Abstract
High-level expression of soluble recombinant human hemoglobin (rHb) in Escherichia coli was obtained with several hemoglobin variants. Under identical conditions, two rHbs containing the Presbyterian mutation (Asn-108→Lys) in β-globin accumulated to approximately twofold less soluble globin than rHbs containing the corresponding wild-type β-globin subunit accumulated. The β-globin Providence(asp) mutation (Lys-82→Asp) significantly improved soluble rHb accumulation compared to the wild-type β-globin subunit and restored soluble accumulation of rHbs containing the Presbyterian mutation to wild-type levels. The Providenceasp substitution introduced a negatively charged residue into the normally cationic 2,3-bisphosphoglycerate binding pocket, potentially reducing the electrostatic repulsion in the absence of the polyanion. The average soluble globin accumulation when there was coexpression of di-α-globin and β-Lys-82→Asp-globin (rHb9.1) and heme was present in at least a threefold molar excess was 36% ± 3% of the soluble cell protein in E. coli. The average total accumulation (soluble globin plus insoluble globin) was 56% ± 7% of the soluble cell protein. Fermentations yielded 6.0 ± 0.3 g of soluble rHb9.1 per liter 16 h after induction and 6.4 ± 0.2 g/liter 24 h after induction. The average total globin yield was 9.4 g/liter 16 h after induction. High-level accumulation of soluble rHb in E. coli depends on culture conditions, the protein sequence, and the molar ratio of the heme cofactor added.
Unique among the hemoglobin-based oxygen carriers currently undergoing clinical evaluation is rHb1.1, a recombinant human hemoglobin (rHb) produced in Escherichia coli. This hemoglobin contains two modifications that increase its suitability as a hemoglobin-based oxygen carrier. The first modification is a genetically linked di-α-globin molecule that prevents dissociation of hemoglobin into αβ dimers, thus reducing renal filtration and extending the intravascular half-life (10, 18). And second, rHb1.1. contains the Presbyterian mutation (Asn-108→Lys) (21) in β-globin which reduces the oxygen affinity to a level roughly comparable to that of native hemoglobin bound to 2,3-biphosphoglycerate (DPG). DPG naturally modifies the oxygen affinity of native hemoglobin in erythrocytes.
Overproduction of a heterologous protein in E. coli is especially challenging when the protein must be soluble and functional and is composed of multiple subunits. In addition, heterologous proteins like rHbs require the enhanced presence of essential cofactors (prosthetic groups), such as heme and flavins, which occurs through supplementation or increased endogenous production (reviewed in reference 29). In E. coli, accumulation of soluble rHb (11, 26), human cystathionine β-synthase (12), rat cytochrome b5 (32), and Vitroscilla hemoglobin (VHb) (9) is limited by heme availability, as indicated by the fact that δ-aminolevulinic acid, a heme precursor, is required to increase heme and protein accumulation (12, 26, 32). Insoluble VHb aggregates do not contain heme, suggesting that heme may stabilize VHb and thus prevent aggregation (9).
High-level production of functional recombinant hemoglobin in E. coli was achieved when there was concomitant expression of both α-globin (or di-α-globin) and β-globin subunits and exogenous heme was provided (10, 18). Accumulation of soluble rHb1.1 in E. coli can account for as much as 15% of the soluble cell protein when heme is added to the culture medium (30). Production of rHb in E. coli can also result in insoluble aggregate formation (10, 18, 28), which also occurs when many other recombinant proteins are overexpressed in E. coli (reviewed in references 20 and 25). The presence of heme in the globin protein is a major factor in partitioning of the globin protein between soluble and insoluble fractions in vivo (28). This is consistent with the hypothesis that an absence of heme during globin chain biosynthesis results in insoluble aggregates composed of partially folded apoprotein (20). Removal of heme from human hemoglobin in vitro results in partial unfolding and severely reduced solubility (16).
In addition to cofactors such as heme, the protein sequence can influence aggregation. Aggregation of protein in vivo during bacterial production is sometimes decreased by mutations which diminish the kinetic trap of self-association of folding intermediates (reviewed in references 13 and 31). Myoglobin mutants which have high soluble protein yields also have relatively stable apoproteins (8).
In the study described here we examined the effects of mutations and heme concentration on soluble rHb accumulation. Our results indicate that high-level accumulation of soluble rHb requires excess heme and depends on the amino acid sequence of the globin protein itself. Although the di-alpha linkage may slightly improve soluble rHb accumulation, the most significant improvements in soluble expression were observed with rHbs containing amino acid substitutions in beta-globin.
MATERIALS AND METHODS
Strain and plasmid construction and sequencing.
The strains and plasmids used in this study are shown in Table 1. In general, strains were constructed by transforming plasmid DNAs into E. coli strains lacking plasmids by using the procedure of Chung et al. (5) or Hanahan (6). Transformants were selected by plating cells onto Luria-Bertani medium supplemented with 15 μg of tetracycline per ml and incubating the preparations at 37°C for 12 to 24 h. pSGE720 contains a synthetic operon composed of the di-α-globin and βPresbyterian-globin genes transcribed from the tac promoter on a tetracycline-resistant plasmid with the pUC high-copy-number origin of replication (28).
TABLE 1.
Strains and plasmids used in this study
| Strain | Plasmid | rHb | Host description | Source or reference |
|---|---|---|---|---|
| SGE1675 | None | None | gyrA96 (Nal) lacIQ1 endA hsdR17 relA1 supE44 recJ (a derivative of JM107) | 28 |
| SGE1464 | pSGE720 | rHb1.1 | SGE1675 | 28 |
| SGE1480 | pSGE726 | rHb1.0 | SGE1675 | This study |
| SGE1483 | pSGE728 | rHb0.0 | SGE1675 | This study |
| SGE2706 | pSGE733 | rHb0.1 | SGE1675 | This study |
| SGE2761 | None | None | SGE1675 + rpsL (strR) by P1 transduction | This study |
| SGE2782 | pSGE767 | rHb9+1.1 | SGE1675 | This study |
| SGE2784 | pSGE768 | rHb9.1 | SGE1675 | This study |
| SGE3083 | pSGE1001 | Mutant | SGE2761 with rHb di-αK158C/βN108K | This study, J. Davidson |
| SGE3084 | pSGE1222 | Mutant | SGE2761 with rHb di-αK158C/βK82D | This study |
| SGE3138 | pMON7124K | None | gyrA96 (Nal) lacIQ1 endA relA1 supE44 recJ (a derivative of SGE1675) | E. Best and L. Lucast |
| SGE3172 | pSGE1237 | Mutant | SGE2761 with rHb di-αK158C/βN108K; K82D | This study |
| SGE3261 | pSGE768 | rHb9.1 | SGE3138 | E. Best and L. Lucast |
pSGE728 was constructed by XhoI digestion and deletion from pSGE720 of one alpha-globin subunit and the di-alpha-globin glycine linker. The resulting plasmid, pSGE726, contained a single alpha-globin gene rather than a di-alpha-globin gene (rHb1.0). The Presbyterian mutation in beta-globin was replaced by digestion with BglII and HindIII and ligation which introduced wild-type beta-globin in order to create pSGE728 (rHb0.0). The Presbyterian mutation in the beta-globin gene of pSGE720 was replaced by digestion and ligation as described above for pSGE728 in order to introduce wild-type beta-globin and create pSGE733 (di-alpha-globin and wild-type beta-globin; rHb0.1).
