Understanding of antibody responses to SARS-CoV-2 natural infection (NI) and/or vaccination is constantly evolving, with most researchers reporting waning immune responses between six to eleven months [[1], [2], [3], [4]]. Studies have previously investigated antibody kinetics following COVID-19 and/or vaccination in various cohorts and at different periods during the pandemic. However, these studies have primarily relied on binding antibody responses, or pseudovirus-based neutralization assays, rather than authentic-virus neutralization assays evaluating functional antibody responses. Here we characterize neutralizing antibody (NAb) responses using authentic-virus plaque reduction neutralization test (PRNT) among individuals with SARS-CoV-2 NI and/or vaccination.
A total of 449 blood samples were collected from 222 human subjects after obtaining informed consent between July 2020 and December 2021 in Honolulu, Hawaii, USA (IRB#2020-00406). An additional 44 de-identified blood samples were collected from individuals with history of SARS-CoV-2 NI (Table 1).
Table 1.
Clinicoepidemological characteristics of individuals with a history of SARS-CoV-2 natural infection and/or COVID-19 vaccination.
| NI (n = 125) | VX (n = 129) | |||||
|---|---|---|---|---|---|---|
| De-Identifieda | n = | 44 | (35%) | ––– | ||
| NI (n = 81) | VX (n = 129) | |||||
| Gender | ||||||
| Male | n = | 35 | (43%) | n = | 54 | (42%) |
| Female | n = | 46 | (57%) | n = | 75 | (58%) |
| Age in years | ||||||
| Mean ± SD (range) | 47.35 ± 15.78 (20 - 83) | 44.86 ± 19.45 (20 - 85) | ||||
| Median (IQR) | 48.00 (34.50 - 59.25) | 40.00 (27.00 - 60.00) | ||||
| Days Post Symptom Onsetb | ||||||
| n = | 67 | (83%) | ––– | |||
| Mean ± SD (range) | 122.51 ± 125.24 (13 - 558) | ––– | ||||
| Median (IQR) | 75.00 (24.00 - 175.50) | ––– | ||||
| Days Post SARS-CoV-2 PCR Positivityb | ||||||
| n = | 64 | (79%) | ––– | |||
| Mean ± SD (range) | 93.09 ± 84.43 (11 - 348) | ––– | ||||
| Median (IQR) | 53.50 (22.00 - 150.75) | ––– | ||||
| COVID-19 Vaccination with and without History of Natural Infection | ||||||
| n = | 42 | (52%) | n = | 129 | (100%) | |
| Pfizer-BioNTech | n = | 28 | (67%) | n = | 66 | (51%) |
| Moderna | n = | 12 | (29%) | n = | 59 | (46%) |
| J&J/Janssen | n = | 2 | (5%) | n = | 3 | (2%) |
| Days Post COVID-19 Vaccination | ||||||
| No. Samples | n = | 61 | n = | 271 | ||
| Dose 1 (≥ 10 and ≤ 42 days) | n = | 20 | (33%) | n = | 56 | (21%) |
| Mean ± SD (range) | 18.60 ± 8.45 (10 - 39) | 19.61 ± 6.07 (13 - 41) | ||||
| Median (IQR) | 15.50 (14.00 - 21.00) | 19.00 (14.00 - 23.25) | ||||
| Dose 2c (≥ 10 days) | n = | 41 | (67%) | n = | 199 | (73%) |
| Mean ± SD (range) | 41.88 ± 42.28 (10 - 212) | 87.96 ± 81.87 (10 -323) | ||||
| Median (IQR) | 26.00 (16.00 - 48.00) | 47.00 (19.00 - 145.00) | ||||
| Dose 3 (≥ 10 days) | n = | 0 | (0%) | n = | 16 | (6%) |
| Mean ± SD (range) | ––– | 32.13 ± 31.05 (13 - 135) | ||||
| Median (IQR) | ––– | 20.00 (17.00 - 28.00) | ||||
NI = history of SARS-CoV-2 natural infection; VX = COVID-19 vaccination without a history of SARS-CoV-2 natural infection; SD = standard deviation; IQR = interquartile range. aDe-identified/anonymized medical waste with clinical history of SARS-CoV-2 natural infection, with no associated clinicoepidemiological data were available. bVaried reporting for days post symptom onset and days post PCR positivity due to asymptomatic infection, and lack of access to SARS-CoV-2 testing. cDose 2 = complete COVID-19 vaccinaton, two doses of a Pfizer-BioNTech or Moderna COVID-19 vaccine or one dose of J&J/Janssen COVID-10 vaccine.
