Abstract
Translocator Protein 18 kDa (TSPO), previously named Peripheral Benzodiazepine Receptor, is a well-validated and widely used biomarker of neuroinflammation to assess diverse central nervous system (CNS) pathologies in preclinical and clinical studies. Many studies have shown that in animal models of human neurological and neurodegenerative disease and in the human condition, TSPO levels increase in the brain neuropil, and this increase is driven by infiltration of peripheral inflammatory cells and activation of glial cells. Therefore, a clear understanding of the dynamics of the cellular sources of the TSPO response is critically important in the interpretation of Positron Emission Tomography (PET) studies and for understanding the pathophysiology of CNS diseases.
Within the normal brain compartment, there are tissues and cells such as the choroid plexus, ependymal cells of the lining of the ventricles, and vascular endothelial cells that also express TSPO at even higher levels than in glial cells. However, there is a paucity of knowledge if these cell types respond and increase TSPO in the diseased brain. These cells do provide a background signal that needs to be accounted for in TSPO-PET imaging studies. More recently, there are reports that TSPO may be expressed in neurons of the adult brain and TSPO expression may be increased by neuronal activity. Therefore, it is essential to study this topic with a great deal of detail, methodological rigor, and rule out alternative interpretations and imaging artifacts.
High levels of TSPO are present in the outer mitochondrial membrane. Recent studies have provided evidence of its localization in other cellular compartments including the plasma membrane and perinuclear regions which may define functions that are different from that in mitochondria. A greater understanding of the TSPO subcellular localization in glial cells and infiltrating peripheral immune cells and associated function(s) may provide an additional layer of information to the understanding of TSPO neurobiology. This review is an effort to outline recent advances in understanding the cellular sources and subcellular localization of TSPO in brain cells and to examine remaining questions that require rigorous investigation.
Keywords: Translocator protein 18 kDa (TSPO), Microglia, Astrocytes, Neuroinflammation, Positron emission tomography – immunofluorescence, Confocal imaging
1. Introduction
For more than three decades, Translocator Protein 18 kDa (TSPO) has been interrogated as a biomarker of neuroinflammation mediated by infiltrating peripheral inflammatory cells and activation of resident microglia and astrocytes, the brain cell types that mount an inflammatory response to a variety of brain insults and pathologies (Kreisl et al., 2020; Kwon & Koh, 2020; Lahooti, Chhibber, Bagchi, Varahachalam, & Jayant, 2021). In this review, whenever we indicate microglia in brain disease it also includes the possibility of infiltrating peripheral inflammatory cells into the brain that contribute to the TSPO signal (Kreisl et al., 2020). Today, many neurological, neurodegenerative, and psychiatric diseases have been examined by TSPO Positron Emission Tomography (PET) imaging and there are many reviews on this topic (Chen & Guilarte, 2008; Guilarte, 2019; Kreisl et al., 2020; Meyer et al., 2020). Interestingly, the early TSPO-PET imaging studies were described in brain tumors such as glioblastoma that express high TSPO levels and can be easily detected from the surrounding brain neuropil with low TSPO expression (Junck et al., 1989; Pappata et al., 1991). This is one of the advantages of TSPO as a biomarker in that TSPO levels are low in the normal brain neuropil and increase specifically at primary and secondary sites of disease based on the degree of injury, making it relatively easy to detect following an acute or chronic pathological event or in brain cancer. Another advantage has been the significant advances in the development of new classes of radioligands with improved pharmacokinetics, with low levels of non-specific binding (Alam, Lee, & Lee, 2017; Werry et al., 2019; Zhang et al., 2021), and even the recent possibility of new ligands with low sensitivity to the TSPO polymorphism known to alter the affinity of TSPO to second-generation radioligands (Kim et al., 2020; Mattner et al., 2021; Vo et al., 2020). Recent studies also provide the prospect of new TSPO radioligands that are selective for glial cells with no sensitivity to vascular endothelial cells (Ji et al., 2021). As a biomarker of neuroinflammation, TSPO has been studied and validated in a multitude of preclinical animal models of diverse CNS pathology and neurodegenerative disorders (Chen & Guilarte, 2008; Guilarte, 2019; Kreisl et al., 2020). Furthermore, it can be used to track neuroinflammation prospectively at multiple time points in the life course or the evolution of a disease (Focke et al., 2019; Kang, Pandya, Zinger, Michaelson, & Gauthier, 2021; Sacher et al., 2019; Sucksdorff et al., 2020).
One of the perceived disadvantages of TSPO-PET imaging is that there is still a lack of knowledge on the temporal profile of the cell types responsible for the TSPO response in many brain diseases. While an increasing number of preclinical studies are beginning to provide this vital information, less is known in human studies. Furthermore, there is emerging evidence that the TSPO response in preclinical animal models may differ from the human condition (Owen et al., 2017). This too requires further investigation and see Table 1. Other disadvantages of TSPO-PET neuroimaging studies are related to the second-generation radioligands that are sensitive to a TSPO polymorphism (rs6971) which alters the affinity of the protein for the radioligand (Owen et al., 2012). While genotyping provides a suitable solution to this problem for comparison of normal versus disease subjects, the role of the polymorphism on the function of TSPO in a specific glial cell type or peripheral immune cell is not known. Previous and recent studies are beginning to provide novel information on the impact of the polymorphism on function and disease outcome (Colasanti et al., 2013; Costa et al., 2009; Costa et al., 2009; Owen et al., 2012; Troike et al., 2021).
Table 1.
Studies examining the cellular localization ofTSPO in brain cells.
| Reference | Disease Model | Age/Time Point | Brain Regions | TSPO Cellular Source |
|---|---|---|---|---|
| Tournier et al. (2019) |
Alzheimer’s
Disease |
|||
| 3xTgAD mouse | 1, 6, 12, 21 months | Anterior dorsal hippocampus Subiculum Dorsal hippocampus Ventral hippocampus |
21 month mice: Microglia (main source) Astrocytes (less) |
|
| Human tissue | Choroid plexus | Epithelium Endothelial cells (some) |
||
| Tournier et al. (2020) |
Alzheimer’s
Disease |
|||
| TgF334-AD rat | 12 months 24 months |
Hippocampus Cortex Striatum Cerebellum Rest of brain |
12 months: — Microglia ↑ Astrocytes — Endothelial cells 24 months: ↑ Microglia ↑ Astrocytes — Endothelial cells |
|
| Human tissue | Avg age: Control 85.6 years AD 89.1 years |
Temporal Cortex | ↑ Microglia ↑ Astrocytes — Endothelial cells |
|
| Gui, Marks, Das, Hyman, and Serrano-Pozo (2020) |
Alzheimer’s
Disease |
|||
| Human tissue | Multiple Ages | Temporal Pole Temporal Association Cortex Frontal Association Cortex Cerebellum |
↑ Microglia ↑ Astrocytes — Endothelial Cells — Vascular Smooth Muscle Cells |
|
| Ji et al. (2021) |
Alzheimer’s
Disease |
|||
| PS19 mouse | Hippocampus | ↑ Microglia ↑ Astrocytes |
||
| Human tissue | Frontal Cortex | ↑ Microglia ↑ Astrocytes |
||
| Nacket al.(2019) |
Multiple Sclerosis Cuprizone mouse |
1, 3, 5 wks Cuprizone | Corpus Callosum Cortex |
Microglia (most) Astrocytes (1/3) |
| Zinnhardt et al. (2019) |
Multiple Sclerosis Cuprizone mouse |
4 wks Cuprizone -demyelination 6 wks Cuprizone -remyelination (spontaneous) |
Corpus Callosum Hippocampus Thalamus |
Microglia: ↑ 4 wks; ↓ 6 wks Astrocytes: ↑ 4 wks; ↑↑ 6 wks |
| Nutma et al. (2019) |
Multiple Sclerosis Human tissue |
Multiple Ages | Multiple (areas with lesions) Normal appearing white matter (NAWM) |
Microglia: 11–14 fold ↑ in active lesion/chronic active rim; 16–21 |
| Normal appearing white matter (NAWM) |
fold ↑ in number from NAWM | |||
| Normal appearing gray matter (NAGM) |
Astrocytes: 15–20 fold ↑ in all lesion subtypes; 5–7 fold ↑ in number from NAWM |
|||
| Total TSPO+ cells in active lesions/chronic active rims: 40% microglia/macrophages 25% astrocytes <5% vascular endothelial cells <1% lymphocytes |
||||
| TSPO+ cells in center of chronic active and in active lesions: 65% TSPO+GFAP+ astrocytes |
||||
| Pannell et al. (2020) |
Neuroinflammation AdTNF Injection - mouse |
Day 3, Day 5 post AdTNF intracranial injection |
Striatum Cortex |
Microglia: ↑ Day 5 Astrocytes: ↑ Day 3 Endothelial cells: No +TSPO |
| Vicente-Rodriguez et al. (2021) |
Neuroinflammation LPS Injection - rat |
Adult | Hippocampus Substantia Nigra |
↑ Microglia ↑ Astrocytes |
Another aspect that has been noted as a disadvantage of TSPO-PET studies is that TSPO is widely expressed throughout the brain and there is no brain region with a complete lack of the protein, making the modeling of PET studies more complicated since there is no reference region (Kreisl et al., 2020). In fact, as it will be shown in this review, the expression of TSPO in blood vessels is significant throughout the neuropil in the normal brain. Lastly, the fact that the TSPO response to brain injury and disease originates from microglia and astrocytes is sometimes perceived as a problem because it is not specific for microglia only nor for astrocytes only. However, the TSPO response does follow the brain cells (microglia and astrocytes) that are responsible for the neuroinflammatory response, therefore, a biomarker of neuroinflammation. In summary, TSPO is a robust and well-validated biomarker of neuroinflammation for use in preclinical and clinical neuroimaging studies.
2. The evolution of studies on the cellular sources of the TSPO response in brain pathologies
There are several extensive and critical reviews on the cellular sources of the TSPO response to diverse CNS pathologies (Chen & Guilarte, 2008; Guilarte, 2019; Kreisl et al., 2020; Nutma et al., 2019; Rupprecht et al., 2010; Tournier, Tsartsalis, Ceyzeriat, Garibotto, & Millet, 2020). While the early dogma that TSPO is solely a “microglia response” rather than a glial response (microglia and astrocytes) has been proven scientifically invalid in the last few years, new uncertainties are appearing in the literature including neurons of the adult brain being a source of TSPO that could potentially influence TSPO-PET imaging studies. Therefore, rather than provide a repetition of what has been published in the past, this review will focus on new studies that are providing valuable information and methodological approaches to study TSPO.
Microglia and astrocytes were one of the first brain cell types that were implicated to express TSPO and to contribute to the TSPO response to brain injury and disease in preclinical animal models (reviewed in Chen & Guilarte, 2008; Guilarte, 2019). While early in the history of TSPO it was thought that the origin of the signal in CNS pathologies was exclusively from microglia (reviewed in Chen & Guilarte, 2008; Guilarte, 2019), contemporary studies that have rigorously examined this question have shown that the TSPO signal in CNS pathologies is derived from both microglia and astrocytes (see Table 1 and (Guilarte, 2019)). What is also known is that the contribution of microglia and astrocytes to the TSPO signal is not possible to define in the continuum of any disease from a PET scan. Furthermore, the temporal profile of a microglia and/or astrocytic TSPO response is brain region- and disease-specific (Chen & Guilarte, 2008; Guilarte, 2019). Therefore, preclinical animal models of human brain disease are relevant to address these important questions. Nevertheless. It should be noted that studies focused in understanding the cellular sources of the TSPO response in human brain tissue have been limited. A greater effort to validate the experimental animal data and its relevance to the human disease condition is needed (See Table 1). A review of the literature since 2019, that at a minimum examined the cellular sources of the TSPO response in microglia and astrocytes, in preclinical studies or human postmortem brain tissue are provided in Table 1. Using these criteria there were a total of 9 studies using experimental animals and/or human tissue. These studies examined animal models of Alzheimer’s disease (AD) and human AD tissue (n = 4), multiple sclerosis (MS) based on cuprizone treatment (n = 2), MS human tissue (n = 1), lipopolysaccharide (LPS)-induced inflammation (n = 1), and injection of Tumor Necrosis Factor (TNF) (n = 1).
In different animal models of AD, there is evidence that TSPO upregulation is derived from microglia and astrocytes. A study that examined TSPO and amyloid deposits in sub-regions of the hippocampus in the 3xTgAD mouse model showed that increased TSPO levels appeared prior to amyloid deposits in different regions of the hippocampus (Tournier et al., 2019). Increases in TSPO expression and amyloid were observed first in the subiculum, then dorsal hippocampus, and later in the ventral hippocampus. Furthermore, immunofluorescence imaging of TSPO with glial cell markers indicates that TSPO was primarily associated with microglia and exhibited a lower level of expression in astrocytes. However, the TSPO cellular source component of the study was only performed in animals at 21 months of age. This is important because the study was performed with 3xTgAD mice at 1, 6, 12, and 21 months of age (Tournier et al., 2019) and it is known that the cellular sources of the TSPO response change dynamically at different stages of the disease (Guilarte, 2019). Relevant to this review, in this same publication, the authors state the lack of TSPO immunostaining in pyramidal neurons of the hippocampus (Tournier et al., 2019). In the context of comparing the cellular sources of TSPO in animal models and the human condition, it was shown that TSPO is highly expressed in human choroid plexus as has been shown in mice (Tournier et al., 2019)(Also see Fig. 1 demonstrating high TSPO levels in the choroid plexus of mice).
