Abstract
People with functional neurological disorder (FND) are frequent in neurological practice. A new approach to the positive diagnosis of FND focuses on recognisable patterns of genuinely experienced symptoms with signs that show variability within and between tasks over time. Psychological stressors are common risk factors but may be absent. We review four entities, functional seizures, functional movement disorders, persistent perceptual postural dizziness, and functional cognitive disorder, outlining similarities and arguing they are variants of a disorder at the interface between neurology and psychiatry. All four have distinctive features and are increasingly possible to diagnose with the support of clinical neurophysiology and other biomarkers. Current understanding of FND pathophysiology includes overactivity of the limbic system, symptom modelling as part of a predictive coding framework, and dysfunction of brain networks that gives movement the sense of voluntariness. Evidence increasingly supports tailored multidisciplinary treatment that may involve physical and psychological therapy approaches.
Keywords: Functional Neurological Disorder, Conversion Disorder, Psychogenic, Functional movement disorder, Dissociative seizure, Psychogenic non-epileptic seizure, Persistent Postural Perceptual Dizziness, Functional Cognitive Disorder
Introduction
The name “functional neurological disorder” (FND) conveys the idea of a condition in which the primary pathophysiologic processes are alterations in functioning of brain networks rather than abnormalities of brain structures. Functional disorders though long recognized, were largely neglected in mid-20th century, and given various diagnostic labels including conversion, psychogenic, and dissociative disorders. There are psychiatric diagnostic criteria in DSM-5 where it is called "Conversion disorder (Functional Neurological Symptom Disorder)" but also diagnostic criteria created by neurologists, psychiatrists, and other healthcare professionals in partnership1,2-4. Here we define the term FND to denote clinical syndromes consisting of symptoms and signs of genuinely experienced alterations in motor, sensory, or cognitive performance that are distressing or impairing and manifest: 1) one or more patterns of deficits consistent predominantly with dysfunction of the nervous system and 2) variability in performance within and between tasks.
The most common presentations of FND are functional seizures (also called dissociative or psychogenic non-epileptic seizures) and functional movement disorders including paresis. Other common manifestations are somatosensory or visual symptoms, and speech disorders, which we exclude here due to space constraints. Chronic dizziness and cognitive dysfunction as part of a functional disorder have occupied an uncertain place in relation to FND, and we review them below. We discuss possible shared mechanisms and distinguishing processes as well as the observation that persons with FND may present more than one phenotype and/or alternate from one to another over time. We include evidence from neuroimaging, neurophysiologic, and genetic studies and new concepts of voluntary motor and sensory control to explain the unique alterations in neurological functioning that identify FND as a bona fide disorder. We also provide a review of improved practical diagnostic techniques, potential biomarkers, and emerging evidence for improved treatments based on a better understanding of mechanisms.
Epidemiology
FND has been reported from age 4-94 years.5,6 There is a striking female preponderance of 60-80%, although the gender gap is narrower in early and late life.7,8 Functional movement disorders have a lower female predominance than functional seizures, which may relate in part to their later median onset (late 30s for movement disorders,7 late 20s for seizures8).
Global and historical data, though of limited quality, suggest that FND occurs at similar rates across geographical regions9 and eras10. The spectrum of symptoms is similar in North America, Europe, Korea, India, and Brazil. FND is one of the commonest reasons for new outpatient neurological consultations, comprising 1 in 6 referrals11. Population studies suggest an incidence of 10-15/100,00012,14, translating to an estimated prevalence of 250,000-300,000 people in the USA alone. Levels of disability11, caregiver burden, and adverse effects on quality of life 15 are similar to other neurological conditions. Healthcare costs for inpatient treatment of adults with FND in the US exceed $1 billion annually16. Access to accurate diagnosis and treatment is problematic worldwide,17 and the evaluation process is often stigmatizing18.
Predisposition, precipitants, perpetuants, and outcomes
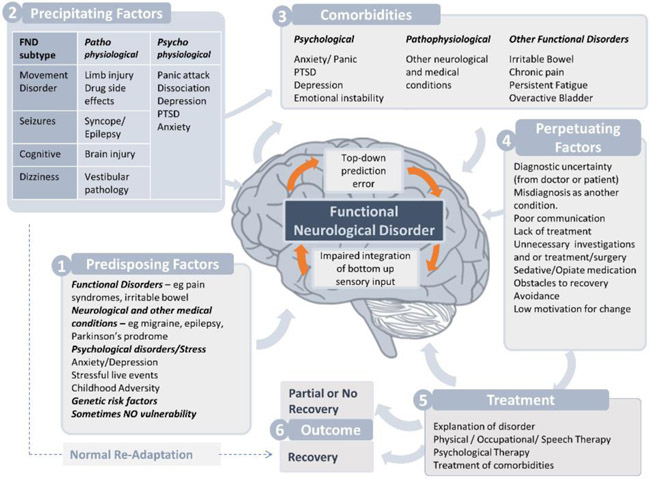
FND is multifactorial. Risk factors for FND in adults include exposure to recent psychological stressors and histories of childhood adversity, particularly neglect19, with odds ratios centred about 3-4. Such events were not reported in over 50% of people with FND in most published studies (Fig. 1). Female preponderance in FND is likely to be partly attributable to their higher frequency of childhood adversity20, although other disorders such as multiple sclerosis are more common in women, and women are more likely to present to health services generally. Risk factors in children include family dysfunction, bullying, and perceived peer pressure, less often abuse21. FND frequently co-exists with depression, anxiety, and traumatic stress disorders, and cluster B personality traits22,23. FND also co-exists with other functional somatic disorders including chronic pain and irritable bowel syndrome suggesting common risk factors or mechanisms24,25,26. Fatigue and pain may have more effect on quality of life than FND symptoms themselves27,15. Psychiatric comorbidities are not unique to FND and are also common in structural neurologic disorders.
Figure 1.
Functional Neurological Disorder can be triggered by pathophysiological and/or psychophysiological events. Acknowledgements to Stoyan Popkirov for the graphic concept.
Predisposition may include genetic factors. In a study of 18 single-nucleotide polymorphisms from 14 candidate genes among 69 patients with functional movement disorder28, the G703T polymorphism of the tryptophan hydroxylase 2 gene significantly predicted clinical manifestations and alterations in neurocircuitry. Compared with GG homozygotes, carriers of a T allele had earlier age at symptom onset, an interactive effect with childhood trauma in predicting symptom severity, and decreased connectivity between right amygdala and middle frontal gyrus. Epigenetic processes such as methylation may underlie relationships among genes, physiologic reactivity, and environmental exposures, including childhood adversity. Patients with FND may have less resilient responses to stress as indicated by decreased 24-hour heart rate variability29. A pilot study of 15 patients with FND found increased methylation of the oxytocin receptor gene, which plays a role in regulating responsivity to stress30.
Many clinicians associate FND exclusively with psychiatric morbidity, but observations beginning in the 19th century found that structural illnesses and injuries may predispose or precipitate FND, as in epilepsy predating functional seizures31 or migraine preceding functional limb weakness25. Physical injury, acute illness, and drug side effects may precipitate FND by causing novel nociceptive or unexpected sensory experience25. Certain neurologic disorders are more likely than others to trigger comorbid FND. For example, Parkinson disease is a likelier precipitant than Alzheimer disease32, perhaps due to specific effects on motor-linked networks.
Clinicians often fear making diagnostic errors in patients who present with FND, but misdiagnosis of FND in neurologic clinics is no more common than misdiagnosis of other neurological and psychiatric disorders33. In fact, erroneous diagnosis of FND as epilepsy34 or multiple sclerosis35 may be more frequent than the other way round.
The prognosis of FND is poorer than most clinicians expect. In one study, 40% of patients were unchanged or worse after a mean of 7 years. Only 20% achieved remission36. Remission rates of functional seizures may be higher (40-50%) than other FND symptoms, but considerable variability exists among studies36. Mortality also may be higher (e.g., 2-3 fold increase in patients with functional seizures), although the causes are unclear37.
