Abstract
Current situation
The global influenza surveillance and response system (GISRS), coordinated by the World Health Organization (WHO), is a global framework for surveillance of influenza and other respiratory viruses, data collection, laboratory capacity building, genomic data submission and archival, standardization, and calibration of reagents and vaccine strains, production of seasonal influenza vaccines and creating a facilitatory regulatory environment for the same.
Gaps
WHO-designated national influenza centers (NICs) are entrusted with establishing surveillance in their respective countries. National and subnational surveillance remains weak in most parts of the world because of varying capacities of the NICs, lack of funds, poor human and veterinary surveillance mechanisms, lack of intersectoral coordination, and varying commitments of the local government.
Way forward
As influenza viruses have a wide variety of nonhuman hosts, it is critical to strengthen surveillance at local levels for timely detection of untypable or novel strains with potential to cause epidemics or pandemics. In this article, we have proposed possible strategies to strengthen and expand local capacities for respiratory virus surveillance through the designated NICs of the WHO.
Keywords: Respiratory virus surveillance, GISRS, National influenza centers, Untypable influenza virus, Influenza surveillance network
Graphical Abstract
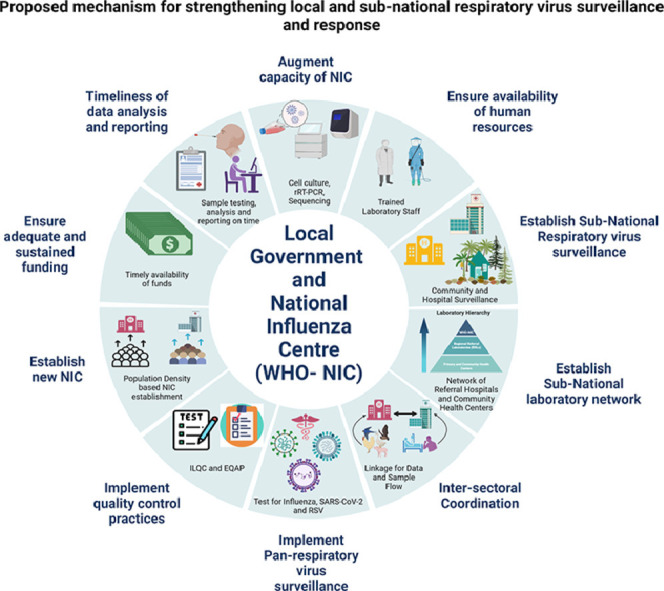
Proposed mechanism for strengthening local or subnational respiratory virus surveillance and response.
Introduction
Recurrent outbreaks of respiratory virus disease, primarily caused by influenza and coronaviruses (CoVs), have been witnessed across the world (Baber, 2020). The global history of pandemics over the past century indicates the maximum potential of influenza viruses to mutate, reassort, and evolve to cause outbreaks. This is exemplified by repeated influenza A virus (IAV) outbreaks starting from H1N1 Spanish Flu in 1918, H2N2 Asian Flu in 1957, H3N2 Hong Kong Flu in 1968, and H1N1 swine flu in 2009 (Peteranderl et al., 2016). Intermittent focal outbreaks of avian influenza virus (AIV) in Asian countries have affected poultry, migratory, and wild birds (Shao et al., 2017), with reported human infections in poultry handlers or their close contacts. CoVs are also known to evolve gradually and cause zoonotic outbreaks of respiratory infections. So far, three CoV outbreaks have been reported, of which the 2019 SARS-CoV-2 outbreak in China rapidly spread across the world and was declared a pandemic within a few months (Louca, 2021).
Overall, repeated outbreaks of respiratory viral infections have adversely impacted the health of people all across the world, health care systems, frontline workers, the global community, the economy, and development. In view of the prolonged devastating impact of the COVID-19 pandemic, it is now critical to strengthen public health surveillance for early detection and control of emerging outbreaks of influenza and other respiratory viruses. As per global recommendations, COVID-19 and influenza surveillance should be integrated through influenza-like illness/severe acute respiratory illness (ILI/SARI) surveillance platforms (Global influenza strategy 2019–2030, n.d.). In this article, we have described the existing global and regional networks for surveillance of circulating and emerging strains of respiratory viruses, highlighted gaps, and proposed strategies to strengthen and expand respiratory virus surveillance through the designated national influenza centers (NICs) of the World Health Organization (WHO).
