Abstract
The lantibiotic bacteriocin mutacin II is produced by the group II Streptococcus mutans. The mutacin II biosynthetic locus consists of seven genes, mutR, -A, -M, -T, -F, -E, and -G, organized as two operons. The mutAMTFEG operon is transcribed from the mutA promoter 55 bp upstream of the translation start codon for MutA, while the mutR promoter is 76 bp upstream of the mutR structural gene. Expression of the mutA promoter is regulated by the components of the growth medium, while the mutR promoter activity does not seem to be affected by these conditions. Inactivation of mutR abolishes transcription of the mutA operon but does not affect its own promoter activity. The expressions of both mutA and mutR promoters are independent of the growth stage, while the production of mutacin II is only elevated at the early stationary phase. Taken together, these results suggest that expression of the mutacin operon is regulated by a complex system involving transcriptional and posttranscriptional or posttranslational controls.
A wide range of bacteria produce antimicrobial peptides called bacteriocins (13). Bacteriocins have attracted tremendous attention from the biomedical research community in the last few years, due to the increase in the number of multidrug-resistant pathogens. Mutacin II, elaborated by group II Streptococcus mutans, has been purified and partially characterized (6, 13, 17, 18). It belongs to the lantibiotic family of bacteriocins, which are characterized by the presence of lanthionines, β-methyllanthionines, and dehydrated amino acid residues (14, 28). Based on the peptide tertiary structure imposed by the position of the thioether bridges, lantibiotics are divided into two groups: group A (linear) and group B (globular) (14, 28). Two or three subgroups are distinguished within group A according to homologies of the prepropeptide sequences and the positions of the modified residues (8, 29). Mutacin II has strong amino acid sequence similarity to subgroup A II lantibiotics, including lacticin 481, streptococcin A-FF22, salivaricin A, streptococcin A-M49, and variacin (2, 12, 21, 22, 24, 26).
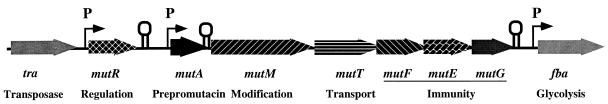
Mutacin II, like other lantibiotics, has the potential to be used in antibiotic therapies because of its wide spectrum of activity against gram-positive bacterial pathogens (19). In order to build a foundation for future large-scale production and application, the basic mechanism(s) controlling the expression of the mutacin biosynthetic genes needs to be elucidated. We recently demonstrated, by en bloc transformation, chromosomal walking, and DNA sequencing, that the mutacin II biosynthetic genes are clustered on the chromosome of the producer strain (5). The locus consists of seven genes in the order mutR, -A, -M, -T, -F, -E, and -G, flanked by a silent transposase gene (tra) and a fructose bisphosphate aldolase gene (fba), respectively (2, 5) (Fig. 1). The first gene, mutR, is a homolog of the transcription regulator of glucosyltransferase G (rgg) of Streptococcus gordonii (30). The structural genes for prepromutacin (mutA) and the modifying enzyme (mutM) have been described previously (32). The fourth gene, mutT, is presumably the ABC (ATP binding cassette) transporter for premutacin processing and secretion. The mutF, -E, and -G genes are thought to be involved in immunity (5).
FIG. 1.
Genomic map of mutacin locus. Shown here is an ∼12-kb DNA fragment containing the silent transposase gene (tra), the putative transcription regulator gene (mutR), the prepromutacin gene (mutA), the modifying-enzyme gene (mutM), the transporter gene (mutT), the immunity genes (mutF, -E, and -G), and the fructose bisphosphate aldolase gene (fba). The rho-independent transcription terminator for mutR and the possible transcription attenuator at the mutA-mutM junction are illustrated. The arrows represent promoters.
DNA sequence analyses revealed promoter-like structures upstream of mutR and mutA and a ρ-independent transcription terminator-like sequence between mutR and mutA. No definitive promoter-like sequence was found in the intergenic region between mutA and mutM or between mutM and mutT, -F, -E, and -G, whose open reading frames overlapped. The aim of this study was to determine the transcription units in the mutacin locus and to gain insights into the regulatory mechanisms for mutacin II production.
MATERIALS AND METHODS
Bacterial strains and media.
A mutacin II-producing strain, S. mutans T8 (25), was used for RNA isolation and strain construction. Escherichia coli DH5α was used for the cloning and propagation of plasmids. For RNA isolation, S. mutans T8 and its derivative strains were grown anaerobically at 37°C in CDM-TSBY medium containing a 1:1 mixture of chemically defined medium (CDM) (JRH Biosciences, Lenexa, Kans.) and trypticase soy broth (BBL Becton Dickinson, Cockeysville, Md.) plus yeast extract (Difco, Detroit, Mich.) (TSBY) (17) or as otherwise indicated (see the legend for Fig. 8). The overnight culture was diluted 1:50 in the same medium and then incubated under the same conditions. Cell growth was followed by densitometry (optical density at 600 nm [OD600]) with a Beckman (Fullerton, Calif.) DU7400 spectrophotometer. Samples were taken at each designated time point during the course of growth. The cells were harvested by centrifugation and frozen at −70°C until needed.
FIG. 8.
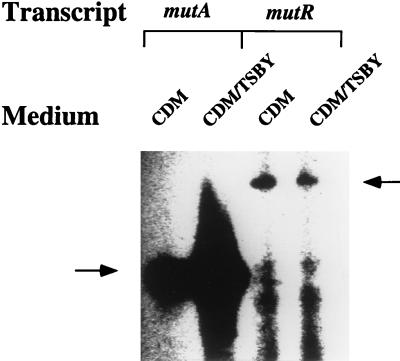
Expression of the mutA and mutR promoters in cells grown in CDM or CDM plus TSBY medium. S. mutans T8 cells were grown anaerobically in either CDM or CDM plus TSBY medium to mid-log phase (the doubling time in CDM was 2 h, and the growth curve levels off at an OD600 of 2.0; in CDM-TSBY medium, the doubling time was 1 h and the growth curve levels off at an OD600 of 4.5). “Mid-log” refers to an OD600 of 1.0 in CDM and 2.0 in CDM-TSBY medium. RNA was isolated, and equal amounts (20 μg) were used to hybridize with the same amount of 32P-labeled mutA or mutR primer. An autoradiograph of the sequencing gel is shown; each lane contained equal amounts of the reaction mixture. The arrow on the left indicates the mutA transcripts, and the arrow on the right indicates the mutR transcripts. The difference between the levels of the mutA transcripts synthesized in the two different media is about sevenfold.