Seizures
Functional seizure(s), also known as psychogenic nonepileptic seizures (PNES) and dissociative seizures, manifest episodes that resemble epilepsy or syncope. Accurate diagnosis can be challenging and often delayed because symptoms are transient and complete histories may be available only from witnesses (Tables 1 and 2).
Table 1.
Positive Diagnostic Features and Biomarkers of Functional Movement Disorder and Functional Seizures.
| Functional Movement Disorders | |
|---|---|
| Commonly used | Newer diagnostic tools and research biomarkers |
| Tremor | |
| Tremor entrainment or cessation to externally cued rhythm | Whack-a-mole sign: holding down a tremulous body part38 |
| Variability of frequency and amplitude | Coherence between antagonist muscles measured with standard coherence or wavelets 39 |
| Dystonia | |
| Fixed inverted and/or plantarflexed ankle | Dystonia of the face: downward lip pulling, orbicularis oculi spasm, platysma spasm40 |
| Fixed clenched fist | Sustained facial movement to evoke a spasm40 |
| Functional hemifacial spasm lacks the "Other Babinski sign" (raising of eyebrow on affected side) | |
| Gait and Balance | |
| Variability of gait performance | Classification of gait types into 7 types: ataxic, spastic, weak gait, antalgic, parkinsonian, hemiparetic, dystonic41 |
| Gait performance shows excellent balance | "Huffing and Puffing" sign: huffing, grunting, grimacing, and breath holding after small amounts of exercise38 |
| ‘Walking on ice gait’, Dragging monoplegic gait, or knee-buckling gait | Posturographic improvement with distraction (guessing numbers written on back or cognitive task)42 |
| Jerks/Myoclonus | |
| Truncal jerking – especially with facial movement* | Increased startle43 |
| Positive Bereitschaftspotential (BP) before movement using back-averaging | Event related desynchronization (ERD) using back averaging42 |
| Limb Weakness and generic motor | |
| Hoover’s sign | Absence of amplitude suppression of median nerve somatosensory evoked potential42 |
| Hip Abductor Sign | Decreased prepulse inhibition of the blink reflex by stimulation of the index finger44 |
| Drift without pronation | Absence of contingent negative variation in reaction time task45 |
| Seizures | |
| Eyes closed | Transparent suggestive seizure induction46 |
| Prolonged attacks | Qualitative conversation analysis47 |
| Hyperventilation | Use of smartphone video48 |
| Awareness during generalised shaking | Wrist-worn accelerometers49 |
| Ictal or post-ictal weeping | Post-ictal plasma proteins50 |
the diagnosis of functional jerks can be difficult, but 58% of 179 patients with truncal myoclonus had FND in one series43.
Table 2.
Diagnostic and Treatment Pitfalls in FND.
| Diagnostic Pitfalls in FND | |
|---|---|
| Pitfalls that can lead to a wrong FND Diagnosis | Pitfalls that can lead to failure to make a correct FND diagnosis |
| Jumping to conclusions based on psychological history/stress | Absence of psychological risk factors/stress |
| Failure to consider an additional medical/neurological cause | Failure to consider FND in presence of additional medical/neurological condition |
| Not basing diagnosis on presence of positive diagnosis signs | Lack of awareness of positive features of FND |
| Diagnosis based on ‘bizarre’ presentation alone | Male, older patient |
| Reliance on normal investigations | Placing too much weight on incidental imaging findings (e.g., white matter lesions) |
| Treatment Pitfalls in FND | |
| Do’s | Don’ts |
| Explain the diagnosis on the basis of positive clinical features of FND | Only describe normal investigations or frame as a medical mystery |
| Where possible demonstrate positive clinical signs supporting the diagnosis. Explain to family and friends as well. | Jump straight to risk factors (‘stress’, ‘psychological’) when discussing possible causes of symptoms |
| Check and consolidate understanding of the diagnosis. Consider copying correspondence to patients. Provide written information. Signpost to online information and support organizations. | Only provide written/online information without also providing or referring on for treatment |
| Encourage early and active goal-directed rehabilitation. Engage family and friends with that process. | Encourage unrealistic expectations. Improvement is a gradual active process, and many patients do not improve. |
| Refer for appropriate therapies (physiotherapy, psychology, speech-language therapy, occupational therapy) | Discharge without any plan for follow-up or further treatment while patient remains significantly symptomatic and/or disabled by symptoms |
| Treat comorbidities - e.g., depression, anxiety disorders (including PTSD), sleep disorders. Refer to psychiatry if necessary. | Neglect to treat comorbid psychiatric disorders |
| Address unhelpful medication regimes; opiates, benzodiazepines, and other sedative medications can worsen symptoms of FND | Suddenly withdraw medications without warning |
| Connect with other professionals to prevent unnecessary and potentially harmful investigations or treatments. Support with training. | Assume that all new symptoms are FND; FND may be comorbid with or precede other neurological disorders. Assess new symptoms on their own merits. |
No single symptom is pathognomonic of functional seizures. Studies have highlighted an overlap between pre-ictal panic-like symptoms and functional seizures51, in which dissociation may occur in response to autonomic arousal and be perpetuated by classical and operant conditioning. Qualitative conversational analysis of seizure descriptions distinguished functional seizures from epilepsy with surprisingly high sensitivity and specificity47. A machine learning algorithm processing clusters of patient/observer responses to questionnaires showed promise in distinguishing epilepsy from syncope, but this is not yet ready for diagnosing individual patients52.
The “gold standard” for diagnosis is recording patients’ typical episodes consistent with functional seizures on video EEG (vEEG)53. Events occur spontaneously within 2 days of monitoring in 81% of those receiving a definitive diagnosis 54. Signs with high sensitivity or specificity for functional seizures are long duration of events, fluctuating asynchronous limb or side to side head movements, pelvic thrusting, ictal eye closure, ictal crying, post-ictal memory recall,53 and peri-ictal responsiveness55. vEEG results require experienced interpretation because they may be normal with certain focal types of epilepsy, e.g., hypermotor frontal lobe seizures. In addition, interictal non-epileptiform abnormalities in functional seizures are still commonly misinterpreted by non-experts56. Many patients lack access to specialized monitoring units or have “indeterminate” diagnosis due to equivocal or uneventful monitoring. They can be managed successfully using published minimum criteria from the International League Against Epilepsy (ILAE) for less than certain diagnosis1. Video only recordings interpreted by experts, including videos captured by smartphone48, appear acceptably reliable compared to vEEG57. Ambulatory EEG can be useful when routine EEG is equivocal or when medication withdrawal is not needed58.
Suggestive seizure induction, using transparent and consented procedures, avoided expensive vEEG in 21% of patients with functional seizures and decreased indeterminate testing by 13% in one study46.
Research on biomarkers to differentiate epileptic from functional seizures is ongoing, but history, risk factors, and ictal data currently are more reliable. Prolactin and lactate levels may be elevated after epileptic convulsions but need to be drawn within 1-2 hours of events, can be normal after focal seizures and sometimes abnormal after functional seizures59. A study of four plasma proteins sampled within 24 hours in 137 patients showed promise50. Wrist-worn accelerometers distinguished convulsive epileptic seizures from functional seizures with 70% sensitivity and 86% specificity49 Neuropsychological assessment did not distinguish functional seizure(s) from epilepsy but may be helpful with other aspects of assessment and formulation53.
Movement disorders including limb weakness
Functional movement disorders (FMD) may manifest with any type of abnormal movement38. Most series found that tremor is most common, followed by dystonia24, myoclonus43, and gait disorders41. Less common are parkinsonism32, tic60, stereotypy61, facial movements like hemifacial spasm40, and chorea. In a multicentre study of 410 patients with FMD, the most common clinical presentation was a ‘mixed’ phenotype (46% of patients)26. Paresis and paralysis merit inclusion among manifestations of FMD because they commonly overlap with hyperkinetic movements and may share similar pathophysiologic alterations of higher control of voluntary movement. In FMD, disruptions of voluntary movement contrast with preserved habitual or reflexive movements of the same body part, which is helpful in distinguishing FMD from isolated dystonia.