The Providence(asp) mutation (βLys-82→Asp) (3) was introduced into the rHb1.1 background to create rHb9+1.1. The Lys-82→Asp mutation was created by PCR amplification of a portion of the β-globin gene performed with an oligonucleotide containing an Asp codon in place of Lys-82 (CBG119; 5′-AGCGAAGGTACCGTCCAGGTT-3′) and CBG124 (5′-CCTGACTCCGGAAGAAAAATCC-3′). The PCR product and vector were digested with BspEI and Asp718 and ligated. DNA sequencing of plasmids isolated from transformants was performed by using Sequenase kit reagents and protocols (United States Biochemical Corp.), 33P (Amersham, Inc.), and primers synthesized with an Applied Biosystems model 380B DNA synthesizer. Sequencing confirmed the identities of the Providence(asp) and Presbyterian mutations and that plasmid pSGE767 was transformed into SGE1675 to produce SGE2782.
The Providence(asp) mutation was introduced into the rHb0.1 background to create rHb9.1. A BamHI-Asp718 fragment from pSGE767 was isolated and ligated into digested pSGE733 (rHb0.1). Sequencing confirmed the identity of the mutation and that plasmid pSGE768 was transformed into SGE1675 to produce SGE2784 and into SGE3138 to produce SGE3261. Similar procedures were used to introduce Providence(asp) and Providence(asp) plus Presbyterian into a plasmid with a di-alpha-globin Lys-185→Cys mutation (SGE3083, SGE3084, and SGE3172 [Table 1]).
Fermentations, rHb purification, and analysis.
Fermentations were performed by using a defined medium in 15-liter Biolaffite fermentors as described by Looker et al. (17), except that induction was for 16 h at 28°C (30) unless indicated otherwise. Expression was induced by adding IPTG (isopropyl-β-d-thiogalactopyranoside) to a concentration between 10 and 200 μM as indicated below. IPTG was added during the log phase at an optical density at 600 nm that was approximately one-third the final cell density (optical density at 600 nm, ∼30). Various concentrations of hemin were added as noted below; typically, the hemin was distributed into three to five aliquots and added to the fermentation preparations at 3-h intervals starting at induction. Different concentrations of hemin did not affect the final cell densities. When possible, fermentations in which different strains were compared under the same conditions were performed in parallel to minimize variability. Purification and functionality were determined as described by Plomer et al. (23), and norvaline contents were measured as described by Apostol et al. (1).
Determination of percentages of soluble and insoluble rHb.
We measured the accumulation of soluble and insoluble rHbs for at least two and usually three or more independent fermentations per condition examined. One-milliliter samples were withdrawn into 1.7-ml Eppendorf tubes at appropriate times after induction. These 1-ml samples were centrifuged in an Eppendorf centrifuge at 4°C for 3 min, and the supernatants were removed. The pellets were stored at −80°C until they were assayed. Soluble and insoluble rHb contents were determined as described previously (30), except that after sodium dodecyl sulfate-polyacrylamide gel electrophoresis, the rHb was detected by either silver staining or Western blotting (30). The gels were silver stained by using the reagents and protocol recommended by Daiichi Pure Chemicals Co., Ltd. (Tokyo, Japan).
Hemin concentration estimation.
The hemin concentration in a fermentation broth was estimated as follows. Samples of the fermentation broth were centrifuged, and an aliquot of the supernatant was added to a solution containing 300 μM human serum albumin (Baxter) in 50 mM Tris (pH 7.5) such that the concentration of hemin did not exceed 50 μM. In the presence of excess human serum albumin hemin forms a 1:1 complex with a well-defined absorbance spectrum (2), and the hemin concentration was estimated from the absorbance at 625 nm. By subtracting the new hemin concentration from the hemin concentration of the previous sample, the quantity of hemin bound by or taken up by the cells was calculated during the entire time course of rHb accumulation.
RESULTS
Improving soluble rHb0.0 accumulation by hemin addition.
In 30°C fermentations containing 15 μM IPTG to induce rHb expression, the levels of soluble rHb0.0 that accumulated were consistently higher than the levels of soluble rHb1.1 (averages, 10.0% ± 1.1% and 6.5% ± 1.6% of the of the soluble cell protein for rHb0.0 and rHb1.1, respectively, in 10 h) (Table 2). At 28°C in the presence of 100 μM IPTG, the accumulation of soluble rHb0.0 and the accumulation of soluble rHb1.1. increased similarly (Table 2). Increasing the length of the induction period from 10 to 16 h increased the accumulation of soluble rHb1.1 to 10.9% ± 1.9% of the soluble cell protein but resulted in the yield of soluble rHb0.0 declining from 16.6% ± 0.3% at 10 h after induction to 11.0% ± 0.3% at 16 h after induction (Table 2). The amount of globin remained relatively constant (Table 2), suggesting that there was a decrease in globin solubility (the proportion of the total globin present in soluble form).
TABLE 2.