Transformation.
Transformation of S. mutans T8 was performed essentially as described previously (31), with minor modifications. Briefly, 1 ml of Todd-Hewitt broth (Difco Laboratories) supplemented with 0.2% bovine serum albumin (Fraction V; United States Biochemical Corp., Cleveland, Ohio) was inoculated with two or three single colonies of S. mutans from an overnight Todd-Hewitt agar plate, and the culture was then incubated anaerobically at 37°C overnight. The overnight culture was diluted 1:40 into 3 to 10 ml (depending on the number of transformations performed) of the same medium and incubated under the same conditions for 3 h. A 0.35-ml aliquot of this culture was transferred to a 1.5-ml Eppendorf tube, and 0.1 to 0.5 μg of DNA was added. The mixture was incubated at 37°C for 1 to 1.5 h, and 10 to 100 μl was plated onto the appropriate selective plate.
RNA isolation.
Total RNA from S. mutans T8 and its derivatives was isolated from 10 to 50 ml of culture (depending on the cell density) at each designated time point. Each sample was transferred without delay to a centrifuge tube containing crushed ice to stop cell growth, and the cells were precipitated by centrifugation at 20,000 × g for 10 min at 4°C. The cell pellets were washed with buffer A (20 mM sodium acetate, pH 4.5, 1 mM Na-EDTA) and frozen at −70°C until needed. The cell pellets were resuspended in 0.7 ml of buffer A and sonically dispersed for 10 s; 70 μl of 10% sodium dodecyl sulfate (SDS) was then added. The suspension was mixed vigorously with 0.7 ml of hot acidic phenol (70°C; pH 4.3) and incubated at 70°C for 10 min with frequent mixing. The samples were chilled on ice for 5 min and then spun down in a microcentrifuge at room temperature for 5 min. The aqueous phase was removed and extracted once with an equal volume of hot phenol, once with an equal volume of phenol-chloroform (1:1), and once with an equal volume of chloroform-isoamyl alcohol (24:1). RNA was precipitated with 2.5 volumes of 100% ethanol in the presence of 0.3 M sodium acetate (pH 5.2). The RNA pellet was washed with 70% ethanol, air dried, and resuspended in diethyl pyrocarbonate-treated water. RNA concentrations were determined spectrophotometrically at 260 nm and affirmed by agarose gel electrophoresis and densitometry after ethidium bromide staining of the gel.
Quantitative primer extension mapping.
Quantitative primer extension mappings were performed with 10 or 20 μg of total RNA isolated from cells in different growth stages. The primer was 5′ end labeled with [γ-32P]ATP and separated from the free isotope by ethanol precipitation. The radioactivity of the probe was measured by using a scintillation counter, and 1.5 × 106 cpm was used for each primer extension reaction. The reactions were carried out with avian myeloblastosis virus reverse transcriptase (Boehringer Mannheim Biochemicals, Indianapolis, Ind.) essentially as described previously (15). The same primer was also used to generate sequencing ladders as size markers. The extended products were separated on an 8% polyacrylamide sequencing gel, visualized by autoradiography, and quantified (if necessary) by using a Molecular Dynamics (Sunnyvale, Calif.) PhosphorImager.
Northern and dot blot analyses.
For Northern blot analysis, 10 to 20 μg of RNA was precipitated and resuspended in 5 μl of diethyl pyrocarbonate-treated H2O. To the RNA suspension, the following reagents were added: 1 μl of ethidium bromide (10 mg/ml), 7 μl of 2× RNA loading buffer (95% deionized formamide, 2% formaldehyde, and 2× MOPS (morpholinepropnaesulfonic acid) buffer [10× MOPS buffer is 0.4 M MOPS, 0.1 M Na acetate, 10 mM EDTA, pH 7.0]), and 1 μl of loading dye (30% glycerol, 0.1 mM EDTA, 0.05% bromophenol blue and xylene cyanol). The mixture was heated at 70°C for 5 min, chilled on ice, and loaded into a 1% agarose gel containing 6.7% formaldehyde and 1× MOPS buffer. An RNA size standard (Gibco BRL, Grand Island, N.Y.) was loaded alongside the RNA samples. After gel electrophoresis, the RNA was blotted onto a nylon membrane by pressure transfer in 20× SSC (3 M NaCl, 0.3 M Na citrate, pH 7.0) and cross-linked in an automatic cross-linker (Stratagene, La Jolla, Calif.). For dot blotting, 5 μg of the pretreated RNA was applied to the nylon membrane with a dot blot apparatus (Gibco BRL).
Radioactive probes were generated by PCR with [α-32P]dATP (Amersham Life Sciences Inc., Arlington Heights, Ill.) under the following conditions. A 50-μl reaction mixture contained 1× standard PCR buffer; 4 ng of DNA template; 100 μM (each) dGTP, dCTG, and dTTP; 2.5 μM (each) “cold” and “hot” dATP; and 2 U of Taq DNA polymerase. The reaction was performed in an automatic thermocycler (Perkin-Elmer, Norwalk, Conn.) for 40 cycles at 94°C for 1 min, 50°C for 1 min, and 72°C for 1 min. After PCR, the labeled probe was separated from the free isotope by ethanol precipitation and the radioactivity of the probe was measured with a scintillation counter.