Other common features of FMD included sudden onset of symptoms (70%) and high rates of comorbidity with anxiety (52%), fatigue (45%), and pain (42%)26. The sudden onset of FMD with weakness makes it a common mimicker of stroke, after migraine and seizure, especially in patients being considered for thrombolysis or needing hospitalization. Among 1165 people in one study admitted for acute stroke-like presentations, 8.4% had FMD, 14% other medical conditions, and 78% stroke14.
Diagnosis of FMD rests on key elements of clinical history and characteristic signs on examination (Tables 1 and 2). Although emphasis has been placed on examination, historical features can provide guidance for differential diagnosis. In a study of 874 patients with hyperkinetic movement disorders, 91% were correctly identified using an algorithm of historical features62. Those suggesting FMD included early age and abrupt onset, more than one movement type, fluctuations during the day, waxing and waning longitudinal course, presence of pain or fatigue, positive psychiatric features, and family history. In 99 patients with functional dystonia, an algorithm of similar historical features had good specificity/sensitivity against other types of dystonia63.
In FMD, there are movement patterns that can be diagnostic (Table 1) 38. Variability may be detected by comparing movements initiated with patients’ full attention, movements performed with distraction, habitual movements (e.g., shifting position in a chair), and movements induced by tendon reflexes. Various tests may demonstrate these differences including Hoover’s sign or the hip abductor sign of functional leg weakness and the tremor entrainment test.38 Techniques of suggestibility and deception run counter to principles of patient autonomy unless undertaken after informed consent and may add little to well- gathered histories and transparent examinations.
Clinical neurophysiological tests may aid diagnosis. Specific tests are phenotype specific and can be found in reviews and in Table 138,42. New methods may prove useful if validated (Table 1). Functional and structural neuroimaging studies have identified group differences between patients with FMD and comparison groups but cannot be used diagnostically for individual patients42.
Persistent Postural-Perceptual Dizziness (PPPD)
The Bárány Society defined the functional vestibular disorder of persistent postural- perceptual dizziness (PPPD) for the International Classification of Vestibular Disorders (Box 1)2. Key symptoms of PPPD are chronic dizziness, unsteadiness, and swaying or rocking (non-spinning) vertigo exacerbated by patients’ own movements and exposure to visually complex or motion-rich environments (Case 1) (Table 3). Earlier descriptions of phobic postural vertigo, space motion discomfort, visual vertigo, and chronic subjective dizziness, informed the definition of PPPD2.
Table 3.
Diagnostic criteria for Persistent Postural Perceptual Dizziness (PPPD) and Functional Cognitive Disorder
| Persistent Postural Perceptual Dizziness Bárány Society diagnostic criteria |
Functional Cognitive Disorder proposed diagnostic criteria4 |
|---|---|
|
|
”Red Flags” discussed in the source document4 included: 1) Inconsistency between cognitive domains – e.g. impaired single word vs sentence comprehension in semantic dementia, difficulties relating to visual comprehension (such as posterior cortical atrophy) that can produce effects similar to internal inconsistency, e.g. the reverse size effect, effects of apathy or low mood, intact implicit memory with defective conscious memory (e.g. Korsakoff’s); 2) Variability over time can occur in other disorders such as Lewy Body Dementia, delirium and obstructive sleep apnoea.
Studies from hospital and university-based neurology clinics found the prevalence of PPPD to be 20% among all patients with vestibular symptoms rising to 40% in a dedicated dizziness centre making PPPD the most common cause of chronic dizziness in those settings64. The median patient age was mid-50s with a 2:1 female predominance. Illnesses precipitating PPPD are similar to its predecessors and include structural vestibular conditions such as benign paroxysmal positional vertigo and unilateral peripheral vestibulopathies (23-25% of cases), vestibular migraine (11-20%), panic and generalized anxiety disorders (15% each), mild traumatic brain injuries (3-15%), stroke (2%), dysautonomias (1-7%), and other medical conditions (3-6%)64. Neuroticism may predispose to PPPD and high levels of body vigilance and aberrant illness-related beliefs at the time of precipitating events may predict persistent dizziness64.
Physiologic investigations of patients with PPPD and its predecessors have identified stiffened postural control sometimes accompanied by excessive upper body sway65. Gait changes include widened base of support, shorter stride length, and momentary two-footed stance mid-stride65. Patients with PPPD also showed overreliance on visual versus vestibular and somatosensory inputs (i.e., visual dependence), making them susceptible to degradation of dynamic visual acuity on exposure to moving visual stimuli65. Spatial navigation may be impaired.
Functional cognitive disorder
Cognitive symptoms traditionally have been excluded from definitions of FND, but recent work highlighted how similar principles of ‘ruling in’ positively identifiable symptoms and signs can be applied to this area (Case 2) (Table 3). Recent consortium diagnostic criteria defined functional cognitive disorder as a condition of cognitive symptoms with clear evidence of internal inconsistency, not better explained by another disorder, and causing distress or impairment or warranting medical evaluation4. Similar to other FND, “internal inconsistency” referred to differences between occupational functioning and observed performance during interview, and variable patterns within cognitive tests. Analysis of behaviour and language during consultation helps discriminate functional from neurodegenerative disorders. Patients with functional cognitive symptoms give detailed and specific descriptions of episodes of memory failure, are more likely to attend alone, and are more concerned about their symptoms than others around them66,67. Validity tests on cognitive batteries may not be helpful as often thought. Single tests are commonly positive in conditions like epilepsy, and mild cognitive impairment.68
Functional cognitive disorder may account for symptoms in a proportion of patients with subjective cognitive decline or mild cognitive impairment who do not ‘convert’ to dementia. A recent meta-analysis of patients attending memory clinics found that 24% received descriptive diagnoses consistent with functional cognitive disorder69.
Functional cognitive disorder may also coexist with structural and metabolic causes of cognitive decline. The relationship may be comorbid, with both disorders contributing to disability or change over time with anxiety-related prodromal functional symptoms predating onset of dementia. In some cases, a disease biomarker such as an amyloid PET scan may prove to be a false positive, never progressing to degenerative illness.
Abnormal metacognition is prominent in functional cognitive disorder70. Individuals with functional cognitive disorder describe cognitive failures experienced by healthy people in everyday life71. They may perceive severe impairment despite good performance on tests or in occupational settings. A bias toward ‘good old days’ has been noted, although it remains unclear whether this is a risk factor or consequence.
Phenotypes of functional cognitive disorder have been proposed69 including isolated cognitive symptoms, illness anxiety about dementia, cognitive symptoms in mixed FND72, and dissociative amnesia. A subtype for cognitive symptoms caused by anxiety or depressive disorders was suggested, but anxiety and depression may also be viewed as comorbidities that exacerbate cognitive symptoms73.
Distinguishing FND from factitious disorder and malingering
Unfortunately, many patients with FND are still commonly subject to suspicion that they may feigning symptoms74 and this is the source of significant stigma18. Actually, there is no more reason for this concern with FND than for many other neurological complaints such as pain or fatigue75.
Feigning is often divided into factitious disorder (wilfully simulating symptoms for medical care) or malingering (simulating symptoms for other gain). Both require evidence of conscious deceptions and actions75, primarily a major discrepancy between reported and observed function - for example someone claiming persistent leg paralysis whose family member provides a video clip of them dancing. Discrepancy between reported symptoms and day to day activity, or variability in performance that the patient does report, do not provide evidence of feigning.
An expanding range of evidence supports FND as a genuinely experienced disorder. Its semiologic manifestations, aetiological risk factors, and co-existence with other medical and psychiatric conditions are similar around the world and throughout history, allowing for cultural differences in attributions of illness. Neurophysiological studies including those looking at event related potentials and sensory attenuation76 77 as well as functional neuroimaging studies78 79 80 81have identified differences between FND and feigning. Differential positive treatment responses obtained in randomized controlled trials of therapeutic interventions designed specifically for patients with FND82,83 are hard to explain if such studies were contaminated by inclusion of individuals feigning illness by unknowing investigators.
Pathophysiology
The fundamental pathophysiology of all the entities reviewed here can be considered in a similar way, a dysfunction of sensory processing, motor or thought output, or both. FMD, functional seizures, and functional cognitive disorder have prominent output abnormalities, whereas PPPD has both sensory and motor dysfunction. Our understanding of the model is best explained in terms of movement and will be described that way.