Effects of temperature and fermentation duration on accumulation of soluble rHba
| Strain | rHb | IPTG concn (μM) | Temp (°C) | Duration of fermentation (h) | Avg % of soluble proteinb | Avg % of total proteinc | No. of fermentationsd |
|---|---|---|---|---|---|---|---|
| SGE1464 | rHb1.1 | 15 | 30 | 10 | 6.5 ± 1.6 | 13.5 ± 6.0 | 38 |
| SGE1483 | rHb0.0 | 15 | 30 | 10 | 10.0 ± 1.1 | 16.8 ± 1.9 | 3 |
| SGE1464 | rHb1.1 | 100 | 28 | 10 | 9.2 ± 2.8 | 13.0 ± 5.4 | 7 |
| SGE1483 | rHb0.0 | 100 | 28 | 10 | 16.6 ± 0.3 | 23.9 ± 5.1 | 3 |
| SGE1464 | rHb1.1 | 100 | 28 | 16 | 10.9 ± 1.9 | 21.8 ± 3.9 | 12 |
| SGE1483 | rHb0.0 | 100 | 28 | 16 | 11.0 ± 0.3 | 22.5 ± 4.0 | 3 |
Three aliquots of hemin were added to the fermentations. The final hemin concentration was 0.34 mM.
Average percentage of the soluble protein present as soluble rHb.
Average percentage of the total protein present as total globin protein (soluble globin plus insoluble globin).
Number of fermentations used to determine average soluble and total expression.
The possibility that additional heme could support continued soluble rHb0.0 accumulation was tested by adding hemin two more times, at 9 and 12 h after induction, which increased the final heme concentration 85%, from 0.34 to 0.63 mM. These additions prevented the decline in the soluble rHb0.0 level observed late in the fermentations described above. Soluble rHb0.0 accumulated to an average of 19.2% ± 2.1% of the soluble cell protein, and total globin accumulated to 28.4% ± 8.5% of the soluble cell protein (Table 3). This 75% increase in soluble hemoglobin accumulation at 16 h after induction was highly significant (P = 0.0005; as determined by a t test of the means). Although an increase in the soluble rHb1.1 accumulation was also observed in fermentations supplemented with higher levels of heme, the magnitude of the improvement was much less than the magnitude of the improvement observed with rHb0.0 (Table 3).
TABLE 3.
Effect of heme concentration on accumulation of soluble rHb1.1 and rHb0.0a
| Final hemin concn (mM) | rHb0.0 (SGE1483)
|
rHb1.1 (SGE1464)
|
||||
|---|---|---|---|---|---|---|
| Avg % of soluble proteinb | Avg % of total proteinc | No. of fermentationsd | Avg % of soluble proteinb | Avg % of total proteinc | No. of fermentationsd | |
| 0.34 | 11.0 ± 0.3 | 22.5 ± 4.0 | 3 | 12.7 ± 2.9 | 21.7 ± 6.3 | 64 |
| 0.63 | 19.2 ± 2.1 | 28.4 ± 8.5 | 3 | 14.5 ± 3.0 | 23.8 ± 5.5 | 5 |
Fermentations were incubated at 28°C, induced for 16 h with 100 μM IPTG, and supplemented with hemin.
Average percentage of the soluble protein present as soluble rHb. The samples were obtained 16 h after induction.
Average percentage of the total protein present as total globin protein (soluble globin plus insoluble globin).
Number of fermentations used to determine average soluble and total expression.
Comparison of expression of four rHbs when a higher hemin concentration was used.
Using the higher hemin concentration (0.63 mM) established for rHb0.0 in 15-liter fermentations at 28°C, we examined the soluble expression of four different recombinant hemoglobins in parallel fermentations (Table 4). All four strains were isogenic and contained identical high-copy-number plasmids except for the following two globin gene differences: (i) the covalent linkage which forms di-α-globin and (ii) the presence of the βPresbyterian-globin (Asn-108→Lys) mutation.
TABLE 4.
Effects of di-alpha-globin and beta-globin subunits on accumulation of soluble rHba
| Strain | rHb | Alpha-globin | Beta-globin | Soluble rHb yield (g/liter) | Avg % of soluble proteinb | Avg % of total proteinc | No. of fermentationsd | % Increase in soluble expression with:
|
|
|---|---|---|---|---|---|---|---|---|---|
| Di-alpha-globin linkere | Wild-type beta-globinf | ||||||||
| SGE1480 | rHb1.0 | Mono | Asn-108→Lys | 1.1 ± 0.2 | 9.6 ± 0.5 | 21.8 ± 1.5 | 3 | ||
| SGE1464 | rHb1.1 | Di | Asn-108→Lys | 1.4 ± 0.4 | 11.2 ± 4.5 | 21.8 ± 0.7 | 3 | 17 | |
| SGE1483 | rHb0.0 | Mono | Wild type | 2.4 ± 0.6 | 19.8 ± 1.4 | 32.4 ± 8.5 | 3 | 106 | |
| SGE2706 | rHb0.1 | Di | Wild type | 3.1 ± 0.5 | 21.2 ± 3.4 | 54.0 ± 15.0 | 3 | 7 | 89 |
Fermentations were incubated at 28°C, induced for 16 h with 100 μM IPTG, and supplemented with five aliquots of hemin. The final concentration of hemin was 0.63 mM.
Average percentage of the soluble protein present as soluble rHb. The samples were obtained 16 h after induction.
Average percentage of the total protein present as total globin protein (soluble globin plus insoluble globin).
Number of fermentations used to determine average soluble and total expression.
Percent increase in soluble expression with the di-alpha-globin linker compared to the soluble expression with the unlinked alpha-globin.
Percent increase in soluble expression with the wild-type beta-globin compared to the soluble expression with the Asn-108→Lys beta-globin.
A significant increase in soluble expression was observed with the two strains expressing a wild-type β-globin subunit rather than βPresbyterian-globin (P < 0.05 as determined by multiple-range tests). The average increase in the soluble rHb level was approximately twofold, from ∼10% of the soluble cell protein to ∼20% or more of the soluble cell protein (Table 4). A slight increase (∼7 to 17%) in soluble globin protein accumulation was correlated with expression of the di-α-globin subunit with both wild-type and βPresbyterian-globin subunits, but the sample number was too small to determine statistical significance.
Soluble accumulation of βLys-82→Asp (Providence) rHb variants.
