Abstract
As the number of cancer survivors has increased significantly over the last decades due to aging of population and development of effective cancer therapies, side effects from cancer therapies have been increasingly recognized. High-dose anthracyclines, immunotherapies, and concurrent radiation, as well as traditional cardiovascular risk factors such as smoking, hypertension, diabetes, hyperlipidemia, and obesity increase risks for unintended cardiovascular toxicity. However, these factors do not fully explain why only a subset of patients develop adverse cardiovascular sequelae from cancer therapies. Recent studies demonstrate that genetics play a substantial role in susceptibility to development of cardiovascular toxicities from cancer therapies. Common single nucleotide polymorphisms in multiple genes involved in various cellular pathways including membrane transport, stress response, and sarcomeres are recognized to increase risks for these toxicities. Pathogenic variants in the genes encoding proteins that comprise sarcomeres also contribute to cardiomyopathy following cancer therapies. Furthermore, genetic manipulations of model systems indicate mechanisms by which cardiotoxicities emerge following cancer immunomodulatory therapies. Continued efforts are needed to enable insights into cardiovascular responsiveness to these multi-targeted therapies, improve risk stratification of patients, and enable therapeutic interventions that limit these unintended adverse consequences from life-saving cancer treatments.
Keywords: Genetics, Cardiomyopathy, Cancer Therapy, Cardiotoxicity
Introduction
Prevalence of cancer has been increasing due to growth and aging of populations, and estimated number of new cancer cases is projected to reach approximately 26 million per year by 2030 [1]. Moreover, development of effective cancer therapy has led to a decline in cancer-related mortality over the past three decades [2]. As the number of cancer survivors has increased, adverse effects of cancer therapies have been increasingly recognized.
Estimates indicate that up to 10% of patients, who receive anthracyclines, one of the most widely used effective chemotherapeutic agents, develop cardiotoxicity [3]. The risks for developing cardiotoxicity escalates with the cumulative anthracycline dose [4], concomitant use of additional cardiotoxic cancer therapies such as trastuzumab and paclitaxel [5,6], chest radiation therapy [7], advanced age [8], pre-existing heart disease, and presence of cardiovascular risk factors such as diabetes or hypertension [9,10]. Based on these observations, the American Society of Clinical Oncology expert consensus guidelines propose the following patient characteristics and treatment regimens as high risks for cardiotoxicity: high-dose anthracycline (e.g., doxorubicin ≥ 250 mg/m2), high-dose radiation therapy (≥ 30 Gy) with cardiac exposure, sequential anthracycline and trastuzumab therapy, and combination low-dose anthracycline and radiation therapies in patients with underlying heart disease or ≥ 2 cardiovascular risk factors (including smoking, hypertension, diabetes, hyperlipidemia, obesity, and age ≥ 60 years) [11]. Unfortunately identifying high-risk patients with these guidelines and prediction models remains difficult. Moreover, the current parameters fail to explain why only a portion of at-risk patients develop cardiotoxicity after receiving antineoplastic agents [12-14].
Recent developments of novel cancer therapeutics have added further complexity to risk stratification of patients. Many of these new medicines target specific molecules with broad tissue expression beyond cancer cells, and thereby increase potential toxicity in patient subsets. For example, data indicates that cardiovascular toxicity occurs in 1-15% of cancer patients [15-17], who are treated with inhibitors of vascular endothelial growth factor (VEGF) (e.g., sunitinib and bevacizumab) and inhibitors of breakpoint cluster region (BCR) protein-activator of RhoGEF and GTPase (BCR)-ABL proto-oncogene 1, or the non-receptor tyrosine kinase (ABL1) (e.g., dasatinib and imatinib). The risks for toxicity from these therapies appear highest among patients with pre-existing vascular disease such as coronary artery disease or hypertension. In addition to directly damaging vascular beds, these medicines can cause myocardial dysfunction, perhaps by impairing vascular reserve and causing perfusion-contraction mismatch [18]. Together these observations imply a mechanistic link between the inhibitor’s therapeutic target and the target’s physiologic expression and function in cardiovascular tissues.
A second novel class of cancer therapies are immune checkpoint inhibitors (ICI; e.g., nivolumab and pembrolizumab that target programmed cell death 1 (PD1) and its ligand (PDL1), or ipilimumab targeting cytotoxic-T-lymphocyte-associated antigen 4 (CTLA4)). ICIs can incite myocarditis with associated QT prolongation and life-threatening arrhythmias [19,20]. Although predisposing factors for cardiotoxicities from these cancer therapies are poorly understood, experimental studies indicate key roles for PD1 and PDL1 in the maintenance of self-tolerance. In the mouse, Pdl1 is expression on cardiac vascular endothelium and induced by inflammatory stimuli [21]. By contrast, mice with biallelic genetic deletion of Pd1 or Pdl1 spontaneously develop dilated cardiomyopathy with troponin I antibodies and myocarditis, respectively [22,23]. The remarkable phenotypic resemblance between experimental models and human cardiotoxicities associated with ICIs implies that depletion of immunomodulatory molecules renders the heart susceptible to autoimmune damage.
Human genotypes provide additional opportunities, beyond clinical profiles, to further identify cancer patients at risk for cardiovascular toxicities. The discovery of genes that influence pharmacokinetics of therapeutic agents or that harbor variants that influence cardiomyocyte, vascular, and immune cell biology, combined with the rapid advances in clinical genome sequencing provide new opportunities to explore how genotypes influence adverse cardiovascular responses to cancer therapies in patients and in experimental models.
Genetic variants generally are classified into two categories based on their minor allele frequency (MAF): common genetic variants and rare monogenic variants. Common genetic variants typically have MAF > 5% and rare monogenic variants generally are defined as having MAF < 0.1%. Variant assessment is also considered in the context of disease prevalence and penetrance [24,25]. For instance, within a reference population, a variant that causes a dominant monogenic disorder would be far less than the prevalence of the disease, and even lower when the disease is incompletely penetrant. This review provides an overview of common genetic variants such as single nucleotide polymorphisms (SNPs) and rare monogenic variants that are associated with risk of developing cancer therapy-induced cardiovascular toxicity. While still emerging, these early insights are poised to improve mechanistic studies that may illuminate both pathogenesis and strategies to combat cardiovascular toxicities in cancer patients.
Common genetic variants are associated with cancer therapy-induced cardiotoxicity
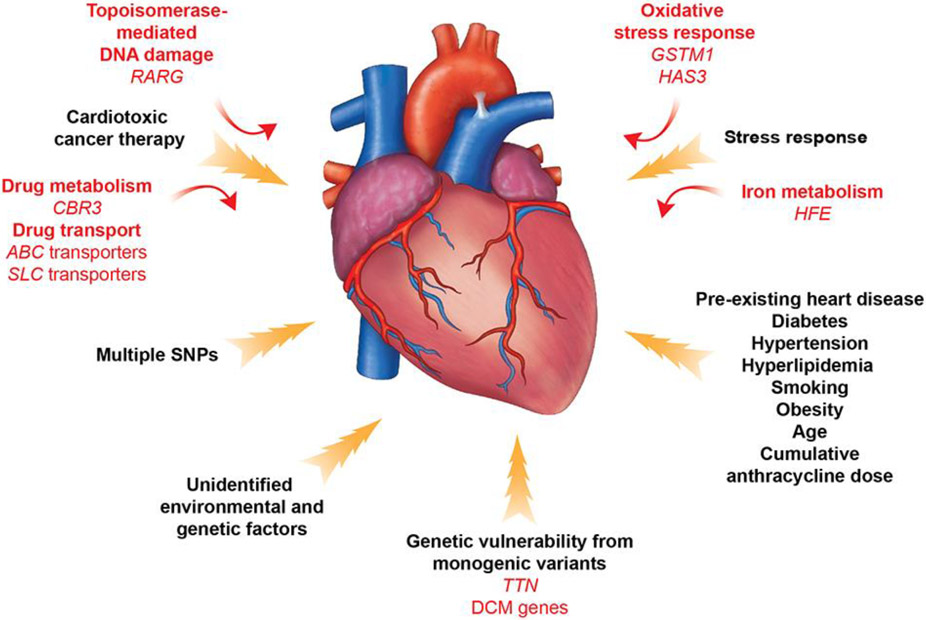
To identify common variants within the general population that convey risks for disease, DNA samples from patients are studied using genotyping arrays containing SNPs in proximity to candidate genes or arrays with SNPs across the entire genome. With the latter approach, genome-wide association studies (GWAS) are performed in combination with imputation to deduce SNPs that were not directly genotyped, using statistical analyses of haplotypes from a genotype reference panel. These strategies have identified SNPs with appreciable frequency in the general population that contribute to the pathogenesis of common disease, including diabetes and coronary artery disease [26-28]. Parallel analyses have identified SNPs in proximity to genes with encoded proteins involved in drug transport and metabolism, oxidative stress responses, topoisomerase-mediated DNA damage, and cardiac contractility that either increase or reduce risks for toxicities from cancer therapies (Figure and Table 1).