The membrane was prehybridized at 68°C for 1 h in 5 ml of the hybridization solution: 7% SDS, 0.5 M Na phosphate buffer [pH 7.0] and 1 mM EDTA. About 106 cpm of the labeled probe was then added to the prehybridization bag, and the hybridization continued at 68°C overnight. The membrane was washed twice with 2× SSC plus 0.1% SDS at room temperature for 15 min each time and then twice with 0.2× SSC plus 0.1% SDS at 62°C for 20 min each time. RNA was localized on the gel by using X-OMAT AR film (Eastman Kodak, New Haven, Conn.) exposed to the membrane.
Plasmid and strain construction.
The plasmid for gene replacement in S. mutans, pCBM6, was constructed previously (3). To construct the mutA promoter deletion mutation, pCBM6 was used as the template in an inverse PCR with two phosphorylated primers: mut14 (5′-GTAGTTCTAGACCTTTTATCGTCCTTAGG-3′) and 007 (5′-AGCAATAAAGTGAGGTG-3′), which bound upstream and downstream of the mutA promoter region, respectively. The PCR was performed in standard PCR buffer with Pfu polymerase (Stratagene) and the following parameters: 94°C for 1 min, 40°C for 1 min, and 72°C for 14 min for 25 cycles. The PCR product was then treated with DpnI restriction enzyme (New England Biolabs Inc., Beverly, Mass.) for 2 h, ligated, and transformed into E. coli DH5α to obtain plasmid pCBM6ΔP. To construct the mutA promoter deletion strain, the insert in pCBM6ΔP was released by BamHI restriction enzyme digestion, transformed into competent S. mutans T8, and integrated into the chromosome via double-crossover exchange. The resultant strain, T8ΔP, was confirmed by PCR analyses as well as by a mutacin activity assay.
Construction of the mutR mutant strain, T8mutR, was achieved by plasmid insertional inactivation. Briefly, a ∼300-bp DNA fragment from the middle of the mutR gene was amplified by PCR and cloned into pVA891, a derivative of pVA838 (16) from which the streptococcal origin of replication was removed. The resulting plasmid, pVA891R, was then transformed into T8 and integrated into the chromosome through a single-crossover recombination at the mutR locus. Since the mutR gene carried on plasmid pVA891R was truncated at both the 5′ and 3′ ends, the chromosomal copy of mutR became truncated after pVA891R insertion, thereby resulting in the MutR− strain, T8mutR. The mutant strain was confirmed by PCR analyses.
Mutacin II activity assay.
The mutacin II activity assays used in the time course experiment (see Fig. 4) were performed as follows. A 10- to 50-ml culture of T8 taken at each designated time point was spun down in a centrifuge. The supernatant was removed and extracted with an equal volume of chloroform. The proteinous interface was then collected by centrifugation and dried under a stream of air. The pellet was washed with 0.5 ml of double-distilled H2O and resuspended in 100 μl of 6 M urea (crude extract). The crude extract was diluted with phosphate-buffered saline, and 20 μl of each of the dilutions was spotted on top of an overlay of the indicator strain, Streptococcus sobrinus OMZ176. One arbitrary unit of mutacin II was defined as the reciprocal of the highest dilution that produced a clear zone of inhibition.
FIG. 4.
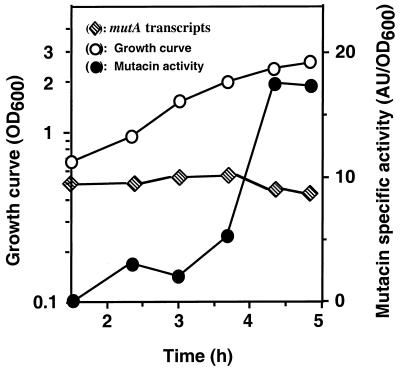
Time course of mutA promoter expression and mutacin production. The levels of mutA transcript synthesis during the course of cell growth were measured by quantifying the reverse transcripts shown in Fig. 3A with a PhosphorImager. The relative transcript levels were calculated by arbitrarily assigning the highest PhosphorImager counts as 100%. To measure the levels of mutacin production, supernatants from the same samples used for the primer extension mapping shown in Fig. 3 were subjected to mutacin extraction and activity assays, as described in Materials and Methods. To ensure reproducibility of the data, the experiments were repeated at least three times with RNA isolated from three independent cultures. The data presented here are from a typical experiment. AU, arbitrary units.
Nucleotide sequence accession numbers.
The sequences of the genes have been deposited in GenBank with accession no. U40620 (mutA-M), AF007761 (mutR), AF026468 (mutT), and AF082183 (mutFEG).
RESULTS
Northern blot analysis of the transcripts from the mutacin biosynthetic locus.
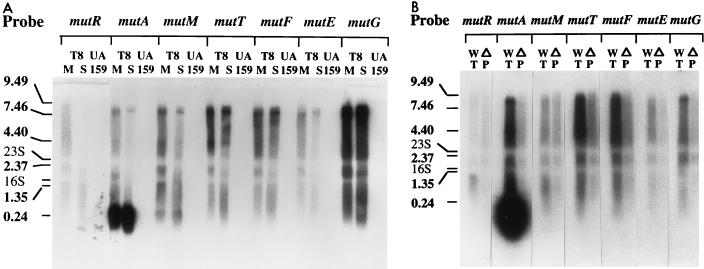
To determine how many transcription units comprise the mutacin biosynthetic locus, we performed Northern blot analyses with 32P-labeled DNA probes (200 to 300 bp) specific to each of the seven genes (Fig. 2A). Hybridization with the mutA probe detected two putative transcripts of about 0.24 and 8 to 9 kb in size. The 0.24-kb RNA was the most abundant transcript and hybridized only with the mutA probe. This result suggested that the majority of the transcripts terminated at the end of the mutA gene. In contrast to the 0.24-kb RNA, the 8- to 9-kb transcript hybridized with every probe except mutR. The size of the transcript agreed with the length of the DNA from mutA to the end of mutG, indicating that this transcript arose from the mutAMTFEG polycistronic operon. The mutR probe, on the other hand, hybridized with transcripts >9 kb in size as well as smaller ones around 1 kb. As a control, the RNA from a mutacin-nonproducing strain, S. mutans UA159, did not hybridize with any of the probes. Taken together, these data indicate that there are only two transcription units in the mutacin biosynthetic locus: mutR and mutAMTFEG.
