Abstract
Despite their low abundance, phosphoinositides play a central role in membrane traffic and signalling. PtdIns(3,4,5)P3 and PtdIns(3,4)P2 are uniquely important, as they promote cell growth, survival, and migration. Pathogenic organisms have developed means to subvert phosphoinositide metabolism to promote successful infection and their survival within host organisms. We demonstrate that PtdIns(3,4)P2 is a major product generated in host cells by effectors of the enteropathogenic bacteria Salmonella and Shigella. Pharmacological, gene silencing and heterologous expression experiments revealed that, remarkably, the biosynthesis of PtdIns(3,4)P2 occurs independently of phosphoinositide 3-kinases. Instead, we found that the Salmonella effector SopB, heretofore believed to be a phosphatase, generates PtdIns(3,4)P2 de novo via a phosphotransferase/phosphoisomerase mechanism. Recombinant SopB is capable of generating PtdIns(3,4,5)P3 and PtdIns(3,4)P2 from PtdIns(4,5)P2 in a cell-free system. Through a remarkable instance of convergent evolution, bacterial effectors acquired the ability to synthesize 3-phosphorylated phosphoinositides by an ATP- and kinase-independent mechanism, thereby subverting host signaling to gain entry and even provoke oncogenic transformation.
Keywords: PtdIns(3,4)P2; PtdIns(3,4,5)P3; phosphoinositide 3-kinases; phosphotransferase; inositol lipids; phosphoinositides; SopB; IpgD; host-pathogen interactions; lipid signaling; photoactivation
Introduction
By directing vesicular traffic, gating ion channels, and orchestrating numerous signal transduction pathways, polyphosphoinositides (PPIns) shape organellar identity and function1. The seven PPIns species formed by combinatory phosphorylation of the inositol ring are in constant flux. Kinases and phosphatases catalyze the addition or removal, respectively, of phosphates at the 3-, 4-, or 5-position of the inositol ring while phospholipase C (PLC) detaches the phospho-inositol ring from its glycerol backbone. That numerous human pathologies are the result of mutations in these enzymes2,3 is a testament to the central role of PPIns in cellular homeostasis.
PtdIns(3,4,5)P3 and PtdIns(3,4)P2 are among the least abundant species in quiescent cells, constituting <0.1% of the total PPIns. However, in response to hormones, growth factors, and chemokines, phosphoinositide 3-kinases (PI3Ks) can increase their abundance by as much as 100-fold4. The PI3K family comprises 15 proteins; a subset –class I and class II PI3Ks– are the sole pathway to generate PtdIns(3,4,5)P3 and PtdIns(3,4)P2. When activated, class I PI3Ks phosphorylate PtdIns(4,5)P2 to yield PtdIns(3,4,5)P3. Class II isoforms phosphorylate the 3-position of PtdIns(4)P to yield PtdIns(3,4)P2 in the plasma membrane (PM), but can also modify the 3-position of PtdIns to yield PtdIns(3)P in endosomes5. Stereospecific interactions of 3-phosphorylated inositides with cognate protein domains coordinate cell migration, mitogenesis, growth, metabolism, and autophagy via downstream effectors including Rac, AKT, and mTOR5,6. However, the inappropriate accumulation of these lipids is a powerful anabolic signal that can drive oncogenesis7,8.