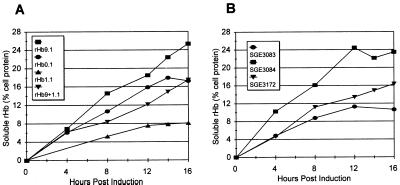
Since a mutation in the beta-globin subunit had a profound negative effect on the soluble expression of a recombinant hemoglobin, a mutation that improved rather than impaired soluble expression was sought. One such mutation, Providence(asp) (Lys-82→Asp), was identified. When coexpressed with di-α-globin (rHb9.1) (strain SGE2784), this mutation improved the expression of soluble rHb by 47% to 25.3% ± 5.4% of the soluble cell protein, and the level of total globin expression increased to 44.4% ± 12.4% (Table 5). This improvement was apparent throughout the entire induction period during fermentation (Fig. 1A) and resulted in an average soluble yield of 3.1 ± 0.7 g/liter. The accumulation of soluble and total rHb9.1 was significantly greater than the accumulation of soluble and total rHb0.1 (P < 0.05, as determined by multiple range tests) under identical conditions (Fig. 1A). Addition of the Providence(asp) mutation to the Presbyterian β-globin subunit (rHb9+1.1) resulted in a 116% increase in soluble accumulation (Table 5 and Fig. 1A) compared to the soluble accumulation with the Presbyterian subunit alone (P < 0.02, as determined by a t test of the means). This rescued rHb1.1 soluble expression to a level statistically indistinguishable from the level of wild-type rHb0.1 (Table 5). Molecules that accumulated to higher levels did so in part because a greater proportion of the protein accumulating within the cells remained soluble (Table 5). The rHbs with higher soluble expression levels (Table 5) were correlated with higher total globin levels (Table 5) (R2 = 0.99) and with higher percentages of the total globin present as soluble rHb (Table 5) (R2 = 0.97).
TABLE 5.
Effects of beta-globin mutations on accumulation of soluble rHba
| Strain | rHb | Beta-globin | Soluble rHb yield (g/liter) | Avg % of soluble proteinb | Avg % of total proteinc | Solubility (%)d | No. of fermentationse | % Increase in soluble expression with Lys-82→Aspf |
|---|---|---|---|---|---|---|---|---|
| SGE1464 | rHb1.1 | Asn-108→Lys | 1.0 ± 0.1 | 8.1 ± 1.4 | 20.6 ± 1.6 | 44.1 ± 4.0 | 3 | |
| SGE2782 | rHb9+1.1 | Asn-108→Lys + Lys-82→Asp | 2.6 ± 0.5 | 17.5 ± 4.0 | 35.7 ± 7.5 | 52.4 ± 10.2 | 5 | 116 |
| SGE2706 | rHb0.1 | Wild type | 2.1 ± 0.5 | 17.2 ± 2.2 | 37.8 ± 5.1 | 50.8 ± 3.7 | 3 | |
| SGE2784 | rHb9.1 | Lys-82→Asp | 3.1 ± 0.7 | 25.3 ± 5.4 | 44.4 ± 12.4 | 58.2 ± 5.1 | 3 | 47 |
Five aliquots of hemin were added to the fermentations. The final concentration of hemin was 0.63 mM.
Average percentage of the soluble protein present as soluble rHb.
Average percentage of the total protein present as total globin protein (soluble globin plus insoluble globin).
Percentage of the total globin present in the soluble form. Values were determined independently for each sample. The values are not necessarily equivalent to the values obtained by dividing the percentage of soluble rHb1.1 by the percentage of total globin since fermentations with greater globin accumulation would have greater weight in an average value.
Number of fermentations used to determine average soluble and total expression.
Percent increase in soluble expression with the Lys-82→Asp mutation in beta-globin compared to the soluble expression with the identical beta-globin without Lys-82→Asp.
FIG. 1.
Effects of mutations on soluble rHb accumulation. Fermentations were incubated at 28°C, induced for 16 h with 100 μM IPTG, and supplemented with hemin (final concentration, 0.63 mM) at 0, 3, 6, 9, and 12 h after induction. (A) Soluble accumulation of four variants, rHb0.1 (SGE2706), rHb1.1 (SGE1464), rHb9+1.1 (SGE2782), and rHb9.1 (SGE2784), for 16 h after induction. Most symbols represent the average from three fermentations; the only exception is the SGE2782 symbols, which represent the average from five fermentations. (B) Accumulation of three variants of soluble rHb with a di-α K158C mutations, as described above. Most symbols represents the average from four fermentations; the only exception is the SGE3083 symbols, which represent the average from two fermentations.
The presence of the beta-globin mutations had similar effects on accumulation of a soluble recombinant hemoglobin having a Lys-158→Cys mutation in di-alpha-globin. The presence of Providence(asp) improved soluble expression from a maximum of 11.3% with the Presbyterian beta-globin to 24.4% (Fig. 1C). The combination of the Providence(asp) and Presbyterian mutations in beta-globin resulted in an intermediate level of expression with a maximum of 16.4% soluble globin accumulation (Fig. 1C).
Improving soluble rHb9.1 accumulation by hemin addition.
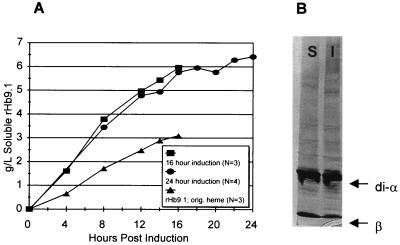
A 69% increase in the hemin concentration to 1.07 mM significantly (P = 0.001) increased the rate of soluble rHb9.1 accumulation (strain SGE3261) (Fig. 2A), and an average of 35.6% ± 2.5% of the soluble cell protein was soluble rHb9.1. These fermentations resulted in an average soluble yield of 6.0 ± 0.3 g of rHb9.1 per liter, which was almost twice the yield (3.1 ± 0.3 g/liter) obtained with the lower heme concentration (Fig. 2A). The average total rHb9.1 globin accumulation was 55.6% ± 6.9% of the soluble cell protein, and the total globin yield from 16-h fermentation was 9.4 g/liter. A maximum accumulation of soluble rHb9.1 corresponding to 39% of the soluble cell protein and a maximum total globin accumulation corresponding to 65% of the total cell protein were observed in an individual fermentation. Silver-stained gels of the soluble and insoluble fractions of cell lysates obtained from fermentations contained prominent di-α-globin and βProvidence(asp)-globin bands that accounted for the majority of the protein in each of the lysate fractions (Fig. 2B).
FIG. 2.
rHb9.1 accumulation. (A) Soluble rHb9.1 (SGE3261) fermentation yields under two different heme conditions and with two different lengths of induction. Symbols: ▴, average soluble rHb9.1 concentrations obtained with the original heme concentration (0.63 mM); •, average soluble rHb9.1 concentrations in three fermentations supplemented with heme (final concentration, 1.07 mM) at 0, 3, 6, 9, and 12 h; ■, soluble rHb9.1 concentrations in four fermentations induced for 24 h and supplemented with eight aliquots of hemin (final concentration, 1.71 mM). (B) Globin protein from soluble and insoluble cell lysate fractions from one of the SGE3261 fermentations in panel A induced for 24 h. The globin bands are indicated by the arrows (upper arrow, di-α-globin; lower arrow, β-globin). Lane S, soluble lysate fraction; lane I, insoluble lysate fraction.
