girija Natarajan, M.D., Isabell B. Purdy, Ph.D., C.P.N.P., Jean R. Lowe, Ph.D., Nathalie L. Maitre, M.D., Ph.D., Heidi M. Harmon, M.D., Laurie A. Hogden, M.D., Ira Adams-Chapman, M.D., Sarah Winter, M.D., William F. Malcolm, M.D., and Rosemary D. Higgins, M.D.
The authors’ affiliations are as follows: the University of New Mexico Health Sciences Center, Albuquerque (K.L.W., C.B.-L., J.R.L.); the Department of Pediatrics, Rainbow Babies and Children’s Hospital, Case Western Reserve University, Cleveland (M.C.W., A.M.H., D.E.W.-C.), Cincinnati Children’s Hospital Medical Center, Department of Pediatrics, University of Cincinnati College of Medicine, Cincinnati (B.B.P., S.M.), and the Department of Pediatrics, Nationwide Children’s Hospital, Ohio State University College of Medicine, Columbus (M.M., N.L.M.) — all in Ohio; the Social, Statistical, and Environmental Sciences Unit, RTI International, Research Triangle Park (L.L.), the Department of Pediatrics, Duke University, Durham (R.N.G., C.M.C., W.F.M.), and the Division of Neonatal–Perinatal Medicine, Department of Pediatrics, University of North Carolina at Chapel Hill, Chapel Hill (M.M.L.) — all in North Carolina; the Department of Pediatrics, Wayne State University (S.C., G.N.), and the Department of Pediatrics, Central Michigan University (S.C.) — both in Detroit; the University of Rochester School of Medicine and Dentistry, Rochester (C.T.D., P.R.C.), and the Department of Pediatrics, University of Buffalo Women’s and Children’s Hospital of Buffalo, Buffalo (A.M.R.) — both in New York; the Department of Pediatrics, Division of Neonatal and Developmental Medicine, Stanford University School of Medicine and Lucile Packard Children’s Hospital, Palo Alto (S.R.H., K.P.V.M.), and the Department of Pediatrics, University of California, Los Angeles, Los Angeles (M.G., I.B.P.) — both in California; the Department of Pediatrics, Division of Neonatology, University of Utah School of Medicine, Salt Lake City (B.A.Y., S.W.); the Department of Pediatrics, McGovern Medical School at the University of Texas Health Science Center at Houston, Houston (K.A.K., G.E.M., A.M.K., R.A.M.), and the Department of Pediatrics, University of Texas Southwestern Medical Center, Dallas (M.H.W., R.J.H.) — both in Texas; the Social, Statistical, and Environmental Sciences Unit, RTI International, Rockville (A.D., M.M.C.), and the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Bethesda (R.D.H.) — both in Maryland; the Department of Pediatrics, Women and Infants Hospital, Brown University, Providence, RI (M.K., E.C.M.); the Department of Pediatrics, Indiana University School of Medicine, Indianapolis (G.M.S., A.C.H.); Emory University School of Medicine, Department of Pediatrics, Children’s Healthcare of Atlanta, Atlanta (B.B.P., R.M.P., N.L.M., I.A.-C.); the Division of Neonatology, University of Alabama at Birmingham, Birmingham (N.A., M.P.-C.); the Department of Pediatrics, Children’s Mercy Hospital, Kansas City, MO (W.E.T., H.W.K.); the Department of Pediatrics, University of Pennsylvania Perelman School of Medicine, Philadelphia (B.S., S.B.D.); the Department of Pediatrics, University of Iowa, Iowa City (E.F.B., H.M.H.); the Department of Pediatrics, Sanford School of Medicine, University of South Dakota, Sioux Falls (L.R.M., L.A.H.); and the College of Health and Human Services, George Mason University, Fairfax, VA (R.D.H.).
Footnotes
A complete list of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Neonatal Research Network investigators is provided in the Supplementary Appendix, available at NEJM.org.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
A data sharing statement provided by the authors is available with the full text of this article at NEJM.org.