Fig. 1.
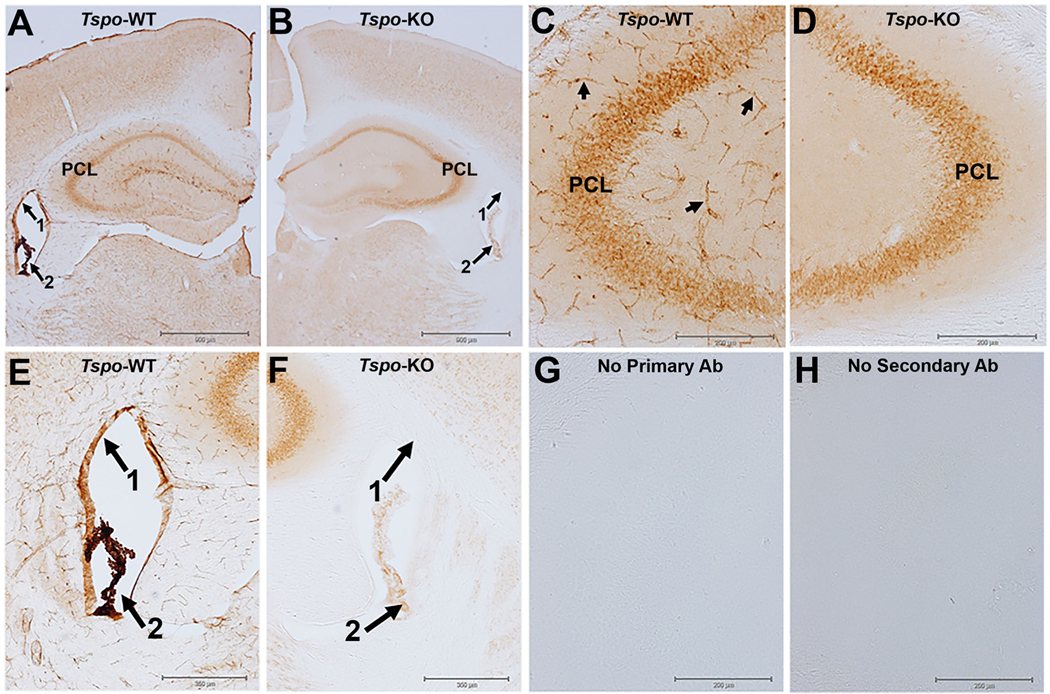
TSPO immunohistochemistry with diaminobenzidine (DAB) visualization in the hippocampus of wildtype and TSPO global knockout mice.
A) TSPO immunostaining in a normal wildtype (WT) animal at the level of the hippocampus with high expression of TSPO in the ependymal cells of the lining of the ventricles (arrow 1), in the choroid plexus (arrow 2), in blood vessels throughout the brain neuropil, and in pyramidal neurons (PCL) of the hippocampus. B) Image of TSPO immunostaining at the same level of the hippocampus as in panel A but the brain tissue is from a global TSPO-KO mouse that was processed at the same time and under the same experimental conditions as in panel A. The image in the TSPO-KO tissue shows the complete loss of TSPO staining in the ependymal cells of the ventricles, blood vessels, and a nearly complete loss in the choroid plexus. However, there was essentially no loss in pyramidal neurons (PCL) of the hippocampus indicative of non-specific TSPO staining since the tissue is from a TSPO-KO mouse. C and D) are higher magnification images at the level of the CA3 region of the hippocampus. Arrows in Panel C point to TSPO staining of blood vessels in the WT tissue with the TSPO staining disappearing in blood vessels of the TSPO-KO mouse. E and F) are higher magnification images of the lateral ventricles. G and H) are images from the no primary or no secondary antibody controls. Brain slices from wildtype (WT) and TSPO knockout (KO) mice were processed using standard methods with the TSPO primary antibody (ab109497 Abcam) which has been extensively validated using multiple approaches including TSPO-KO cells and tissue (Loth et al., 2020 and supplementary materials within). Experimental methods are described in supplementary materials section.
Using a different AD animal model, in this case the TgF344-AD rat model and a quantitative new approach to assess the cellular sources of the TSPO response, the authors reach a different conclusion (Tournier et al., 2020). TSPO was increased in the hippocampus at 12 and 24 months of age in TgF344-AD rats compared to wildtype rats. Importantly, at 12 months of age, the TSPO signal was exclusively from astrocytes while at 24 months it was from both astrocytes and microglia providing a different perspective than in their previous study. They also found that vascular endothelial cells expressed significant radioligand binding, but they do not contribute to the increased TSPO ligand binding in the TgF344-AD rat model. In the same study, the authors also performed analysis of temporal cortex tissue from AD patients. Consistent with the animal model of AD astrocytes and microglia, but not endothelial cells, were the main source of the increased TSPO binding in the temporal cortex tissue from AD subjects relative to controls.
Another study by Gui and colleagues (Gui et al., 2020) using postmortem brain tissue from normal and AD brains found that TSPO is expressed not just in microglia but also in astrocytes, endothelial cells, and vascular smooth muscle cells. In this human study, there was also no TSPO labeling of neurons. Finally, Ji and colleagues found that TSPO binding increased in the brain of PS19 (tau AD mice) mice, and the increase was driven by microglia and astrocytes (Ji et al., 2021). They further show that TSPO appears to be more prominent in microglia from AD tissue than astrocytes. From these studies in preclinical animal models when compared to human AD tissue, it suggests that the sources of the TSPO response in AD animal models and human tissue are from microglia and astrocytes.
Studies using animal models of MS also examined the cellular sources of the TSPO response. In a study by Nack and colleagues, cuprizone-induced demyelination produced an increase in TSPO levels associated with microglia and astrocyte activation and axonal injury (Nack et al., 2019). Most of the microglia and approximately one-third to one-half of the astrocytes expressed TSPO based on the temporal profile of the injury. Another study by Zinnhardt and colleagues (Zinnhardt et al., 2019) also used a cuprizone model of MS. In the latter study, peak levels of TSPO radioligand binding occurred at 4 weeks of cuprizone treatment in the corpus callosum, hippocampus, and thalamus and declined after 6 weeks of cuprizone treatment. Cuprizone-induced demyelination corresponded with the increase in TSPO levels in these brain regions. In agreement with previous studies from our laboratory (Chen, Baidoo, Verina, & Guilarte, 2004; Chen & Guilarte, 2008), the number of microglia increased after 4 weeks of cuprizone treatment but then decreased at 6 weeks relative to control tissue. On the other hand, there was a steady increase in astrocytes at 4 and 6 weeks of cuprizone treatment. Therefore, the TSPO response was mainly associated with microglia at 4 weeks of cuprizone treatment, while at 6 weeks of treatment TSPO expressed in microglia declines with a greater contribution of astrocytes to the TSPO signal, indicating the temporal dynamics of the cellular sources of the TSPO response.
Finally, a study by Nutma and colleagues (Nutma et al., 2019), using brain samples from MS patients and age-matched controls examined markers for microglia, astrocytes, and lymphocytes to assess the sources of the TSPO response. Cells double-labeled for TSPO and the microglia marker HLA-DR increased 20-fold in the active core and the rim of chronic active lesions relative to normal white matter. TSPO was increased 20-fold in astrocytes labeled with glial fibrillary protein (GFAP) across all lesions in active and inactive lesions relative to white matter from control subjects and this counted for 25% of the TSPO signal in these lesions. They also found that TSPO was in vascular endothelial cells (<5%) and in lymphocytes (<1%), but their total contribution was a small fraction of the total TSPO signal. Again, in MS, the animal data is consistent with the human data, microglia and astrocytes are the main contributors to the TSPO signal.
Other studies examining TSPO expression in the neuroinflammatory response associated with LPS and cytokine administration provide a similar picture. Pannell and colleagues (Pannell et al., 2020) injected TNF-inducing adenovirus (Ad-TNF) or IL-4 in mouse striatum followed by TSPO-PET imaging 48 h (IL-4) or 5 days (Ad-TNF) after injection. Using immunohistochemical staining, they found that TSPO expression was significantly increased in the injected hemisphere relative to the contralateral side after Ad-TNF but not IL-4. A significant increase in astrocytes was observed at 3 days and microglia increased significantly at 5 days after Ad-TNF injection. The authors also showed that TSPO expression increased in both astrocytes and microglia after Ad-TNF injection. Consistent with previous studies noted above in AD and in MS, vascular endothelial cells isolated from Ad-TNF injected animals showed no increase in TSPO expression concluding that the contribution of vascular endothelial cells to the TSPO signal in the injured brain is not increased.
Another study examined the cellular sources of the TSPO response following injections of LPS. Vicente-Rodriquez and colleagues (Vicente-Rodriguez et al., 2021) used adult rats with intracerebral (striatum) and intraperitoneal LPS injections. Four days after intracerebral or 24 h after systemic LPS administration, TSPO-PET studies were performed. In the intracerebral LPS injections, there was a significant TSPO increase in the ipsilateral versus the contralateral injected site in the TSPO-PET which was confirmed using ex-vivo TSPO autoradiography. A similar effect was found in multiple brain regions of animals injected with LPS systemically. Examining the brain of the animals for the cellular sources of the TSPO response using double-label immunofluorescence confocal imaging they found that microglia together with astrocytes are the major contributors to the increased TSPO signal in the PET and autoradiography studies (Table 1). In this study, they also examined the TSPO signal in neurons and vascular endothelial cells using triple-label immunofluorescence. Although they describe the presence of TSPO in neurons and vascular endothelial cells, these cells did not increase TSPO levels after LPS administration. They conclude that although TSPO is expressed in various brain cell types, microglia and astrocytes are the only cells that increase the TSPO signal after LPS administration.
In conclusion, the most recently published studies in which the cellular sources of the TSPO response were examined in diverse CNS disease conditions indicates that while TSPO is expressed in multiple brain cell types, microglia and astrocytes appear to be the only cells that increase TSPO in pathological conditions. Importantly, the expression of neuronal TSPO remains controversial, with studies indicating TSPO expression in adult neurons (Gong et al., 2019; Notter et al., 2020; Nutma et al., 2019; Tournier, Tsartsalis, Ceyzeriat, Garibotto, & Millet, 2020; Wang, Fan, & Papadopoulos, 2012) and other studies indicating no TSPO expression in adult neurons (Betlazar, HarrisonBrown, Middleton, Banati, & Liu, 2018; Gui et al., 2020; Tournier et al., 2019), including findings from our own laboratory provided in this review.
3. A new approach to assess the cellular sources of the TSPO response in CNS pathologies
Defining the cell types that are responsible for the TSPO signal in the healthy and diseased brain is an important undertaking for the interpretation of PET brain imaging studies and to understand the pathophysiology of a given brain disease. Until recently, the typical approach has been to perform double- or triple-label immunofluorescence confocal imaging with cell-specific markers and TSPO. Tournier and colleagues have described an important methodological advancement that can provide quantitative information not only about the cellular sources of TSPO, but also which cell types increase TSPO in the diseased brain (Tournier et al., 2020). The authors used antibody-based fluorescence-activated cell sorting to select specific cell types from dissociated brain tissue using flow cytometry combined with TSPO radioligand binding. Based on the combination of these two techniques, they were able to determine the level of [125I]-CLINDE binding to TSPO that originates from a specific brain cell type isolated using flow sorting (Tournier, Tsartsalis, Ceyzeriat, Medina, et al., 2020). This can be done in two ways. First, one can administer a dose of a radioactive TSPO ligand to the animal, excise the radioactive brain, dissociate cells, select the different cell populations using antibody-based cell sorting and then count the radioactivity associated with each cell type. The second approach is to extract the brain, dissociate the cells, exposed them to a radioactive TSPO ligand in vitro, and then sort the cell types with an antibody-based cell sorting and count the radioactivity associated with each cell type. Overall, this is an important quantitative approach to provide disease-specific information about the cellular sources of the TSPO response.
Tournier and colleagues used this approach to examine the cellular sources of the TSPO response in an animal model of AD, the TgF344AD rat model, and in brain tissue from AD subjects (Tournier, Tsartsalis, Ceyzeriat, Fraser, et al., 2020). Based on TSPO radioligand counting, there was a significant TSPO increase in the hippocampus of the TgF344-AD rat at 12 months while at 24 months of age increases were measured in the hippocampus, frontal cortex, striatum, cerebellum, and residual brain tissue. Importantly, there was also increased TSPO binding in the eyes at 24 months but not in any other peripheral organs analyzed. Identification of the cell types in brain tissues from the TgF344-AD rat and wildtype was performed using fluorescence-activated cell sorting of radioligand treated tissue. It was found that the increased level of [125I]-CLINDE binding to TSPO in the hippocampus of 12-month TgF344-AD rat was associated with astrocytes and not with microglia and this was followed by astrocytes and microglia in the 24-months old TgF344-AD rat brain. Although TSPO binding was measured in endothelial cells, there was no increase in the TSPO binding to endothelial cells in the TgF344-AD rat relative to control indicative of a lack of contribution of endothelial cells to the increase in radioligand binding in the TgF344-AD rat brain. Therefore, the increase in TSPO binding in the TgF344-AD rat was associated with an astrocytic response followed by a “glial response” (microglia and astrocytes) in which there is a distinct temporal profile and an upregulation in the number of TSPO binding sites per cell without an expansion of the number of glial cells (Tournier, Tsartsalis, Ceyzeriat, Fraser, et al., 2020).