Pathogenic organisms have evolved means to enter eukaryotic cells by receptor-independent pathways9,10. The delivery of effector proteins into host cells can target the actin cytoskeleton to re-shape the plasmalemma, driving bacterial internalization and formation of vacuoles that serve as an intracellular niche for pathogen survival. Remarkably, signaling associated with 3-phosphorylated PPIns has long been noted during entry of several enteropathogenic bacteria11-14, but the mechanism(s) underlying their formation remains elusive. It has been assumed that this is a direct consequence of altered PI3K activity, but the putative kinase has not been identified. The precise nature of the PPIns generated has also remained uncertain due to the use of dual-specificity biosensors such as the pleckstrin-homology (PH) domain of AKT15,16.
New genetically-encoded biosensors with high specificity for PtdIns(3,4)P2 or PtdIns(3,4,5)P3 have recently been devised17,18. We took advantage of such improved probes, in combination with live cell imaging, optogenetics, and in vitro lipid analyses to revisit the mechanism underlying 3-PPIns generation by enteropathogens. Our studies identified an unprecedented mechanism of 3-PPIns generation, independent of PI3Ks.
Results
Formation of 3-phosphorylated PPIns during pathogen entry
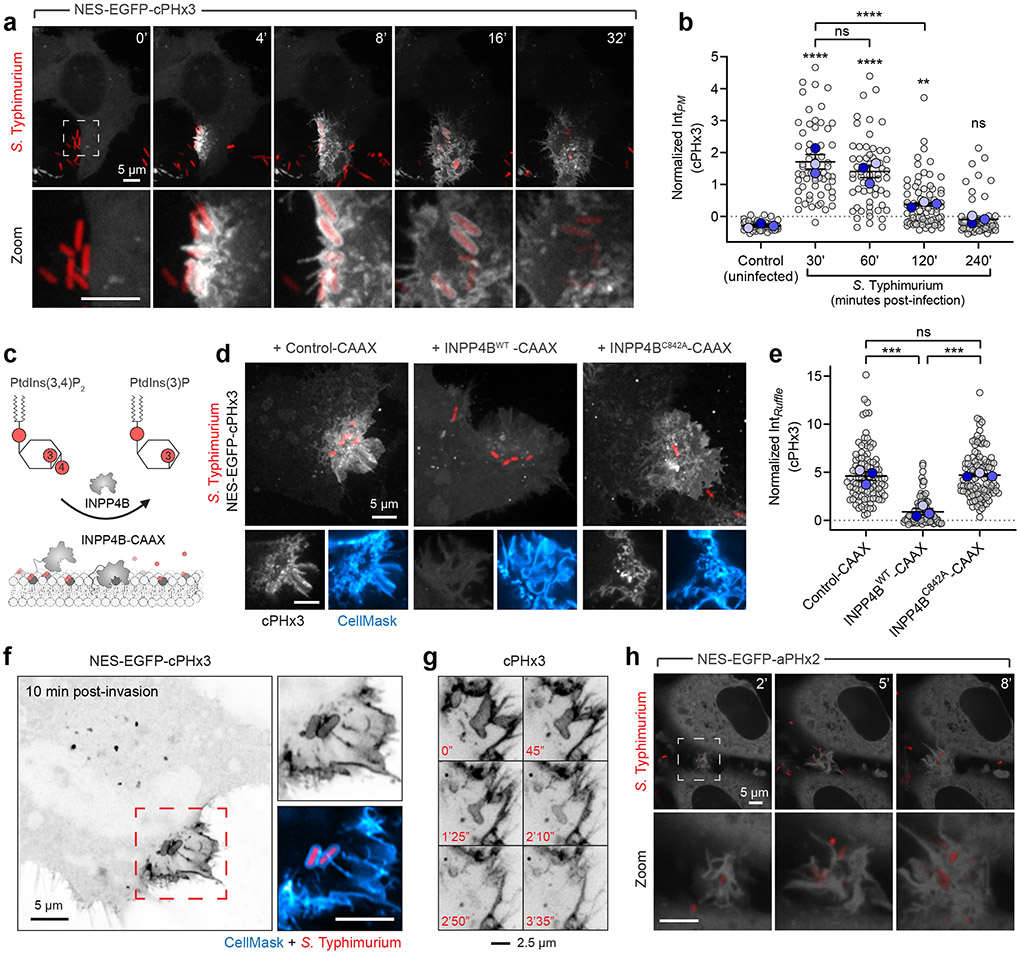
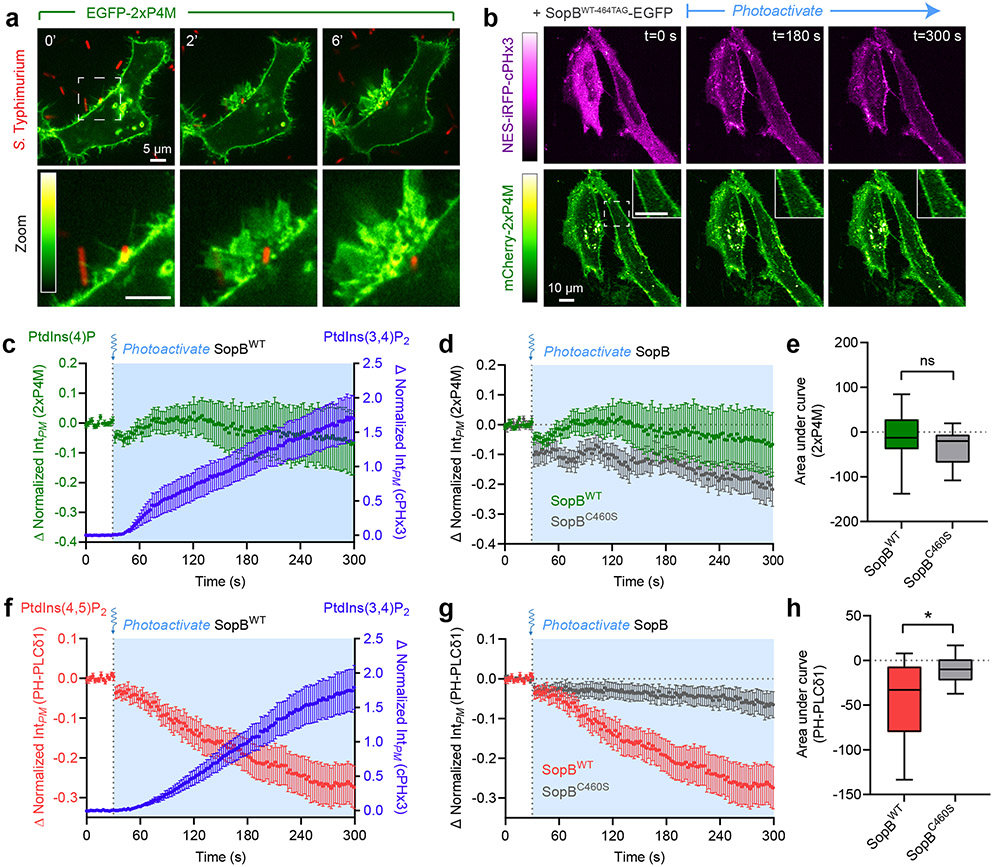
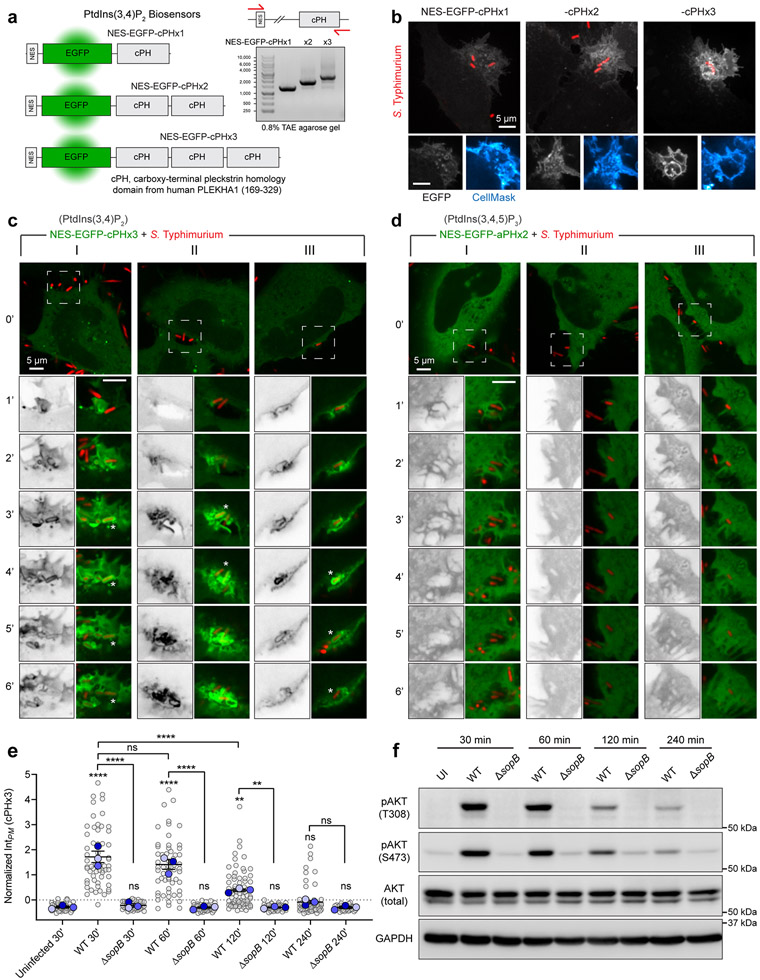
To investigate the possible formation and distribution of PtdIns(3,4)P2 during bacterial invasion, we employed a genetically-encoded biosensor based on the carboxy-terminal PH domain of TAPP119,20, recently modified to improve its sensitivity17 (Extended Data Fig. 1a). As a model of enteropathogenic invasion, we exposed epithelial cells to Salmonella enterica (serovar Typhimurium, hereafter Salmonella) –a prevalent world-wide threat to humans21,22. In the absence of serum, levels of PtdIns(3,4)P2 in epithelial cells are low, resulting in a cytosolic distribution of the biosensor NES-EGFP-cPHx3 (hereafter cPHx3; Fig. 1a)17. In contrast, the addition of Salmonella was associated with a remarkable enrichment of cPHx3 at sites of contact between bacteria and the host PM; this was followed by the generation of extensive cPHx3-labeled membrane ruffles and the internalization of bacteria into plasmalemmal-derived vacuoles (Fig. 1a, Extended Data Fig. 1a-c, Supplementary Video 1 and 2). Quantification of cPHx3 in the PM revealed enrichment of the biosensor that persisted long after pathogen entry (Fig. 1b, Extended Data Fig. 1e). Even though Salmonella entered cells within 10 min of addition, elevated levels of plasmalemmal cPHx3 were detected for >1 hour post-infection, despite removal of extracellular bacteria. The phosphorylation of the sentinel kinase AKT, which senses 3-PPIns, tracked temporally the increase in membrane cPHx3 (Extended Data Fig. 1e,f).