Figure. Contributors to cancer therapy-induced cardiovascular toxicity.
A combination of clinical and genetic risk factors leads to increased risk of developing toxicity upon cancer therapy treatment. Elucidation of genetic contributors of cancer therapy-induced cardiovascular toxicity facilitates understanding of its molecular mechanism and development of its therapeutic strategies.
Table 1.
Common genetic variants that are associated with risk of cancer therapy-induced cardiotoxicity
| Proposed mechanism | Gene | Reference SNP ID | Odds ratio | p-value |
|---|---|---|---|---|
| Drug transport | ABCB1 | rs1045642 [34] | 0.48 | 0.049 |
| ABCB4 | rs1149222 [33] | 1.87 | 0.005 | |
| ABCC1 | rs246221 [30] | 1.59 | 0.02 | |
| rs4148350 [33] | 3.44 | 0.001 | ||
| ABCC2 | rs8187694 [31] | 2.3 | 0.049 | |
| rs8187710 [32] | 4.33 | < 0.01 | ||
| SLC10A2 | rs9514091 [33] | 0.43 | 0.003 | |
| SLC28A3 | rs4877847 [33] | 0.60 | 0.009 | |
| rs7853758 [33] | 0.31 | 1.0 x 10e-4 | ||
| SLC22A7 | rs4149178 [36] | 0.45 | 0.001 | |
| SLC22A17 | rs4982753 [36] | 0.50 | 4.4 x 10e-4 | |
| Drug metabolism | CBR3 | rs1056892 [39] | 3.3 (G/G genotype) | 0.006 (G/G genotype) |
| Oxidative stress response | HAS3 | rs2232228 [42] | 5.2 (G/A genotype) 9.9 (A/A genotype) |
0.007 (G/A genotype) 0.002 (A/A genotype) |
| GSTM1 | N/A (Homozygous deletion) [44] | 2.7 | 0.007 | |
| Iron metabolism | HFE | rs1799945 [51] | 3.44 | 0.005 |
| Topoisomerase-mediated DNA damage | RARG | rs2229774 [57] | 4.7 | 5.9 x 10e-8 |
| Splicing of a sarcomere gene | CELF4 | rs1786814 [60] | 2.3 | 0.006 |
The most common group of genes that have been implicated in cardiovascular toxicities to date participate in drug transport. Adenosine triphosphate-binding cassette transporter (ABC) proteins serve as active cellular transporters of multiple drugs including anthracyclines [29]. Polymorphisms in this family of genes, including ABCB4 (rs1149222), ABCC1 (rs246221 and rs4148350), and ABCC2 (rs8187694 and rs8187710), are associated with increased risk of cardiac dysfunction following anthracycline therapies in pediatric and adult cancer cohorts with both hematological and solid tumors [30-33].
In contrast, a genetic variant in ABCB1 (rs1045642) appears to have cardioprotective effects (odds ratio (OR) 0.48 and p = 0.049) [34]. As this gene encodes an efflux transporter, a working model to explain protective effect is that the SNP increases drug clearance in cardiomyocytes. Genetic variants in a gene family of soluble carriers (SLCs) also show a protective influence on anthracycline-induced cardiomyopathy. These include SNPs in SLC22A7 (rs4149178; OR 0.45 and p = 0.001), SCL22A17 (rs4982753; OR 0.5 and p = 4.4 x 10e-4), SLC10A2 (rs9514091; OR 0.43 and p = 0.003) and SLC28A3 (rs4877847; OR 0.60 and p = 0.009; rs7853758; OR 0.31 and p = 1.0 x 10e-4). Magdy et al. recently recapitulated the cardioprotective effect of the SLC28A3 locus (rs7853758) in human-induced pluripotent stem cell-derived cardiomyocytes and demonstrated that the SLC competitive inhibitor desipramine is protective against doxorubicin-induced cardiotoxicity [35]. Notably, these transporter are expressed in cardiovascular cells and transport doxorubicin and nucleoside-based therapeutics as well as physiologic substrates [33,36].
Genes that function in drug metabolism can also influence the risk of cancer therapy-induced cardiovascular toxicities. For example, CBR3 encodes carbonyl reductase 3, which catalyzes reduction of anthracyclines to the cardiotoxic alcohol metabolite doxorubicinol [37]. Among childhood cancer survivors and adult patients with breast cancer, a homozygous missense variant in CBR3 (Val244Met; rs1056892) increased risk of cardiomyopathy when patients receive low to moderate doses of doxorubicin (< 250 mg/m2; OR 3.3 and p = 0.006) [38]. Additionally, this SNP was associated with cardiotoxicity in adult cohorts with breast cancer treated with anthracyclines (4 x 60 mg/m2) with or without trastuzumab [34,39]. However, among patients receiving high dose doxorubicin (≥ 250 mg/m2; OR 1.37 and p = 0.40) this SNP (CBR3 Val244Met; rs1056892) did not influence susceptibility to cardiotoxicity [38], an observation which implies that damaging pathways activated by doxorubicin are dose-dependent.
Reactive oxygen species mediate pathological response to many common cardiovascular disorders [40]. Metabolites of anthracyclines also generate excessive reactive oxygen species and oxygen free radicals [41]. Recognizing that these shared signals may account for the clinical association between underlying cardiovascular disorders and increased risks for cancer therapy toxicities, Wang et al. studied SNPs associated with 2,100 curated cardiovascular disease genes. These analyses identified a SNP in hyaluronan synthase 3 (HAS3; rs2232228) that was significantly associated with cardiomyopathy following high doses of anthracyclines (> 250 mg/m2; OR = 5.2 and p = 0.007 for G/A genotype; OR 9.9 and p = 0.002 for A/A genotype) [42]. HAS3 encodes an enzyme involved in the synthesis of hyaluronan, a component of extracellular matrix that serves as a scaffold for organizing the cardiac cells, particularly during remodeling after injury. Hyaluronan also has anti-oxidant properties that promotes cardiac survival from oxidative stresses that can incite myocardial dysfunction [43]. Notably, human heart samples with the rs2232228 A/A genotype have markedly decreased expression (76%) of HAS3 mRNA levels in comparison to hearts with the heterozygous G/A genotype, albeit without achieving statistical significance [42]. Thus, the HAS3 A/A genotype and associated lower hyaluronan levels could increase sensitivity to reactive oxygen species after anthracycline exposure and greater risk for cardiotoxicity.