In the same study, they also examined temporal cortex tissue from AD and control subjects. There was a significant increase in TSPO binding that was higher in astrocytes than in microglia with no change in endothelial cells (Tournier, Tsartsalis, Ceyzeriat, Fraser, et al., 2020). In this study, there was no apparent increase in the number of astrocytes or endothelial cells but there was an expansion of microglia. Combined, these studies show that in the AD temporal cortex, the increase in TSPO is associate with astrocytes and microglia with no involvement of endothelial cells. It supports the notion that endothelial cells provide a “background” of TSPO binding that needs to be considered for in vivo PET imaging studies but the increase in TSPO in the diseased brain is a “glial response” in which the contribution of each glial cell type needs to be confirmed under each disease condition and brain region. That is, the glial response at any time point in the disease progression could be solely due to astrocytes, microglia, or a mixed astrocyte/microglia response and it is dependent upon the disease, the disease stage, and the brain region involved (Chen & Guilarte, 2008; Guilarte, 2019).
4. Is TSPO expressed in adult neurons?
There are reports indicating that TSPO is expressed in neurons in the normal rodent adult brain (Gong et al., 2019; Notter et al., 2020; Wang et al., 2012). These studies indicate that TSPO is expressed in Purkinje cells of the cerebellum (Betlazar et al., 2018; Nutma et al., 2019; Tournier, Tsartsalis, Ceyzeriat, Garibotto, & Millet, 2020; Wang et al., 2012), in pyramidal and dentate granule cells of the hippocampus (Notter et al., 2020; Wang et al., 2012) and in dopaminergic neurons of the substantia nigra (Gong et al., 2019). On the other hand, other studies provide no evidence of TSPO expression in neurons of the normal adult rodent brain (Betlazar et al., 2018; Tournier et al., 2019). Therefore, there is uncertainty in the published literature about the presence of TSPO in adult neurons and their potential contribution to PET imaging. Furthermore, a recent report suggests a selective increase in the putative TSPO neuronal signal following different neuronal activating paradigms in brain regions of the normal mouse brain (Notter et al., 2020). The latter findings put forth the possibility that a TSPO neuronal signal may contribute to the overall signal in TSPO-PET imaging studies. The contribution of neurons to the TSPO signal has not been considered previously because, although neuronal progenitor stem cells express high levels of TSPO, there is a marked decrease in TSPO expression with neuronal differentiation with no detectable TSPO expression in postmitotic, differentiated adult neurons (Tanimoto et al., 2020; Varga et al., 2009). Nevertheless, the fact that TSPO has been described to be expressed in adult neurons and its expression can be induced exclusively in neurons and not in glial cells by different neuronal activating paradigms requires significant attention and rigorous validation. It suggests that normal variations in daily human activities may modulate neuronal TSPO expression and thus be a source of variation in TSPOPET imaging studies in normal subjects.
5. Neuronal activating paradigms selectively increase neuronal and not glial TSPO: a critical analysis
The study by Notter and colleagues describing significant TSPO expression in neurons of the normal adult mouse brain used single-cell RNA sequencing (scRNA-seq) from normal adult mouse hippocampus (Notter et al., 2020). This was done to generate gene sets to identify specific brain cell type in the hippocampus. The degree of TSPO gene expression was then attributed to each individual cell type based on the scRNA-seq results. It was found that in the normal adult mouse hippocampus, 3% of neurons express TSPO relative to 40% of ependymal cells, 20% of endothelial cells, 15% of microglia, and 3% of astrocytes. Based on this information, approximately 60% of the TSPO signal in the normal adult mouse hippocampus is provided by ependymal and endothelial cells. Importantly, the authors further characterize neurons into four subclusters and show that amongst these neuronal subclusters, subcluster 3 accounts for most of the TSPO relative to subclusters 1/2/4. These neuronal subclusters were not identified, but it is possible that neuronal subcluster 3 is associated with neural stem/progenitor cells that are Nestin-positive and reside in the hippocampus, a brain region with a very active adult neurogenesis niche in the subgranular zone of the dentate gyrus (Niklison-Chirou, Agostini, Amelio, & Melino, 2020; Vicidomini, Guo, & Sahay, 2020). These neuronal progenitor stem cells are known to express high levels of TSPO (Betlazar et al., 2018; Varga et al., 2009).
One of the goals of the study was to assess the source of the TSPO signal following different neuronal activating paradigms (Notter et al., 2020). To this aim, a critical experiment using scRNA-seq was not performed. That is, the authors did not compare cells from the brain of animals that experienced the neuronal activating paradigms to sham-controlled animals using the same scRNA-seq approach. This critical experiment would have provided a direct comparison of the TSPO expression attributed to each cell type using the same gene sets defined by scRNA-seq and would have been able to determine the cell type (s) or neuronal subcluster(s) from which the putative increase in the TSPO signal originated following the different neuronal activating paradigms relative to sham-control animals.
For the neuronal activating studies, the authors used TSPO immunofluorescence confocal imaging of phenotypically defined cells using cell-specific markers and provided images of apparent TSPO-positive puncta in neuronal perikarya and in glial cells from normal mouse adult brain tissue (Notter et al., 2020). Based on the images provided, it appears that neurons from the normal adult hippocampus express more TSPO-positive puncta per cell than microglia or astrocytes. However, this is difficult to assess because the magnification of the images provided in the different panels are not the same. That is, the neuronal panels have the highest magnification compared to the other cell types. Importantly, the signal intensity settings in the confocal images were not provided and if these are increased to improve image quality, it can introduce imaging artifacts.
An alternative interpretation of the images of TSPO-positive puncta in neurons that was not interrogated in the study is the possibility that TSPO-positive puncta that appear to be localized within neuronal cell bodies based on the double-label study may be localized to glial processes or blood vessels contacting or in near proximity of neuronal soma and processes (see sections 10 and 11). Finally, an important experiment that was not performed to verify that the TSPO-positive puncta in neurons is a specific signal, was to perform the same study in global TSPO knockout (KO) mice. The latter would have provided an important additional layer of confirmation that the putative TSPO signal originating from adult neurons is specific for TSPO and not an imaging artifact or antibody non-specific binding (see Fig. 1).
In the same study, for the neuronal activating paradigms, the authors used DREADD-based chemo-activation of neuronal activity in the medial prefrontal cortex and in the CA1 region of the hippocampus. The results seem to suggest a specific increase in TSPO gene and protein expression in neurons with no change in glial cells (Notter et al., 2020). The same neuronal selective increases in TSPO gene and protein expression were observed in the hippocampus when adult mice were briefly exposed to a novel environment (15 min) and in neurons of the nucleus accumbens following D-amphetamine administration (single injection 2 mg/kg). In this part of the study, the authors used the expression of the activity-regulated genes cFos, Arc, and Zif268 measured by qRT-PCR as an indication that the increase in TSPO following the neuronal activation paradigms is neuronal in nature (Notter et al., 2020). However, cFos, Arc, and Zif268 are not selectively expressed nor selectively activated in neurons since these activity-regulated genes are also present in astrocytes and these same genes are upregulated in astrocytes following neuronal activation (Adamsky et al., 2018; Edling, Ingelman-Sundberg, & Simi, 2007; Hung et al., 2000; Rodriguez et al., 2005; Rodriguez et al., 2008). Therefore, the designation of the origin of the TSPO mRNA signal measured by qRT-PCR to be selective for neurons based on the increased expression of these activity-regulated genes is confounded by a putative contribution from astrocytes.
The authors also point out the possibility that neuronal activation or decreased neuronal activity may alter neuron-specific TSPO expression and this signal may contribute to changes in TSPO levels in PET studies of psychiatric disorders (Notter et al., 2020). The putative presence of TSPO protein in neurons based on immunofluorescence imaging does not equate with the level of functional TSPO radioligand binding that would ultimately determine if the putative neuronal TSPO signal is detectable using sensitive ex vivo imaging methods such as emulsion- or film-autoradiography or in PET imaging studies. In other words, is the putative neuronal TSPO signal sufficiently robust to be detected in imaging studies? While film or emulsion autoradiography was not performed in this study (Notter et al., 2020), the same group of investigators did compare TSPO immunofluorescence confocal imaging with TSPO autoradiography in a previous study using a different experimental paradigm (Notter et al., 2018). In the latter study, the activating paradigm was immune activation in a mouse model of schizophrenia to determine if there was a decrease in TSPO binding as has been observed in PET studies of schizophrenia subjects (Notter et al., 2020). They found that there was no change in TSPO radioligand binding using autoradiography in the brain of immune-activated animals relative to controls, although they described a decrease in TSPO using immunofluorescence in the immune-activated animals relative to controls. The lack of confirmation of the decrease in TSPO immunofluorescence by TSPO autoradiography questions the interpretation of the immunofluorescence results as immunofluorescence can be influenced by a variety of different methodological variables including imaging artifacts.
6. A critical examination of TSPO immunostaining studies in adult neurons: real or artifacts?
One of the first publications describing the putative presence of TSPO in neurons of the hippocampus and in Purkinje cells using TSPO immunohistochemistry with diaminobenzidine (DAB) visualization was related to the generation of a transgenic mouse model expressing green fluorescent protein (GFP) under the control of the Tspo promoter (Tspo-AcGFP) (Wang et al., 2012). Examination of the TSPO-DAB immunohistochemistry images from the dentate gyrus and pyramidal cell layer of the hippocampus as well as the Purkinje cell layer in the cerebellum shows that all neurons in the dentate gyrus and pyramidal cells of the hippocampus stain strongly for TSPO (Fig. 4 in (Wang et al., 2012)). Further, they also show TSPO immunostaining of presumed Purkinje cells in the cerebellum. The fact that every single neuron in the hippocampus strongly labels with TSPO suggests nonspecific TSPO staining or an artifact of the staining procedure (Fig. 1). The possibility that the putative TSPO neuronal staining is an artifact in the study conducted by Wang and colleagues (Betlazar et al., 2018; Wang et al., 2012) can be surmised from the fact that the TSPO-DAB signal is not congruent with the Tspo-AcGFP signal. In Fig. 4 of their publication, panels a,d,g are Nissl stain to identify the neuronal population in the dentate gyrus (panel a), in pyramidal cells (panel d) and the Purkinje cells of the cerebellum (panel g). In the next row, there are representative images of TSPO-DAB immunostaining (Panels b,e,h). Lastly, panels c,f,i are from the same regions of the hippocampus and cerebellum but now providing the green fluorescence signal in the Tspo-AcGFP mouse hippocampus and cerebellum. From these images, the TSPO-GFP signal is not congruent to the TSPO-DAB signal, and very few cell express that express TSPO-DAB express TSPO-GFP suggesting that the “apparent” TSPO-DAB signal in panels b,e,h are highly likely to be nonspecific staining (see Fig. 1 for confirmation of non-specific immunohistochemistry staining in hippocampal neurons). In this study, there was no double-label immunostaining of TSPO with neuronal or glial markers to confirm the cellular sources of the TSPO signal. The authors compared the pattern of staining and presumed that it originated from neurons. However, the TSPO labeling pattern did not match between TSPO-DAB staining and Tspo-AcGFP. In fact, most of the Tspo-AcGFP signal is present outside of the granule cell neurons in the dentate gyrus (panel b), and outside of the pyramidal cell layer (panel e). Furthermore, the presumed Tspo-AcGFP signal from Purkinje cells (panel i) could be from Bergmann glia whose cell bodies are located within the Purkinje cell layer and have an intimate physical relationship with Purkinje cells or even from the significant amount of TSPO found in blood vessels.
7. Assessment of the specificity of TSPO immunostaining
Studies using TSPO-DAB immunohistochemistry in the hippocampus of TSPO wildtype (WT) or TSPO global KO mice with a TSPO antibody that has been well validated in our lab using a variety of different approaches including TSPO-KO mice (Loth et al., 2020 and supplementary materials within) shows that there is prominent TSPO immunostaining in blood vessels, in neurons of the pyramidal cell layer of the hippocampus, as well as in the choroid plexus and ependymal cells of the lining of the ventricles in WT mice (Fig. 1). To confirm the specificity of the staining protocol and the TSPO antibody used, TSPO staining was also performed in TSPO-KO brain tissue at the same time and under the same experimental conditions. Fig. 1 shows that in blood vessels, choroid plexus, and ependymal cells of the lining of the ventricles there is a complete loss of TSPO staining in the TSPO-KO mice relative to WT. However, the TSPO staining in neurons of the hippocampus remains in the TSPO-KO mice (compare panel A to B, C to D, and E to F in Fig. 1). The latter is a clear indication that the TSPO staining in neurons of the hippocampus is an artifact of the staining procedure and/or antibody cross reactivity. Therefore, even with well-validated TSPO antibodies, there is the possibility of imaging artifacts generated by the immunostaining procedure and validation of the staining method in TSPO-KO mice provides an important layer of confirmation to the specificity of the signal. Therefore, previous studies using TSPO-DAB (Wang et al., 2012) indicative of neuronal TSPO staining are most likely due to non-specific staining or antibody cross-reactivity.