FIG. 2.
Northern blot analysis of transcripts of mutacin locus. (A) RNAs isolated from the mid-log-phase (lanes M) and stationary-phase (lanes S) cultures of S. mutans T8 and RNA from the nonproducer strain UA159 (lanes 159) were hybridized separately with 32P-labeled probes specific to the seven genes in the mutacin locus. “Mid-log-phase cultures” refers to samples taken at an OD600 of 2.0 in CDM-TSBY medium, and stationary-phase cultures are samples taken at an OD600 of 4.5. S. mutans T8 and its derivatives grown in CDM-TSBY medium have a doubling time of 1 h, and the growth curve levels off at an OD600 of 4.5, as measured on a Beckman DU7400 spectrophotometer. (B) To confirm that mutA is the only promoter in the mutAMTFEG operon, the same gene probes were used to hybridize with RNAs isolated from the wild-type strain (WT) and the promoter deletion mutant strain (ΔP). The RNA molecular weight markers are labeled on the left. Note that in both autoradiographs the positions of the 23S and 16S RNAs are shown as light bands due to the existence of an excessive amount of the RNA, which blocked hybridization of the probes to their respective target RNAs.
To provide further support for the above assumption, we constructed a mutA promoter deletion strain (T8Δp) and compared the transcripts produced by this strain with those of the wild-type strain by Northern blot analysis. We reasoned that if the mutAMTFEG genes were cotranscribed from the mutA promoter, then deletion of the promoter would abolish transcription of the mutA as well as the mutMTFEG genes. In comparison, the mutA promoter deletion should not affect transcription of the mutR gene. As shown in Fig. 2B, similar hybridization patterns were observed in the wild-type and mutant strains with the mutR probe. In contrast, the 8- to 9-kb transcripts were not detected in the mutant strain with the mutA, -M, -T, -F, -E, and -G probes, nor was the 0.24-kb transcript.
Localization of the mutA promoter.
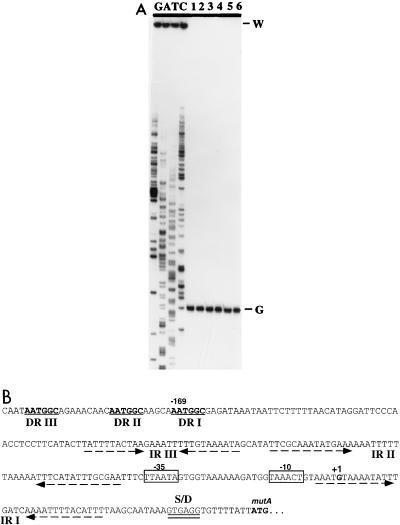
To locate the promoter for the mutAMTFEG operon, we used a 5′ end-labeled primer complementary to codons 6 through 15 of the mutA structural gene. Primer extension mapping detected a single transcript, which was initiated at a G residue 55 bp upstream of the translation start codon for MutA (Fig. 3). Inspection of the DNA sequence upstream of the transcription start site revealed a putative −10 region with the sequence TAAACT and a putative −35 region with the sequence TTAATA, separated by a 16-bp spacer (Fig. 3B).
FIG. 3.
Primer extension mapping of the mutA transcripts. (A) Autoradiograph of the sequencing gel used to analyze the reverse transcripts. Lanes G, A, T, and C, sequencing ladders generated by the same primer used in the primer extension reaction; lanes 1 through 6, RNA samples isolated from early-log-phase to stationary-phase cultures at about 45-min intervals (Fig. 4). The initiation nucleotide (G) and the position of the loading well on the gel (W) are labeled on the right. Note that the DNA template used for the sequencing reaction contained an A-to-G point mutation in the promoter −10 region. (B) DNA sequence of the mutA promoter region. The −35 and −10 regions are boxed. The transcription initiation site for the mutA promoter (+1), the Shine-Dalgarno sequence (S/D), and the initiation codon for MutA (mutA) are indicated above the sequence. The arrows represent the three inverted repeats (IR I to III), and the underlined bold letters denote the three direct repeats (DR I to III).
Time course of the mutA transcript synthesis and mutacin II production.
To determine the temporal expression pattern of the mutA promoter, we used a quantitative method for the primer extension mapping described above (see Materials and Methods). The reverse transcripts generated from the primer extension mapping were then quantified by using a PhosphorImager. The results, shown in Fig. 4, demonstrated that mutA promoter expression was independent of the growth stage. To ensure that this quantitation method was reliable, a parallel dot blot analysis was performed with 5 μg of total RNA from each time point and a 32P-labeled mutA probe. Similar results were obtained (data not shown). To determine the correlation between the transcription of mutA and the production of mutacin, the supernatants saved from the samples used in the primer extension mapping were subjected to mutacin isolation and activity assays as described in Materials and Methods. When the levels of mutA transcripts and mutacin activity were plotted in parallel as a function of cell growth, a significant difference was observed between the patterns of mutA transcript synthesis and mutacin activity (Fig. 4). While the levels of steady-state mutA transcripts were constantly high during growth, the levels of mutacin activity were very low (∼5 to 10% of the peak level) during the same period. At early stationary phase, the transcript level decreased slightly, presumably due to the increased turnover rate of RNA, and the level of mutacin activity increased dramatically. Similar results were also obtained with two other independent experiments (data not shown). These results suggest that mutacin production is not only regulated at the transcriptional level but also at the posttranscriptional or posttranslational level.
Primer extension mapping of mutM transcripts.