A recent study also suggested that another detoxification enzyme and free radical scavenger, glutathione S-transferase Mu-1 (GSTM1), is associated with cancer therapy-induced cardiotoxicity. GSTM1 genotypes in 75 subjects with and 92 subjects without cardiotoxicity showed a significant association between homozygous deletion of GSTM1 and cancer therapy-related cardiomyopathy (OR 2.7 and p = 0.007). Concurrent studies of human-induced pluripotent stem cell-derived cardiomyocytes from childhood cancer survivors with anthracycline-induced cardiomyopathy confirmed reduced GSTM1 expression [44].
Inadequate protection of the heart from stress can ultimately result in cell death, an outcome that emerges from regulated signaling pathways that activate apoptosis, necrosis, or autophagy [45]. By contrast, the chemical properties of anthracyclines trigger cell death through ferroptosis, a pathway characterized by iron accumulation that induces lethal lipid peroxidation and mitochondrial dysfunction and demise [46-48]. Anthracyclines bind iron and impair iron metabolism, while dexrazoxane, a derivative of EDTA that chelates iron and reduces anthracycline-iron complexes, limits anthracycline toxicities [49,50]. Genetic analyses support the importance of these biochemical interactions. HFE encodes a cell surface molecule that regulates iron uptake and is mutated in the iron-storage disorder hemochromatosis. A missense SNP in HFE (His63Asp; rs1799945) is significantly enriched (p < 0.005) among anthracycline-treated breast cancer patients, who developed cardiomyopathy compared to those without cardiotoxicity [51]. Moreover, anthracyclines significantly increased iron accumulation across multiple organs in mice lacking Hfe, causing greater mitochondrial damage and earlier death compared to untreated mutant mice [52].
Anthracyclines also trigger cell death by forming the ternary complex with DNA and topoisomerase II isoenzymes, which introduce single or double strand DNA breaks [53]. Topoisomerase IIα (TOP2A) is active during the cell cycle and is highly expressed in dividing tumor cells, but when bound by anthracyclines, cell proliferation is inhibited. Topoisomerase IIβ (TOP2B) is expressed in quiescent cells including adult cardiomyocytes [54]. Anthracyclines bound to TOB2B suppress expression of the peroxisome proliferator-activated receptor, impair mitochondrial oxidative metabolism, and activate multifaceted downstream abnormalities. Cardiac-specific deletion of Top2b in mice are resistant to doxorubicin-induced DNA double-strand breaks and were protected from development of heart failure [55]. Notably, an additional cardioprotective effect of dexrazoxane includes proteasomal degradation of Top2b [56].
TOP2B expression likely provides the mechanism by which SNPs in the retinoic acid receptor g (RARG) convey risks for anthracycline-induced cardiotoxicity risk. A GWAS of 280 childhood cancer patients, including 32 with cardiotoxicity, identified a RARG missense variant (Ser247Leu; rs2229774) that conveyed increased risk (OR 4.7 and p = 5.9 x 10e-8) and was confirmed in a replication cohort [57]. Ligand-activated retinoic acid receptors are known to bind DNA regulatory elements and alter the expression of multiple downstream genes [58]. Experimental studies showed that wild-type Rarg protein expressed in a rodent cardiomyoblast line repressed Top2b levels; while Rarg protein harboring the Ser247Leu variant attenuated Top2b repression [57]. Together these indicated that RARG SNPs convey risk for cardiotoxicity by influencing TOP2B expression.
Genotypes that influence the structure of sarcomeres, the basic contractile units of cardiomyocytes, can also contribute to the development of cardiotoxicity after cancer therapies. A polymorphism in the RNA-binding protein, CUGBP Elav-like family member 4 (CELF4), which regulates developmental splicing of the sarcomere thin filament gene encoding cardiac troponin T (TNNT2) [59] was identified in a GWAS of childhood cancer survivors. The CELF4 SNP (rs1786814) was enriched in cancer survivors with cardiomyopathy (n = 112) and compared to those without (n = 219) cardiomyopathy (OR 2.3 and p = 0.006) [60]. Strikingly, OR increased to 10.2 (p < 0.001) in subjects with the G/G genotype compared to the G/A or A/A genotypes among patients, who received more than 300 mg/m2 of anthracyclines, demonstrating a dose-dependent association. This study further assessed the impact of the CELF4 SNP on TNNT2 splicing. Among 33 human hearts with the rs1786814 genotype, both the embryonic and adult TNNT2 isoforms were identified, an admixture that perturbed calcium responses and diminished contractility, thereby implying a direct mechanism by which this SNP could increase risk of cardiac dysfunction after chemotherapy.
Rare pathogenic variants increase risk of cancer therapy-induced cardiotoxicity
Hypertrophic cardiomyopathy (HCM), dilated cardiomyopathy (DCM), and arrhythmogenic cardiomyopathy (ACM) are monogenic disorders that are caused by damaging variants within protein coding sequences. A genetic etiology accounts for approximately 30-50% of HCM and 10-30% of DCM patients [61,62]. Pathogenic variants that cause HCM typically occur in eight sarcomere genes encoding: cardiac myosin binding protein C (MYBPC3), β-myosin heavy chain (MYH7), myosin regulatory light chain (MYL2), myosin essential light chain (MYL3), cardiac troponin I (TNNI3), TNNT2, α-tropomyosin (TPM1), and cardiac α -actin (ACTC1) [63-67]. However, pathogenic variants predominate in MYBPC3 and MYH7, accounting for up to 50% of all HCM cases and 75% of cases with a defined genetic cause [62,68,69].
Genetic causes of DCM are more varied and scores of putative DCM genes have been reported [70]. Despite this considerable genetic heterogeneity, causal variants are most often identified in a subset of all DCM genes. Pathogenic variants in gene titin (TTN) is the most common genetic cause of adult-onset DCM, accounting for 15 to 20% of the affected individuals, but are rarely identified in childhood-onset DCM [71,72]. Titin is a giant protein that spans a half of a sarcomere and serves as a scaffold to support formation of the contractile apparatus and also as a molecular spring that influences contractility and relaxation. Pathogenic variants in TTN that cause DCM by creating premature truncation of the protein are denoted TTNtv [73]. The second most common genetic cause of DCM are rare pathogenic variants in LMNA, which encodes a ubiquitously expressed inner nuclear membrane protein, lamin A/C, that participates in the maintenance of nuclear structure [74]. LMNA variants account for approximately 6% of DCM cases and have a higher prevalence among DCM patients with cardiac conduction abnormalities [75].
HCM and ACM can cause variable symptoms late in childhood, adolescence, or not until adulthood while DCM most typically presents in adults. The considerable variability in the age of onset and severity of manifestations contributes to the lack of clinical recognition of pathogenic variants in asymptomatic individuals. However, additional stresses can unmask cardiac dysfunction that results from these variants. For example, in addition to causing DCM, pathogenic variants in TTN contribute to ~15% of patients with peripartum cardiomyopathy [76] and ~10% of patients alcohol-induced cardiomyopathy [77].
These insights have propelled analyses of selected cardiomyopathy genes in patients with cancer therapy cardiotoxicity. In 2010, van den Bert et al. reported sequencing analysis of LMNA, TNNT2, and MYH7 in six DCM families that included a cancer patient with anthracycline-associated cardiomyopathy. Two cancer patients were found to have a pathogenic missense variant and a premature stop codon in MYH7 [78]. In follow-up analysis of 48 cardiomyopathy genes, 11 missense variants were identified in patients with cancer therapy cardiotoxicities [79] Although nine of 11 these missense residues are now classified as variants of unknown significance based on large normative databases that provide more accurate population-based variant prevalence, these studies support the potential role for monogenic variants in the genetic susceptibility to cardiotoxicity after cancer therapy. Subsequent analysis that harnessed next-generation sequencing of cardiomyopathy genes identified TTNtv in two breast cancer patients, who developed cardiomyopathy five to seven months after receiving either doxorubicin alone or combined with trastuzumab [80].