8. TSPO immunofluorescence confocal imaging in Purkinje cells of the cerebellum
Purkinje cells of the cerebellum have been described to express TSPO (Betlazar et al., 2018; Wang et al., 2012). As noted, the original observation was presented in the Tspo-AcGFP transgenic mouse study noted above (Wang et al., 2012). In this communication, they observed TSPO-positive staining in what appears to be Purkinje cell soma based on morphological criteria. However, we provide evidence in Fig. 1 that the TSPO-DAB staining generates non-specific labeling of neurons. On the other hand, TSPO immunofluorescence studies do not appear to have the same problem. Nevertheless, with either method, it is important to perform validation studies on the localization of the TSPO signal with cell specific markers. This is due to the fact that Purkinje cells have a very close relationship with Bergmann glia which are unipolar astrocytes that are positive for the astrocyte marker GFAP (Yamada & Watanabe, 2002) (Fig. 2). In fact, multiple Bergmann glia cell bodies are located alongside in intimate contact with Purkinje cell bodies in the Purkinje cell layer (Fig. 2) (Reeber, Arancillo, & Sillitoe, 2018; Yamada & Watanabe, 2002). Bergmann glia cell bodies wrap around Purkinje cell soma and processes (Fig. 2). They can be identified with GFAP (Reeber et al., 2018). GFAP-positive staining in Bergmann glia occurs from the cell bodies in the Purkinje cell layer, all the way to the pial surface (Reeber et al., 2018)(Fig. 2). Thus, Bergmann glia could easily be misidentified as Purkinje cells if a single staining with TSPO is performed. Therefore, a general concept that needs to be considered in the interpretation of TSPO immunostaining studies is the possibility that TSPO is localized with glia processes or cell bodies or even with blood vessels that are intimately contacting neuronal soma and processes. Furthermore, TSPO immunostaining by itself is highly unlikely to provide the identification of the cell type that is providing the signal.
Fig. 2.
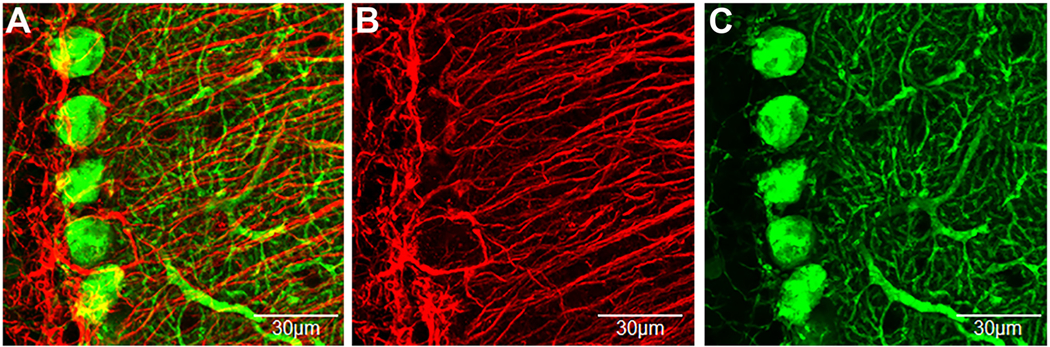
Immunofluorescent confocal imaging of Purkinje cells and Bergmann glia in the cerebellar cortex.
A) Top-view of a 3D condensed z-stack of double label immunofluorescence confocal imaging of Purkinje cells (calbindin-green) and Bergmann glia (GFAP-red). B and C) are the presentation of the individual channels. Bergmann glia cell bodies closely contact and wrap Purkinje cell soma and processes. One distinguishing anatomical feature of Bergmann glia is that they have processes that originate at their soma in the Purkinje cell layer and have a relatively straight and lengthy trajectory to the pial surface of the molecular layer (Reeber et al., 2018). On the other hand, Purkinje cell processes are shorter and radiate at all angles throughout the molecular layer. Green = Purkinje cell; Red = Bergmann glia. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Another study in normal mouse brain tissue did perform double label immunofluorescence confocal imaging of the Purkinje cell marker calbindin with TSPO (Betlazar et al., 2018). They show an apparent colocalization of TSPO with calbindin in Purkinje cell soma. However, even though in this study it may look like TSPO colocalization is with Purkinje cells, the possibility that TSPO cellular localization is in Bergmann glia, microglia, or blood vessels that are closely contacting Purkinje cell soma and processes was not ruled out. This possibility can offer an alternative explanation to the cellular origin of the TSPO signal and requires close investigation (see section 11 and 12). Considering that TSPO is localized in glial and vascular endothelial cells, and they have a close relationship with neurons, endothelial cell and glia staining should always be incorporated in studies that examine the putative labeling of TSPO in neurons.
9. Microglia and astrocyte processes contact neuronal cell bodies:
Several publications suggest that TSPO is expressed in neurons of the normal mouse brain. However, an alternative explanation that needs to be ruled out is the possibility that the apparent TSPO neuronal signal may be due to the presence of TSPO in glia (microglia and/or astrocytes) processes contacting the neuronal cell body. What is the evidence for this possibility? In the cerebellum, there is evidence that cerebellar microglia interact in a dynamic way with the dendritic arbors and cell bodies of Purkinje cells (Stowell et al., 2018). In vivo two-photon imaging shows that in the Purkinje cell layer, microglia cell soma and processes are interspersed between Purkinje neuron cell bodies and could be observed moving around the Purkinje cell body and wrap around it (Stowell et al., 2018). Microglia arbors can contact 4–8 Purkinje cell bodies and sometimes they create a webbing over the cell body covering the entire side of the Purkinje cell (Stowell et al., 2018). These findings demonstrate that besides Bergmann glia, microglia in the Purkinje cell layer are closely associated with Purkinje cells and make contacts with their cell bodies and dendrites (Stowell et al., 2018). Similarly, another study (Refaeli et al., 2021) using large number of astrocytes made transparent by CLARITY and fluorescence labeling of astrocytes and pyramidal cells via viral vectors and two-photon imaging was able to determine the elaborate structure, distribution, and neuronal content of astrocytic domains in the CA1 region of the hippocampus. This study shows a large but varying number of pyramidal neurons in astrocytic domains in the CA1 pyramidal cell region. That is, pyramidal cell soma in the hippocampus, are highly innervated by astrocytic processes. Therefore, there is significant scientific evidence of the close interactions between glial cell bodies and processes with neurons. Therefore, the TSPO-positive puncta described in hippocampal and cerebellar neurons may be directly attributed to glial processes, either from microglia, astrocytes, or both and this possibility needs to be ruled out.
10. Immunofluorescent confocal imaging demonstrates TSPO colocalization with glial processes and blood vessels but not with neurons in the normal adult mouse brain
To understand the sources of TSPO immunofluorescent staining in the Purkinje cell layer in the normal mouse brain, and to provide a picture of the complex interaction between Purkinje cells, Bergmann glia, and microglia, we performed quadruple-label immunofluorescent confocal imaging in the Purkinje cell layer of the cerebellum to visualize the relationship between Purkinje cells (stained with calbindin), astrocytes (stained with GFAP - Bergmann glia), microglia (stained with Iba1) and TSPO. Panel A in Fig. 3 shows a top-down view of a 3D condensed z-stack image of quadruple labeling of Purkinje cells (gray), Bergmann glia (green), microglia (blue) and TSPO (red). This image was generated using an experimental staining and image acquisition protocol described in the experimental protocol in the supplementary materials. In the image in Fig. 3 panel A, it appears that TSPO (red) positive puncta may be localized in Purkinje cells (gray). The areas of apparent colocalization of TSPO with Purkinje cells are circled in yellow for ease of identification. Because of the complexity of the composite image with all four signals merged in panel A, and to provide a clear picture of the TSPO staining (red) in the presence of Purkinje cells (this time in green), we removed the astrocyte (GFAP) and microglia (Iba-1) signals to generate panel B. Thus, panel B shows the same image as in panel A but this time only representing TSPO (red) and Purkinje cell (green) under the same imaging acquisition conditions as in panel A. One can see from panel B, that the level of TSPO staining in the normal cerebellum is low and provides the appearance that TSPO is associated with Purkinje cells in the yellow circles. In panel C, we used the same image as in panel B, but this time we only present the TSPO (red). Furthermore, the red signal intensity for TSPO was artificially enhanced in order to visualize the low levels of the TSPO signal. See supplementary Fig. 1-panel C for change in red signal intensity relative to the same red signal in panel A and B. The resulting image shows that the TSPO staining has a tubular shape consistent with the possibility that it is within blood vessels. In panel D, TSPO and the Purkinje cell fluorescent signals are both enhanced (see change in signal intensity in supplementary Fig. 1). By performing this manipulation in the signal intensity of the image, it now appears that in the regions circled in yellow, there is TSPO colocalization (red) with Purkinje cells (green). However, analysis of the TSPO puncta in the Purkinje cell in panel D using intensity profile, shows that TSPO does not colocalize within the Purkinje cell. In panel E, the microglia (blue) signal is now added to the composite image and it shows that the TSPO (red) positive puncta in the smaller yellow circle is associated with microglia (blue) generating a magenta color and its colocalization is confirmed using signal intensity profiles showing that the TSPO (red) peak corresponds with the microglia (blue) signal peak and not with the Purkinje cell (green) signal. Finally, in panel F, the GFAP (green – Bergmann glia) and TSPO (red) are presented without the microglia and Purkinje cells signals. The images show several areas of TSPO colocalization with Bergmann glia processes. The colocalization was confirmed using signal intensity profiles. The colocalization is most likely representative of contact points of TSPO in blood vessels and Bergmann glia processes. Overall, in the Purkinje cell layer of the normal mouse cerebellum, TSPO is present at low levels, and it is associated with blood vessels and to a lesser extent with microglia and Bergmann glia. There is no TSPO colocalization with Purkinje cells. Supplementary Fig. 2 and 3 provide additional evidence of the colocalization of TSPO with microglia and Bergmann glia but not with Purkinje cells in another area of the image in Fig. 3.
Fig. 3.
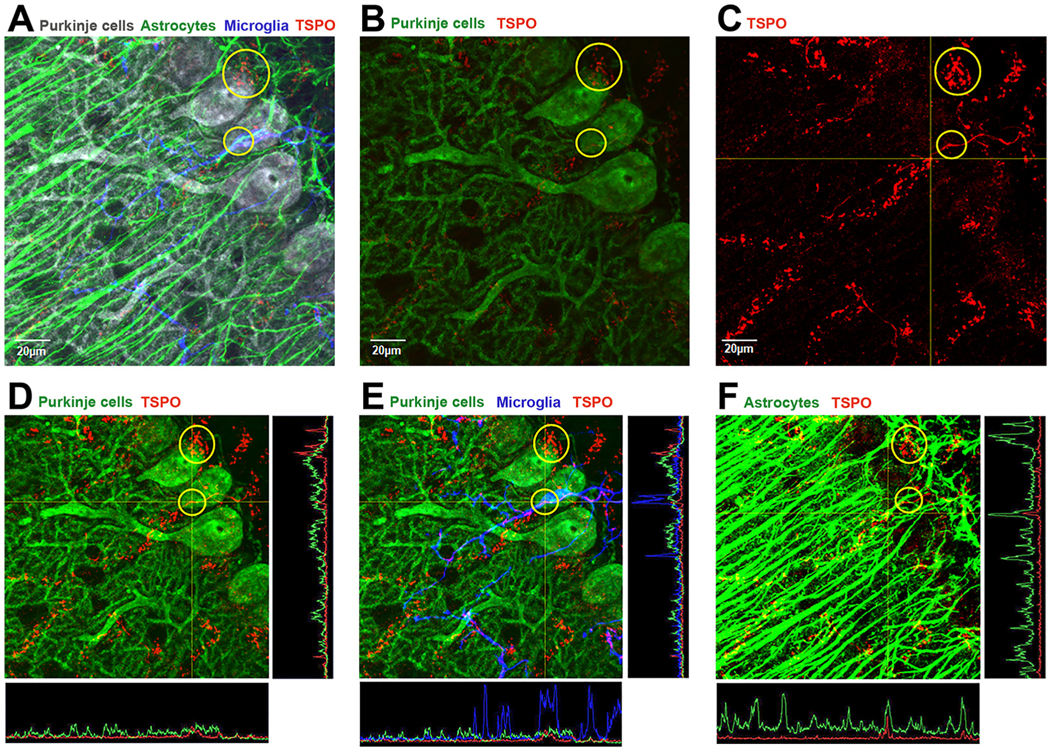
TSPO expression in the Purkinje cell layer of the cerebellum.