Existing global surveillance mechanisms for influenza and other respiratory viruses
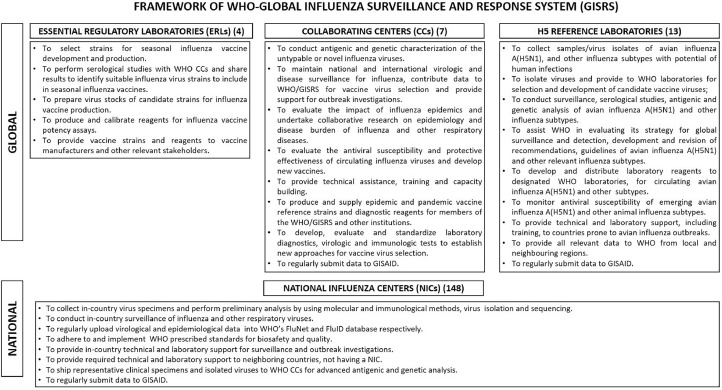
The global influenza program (GIP) was established in 1947, expanded with the establishment of WHO, and renamed as global influenza surveillance and response system (GISRS) (Ziegler et al., 2018). The WHO-GISRS framework includes four WHO essential regulatory laboratories (WHO ERLs), seven WHO coordinating centers (WHO CCs) and 13 WHO H5 reference laboratories (WHO H5 RLs) at the global level, and 148 NICs in 124 WHO member states at the national level (World Health Organization, 2022a). All WHO network laboratories are mandated to regularly submit sequencing data to the global initiative on sharing avian influenza data (GISAID) (Hay and McCauley, 2018). Figure 1 describes the roles and responsibilities of the WHO-GISRS laboratory network.
Figure 1.
Detailed overview of designated roles and responsibilities of various laboratories at global and national levels in the World Health Organization (WHO) global influenza surveillance and response system (GISRS).
NICs are the national reference laboratories and serve as a backbone for influenza testing, provision of vaccine strains/clinical samples, surveillance, and outbreak response in accordance with WHO protocols. They undertake ILI, acute respiratory illness (ARI), and SARI surveillance for detecting circulating strains of influenza and other respiratory viruses. Training for real-time reverse transcription-polymerase chain reaction (rRT-PCR), cell culture–based virus isolation, and genome sequencing is also provided to NICs by WHO. The laboratory and epidemiological data are required to be submitted to WHO FluNet and WHO FluID databases, respectively. Untypable influenza strains are to be referred to WHO CCs for characterization.
WHO CCs are global reference laboratories assigned with the task of maintaining national and international virologic and disease surveillance data, advanced viral antigenic and genetic characterization, vaccine strain selection, maintaining a repository of virus isolates, providing resources to WHO partner laboratories, conducting required epidemiological and laboratory studies, training, and capacity building. One WHO CC is also involved in animal influenza ecological studies (World Health Organization Collaborating Centers, n.d. [a]). CCs undertake specialized laboratory and field research studies as per global requirements of the GISRS (World Health Organization Collaborating Centers, n.d. [b]).
WHO ERLs undertake influenza vaccine strain selection, calibration of reagents, and provide strains and reagents to vaccine manufacturers and relevant stakeholders.
WHO H5 reference laboratories work on influenza A (H5N1) and other avian/animal influenza viruses, which may cause poultry outbreaks and human infections. They are involved in surveillance, virus isolation, antigenic and genetic characterization, antiviral susceptibility testing, conducting laboratory studies, providing technical and laboratory support to partner institutions, and offer required support to WHO for formulating and revising guidelines periodically.
Gaps in surveillance of influenza and other respiratory viruses
NICs are entrusted with the task of conducting national surveillance. However, the national and subnational framework for surveillance of influenza and other respiratory viruses remains weak in most parts of the world because of varying capacities of NICs (El Guerche-Séblain et al., 2021), workforce constraints (Abubakar et al., 2020), inadequate human and veterinary surveillance (Bailey et al., 2018; Rabinowitz et al., 2012), poor intersectoral coordination (Buse et al., 2022; Perez Arredondo et al., 2021), funding constraints, and low commitments of the local governments (World Health Organization, 2014). Because influenza viruses have the capacity to mutate and evolve rapidly, it is critical to strengthen surveillance at local levels for improving vaccine strain selection and timely detection of untypable or novel human or AIVs, which may lead to outbreaks.