REFERENCES
- 1.Stoll BJ, Hansen NI, Bell EF, et al. Trends in care practices, morbidity, and mortality of extremely preterm neonates, 1993–2012. JAMA 2015; 314: 1039–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nakashima T, Inoue H, Sakemi Y, Ochiai M, Yamashita H, Ohga S. Trends in bronchopulmonary dysplasia among extremely preterm infants in Japan, 2003–2016. J Pediatr 2021; 230: 119–125. e7. [DOI] [PubMed] [Google Scholar]
- 3.Higgins RD, Jobe AH, Koso-Thomas M, et al. Bronchopulmonary dysplasia: executive summary of a workshop. J Pediatr 2018; 197: 300–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yoder BA, Harrison M, Clark RH. Time-related changes in steroid use and bronchopulmonary dysplasia in preterm infants. Pediatrics 2009; 124: 673–9. [DOI] [PubMed] [Google Scholar]
- 5.Doyle LW, Carse E, Adams A-M, Ranganathan S, Opie G, Cheong JLY. Ventilation in extremely preterm infants and respiratory function at 8 Years. N Engl J Med 2017; 377: 329–37. [DOI] [PubMed] [Google Scholar]
- 6.Natarajan G, Pappas A, Shankaran S, et al. Outcomes of extremely low birth weight infants with bronchopulmonary dysplasia: impact of the physiologic definition. Early Hum Dev 2012; 88: 509–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheong JLY, Doyle LW. An update on pulmonary and neurodevelopmental outcomes of bronchopulmonary dysplasia. Semin Perinatol 2018; 42: 478–84. [DOI] [PubMed] [Google Scholar]
- 8.Avery GB, Fletcher AB, Kaplan M, Brudno DS. Controlled trial of dexamethasone in respirator-dependent infants with bronchopulmonary dysplasia. Pediatrics 1985; 75: 106–11. [PubMed] [Google Scholar]
- 9.Cummings JJ, D’Eugenio DB, Gross SJ. A controlled trial of dexamethasone in preterm infants at high risk for bronchopulmonary dysplasia. N Engl J Med 1989; 320: 1505–10. [DOI] [PubMed] [Google Scholar]
- 10.Yeh TF, Lin YJ, Lin HC, et al. Outcomes at school age after postnatal dexamethasone therapy for lung disease of prematurity. N Engl J Med 2004; 350: 1304–13. [DOI] [PubMed] [Google Scholar]
- 11.Barrington KJ. The adverse neurodevelopmental effects of postnatal steroids in the preterm infant: a systematic review of RCTs. BMC Pediatr 2001; 1: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walsh MC, Yao Q, Horbar JD, Carpenter JH, Lee SK, Ohlsson A. Changes in the use of postnatal steroids for bronchopulmonary dysplasia in 3 large neonatal networks. Pediatrics 2006; 118(5): e1328–e1335. [DOI] [PubMed] [Google Scholar]
- 13.Zhang B, Xu X, Niu F, et al. Corticosterone replacement alleviates hippocampal neuronal apoptosis and spatial memory impairment induced by dexamethasone via promoting brain corticosteroid receptor rebalance after traumatic brain injury. J Neurotrauma 2020; 37: 262–72. [DOI] [PubMed] [Google Scholar]
- 14.Feng Y, Kumar P, Wang J, Bhatt AJ. Dexamethasone but not the equivalent doses of hydrocortisone induces neurotoxicity in neonatal rat brain. Pediatr Res 2015; 77: 618–24. [DOI] [PubMed] [Google Scholar]
- 15.Sze C-I, Lin Y-C, Lin Y-J, Hsieh T-H, Kuo YM, Lin C-H. The role of glucocorticoid receptors in dexamethasone-induced apoptosis of neuroprogenitor cells in the hippocampus of rat pups. Mediators Inflamm 2013; 2013: 628094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Halbmeijer NM, Onland W, Cools F, et al. Effect of systemic hydrocortisone initiated 7 to 14 days after birth in ventilated preterm infants on mortality and neurodevelopment at 2 years’ corrected age: follow-up of a randomized clinical trial. JAMA 2021; 326: 355–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Watterberg KL. Hydrocortisone dosing for hypotension in newborn infants: less is more. J Pediatr 2016; 174: 23–26. e1. [DOI] [PubMed] [Google Scholar]
- 18.Shaffer ML, Baud O, Lacaze-Masmonteil T, Peltoniemi OM, Bonsante F, Watterberg KL. Effect of prophylaxis for early adrenal insufficiency using low-dose hydrocortisone in very preterm infants: an individual patient data meta-analysis. J Pediatr 2019; 207: 136–142. e5. [DOI] [PubMed] [Google Scholar]