A) Quadruple immunofluorescence confocal imaging represented in a top-down view of a 3D condensed z-stack of Purkinje cells (calbindin-gray color), Bergmann glia(GFAP-green color), microglia (Iba1-blue color) and TSPO (red color). The image shows the complex network of glial processes and cell bodies intimately touching the Purkinje cell soma and processes. B) Same image as panel A but the Bergmann glia and microglia channels are not presented to show the relationship of TSPO (red) with Purkinje cells (calbindin-green). Based on this view, in the yellow circles it appears that TSPO may be localized in Purkinje cells. C) In this image, only the TSPO signal is presented and enhanced to be able to visualize since TSPO levels are low in the normal brain [see LUT settings in supplementary Fig. 1]. D) This image again shows the Purkinje cell (calbindin-green) and TSPO (red) but in this image both signals are enhanced [see LUT settings in supplementary Fig. 1]. With signal enhancement one can see a perceived colocalization of TSPO with the Purkinje cell. However, analysis of the TSPO and Purkinje cell signals in the yellow circle using line intensity profiles indicates they do not colocalize as the peaks do not correspond with each other. E) The image provided is the same as D, but the microglia (blue – Iba1) signal is also added. One can see based on the intensity profile that TSPO colocalizes with microglia and not with the Purkinje cell. F) This image represents GFAP (green) staining of Bergmann glia process which in different areas colocalize with TSPO (red) generating a yellow color. Intensity profiles indicate that TSPO (red) colocalizes with Bergmann glia processes (green). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Another example of quadruple-label localization of neuronal staining with NeuN, the astrocyte marker GFAP, the microglia marker Iba1, and TSPO in pyramidal cells of the CA1 region of the adult hippocampus is presented in Fig. 4. In panel A of Fig. 4, pyramidal cell neurons were stained with NeuN (gray), astrocytes with GFAP (green), microglia with Iba-1 (blue), and TSPO (red). We observed a very low level of TSPO staining overall in most regions of the pyramidal cell layer in the normal mouse brain. In panel B1, pyramidal neurons are displayed in green (NeuN) and TSPO in red. Unlike in Purkinje cells, most of the low level of TSPO staining was present outside of pyramidal neurons or were between pyramidal neurons but never colocalized with pyramidal neurons. Panel B2 represents a 67.5° rotation of the z-stack in panel A showing that the TSPO staining is not localized within neurons. In panel C, one of the TSPO positive immunostaining is presented with the astrocyte marker GFAP (blue) outside of the pyramidal cell layer. The image shows that TSPO is colocalized with astrocytes both in the sideview as well as in the intensity profile in the bottom of the panel. In panel D, the same is done but in this case with the microglia marker Iba1 (blue color). Similarly, the colocalization shows TSPO within microglia dispersed between neurons. In summary, Figs. 3 and 4 provide no evidence using TSPO immunofluorescent confocal imaging that TSPO is present in neurons of the cerebellum or hippocampus. Overall, the above studies provide important information on the careful interpretation of TSPO immunostaining studies and the necessity to perform rigorous experimental protocols to eliminate potential artifacts that can confound the interpretation of the cellular sources of TSPO.
Fig. 4.
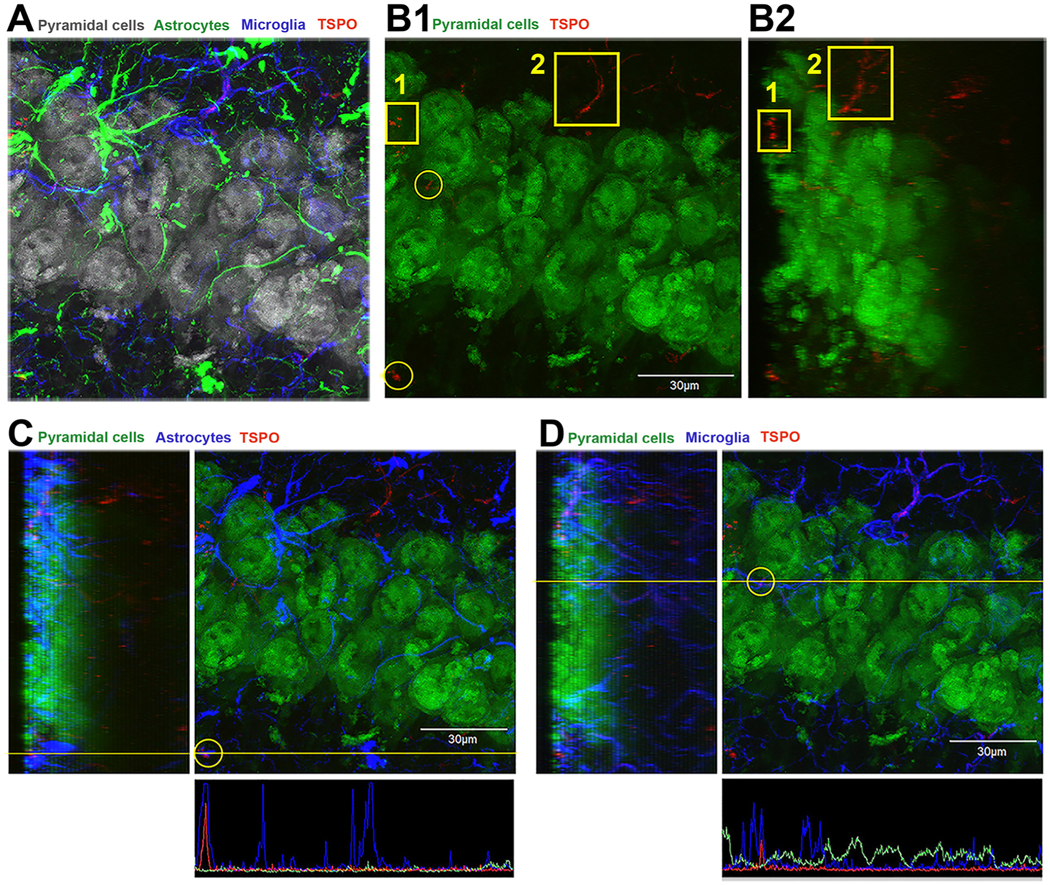
TSPO expression in the CA1 region of the hippocampus is not associated with neurons.
A) Quadruple immunofluorescence confocal imaging represented in a top-down view of a 3D condensed z-stack of pyramidal neurons (NeuN-gray color), astrocytes (GFAP-green color), microglia (Iba1-blue color) and TSPO (red color). The image shows the complex network of glial processes and cell bodies intimately touching pyramidal neurons and processes. B1) This image is the same as in panel A, but now pyramidal neurons labeled with NeuN are displayed in green and TSPO in red. The microglia and astrocyte signal were removed to visualize the spatial relationship between TSPO and neurons. Areas of TSPO are noted in the yellow boxes and circles. There is a low level of TSPO expression that is outside or interspersed between pyramidal neurons. B2) This image is the same as in panel B1, but it represents a 67.5 degree rotation of the z-stack showing that the TSPO staining is not localized with neurons. C) Same image as B1 but now the astrocyte (blue signal) is added and the colocalization of TSPO with astrocyte in the yellow circle is analyzed. The left side of the image is the side view of the zstack and the bottom is the intensity profile (yellow line) indicating signal colocalization of TSPO and astrocyte. D) A similar approach was used in panel D as in panel C, but in this case, the TSPO signal inside the yellow circle appears to colocalized with the microglia marker Iba1 (blue). Again, the side view of the z-stack shows that the TSPO signal colocalizes with microglia which is confirmed in the intensity profile (yellow line) of the signals in the bottom of panel D. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
11. TSPO in blood vessels of the normal adult brain
To confirm that that tubular structures in which TSPO is localized in the normal Purkinje cell layer of the adult cerebellum and in the hippocampus are blood vessels (see Figs. 1, 3, and 4), we performed quadruple label immunofluorescence confocal imaging using the vascular endothelial cell marker CD31 (blue), TSPO (red), NeuN or calbindin (cyan), and GFAP (green). Fig. 5, Panel A shows that nearly all of the TSPO label is within the bounds of the CD31 positive blood vessel with no TSPO in the granule cells of the dentate gyrus of the hippocampus. The side view of the image in panel A shows that the blood vessel is in a different plane than the NeuN-positive granule cells. Panel B of the same figure shows the different individual signals. Fig. 5, panel C depicts the same quadruple label in the Purkinje cell layer. In panel C, the Purkinje cell is represented by the cyan color, TSPO is red, and CD31 in blue. The lower images in panel C indicate the close association of TSPO with CD31-positive blood vessels. These findings indicate that TSPO in blood vessels represent a significant percentage of the total TSPO signal in the normal adult brain neuropil.
Fig. 5.
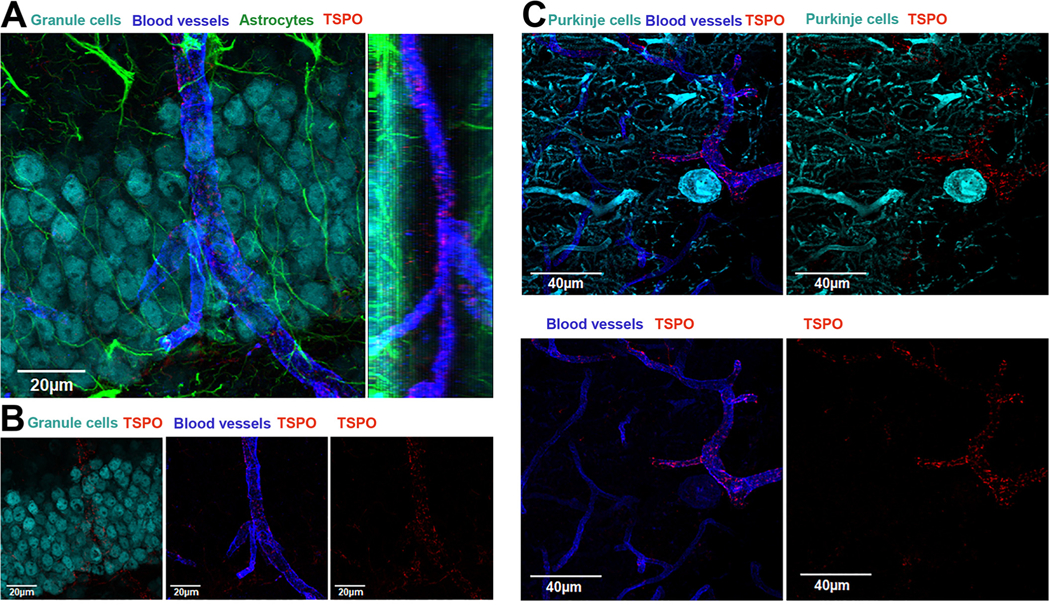
TSPO is highly expressed in blood vessels in the hippocampus and cerebellum of the normal mouse brain.
A) Quadruple immunofluorescence confocal imaging represented in a top-down view of a 3D condensed z-stack of neurons in the dentate gyrus in the hippocampus (NeuN-cyan), blood vessels (CD31-blue), astrocytes (GFAP-green), and TSPO (red). The image shows the presence TSPO in a blood vessel in close contact with neurons in the granule cell layer. To the right is the side view of the z-stack showing the blood vessel with several TSPO positive puncta. B) the different panels show the individual signals showing that TSPO is present in blood vessels. C) Triple immunofluorescence confocal imaging represented in a top-down view of a 3D condensed z-stack of Purkinje cells (calbindin – green), blood vessels (CD31 – blue), and TSPO (red). Left panel shows the 3 signals and the right panel shows Purkinje cell and TSPO. Lower left panel shows the presence of TSPO in the blood vessel and the lower right panel is TSPO only. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
12. TSPO subcellular localization: does location define function?
Understanding the cellular sources of the TSPO response in diverse CNS pathologies is an important scientific endeavor. However, there is a paucity of knowledge on the function(s) of TSPO in glial and peripheral immune cells that contribute to the TSPO signal in PET studies of CNS pathologies. Furthermore, the scientific literature has attributed a variety of different functions to TSPO, many of which have not been confirmed, and some of which have been recently questioned with the advent of global and conditional TSPO-KO mice. Based on the studies provided in the previous sections of this review, it is evident that microglia and astrocytes are the principal cells that upregulate TSPO in diverse pathologies. What is not fully understood are the function(s) of TSPO in microglia or astrocytes. In fact, there is very little data on the function and the subcellular localization of TSPO in glial cells in the normal and disease brain. A greater understanding of these parameters could provide useful information for understanding disease pathophysiology. Furthermore, while TSPO is clearly increased in diverse CNS pathologies, it is not known whether this increase in microglia and/or astrocyte is beneficial or detrimental to the disease condition. Here, we will examine what is known about the localization of TSPO in glial cells to understand its function and potentially advance the development of therapeutic strategies.