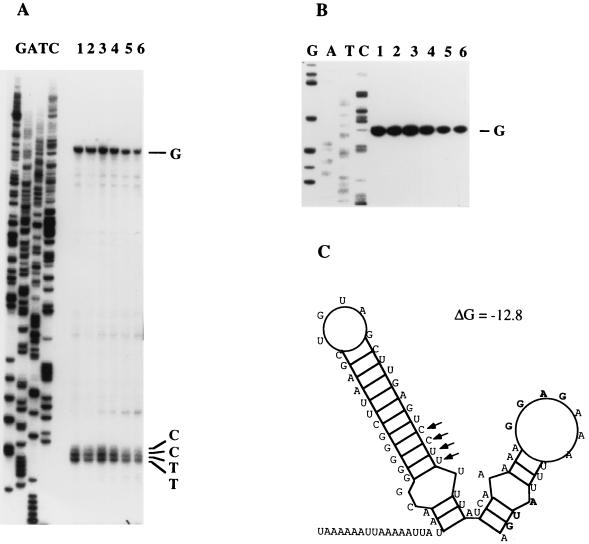
The Northern blot analyses described above suggested that the mutMTFEG genes were transcribed from the mutA promoter (Fig. 2); however, convincing evidence was lacking due to the extensive degradation of the full-length RNA and the low resolution of the agarose gel. To unambiguously resolve the question of whether mutM was indeed transcribed from the mutA promoter, we determined the 5′ ends of the mutM transcripts by primer extension mapping with a primer complementary to codons 7 through 13 of the mutM structural gene. The results are shown in Fig. 5. Unlike the results obtained with the mutA primer (Fig. 3), extension with the mutM primer generated several transcripts, as shown on the autoradiograph (Fig. 5A). The four smaller bands corresponded to RNA transcripts with their 5′ ends as C, C, U, and U, respectively. Inspection of the DNA sequence upstream of the four nucleotides did not reveal any promoter-like sequences. Instead, the four nucleotides constituted the lower part of the stem in a proposed stem-loop structure in the RNA (Fig. 5C). Accordingly, we assumed that the four smaller transcripts arose from the premature termination products of reverse transcriptase due to the secondary structure in the template RNA.
FIG. 5.
Primer extension mapping of the mutM transcripts. RNA was isolated from S. mutans T8 cell cultures from mid-log to stationary phase at 1-h intervals. (A) Autoradiograph showing the reverse transcripts generated from the mutM primer. The nucleotides at the 5′ ends of the four smaller transcripts are labeled on the right (CCTT). These four nucleotides correspond to the nucleotides at the lower part of the proposed stem-loop structure in the RNA (indicated by the arrows in panel C). (B) With prolonged electrophoresis, the upper band on the autoradiograph was resolved and was determined to correspond to a transcript initiated at the same G residue as that of the mutA transcript. (C) Proposed secondary structure of RNA in the mutA-mutM intergenic region. The Shine-Dalgarno sequence and the translation start codon for MutM are shown by bold letters. The secondary structure was generated by using the RNA Fold program (Genetics Computer Group). See the legend to Fig. 3A for an explanation of the lane labels.
In addition to the four smaller transcripts, a larger transcript was evident (Fig. 5A). With prolonged electrophoresis, the 5′ end of this transcript proved to be the same G residue as that of the mutA transcript (Fig. 5B).
Primer extension mapping of the mutR transcript.
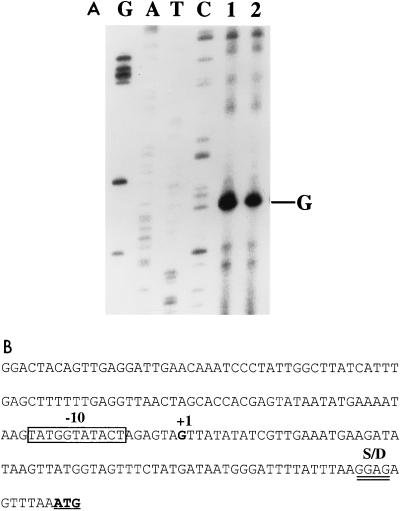
Northern blot analysis suggested that mutR may be transcribed separately from the mutAMTFEG genes. To locate the mutR promoter, we performed primer extension mapping with a primer complementary to codons 5 through 11 of the mutR structural gene and RNA isolated from mid-log- and early-stationary-phase cultures of S. mutans T8. Analysis of the polyacrylamide sequencing gel revealed a major band corresponding to a transcript initiated at a G residue 76 bp upstream of the initiation codon for MutR (Fig. 6). In addition, numerous larger bands were observed, suggesting a substantial read-through from the upstream gene(s). This result confirmed the observations made in the Northern blot analysis described above (Fig. 2). Examination of the DNA sequence upstream of the transcription start site revealed an extended −10 region with the sequence TATGGTATACT. Interestingly, no −35 sequence was present in the expected location. Instead, a consensus −10 sequence (TATAAT) was located 16 bp upstream of the authentic −10 hexamer (Fig. 6B). This result suggests that the mutR promoter may belong to a family of promoters with extended −10 regions and without canonical −35 sequences (27).
FIG. 6.
Primer extension mapping of the mutR transcripts. (A) RNA was isolated from mid-log- and early-stationary-phase cultures (lanes 1 and 2, respectively) of S. mutans T8 and hybridized with the mutR primer labeled at its 5′ end. The same primer was used to generate sequencing ladders (lanes G, A, T, and C). The 5′ end of the major transcript corresponds to a G residue. Note the extensive read-through from the upstream gene(s). (B) Sequence of the mutR promoter region. The extended −10 region and the transcription start site (+1) are indicated. The Shine-Dalgarno (S/D) sequence and the translation start codon for MutR are double underlined and underlined, respectively.
Effects of mutR mutation on transcription of mutA and mutR promoters.