Extending the induction period to 24 h and adding hemin every 3 h resulted in a marginal increase in the average soluble rHb9.1 yield, from 6.0 ± 0.3 to 6.4 ± 0.2 g/liter, but did not result in any increase in the percentage of soluble or total rHb9.1 (Fig. 2A). However, this treatment resulted in the highest yield of soluble rHb9.1 from an individual fermentation (6.8 g/liter). We also tested the effect on soluble rHb9.1 accumulation of adding at induction the entire amount of hemin typically distributed over the entire fermentation period. We observed no significant difference in the soluble rHb9.1 accumulation when this strategy was used.
Heme-to-rHb9.1 stoichiometry.
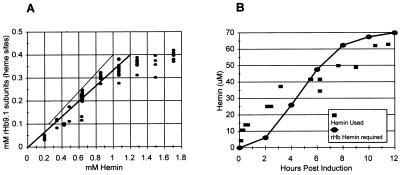
The molar concentration of hemin was compared to the molar accumulation of soluble rHb9.1 on a per heme basis. The molar concentration of soluble rHb9.1 was calculated from the soluble yields. The molar concentration of hemin supplied was calculated from the known mass of hemin added. One mol of hemoglobin contains 4 mol of globin subunits (2 mol of α subunits and 2 mol of β subunits), and each globin subunit accommodates heme; therefore, 1 mol of hemoglobin contains 4 mol of heme. The accumulation of soluble rHb9.1 was strongly correlated with the concentration of hemin supplied, and there was an apparently linear relationship up to a hemin concentration of about 1 mM (Fig. 3A). Addition of hemin to a concentration of >1 mM did not improve the soluble rHb9.1 accumulation, indicating that hemin was not limiting under these conditions (Fig. 3A). The correlation between the accumulation of soluble rHb9.1 subunits and the concentration of hemin supplied was very strong over the initial concentration range used (Fig. 3A) (R2 = 0.90; 50 samples). The comparison revealed that an approximately threefold molar excess of hemin was required for accumulation of soluble rHb9.1 over a range of concentrations (Fig. 3A). We did not observe a single sample among the 70 samples examined that was accompanied by less than a 2.5-fold molar excess of hemin (Fig. 3A). This value was therefore considered the lower limit of hemin excess required for maximal soluble rHb9.1 accumulation.
FIG. 3.
(A) Comparison of accumulation of soluble rHb9.1 heme sites with the concentration of hemin supplied. The symbols represent 70 individual samples. One heme site is present in each α subunit and β subunit; therefore, there are four sites per hemoglobin molecule. The heavy line represents a threefold molar excess of hemin with respect to heme binding sites in hemoglobin. The light line represents a 2.5-fold molar excess of hemin. (B) Hemin uptake and hemoglobin production. Symbols: ■, independent measurements of hemin loss from the fermentation broth; •, time course for rHb production converted to the amount of hemin required for each level of rHb accumulation, assuming that there are four heme molecules per rHb molecule.
To determine the relative importance of time of accumulation and hemin concentration, we compared two sets of data from different hemin supplementation experiments in which the concentrations of hemin were nearly identical at different times during accumulation. In one experiment a total of 0.64 mM hemin was added by 6 h after induction, and in the other experiment 0.63 mM hemin was added by 12 h after induction. We compared the concentrations of soluble rHb9.1 on a per heme basis at 8 h after induction in the first experiment and at 14 and 16 h after induction in the second experiment. Regardless of the time since induction, the soluble rHb9.1 accumulations were indistinguishable (0.22 ± 0.02 and 0.19 ± 0.04 mM, respectively), suggesting that under the conditions used hemin was more important than time of rHb accumulation for achieving high soluble yields.
We examined the heme lost from the medium in the 15-liter fermentor incubated at 28°C in which rHb1.1 expression was induced by adding 100 μM IPTG and correlated it with the accumulation of soluble rHb. An approximately stoichiometric relationship was observed, indicating that only the heme required to support soluble rHb accumulation disappeared from the medium (Fig. 3B).
Accumulation of soluble rHb0.1 in heme-free fermentations.
We tested whether rHb0.1 accumulated to higher soluble levels than rHb1.1 accumulated in fermentations without hemin. When two fermentations were incubated at 28°C and induced with 100 μM IPTG, the average soluble rHb0.1 yield was just 2.5% ± 0.2% of the soluble cell protein, a value similar to the value of obtained for rHb1.1 (26). When 10 μM IPTG was used for induction, an average of 1.6% ± 0.3% of the soluble cell protein was soluble rHb0.1. By dividing the soluble rHb0.1 accumulation in the absence of hemin by the accumulation in the presence of hemin, we estimated that a maximum of 12 to 15% of the accumulation in the presence of hemin was due to E. coli heme biosynthesis.
Functionality of rHbs.
Samples of six variants of recombinant hemoglobins were purified. Two parameters of functionality, oxygen binding affinity and cooperativity, were measured (Table 6). As expected, the hemoglobin mutations affected oxygen binding in the recombinant molecules to approximately the same degree that they affected oxygen binding in the native hemoglobin molecules (3, 21). The correlations between the two functional parameters and between each parameter and the soluble expression levels were examined. The two functional parameters were not correlated with each other (R2 = 0.04; P = 0.69). In spite of this, these parameters were equally correlated with soluble expression (R2 = 0.46 for oxygenbinding affinity [P = 0.14] and R2 = 0.47 for cooperativity [P = 0.13]). This indicates that low oxygen binding affinity and/or cooperativity may be correlated with higher soluble expression. However, since the P values were greater than 0.1, the correlation was not statistically significant, and additional testing would be required to verify this observation.
TABLE 6.