12.1. TSPO localization in the Outer Mitochondrial Membrane (OMM)
Early studies on the subcellular fractionation and detection of TSPO using radioligand binding showed that TSPO is highly enriched in the outer mitochondrial membrane (OMM) (Anholt, Pedersen, De Souza, & Snyder, 1986). One of the first reported biological roles for TSPO was as a cholesterol transporter in steroidogenesis and the rate-limiting step in the transfer of cholesterol from the outer to the inner mitochondria membrane (Lacapere et al., 2001; Mukhin, Papadopoulos, Costa, & Krueger, 1989; Papadopoulos et al., 1997; Veenman, Papadopoulos, & Gavish, 2007). However, this function has come into question as studies utilizing TSPO-KO mice and cell-specific conditional TSPO knockdown have shown that cholesterol transport, as well as cholesterol-dependent steroid synthesis, are largely unaffected in TSPO-KO tissues, arguing against TSPO being necessary for those functions (Morohaku et al., 2014; Tu et al., 2014). Other studies have suggested a biological function of TSPO in the generation of cellular reactive oxygen species (ROS) (Choi, Ifuku, Noda, & Guilarte, 2011; Veenman et al., 2007). This is primarily due to direct evidence of subcellular localization of TSPO with cellular components involved in ROS production, such as NADPH oxidases (Loth et al., 2020), as well as extra-mitochondrial spaces such as vesicles and peroxisomes (Boujrad, Vidic, Gazouli, Culty, & Papadopoulos, 2000; Gatliff et al., 2017; Guilarte, Loth, & Guariglia, 2016). This is particularly important for microglia and infiltrating macrophages, which are a significant source of ROS production in a variety of different neurodegenerative conditions.
12.2. Mitochondrial associated ER membrane (MAM)
TSPO immune-gold electron microscopy in primary microglia has confirmed that TSPO is present in the OMM (Loth et al., 2020). This same study found that TSPO is also present in the endoplasmic reticulum (ER), mitochondria-associated ER membrane (MAM), and the plasma membrane (Loth et al., 2020). Since the MAM is a site of ER-mitochondria communication, we hypothesize (Guilarte et al., 2016) that it may be a subcellular site where TSPO binds heme and associates with NADPH oxidase (NOX) subunits (Paillusson et al., 2016; Taketani, Kohno, Furukawa, & Tokunaga, 1995; Verma, Nye, & Snyder, 1987). NOX2 is a major source of ROS in the central nervous system and our recent finding of a TSPO-NOX2 interaction in primary microglia supports the hypothesis that TSPO could be modulating ROS generation in microglia, particularly following an inflammatory stimulus (Loth et al., 2020). Evidence for a putative TSPO localization at the MAM also comes from a study in hormone-treated steroidogenic cells. Hormone treatment of MA-10 (clonal strain of mouse Leydig tumor cells) cells increased the formation of MAM contact sites (Issop et al., 2015). High concentrations of cholesterol in MAM’s lipid rafts could also serve as a platform for the transfer of cholesterol to mitochondria and facilitate the efflux of mitochondria-synthesized steroids products and possibly heme out of mitochondria to the ER (Fujimoto & Hayashi, 2011). Therefore, MAMs are subcellular sites in which TSPO, cholesterol, and heme may be found in lipid rafts that could serve as an assembly platform.
12.3. Plasma membrane
Although cell fractionation studies determined that TSPO was mostly associated with the OMM (Krueger & Papadopoulos, 1990), there are reports of TSPO also present in the plasma membrane, albeit at lower levels than in mitochondria (Olson, Ciliax, Mancini, & Young, 1988; Woods & Williams, 1996). Plasma membrane TSPO has been described in the heart, liver, adrenal, and testis, and on hematopoietic cells (Batoko, Veljanovski, & Jurkiewicz, 2015; Oke, Suarez-Quian, Riond, Ferrara, & Papadopoulos, 1992). TSPO has also been observed in the plasma membrane of erythrocytes (Olson et al., 1988). Recently, TSPO was shown to be ubiquitously expressed on the cell surface of various immune cell types using flow cytometry, with monocytes and neutrophils having the highest frequency of TSPO surface expression (Blevins, Crawford, Azzam, Guilarte, & Kaminski, 2020). Since these cell types can infiltrate the brain and contribute to the TSPO signal, they are an essential cell type to consider. Furthermore, activating monocytes with pro-inflammatory LPS increased the frequency of TSPO surface expression in the absence of altered TSPO gene expression. The authors also observed an increased frequency of TSPO surface localization in monocytes from a chronic inflammatory disease such as chronic HIV infection. This novel finding in myeloid lineage cells (Blevins et al., 2020) has also been confirmed in primary microglia using flow cytometry (Loth et al., 2020). In addition, BV2 cells, a microglia cell line, express plasma membrane TSPO that can be increased by LPS activation (Shimoyama et al., 2019). In that study, LPS treatment increased TSPO expression in the mitochondrial and plasma membrane fractions in BV2 cells using subcellular fractionation. TSPO was localized in the plasma membrane fraction in the control group and was almost 2-fold higher in the LPS-treated group (Shimoyamaetal.,2019).These studies confirm that TSPO is found on the surface of virtually all immune cells including microglia and can be modulated by LPS, however, the biological implications of this subcellular localization are still unknown. The potential role of TSPO in cholesterol transport could have biological implications for immune cells with TSPO on their plasma membrane. Given the crucial role of cholesterol in immune cell function, it has been speculated a role of TSPO in the plasma membrane (Blevins et al., 2020). Further studies are needed to gain a better understanding of the role of TSPO in the plasma membrane.
12.4. Perinuclear region
Evidence that TSPO is located in the perinuclear region in microglia and astrocytes were shown after trimethyltin (TMT) exposure to induce brain injury in rats (Kuhlmann & Guilarte, 2000). Using double-labeling fluorescence histochemistry of GSI-B4 to detect activated microglia or GFAP for activated astrocytes in combination with TSPO labeling, TSPO expression was found to be abundant not only in the cytoplasm but also in the perinuclear area in both glial cell types following TMT-induced neurotoxicity (Kuhlmann & Guilarte, 2000). Thus, the perinuclear localization of the TSPO in microglia and astrocytes might be associated with the differentiation, proliferation, and migration potential of these cells following a brain insult. However, the functional role of TSPO in the perinuclear compartment has yet to be determined. Previous evidence of perinuclear TSPO localization in cultured astrocytes has also been shown in primary cultures of enriched astrocytes obtained from newborn rat cerebella (Alho, Varga, & Krueger, 1994). Using electron microscopy, the authors observed localization of TSPO around the mitochondria and enlarged endoplasmic reticulum, nuclear membrane, cell membrane, and centrioles. TSPO was also located in cell division structures in dividing cells, indicating that TSPO may play a role in cell growth and division. However, no functional experiments were performed.
Similarly, a prominent location of TSPO in the perinuclear region was also shown primarily on microglia/macrophages following intracerebral hemorrhage (ICH) in brain sections of male mice (Bonsack, Alleyne Jr., & Sukumari-Ramesh, 2016). Using double immunehistochemical labeling of proliferating cell nuclear antigen (PCNA) combined with TSPO, the authors showed a marked perinuclear expression in activated microglia. The authors suggest a role of TSPO in microglial proliferation after ICH, which can be associated with neurodegenerative conditions. However, the functional role of TSPO was also not validated in this study. Consistent with this observation, in vitro studies have shown that TSPO is acutely upregulated in retinal microglia in mouse models of retinal inflammation and injury at mRNA and protein levels and shows a perinuclear distribution (Wang et al., 2014). Double immunohistochemical analyses of CD11b, as a marker of activated microglia, and TSPO, showed a concentrated subcellular localization of TSPO in the perinuclear region in cultured retinal microglia after LPS exposure. On the contrary, GFAP-labeled astrocytes and GS-labeled Muller cells did not show TSPO expression after LPS activation. Cultured retinal microglia showed that TSPO expression is increased after LPS activation at mRNA and protein levels in activated microglia and is concentrated in the perinuclear region, using immunohistochemical analyses. Again, the authors did not evaluate the functional role that TSPO may play in cultured activated retinal microglia in the perinuclear region. Finally, a newly reported function of TSPO in nucleus associated mitochondria (NAM) has been identified where TSPO links mitochondria and nucleus during the mitochondrial-retrograde response, allowing for redistribution of cholesterol that deacetylases NF-KB and promotes a pro-survival response (Desai et al., 2020; Fan & Papadopoulos, 2020; Yasin et al., 2017). This study suggests that TSPO may play a role in regulating nuclear gene expression through intracellular signaling. However, the mediators of mitochondrial to nuclear signaling induced by TSPO and its ligands are still unknown.
Overall, the studies reviewed here evaluate the subcellular localization of TSPO in glial and other cells using microscopy-based approaches, which could have potential limitations such as off-target binding of antibodies due to cross-reactivity to other proteins, and potential artifacts due to fixation and permeabilization of the cells that can potentially affect subcellular localization. Moreover, it is also worth noting that even using high-quality reagents; it may be challenging to determine precisely whether a signal represents all or a subset of the protein of interest because of the relative accessibility of the anti body to different cellular compartments and the tendency to focus on fluorescence signal that have higher intensities and punctate pattern, versus lower intensities but diffuse patterns. Therefore, to appropriately determine the subcellular localization of a protein, imaging techniques should also be combined with subcellular fractionation methods that can separate cellular structures, typically by centrifugation based on their sedimentation coefficients, for further separation and confirmation of the subcellular localization.
13. TSPO oligomerization: does it alter function?
TSPO has been reported in several studies to exist as a monomer, dimer, or higher-order oligomeric form (Korkhov, Sachse, Short, & Tate, 2010; Li et al., 2016). The oligomerization may vary between species and tissues, so the oligomeric forms of TSPO may not be conserved between species but may be associated with different functions (Hiser, Montgomery, & Ferguson-Miller, 2021; Li, Liu, Garavito, & FergusonMiller, 2015). In vivo studies have shown that TSPO exists in multimers up to six units (Boujrad, Vidic, & Papadopoulos, 1996; Delavoie et al., 2003). Cholesterol is shown to bind better to TSPO monomers than polymers, whereas TSPO drug ligands exhibit higher binding to TSPO polymers (Jaipuria et al., 2017; Rone et al., 2012; Si Chaib et al., 2020). TSPO monomer to polymer transition may also be driven by hormones and ROS (Lacapere & Papadopoulos, 2003). In response to ROS, TSPO forms covalent polymers in Leydig and breast cancer cells both in vitro and in vivo (Delavoie et al., 2003). A study using colonic cells showed that the TSPO ligand PK11195 could induce TSPO polymerization by stabilizing the dimeric form (Issop et al., 2016). High molecular weight TSPO was also observed in the retina after laser injury in vivo (Wolf, Herb, Schramm, & Langmann, 2020). Thus, evidence so far show that the oligomerization state and ligand binding specificity for mammalian TSPO may be affected by different binding partners (Hiser et al., 2021). However, more work remains to be done to understand the functional significance of the different oligomeric forms of TSPO. Furthermore, most of these studies were not performed with glial cells, thus, their relevance to glial cell biology remains to be discovered.
14. Summary
The recent peer-reviewed literature has described significant advances in understanding the cellular sources of the TSPO response in neuroinflammation and the development of methodologies to study the function of this ancient and biologically important protein. There is yet much more to be done from many different perspectives including the development of better and more cell selective radioligands, improved mathematical modeling to quantify TSPO in PET studies, and a greater understanding of its function in the brain cells that express TSPO. While TSPO is highly expressed in mitochondria, and this has been the most widely studied subcellular compartment associated with function, other subcellular compartments also expressed TSPO although at lower levels, but not necessarily of a lesser functional significance to a specific cell type. From a methodological perspective, more quantitative and better approaches are being developed to study the sources and the degree of induction of TSPO in glial cells. When in vitro immunostaining methods are used for this purpose, careful validation of the techniques are essential to eliminate imaging artifacts which are common using this type of methodology. Finally, an important validation of TSPO imaging is provided by the use of readily available global or conditional TSPO knockout cells, tissues, and animals.
Supplementary Material
Acknowledgments
This work was supported by funding from National Institute of Environmental Health Sciences (NIEHS) Grant R01-ES007062 to TRG.
Abbreviations:
- 3xTgAD
Triple transgenic mouse model of Alzheimer’s disease
- AD
Alzheimer’s disease
- Ad-TNF
Tumor Necrosis Factor-inducing adenovirus
- BV2
Microglia cell line
- CD11b
Cluster of Differentiation Molecule 11B, microglia marker
- CD31
Cluster of Differentiation 31, blood vessel marker
- CLARITY
Clear Lipid-Exchanged Acrylamide-Hybridized Rigid Imaging/Immunostaining/In Situ-Hybridization Compatible Tissue Hydrogel
- CNS
Central Nervous System
- DAB
Diaminobenzidine
- DREADD
Designer Receptors Exclusively Activated by Designer Drugs
- ER
Endoplasmic reticulum
- GFAP
Glial Fibrillary Acidic Protein, astrocyte marker
- GFP
Green Fluorescent Protein
- GSI-B4
Griffonia Simplicifolia 1 Isolectin B4, microglia marker
- HLA-DR
Human Leukocyte Antigen-DR isotype, microglia marker
- Iba1
Allograft Inflammatory Factor 1, microglia marker
- ICH
Intracerebral hemorrhage
- IL-4
Interleukin 4
- KO
Knockout
- LPS
Lipopolysaccharide
- MA-10
Clonal strain of mouse Leydig tumor cells
- MAM
Mitochondria-associated ER membrane
- MS
Multiple sclerosis
- NADPH
Nicotinamide Adenine Dinucleotide Phosphate
- NAM
Nucleus associated mitochondria
- NeuN
Neuronal Nuclei, neuron marker
- NF-KB
Nuclear factor kappa-light-chain-enhancer of activated B cells
- NOX
NADPH oxidase
- OMM
Outer mitochondrial membrane
- PCNA
Proliferating cell nuclear antigen
- PET
Positron Emission Tomography
- PS19
Transgenic Tau mouse model of Alzheimer’s disease
- qRT-PCR
Real time Quantitative Reverse Transcription Polymerase Chain Reaction
- ROS
Reactive oxygen species
- scRNA-seq
Single-cell RNA sequencing
- TgF344-AD
Double transgenic rat model of Alzheimer’s disease
- TMT
Trimethyltin
- TNF
Tumor Necrosis Factor
- TSPO
Translocator Protein 18 kDa
- WT
Wildtype
Footnotes
Declaration of Competing Interest
The authors declare that there are no conflicts of interest.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.pharmthera.2021.108048.