Fig. 1∣. Rapid and sustained PtdIns(3,4)P2 synthesis during Salmonella entry and maturation.
(a) Confocal imaging of cPHx3 during invasion by RFP-expressing Salmonella. Maximum intensity projections (main panels) and enlargements of the boxed region (bottom panels) are presented where 0 min indicates time of bacterial contact with the membrane.
(b) Cells serum-starved for 3 h were infected by Salmonella. Extracellular bacteria were removed by washing, and cells returned to serum-free medium containing gentamycin. PM cPHx3 intensities were quantified across 59 (control), 60 (30 min), 58 (60 min), 75 (120 min), and 66 cells (240 min) from n=3 independent experiments. Data are trial means ± SEM (blue, foreground) overlaid on cell measurements (gray, background). ****P < 0.0001; **P = 0.0027; ns, not significant.
(c) Model of PtdIns(3,4)P2 depletion by INPP4B-CAAX.
(d,e) INPP4B impaires recruitment of the biosensor cPHx3 to Salmonella-induced ruffles. Membrane cPHx3 intensity in invasion ruffles was analyzed 10 min post-infection in cells co-transfected with TagBFP2-CAAX (Control-CAAX, 106 ruffles), TagBFP2-INPP4BWT-CAAX (112 ruffles), or catalytically inactive TagBFP2-INPP4BC842A-CAAX (113 ruffles) from n=3 independent experiments. Maximum intensity projections are presented (main), and panels below are corresponding confocal sections of the invasion ruffle. CellMask identifies the PM. TagBFP2 fluorescence is not presented. Data are trial means ± SEM (blue, foreground) overlaid on cell measurements (gray, background). ***P = 0.0006; ns, not significant.
(f) Airyscan microscopy of cPHx3 within the invasion ruffle induced by Salmonella (10 min post-infection). Note the continuous cPHx3 (inverted gray) labelling that coincides with a CellMask (right panel)-stained membrane compartment.
(g) Confocal time lapse of cPHx3 during bacterial entry and constriction of the invaginating membrane. Images are maximum intensity projections of a 2 μm optical slice. Bacterial fluorescence is not presented.
(h) PtdIns(3,4,5)P3 during invasion. HeLa cells expressing aPHx2 were imaged live during invasion with Salmonella. Confocal sections are presented where 0 min marks the first indication of membrane ruffling induced by the bacteria. Source numerical data are available in source data.
To validate if cPHx3 faithfully maps PtdIns(3,4)P2, we analyzed infection of cells overexpressing PM-targeted inositol polyphosphate 4-phosphatase type II (INPP4B-CAAX)17,23, which specifically hydrolyzes PtdIns(3,4)P2 (Fig. 1c)24,25. The enrichment of cPHx3 within Salmonella-induced ruffles was largely eliminated by co-expressed active INPP4B-CAAX, but not by a mutant (C482A) of the phosphatase activity (Fig. 1d,e). Further analyses of cPHx3 revealed that during pathogen entry, PtdIns(3,4)P2 became highly enriched on the forming Salmonella-containing vacuole (Fig. 1a,f). Loss of vacuolar cPHx3 correlated tightly with its disengagement from the plasmalemma (Fig. 1g and Extended Data Fig. 1c). Labeling of bystander macropinocytic compartments by cPHx3 was also evident (Supplementary Video 2).
We also monitored PtdIns(3,4,5)P3 distribution utilizing tandem PH domains of ARNO (Cytohesin-2)26-28. This biosensor, NES-EGFP-aPHI303Ex2 (Extended Data Fig. 2a) called aPHx217, evinced modest enrichment in invasion ruffles (Fig. 1h, Extended Data Fig. 1d and 2b-c, and Supplementary Video 3).
SopB promotes the acute synthesis of PtdIns(3,4)P2
Salmonella expresses a complement of virulence factors, pre-synthesized and primed for delivery into the host cytosol for invasion. The type III secretion system, a ‘molecular needle’ expressed on the bacterial surface, penetrates the host membrane to translocate the effector load29. Salmonella outer protein B (SopB), one such effector targeted to the host membrane surrounding the forming vacuole (Extended Data Fig. 3a,b)30,31, is a key virulence determinant of Salmonella infection32-36. Remarkably, nearly half of the ≈9500 non-redundant host phosphorylation events during invasion are SopB-dependent37. The functions of SopB have been tightly linked to its enzymatic activity, presumed to be a phosphatase that acts on host cell inositol phosphates and PPIns12,33,38.
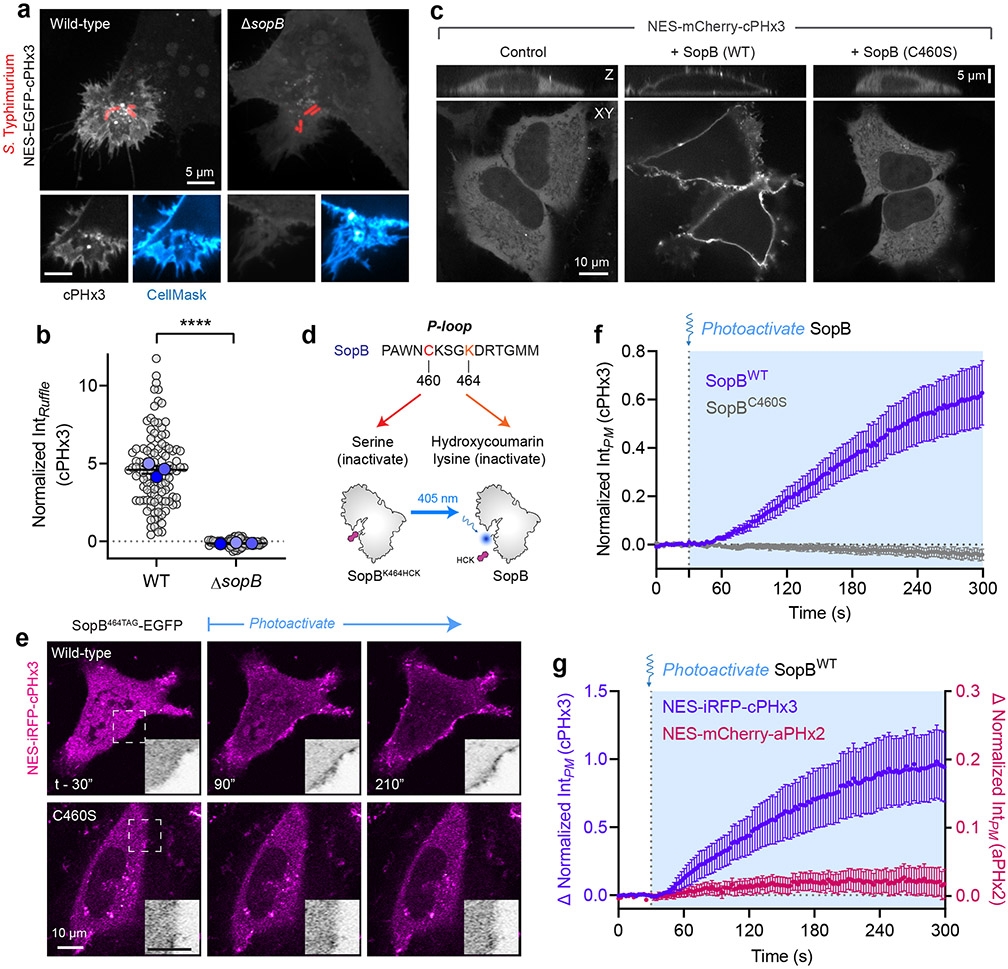
We tested the requirement of SopB for generation of PtdIns(3,4)P2 by comparing host responses to wild-type Salmonella or an isogenic strain devoid of this effector (ΔsopB). Invasion of epithelial cells by wild-type bacteria consistently induced recruitment of cPHx3 to the invasion site; however, ruffles induced by bacteria lacking SopB were completely devoid of this enrichment (Fig. 2a,b). Further, the overall increase in plasmalemmal PtdIns(3,4)P2 following invasion (Fig. 1b) and the elevated phosphorylation of AKT also required SopB (Extended Data Fig. 1e,f), consistent with earlier findings11,39. Similarly, the modest enrichment of PtdIns(3,4,5)P3 was absent during invasion by ΔsopB bacteria (Extended Data Fig. 2b,c).