Effects of beta-globin mutations on rHb functionality
| Alpha-globin | rHb | Beta-globin | Oxygen binding affinity (P50) | Cooperativity (nMax) |
|---|---|---|---|---|
| Di | rHb0.1 | Wild type | 10 | 2.2 |
| rHb9.1 | Lys-82→Asp | 15 | 2.0 | |
| rHb1.1 | Asn-108→Lys | 31 | 2.4 | |
| rHb9+1.1 | Asn-108→Lys + | 44 | 2.2 | |
| Lys-82→Asp | ||||
| Mono | rHb0.0 | Wild type | 15 | 2.6 |
| rHb1.0 | Asn-108→Lys | 36 | 2.5 |
In addition, a sample of soluble rHb9.1 from a fermentation yielding 3.7g/liter was examined for norvaline substitution, which was observed previously in rHb1.1 (1, 27). The level of norvaline was below the limit of quantitation, indicating that high-level soluble expression did not increase the level of misincorporation of this amino acid.
DISCUSSION
Measurements of the insoluble protein contents in fermentations yielded evidence that reductions in soluble hemoglobin levels were due to conversion into insoluble globin. Loss of heme as a mechanism for turnover of soluble hemoglobin into insoluble globins has been well-characterized (20). The rate of turnover was consistent with an 11-h half-life for rHb1.1. in E. coli flask cultures (28), raising the possibility that when heme became limiting (when the level dropped below a required stoichiometric level), soluble accumulation ceased and turnover of rHb was driven primarily by heme loss, followed by insoluble aggregation.
Many unstable human hemoglobin mutants that were found in vivo in inclusion bodies contained heme, as did in vitro heat-precipitated forms of mutant hemoglobins (24). Heme may stabilize some globin conformations even in insoluble aggregates, and the absence of heme from one or more of the four hemoglobin subunits may lead to insolubility and rapid turnover of the subunits (28). Although globin can accept heme before it is released from the ribosome in eukaryotic cell-free translation systems (15), it is unclear whether this occurs in bacteria. Unlike the situation in eukaryotes, globin probably cannot complete folding into its native configuration until it is released from the ribosome in E. coli (22). Therefore, without heme, apo-globin can be likened to a trapped folding intermediate.
In fermentations supplemented with increased heme, several globin variants exhibited greater soluble hemoglobin accumulation than seen with rHb1.1. The abilities of different globins to accumulate to different levels may be understood in terms of the stability of the apo-globin. In myoglobin, the levels of expression of a series of mutants were shown to be related to the stability of the folded apo-globin (7, 8). Thus, the rate of formation of functional myoglobin or hemoglobin is a function of the concentration of heme and the concentration of apo-globin in a classical bimolecular manner (Fig. 4).
FIG. 4.
A simplified reaction for the formation of hemoglobin. The reversible biomolecular reaction combining apoglobin and heme depends on the concentration of the reactants (concentration denoted by brackets). Irreversible aggregants compete as side reactions, and the concentration of the reactant heme inside the cell is driven by the extracellular heme concentration.
The reversible bimolecular reaction combining apo-globin and heme to form hemoglobin can best be encouraged by increasing the concentration of one or both reactants, but this reaction competes with aggregation of apo-globin, a terminal side reaction. Either hemoglobin can aggregate into insoluble hemoglobin that still contains heme or dissociation of heme from one or more subunits may be a prerequisite. Accumulation of soluble hemoglobin is very sensitive to (i) the rate of globin synthesis, (ii) the heme pool within the cells, and (iii) the stability of the apo-globin (30), as discussed below.
In each experiment in this study, the expression strains and plasmids were identical, as were the fermentation and induction conditions. Therefore, approximately the same amount of protein synthesis occurred for each rHb variant. No significant differences in cell growth were observed, suggesting that the rHb variants did not have different toxic effects on the cells, which could have accounted for the differences in the percentages of the soluble protein accumulating as soluble rHb.
The extracellular heme concentration was manipulated in order to influence the intracellular heme concentration. The use of identical expression strains imposed the same heme transport (diffusion) and biosynthetic capacities on the cells expressing all rHb variants. The E. coli strains were sufficiently heme permeable to allow a heme protein, rHb9.1, to accumulate to levels that were equivalent to almost 40% of the soluble protein in the cell. Since very high soluble expression of rHbs always required a >2.5-fold molar excess of heme, this excess may be required for sufficient diffusion across the cell membrane to maintain an intracellular heme concentration high enough to drive the bimolecular reaction of heme with globin. Fermentations not supplemented with heme accumulated only ∼12 to 15% of the soluble rHb0.1 accumulated when hemin was added, indicating that biosynthesis of heme contributes little to the soluble globin and therefore to the heme pool within the cells. In addition, exogenous hemin probably suppresses endogenous heme production in E. coli, so the actual contribution of endogenous heme to soluble rHb accumulation is probably much less than the estimated contribution.
Mutations which influence protein solubility were presumed to affect apo-globin stability and therefore, concentration by reducing the terminal aggregation side reaction of apo-globin. More than 29% the more than 200 human hemoglobin beta-chain variants investigated previously were unstable (4). Instability of hemoglobin protein containing the Presbyterian mutation has been observed previously (14). This instability probably had an impact on the accumulation of soluble rHb1.1 in E. coli, which was approximately one-half the accumulation of rHb0.1 containing wild-type Asn-108 instead of lysine. With the Presbyterian mutant, the apo-globin aggregation reaction most likely dominates the soluble accumulation rate (30). However, no significant difference in the stabilities of these globin proteins was observed in the gas phase by mass spectroscopy (1a).
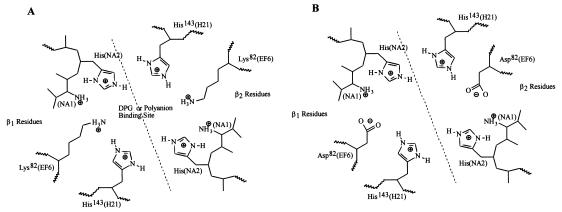
The Providence(asp) mutation occurs at a key residue in the DPG binding cleft (Lys-82; EF6) between the two beta-globin subunits in the hemoglobin tetramer (Fig. 5). This anion-binding cleft is lined with at least six positively charged residues, three from each beta-globin (His-2, His-143, and Lys-82). In the absence of DPG or inositol hexaphosphate, such as during accumulation of rHbs in E. coli, electrostatic repulsion between these residues may destabilize or even partially denature the beta-globin structure. Replacement of the normally occurring positively charged lysine by a negatively charged aspartate introduces a counterion into the DPG pocket, which may form an electrostatic interaction with His-143 (3), which is likely to stabilize the beta chain. This could account for the improved accumulation of soluble rHb9.1 compared with the accumulation of soluble rHb0.1, which contains the wild-type lysine at position 82. The charge substitution may also increase the rate of hemoglobin assembly by reducing the electrostatic repulsion in this region. Differences in accumulation of stable hemoglobin variants in humans appear to be correlated with differences in subunit assembly rates (4).