References
- Adamsky A, Kol A, Kreisel T, Doron A, Ozeri-Engelhard N, Melcer T, … Goshen I (2018). Astrocytic activation generates de novo neuronal potentiation and memory enhancement. Cell 174(59–71), Article e14. [DOI] [PubMed] [Google Scholar]
- Alam MM, Lee J, & Lee SY (2017). Recent progress in the development of TSPO PET ligands for neuroinflammation imaging in neurological diseases. Nuclear Medicine and Molecular Imaging 51, 283–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alho H, Varga V, & Krueger KE (1994). Expression of mitochondrial benzodiazepine receptor and its putative endogenous ligand diazepam binding inhibitor in cultured primary astrocytes and C-6 cells: Relation to cell growth. Cell Growth & Differentiation 5, 1005–1014. [PubMed] [Google Scholar]
- Anholt RR, Pedersen PL, De Souza EB, & Snyder SH (1986). The peripheral-type benzodiazepine receptor. Localization to the mitochondrial outer membrane. J Biol Chem 261, 576–583. [PubMed] [Google Scholar]
- Batoko H, Veljanovski V, & Jurkiewicz P (2015). Enigmatic translocator protein (TSPO) and cellular stress regulation. Trends in Biochemical Sciences 40, 497–503. [DOI] [PubMed] [Google Scholar]
- Betlazar C, Harrison-Brown M, Middleton RJ, Banati R, & Liu GJ (2018). Cellular sources and regional variations in the expression of the neuroinflammatory marker translocator protein (TSPO) in the normal brain. International Journal of Molecular Sciences 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blevins LK, Crawford RB, Azzam DJ, Guilarte TR, & Kaminski NE (2020). Surface translocator protein 18 kDa (TSPO) localization on immune cells upon stimulation with LPS and in ART-treated HIV(+) subjects. Journal of Leukocyte Biology 110(1), 123–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonsack F. t., Alleyne CH Jr., & Sukumari-Ramesh S (2016). Augmented expression of TSPO after intracerebral hemorrhage: A role in inflammation? Journal of Neuroinflammation 13, 151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boujrad N, Vidic B, Gazouli M, Culty M, & Papadopoulos V (2000). The peroxisome proliferator perfluorodecanoic acid inhibits the peripheral-type benzodiazepine receptor (PBR) expression and hormone-stimulated mitochondrial cholesterol transport and steroid formation in Leydig cells. Endocrinology 141, 3137–3148. [DOI] [PubMed] [Google Scholar]
- Boujrad N, Vidic B, & Papadopoulos V (1996). Acute action of choriogonadotropin on Leydig tumor cells: Changes in the topography of the mitochondrial peripheraltype benzodiazepine receptor. Endocrinology 137, 5727–5730. [DOI] [PubMed] [Google Scholar]
- Chen MK, Baidoo K, Verina T, & Guilarte TR (2004). Peripheral benzodiazepine receptor imaging in CNS demyelination: Functional implications of anatomical and cellular localization. Brain 127, 1379–1392. [DOI] [PubMed] [Google Scholar]
- Chen MK, & Guilarte TR (2008). Translocator protein 18 kDa (TSPO): Molecular sensor of brain injury and repair. Pharmacology & Therapeutics 118, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J, Ifuku M, Noda M, & Guilarte TR (2011). Translocator protein (18 kDa)/peripheral benzodiazepine receptor specific ligands induce microglia functions consistent with an activated state. Glia 59, 219–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colasanti A, Owen DR, Grozeva D, Rabiner EA, Matthews PM, Craddock N, & Young AH (2013). Bipolar disorder is associated with the rs6971 polymorphism in the gene encoding 18 kDa translocator protein (TSPO). Psychoneuroendocrinology 38, 2826–2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa B, Pini S, Gabelloni P, Da Pozzo E, Abelli M, Lari L, … Martini C (2009). The spontaneous Ala147Thr amino acid substitution within the translocator protein influences pregnenolone production in lymphomonocytes of healthy individuals. Endocrinology 150, 5438–5445. [DOI] [PubMed] [Google Scholar]
- Costa B, Pini S, Martini C, Abelli M, Gabelloni P, Landi S, … Cassano GB (2009). Ala147Thr substitution in translocator protein is associated with adult separation anxiety in patients with depression. Psychiatric Genetics 19, 110–111. [DOI] [PubMed] [Google Scholar]
- Delavoie F, Li H, Hardwick M, Robert JC, Giatzakis C, Peranzi G, … Papadopoulos V (2003). In vivo and in vitro peripheral-type benzodiazepine receptor polymerization: Functional significance in drug ligand and cholesterol binding. Biochemistry 42, 4506–4519. [DOI] [PubMed] [Google Scholar]
- Desai R, East DA, Hardy L, Faccenda D, Rigon M, Crosby J, … Campanella M (2020). Mitochondria form contact sites with the nucleus to couple prosurvival retrograde response. Science Advances 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edling Y, Ingelman-Sundberg M, & Simi A (2007). Glutamate activates c-fos in glial cells via a novel mechanism involving the glutamate receptor subtype mGlu5 and the transcriptional repressor DREAM. Glia 55, 328–340. [DOI] [PubMed] [Google Scholar]
- Fan J, & Papadopoulos V (2020). Mitochondrial TSPO deficiency triggers retrograde Signaling in MA-10 mouse tumor Leydig cells. International Journal of Molecular Sciences 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Focke C, Blume T, Zott B, Shi Y, Deussing M, Peters F, … Brendel M (2019). Early and longitudinal microglial activation but not amyloid accumulation predicts cognitive outcome in PS2APP mice. Journal of Nuclear Medicine 60, 548–554. [DOI] [PubMed] [Google Scholar]
- Fujimoto M, & Hayashi T (2011). New insights into the role of mitochondria-associated endoplasmic reticulum membrane. International Review of Cell and Molecular Biology 292, 73–117. [DOI] [PubMed] [Google Scholar]
- Gatliff J, East DA, Singh A, Alvarez MS, Frison M, Matic I, … Campanella M (2017). A role for TSPO in mitochondrial Ca(2+) homeostasis and redox stress signaling. Cell Death & Disease 8, Article e2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong J, Szego EM, Leonov A, Benito E, Becker S, Fischer A, … Schneider A (2019). Translocator protein ligand protects against neurodegeneration in the MPTP mouse model of parkinsonism. The Journal of Neuroscience 39, 3752–3769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gui Y, Marks JD, Das S, Hyman BT, & Serrano-Pozo A (2020). Characterization of the 18 kDa translocator protein (TSPO) expression in post-mortem normal and Alzheimer’s disease brains. Brain Pathology 30, 151–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilarte TR (2019). TSPO in diverse CNS pathologies and psychiatric disease: A critical review and a way forward. Pharmacology & Therapeutics 194, 44–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilarte TR, Loth MK, & Guariglia SR (2016). TSPO finds NOX2 in microglia for redox homeostasis. Trends in Pharmacological Sciences 37, 334–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiser C, Montgomery BL, & Ferguson-Miller S (2021). TSPO protein binding partners in bacteria, animals, and plants. Journal of Bioenergetics and Biomembranes 53, 463–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung AC, Huang HM, Tsay HJ, Lin TN, Kuo JS, & Sun SH (2000). ATP-stimulated c-fos and zif268 mRNA expression is inhibited by chemical hypoxia in a rat brainderived type 2 astrocyte cell line, RBA-2. Journal of Cellular Biochemistry 77, 323–332. [DOI] [PubMed] [Google Scholar]
- Issop L, Fan J, Lee S, Rone MB, Basu K, Mui J, & Papadopoulos V (2015). Mitochondria-associated membrane formation in hormone-stimulated Leydig cell steroidogenesis: Role of ATAD3. Endocrinology 156, 334–345. [DOI] [PubMed] [Google Scholar]
- Issop L, Ostuni MA, Lee S, Laforge M, Peranzi G, Rustin P, … Lacapere JJ (2016). Translocator protein-mediated stabilization of mitochondrial architecture during inflammation stress in colonic cells. PLoS One 11, Article e0152919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaipuria G, Leonov A, Giller K, Vasa SK, Jaremko L, Jaremko M, … Zweckstetter M (2017). Cholesterol-mediated allosteric regulation of the mitochondrial translocator protein structure. Nature Communications 8, 14893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji B, Ono M, Yamasaki T, Fujinaga M, Zhang MR, Seki C, … Higuchi M (2021). Detection of Alzheimer’s disease-related neuroinflammation by a PET ligand selective for glial versus vascular translocator protein. Journal of Cerebral Blood Flow and Metabolism 41, 2076–2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junck L, Olson JM, Ciliax BJ, Koeppe RA, Watkins GL, Jewett DM, … Starosta Rubinstein S, et al. (1989). PET imaging of human gliomas with ligands for the peripheral benzodiazepine binding site. Annals of Neurology 26, 752–758. [DOI] [PubMed] [Google Scholar]
- Kang Y, Pandya S, Zinger N, Michaelson N, & Gauthier SA (2021). Longitudinal change in TSPO PET imaging in progressive multiple sclerosis. Annals of Clinical Translational Neurology 8, 1755–1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K, Kim H, Bae SH, Lee SY, Kim YH, Na J, … Youn H (2020). [(18)F]CB251 PET/ MR imaging probe targeting translocator protein (TSPO) independent of its polymorphism in a neuroinflammation model. Theranostics 10, 9315–9331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korkhov VM, Sachse C, Short JM, & Tate CG (2010). Three-dimensional structure of TspO by electron cryomicroscopy of helical crystals. Structure 18, 677–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreisl WC, Kim MJ, Coughlin JM, Henter ID, Owen DR, & Innis RB (2020). PET imaging of neuroinflammation in neurological disorders. Lancet Neurology 19, 940–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger KE, & Papadopoulos V (1990). Peripheral-type benzodiazepine receptors mediate translocation of cholesterol from outer to inner mitochondrial membranes in adrenocortical cells. The Journal of Biological Chemistry 265, 15015–15022. [PubMed] [Google Scholar]
- Kuhlmann AC, & Guilarte TR (2000). Cellular and subcellular localization of peripheral benzodiazepine receptors after trimethyltin neurotoxicity. Journal of Neurochemistry 74, 1694–1704. [DOI] [PubMed] [Google Scholar]
- Kwon HS, & Koh SH (2020). Neuroinflammation in neurodegenerative disorders: The roles of microglia and astrocytes. Transl Neurodegener 9, 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacapere JJ, Delavoie F, Li H, Peranzi G, Maccario J, Papadopoulos V, & Vidic B (2001). Structural and functional study of reconstituted peripheral benzodiazepine receptor. Biochemical and Biophysical Research Communications 284, 536–541. [DOI] [PubMed] [Google Scholar]
- Lacapere JJ, & Papadopoulos V (2003). Peripheral-type benzodiazepine receptor: Structure and function of a cholesterol-binding protein in steroid and bile acid biosynthesis. Steroids 68, 569–585. [DOI] [PubMed] [Google Scholar]
- Lahooti B, Chhibber T, Bagchi S, Varahachalam SP, & Jayant RD (2021). Therapeutic role of inflammasome inhibitors in neurodegenerative disorders. Brain, Behavior, and Immunity 91, 771–783. [DOI] [PubMed] [Google Scholar]
- Li F, Liu J, Garavito RM, & Ferguson-Miller S (2015). Evolving understanding of translocator protein 18 kDa (TSPO). Pharmacological Research 99, 404–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Liu J, Liu N, Kuhn LA, Garavito RM, & Ferguson-Miller S (2016). Translocator protein 18 kDa (TSPO): An old protein with new functions? Biochemistry 55, 2821–2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loth MK, Guariglia SR, Re DB, Perez J, de Paiva VN, Dziedzic JL, … Guilarte TR (2020). A novel interaction of translocator protein 18 kDa (TSPO) with NADPH oxidase in microglia. Molecular Neurobiology 57, 4467–4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattner F, Katsifis A, Bourdier T, Loc’h C, Berghofer P, Fookes C, … Fulham M (2021). Synthesis and pharmacological evaluation of [(18)F]PBR316: A novel PET ligand targeting the translocator protein 18 kDa (TSPO) with low binding sensitivity to human single nucleotide polymorphism rs6971. RSC Med Chem 12, 1207–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer JH, Cervenka S, Kim MJ, Kreisl WC, Henter ID, & Innis RB (2020). Neuroinflammation in psychiatric disorders: PET imaging and promising new targets. Lancet Psychiatry 7, 1064–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morohaku K, Pelton SH, Daugherty DJ, Butler WR, Deng W, & Selvaraj V (2014). Translocator protein/peripheral benzodiazepine receptor is not required for steroid hormone biosynthesis. Endocrinology 155, 89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhin AG, Papadopoulos V, Costa E, & Krueger KE (1989). Mitochondrial benzodiazepine receptors regulate steroid biosynthesis. Proceedings of the National Academy of Sciences of the United States of America 86, 9813–9816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nack A, Brendel M, Nedelcu J, Daerr M, Nyamoya S, Beyer C, … Kipp M (2019). Expression of translocator protein and [18F]-GE180 ligand uptake in multiple sclerosis animal models. Cells 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niklison-Chirou MV, Agostini M, Amelio I, & Melino G (2020). Regulation of adult neurogenesis in mammalian brain. International Journal of Molecular Sciences 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notter T, Coughlin JM, Gschwind T, Weber-Stadlbauer U, Wang Y, Kassiou M, … Meyer U (2018). Translational evaluation of translocator protein as a marker of neuroinflammation in schizophrenia. Molecular Psychiatry 23, 323–334. [DOI] [PubMed] [Google Scholar]