Fig. 2∣. SopB is necessary and sufficient for PtdIns(3,4)P2 biosynthesis in mammalian cells.
(a) Cells expressing cPHx3 were infected for 10 min with wild-type or ΔsopB Salmonella expressing RFP prior to staining the PM with CellMask. Maximum intensity projections (main) and corresponding confocal sections of the invasion ruffle (bottom) are presented.
(b) Membrane cPHx3 intensity was quantified in invasion ruffles across n=3 independent experiments (WT, 113 ruffles; ΔsopB, 87 ruffles). Data are trial means ± SEM (blue, foreground) overlaid on cell measurements (gray, background). ****P < 0.0001.
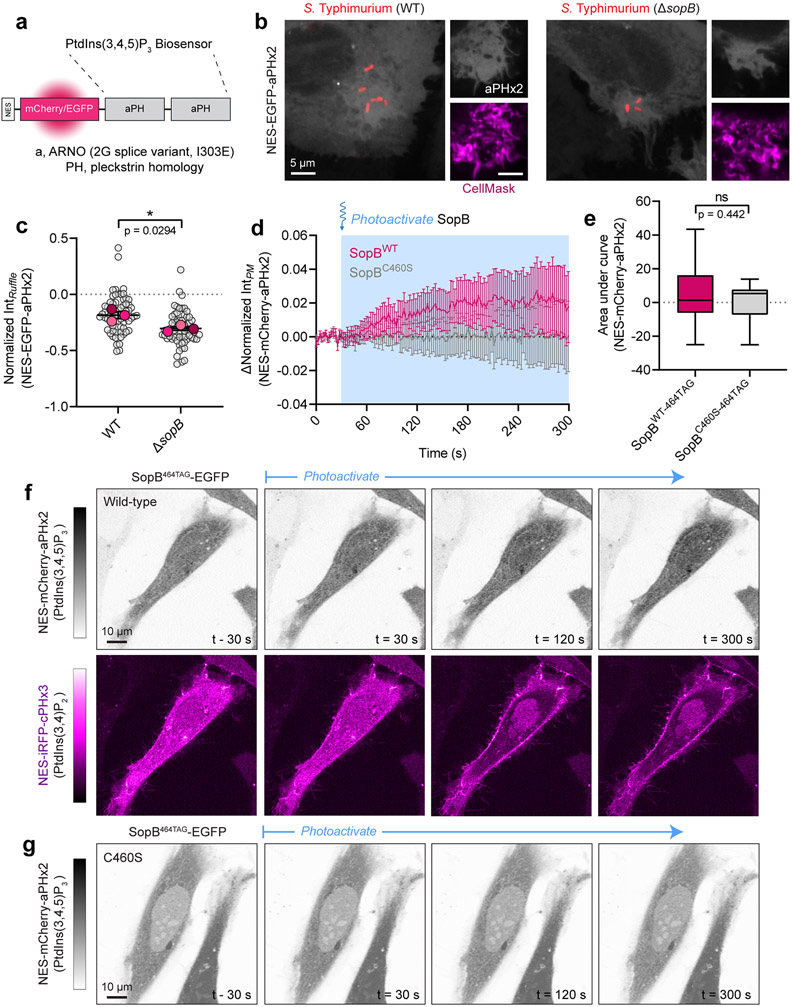
(c) Heterologous expression of SopB generates PtdIns(3,4)P2. Representative XY and Z confocal sections of HeLa expressing mCherry-tagged cPHx3 and co-transfected as indicated prior to imaging live. Control (EGFP-transfected, 122 cells; n=5), SopBWT-EGFP (210 cells, n=5) and SopBC460S-EGFP (107 cells; n=3), where n=independent experiments.
(d) Model of hydroxycoumarin lysine (HCK) incorporation at SopB residue 464 and photolysis. Cells are transfected with SopB464TAG and plasmids that encode an Amber stop codon (UAG)-recognizing tRNA and a tRNA synthase that incorporates HCK during translation. Illumination by 405 nm light photolyzes the hydroxycoumarin to yield wild-type (active) SopB.
(e) Photoactivation of SopB induces acute PtdIns(3,4)P2 formation. Representative confocal time-lapses of cells expressing cPHx3 pre- and post-photoactivation of SopBWT-464TAG or SopBC460S-464TAG. Insets are inverted grayscale images of cPHx3 from the region marked by the box in the left-most frame.
(f) Quantification of PM cPHx3 intensity from experiments like those in (e) examining n=119 cells (SopBWT-464TAG) and n=50 cells (SopBC460S-464TAG) pooled from 7 independent experiments. Blue-shaded background represents 405-nm-illuminated timepoints. Data are the baseline-corrected mean ± SEM of cPHx3 measurements.
(g) Quantification of PM intensity of cPHx3 (left ordinate axis) and aPHx2 (right ordinate axis) following the photoactivation of SopBWT-464TAG. Data are the filtered baseline-corrected mean ± SEM from n=29 cells across 3 independent experiments. Source numerical data are available in source data.