PRNTs were conducted as previously described using the SARS-CoV-2 isolate USA-WA1/2020 [5]. Sigmoidal-dose-response-logistic-regression model was used to determine PRNT titers at 50% neutralization. All calculations were conducted using GraphPad(v9.0).
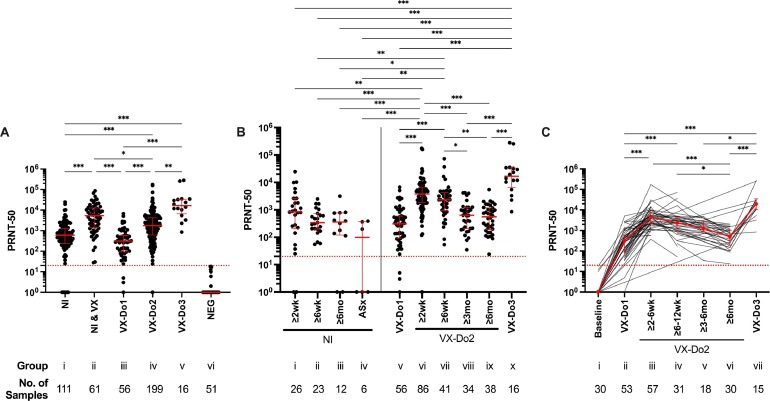
Functional neutralization assay, expressed as median NAb titers and IQR, revealed significantly lower titers among individuals with history of NI alone (608, IQR:241-1,256) compared to NAb titers among individuals with history of NI after at least one dose of COVID-19 vaccination (5,347, IQR:1,433-12,723; p<0.001) or individuals without history of NI after complete COVID-19 vaccination (1,766, IQR:606-4,219; p<0.001). History of previous SARS-CoV-2 NI provided a moderate increase in NAb titers compared to that among SARS-CoV-2 naive individuals following complete COVID-19 vaccination (p=0.033). NAb titers after two dose COVID-19 vaccination series were significantly higher (3,785, IQR:1,837-6,770; p<0.001) than after one dose (310, IQR:93-588), which was further enhanced following booster dose (16,715, IQR:6,671-33,930; p<0.001) (Fig. 1A). Longitudinal analysis following SARS-CoV-2 NI revealed that NAb titers declined most dramatically within the first six weeks (851 at two weeks vs. 341 at six weeks) after NI. Longitudinal analysis in COVID-19 vaccinated individuals without history of NI showed the largest decline in NAb titers at three- and six-months following completion of primary vaccination (3,785 at two weeks vs. 627 at three months, p<0.001; 557 at six months, p<0.001), which was restored after booster dose administration (p<0.001) (Fig. 1B). Similar findings were observed in the longitudinal cohort analysis among individuals for which multiple blood draws were obtained (Fig. 1C).
Fig. 1.
SARS-CoV-2 PRNT-50 antibody titers. (A) Group comparisons among, i) individuals with a history of SARS-CoV-2 natural infection prior to COVID-19 vaccination (NI); ii) individuals with a history of SARS-CoV-2 natural infection and ≥ 10 days following at least one dose of a COVID-19 vaccine (NI & VX); iii) individuals without a history of SARS-CoV-2 natural infection, ≥ 10 and ≤ 42 days following one dose of a mRNA COVID-19 vaccine (VX-Do1), iv) individuals without a history of SARS-CoV-2 natural infection, ≥ 10 days following two doses/complete COVID-19 vaccination (VX-Do2), v) individuals without a history of SARS-CoV-2 natural infection, ≥ 10 days following booster dose (VX-Do3); and vi) SARS-CoV-2 naïve individuals (NEG). (B) Longitudinal analysis among individuals with a history of SARS-CoV-2 natural infection (left) or COVID-19 vaccination without a history of SARS-CoV-2 natural infection (right). Intervals for the SARS-CoV-2 naturally infected individuals (left) were as follows: i) ≥ 10 days to 6 weeks (NI, ≥ 2wk), ii) ≥ 6 weeks to 6 months (NI, ≥ 6wk), iii) ≥ 6 months (NI, ≥ 6mo) following symptom onset and/or PCR positivity, and iv) asymptomatic SARS-CoV-2 natural infection (ASx). Intervals for the individuals without a history of SARS-CoV-2 natural infection following COVID-19 vaccination (right) were as follows: v) ≥ 10 and ≤ 42 days following one dose of a COVID-19 vaccine (VX-Do1), vi) ≥10 days to 6 weeks (VX-Do2, ≥ 2wk), vii) ≥ 6 weeks to 3 months (VX-Do2, ≥ 6wk), viii) ≥ 3 months to 6 months (VX-Do2, ≥ 3 mo), ix) ≥ 6 months (VX-Do2, ≥ 6 months) following two doses/complete COVID-19 vaccination; and x) with complete COVID-19 vaccination and ≥10 days following booster dose (VX-Do3), without a history of SARS-CoV-2 infection. (C) Longitudinal cohort analysis among the individuals following COVID-19 vaccination without a history of SARS-CoV-2 infection for which multiple blood draws (n = 64) were obtained (two blood draws/collection points, n = 16; three blood draws, n = 22; four blood draws, n = 8; five blood draws, n = 7; six blood draws, n = 8; seven blood draws, n = 3). Samples were collected at