Our group undertook genetic analysis of three independent cohorts comprised of 213 adult and pediatric patients with cardiotoxicities after treatment for solid and hematological malignancies [81]. Two of these three cohorts had cardiac assessments to ensure normal function before the initiation of cancer therapies. We prioritized the analysis of nine DCM genes including BCL2-associated athanogene-3 (BAG3), desmoplakin (DSP), LMNA, MYH7, sodium voltage-gated channel, alpha subunit 5 (SCN5A), telethonin (TCAP), cardiac troponin C (TNNC1), TNNT2, and TTN. These studies revealed a significant enrichment of TTNtv among these patients (7.5%) compared to healthy volunteers (0.7% with p = 3.4 x 10e-6) and the Cancer Genome Atlas participants (1.1% with p = 7.4 x 10e-8). Furthermore, the adult cancer patients with TTNtv experienced worse clinical outcomes, including a higher prevalence of heart failure, atrial fibrillation (p = 0.003), and impaired myocardial recovery (p = 0.03) than those without TTNtv.
To further confirm a significant role of TTNtv in development of cardiotoxicity after cancer therapy we treated mice that harbor a heterozygous truncating variant in Ttn (Ttntv/+) with low-dose anthracycline. Both wild-type and Ttntv/+ mice developed cardiac dysfunction within 4 weeks after anthracycline administration. Cardiac function recovered to baseline in wild-type mice by week 8 after anthracycline exposure, but it remained depressed through week 12 in Ttntv/+ mice.
These human and experimental data indicate that a seemingly healthy myocardium with genetic vulnerability can succumb to cardiovascular stress in the context of cardiotoxic cancer therapies (Figure). The widespread availability of clinical genetic testing for cardiomyopathies could allow the identification of these high-risk patients in advance of cancer treatments.
Mechanisms and genetic contributors to cardiovascular toxicities from targeted cancer therapies (Table 2) are areas of active research. Human epidermal growth factor receptor 2 (HER2) is amplified in 25-30% of breast cancers and overexpression is associated with increased tumor aggressiveness [82,83]. Trastuzumab is a monoclonal antibody targeting Erb-B2 receptor tyrosine kinase 2 (commonly called HER2), which is over expressed in multiple malignancies, including breast cancers. When combined with traditional treatments of anthracycline and cyclophosphamide, trastuzumab has demonstrated significantly improved clinical efficacy. However, 27% of patients receiving this therapeutic triad develop cardiac dysfunction [84]. Unexpectedly, cardiac-specific deletion of Her2 in mice demonstrated chamber dilation, wall thinning and decreased contractility, and isolated cardiomyocytes were more susceptible to anthracycline toxicity [85]. As neuregulin, which is released from endothelial cells in response to cardiac stresses binds Her2 and activates compensatory responses [86], trastuzumab-mediated inactivation of HER2 may attenuate pro-survival mechanisms in the heart.
Table 2.
Experimental models to establish mechanisms of cardiovascular toxicities from targeted cancer therapies
| Cancer therapy | Target | Experimental model | Phenotype |
|---|---|---|---|
| Trastuzumab | HER2 | Cardiac-specific deletion of Her2 in mice [85] | Dilated cardiomyopathy |
| Immune checkpoint inhibitors | CTLA | Deletion of Ctla4 in mice [90] Monoallelic loss of Ctla4 in Pd1 null mice [93] |
Fatal autoimmune myocarditis |
| PD1 PDL1 | Deletion of Pd1 in mice [91,92] | Fulminant myocarditis with heart failure and deposition of auto-antibodies to troponin I |
The incidence of cardiotoxicities from ICIs including ipilimumab (targeting CTLA4) and nivolumab and pembrolizumab (targeting PD1 and PDL1) are not well established. A retrospective safety database review by the manufacturer of nivolumab and ipilimumab reported myocarditis in 0.27% of treated patients [19], while a meta-review of case reports identified takotsubo cardiomyopathy in 9.7% of patients receiving ICIs [87]. Pericardial disease, vasculitis, and arrhythmias can also occur and like myocardial toxicities, these are associated with high morbidity and mortality [88,89].
Cancer immunotherapies target the adaptive immune system, which is physiologically controlled by balancing stimulatory and inhibitory pathways that adequately mount an immune response to fight infection yet avoid autoimmunity with unintended tissue inflammation and damage. Presumably, these powerful agents shift the homeostatic balance and result in excessive immune system activation that attacks multiple organs including the cardiovascular system. Experimental model systems to study these mechanisms support this hypothesis. Genetic deletion of Ctla4 in mice results in fatal autoimmune myocarditis, mediated by CD8 T-cells [90]. Deletion of Pd1 caused fulminant myocarditis with heart failure and deposition of autoantibodies to troponin I in wild-type mice, but not mice lacking Rag2, a required gene for immunoglobulin recombination [91,92]. Furthermore, a recent study demonstrated that monoallelic deletion of Ctla4 in the absence of Pd1 leads to development myocarditis, suggesting interaction between Ctla4 and Pd1 in a gene dosage-dependent manner [93]. This result supports clinical observation that incidence of myocarditis is increased when combination ICI therapy is used [19,20]. Understanding these unintended signals for excessive immunoactivation in experimental models and human patients will be critical to establish strategies to reduce these cardiotoxicities.
Summary and future directions
Genetic studies both in humans and animal model systems have provided insights into a mechanism of cancer therapy-induced cardiotoxicity and opportunities to optimize care of patients, who are undergoing cancer treatment. It is currently postulated that multiple genes from distinct, but intertwined pathways, which transport and metabolize drugs, mediate cellular stress response, and regulate myocardial contractile function work together to maintain homeostasis of cardiomyocytes upon cancer-therapy treatment. While these insights are largely from studies of adult cancer patients, emerging analyses of pediatric cases identify the same genes or genes with similar functions. Notably, common SNPs and rare monogenic variants likely play an important role in pathogenesis of not only cancer therapy-induced cardiomyopathy, but also DCM by lowering a threshold when vulnerable myocardium develops dysfunction. Therefore, discovery of the genes that are associated with increased risk of cancer therapy-induced cardiomyopathy will help facilitate elucidation of its pathogenesis and that of DCM and may accelerate establishment of their therapeutic strategies.
The recognition of common and rare monogenic variants that influence susceptibility to cardiomyopathy after receiving cardiotoxic cancer therapies can improve cardiovascular risk stratification of cancer patients. There has been growing interest and attempt to establish risk prediction models using both clinical and genetic variables to provide personalized treatment plan for cancer patients [33,36]. One prediction model, which was built using 5 SNPs from GWAS studies (rs7853758, rs17863783, rs10426377, rs2305364, and rs4148808) and clinical risk factors such as a high cumulative dose of anthracyclines and concurrent radiation therapy has been shown to have area under the curve (AUC) equaling 0.77 [94]. Another prediction model, which was established by also combining clinical and genetic risk factors showed better performance (AUC 0.79) than using clinical (AUC 0.69) or genetic (AUC 0.67) models alone [32]. Identification of further genetic risk factors in addition to clinical risk factors will facilitate establishment of a better prediction score model and improve its predictability for cancer therapy-induced cardiomyopathy.
Genetic susceptibility not only increases risk of cardiotoxicity, but also worsens clinical outcome. For instance, cancer patients harboring TTNtv experienced more heart failure (p = 0.003), atrial fibrillation (p = 0.003), and impaired myocardial function recovery (p = 0.03) compared to those without these variants [81]. Individualization of cancer treatment plan and cardiovascular screening before, during, and after cancer therapy for each patient based on their genetic predisposition may improve clinical outcome and needs further investigation.
In summary, genetic predisposition plays an essential role in both development and clinical outcomes of cardiovascular toxicities following cancer therapies. However, our ability to predict who will develop cancer therapy-induced cardiotoxicity and to personalize the cancer treatment based on known genetic and clinical risk factors remains limited. Ongoing investigation with efforts to identify additional genetic risk factors will enhance identification of cancer patients, who are at the highest risk for adverse cardiovascular outcomes and ultimately improve their clinical outcome by co-optimizing cancer therapies and proactive interventions to minimize or prevent adverse cardiovascular sequalae.