Proposed mechanisms for strengthening local or subnational surveillance and response
The WHO NICs are positioned well to establish and run the subnational respiratory virus surveillance programs in the field. The NICs should be strengthened and leveraged fully to establish a robust subnational respiratory virus surveillance network for quick detection of novel influenza virus strains and signals of impending outbreaks. The following steps are recommended for strengthening the existing influenza surveillance networks at the national level through the NICs.
At national level
-
1
Augment capacity of the NICs: Malik et al. reviewed the evolution of influenza surveillance programs in 22 countries of the WHO East Mediterranean Region from 2011 to 2018. The capacity of NICs regarding their functionality, facilities, and expertise for rRT-PCR, virus isolation, antiviral susceptibility, and sequencing was evaluated along with other parameters. Results revealed significant differences within the baseline capacities of the NICs (Malik et al., 2020). Other studies have also highlighted the need to work on continuous strengthening of the NICs. In addition to rRT-PCR-based detection capacity, the maximum number of NICs should be equipped with expertise and facilities for antigenic and genetic characterization of virus strains, cell culture, virus isolation, and next-generation sequencing (Polansky et al., 2016; World Health Organization, 2014). Strengthening the infrastructure and expertise of NICs will help in their efficient functioning and enable expedited availability of local data to guide policy decisions. During the COVID-19 pandemic, many countries have significantly ramped up their capacity for molecular testing and next-generation sequencing, which must be repurposed and used to fulfill the larger surveillance gaps.
-
2
Ensure availability of human resources: Abubakar et al. evaluated the progress and challenges of influenza surveillance networks in 22 countries of the East Mediterranean region between 2014 and 2017 (Abubakar et al., 2020). Staff attrition and redeployment to other programs were identified as key challenges. A similar problem exists across many countries of the world. NICs suffer from staff attrition and the availability of a trained workforce. The presence of a trained workforce is pivotal to maintaining the quality and timeliness of various laboratory assays and surveillance data. Local governments must realize the paramount importance of encouraging and retaining trained staff and formulate employee-friendly policies to reduce attrition for maintaining high-quality surveillance.
-
3
Establish subnational respiratory virus surveillance by the NICs: Through a structured questionnaire, Polansky et al. evaluated 35 countries that had partnered with the Centers for Disease Control and Prevention (CDC) from 2004 to 2009 to strengthen their influenza surveillance capacities. Data analysis demonstrated a gradual increase in the number of countries undertaking routine influenza surveillance and reporting results. In addition, the time, length, and breadth of the surveillance programs also expanded within countries. Increased use of surveillance data for vaccine strain selection and guiding public health policies were also reported (Polansky et al., 2016). However, WHO member states still have a limited number of NICs with varying surveillance protocols. El Guerche-Séblain et al. evaluated three different national influenza surveillance networks in Australia, China, and Malaysia. Using the WHO-recommended surveillance parameters, a uniform evaluation format was developed, and the three programs were compared. Analysis revealed that the surveillance program of Australia had the broadest scope, followed by China and Malaysia. The type of data captured by the three programs also differed among countries (El Guerche-Séblain et al., 2021). In most places, ILI/SARI surveillance is restricted to only a few hospitals with very limited or no community representation. In large countries such as India, with a single operational NIC, such surveillance may be suboptimal. For timely detection of untypable or novel influenza virus strains, it is essential to conduct ILI and SARI surveillance at the hospital and community level while ensuring geographically representative sampling. Besides selected hospitals, it is desirable to collect samples from defined community catchment areas over a long period of time to depict evolving trends of the virus within the same catchment area. The designated hospital and community surveillance sites must be maintained and optimally funded by the local governments. Samples collected from these sites should be fully characterized, stored, and analyzed systematically. All untypable isolates/samples must be referred for characterization to the designated WHO CCs by the NIC as per the recommended WHO protocol (World Health Organization, 2022b).