FIG. 5.
Principal amino acids in the DPG binding pocket at the interface between the two beta subunits (β1 and β2). (A) Native hemoglobin with a lysine at position 82. (B) Hemoglobin Providence(asp) with an aspartate at position 82. The positions of the amino acids in β-globin are indicated; the designations in parentheses are helical structure designations. The interface between the two β subunits is indicated by a dashed diagonal line.
It is likely that the same Lys-82→Asp (Providence) substitution could stabilize and improve the accumulation of other soluble recombinant hemoglobin molecules, since all such molecules are typically expressed without DPG present. This was observed with a di-α-globin mutant rHb, Lys-158→Cys, in which the presence of Providence(asp) more than doubled the soluble globin accumulation. The addition of the Providence(asp) mutation to rHb1.1 to create rHb9+1.1 resulted in a more than twofold increase in soluble expression, rescuing the Presbyterian mutation by restoring the soluble expression to wild-type levels. In addition to improving soluble globin accumulation, rHb variants also increased total globin accumulation. This implied that soluble hemoglobin is more stable than the insoluble protein; otherwise, an improvement in solubility would not be expected to increase the total globin levels but merely would reallocate more of the total globin to the soluble form. Our interpretation is consistent with the results of pulse-chase experiments in which we observed a much shorter half-life for insoluble globin than for soluble globin (28). Thus, the greater the amount of globin maintained in the soluble form, the greater the total globin accumulation expected, since the insoluble globin is more rapidly removed.
The rHb affinity for heme theoretically could contribute to the difference in soluble rHb accumulation by eliminating or reducing the denaturation of hemoglobin to apo-globin plus heme. While the stability of myoglobin (and by analogy, hemoglobin) depends on the affinity for heme (7), this does not necessarily apply to the forward reaction (i.e., folding and hemoglobin formation). High rates of heme loss from myoglobin were not necessarily correlated with unstable apo-globin and vice versa (8). Although the Providence(asp) mutation resulted in a lower oxygen affinity, this was most likely due to stabilization of the T-state (low affinity) of hemoglobin in the absence of DPG, not to changes in the heme pocket (3). Because of their location relative to the heme pocket, we do not believe that the mutations studied here affected soluble expression because of significant changes in heme affinity.
This study demonstrated that increases in accumulation of soluble mutant rHbs were correlated with increases in the proportion of rHb accumulating as soluble protein rather than insoluble protein. This supports the model in which mutations reduced the terminal aggregation side reaction and thus increased the soluble yield. Increased heme supply supported higher soluble yields of mutant rHbs, which corroborated the bimolecular reaction model. In this study using rHbs, we obtained what we believe are the highest soluble expression yields for a heterogous, complex, multiple-subunit protein with a prosthetic group (heme) ever obtained in E. coli (6.4 g/liter). Maintenance of a threefold molar excess of heme was critical to accumulation of soluble globin. Our study confirmed that rHbs can be very significantly overproduced in E. coli. In addition, the results of the study of recombinant hemoglobin mutants provide new and useful model for the effects of mutations on protein folding and subunit assembly.
ACKNOWLEDGMENTS
The assistance of Izydor Apostol in purification, functionality measurements, and especially norvaline measurements was invaluable. Michael Schick supplied the anti-rHb1.1 antibody for Western blotting, Louise Lucast synthesized the oligonucleotides required to make rHb mutants, Antony Mathews provided the hemin uptake assay to R.B., and Shawn Curry introduced M.P. to insoluble Western blotting and performed several of the initial measurements. We appreciate the technical assistance and/or helpful comments of many individuals in the Somatogen Molecular Biology, Assay Services, and Pilot Operations groups and the contributions of strains or plasmids by Jeff Davidson, Louise Lucast, and Elaine Best. We appreciate the stimulating discussions which we had with John Olson, Antony Mathews, and Doug Lemon and the helpful comments on the manuscript provided by Doug Looker, Izydor Apostol, and Antony Mathews.
REFERENCES
- 1.Apostol I, Levine J, Lippincott J, Leach J, Hess E, Glascock C R, Weickert M J, Blackmore R. Incorporation of norvaline at leucine positions in recombinant human hemoglobin expressed in Escherichia coli. J Biol Chem. 1997;272:28980–28988. doi: 10.1074/jbc.272.46.28980. [DOI] [PubMed] [Google Scholar]
- 1a.Apostol, I. Personal communication.