To determine the role mutR plays in mutacin production, a mutR mutation strain, T8mutR, was constructed by insertional disruption of the mutR gene on the chromosome (see Materials and Methods). The effect of mutR mutation on the production of mutacin II was then assessed by the deferred-antagonism method on TSBY plates with S. sobrinus OMZ176 as the indicator. The result (not shown) showed the absence of an inhibition zone for strain T8mutR compared with the parental strain T8, indicating that mutR was essential for mutacin II production.
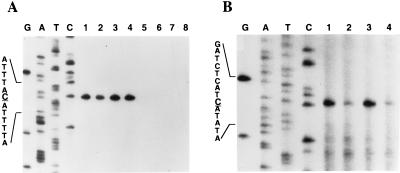
Next, we wondered whether the mutR gene mutation affected the transcription of the mutacin operon. To answer this question, we analyzed the mutA transcript levels in the wild-type and the mutant strains by quantitative primer extension mapping. The wild-type and mutant strains were grown anaerobically in the optimal medium, CDM-TSBY, and cell growth was followed by densitometry (OD600). The mutant strain grew at the same doubling time (1 h) as the wild-type strain. Cell samples were taken at 1-h intervals starting at an OD600 of 0.5, and total RNA was isolated and used as templates for primer extension mapping. As shown in Fig. 7A, with the wild-type T8, the mutA transcript levels were high throughout the growth stages (lanes 1 to 4), consistent with our previous observations (Fig. 3). In contrast, no mutA transcript was detected in the mutR mutant strain (lanes 5 to 8). This result indicates that mutR plays an essential role in mutA promoter activity, possibly as a transcription activator.
FIG. 7.
Effects of mutR mutation on activities of the mutA and mutR promoters. (A) quantitative primer extension mapping of the mutA transcripts. The wild-type strain T8 and the mutant strain T8mutR were grown in CDM-TSBY medium, and samples were taken at 1-h intervals starting at an OD600 of 0.5. Total RNA was extracted and used as a template for primer extension with the mutA primer described in the legend to Fig. 3. Lanes 1 to 4, samples from time points 1 to 4 of the wild-type culture; lanes 5 to 8, samples from time points 1 to 4 of the mutR mutant culture. T8 and T8mutR have the same growth rate. (B) quantitative primer extension mapping of the mutR transcripts. The primer used here was the same as that for Fig. 6. Lanes 1 and 2, samples from time points 2 and 4 of the wild-type culture in panel A; lanes 3 and 4, samples from time points 2 and 4 of the mutant culture in panel A. The sequences around the initiation sites (C) are shown on the left of each panel. Lanes G, A, T, and C contain sequencing ladders.
To determine if mutR also regulated its own promoter activity, RNA samples from time points 2 and 4 (Fig. 7A) were analyzed by primer extension to determine the level of mutR transcripts. As shown in Fig. 7B, the wild-type (lanes 1 and 2) and the mutant (lanes 3 and 4) strains produced nearly identical levels of mutR transcripts, indicating that mutR expression was not autoregulated.
Effect of medium composition on expression of mutA and mutR promoters.
Our previous studies demonstrated that mutacin II production was dependent on the medium composition; a 1:1 mixture of CDM-TSBY medium gave rise to a maximum yield under the conditions that we used (17). To determine whether this medium-dependent mutacin production was controlled at the transcriptional or posttranscriptional level, we measured, by primer extension mapping, the levels of the mutA and mutR transcripts produced in cells grown in either CDM or CDM plus TSBY medium. As shown in Fig. 8, the level of the mutA transcripts synthesized in cells grown in CDM was about sevenfold less than that in cells grown in CDM-TSBY medium. In contrast, the level of the mutR transcripts was not affected by the different medium compositions. This result indicates that the low yield of mutacin II production in CDM is, at least partially, due to low levels of transcription of the mutacin operon. It is also worth noting that the level of mutR transcripts in both media is extremely low compared with the level of the mutA transcripts in the same media.
DISCUSSION
In this study, we demonstrated that the mutacin II biosynthetic locus consists of two operons: the mutR and the mutAMTFEG operons. This conclusion came from Northern blot analysis with probes specific to the seven genes in the mutacin locus and RNAs isolated from the wild-type strain and the mutA promoter deletion strain, T8ΔP. With the wild-type strain, a transcript of about 8 to 9 kb hybridized with every gene probe except mutR, whereas in the mutant strain, this transcript was not produced (Fig. 2B). Furthermore, the T8ΔP strain was also defective in immunity to mutacin II (data not shown), indicating that the expression of the downstream immunity genes was dependent on the mutA promoter. More direct support came from the primer extension mapping of the mutM transcripts. Data from this experiment clearly revealed that the mutM transcripts were initiated at the same start site as that of the mutA transcripts (Fig. 5). Because the mutM, -T, -F, -E, and -G genes were either overlapping or adjoining, they were likely to be cotranscribed.
The mutA promoter was located by primer extension mapping as a G residue 55 bp upstream of the translation start codon for MutA. Analysis of the mutA promoter region revealed three inverted repeats, IR I, II, and III, and three 6-bp direct repeats, DR I, II, and III (Fig. 3B). IR I overlaps with the transcription start site, whereas IR II and III are located immediately upstream of the −35 region (from −39 to −116). Similar structures are also present in the epidermin biosynthetic operon, in which IR II serves as the binding site for the regulatory protein, EpiQ (20). DR I to III are located between −169 and −195, with 5-bp spacing between DR I and II and 9-bp spacing between DR II and III. No similar structures were reported with other lantibiotic genes and operons. The presence of these structural features in the mutA promoter region suggests a complex system for transcription regulation of the mutA operon, possibly involving both cis- and trans-acting elements. Data presented in Fig. 7 clearly demonstrate the involvement of MutR in transcription activation of the mutA promoter. Then does MutR bind to the IR region as EpiQ does? Protein sequence analysis did not reveal any obvious DNA binding motifs, nor are similar structural domains found in the regulators of the two-component signal transduction system. However, the fact that MutR bears strong homology to the transcription regulator, Rgg, suggests that it may be directly involved in the activation of the mutA promoter. Gel shift and DNA footprint analyses with purified MutR are needed to answer this question.