Highlights.
Survivors of cancer therapies have life-long increased risks for heart disease.
Both common and rare damaging gene variants convey cardiotoxicity risks.
Emerging cancer immunotherapies can evoke cardiotoxic autoimmunity.
Improving cancer care requires identification and treatment of cardiotoxicity risks.
Acknowledgements
This work was support in part by grants from the Brigham and Women’s Hospital (Khoury Innovation Award to Y.K.), NIH (KL2 TR002542 to Y.K, HL080494 and HL084553 to J.G.S. and C.E.S) and the Howard Hughes Medical Institute (C.E.S.).
Footnotes
Declaration of competing interest
J.G.S. and C.E.S are founders of Myokardia (a Bristol-Myers-Squibb Subsidiary) and Consultants for Maze and BridgeBio. C.E.S serves on the Merck Board of Directors. None of these companies provided any support or input into this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Thun MJ, DeLancey JO, Center MM, Jemal A, Ward EM, The global burden of cancer: priorities for prevention, Carcinogenesis. 31 (2010) 100–110. 10.1093/carcin/bgp263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Siegel RL, Miller KD, Jemal A, Cancer statistics, 2020, CA: A Cancer Journal for Clinicians. 70 (2020) 7–30. 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- [3].Cardinale Daniela, Colombo Alessandro, Bacchiani Giulia, Tedeschi Ines, Meroni Carlo A., Veglia Fabrizio, et al. , Early Detection of Anthracycline Cardiotoxicity and Improvement With Heart Failure Therapy, Circulation. 131 (2015) 1981–1988. 10.1161/CIRCULATIONAHA.114.013777. [DOI] [PubMed] [Google Scholar]
- [4].Von Hoff DD, Layard MW, Basa P, Davis HL, Von Hoff AL, Rozencweig M, et al. , Risk factors for doxorubicin-induced congestive heart failure, Ann Intern Med. 91 (1979) 710–717. 10.7326/0003-4819-91-5-710. [DOI] [PubMed] [Google Scholar]
- [5].Slamon D, Eiermann W, Robert N, Pienkowski T, Martin M, Press M, et al. , Adjuvant trastuzumab in HER2-positive breast cancer, N Engl J Med. 365 (2011) 1273–1283. 10.1056/NEJMoa0910383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Gianni L, Munzone E, Capri G, Fulfaro F, Tarenzi E, Villani F, et al. , Paclitaxel by 3-hour infusion in combination with bolus doxorubicin in women with untreated metastatic breast cancer: high antitumor efficacy and cardiac effects in a dose-finding and sequence-finding study, J Clin Oncol. 13 (1995) 2688–2699. 10.1200/JCO.1995.13.11.2688. [DOI] [PubMed] [Google Scholar]
- [7].Steinherz LJ, Steinherz PG, Tan CT, Heller G, Murphy ML, Cardiac toxicity 4 to 20 years after completing anthracycline therapy, JAMA. 266 (1991) 1672–1677. [PubMed] [Google Scholar]
- [8].Swain SM, Whaley FS, Ewer MS, Congestive heart failure in patients treated with doxorubicin: a retrospective analysis of three trials, Cancer. 97 (2003) 2869–2879. 10.1002/cncr.11407. [DOI] [PubMed] [Google Scholar]
- [9].Hershman DL, McBride RB, Eisenberger A, Tsai WY, Grann VR, Jacobson JS, Doxorubicin, cardiac risk factors, and cardiac toxicity in elderly patients with diffuse B-cell non-Hodgkin’s lymphoma, J Clin Oncol. 26 (2008) 3159–3165. 10.1200/JCO.2007.14.1242. [DOI] [PubMed] [Google Scholar]
- [10].Lotrionte M, Biondi-Zoccai G, Abbate A, Lanzetta G, D’Ascenzo F, Malavasi V, et al. , Review and Meta-Analysis of Incidence and Clinical Predictors of Anthracycline Cardiotoxicity, The American Journal of Cardiology. 112 (2013) 1980–1984. 10.1016/j.amjcard.2013.08.026. [DOI] [PubMed] [Google Scholar]
- [11].Armenian SH, Lacchetti C, Barac A, Carver J, Constine LS, Denduluri N, et al. , Prevention and Monitoring of Cardiac Dysfunction in Survivors of Adult Cancers: American Society of Clinical Oncology Clinical Practice Guideline, J Clin Oncol. 35 (2017) 893–911. 10.1200/JCO.2016.70.5400. [DOI] [PubMed] [Google Scholar]
- [12].Ezaz Ghideon, Long Jessica B., Gross Cary P., Chen Jersey, Risk Prediction Model for Heart Failure and Cardiomyopathy After Adjuvant Trastuzumab Therapy for Breast Cancer, Journal of the American Heart Association. 3 (n.d.) e000472. 10.1161/JAHA.113.000472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Chow EJ, Chen Y, Kremer LC, Breslow NE, Hudson MM, Armstrong GT, et al. , Individual prediction of heart failure among childhood cancer survivors, J Clin Oncol. 33 (2015) 394–402. 10.1200/JCO.2014.56.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Upshaw JN, Ruthazer R, Miller KD, Parsons SK, Erban JK, O’Neill AM, et al. , Personalized Decision Making in Early Stage Breast Cancer: Applying Clinical Prediction Models for Anthracycline Cardiotoxicity and Breast Cancer Mortality Demonstrates Substantial Heterogeneity of Benefit-Harm Trade-off, Clin Breast Cancer. 19 (2019) 259–267.e1. 10.1016/j.clbc.2019.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Choueiri TK, Mayer EL, Je Y, Rosenberg JE, Nguyen PL, Azzi GR, et al. , Congestive Heart Failure Risk in Patients With Breast Cancer Treated With Bevacizumab, JCO. 29 (2011) 632–638. 10.1200/JCO.2010.31.9129. [DOI] [PubMed] [Google Scholar]
- [16].Telli ML, Witteles RM, Fisher GA, Srinivas S, Cardiotoxicity associated with the cancer therapeutic agent sunitinib malate, Ann Oncol. 19 (2008) 1613–1618. 10.1093/annonc/mdn168. [DOI] [PubMed] [Google Scholar]
- [17].Atallah E, Durand J-B, Kantarjian H, Cortes J, Congestive heart failure is a rare event in patients receiving imatinib therapy, Blood. 110 (2007) 1233–1237. 10.1182/blood-2007-01-070144. [DOI] [PubMed] [Google Scholar]
- [18].Touyz RM, Herrmann J, Cardiotoxicity with vascular endothelial growth factor inhibitor therapy, Npj Precision Onc. 2 (2018) 1–11. 10.1038/s41698-018-0056-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Johnson DB, Balko JM, Compton ML, Chalkias S, Gorham J, Xu Y, et al. , Fulminant Myocarditis with Combination Immune Checkpoint Blockade, New England Journal of Medicine. 375 (2016) 1749–1755. 10.1056/NEJMoa1609214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Mahmood SS, Fradley MG, Cohen JV, Nohria A, Reynolds KL, Heinzerling LM, et al. , Myocarditis in Patients Treated With Immune Checkpoint Inhibitors, J Am Coll Cardiol. 71 (2018) 1755–1764. 10.1016/j.jacc.2018.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Grabie N, Gotsman I, DaCosta R, Pang H, Stavrakis G, Butte MJ, et al. , Endothelial programmed death-1 ligand 1 (PD-L1) regulates CD8+ T-cell mediated injury in the heart, Circulation. 116 (2007) 2062–2071. 10.1161/CIRCULATIONAHA.107.709360. [DOI] [PubMed] [Google Scholar]