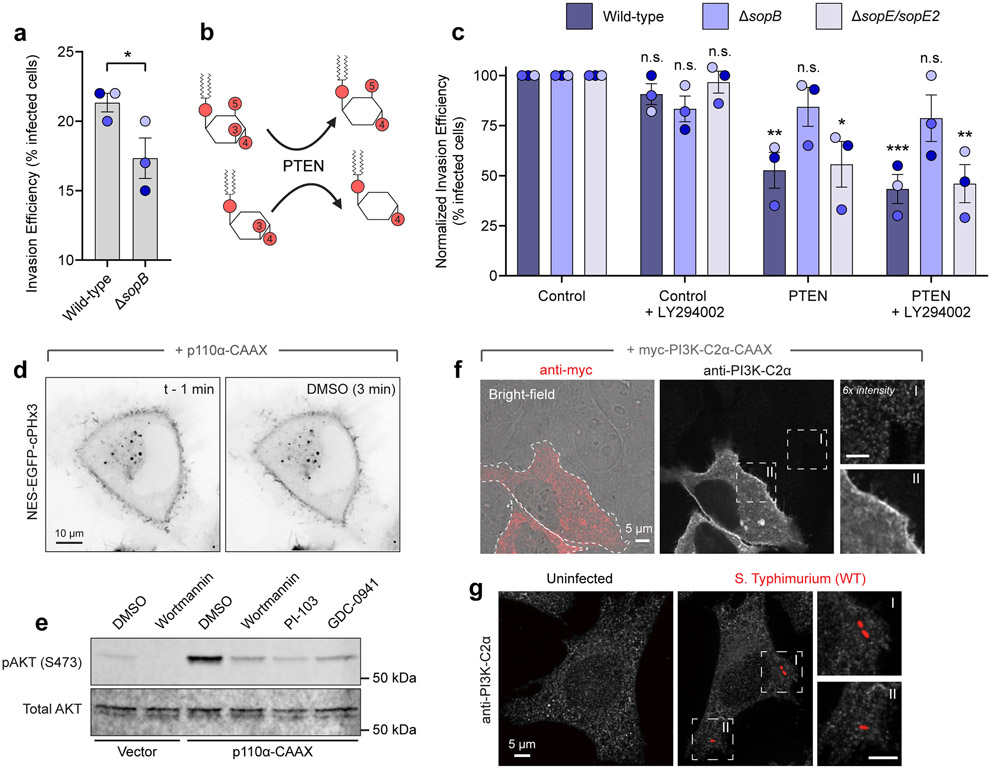
Given that ΔsopB bacteria have reduced invasion efficiency (Extended Data Fig. 4a)40,41, we assessed the possible role of PtdIns(3,4)P2 and PtdIns(3,4,5)P3 in promoting invasion by depleting these lipids enzymatically with PTEN (Extended Data Fig. 4b). Expression of an engineered version of PTEN with enhanced membrane-association and activity42 led to a marked (47.3 ± 8.9%) decrease in invasion efficiency for wild-type bacteria; this PTEN-induced reduction was not seen for ΔsopB bacteria (Extended Data Fig. 4c). Thus, SopB is required to generate 3-PPIns species, and these lipids promote host cell invasion.
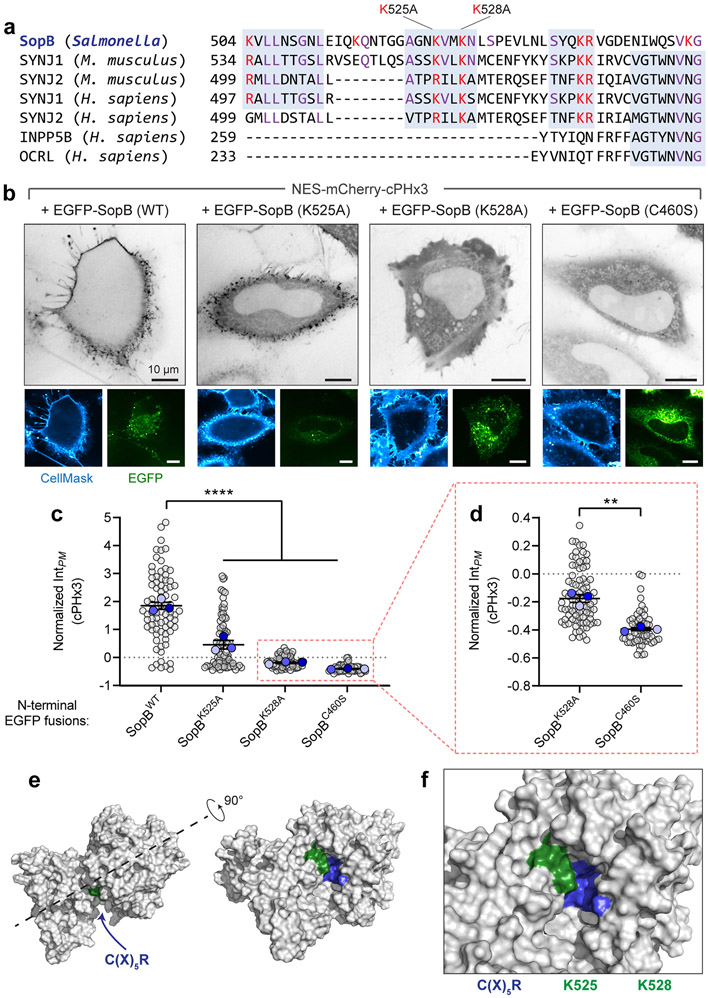
Whether SopB requires other virulence factors to initiate this response was examined next. To exclude the possible contribution of other bacterial effectors, we transfected epithelial cells with a plasmid encoding SopB. Expression of SopB alone led to a marked translocation of cPHx3 to the PM of the transfected cells (Fig. 2c and Extended Data Fig. 3c), implying that PtdIns(3,4)P2 synthesis occurs independently of other bacterial effectors. SopB shares homology with the Cys-X5-Arg active site consensus sequence of the mammalian 4-phosphatases INPP4A/4B and the 3-phosphatase PTEN33. Mutation of the homologous cysteine in SopB to serine (C460S), reported to block its phosphatase activity12,33, also completely blocked its effect on cPHx3 translocation (Fig. 2c, right). Deletion or mutation of other residues that support optimal phosphatase activity of SopB12 also reduced cPHx3 translocation (Extended Data Fig. 3d and Extended Data 5a-d). In contrast, although the localization of SopB is modulated by its interaction with host Cdc4230, this GTPase was not required for PtdIns(3,4)P2 generation by SopB (Extended Data Fig. 3e-g).
Unlike its acute response during bacterial invasion, manifestation of the effects of SopB on PtdIns(3,4)P2 in the case of heterologous SopB expression requires hours, raising the possibility that they may arise indirectly through slow intermediate events. To investigate this possibility, we turned to acute optogenetic activation43,44 of SopB. We selected Lys464 of SopB, whose codon was mutated to UAG and co-expressed with a plasmid to facilitate the incorporation of the unnatural, caged amino acid, hydroxycoumarin lysine (HCK) during translation (Fig. 2d). We predicted that the active site would be occluded –and thus non-functional– by the bulky hydroxycoumarin derivative. As predicted, transfection of SopB464TAG did not support the generation of PtdIns(3,4)P2 (Fig. 2e, top left). However, light-induced photolysis of the HCK group by 405-nm illumination restored the Lys464 residue (Fig. 2d), leading to rapid (<1 min) SopB-mediated formation of PM PtdIns(3,4)P2 (Fig. 2e-f, Supplementary Video 4). Importantly, the increase in PtdIns(3,4)P2 was not observed when the C460S mutation was introduced into SopB464TAG (Fig. 2e,f), ruling out a spurious effect of light exposure. Using this approach we compared the dynamics of PtdIns(3,4)P2 and PtdIns(3,4,5)P3 synthesis during the acute photoactivation. A relatively minor PtdIns(3,4,5)P3 response was observed in cells exhibiting robust PtdIns(3,4)P2 generation (Fig. 2g and Extended Data Fig. 2d-g; note expanded scale of aPHx2 plot). Thus, SopB is both necessary and sufficient to generate a rapid and robust PtdIns(3,4)P2 response in human cells.