The mutR promoter was mapped to the position 76 bp upstream of the mutR structural gene (Fig. 6). Unlike the mutA promoter, the mutR promoter has an extended −10 region but lacks a −35 sequence. This feature is commonly seen among other streptococcal promoters (27). Interestingly, the nisR promoter also lacks a canonical −35 sequence, and both the mutR and nisR promoters seem to be expressed constitutively (Fig. 6A) (7). Inspection of the mutR promoter region did not divulge any obvious inverted repeats or direct repeats.
A stem-loop structure between the structural gene (lanA) and the modifying-enzyme gene (lanB) is highly conserved among all lantibiotic operons so far characterized (11). This structure is presumed to serve as a transcription attenuator or RNA-processing site for differential gene expression (11). Data obtained from Northern blot analysis (Fig. 2) revealed the 0.24-kb RNA as the most abundant transcript, suggesting that the majority of transcripts initiated at the mutA promoter terminated after the mutA structural gene, presumably at the stem-loop structure. While this explanation seems plausible, we cannot exclude the possibility that the 0.24-kb RNA was the product of an RNA-processing event, in which the downstream RNA moiety was degraded due to a lack of stability.
By using quantitative primer extension mapping and dot blot analyses, we demonstrated that mutacin operon expression is independent of the growth stage. This pattern of expression is in contrast to the pattern of mutacin production, which peaks at the end of the exponential phase (Fig. 4). This discrepancy raises several questions. (i) What happens to the mutA transcripts during exponential growth? Are they translated? (ii) If they are, then are the prepromutacin peptides processed? (iii) Is mutacin production controlled by posttranscriptional or posttranslational regulation? These questions are being investigated with antibodies to the premutacin and mature mutacin peptides and by immunological assays.
The production levels of antibiotics are, in general, affected by medium composition (1, 9, 10). Mutacin II production is maximal in CDM plus TSBY medium and nearly undetectable in CDM alone. We show here that this difference in mutacin production is due to suppression of transcription of the mutacin operon in cells grown in CDM (Fig. 8), which means that some signal(s) present in the complex medium is required for activation of the mutA promoter. This finding is significant from a practical point of view. Since purification of mutacin II from the complex medium is laborious and time consuming, it is only feasible under laboratory conditions. Producing mutacin in CDM will be a prerequisite for large-scale production in an industrial setting. The findings of this study undoubtedly provide a foundation for increasing mutacin production in CDM by genetic manipulations of the promoter region. For example, the mutA promoter can be changed so that it no longer requires activation by accessory factors to be transcribed at high levels.
ACKNOWLEDGMENTS
We thank Casey Morrow for providing the scintillation-counting facilities.
This research was supported by NIH grant DE09082 and a grant from Unilever.
REFERENCES
- 1.Bibb M. The regulation of antibiotic production in Streptomyces coelicolor A3(2) Microbiology. 1996;142:1335–1344. doi: 10.1099/13500872-142-6-1335. [DOI] [PubMed] [Google Scholar]
- 2.Caufield P W, Chen P, Qi F, Novak J. Presented at the 5th Conference on Streptococcal Genetics, Vichy, France. 1998. [Google Scholar]
- 3.Chen P, Novak J, Kirk M, Barnes S, Qi F, Caufield P W. Structure-activity study of the lantibiotic mutacin II from Streptococcus mutans T8 by a gene replacement strategy. Appl Environ Microbiol. 1998;64:2335–2340. doi: 10.1128/aem.64.7.2335-2340.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen P, Novak J, Qi F, Caufield P W. Diacylglycerol kinase is involved in regulation of expression of the lantibiotic mutacin II of Streptococcus mutans. J Bacteriol. 1998;180:167–170. doi: 10.1128/jb.180.1.167-170.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen P, Novak J, Qi F, Caufield P W. Presented at the Third International Workshop on Lantibiotics and Related Modified Antibiotic Peptides, Blaubeuren, Germany. 1998. [Google Scholar]
- 6.Chikindas M L, Novak J, Driessen A J M, Konings W N, Schilling K M, Caufield P W. Mutacin II, a bactericidal lantibiotic from Streptococcus mutans. Antimicrob Agents Chemother. 1995;39:2656–2660. doi: 10.1128/aac.39.12.2656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Ruyter P G G, Kuipers O P, Beerthuyzen M M, van Alen-Boerrigter I, de Vos W M. Functional analysis of promoters in the nisin gene cluster of Lactococcus lactis. J Bacteriol. 1996;178:3434–3439. doi: 10.1128/jb.178.12.3434-3439.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Vos W M, Kuipers O P, van der Meer J R, Siezen R J. Maturation pathway of nisin and other lantibiotics: post-translationally modified antimicrobial peptides exported by Gram-positive bacteria. Molecular Microbiol. 1995;17:427–437. doi: 10.1111/j.1365-2958.1995.mmi_17030427.x. [DOI] [PubMed] [Google Scholar]
- 9.de Vuyst L, Callewaert R, Crabbé K. Primary metabolite kinetics of bacteriocin biosynthesis by Lactobacillus amylovorus and evidence for stimulation of bacteriocin production under unfavourable growth conditions. Microbiology. 1996;142:817–827. doi: 10.1099/00221287-142-4-817. [DOI] [PubMed] [Google Scholar]