- 19.Walsh MC, Yao Q, Gettner P, et al. Impact of a physiologic definition on bronchopulmonary dysplasia rates. Pediatrics 2004; 114: 1305–11. [DOI] [PubMed] [Google Scholar]
- 20.Bayley N Bayley Scales of Infant and Toddler Development. 3rd ed. San Antonio, TX: Harcourt Assessment, 2006. [Google Scholar]
- 21.Palisano RJ, Hanna SE, Rosenbaum PL, et al. Validation of a model of gross motor function for children with cerebral palsy. Phys Ther 2000; 80: 974–85. [PubMed] [Google Scholar]
- 22.Newman JE, Bann CM, Vohr BR, Dusick AM, Higgins RD. Improving the Neonatal Research Network annual certification for neurologic examination of the 18–22 month child. J Pediatr 2012; 161: 1041–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peltoniemi OM, Lano A, Puosi R, et al. Trial of early neonatal hydrocortisone: two-year follow-up. Neonatology 2009; 95: 240–7. [DOI] [PubMed] [Google Scholar]
- 24.Watterberg KL, Shaffer ML, Mishefske MJ, et al. Growth and neurodevelopmental outcomes after early low-dose hydrocortisone treatment in extremely low birth weight infants. Pediatrics 2007; 120: 40–8. [DOI] [PubMed] [Google Scholar]
- 25.Pocock SJ. Group sequential methods in the design and analysis of clinical trials. Biometrika 1977; 64: 191–9. [Google Scholar]
- 26.Lachin JM. A review of methods for futility stopping based on conditional power. Stat Med 2005; 24: 2747–64. [DOI] [PubMed] [Google Scholar]
- 27.SAS Institute. SAS/STAT v14.3 user’s guide. Cary, NC: SAS Institute, 2021. [Google Scholar]
- 28.Spiegelman D, Hertzmark E. Easy SAS calculations for risk or prevalence ratios and differences. Am J Epidemiol 2005; 162: 199–200. [DOI] [PubMed] [Google Scholar]
- 29.Agresti A Categorical data analysis. 2nd ed. New York: John Wiley, 2002. [Google Scholar]
- 30.Gail M, Simon R. Testing for qualitative interactions between treatment effects and patient subsets. Biometrics 1985; 41: 361–72. [PubMed] [Google Scholar]
- 31.Fenton TR, Kim JH. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatr 2013; 13: 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958; 53: 457–81. [Google Scholar]
- 33.Jensen EA, Dysart K, Gantz MG, et al. The diagnosis of bronchopulmonary dysplasia in very preterm infants. An evidence-based approach. Am J Respir Crit Care Med 2019; 200: 751–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bax M, Goldstein M, Rosenbaum P, et al. Proposed definition and classification of cerebral palsy, April 2005. Dev Med Child Neurol 2005; 47: 571–6. [DOI] [PubMed] [Google Scholar]
- 35.Jensen EA, DeMauro SB, Kornhauser M, Aghai ZH, Greenspan JS, Dysart KC. Effects of multiple ventilation courses and duration of mechanical ventilation on respiratory outcomes in extremely lowbirth-weight infants. JAMA Pediatr 2015; 169: 1011–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Onland W, Cools F, Kroon A, et al. Effect of hydrocortisone therapy initiated 7 to 14 days after birth on mortality or bronchopulmonary dysplasia among very preterm infants receiving mechanical ventilation: a randomized clinical trial. JAMA 2019; 321: 354–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Watterberg KL, Scott SM, Backstrom C, Gifford KL, Cook KL. Links between early adrenal function and respiratory outcome in preterm infants: airway inflammation and patent ductus arteriosus. Pediatrics 2000; 105: 320–4. [DOI] [PubMed] [Google Scholar]
- 38.Hagman C, Björklund LJ, Bjermer L, Hansen-Pupp I, Tufvesson E. Perinatal inflammation relates to early respiratory morbidity and lung function at 12 years of age in children born very preterm. Acta Paediatr 2021; 110: 2084–92. [DOI] [PubMed] [Google Scholar]
- 39.Doyle LW, Cheong JL, Hay S, Manley BJ, Halliday HL. Late (≥ 7 days) systemic postnatal corticosteroids for prevention of bronchopulmonary dysplasia in preterm infants. Cochrane Database Syst Rev 2021; 11: CD001145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Onland W, De Jaegere AP, Offringa M, van Kaam A. Systemic corticosteroid regimens for prevention of bronchopulmonary dysplasia in preterm infants. Cochrane Database Syst Rev 2017; 1: CD010941. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