PI3Ks are not required for SopB-driven PtdIns(3,4)P2
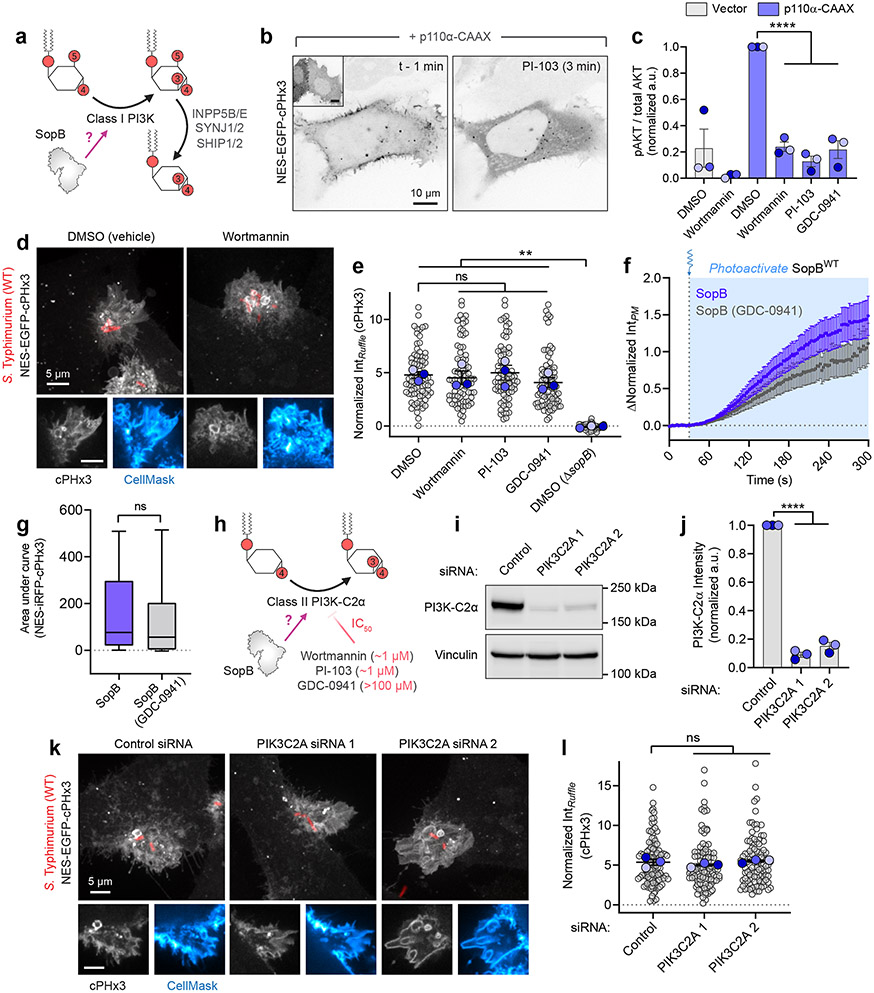
Hydrolysis of the predominant cellular phosphoinositides PtdIns(4)P and PtdIns(4,5)P2 by a phosphatase cannot directly account for the observed PtdIns(3,4)P2 synthesis. Instead, we entertained the possibility that SopB stimulates a host PI3K5,9. In response to the activation G protein-coupled receptors, tyrosine kinases, and small GTPases like Ras, PtdIns(4,5)P2 is phosphorylated to PtdIns(3,4,5)P3 by class I PI3Ks. PtdIns(3,4,5)P3 can be rapidly converted to PtdIns(3,4)P2 by subsequent dephosphorylation by 5-phosphatases1,3 (Fig. 3a). Indeed, the constitutive recruitment of a class I PI3K to the PM (p110α-CAAX) triggered a parallel increase in PtdIns(3,4)P2 levels (Fig. 3b).
Fig. 3∣. Class I PI3Ks and Class II PI3K-C2α are not required for PtdIns(3,4)P2 synthesis during Salmonella entry.
(a) Class I PI3K-mediated, indirect PtdIns(3,4)P2 synthesis.
(b) cPHx3 was co-transfected with p110α-CAAX (main panels) or vector control (inset left). Representative micrographs from n=3 independent experiments are presented pre- and post-PI-103 (500 nM) treatment.
(c) Control or p110α-CAAX-expressing cells were treated for 20 min with DMSO (vehicle), wortmannin (100 nM), PI-103 (500 nM), or GDC-0941 (500 nM) prior to immunoblotting for pAKT (S473) and pan AKT. pAKT/AKT densitometry is presented normalized to p110α-CAAX (DMSO) from n=3 independent experiments. Data are mean ± SEM. ****P < 0.0001.
(d) cPHx3-expressing cells were pre-treated as in (c) prior to 10 min Salmonella exposure. A representative maximum intensity projection of a DMSO- or wortmannin-treated cell is presented (main) with confocal sections of the invasion ruffle (bottom).
(e) cPHx3 intensity quantified in invasions ruffles from (d) following 20 min pre-treatment with PI3K inhibitors. N=3 independent experiments quantifying the following number of ruffles: DMSO, 81; wortmannin, 80; PI-103, 76; GDC-0941, 85; DMSO (ΔsopB), 58. Data are mean ± SEM (foreground) overlaid on cell measurements (background). **P < 0.001.
(f) Photoactivation of SopBWT-464TAG in untreated or GCD-0941 (250 nM, 30 min) treated cells. Data are filtered baseline-corrected mean ± SEM cPHx3 intensity from n=69 cells (Control; 10 experiments, 40 cells pooled from Fig. 2f) and n=39 cells (GDC-0941; 6 experiments).
(g) AUC calculation of cPHx3 intensities from (f). Data are median, box (25th-75th) and whisker (10th-90th) percentiles of n=69 control cells (10 experiments, 40 cells pooled from Fig. 2f) and n=39 GDC-0941 cells (6 experiments). P = 0.0975.
(h) PI3K-C2α-mediated PtdIns(3,4)P2 synthesis and inhibitory IC50 values. (i) Cells treated with the indicated siRNAs and lysates immunoblotted against PI3K-C2α. Vinculin served as a loading control.
(j) Densitometric estimation of PI3K-C2α remaining after RNAi treatment, normalized to vinculin. Data are mean ± SEM from n=3 independent experiments. ****P < 0.0001.
(k) siRNA-treated cells transfected with cPHx3 and infected with Salmonella for 10 min. Representative maximum intensity projections (main) and invasion ruffle sections (bottom) are presented.
(l) Quantification of cPHx3 intensity in the invasion ruffle from (k) in n=3 independent experiments quantifying the following number of ruffles (control, 117; PIK3C2A 1, 105; PIK3C2A 2, 107). Data are mean ± SEM (foreground) overlaid on cell measurements (background). P = 0.6826 (C-vs-1), P > 0.9999 (C-vs-2). Source numerical data and unprocessed blots are available in source data.