- [22].Lucas JA, Menke J, Rabacal WA, Schoen FJ, Sharpe AH, Kelley VR, Programmed death ligand 1 regulates a critical checkpoint for autoimmune myocarditis and pneumonitis in MRL mice, J Immunol. 181 (2008) 2513–2521. 10.4049/jimmunol.181.4.2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Nishimura H, Okazaki T, Tanaka Y, Nakatani K, Hara M, Matsumori A, et al. , Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice, Science. 291 (2001) 319–322. 10.1126/science.291.5502.319. [DOI] [PubMed] [Google Scholar]
- [24].Tayal U, Prasad S, Cook SA, Genetics and genomics of dilated cardiomyopathy and systolic heart failure, Genome Medicine. 9 (2017) 20. 10.1186/s13073-017-0410-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Whiffin N, Minikel E, Walsh R, O’Donnell-Luria AH, Karczewski K, Ing AY, et al. , Using high-resolution variant frequencies to empower clinical genome interpretation, Genetics in Medicine. 19 (2017) 1151–1158. 10.1038/gim.2017.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Morris AP, Voight BF, Teslovich TM, Ferreira T, Segrè AV, Steinthorsdottir V, et al. , Large-scale association analysis provides insights into the genetic architecture and pathophysiology of type 2 diabetes, Nat Genet. 44 (2012) 981–990. 10.1038/ng.2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Voight BF, Scott LJ, Steinthorsdottir V, Morris AP, Dina C, Welch RP, et al. , Twelve type 2 diabetes susceptibility loci identified through large-scale association analysis, Nat Genet. 42 (2010) 579–589. 10.1038/ng.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Wellcome Trust Case Control Consortium, Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls, Nature. 447 (2007) 661–678. 10.1038/nature05911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Januchowski R, Wojtowicz K, Andrzejewska M, Zabel M, Expression of MDR1 and MDR3 gene products in paclitaxel-, doxorubicin- and vincristine-resistant cell lines, Biomed Pharmacother. 68 (2014) 111–117. 10.1016/j.biopha.2013.09.004. [DOI] [PubMed] [Google Scholar]
- [30].Vulsteke C, Pfeil AM, Maggen C, Schwenkglenks M, Pettengell R, Szucs TD, et al. , Clinical and genetic risk factors for epirubicin-induced cardiac toxicity in early breast cancer patients, Breast Cancer Res Treat. 152 (2015) 67–76. 10.1007/s10549-015-3437-9. [DOI] [PubMed] [Google Scholar]
- [31].Wojnowski L, Kulle B, Schirmer M, Schlüter G, Schmidt A, Rosenberger A, et al. , NAD(P)H oxidase and multidrug resistance protein genetic polymorphisms are associated with doxorubicin-induced cardiotoxicity, Circulation. 112 (2005) 3754–3762. 10.1161/CIRCULATIONAHA.105.576850. [DOI] [PubMed] [Google Scholar]
- [32].Armenian SH, Ding Y, Mills G, Sun C, Venkataraman K, Wong FL, et al. , Genetic susceptibility to anthracycline-related congestive heart failure in survivors of haematopoietic cell transplantation, Br J Haematol. 163 (2013) 205–213. 10.1111/bjh.12516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Visscher H, Ross CJD, Rassekh SR, Barhdadi A, Dubé M-P, Al-Saloos H, et al. , Pharmacogenomic Prediction of Anthracycline-Induced Cardiotoxicity in Children, JCO. 30 (2011) 1422–1428. 10.1200/JCO.2010.34.3467. [DOI] [PubMed] [Google Scholar]
- [34].Hertz DL, Caram MV, Kidwell KM, Thibert JN, Gersch C, Seewald NJ, et al. , Evidence for association of SNPs in ABCB1 and CBR3, but not RAC2, NCF4, SLC28A3 or TOP2B, with chronic cardiotoxicity in a cohort of breast cancer patients treated with anthracyclines, Pharmacogenomics. 17 (2016) 231–240. 10.2217/pgs.15.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Magdy T, Jouni M, Kuo H-H, Weddle CJ, Lyra-Leite D, Fonoudi H, et al. , Identification of Drug Transporter Genomic Variants and Inhibitors That Protect Against Doxorubicin-Induced Cardiotoxicity, Circulation. 145 (2022) 279–294. 10.1161/CIRCULATIONAHA.121.055801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Visscher H, Rassekh SR, Sandor GS, Caron HN, van Dalen EC, Kremer LC, et al. , Genetic variants in SLC22A17 and SLC22A7 are associated with anthracycline-induced cardiotoxicity in children, Pharmacogenomics. 16 (2015) 1065–1076. 10.2217/pgs.15.61. [DOI] [PubMed] [Google Scholar]
- [37].Schaupp CM, White CC, Merrill GF, Kavanagh TJ, Metabolism of Doxorubicin to the Cardiotoxic Metabolite Doxorubicinol Is Increased in a Mouse Model of Chronic Glutathione Deficiency: A Potential Role for Carbonyl Reductase 3, Chem Biol Interact. 234 (2015) 154–161. 10.1016/j.cbi.2014.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Blanco JG, Sun C-L, Landier W, Chen L, Esparza-Duran D, Leisenring W, et al. , Anthracycline-related cardiomyopathy after childhood cancer: role of polymorphisms in carbonyl reductase genes--a report from the Children’s Oncology Group, J Clin Oncol. 30 (2012) 1415–1421. 10.1200/JCO.2011.34.8987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Serie D, Crook J, Necela B, Dockter T, Wang X, Asmann Y, et al. , Genome-wide association study of cardiotoxicity in NCCTG N9831 (Alliance) Adjuvant Trastuzumab Trial, Pharmacogenet Genomics. 27 (2017) 378–385. 10.1097/FPC.0000000000000302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Forrester SJ, Kikuchi DS, Hernandes MS, Xu Q, Griendling KK, Reactive Oxygen Species in Metabolic and Inflammatory Signaling, Circulation Research. 122 (2018) 877–902. 10.1161/CIRCRESAHA.117.311401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Doroshow JH, Effect of anthracycline antibiotics on oxygen radical formation in rat heart, Cancer Res. 43 (1983) 460–472. [PubMed] [Google Scholar]
- [42].Wang X, Liu W, Sun C-L, Armenian SH, Hakonarson H, Hageman L, et al. , Hyaluronan synthase 3 variant and anthracycline-related cardiomyopathy: a report from the children’s oncology group, J Clin Oncol. 32 (2014) 647–653. 10.1200/JCO.2013.50.3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].NOBLE PW, LIANG J, JIANG D, Hyaluronan as an Immune Regulator in Human Diseases, Physiol Rev. 91 (2011) 221–264. 10.1152/physrev.00052.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Singh P, Wang X, Hageman L, Chen Y, Magdy T, Landier W, et al. , Association of GSTM1 null variant with anthracycline-related cardiomyopathy after childhood cancer—A Children’s Oncology Group ALTE03N1 report, Cancer. 126 (2020) 4051–4058. 10.1002/cncr.32948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Kist M, Vucic D, Cell death pathways: intricate connections and disease implications, EMBO J. 40 (2021) e106700. 10.15252/embj.2020106700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Fang X, Wang H, Han D, Xie E, Yang X, Wei J, et al. , Ferroptosis as a target for protection against cardiomyopathy, PNAS. 116 (2019) 2672–2680. 10.1073/pnas.1821022116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Ichikawa Y, Ghanefar M, Bayeva M, Wu R, Khechaduri A, Prasad SVN, et al. , Cardiotoxicity of doxorubicin is mediated through mitochondrial iron accumulation, J Clin Invest. 