i) baseline, ii) ≥ 10 and ≤ 42 days following one dose of a mRNA COVID-19 vaccine (VX-Do1), iii) ≥10 days to 6 weeks (VX-Do2, ≥ 2wk), iv) ≥ 6 weeks to 3 months (VX-Do2, ≥ 6wk), v) ≥ 3 months to 6 months (VX-Do2, ≥ 3mo), vi) ≥ 6 months (VX-Do2, ≥ 6 months) following two doses/complete COVID-19 vaccination; or vii) after complete COVID-19 vaccination and ≥10 days following booster/third dose (VX-Do3). Kruskal-Wallis ANOVA test for non-parametric data was used to evaluate group differences. Dotted red line is the limit of detection of the neutralizing antibody titer (PRNT ≥ 20). *p≤0.033, **p≤0.002, ***p<0.001.
Based on functional neutralization assay, we report (i) potent COVID-19 vaccine-induced NAb responses are superior to responses following NI (p<0.001); (ii) NI followed by vaccination has a modest advantage in NAb levels when compared to administration of two-dose vaccine series (p=0.033); (iii) NAb responses decline at three- and six-months following the second dose of COVID-19 vaccine (p<0.001); and (iv) NAb responses are restored within two weeks following booster dose of COVID-19 vaccine (p<0.001). SARS-CoV-2 antibody responses characterized in this and other studies are similar to those observed following common cold coronavirus infection, which produces waning antibody responses that results in frequent re-infection [6]. These data paired with morbidity and mortality data associated with the onslaught of new SARS-CoV-2 variants will help guide the administration of future booster vaccination.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This research was supported by a grant (P30GM114737) from the Pacific Center for Emerging Infectious Diseases Research, COBRE, a grant (3P20GM103466-20S1) from INBRE, National Institute of General Medical Sciences, a grant (U54MD007601) from Ola Hawaii, a grant (T37MD008636) from the Minority Health Research Training Program, National Institute on Minority Health and Health Disparities, NIH, a grant from the Myra W. and Jean Kent Angus Foundation, and Institutional funds. We thank Hawaii Center for AIDS, and the Tropical Medicine Clinical Laboratory, JABSOM, UH Manoa nurses and staff for assistance with sample collection, and the patients for their participation in this study.
References
- 1.Dan JM, Mateus J, Kato Y, et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science. 2021;371(6529):eabf4063. doi: 10.1126/science.abf4063. Published online January 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Legros V, Denolly S, Vogrig M, et al. A longitudinal study of SARS-CoV-2-infected patients reveals a high correlation between neutralizing antibodies and COVID-19 severity. Cell. Mol. Immunol. 2021;18(2):318–327. doi: 10.1038/s41423-020-00588-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.L’Huillier AG, Meyer B, Andrey DO, et al. Antibody persistence in the first 6 months following SARS-CoV-2 infection among hospital workers: a prospective longitudinal study. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2021;27(5):784.e1–784.e8. doi: 10.1016/j.cmi.2021.01.005. Published online January 20S1198-743X(21)00031-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Giorgi V, West KA, Henning AN, et al. Naturally acquired SARS-CoV-2 immunity persists for up to 11 months following infection. J. Infect. Dis. 2021;224(8):1294–1304. doi: 10.1093/infdis/jiab295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsai WY, Ching LL, Hsieh SC, Melish ME, Nerurkar VR, Wang WK. A real-time and high-throughput neutralization test based on SARS-CoV-2 pseudovirus containing monomeric infrared fluorescent protein as reporter. Emerg. Microbes Infect. 2021;10(1):894–904. doi: 10.1080/22221751.2021.1925163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Edridge AWD, Kaczorowska J, Hoste ACR, et al. Seasonal coronavirus protective immunity is short-lasting. Nat. Med. 2020;26(11):1691–1693. doi: 10.1038/s41591-020-1083-1. [DOI] [PubMed] [Google Scholar]