We employed several potent pan-PI3K inhibitors to probe the role of this pathway: wortmannin, which irreversibly modifies the active site of multiple PI3Ks45; PI-103, a multi-target PI3K and mTOR inhibitor46; and Pictilisib (GDC-0941) which exhibits selectivity towards class I PI3Ks47. Addition of nanomolar concentrations of these compounds to cells expressing p110α-CAAX led to the complete and rapid (<3 min) release of cPHx3 from the PM (Fig. 3b and Extended Data Fig. 4d) and suppressed phosphorylation of AKT (Fig. 3c and Extended Data Fig. 4e), validating their potency towards PI3Ks. Remarkably, despite their obvious efficacy towards PI3Ks, pre-incubation of cells with any of the above inhibitors failed to reduce PtdIns(3,4)P2 biogenesis at sites of Salmonella invasion (Fig. 3d,e) or to block synthesis of PM PtdIns(3,4)P2 following photoactivation of SopB (Fig. 3f,g).
As an alternative to SopB stimulating a class I PI3K, we tested the role of class II PI3Ks which can directly phosphorylate PtdIns(4)P to generate PtdIns(3,4)P2 (Fig. 3h). It is noteworthy that of the class II isoforms, only PI3K-C2α is (somewhat) refractory to classical inhibitors of PI3Ks46,48,49 and may have resisted inhibition in our pharmacological screen. To test the role of PI3K-C2α, two independently-targeting siRNA sequences against PIK3C2A (encoding PI3K-C2α) were introduced into the cells in conjunction with the biosensor cPHx3. Despite depletion of 85-95% of PI3K-C2α determined by immunoblotting (Fig. 3i,j), the robust recruitment of cPHx3 to Salmonella-induced ruffles persisted (Fig. 3k,l). Moreover, endogenous PI3K-C2α showed no enrichment at invasion ruffles (Extended Data Fig. 4f,g). Together, these observations raised the possibility that conventional biosynthetic pathways are not responsible for the PtdIns(3,4)P2 synthesis induced by SopB.
IpgD generates PtdIns(3,4)P2 independently of PI3Ks
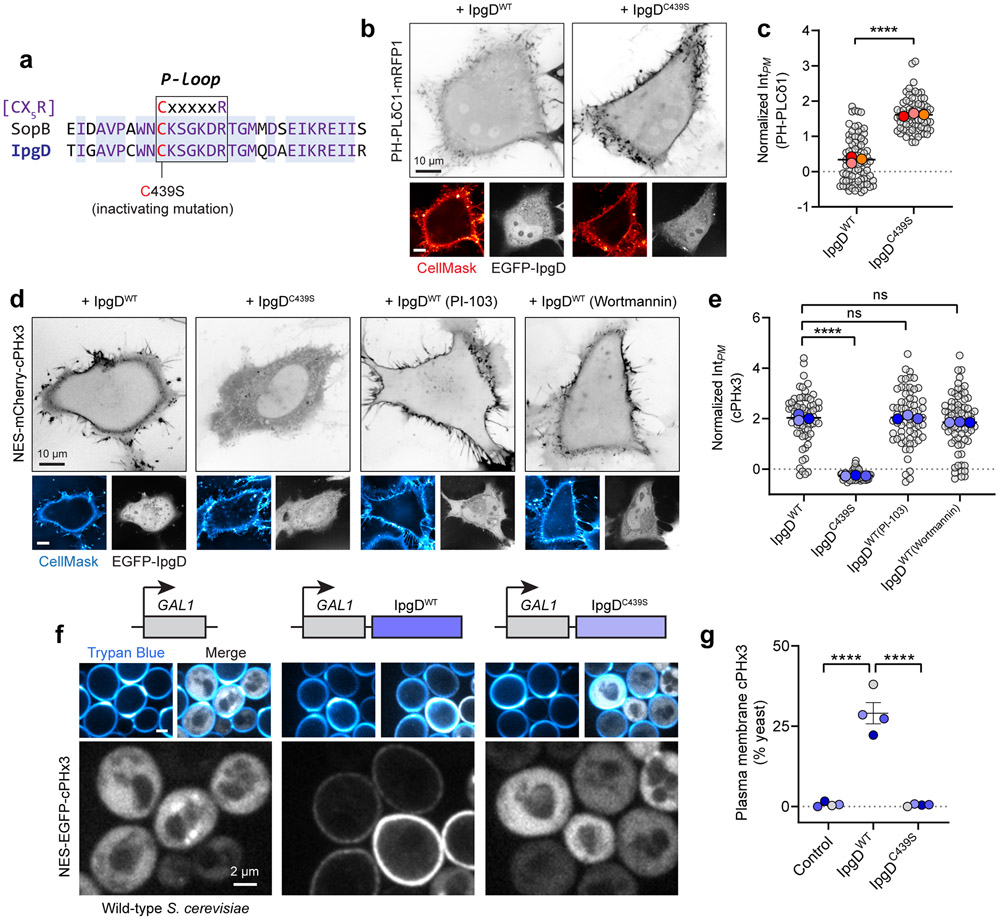
Based on sequence homology (Extended Data Fig. 6a), we hypothesized that SopB and IpgD, a Shigella flexneri effector, share analogous enzymatic activities. We monitored cPHx3 localization when co-expressed with IpgD. Indeed, expression of wild-type IpgD –but not IpgD with cysteine 439 mutated to serine– led to a robust relocalization of cPHx3 to the PM (Extended Data Fig. 6d). As in the case of SopB, IpgD-mediated generation of PtdIns(3,4)P2 was unaffected by PI3K inhibition by PI-103 or wortmannin (Extended Data Fig. 6d,e). Thus, Shigella’s IpgD and Salmonella’s SopB likely share their mode of action.
SopB and IpgD activity in S. cerevisiae devoid of PI3Ks
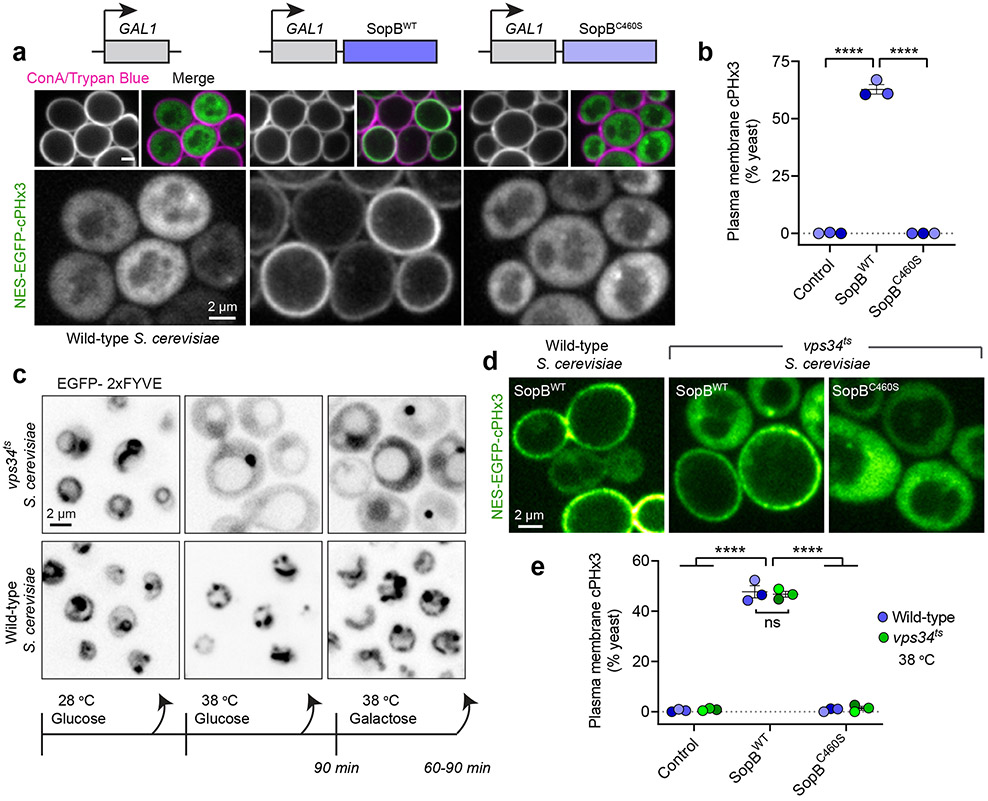
The inhibitor studies and silencing of PI3K-C2α suggested that neither class I nor II PI3Ks are required for SopB- or IpgD-mediated PtdIns(3,4)P2 generation. To assess this conclusion more definitively, we examined the effect of these effectors in Saccharomyces cerevisiae, an organism lacking class I and class II PI3Ks50,51. In otherwise untreated cells, the cPHx3 probe was entirely cytosolic (Fig. 4a, left) consistent with the inability of yeast to intrinsically synthesize PtdIns(3,4)P2. SopB was expressed acutely under the control of a galactose-inducible promoter because its prolonged expression is deleterious to yeast30,52. Remarkably, induction of SopB in the yeast yielded a robust translocation of cPHx3 to the PM (Fig. 4a). Importantly, SopB cysteine 460 was strictly required for this translocation (Fig. 4a,b). Like SopB, IpgD also caused robust translocation in S. cerevisiae, an effect that required cysteine 439 of the Shigella effector (Extended Data Fig. 6f,g).