-
4
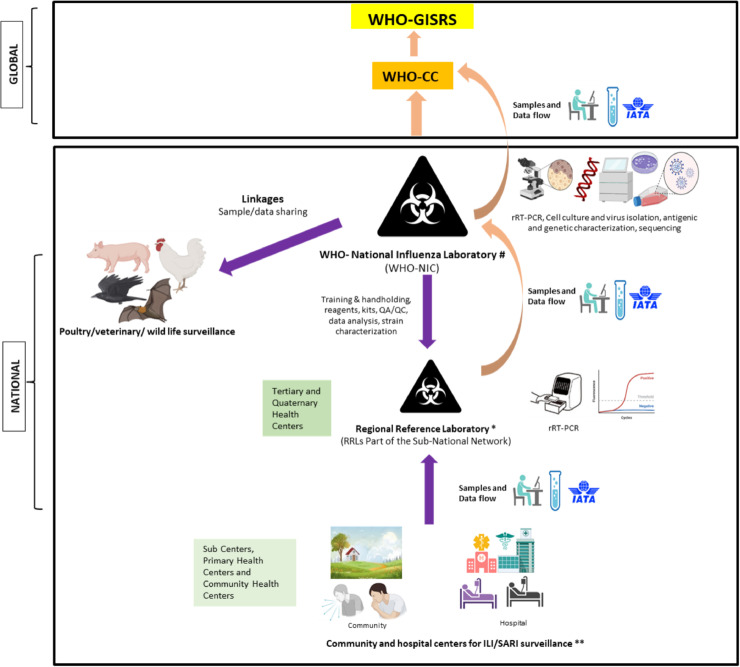
Establish a subnational laboratory network under the NICs: While strengthening hospital and community-based ILI/SARI surveillance, it is important to establish a geographically representative network of laboratories under the NICs. The CDC CC has an influenza surveillance network that spans 100 public health and 300 clinical laboratories (Centers for Disease Control and Prevention, n.d.). Such subnational networks are critical for drawing representation from all parts of the country. However, such systems are required not only under the CCs but also under the NICs, where they are practically nonexistent in many parts of the world. The subnational network of laboratories should be geographically representative and have adequately trained and equipped staff for sample collection and testing using a standard algorithm, data capture, and transport samples to NICs for further characterization and quality control purposes. In Figure 2 , we have suggested a broad framework for subnational surveillance, which can be established under the NICs.
-
5
Intersectoral coordination: Because influenza has a wide array of hosts, including avian, human, and other animal reservoirs, influenza surveillance programs should operate on the policy of “One Health,” wherein linkages with poultry, avian, and animal surveillance are absolutely critical. Though challenging (Buse et al., 2022), it is imperative to inculcate practices of data sharing and promote joint surveillance & research to detect new influenza subtypes with pandemic potential. NICs should be encouraged to collaborate with other sectors, including veterinary laboratories. In a recent comparative analysis of intersectoral collaboration to achieve the One Health goal in India and Ghana, it was documented that both countries have laid down frameworks and taken initial implementation steps. However, scaling up the effort and engagement with key stakeholders needs to be undertaken (Perez Arredondo et al., 2021).
-
6
Implement pan-respiratory virus surveillance at subnational levels: The onslaught of the COVID-19 pandemic has compromised influenza surveillance globally, mainly because of the diversion of the public health resources toward SARS-CoV-2 detection and containment. This has increased the possibility of unchecked transmission of new influenza strains giving rise to future epidemics/pandemics. Therefore, in line with the recent WHO recommendations (World Health Organization, 2022c), it is critical to integrate influenza and SARS-CoV-2 surveillance and laboratory detection capacities. It is important to realize the need for establishing and maintaining local surveillance networks for respiratory viruses, particularly influenza, SARS-CoV-2, and also respiratory syncytial virus (RSV). The network laboratories should conduct year-round surveillance for these infections pathogens and depict local trends to trigger policy decisions/interventions. The laboratories must be maintained under the continuous supervision of the NICs.
Figure 2.
Suggested framework of the subnational respiratory virus surveillance under the WHO national influenza centers (NIC) and its linkages with the WHO collaborative centers (CCs) and global influenza surveillance and response system (GISRS).
#Number of NICs may be one or more depending on the country's population density.
*Several RRLs to be operational under the NIC. Number will depend on the country's population density.
**Number of community and hospital centers operational under RRLs for ILI/SARI surveillance will vary depending upon the size of the province and population density in the particular area.
To successfully scale up pan-respiratory virus surveillance, WHO has recommended the adoption of multiplex PCR assays for detection of at least SARS-CoV-2 and influenza (World Health Organization, 2020). It may be desirable to expand the scope of multiplex assays to include RSV, as vaccines are on the horizon for RSV, and surveillance data would be critical to prioritize vaccine introduction. However, cost-effectiveness, reliability in terms of sensitivity & specificity, and upscaling such assays for widespread availability within countries are practical challenges.