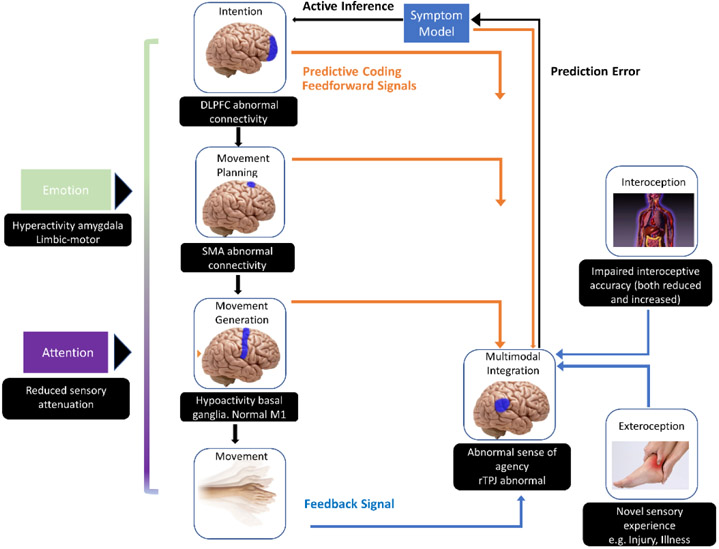
A key element of FND is a partial loss of voluntary control over the body: patients do not feel they are the agents of their abnormal movements. This can be understood in the frame of predictive coding models on how the brain generates the sense that we are the agent of what happens in our body, in particular generating movements (Figure 2). When a movement is planned, a motor command is sent to the motor cortex which will execute the movement. In parallel, a feedforward signal goes to the so-called agency network (an important hub being the right temporo-parietal junction). Once the movement is executed, feedback information goes to the agency network and a comparison between the feedforward and feedback data occurs; when there is a good match, the sense of agency arises38. A similar model has been developed to explain functional seizures and functional somatic symptoms in general84,85. Many recent studies converge to a mechanism of abnormal sense of agency, with neuroimaging studies pointing to abnormal activation of the right temporo-parietal junction38,42 A pilot uncontrolled study found clinical improvement after non-invasive stimulation of this area in seven patients with functional seizures86.
Figure 2. Neural mechanisms of FND.
This scheme relates to FMD but its principles are applicable to all FNDs. Movements are generated by motor cortex after planning/preparation in SMA. This produces feedforward signals to be compared to feedback from interoceptive and external signals after action. If signals don’t match, movement will not be appreciated as voluntary. The brain has a model of the body and world which adds predictive coding to this multimodal integration. Feedback signals that don’t match predictive coding create prediction error, which modifies the model so that predictive coding matches subsequent feedback. In FND, it is hypothesized that prediction error is not accurately updated, perpetuating dysfunction.
FMD = functional movement disorder; DLPFC=Dorsolateral Prefrontal Cortex; SMA=Supplementary Motor Area; M1= Primary Motor Area; rTPJ=Right Temporoparietal Junction
In FND, evidence points to overweighting the feedforward message under the influence of prior expectations, attention, and emotion (Figure 2). A cognitive bias -“jumping to conclusion” - found in FND patients supports this hypothesis: patients tend to favour their prior hypothesis/expectation of an outcome over objective data regarding the future outcome87. A recent electrophysiological experiment confirmed slow sensory information processing in FND patients suggesting a reduced attention allocation to objective body signals88 and this could explain this shift toward too much emphasis on the feedforward signal. In chronic functional dystonia, increased pain tolerance has been found, despite normal pain threshold indicating a dissociation between the discriminative and affective components of pain89. It can be postulated that here also attention plays a role in “filtering” the feedback signal during multimodal integration including emotional salience. Abnormal increased attention to the symptom found in FND also explains why explicit/deliberate movements (lifting the leg during examination) are harder to execute in FND patients than implicit/automatic movements: a different motor program is involved38.
Increased activity in the limbic system found in neuroimaging (fMRI) during motor and emotional tasks90, increased cortisol levels, and abnormal heart rate variability91 all point to abnormal stress reactivity and emotion regulation in FND90. Such abnormalities can be important precipitating and perpetuating factors.
Findings in functional seizures and PPPD, although less extensively studied, suggest similar abnormalities in agency and emotional networks as seen in FMD. Functional neuroimaging studies in people with functional seizures highlight increased connectivity between the insula, motor, and parietal areas92. Small studies of ictal SPECT in functional seizures highlighted abnormalities of agency and limbic networks93. Changes in PPPD were more nuanced. Connectivity in parietal areas was increased on resting state fMRI in proportion to symptom severity,94 but cortical folding was decreased in right temporal- parietal regions which showed reduced activity and connectivity in response to vestibular stimulation.95
Several studies have demonstrated brain anatomical differences (grey matter and basal ganglia volumes) in FND96. Recent machine-learning classifying paradigms have been able to correctly identify FND patients and healthy controls with good accuracies, both with resting state functional and structural brain images78. Correlation between structural changes and clinical data (symptom severity, emotion/mood, dissociation, gender, childhood trauma78) strongly suggest a link between symptoms of FND and brain anatomical difference but longitudinal studies are needed to address the question of causality.
Treatment
Effective treatment for all types of FND begins with establishing therapeutic two-way communication to understand and engage patients in their own recovery process (Table 2). Neurologists have traditionally avoided taking responsibility for people with FND, although are often most appropriate to engage patients in treatment. Explaining the diagnosis with clarity, confidence, using the principles of a ‘rule in’ process, is a key step in treatment97 Patients should be provided written information, although a randomised trial showed that this alone is insufficient to improve outcome98.
Multidisciplinary treatment which individualises the patient’s problems has gained more support than a purely psychological approach33,99. A common theme of such approaches is treatment based on a new understanding of FND symptoms, and not just the individual’s aetiological risk factors. For example, therapy for functional seizures now commonly focuses on psychophysiological mechanism of the events. Therapy for FMD builds on the differences between automatic and voluntary movement that form the diagnosis and use these in physical therapy. Clinical experience suggests that treatment for co-existing conditions such as migraine, orthostatic intolerance, and anxiety or depression is also important.
New approaches to physiotherapy for FMD have some of the strongest evidence for treatment33. Whereas someone with stroke may be encouraged to focus on their affected limb, in FMD physiotherapy, explicit use of distraction and preserved automatic movements are used to ‘retrain’ aberrant motor function. A randomized study compared 29 patients with physical therapy to 28 controls with similar amount of conventional physiotherapy82. At 6 months, 72% of the physical therapy group rated their symptoms as improved compared with 18% in the controls. A physical therapy program can be managed by telemedicine and still be successful100 . In PPPD, studies of vestibular rehabilitation 101 reported reductions in sensitivity to motion and dizziness. Occupational therapy also has an important role in FND, and consensus recommendations have been published102, but has generally only been tested as part of multidisciplinary treatment.
Psychological therapy has been tested in many forms of FND103 and is the treatment of choice for functional seizures. Controlled studies and case series found that 82% of patients had 50% reduction in seizure frequency immediately following treatment104. In the past decade, f a limited number of randomized controlled trials (RCT) showed benefit of cognitive behavioral therapy (CBT) informed psychotherapy over standard medical treatment for seizure frequency over periods up to 6 months103. In a RCT of 368 patients with functional seizures both groups improved but there was no difference in seizure frequency from adding CBT to standardised therapy. CBT did however lead to improvement in 8 out of 13 secondary outcomes such as general functioning, distress, somatic symptoms, and duration of seizure freedom83. Other types of therapy such as mindfulness-based psychotherapy, psychodynamic psychotherapy, and prolonged exposure therapy for those with PTSD show promising outcomes105. Self-help treatments for stress reduction106 have been shown to be safe and acceptable to patients and may be helpful for patients with limited access to treatment. There is less evidence for psychological therapies for functional movement disorder,103 and there is only one such study for functional cognitive disorder107. For PPPD, CBT enhanced response to sertraline108 and acceptance and commitment therapy produced significant benefits when combined with vestibular rehabilitation.109
Trials of psychopharmacologic treatments have been small and inconclusive for FND seizure and motor symptoms, although treatment of comorbidity is less controversial110. In PPPD, eight uncontrolled trials, totalling >300 patients, found that selective serotonin reuptake inhibitors and serotonin norepinephrine reuptake inhibitors reduced mean severity of PPPD or previous syndromes of persistent dizziness by 50% in 6-12 weeks65. For functional seizures, removing any anti-seizure medications that are not treating common comorbidities such as anxiety or depression (or epilepsy) has been shown to be helpful and safe111.