124 (2014)617–630. 10.1172/JCI72931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Tadokoro T, Ikeda M, Ide T, Deguchi H, Ikeda S, Okabe K, et al. , Mitochondriadependent ferroptosis plays a pivotal role in doxorubicin cardiotoxicity, JCI Insight. 5 (2020). 10.1172/jci.insight.132747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Ichikawa Y, Ghanefar M, Bayeva M, Wu R, Khechaduri A, Prasad SVN, et al. , Cardiotoxicity of doxorubicin is mediated through mitochondrial iron accumulation, J Clin Invest. 124 (2014)617–630. 10.1172/JCI72931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Swain SM, Vici P, The current and future role of dexrazoxane as a cardioprotectant in anthracycline treatment: expert panel review, J Cancer Res Clin Oncol. 130 (2004) 1–7. 10.1007/s00432-003-0498-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Vaitiekus D, Muckiene G, Vaitiekiene A, Sereikaite L, Inciuraite R, Insodaite R, et al. , HFE Gene Variants’ Impact on Anthracycline-Based Chemotherapy-Induced Subclinical Cardiotoxicity, Cardiovasc Toxicol. 21 (2021) 59–66. 10.1007/s12012-020-09595-1. [DOI] [PubMed] [Google Scholar]
- [52].Miranda CJ, Makui H, Soares RJ, Bilodeau M, Mui J, Vali H, et al. , Hfe deficiency increases susceptibility to cardiotoxicity and exacerbates changes in iron metabolism induced by doxorubicin, Blood. 102 (2003) 2574–2580. 10.1182/blood-2003-03-0869. [DOI] [PubMed] [Google Scholar]
- [53].Nitiss JL, DNA topoisomerase II and its growing repertoire of biological functions, Nat Rev Cancer. 9 (2009) 327–337. 10.1038/nrc2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Capranico G, Tinelli S, Austin CA, Fisher ML, Zunino F, Different patterns of gene expression of topoisomerase II isoforms in differentiated tissues during murine development, Biochimica et Biophysica Acta (BBA) - Gene Structure and Expression. 1132 (1992) 43–48. 10.1016/0167-4781(92)90050-A. [DOI] [PubMed] [Google Scholar]
- [55].Zhang S, Liu X, Bawa-Khalfe T, Lu L-S, Lyu YL, Liu LF, et al. , Identification of the molecular basis of doxorubicin-induced cardiotoxicity, Nature Medicine. 18 (2012) 1639–1642. 10.1038/nm.2919. [DOI] [PubMed] [Google Scholar]
- [56].Lyu YL, Kerrigan JE, Lin C-P, Azarova AM, Tsai Y-C, Ban Y, et al. , Topoisomerase IIβ–Mediated DNA Double-Strand Breaks: Implications in Doxorubicin Cardiotoxicity and Prevention by Dexrazoxane, Cancer Res. 67 (2007) 8839–8846. 10.1158/0008-5472.CAN-07-1649. [DOI] [PubMed] [Google Scholar]
- [57].Aminkeng F, Bhavsar AP, Visscher H, Rassekh SR, Li Y, Lee JW, et al. , A Coding Variant in RARG Confers Susceptibility to Anthracycline-Induced Cardiotoxicity in Childhood Cancer, Nat Genet. 47 (2015) 1079–1084. 10.1038/ng.3374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Tang Q, Chen Y, Meyer C, Geistlinger T, Lupien M, Wang Q, et al. , A Comprehensive View of Nuclear Receptor Cancer Cistromes, Cancer Res. 71 (2011) 6940–6947. 10.1158/0008-5472.CAN-11-2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Ladd AN, Charlet-B N, Cooper TA, The CELF Family of RNA Binding Proteins Is Implicated in Cell-Specific and Developmentally Regulated Alternative Splicing, Molecular and Cellular Biology. 21 (2001) 1285–1296. 10.1128/MCB.21.4.1285-1296.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Wang X, Sun C-L, Quiñones-Lombraña A, Singh P, Landier W, Hageman L, et al. , CELF4 Variant and Anthracycline-Related Cardiomyopathy: A Children’s Oncology Group Genome-Wide Association Study, J Clin Oncol. 34 (2016) 863–870. 10.1200/JCO.2015.63.4550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Pugh TJ, Kelly MA, Gowrisankar S, Hynes E, Seidman MA, Baxter SM, et al. , The landscape of genetic variation in dilated cardiomyopathy as surveyed by clinical DNA sequencing, Genetics in Medicine. 16 (2014) 601–608. 10.1038/gim.2013.204. [DOI] [PubMed] [Google Scholar]
- [62].Alfares AA, Kelly MA, McDermott G, Funke BH, Lebo MS, Baxter SB, et al. , Results of clinical genetic testing of 2,912 probands with hypertrophic cardiomyopathy: expanded panels offer limited additional sensitivity, Genet Med. 17 (2015) 880–888. 10.1038/gim.2014.205. [DOI] [PubMed] [Google Scholar]
- [63].Thierfelder L, Watkins H, MacRae C, Lamas R, McKenna W, Vosberg HP, et al. , Alpha-tropomyosin and cardiac troponin T mutations cause familial hypertrophic cardiomyopathy: a disease of the sarcomere, Cell. 77 (1994) 701–712. 10.1016/0092-8674(94)90054-x. [DOI] [PubMed] [Google Scholar]
- [64].Geisterfer-Lowrance AA, Kass S, Tanigawa G, Vosberg HP, McKenna W, Seidman CE, et al. , A molecular basis for familial hypertrophic cardiomyopathy: a beta cardiac myosin heavy chain gene missense mutation, Cell. 62 (1990) 999–1006. 10.1016/0092-8674(90)90274-i. [DOI] [PubMed] [Google Scholar]
- [65].Poetter K, Jiang H, Hassanzadeh S, Master SR, Chang A, Dalakas MC, et al. , Mutations in either the essential or regulatory light chains of myosin are associated with a rare myopathy in human heart and skeletal muscle, Nat Genet. 13 (1996) 63–69. 10.1038/ng0596-63. [DOI] [PubMed] [Google Scholar]
- [66].Watkins H, Conner D, Thierfelder L, Jarcho JA, MacRae C, McKenna WJ, et al. , Mutations in the cardiac myosin binding protein-C gene on chromosome 11 cause familial hypertrophic cardiomyopathy, Nat Genet. 11 (1995) 434–437. 10.1038/ng1295-434. [DOI] [PubMed] [Google Scholar]
- [67].Kimura A, Harada H, Park JE, Nishi H, Satoh M, Takahashi M, et al. , Mutations in the cardiac troponin I gene associated with hypertrophic cardiomyopathy, Nat Genet. 16 (1997) 379–382. 10.1038/ng0897-379. [DOI] [PubMed] [Google Scholar]
- [68].Richard P, Charron P, Carrier L, Ledeuil C, Cheav T, Pichereau C, et al. , Hypertrophic cardiomyopathy: distribution of disease genes, spectrum of mutations, and implications for a molecular diagnosis strategy, Circulation. 107 (2003) 2227–2232. 10.1161/01.CIR.0000066323.15244.54. [DOI] [PubMed] [Google Scholar]
- [69].Andersen PS, Havndrup O, Hougs L, Sørensen KM, Jensen M, Larsen LA, et al. , Diagnostic yield, interpretation, and clinical utility of mutation screening of sarcomere encoding genes in Danish hypertrophic cardiomyopathy patients and relatives, Hum Mutat. 30 (2009) 363–370. 10.1002/humu.20862. [DOI] [PubMed] [Google Scholar]
- [70].Rosenbaum AN, Agre KE, Pereira NL, Genetics of dilated cardiomyopathy: practical implications for heart failure management, Nat Rev Cardiol. 17 (2020) 286–297. 10.1038/S41569-019-0284-0. [DOI] [PubMed] [Google Scholar]