-
7
Implement quality control practices: All NICs undergo an annual External Quality Assurance Program (EQAP) implemented by WHO under GISRS (World Health Organization, n.d.). Handholding and mentoring by WHO are essential to upgrade the quality standards of NICs. The NICs should be further trained to plan and implement quality control programs for the subnational laboratory network operational under them. The in-country quality initiatives may be started through Inter-Laboratory-Quality Control (ILQC) programs and can be expanded gradually to EQAP, depending on the capacity of NICs to prepare proficiency testing panels.
-
8
Establish new NICs: With concerted efforts of WHO-GISRS and global stakeholders, the number of WHO-designated NICs has steadily increased from 62 in 1972 to 143 in 2016 (Ziegler et al., 2018). In 2022, WHO reported the presence of 148 NICs in 124 countries (World Health Organization, 2022a). However, more than 70 countries still do not have designated NICs. Each country should attempt to establish at least one NIC for instituting systematic mechanisms of collaboration and support from WHO. In large and densely populated countries, one NIC may not be able to effectively coordinate and manage surveillance for the whole country. In such circumstances, it may be appropriate to establish more than one NIC, depending upon the population of the country, its size, availability of resources, and equipped laboratories.
-
9
Ensure adequate and sustained funding: Funding is a key factor for establishing and sustaining surveillance, laboratory testing, maintaining desired data quality, and retaining a trained workforce. Self-assessment of the GISRS was undertaken as per recommendations of the WHO Advisory Group. Findings revealed that many NICs suffer because of poor fund allocation and low commitment of the local governments (World Health Organization, 2014). Polansky et al., in their survey of 35 countries supported by the CDC for developing influenza surveillance, found that 29 (81%) countries identified funding as the most critical requirement to sustain surveillance, followed by staff training and technical advice (Polansky et al., 2016). Often developing countries face challenges in funding and maintaining public health surveillance, thus making them vulnerable to large outbreaks and economic losses. It is pivotal for the local governments to establish mechanisms for funding surveillance by collaborating with relevant international stakeholders and partners. CDC continues to undertake such capacity building in under-represented areas (Polansky et al., 2016). However, more international support and collaborations must be fostered to keep the world safe by early detection and effective containment of pathogens of pandemic potential and appropriate selection of influenza vaccine strains. The local policymakers should also be proactive in seeking international financial and technical support for strengthening public health surveillance in their countries.
-
10
Timeliness of data analysis and reporting: Malik et al. reported a substantial increase in data reporting to the FluNet database from the WHO East Mediterranean Region countries between 2011 to 2018, with 16 of 22 countries reporting data on a regular basis (Malik et al., 2020). Newman et al. analyzed influenza surveillance data generated between 2011 and 2016 in six WHO regions. It was seen that only 32%–79% of the countries in the six WHO regions reported data to WHO FluNet within the particular duration (Newman et al., 2018). Maximum reporting was from the European region, and the least reporting was from the West Pacific region. Timeliness of data collection, submission, and analysis are pivotal for the GISRS network. The epidemiological and rRT-PCR testing data should be collected on a real-time basis from the subnational laboratory network. The NICs should regularly submit the data to WHO FluNet and FluID databases, respectively. This will help in the appropriate and timely selection of the influenza vaccine strains. Besides, prompt detection of novel respiratory viruses is key to the prevention and control of impending epidemics or pandemics.
At global level
The world organization for animal health (formerly OIE) works with various member states for avian influenza data collection and analysis to inform local and global policymakers (World Health Organization, 2019). Intersectoral collaboration between OIE and GISRS network needs to be strengthened at the global and national levels (World Health Organization, 2013). Formulation and implementation of a One Health action plan along with the establishment of national and subnational networks of animal/avian surveillance laboratories is required for timely detection of novel influenza strains with the potential of human transmission. (World Organization for Animal Health, 2016).
Way forward
The recent COVID-19 pandemic and past pandemics of influenza viruses have underscored the critical importance of not only formulating but also implementing appropriate pandemic preparedness activities in the immediate future. Strong field surveillance systems backed up with commensurate laboratory capacity, reliable diagnostics, and required intersectoral coordination are pivotal to picking up early warning signals of emerging epidemics. Establishing, maintaining, and strengthening the national laboratory surveillance networks will enable early detection of evolving virus strains and trigger national and international alerts as per the International Health Regulations 2005. These initiatives need to be simultaneously accompanied by timely collection and reporting of good quality clinical and epidemiological data to help in understanding the infection transmission trends and clinical course of the disease. Good quality data can be effectively used for generating reliable future predictions through mathematical modeling. Data sharing and increased data transparency will facilitate learnings from the experiences of other countries and will benefit the global community.