Repetitive transcranial magnetic stimulation has had mixed results in FMD112 . A large placebo controlled trial of botulinum toxin therapy for tremulous and jerky FMD showed no benefit113. Treatments such as hypnotherapy, therapeutic sedation and intensive inpatient treatment may help some patients114.
Future Directions and Conclusions
For decades, FND has been a disorder restricted strictly to conditions involving the voluntary motor and sensory nervous system. We have not had space here to discuss sensory disorders, such as functional anaesthesia or blindness or other motor disorders such as speech and swallowing symptoms, which all have their own diagnostic features based on similar principles. Functional seizures are paroxysmal motor events, one strong reason to keep them in the same category, along with the fact they commonly co-occur with other FNDs 26. We have included two other common FND subtypes involving dizziness and cognition to explore their overlap (Figure 1)
A striking feature of many functional disorders is their triggering by and co-existence with recognised pathophysiological events. FMD and functional seizures are often triggered by injury or other neurological disease. For PPPD, an initial, usually vestibular event is part of the definition. Functional cognitive disorder commonly follows mild traumatic brain injury after the point when natural recovery might have been expected to occur. Psychological factors, such as adversity, personality traits, or psychiatric disorder may be relevant as predisposing, precipitating, and perpetuating factors at all stages of FND but contrary to previous conversion disorder models, are not a prerequisite. The strength of these triggering pathophysiological events, which themselves often shape the symptom of the subsequent functional disorder, may partly explain why there is such heterogeneity in background risk.
Figure 2 illustrates overlapping physiological processes. In all entities reviewed here, the person with FND develops an abnormal model of the way his or her brain and body function. The model confirms and reinforces the abnormal function. The underlying push toward the abnormal model comes from a physical or emotional experience that cannot be subsumed into normal function. FND is arguably what might be expected to happen when “predictive processing” in the brain goes awry.
There are also commonalities in our best therapeutic approaches to all these disorders. Physical therapy in FMD promotes automatic movements over abnormal overlearnt impaired voluntary ones. In PPPD habituation exercises aim to ‘desensitise’ a similarly aberrant sensory and motor system where there is an overlearnt abnormal “attentional spotlight” on the symptom of dizziness. Similarly, psychotherapies like CBT for FND, aim to modify, among many targets, the various inputs in Figures 1 and 2 that may contribute to the disorder, including interoception, attentional style, cognitions, emotions, and psychological comorbidities.
Clearly there are important differences between these diverse symptoms. Seizures are paroxysmal, PPPD and FMD are typically continuous and are arguably as different as panic disorder and depression. As with panic and depression, they co-exist more often than by chance and treatment typically improves with an understanding of their overlap.
The new principles of making a ‘rule in’ diagnosis based on clinical signs map on to a new understanding of pathophysiology and treatment and allow diagnosis in people with other neurological conditions. Establishing reliability in positive diagnosis of PPPD and functional cognitive disorder will be especially important in evaluating their relevance to controversial syndromes such as those arising in Havana and after Covid-19 infection. With increasing evidence-based treatment, the diagnosis of FND should be seen as a process of looking for potentially reversible cause of disability and distress whether or not an individual has abnormalities on conventional laboratory or radiological testing.
Supplementary Material
Acknowledgements:
M.H. is supported by the NINDS Intramural Program
B.A.D. is supported by the Andrew J. Trustey Research Fund.
Jo.S. is supported by an NHS Scotland Research Career Fellowship
Je.S. is supported by grant number W81XWH1810760 from the U.S. Army Medical Research and Development Command via the Congressionally Directed Medical Research Program.
S.A. is supported by the Swiss National Science Foundation (grant PP00P3_176985)
L.M. is supported by a Baillie Gifford Clinical Research Fellowship and the NHS Scotland Chief Scientist’s Office.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures (full)
Jo.S, reports royalties from UpToDate for articles on FND and runs a free self-help website for people with FND. He carries out independent expert medicolegal work including in relation to FND. He is on the medical advisory board for FND Hope and FND Action.
B.A.D. receives royalties from Oxford University Press on her book entitled "Psychogenic Nonepileptic Seizures: Toward the Integration of Care."
L.M. reports expert witness work in personal injury and negligence cases including Functional Neurological Disorder.
The other authors declared no conflicts of interest.
Search Strategy and Selection Criteria
References for this Review were identified by searches of PubMed between January 2016 to March 2021), and references from relevant articles. The search terms “Functional Neurological Disorder”, “Conversion Disorder”, “Functional movement disorder”, “Psychogenic movement disorder”, “(Psychogenic OR Dissociative OR Nonepileptic) AND (Seizure OR attack OR spell)”), ““Persistent Postural Perceptual Dizziness”, and “Functional Cognitive Disorder” were used. Reference lists were searched. There were no language restrictions. The final reference list was generated on the basis of relevance to the topics covered in this Review.
References
- 1.LaFrance WC, Baker G a, Duncan R, Goldstein LH, Reuber M. Minimum requirements for the diagnosis of psychogenic nonepileptic seizures: a staged approach: a report from the International League Against Epilepsy Nonepileptic Seizures Task Force. Epilepsia 2013; 54: 2005–18. [DOI] [PubMed] [Google Scholar]
- 2.Staab JP, Eckhardt-Henn A, Horii A, et al. Diagnostic criteria for persistent postural-perceptual dizziness (PPPD): Consensus document of the committee for the classification of vestibular disorders of the barany society. J Vestib Res Equilib Orientat 2017; 27: 191–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gupta A, Lang AE. Psychogenic movement disorders. Curr Opin Neurol 2009; 22: 430–6. [DOI] [PubMed] [Google Scholar]
- 4.Ball HA, McWhirter L, Ballard C, et al. Functional cognitive disorder: dementia’s blind spot. Brain 2020; 143: 2895–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harris SR. Psychogenic movement disorders in children and adolescents: an update. Eur J Pediatr 2019; 178: 581–5. [DOI] [PubMed] [Google Scholar]
- 6.Chouksey A, Pandey S. Functional movement disorders in elderly. Tremor and Other Hyperkinetic Movements 2019; 9: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baizabal-Carvallo JF, Jankovic J. Gender Differences in Functional Movement Disorders. Mov Disord Clin Pract 2020; 7: 182–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldstein LH, Robinson EJ, Reuber M, et al. Characteristics of 698 patients with dissociative seizures: A UK multicenter study. Epilepsia 2019; 60: 2182–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kanemoto K, LaFrance WC, Duncan R, et al. PNES around the world: Where we are now and how we can close the diagnosis and treatment gaps-an ILAE PNES Task Force report. Epilepsia Open 2017; 2: 307–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stone J Neurologic approaches to hysteria, psychogenic and functional disorders from the late 19th century onwards. Handb Clin Neurol 2016; 139: 25–36. [DOI] [PubMed] [Google Scholar]