- 10.De Vuyst L, Vandamme E J. Influence of carbon source on nisin production in Lactococcus lactis subsp. lactis batch fermentations. J Gen Microbiol. 1992;138:571–578. doi: 10.1099/00221287-138-3-571. [DOI] [PubMed] [Google Scholar]
- 11.Gilmore M S, Skaugen M, Nes I. Enterococcus faecalis cytolysin and lactocin S of Lactobacillus sake. Antonie Leeuwenhoek. 1996;69:129–138. doi: 10.1007/BF00399418. [DOI] [PubMed] [Google Scholar]
- 12.Hynes W L, Ferretti J P, Tagg J R. Cloning of the gene encoding streptococcin A-FF22, a novel lantibiotic produced by Streptococcus pyogenes, and determination of its nucleotide sequence. Appl Environ Microbiol. 1993;59:1969–1971. doi: 10.1128/aem.59.6.1969-1971.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jack R W, Tagg J R, Ray B. Bacteriocins of gram-positive bacteria. Microbiol Rev. 1995;59:171–200. doi: 10.1128/mr.59.2.171-200.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jung G. Lantibiotics: a survey. In: Jung G, Sahl H G, editors. Nisin and novel lantibiotics. Leiden, The Netherlands: ESCOM Scientific Publishers; 1991. pp. 1–34. [Google Scholar]
- 15.Liu J, Turnbough C L J. Effects of transcriptional start site sequence and position on nucleotide-sensitive selection of alternative start sites at the pyrC promoter in Escherichia coli. J Bacteriol. 1994;176:2938–2945. doi: 10.1128/jb.176.10.2938-2945.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Macrina F L, Tobian J A, Jones K R, Evans R P, Clewell D B. A cloning vector able to replicate in Escherichia coli and Streptococcus sanguis. Gene. 1982;19:345–353. doi: 10.1016/0378-1119(82)90025-7. [DOI] [PubMed] [Google Scholar]
- 17.Novak J, Caufield P W, Miller E J. Isolation and biochemical characterization of a novel lantibiotic mutacin from Streptococcus mutans. J Bacteriol. 1994;176:4316–4320. doi: 10.1128/jb.176.14.4316-4320.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Novak J, Kirk M, Caufield P W, Barnes S, Morrison K, Baker J. Detection of modified amino acids in lantibiotic peptide mutacin II by chemical derivatization followed by electrospray ionization mass spectroscopic analysis. Anal Biochem. 1996;236:358–360. doi: 10.1006/abio.1996.0181. [DOI] [PubMed] [Google Scholar]
- 19.Parrot M, Caufield P W, Lavoie M C. Preliminary characterization of four bacteriocins from Streptococcus mutans. Can J Microbiol. 1990;36:123–130. doi: 10.1139/m90-022. [DOI] [PubMed] [Google Scholar]
- 20.Peschel A, Augustin J, Kupke T, Stevanovic S, Götz F. Regulation of epidermin biosynthetic genes by EpiQ. Mol Microbiol. 1993;9:31–39. doi: 10.1111/j.1365-2958.1993.tb01666.x. [DOI] [PubMed] [Google Scholar]
- 21.Piard J-C, Kuipers O, Rollema H S, Desmazeaud M J, de Vos W M. Structure, organization, and expression of the lct gene for lacticin 481, a novel lantibiotic produced by Lactococcus lactis. J Biol Chem. 1993;268:16361–16368. [PubMed] [Google Scholar]
- 22.Pridmore D, Rekhif N, Pittet A-C, Suri B, Mollet B. Variacin, a new lanthionine-containing bacteriocin produced by Micrococcus varians: comparison to lacticin 481 of Lactococcus lactis. Appl Environ Microbiol. 1996;62:1799–1802. doi: 10.1128/aem.62.5.1799-1802.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qi F, Chen P, Novak J, Caufield P W. Presented at the 5th Conference on Streptococcal Genetics, Vichy, France. 1998. [Google Scholar]
- 24.Rince A, Dufour A, Le Pogam S, Thuault D, Bourgeois C M, Le Pennec J P. Cloning, expression, and nucleotide sequence of genes involved in production of lactococcin DR, a bacteriocin from Lactococcus lactis subsp. lactis. Appl Environ Microbiol. 1994;60:1652–1657. doi: 10.1128/aem.60.5.1652-1657.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rogers A H. Bacteriocin types of Streptococcus mutans in human mouths. Arch Oral Biol. 1975;20:853–858. doi: 10.1016/0003-9969(75)90066-7. [DOI] [PubMed] [Google Scholar]
- 26.Ross K F, Ronson C W, Tagg J R. Isolation and characterization of the lantibiotic salivaricin A and its structural gene salA from Streptococcus salivarius 20P3. Appl Environ Microbiol. 1993;59:2014–2021. doi: 10.1128/aem.59.7.2014-2021.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sabelnikov A G, Greenberg B, Lacks S A. An extended −10 promoter alone directs transcription of the dpnII operon of Streptococcus pneumoniae. J Mol Biol. 1995;250:144–155. doi: 10.1006/jmbi.1995.0366. [DOI] [PubMed] [Google Scholar]
- 28.Sahl H-G, Bierbaum G. LANTIBIOTICS: biosynthesis and biological activities of uniquely modified peptides from Gram-positive bacteria. Annu Rev Microbiol. 1998;52:41–79. doi: 10.1146/annurev.micro.52.1.41. [DOI] [PubMed] [Google Scholar]
- 29.Sahl H-G, Jack R W, Bierbaum G. Biosynthesis and biological activities of lantibiotics with unique post-translational modifications. Eur J Biochem. 1995;230:827–853. doi: 10.1111/j.1432-1033.1995.tb20627.x. [DOI] [PubMed] [Google Scholar]
- 30.Salavik M C, Clewell D B. Rgg is a positive transcriptional regulator of the Streptococcus gordonii gtfG gene. J Bacteriol. 1996;178:5826–5830. doi: 10.1128/jb.178.19.5826-5830.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shah G R, Caufield P W. Enhanced transformation of Streptococcus mutans by modifications in culture conditions. Anal Biochem. 1993;214:343–346. doi: 10.1006/abio.1993.1503. [DOI] [PubMed] [Google Scholar]
- 32.Woodruff W A, Novak J, Caufield P W. Characterization of mutA and mutM genes involved in the biosynthesis of the lantibiotic mutacin II in Streptococcus mutans. Gene. 1998;206:37–43. doi: 10.1016/s0378-1119(97)00578-7. [DOI] [PubMed] [Google Scholar]