In addition, multifaceted pandemic preparedness approaches, including public health system and health care infrastructure strengthening, must be continued. Development of effective risk communication strategies along with social mobilization and strong community engagement plans need to be developed and implemented. Community sensitization toward calibrated use of nonpharmaceutical interventions for disease containment needs to be undertaken when disease clusters are seen to develop rapidly. It is also vital to continue research for developing effective plug-and-play vaccine platforms which can be easily deployed for quick development of vaccines during the pandemic.
Timely response in any outbreak is of utmost importance, as early alert will help in quick contact tracing and disease containment. Besides, successful virus isolation early in the outbreak will enable the development of diagnostic tests for implementing preventive and therapeutic strategies to contain virus transmission. Commitment and support of the international stakeholders, increased international funding, and handholding of local governments by WHO will be instrumental in achieving this goal. Training and capacity building in various domains related to field epidemiological surveillance; laboratory testing, biosafety, biosecurity, and quality adherence; data collection, proper archival, analysis, and timely reporting are of crucial importance. Orientation of the local government authorities and prioritization of respiratory virus surveillance as one of the top public health priorities need to be undertaken by United Nations agencies to protect the world against devastating pandemics in the future. Learning from the experiences of the COVID-19 pandemic and to timely avert such threats in the future, we must aim at early detection, prevention, and control at local levels to prevent future epidemics and pandemics.
Acknowledgments
Conflict of interest
The authors have no competing interest to declare.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethical approval
Approval was not required.
References
- Abubakar A, Elkholy A, Barakat A, Shrestha B, Elhakim M, Malik MR, et al. Pandemic influenza preparedness (PIP) framework: progress challenges in improving influenza preparedness response capacities in the eastern Mediterranean Region, 2014–2017. J Infect Public Health. 2020;13:446–450. doi: 10.1016/j.jiph.2019.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baber R. Pandemics: learning from the past. Climacteric. 2020;23:211–212. doi: 10.1080/13697137.2020.1756586. [DOI] [PubMed] [Google Scholar]
- Bailey ES, Choi JY, Fieldhouse JK, Borkenhagen LK, Zemke J, Zhang D, et al. The continual threat of influenza virus infections at the human-animal interface: what is new from a one health perspective? Evol Med Public Heal. 2018;2018:192–198. doi: 10.1093/emph/eoy013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buse K, Tomson G, Kuruvilla S, Mahmood J, Alden A, van der Meulen M, et al. Tackling the politics of intersectoral action for the health of people and planet. BMJ. 2022:376. doi: 10.1136/bmj-2021-068124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention, Influenza hospitalization surveillance network (FluSurv-NET). https://www.cdc.gov/flu/weekly/influenza-hospitalization-surveillance.htm, n.d. (accessed 27 April 2022).
- El Guerche-Séblain C, Rigoine De Fougerolles T, Sampson K, Jennings L, Van Buynder P, Shu Y, et al. Comparison of influenza surveillance systems in Australia, China, Malaysia and expert recommendations for influenza control. BMC Public Health. 2021;21:1750. doi: 10.1186/s12889-021-11765-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Global influenza strategy 2019–2030, n.d. https://apps.who.int/iris/handle/10665/311184; (accessed 6 March 2022).