- 11.Carson A, Lehn A. Epidemiology. In: Handbook of Clinical Neurology Vol 139: Functional Neurologic Disorders. 2016: 47–60. [DOI] [PubMed] [Google Scholar]
- 12.Villagrán A, Eldøen G, Hofoss D, Ingvar M, Duncan R. Incidence and prevalence of psychogenic nonepileptic seizures in a Norwegian county : A 10- - year population- - based study. 2021; : 1–8. [DOI] [PubMed] [Google Scholar]
- 13.Duncan R, Razvi S, Mulhern S. Newly presenting psychogenic nonepileptic seizures: incidence, population characteristics, and early outcome from a prospective audit of a first seizure clinic. Epilepsy Behav 2011; 20: 308–11. [DOI] [PubMed] [Google Scholar]
- 14.Gargalas S, Weeks R, Khan-Bourne N, et al. Incidence and outcome of functional stroke mimics admitted to a hyperacute stroke unit. J Neurol Neurosurg Psychiatry 2017; 88: 2–6. [DOI] [PubMed] [Google Scholar]
- 15.Jones B, Reuber M, Norman P. Correlates of health-related quality of life in adults with psychogenic nonepileptic seizures: A systematic review. Epilepsia 2016; 57: 171–81. [DOI] [PubMed] [Google Scholar]
- 16.Stephen CD, Fung V, Lungu CI, Espay AJ. Assessment of Emergency Department and Inpatient Use and Costs in Adult and Pediatric Functional Neurological Disorders. JAMA Neurol 2021; 78: 88–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hingray C, El-Hage W, Duncan R, et al. Access to diagnostic and therapeutic facilities for psychogenic nonepileptic seizures: An international survey by the ILAE PNES Task Force. Epilepsia 2018; 59: 203–14. [DOI] [PubMed] [Google Scholar]
- 18.MacDuffie KE, Grubbs L, Best T, et al. Stigma and functional neurological disorder: a research agenda targeting the clinical encounter. CNS Spectr 2020; : 1–6. [DOI] [PubMed] [Google Scholar]
- 19.Ludwig L, Pasman JA, Nicholson T, et al. Stressful life events and maltreatment in conversion (functional neurological) disorder: systematic review and meta-analysis of case-control studies. The Lancet Psychiatry 2018; 5: 307–20. [DOI] [PubMed] [Google Scholar]
- 20.Kletenik I, Sillau SH, Isfahani SA, LaFaver K, Hallett M, Berman BD. Gender as a Risk Factor for Functional Movement Disorders: The Role of Sexual Abuse. Mov Disord Clin Pract 2020; 7: 177–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Watson C, Sivaswamy L, Agarwal R, Du W, Agarwal R. Functional Neurologic Symptom Disorder in Children: Clinical Features, Diagnostic Investigations, and Outcomes at a Tertiary Care Children’s Hospital. J Child Neurol 2019; 34: 325–31. [DOI] [PubMed] [Google Scholar]
- 22.Brown RJ, Reuber M. Psychological and psychiatric aspects of psychogenic nonepileptic seizures (PNES): A systematic review. Clin Psychol Rev 2016; 45: 157–82. [DOI] [PubMed] [Google Scholar]
- 23.Kranick S, Ekanayake V, Martinez V, Ameli R, Hallett M, Voon V. Psychopathology and psychogenic movement disorders. Mov Disord Off J Mov Disord Soc 2011; 26: 1844–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frucht L, Perez DL, Callahan J, et al. Functional Dystonia: Differentiation From Primary Dystonia and Multidisciplinary Treatments. Front Neurol 2021; 11: 1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tinazzi M, Geroin C, Erro R, et al. Functional motor disorders associated with other neurological diseases: Beyond the boundaries of “organic” neurology. Eur J Neurol 2021; 28: 1752–8. [DOI] [PubMed] [Google Scholar]
- 26.Tinazzi M, Morgante F, Marcuzzo E, et al. Clinical Correlates of Functional Motor Disorders: An Italian Multicenter Study. Mov Disord Clin Pract 2020; 7: 920–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Věchetová G, Slovák M, Kemlink D, et al. The impact of non-motor symptoms on the health-related quality of life in patients with functional movement disorders. J Psychosom Res 2018; 115: 32–7. [DOI] [PubMed] [Google Scholar]
- 28.Spagnolo PA, Norato G, Maurer CW, et al. Effects of TPH2 gene variation and childhood trauma on the clinical and circuit-level phenotype of functional movement disorders. J Neurol Neurosurg Psychiatry 2020; 91: 814–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maurer CW, Liu VD, LaFaver K, et al. Impaired resting vagal tone in patients with functional movement disorders. Park Relat Disord 2016; 30: 18–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Apazoglou K, Adouan W, Aubry J-M, Dayer A, Aybek S. Increased methylation of the oxytocin receptor gene in motor functional neurological disorder: a preliminary study. J Neurol Neurosurg Psychiatry 2018; 89: 552–4. [DOI] [PubMed] [Google Scholar]
- 31.Kutlubaev MA, Xu Y, Hackett ML, Stone J. Dual diagnosis of epilepsy and psychogenic nonepileptic seizures: Systematic review and meta-analysis of frequency, correlates, and outcomes. Epilepsy Behav 2018; 89: 70–8. [DOI] [PubMed] [Google Scholar]
- 32.Ambar Akkaoui M, Geoffroy PA, Roze E, Degos B, Garcin B. Functional Motor Symptoms in Parkinson’s Disease and Functional Parkinsonism: A Systematic Review. J Neuropsychiatry Clin Neurosci 2020; 32: 4–13. [DOI] [PubMed] [Google Scholar]
- 33.Espay AJ, Aybek S, Carson A, et al. Current Concepts in Diagnosis and Treatment of Functional Neurological Disorders. JAMA Neurol 2018; 75: 1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu Y, Nguyen D, Mohamed A, et al. Frequency of a false positive diagnosis of epilepsy: A systematic review of observational studies. Seizure 2016; 41: 167–74. [DOI] [PubMed] [Google Scholar]
- 35.Walzl D, Carson AJ, Stone J. The misdiagnosis of functional disorders as other neurological conditions. J Neurol 2019; 266: 2018–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gelauff J, Stone J. Prognosis of functional neurologic disorders. Handb Clin Neurol 2016; 139: 523–41. [DOI] [PubMed] [Google Scholar]
- 37.Nightscales R, McCartney L, Auvrez C, et al. Mortality in patients with psychogenic nonepileptic seizures. Neurology 2020; 95: e643–52. [DOI] [PubMed] [Google Scholar]
- 38.Perez DL, Edwards MJ, Nielsen G, Kozlowska K, Hallett M, LaFrance, Jr WC. Decade of progress in motor functional neurological disorder: continuing the momentum. J Neurol Neurosurg Psychiatry 2021; 92: 668–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kramer G, Van der Stouwe AMM, Maurits NM, Tijssen MAJ, Elting JWJ. Wavelet coherence analysis: A new approach to distinguish organic and functional tremor types. Clin Neurophysiol 2018; 129: 13–20. [DOI] [PubMed] [Google Scholar]
- 40.Stone J, Hoeritzauer I, Tesolin L, Carson A. Functional movement disorders of the face: A historical review and case series. J Neurol Sci 2018; 395: 35–40. [DOI] [PubMed] [Google Scholar]
- 41.Nonnekes J, Růžička E, Serranová T, Reich SG, Bloem BR, Hallett M. Functional gait disorders. Neurology 2020; 94: 1093–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thomsen BLC, Teodoro T, Edwards MJ. Biomarkers in functional movement disorders: A systematic review. J Neurol Neurosurg Psychiatry 2020; 91: 1261–9. [DOI] [PubMed] [Google Scholar]
- 43.Dreissen YEM, Cath DC, Tijssen MAJ. Functional jerks, tics, and paroxysmal movement disorders. In: Handbook of Clinical Neurology. 2016: 247–58. [DOI] [PubMed] [Google Scholar]
- 44.Hanzlíková Z, Kofler M, Slovák M, et al. Prepulse inhibition of the blink reflex is abnormal in functional movement disorders. Mov Disord 2019; 34: 1022–30. [DOI] [PubMed] [Google Scholar]