- [71].Herman DS, Lam L, Taylor MRG, Wang L, Teekakirikul P, Christodoulou D, et al. , Truncations of Titin Causing Dilated Cardiomyopathy, New England Journal of Medicine. 366 (2012) 619–628. 10.1056/NEJMoa1110186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Fatkin D, Lam L, Herman DS, Benson CC, Felkin LE, Barton PJR, et al. , Titin truncating mutations: A rare cause of dilated cardiomyopathy in the young, Progress in Pediatric Cardiology. 40 (2016) 41–45. 10.1016/j.ppedcard.2016.01.003. [DOI] [Google Scholar]
- [73].Roberts AM, Ware JS, Herman DS, Schafer S, Baksi J, Bick AG, et al. , Integrated allelic, transcriptional, and phenomic dissection of the cardiac effects of titin truncations in health and disease, Science Translational Medicine. 7 (2015) 270ra6–270ra6. 10.1126/scitranslmed.3010134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Nikolova V, Leimena C, McMahon AC, Tan JC, Chandar S, Jogia D, et al. , Defects in nuclear structure and function promote dilated cardiomyopathy in lamin A/C–deficient mice, J Clin Invest. 113 (2004) 357–369. 10.1172/JCI19448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Kumar Saurabh, Baldinger Samuel H., Gandjbakhch Estelle, Maury Philippe, Sellal Jean-Marc, Androulakis Alexander F.A., et al. , Long-Term Arrhythmic and Nonarrhythmic Outcomes of Lamin A/C Mutation Carriers, Journal of the American College of Cardiology. 68 (2016) 2299–2307. 10.1016/j.jacc.2016.08.058. [DOI] [PubMed] [Google Scholar]
- [76].Ware JS, Li J, Mazaika E, Yasso CM, DeSouza T, Cappola TP, et al. , Shared Genetic Predisposition in Peripartum and Dilated Cardiomyopathies, N Engl J Med. 374 (2016) 233–241. 10.1056/NEJMoa1505517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Ware JS, Amor-Salamanca A, Tayal U, Govind R, Serrano I, Salazar-Mendiguchía J, et al. , Genetic Etiology for Alcohol-Induced Cardiac Toxicity, J Am Coll Cardiol. 71 (2018) 2293–2302. 10.1016/j.jacc.2018.03.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].van den Berg MP, van Spaendonck-Zwarts KY, van Veldhuisen DJ, Gietema JA, Postma A, van Tintelen JP, Familial dilated cardiomyopathy: another risk factor for anthracycline-induced cardiotoxicity?, Eur J Heart Fail. 12 (2010) 1297–1299. 10.1093/eurjhf/hfq175. [DOI] [PubMed] [Google Scholar]
- [79].Wasielewski M, van Spaendonck-Zwarts KY, Westerink N-DL, Jongbloed JDH, Postma A, Gietema JA, et al. , Potential genetic predisposition for anthracycline-associated cardiomyopathy in families with dilated cardiomyopathy, Open Heart. 1 (2014). 10.1136/openhrt-2014-000116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Linschoten M, Teske AJ, Baas AF, Vink A, Dooijes D, Baars HF, et al. , Truncating Titin (TTN) Variants in Chemotherapy-Induced Cardiomyopathy, J Card Fail. 23 (2017) 476–479. 10.1016/j.cardfail.2017.03.003. [DOI] [PubMed] [Google Scholar]
- [81].Garcia-Pavia P, Kim Y, Restrepo-Cordoba MA, Lunde IG, Wakimoto H, Smith AM, et al. , Genetic Variants Associated With Cancer Therapy–Induced Cardiomyopathy, Circulation. 140 (2019) 31–41. 10.1161/CIRCULATIONAHA.118.037934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Seshadri R, Firgaira FA, Horsfall DJ, McCaul K, Setlur V, Kitchen P, Clinical significance of HER-2/neu oncogene amplification in primary breast cancer. The South Australian Breast Cancer Study Group, J Clin Oncol. 11 (1993) 1936–1942. 10.1200/JCO.1993.11.10.1936. [DOI] [PubMed] [Google Scholar]
- [83].Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL, Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene, Science. 235 (1987) 177–182. 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- [84].Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, et al. , Use of Chemotherapy plus a Monoclonal Antibody against HER2 for Metastatic Breast Cancer That Overexpresses HER2, New England Journal of Medicine. 344 (2001) 783–792. 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- [85].Crone SA, Zhao Y-Y, Fan L, Gu Y, Minamisawa S, Liu Y, et al. , ErbB2 is essential in the prevention of dilated cardiomyopathy, Nat Med. 8 (2002) 459–465. 10.1038/nm0502-459. [DOI] [PubMed] [Google Scholar]
- [86].De Keulenaer GW, Feyen E, Dugaucquier L, Shakeri H, Shchendrygina A, Belenkov YN, et al. , Mechanisms of the Multitasking Endothelial Protein NRG-1 as a Compensatory Factor During Chronic Heart Failure, Circ Heart Fail. 12 (2019) e006288. 10.1161/CIRCHEARTFAILURE.119.006288. [DOI] [PubMed] [Google Scholar]
- [87].Carbone A, Bottino R, Russo V, D’Andrea A, Liccardo B, Maurea N, et al. , Takotsubo Cardiomyopathy as Epiphenomenon of Cardiotoxicity in Patients With Cancer: A Meta-summary of Case Reports, J Cardiovasc Pharmacol. 78 (2021) e20–e29. 10.1097/FJC.0000000000001026. [DOI] [PubMed] [Google Scholar]
- [88].Heinzerling L, Ott PA, Hodi FS, Husain AN, Tajmir-Riahi A, Tawbi H, et al. , Cardiotoxicity associated with CTLA4 and PD1 blocking immunotherapy, Journal for ImmunoTherapy of Cancer. 4 (2016) 50. 10.1186/s40425-016-0152-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Salem J-E, Manouchehri A, Moey M, Lebrun-Vignes B, Bastarache L, Pariente A, et al. Cardiovascular toxicities associated with immune checkpoint inhibitors: an observational, retrospective, pharmacovigilance study, The Lancet Oncology. 19 (2018) 1579–1589. 10.1016/S1470-2045(18)30608-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Love VA, Grabie N, Duramad P, Stavrakis G, Sharpe A, Lichtman A, CTLA-4 ablation and interleukin-12 driven differentiation synergistically augment cardiac pathogenicity of cytotoxic T lymphocytes, Circ Res. 101 (2007) 248–257. 10.1161/CIRCRESAHA.106.147124. [DOI] [PubMed] [Google Scholar]
- [91].Nishimura H, Okazaki T, Tanaka Y, Nakatani K, Hara M, Matsumori A, et al. , Autoimmune Dilated Cardiomyopathy in PD-1 Receptor-Deficient Mice, Science. 291 (2001) 319–322. 10.1126/science.291.5502.319. [DOI] [PubMed] [Google Scholar]
- [92].Okazaki T, Tanaka Y, Nishio R, Mitsuiye T, Mizoguchi A, Wang J, et al. , Autoantibodies against cardiac troponin I are responsible for dilated cardiomyopathy in PD-1-deficient mice, Nature Medicine. 9 (2003) 1477–1483. 10.1038/nm955. [DOI] [PubMed] [Google Scholar]
- [93].Wei SC, Meijers WC, Axelrod ML, Anang N-AAS, Screever EM, Wescott EC, et al. A Genetic Mouse Model Recapitulates Immune Checkpoint Inhibitor-Associated Myocarditis and Supports a Mechanism-Based Therapeutic Intervention, Cancer Discov. 11 (2021)614–625. 10.1158/2159-8290.CD-20-0856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Visscher H, Ross CJD, Rassekh SR, Sandor GSS, Caron HN, van Dalen EC, et al. Validation of variants in SLC28A3 and UGT1A6 as genetic markers predictive of anthracycline-induced cardiotoxicity in children, Pediatric Blood & Cancer. 60 (2013) 1375–1381. 10.1002/pbc.24505. [DOI] [PubMed] [Google Scholar]