- Notter T, Schalbetter SM, Clifton NE, Mattei D, Richetto J, Thomas K, … Hall J (2020). Neuronal activity increases translocator protein (TSPO) levels. Molecular Psychiatry 26(6), 2025–2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutma E, Stephenson JA, Gorter RP, de Bruin J, Boucherie DM, Donat CK, … Amor S (2019). A quantitative neuropathological assessment of translocator protein expression in multiple sclerosis. Brain 142, 3440–3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oke BO, Suarez-Quian CA, Riond J, Ferrara P, & Papadopoulos V (1992). Cell surface localization of the peripheral-type benzodiazepine receptor (PBR) in adrenal cortex. Molecular and Cellular Endocrinology 87, R1–R6. [DOI] [PubMed] [Google Scholar]
- Olson JM, Ciliax BJ, Mancini WR, & Young AB (1988). Presence of peripheral-type benzodiazepine binding sites on human erythrocyte membranes. European Journal of Pharmacology 152, 47–53. [DOI] [PubMed] [Google Scholar]
- Owen DR, Narayan N, Wells L, Healy L, Smyth E, Rabiner EA, … Moore CS (2017). Pro-inflammatory activation of primary microglia and macrophages increases 18 kDa translocator protein expression in rodents but not humans. Journal of Cerebral Blood Flow and Metabolism 37, 2679–2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen DR, Yeo AJ, Gunn RN, Song K, Wadsworth G, Lewis A, … Rubio JP (2012). An 18-kDa translocator protein (TSPO) polymorphism explains differences in binding affinity of the PET radioligand PBR28. Journal of Cerebral Blood Flow and Metabolism 32, 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paillusson S, Stoica R, Gomez-Suaga P, Lau DHW, Mueller S, Miller T, & Miller CCJ (2016). There’s something wrong with my MAM; the ER-mitochondria axis and neurodegenerative diseases. Trends in Neurosciences 39, 146–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannell M, Economopoulos V, Wilson TC, Kersemans V, Isenegger PG, Larkin JR, … Sibson NR (2020). Imaging of translocator protein upregulation is selective for proinflammatory polarized astrocytes and microglia. Glia 68, 280–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulos V, Amri H, Boujrad N, Cascio C, Culty M, Garnier M, … Drieu K (1997). Peripheral benzodiazepine receptor in cholesterol transport and steroidogenesis. Steroids 62, 21–28. [DOI] [PubMed] [Google Scholar]
- Pappata S, Cornu P, Samson Y, Prenant C, Benavides J, Scatton B, … Syrota A (1991). PET study of carbon-11-PK 11195 binding to peripheral type benzodiazepine sites in glioblastoma: A case report. Journal of Nuclear Medicine 32, 1608–1610. [PubMed] [Google Scholar]
- Reeber SL, Arancillo M, & Sillitoe RV (2018). Bergmann glia are patterned into topographic molecular zones in the developing and adult mouse cerebellum. Cerebellum 17, 392–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Refaeli R, Doron A, Benmelech-Chovav A, Groysman M, Kreisel T, Loewenstein Y, & Goshen I (2021). Features of hippocampal astrocytic domains and their spatial relation to excitatory and inhibitory neurons. Glia. 69(10), 2378–2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez JJ, Davies HA, Errington ML, Verkhratsky A, Bliss TV, & Stewart MG (2008). ARG3.1/ARC expression in hippocampal dentate gyrus astrocytes: Ultrastructural evidence and co-localization with glial fibrillary acidic protein. Journal of Cellular and Molecular Medicine 12, 671–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez JJ, Davies HA, Silva AT, De Souza IE, Peddie CJ, Colyer FM, … Stewart MG (2005). Long-term potentiation in the rat dentate gyrus is associated with enhanced Arc/Arg3.1 protein expression in spines, dendrites and glia. The European Journal of Neuroscience 21, 2384–2396. [DOI] [PubMed] [Google Scholar]
- Rone MB, Midzak AS, Issop L, Rammouz G, Jagannathan S, Fan J, … Papadopoulos V (2012). Identification of a dynamic mitochondrial protein complex driving cholesterol import, trafficking, and metabolism to steroid hormones. Molecular Endocrinology 26, 1868–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupprecht R, Papadopoulos V, Rammes G, Baghai TC, Fan J, Akula N, … Schumacher M (2010). Translocator protein (18 kDa) (TSPO) as a therapeutic target for neurological and psychiatric disorders. Nature Reviews. Drug Discovery 9, 971–988. [DOI] [PubMed] [Google Scholar]
- Sacher C, Blume T, Beyer L, Peters F, Eckenweber F, Sgobio C, … Brendel M (2019). Longitudinal PET monitoring of amyloidosis and microglial activation in a secondgeneration amyloid-beta mouse model. Journal of Nuclear Medicine 60, 1787–1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimoyama S, Furukawa T, Ogata Y, Nikaido Y, Koga K, Sakamoto Y, Ueno S, & Nakamura K (2019). Lipopolysaccharide induces mouse translocator protein (18 kDa) expression via the AP-1 complex in the microglial cell line, BV-2. PLoS One 14, Article e0222861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Si Chaib Z, Marchetto A, Dishnica K, Carloni P, Giorgetti A, & Rossetti G (2020). Impact of cholesterol on the stability of monomeric and dimeric forms of the translocator protein TSPO: A molecular simulation study. Molecules 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowell RD, Wong EL, Batchelor HN, Mendes MS, Lamantia CE, Whitelaw BS, & Majewska AK (2018). Cerebellar microglia are dynamically unique and survey Purkinje neurons in vivo. Developmental Neurobiology 78, 627–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sucksdorff M, Matilainen M, Tuisku J, Polvinen E, Vuorimaa A, Rokka J, Nylund M, Rissanen E, & Airas L (2020). Brain TSPO-PET predicts later disease progression independent of relapses in multiple sclerosis. Brain 143, 3318–3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taketani S, Kohno H, Furukawa T, & Tokunaga R (1995). Involvement of peripheraltype benzodiazepine receptors in the intracellular transport of heme and porphyrins. Journal of Biochemistry 117, 875–880. [DOI] [PubMed] [Google Scholar]
- Tanimoto Y, Yamasaki T, Nagoshi N, Nishiyama Y, Nori S, Nishimura S, … Okano H (2020). In vivo monitoring of remnant undifferentiated neural cells following human induced pluripotent stem cell-derived neural stem/progenitor cells transplantation. Stem Cells Translational Medicine 9, 465–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tournier BB, Tsartsalis S, Ceyzeriat K, Fraser BH, Gregoire MC, Kovari E, & Millet P (2020). Astrocytic TSPO upregulation appears before microglial TSPO in Alzheimer’s disease. Journal of Alzheimer’s Disease 77, 1043–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tournier BB, Tsartsalis S, Ceyzeriat K, Garibotto V, & Millet P (2020). In vivo TSPO signal and neuroinflammation in Alzheimer’s disease. Cells 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tournier BB, Tsartsalis S, Ceyzeriat K, Medina Z, Fraser BH, Gregoire MC, … Millet P (2020). Fluorescence-activated cell sorting to reveal the cell origin of radioligand binding. Journal of Cerebral Blood Flow and Metabolism 40, 1242–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tournier BB, Tsartsalis S, Rigaud D, Fossey C, Cailly T, Fabis F, … Millet P (2019). TSPO and amyloid deposits in sub-regions of the hippocampus in the 3xTgAD mouse model of Alzheimer’s disease. Neurobiology of Disease 121, 95–105. [DOI] [PubMed] [Google Scholar]
- Troike KM, Acanda de la Rocha AM, Alban TJ, Grabowski MM, Otvos B, Cioffi G, … Azzam DJ (2021). The Translocator Protein (TSPO) Genetic Polymorphism A147T Is Associated with Worse Survival in Male Glioblastoma Patients. Cancers (Basel) 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu LN, Morohaku K, Manna PR, Pelton SH, Butler WR, Stocco DM, & Selvaraj V (2014). Peripheral benzodiazepine receptor/translocator protein global knock-out mice are viable with no effects on steroid hormone biosynthesis. The Journal of Biological Chemistry 289, 27444–27454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga B, Marko K, Hadinger N, Jelitai M, Demeter K, Tihanyi K, … Madarasz E (2009). Translocator protein (TSPO 18kDa) is expressed by neural stem and neuronal precursor cells. Neuroscience Letters 462, 257–262. [DOI] [PubMed] [Google Scholar]
- Veenman L, Papadopoulos V, & Gavish M (2007). Channel-like functions of the 18-kDa translocator protein (TSPO): Regulation of apoptosis and steroidogenesis as part of the host-defense response. Current Pharmaceutical Design 13, 2385–2405. [DOI] [PubMed] [Google Scholar]
- Verma A, Nye JS, & Snyder SH (1987). Porphyrins are endogenous ligands for the mitochondrial (peripheral-type) benzodiazepine receptor. Proceedings of the National Academy of Sciences of the United States of America 84, 2256–2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicente-Rodriguez M, Singh N, Turkheimer F, Peris-Yague A, Randall K, Veronese M, … Parker CA (2021). Resolving the cellular specificity of TSPO imaging in a rat model of peripherally-induced neuroinflammation. Brain, Behavior, and Immunity 96, 154–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicidomini C, Guo N, & Sahay A (2020). Communication, cross talk, and signal integration in the adult hippocampal neurogenic niche. Neuron 105, 220–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vo SV, Banister SD, Freelander I, Werry EL, Reekie TA, Ittner LM, & Kassiou M (2020). Reversing binding sensitivity to A147T translocator protein. RSC Med Chem 11, 511–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HJ, Fan J, & Papadopoulos V (2012). Translocator protein (Tspo) gene promoter-driven green fluorescent protein synthesis in transgenic mice: An in vivo model to study Tspo transcription. Cell and Tissue Research 350, 261–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Wang X, Zhao L, Ma W, Rodriguez IR, Fariss RN, & Wong WT (2014). Macroglia-microglia interactions via TSPO signaling regulates microglial activation in the mouse retina. The Journal of Neuroscience 34, 3793–3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werry EL, Bright FM, Piguet O, Ittner LM, Halliday GM, Hodges JR, … Kassiou M (2019). Recent developments in TSPO PET imaging as a biomarker of neuroinflammation in neurodegenerative disorders. International Journal of Molecular Sciences 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf A, Herb M, Schramm M, & Langmann T (2020). The TSPO-NOX1 axis controls phagocyte-triggered pathological angiogenesis in the eye. Nature Communications 11, 2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods MJ, & Williams DC (1996). Multiple forms and locations for the peripheraltype benzodiazepine receptor. Biochemical Pharmacology 52, 1805–1814. [DOI] [PubMed] [Google Scholar]
- Yamada K, & Watanabe M (2002). Cytodifferentiation of Bergmann glia and its relationship with Purkinje cells. Anatomical Science International 77, 94–108. [DOI] [PubMed] [Google Scholar]
- Yasin N, Veenman L, Singh S, Azrad M, Bode J, Vainshtein A, … Gavish M (2017). Classical and novel TSPO ligands for the mitochondrial TSPO can modulate nuclear gene expression: Implications for mitochondrial retrograde Signaling. International Journal of Molecular Sciences 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Hu K, Shao T, Hou L, Zhang S, Ye W, … Liang SH (2021). Recent developments on PET radiotracers for TSPO and their applications in neuroimaging. Acta Pharmaceutica Sinica B 11, 373–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinnhardt B, Belloy M, Fricke IB, Orije J, Guglielmetti C, Hermann S, … Jacobs AH (2019). Molecular imaging of immune cell dynamics during de- and remyelination in the cuprizone model of multiple sclerosis by [(18)F]DPA-714 PET and MRI. Theranostics 9, 1523–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