- 45.Teodoro T, Koreki A, Meppelink AM, et al. Contingent negative variation: a biomarker of abnormal attention in functional movement disorders. Eur J Neurol 2020; 27: 985–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Popkirov S, Grönheit W, Jungilligens J, Wehner T, Schlegel U, Wellmer J. Suggestive seizure induction for inpatients with suspected psychogenic nonepileptic seizures. Epilepsia 2020; 61: 1931–8. [DOI] [PubMed] [Google Scholar]
- 47.Jenkins L, Cosgrove J, Chappell P, Kheder A, Sokhi D, Reuber M. Neurologists can identify diagnostic linguistic features during routine seizure clinic interactions: results of a one-day teaching intervention. Epilepsy Behav 2016; 64: 257–61. [DOI] [PubMed] [Google Scholar]
- 48.Tatum WO, Hirsch LJ, Gelfand MA, et al. Assessment of the Predictive Value of Outpatient Smartphone Videos for Diagnosis of Epileptic Seizures. JAMA Neurol 2020; 77: 593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kusmakar S, Karmakar C, Yan B, et al. Novel features for capturing temporal variations of rhythmic limb movement to distinguish convulsive epileptic and psychogenic nonepileptic seizures. Epilepsia 2018; : epi.14619. [DOI] [PubMed] [Google Scholar]
- 50.Gledhill JM, Brand EJ, Pollard JR, St. Clair RD, Wallach TM, Crino PB. Association of Epileptic and Nonepileptic Seizures and Changes in Circulating Plasma Proteins Linked to Neuroinflammation. Neurology 2021; 96: e1443–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Indranada AM, Mullen SA, Duncan R, Berlowitz DJ, Kanaan RAA. The association of panic and hyperventilation with psychogenic non-epileptic seizures: A systematic review and meta-analysis. Seizure 2018; 59: 108–15. [DOI] [PubMed] [Google Scholar]
- 52.Wardrope A, Jamnadas-Khoda J, Broadhurst M, et al. Machine learning as a diagnostic decision aid for patients with transient loss of consciousness. Neurol Clin Pract 2020; 10: 96–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Baslet G, Bajestan SN, Aybek S, et al. Evidence-Based Practice for the Clinical Assessment of Psychogenic Nonepileptic Seizures: A Report From the American Neuropsychiatric Association Committee on Research. J Neuropsychiatry Clin Neurosci 2021; 33: 27–42. [DOI] [PubMed] [Google Scholar]
- 54.Zanzmera P, Sharma A, Bhatt K, et al. Can short-term video-EEG substitute long-term video-EEG monitoring in psychogenic nonepileptic seizures? A prospective observational study. Epilepsy Behav 2019; 94: 258–63. [DOI] [PubMed] [Google Scholar]
- 55.Wardrope A, Wong S, McLaughlan J, Wolfe M, Oto M, Reuber M. Peri-ictal responsiveness to the social environment is greater in psychogenic nonepileptic than epileptic seizures. Epilepsia 2020; 61: 758–65. [DOI] [PubMed] [Google Scholar]
- 56.Amin U, Benbadis SR. The Role of EEG in the Erroneous Diagnosis of Epilepsy. J Clin Neurophysiol 2019; 36: 294–7. [DOI] [PubMed] [Google Scholar]
- 57.Gasparini S, Beghi E, Ferlazzo E, et al. Management of psychogenic non-epileptic seizures: a multidisciplinary approach. Eur J Neurol 2019; 26: 205–15. [DOI] [PubMed] [Google Scholar]
- 58.Syed TU, LaFrance WC, Loddenkemper T, et al. Outcome of ambulatory video-EEG monitoring in a ~10,000 patient nationwide cohort. Seizure 2019; 66: 104–11. [DOI] [PubMed] [Google Scholar]
- 59.Sundararajan T, Tesar GE, Jimenez XF. Biomarkers in the diagnosis and study of psychogenic nonepileptic seizures: A systematic review. Seizure 2016; 35: 11–22. [DOI] [PubMed] [Google Scholar]
- 60.Ganos C, Martino D, Espay AJ, Lang AE, Bhatia KP, Edwards MJ. Tics and functional tic-like movements: Can we tell them apart? Neurology. 2019; 93: 750–8. [DOI] [PubMed] [Google Scholar]
- 61.Baizabal-Carvallo JF, Jankovic J. Functional (psychogenic) stereotypies. J Neurol 2017; 264: 1482–7. [DOI] [PubMed] [Google Scholar]
- 62.Lagrand T, Tuitert I, Klamer M, et al. Functional or not functional; that’s the question. Eur J Neurol 2021; 28: 33–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stephen CD, Perez DL, Chibnik LB, Sharma N. Functional dystonia: A case-control study and risk prediction algorithm. Ann Clin Transl Neurol 2021; 8: 732–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Staab J Behavioural neurotology. In: Bronstein A, ed. Oxford Textbook of Vertigo and Imbalance, 2nd edn. Oxford: Oxford University Press, 2021. [Google Scholar]
- 65.Staab JP. Persistent Postural-Perceptual Dizziness. Semin Neurol 2020; 40: 130–7. [DOI] [PubMed] [Google Scholar]
- 66.Reuber M, Blackburn DJ, Elsey C, et al. An Interactional Profile to Assist the Differential Diagnosis of Neurodegenerative and Functional Memory Disorders. Alzheimer Dis Assoc Disord 2018; 32: 197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Williamson J, Larner A. Attended with and head-turning sign can be clinical markers of cognitive impairment in older adults. Int Psychogeriatrics 2018; 20: 1569–1569. [DOI] [PubMed] [Google Scholar]
- 68.McWhirter L, Ritchie CW, Stone J, Carson A. Performance validity test failure in clinical populations- A systematic review. J Neurol Neurosurg Psychiatry 2020; 91: 945–52. [DOI] [PubMed] [Google Scholar]
- 69.McWhirter L, Ritchie C, Stone J, Carson A. Functional cognitive disorders: a systematic review. The Lancet Psychiatry 2020; 7: 191–207. [DOI] [PubMed] [Google Scholar]
- 70.Bhome R, McWilliams A, Huntley JD, Fleming SM, Howard RJ. Metacognition in functional cognitive disorder- a potential mechanism and treatment target. Cogn Neuropsychiatry 2019; 24: 311–21. [DOI] [PubMed] [Google Scholar]
- 71.McWhirter L, King L, McClure E, Ritchie C, Stone J, Carson A. The frequency and framing of cognitive lapses in healthy adults. CNS Spectr 2021; : 1–8. [DOI] [PubMed] [Google Scholar]
- 72.Teodoro T, Edwards MJ, Isaacs JD. A unifying theory for cognitive abnormalities in functional neurological disorders, fibromyalgia and chronic fatigue syndrome: systematic review. J Neurol Neurosurg Psychiatry 2018; 89: 1308–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bhome R, Huntley JD, Price G, Howard RJ. Clinical presentation and neuropsychological profiles of Functional Cognitive Disorder patients with and without co-morbid depression. Cogn Neuropsychiatry 2019; 24: 152–64. [DOI] [PubMed] [Google Scholar]
- 74.Kanaan RA, Armstrong D, Wessely SC. Neurologists’ understanding and management of conversion disorder. J Neurol Neurosurg Psychiatry 2011; 82: 961–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bass C, Halligan P. Factitious disorders and malingering in relation to functional neurologic disorders. In: Handbook of Clinical Neurology. 2016: 509–20. [DOI] [PubMed] [Google Scholar]
- 76.Parees I, Brown H, Nuruki A, et al. Loss of sensory attenuation in patients with functional (psychogenic) movement disorders. Brain 2014; 137: 2916–21. [DOI] [PubMed] [Google Scholar]
- 77.Stone J Unfeignable biomarkers in functional neurological disorder: drifting back to Pierre Janet. Brain 2020; 143: 393–5. [DOI] [PubMed] [Google Scholar]
- 78.Perez DL, Nicholson TR, Asadi-Pooya AA, et al. Neuroimaging in Functional Neurological Disorder: State of the Field and Research Agenda. NeuroImage Clin 2021; 30: 102623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cojan Y, Waber L, Carruzzo A, Vuilleumier P. Motor inhibition in hysterical conversion paralysis. Neuroimage 2009; 47: 1026–37. [DOI] [PubMed] [Google Scholar]