- Hay AJ, McCauley JW. The WHO global influenza surveillance and response system (GISRS) -A future perspective. Influenza Other Respir Viruses. 2018;12:551–557. doi: 10.1111/irv.12565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louca S. SARS-CoV-2 infections in 165 countries over time. Int J Infect Dis. 2021;111:336–346. doi: 10.1016/j.ijid.2021.08.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik MR, Abubakar A, Kholy AE, Buliva E, Khan WM, Lamichhane J, et al. Improved capacity for influenza surveillance in the WHO eastern Mediterranean Region: progress in a challenging setting. J Infect Public Health. 2020;13:391–401. doi: 10.1016/j.jiph.2019.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman LP, Bhat N, Fleming JA, Neuzil KM. Global influenza seasonality to inform country-level vaccine programs: an analysis of WHO FluNet influenza surveillance data between 2011 and 2016. PLoS ONE. 2018;13 doi: 10.1371/journal.pone.0193263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez Arredondo AM, Yasobant S, Bruchhausen W, Bender K, Falkenberg T. Intersectoral collaboration shaping One Health in the policy agenda: a comparative analysis of Ghana and India. One Health. 2021;13 doi: 10.1016/j.onehlt.2021.100272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peteranderl C, Herold S, Schmoldt C. Human Influenza Virus Infections. Semin Respir Crit Care Med. 2016;37(4):487–500. doi: 10.1055/s-0036-1584801. Aug. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polansky LS, Outin-Blenman S, Moen AC. Improved global capacity for influenza surveillance. Emerg Infect Dis. 2016;22:993–1001. doi: 10.3201/eid2206.151521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinowitz PM, Galusha D, Vegso S, Michalove J, Rinne S, Scotch M, et al. Comparison of human and animal surveillance data for H5N1 influenza A in Egypt 2006–2011. PLoS ONE. 2012;7:e43851. doi: 10.1371/journal.pone.0043851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao W, Li X, Goraya MU, Wang S, Chen JL. Evolution of influenza A virus by mutation and re-assortment. Int J Mol Sci. 2017;18 doi: 10.3390/ijms18081650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization Collaborating Centers, n.d.(a). WHO Collaborating Center for Studies on the Ecology of Influenza in Animals. https://apps.who.int/whocc/Detail.aspx?x58EgLgSW/RHnZjrTQOF2A== (accessed 6 March 2022).
- World Health Organization Collaborating Centers, WHO Collaborating Centers. https://www.who.int/initiatives/global-influenza-surveillance-and-response-system/who-collaboration-center-erl, n.d.(b) (accessed 6 March 2022).
- World Health Organization . WHO; Geneva: 2013. The use of PCR in the surveillance, characterization and diagnosis of influenza. [Google Scholar]
- World Health Organization . WHO; Geneva, Switzerland: 2014. Meeting of the Pandemic Influenza Preparedness (PIP) Framework Advisory Group. [Google Scholar]
- World Health Organization, FAO, OIE, and WHO launch a guide for countries on taking a One Health approach to addressing zoonotic diseases - OIE - World Organisation for Animal Health 2019. https://www.oie.int/en/fao-oie-and-who-launch-a-guide-for-countries-on-taking-a-one-health-approach-to-addressing-zoonotic-diseases, 2019 (accessed 6 March 2022).
- World Health Organization, Maintaining surveillance of influenza and monitoring SARS-CoV-2 – adapting Global Influenza Surveillance and Response System (GISRS) and sentinel systems during the COVID-19 pandemic. https://www.who.int/publications/i/item/maintaining-surveillance-of-influenza-and-monitoring-sars-cov-2-adapting-global-influenza-surveillance-and-response-system-(gisrs)-and-sentinel-systems-during-the-covid-19-pandemic, 2020 (accessed 27 April 2022).
- World Health Organization, National Influenza Centers of World Health Organization. https://www.who.int/initiatives/global-influenza-surveillance-and-response-system/national-influenza-centres, 2022a (accessed 24 April 2022).
- World Health Organization, Regional and global influenza laboratory networks. https://www.euro.who.int/en/health-topics/communicable-diseases/influenza/seasonal-influenza/surveillance-and-lab-network/regional-and-global-influenza-laboratory-networks, 2022b (accessed 28 April 2022).
- World Health Organization, End-to-end integration of SARS-CoV-2 and influenza sentinel surveillance: revised interim guidance. https://www.who.int/publications/i/item/WHO-2019-nCoV-Integrated_sentinel_surveillance-2022.1, 2022c (accessed 27 April 2022).
- World Health Organization, Global Influenza Programme, https://www.who.int/teams/global-influenza-programme/laboratory-network/eqa-project, n.d. (accessed 27 April 2022).
- Ziegler T, Mamahit A, Cox NJ. 65 years of influenza surveillance by a World Health Organization-coordinated global network. Influenza Other Respir Viruses. 2018;12:558–565. doi: 10.1111/irv.12570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Organization for Animal Health, Asia Pacific Workshop on surveillance, prevention, and control of zoonotic influenza. https://rr-asia.oie.int/en/events/zoonotic_flu_paro_aug2016/, 2016 (accessed 8 July 2021).