- 2.Beaven G H, Chen S-H, d’Albis A, Gratzer W B. A spectroscopic study of the haemin-human-serum-albumin system. Eur J Biochem. 1974;41:539–546. doi: 10.1111/j.1432-1033.1974.tb03295.x. [DOI] [PubMed] [Google Scholar]
- 3.Bonaventura J, Bonaventura C, Sullivan B, Ferruzzi G, McCurdy P R, Fox J, Moo-Penn W F. Hemoglobin Providence. Functional consequences of two alterations of the 2,3-diphosphoglycerate binding site at position β82. J Biol Chem. 1976;251:7563–7571. [PubMed] [Google Scholar]
- 4.Bunn E H, Forget B G. Hemoglobin: molecular, genetic and clinical aspects. W. B. Philadelphia, Pa: Saunders Co.; 1986. [Google Scholar]
- 5.Chung C, Niemela S, Miller R. One step preparation of competent Escherichia coli: transformation and storage of bacterial cells in the same solution. Proc Natl Acad Sci USA. 1989;86:2172–2175. doi: 10.1073/pnas.86.7.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hanahan D. Techniques for transformation of E. coli. In: Glover D M, editor. DNA cloning: a practical approach. Vol. 1. Oxford, United Kingdom: IRL Press; 1985. pp. 109–135. [Google Scholar]
- 7.Hargrove M S, Barrick D, Olson J. The association rate constant for heme binding to globin is independent of protein structure. Biochemistry. 1996;35:11293–11299. doi: 10.1021/bi960371l. [DOI] [PubMed] [Google Scholar]
- 8.Hargrove M S, Krzywda S, Wilkinson A J, Dou Y, Ikeda-Saito M, Olson J. Stability of myoglobin: a model for the folding of heme proteins. Biochemistry. 1994;33:11767–11775. doi: 10.1021/bi00205a012. [DOI] [PubMed] [Google Scholar]
- 9.Hart R A, Kallio P T, Bailey J E. Effect of biosynthetic manipulation of heme on insolubility of Vitreoscilla hemoglobin in Escherichia coli. Appl Environ Microbiol. 1994;60:2431–2437. doi: 10.1128/aem.60.7.2431-2437.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoffman S J, Looker D L, Roehrich J M, Cozart P E, Durfee S L, Tedesco J L, Stetler G L. Expression of fully functional tetrameric human hemoglobin in Escherichia coli. Proc Natl Acad Sci USA. 1990;87:8521–8525. doi: 10.1073/pnas.87.21.8521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jessen T H, Komiyama N H, Tame J, Pagnier J, Shih D, Luisi B, Fermi G, Nagai K. Production of human hemoglobin in Escherichia coli using cleavable fusion protein expression vector. Methods Enzymol. 1994;231:347–364. doi: 10.1016/0076-6879(94)31024-6. [DOI] [PubMed] [Google Scholar]
- 12.Kery V, Elleder D, Kraus J P. δ-Aminolevulinate increases heme saturation and yield of human cystathionine β-synthase expressed in Escherichia coli. Arch Biochem Biophys. 1995;316:24–29. doi: 10.1006/abbi.1995.1005. [DOI] [PubMed] [Google Scholar]
- 13.King J, Haase-Pettingell C, Robinson A S, Speed M, Mitraki A. Thermolabile folding intermediates: inclusion body precursors and chaperone substrates. FASEB J. 1996;10:57–66. doi: 10.1096/fasebj.10.1.8566549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kohne E, Behnken L J, Leupold D, Rogge H, Martin H, Kleihauer E. Hemoglobin Presbyterian [β108 (G10) asn→lys] in a German family. Hemoglobin. 1979;3:365–370. doi: 10.3109/03630267908997542. [DOI] [PubMed] [Google Scholar]
- 15.Komar A K, Kommer A, Krasheninnikov I A, Spirin A S. Cotranslational folding of globin. J Biol Chem. 1997;272:10646–10651. doi: 10.1074/jbc.272.16.10646. [DOI] [PubMed] [Google Scholar]
- 16.Leutzinger Y, Beychock S. Kinetics and mechanism of heme-induced refolding of human alpha-globin. Proc Natl Acad Sci USA. 1981;78:780–784. doi: 10.1073/pnas.78.2.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Looker D, Mathews A J, Neway J O, Stetler G L. Expression of recombinant human hemoglobin in Escherichia coli. Methods Enzymol. 1994;231:364–374. doi: 10.1016/0076-6879(94)31025-4. [DOI] [PubMed] [Google Scholar]
- 18.Looker D L, Abbott-Brown D, Cozart P, Durfee S, Hoffman S, Mathews A J, Miller-Roehrich J, Shoemaker S, Trimble S, Fermi G, Komiyama N H, Nagai K, Stetler G L. A human recombinant haemoglobin designed for use as a blood substitute. Nature. 1992;356:258–260. doi: 10.1038/356258a0. [DOI] [PubMed] [Google Scholar]
- 19.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1972. [Google Scholar]
- 20.Mitraki A, King J. Protein folding intermediates and inclusion body formation. Bio/Technology. 1989;7:690–697. [Google Scholar]
- 21.Moo-Penn W F, Wolff J A, Simon G, Vacek M, Jue D L, Johnson M H. Hemoglobin Presbyterian: β108 (G10) asparagine→lysine. A hemoglobin variant with low oxygen affinity. FEBS Lett. 1978;92:53–56. doi: 10.1016/0014-5793(78)80720-0. [DOI] [PubMed] [Google Scholar]
- 22.Netzer W J, Hartl F U. Recombination of protein domains facilitated by co-translational folding in eukaryotes. Nature. 1997;388:343–349. doi: 10.1038/41024. [DOI] [PubMed] [Google Scholar]
- 23.Plomer J J, Ryland J R, Matthews M-A H, Traylor D, Milne E E, Durfee S L, Mathews A J, Neway J O. Purification of hemoglobin. International Patent Application PCT WO96/15151. May 1996. [Google Scholar]
- 24.Rachmilewitz E A. Denaturation of the normal and abnormal hemoglobin molecule. Semin Hematol. 1974;11:441–462. [PubMed] [Google Scholar]
- 25.Schein C H. Production of soluble recombinant proteins in bacteria. Bio/Technology. 1989;7:1141–1149. [Google Scholar]
- 26.Verderberg E, Lucast L, VanDehy J A, Cozart P, Etter J B, Best E A. Role of the hemA gene product and δ-aminolevulinic acid in regulation of Escherichia coli heme synthesis. J Bacteriol. 1997;179:4583–4590. doi: 10.1128/jb.179.14.4583-4590.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weickert M J, Apostol I. High-fidelity translation of recombinant human hemoglobin in Escherichia coli. Appl Environ Microbiol. 1998;64:1589–1593. doi: 10.1128/aem.64.5.1589-1593.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weikert M J, Curry S R. Turnover of recombinant human hemoglobin in Escherichia coli occurs rapidly for insoluble and slowly for soluble globin. Arch Biochem Biophys. 1997;348:337–346. doi: 10.1006/abbi.1997.0410. [DOI] [PubMed] [Google Scholar]
- 29.Weickert M J, Doherty D H, Best E A, Olins P E. Optimization of heterologous protein production in Escherichia coli. Curr Opin Biotechnol. 1996;7:494–499. doi: 10.1016/s0958-1669(96)80051-6. [DOI] [PubMed] [Google Scholar]
- 30.Weickert M J, Pagratis M, Curry S R, Blackmore R. Low temperature improves accumulation of soluble recombinant hemoglobin in Escherichia coli by stabilization of apo-globin. Appl Environ Microbiol. 1997;63:4313–4320. doi: 10.1128/aem.63.11.4313-4320.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wetzel R. Mutations and off pathway aggregation of proteins. Trends Biotechnol. 1994;12:193–198. doi: 10.1016/0167-7799(94)90082-5. [DOI] [PubMed] [Google Scholar]
- 32.Woodward S I, Dailey H A. Regulation of heme biosynthesis of Escherichia coli. Arch Biochem Biophys. 1995;316:110–115. doi: 10.1006/abbi.1995.1016. [DOI] [PubMed] [Google Scholar]