- 80.Hassa T, De Jel E, Tuescher O, Schmidt R, Schoenfeld MA. Functional networks of motor inhibition in conversion disorder patients and feigning subjects. NeuroImage Clin 2016; 11: 719–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Voon V, Gallea C, Hattori N, Bruno M, Ekanayake V, Hallett M. The involuntary nature of conversion disorder. Neurology 2010; 74: 223–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nielsen G, Buszewicz M, Stevenson F, et al. Randomised feasibility study of physiotherapy for patients with functional motor symptoms. J Neurol Neurosurg Psychiatry 2017; 88: 484–90. [DOI] [PubMed] [Google Scholar]
- 83.Goldstein LH, Robinson EJ, Mellers JDC, et al. Cognitive behavioural therapy for adults with dissociative seizures (CODES): a pragmatic, multicentre, randomised controlled trial. The Lancet Psychiatry 2020; 7: 491–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Van den Bergh O, Witthöft M, Petersen S, Brown RJ. Symptoms and the body: Taking the inferential leap. Neurosci Biobehav Rev 2017; 74: 185–203. [DOI] [PubMed] [Google Scholar]
- 85.Brown RJ, Reuber M. Towards an integrative theory of psychogenic non-epileptic seizures (PNES). Clin Psychol Rev 2016; 47: 55–70. [DOI] [PubMed] [Google Scholar]
- 86.Peterson KT, Kosior R, Meek BP, Ng M, Perez DL, Modirrousta M. Right Temporoparietal Junction Transcranial Magnetic Stimulation in the Treatment of Psychogenic Nonepileptic Seizures: A Case Series. Psychosomatics 2018; 59: 601–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Spagnolo PA, Garvey M, Hallett M. A dimensional approach to functional movement disorders: Heresy or opportunity. Neurosci Biobehav Rev 2021; 127: 25–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sadnicka A, Daum C, Meppelink A-M, Manohar S, Edwards M. Reduced drift rate: a biomarker of impaired information processing in functional movement disorders. Brain 2020; 143: 674–83. [DOI] [PubMed] [Google Scholar]
- 89.Morgante F, Matinella A, Andrenelli E, et al. Pain processing in functional and idiopathic dystonia: An exploratory study. Mov Disord 2018; 33: 1340–8. [DOI] [PubMed] [Google Scholar]
- 90.Baizabal-carvallo JF, Hallett M, Jankovic J. Pathogenesis and pathophysiology of functional (psychogenic) movement disorders. Neurobiol Dis 2019; 127: 32–44. [DOI] [PubMed] [Google Scholar]
- 91.Sundararajan T, Tesar GE, Jimenez XF. Biomarkers in the diagnosis and study of psychogenic nonepileptic seizures: A systematic review. Seizure 2016; 35: 11–22. [DOI] [PubMed] [Google Scholar]
- 92.Foroughi AA, Nazeri M, Asadi-Pooya AA. Brain connectivity abnormalities in patients with functional (psychogenic nonepileptic) seizures: A systematic review. Seizure 2020; 81: 269–75. [DOI] [PubMed] [Google Scholar]
- 93.Gallucci-Neto J, Brunoni AR, Ono CR, Fiore LA, Martins Castro LH, Marchetti RL. Ictal SPECT in Psychogenic Nonepileptic and Epileptic Seizures. J Acad Consult Psychiatry 2021; 62: 29–37. [DOI] [PubMed] [Google Scholar]
- 94.Lee J-O, Lee E-S, Kim J-S, et al. Altered brain function in persistent postural perceptual dizziness: A study on resting state functional connectivity. Hum Brain Mapp 2018; 39: 3340–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nigro S, Indovina I, Riccelli R, et al. Reduced cortical folding in multi-modal vestibular regions in persistent postural perceptual dizziness. Brain Imaging Behav 2019; 13: 798–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bègue I, Adams C, Stone J, Perez DL. Structural alterations in functional neurological disorder and related conditions: a software and hardware problem? NeuroImage Clin 2019; 22: 101798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Stone J, Carson A, Hallett M. Explanation as treatment for functional neurologic disorders. In: Handbook of Clinical Neurology: Functional Neurologic Disorders. 2016: 543–53. [DOI] [PubMed] [Google Scholar]
- 98.Gelauff JM, Rosmalen JGM, Carson A, et al. Internet-based self-help randomized trial for motor functional neurologic disorder (SHIFT). Neurology 2020; 95: e1883–96. [DOI] [PubMed] [Google Scholar]
- 99.Schmidt T, Ebersbach G, Oelsner H, et al. Evaluation of Individualized Multi- Disciplinary Inpatient Treatment for Functional Movement Disorders. Mov Disord Clin Pract 2021; : mdc3.13268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Demartini B, Bombieri F, Goeta D, Gambini O, Ricciardi L, Tinazzi M. A physical therapy programme for functional motor symptoms: A telemedicine pilot study. Parkinsonism Relat Disord 2020; 76: 108–11. [DOI] [PubMed] [Google Scholar]
- 101.Nada EH, Ibraheem OA, Hassaan MR. Vestibular Rehabilitation Therapy Outcomes in Patients With Persistent Postural-Perceptual Dizziness. Ann Otol Rhinol Laryngol 2019; 128: 323–9. [DOI] [PubMed] [Google Scholar]
- 102.Nicholson C, Edwards MJ, Carson AJ, et al. Occupational therapy consensus recommendations for functional neurological disorder. J Neurol Neurosurg Psychiatry 2020; 91: 1037–45. [DOI] [PubMed] [Google Scholar]
- 103.Gutkin M, McLean L, Brown R, Kanaan RA. Systematic review of psychotherapy for adults with functional neurological disorder. J Neurol Neurosurg Psychiatry 2021; 92: 36–44. [DOI] [PubMed] [Google Scholar]
- 104.Carlson P, Nicholson Perry K. Psychological interventions for psychogenic nonepileptic seizures: A meta-analysis. Seizure 2017; 45: 142–50. [DOI] [PubMed] [Google Scholar]
- 105.Myers L, Vaidya-Mathur U, Lancman M. Prolonged exposure therapy for the treatment of patients diagnosed with psychogenic non-epileptic seizures (PNES) and post-traumatic stress disorder (PTSD). Epilepsy Behav 2017; 66: 86–92. [DOI] [PubMed] [Google Scholar]
- 106.Novakova B, Harris PR, Rawlings GH, Reuber M. Coping with stress: A pilot study of a self-help stress management intervention for patients with epileptic or psychogenic nonepileptic seizures. Epilepsy Behav 2019; 94: 169–77. [DOI] [PubMed] [Google Scholar]
- 107.Bhome R, Berry AJ, Huntley JD, Howard RJ. Interventions for subjective cognitive decline: Systematic review and meta-analysis. BMJ Open. 2018; 8: e021610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yu Y-C, Xue H, Zhang Y, Zhou J. Cognitive Behavior Therapy as Augmentation for Sertraline in Treating Patients with Persistent Postural-Perceptual Dizziness. Biomed Res Int 2018; 2018: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kuwabara J, Kondo M, Kabaya K, et al. Acceptance and commitment therapy combined with vestibular rehabilitation for persistent postural-perceptual dizziness: A pilot study. Am J Otolaryngol 2020; 41: 102609. [DOI] [PubMed] [Google Scholar]
- 110.Gilmour GS, Nielsen G, Teodoro T, et al. Management of functional neurological disorder. J Neurol 2020; 267: 2164–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Oto M, Espie C, Pelosi A, Selkirk M, Duncan R. The safety of antiepileptic drug withdrawal in patients with non-epileptic seizures. J Neurol Neurosurg Psychiatry 2005; 76: 1682–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Pick S, Hodsoll J, Stanton B, et al. Trial Of Neurostimulation In Conversion Symptoms (TONICS): a feasibility randomised controlled trial of transcranial magnetic stimulation for functional limb weakness. BMJ Open 2020; 10: e037198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Dreissen YEM, Dijk JM, Gelauff JM, et al. Botulinum neurotoxin treatment in jerky and tremulous functional movement disorders: a double-blind, randomised placebo-controlled trial with an open-label extension. J Neurol Neurosurg Psychiatry 2019; 90: 1244–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hallett M, Stone J, Carson A (eds. . Functional Neurologic Disorders: Handbook of Clinical Neurology (Volume 139). Amsterdam: Elsevier, 2016. https://www.sciencedirect.com/handbook/handbook-of-clinical-neurology/vol/139/suppl/C. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.