Fig. 4∣. PtdIns(3,4)P2 generation in S. cerevisiae that are devoid of PI3K activity.
(a) PM PtdIns(3,4)P2 synthesis in S. cerevisiae. Galactose-inducible empty vector (control), SopBWT, or SopBC460S were induced for 2 hours in yeast that expressed cPHx3. Concanavalin A (ConA) and trypan blue staining demarcate both the cell wall and non-viable yeast.
(b) Yeast from (a) were scored for plasmalemmal cPHx3 localization across n=3 independent experiments analyzing the following number of yeast cells (control, 929; SopBWT, 929; SopBC460S, 1116). Data are mean ± SEM. ****P < 0.0001.
(c) PtdIns(3)P loss in vps34ts mutant S. cerevisiae. Cells expressing EGFP-2xFYVE were maintained at 28-30°C (permissive) during growth or shifted where indicated to 38 °C (non-permissive) for 90 min before switching carbon source and continued growth at 38 °C.
(d) PtdIns(3,4)P2 synthesis by SopB persisted despite loss of Vps34p activity. Yeast expressing galactose-inducible plasmids were shifted to a non-permissive temperature as in (c) before imaging. Non-viable cells were excluded using Trypan blue (not shown).
(e) Quantification of cPHx3 translocation to the PM from experiments like (d) across n=3 independent experiments. The following number of yeast cells were scored per condition: Control (WT, 455 cells; ts, 510 cells), SopBWT (WT, 597 cells; ts, 475 cells), SopBC460S (WT, 518 cells; ts, 331 cells). Data are mean ± SEM. ****P < 0.0001. Source numerical data are available in source data.
Despite lacking class I and class II PI3Ks, it was conceivable that the sole PI3K expressed in S. cerevisiae –class III Vps34p– might be involved in the SopB- or IpgD-induced generation of PtdIns(3,4)P2. Deletion of VPS34 (encoding Vps34p) and the resultant loss of PtdIns(3)P severely compromise endo-vacuolar protein sorting and growth of the yeast50,53. We therefore turned to a temperature-sensitive mutant (vps34ts) that exhibits acute loss of PtdIns(3)P only at non-permissive temperatures, which we confirmed using a tandem FYVE domain probe 54 that recognizes PtdIns(3)P (Fig. 4c). SopB expression was induced 90 min after shifting the vps34ts strain to a non-permissive temperature, before monitoring cPHx3 localization. Despite the loss of Vps34p activity and cellular PtdIns(3)P, expression of wild-type –but not of C460S– SopB induced translocation of cPHx3 to the PM (Fig. 4d,e).
SopB requires PtdIns(4,5)P2 to generate PtdIns(3,4)P2
Jointly, the preceding results appear to rule out the involvement of known host PI3Ks in the generation of PtdIns(3,4)P2 induced by SopB and IpgD; a distinct biosynthetic pathway must therefore be invoked. In vitro, SopB can function as a rather promiscuous PPIns and inositol polyphosphate phosphatase12,33, and causes the disappearance of PtdIns(4,5)P2 from the base of invasion ruffles in vivo55. These effects depend on cysteine 460. It is noteworthy that the same residue is also essential for the formation of PtdIns(3,4)P2 by SopB (Fig. 2 and 4). On this basis we hypothesized that, rather than activating host kinases, SopB directly causes phosphorylation of the 3-position of the inositol ring through rearrangement of the phosphate groups of pre-existing cellular lipids.
To test this hypothesis we analyzed the fate of PPIns regio-isomers during SopB-induced generation of PtdIns(3,4)P2. To monitor PtdIns(4)P we transfected human cells with a biosensor based on tandem P4M domains from the Legionella effector SidM56. PtdIns(4)P was abundant in the resting PM as well as in Salmonella-induced ruffles, and could hence potentially serve as a substrate for the formation of PtdIns(3,4)P2 (Fig. 5a). To test the possible involvement of PtdIns(4)P in the formation of PtdIns(3,4)P2, we simultaneously monitored the distribution of the 2xP4M and cPHx3 sensors during optogenetic activation of SopB. Under these conditions, plasmalemmal PtdIns(4)P was largely unperturbed despite robust PtdIns(3,4)P2 generation (Fig. 5b,c). The minute decrease in PtdIns(4)P observed following SopB photo-uncaging was also observed when using C460S SopB, implying that it was unrelated to the generation of PtdIns(3,4)P2 (Fig. 6d,e). Thus, we found no evidence that PtdIns(4)P was directly involved in the process.
Fig. 5∣. PtdIns(4,5)P2 levels correlate inversely with SopB-mediated PtdIns(3,4)P2 formation.
(a) PtdIns(4)P during bacterial invasion. A representative time-lapse of a HeLa cell expressing 2xP4M (inset bar, linear RGB intensity scale where 0=black, 255=white) during wild-type Salmonella invasion (0 min indicates time of bacterial contact with the membrane). Panels at bottom show enlarged region denoted by white box. PtdIns(4)P remains abundant within ruffles but is rapidly cleared from the forming vacuole.
(b) PM PtdIns(4)P is stable during optogenetic activation of SopB. Representative micrographs of cPHx3 and 2xP4M pre- and post-photoactivation of SopBWT-464TAG. Left bars, corresponding linear RGB intensity scales where 0=black, 255=white. Illumination of the sample with 405-nm light began at t=30 sec. Insets show enlarged region denoted by white box in frame 1.
(c) Quantification of experiments like (b) plotting PM 2xP4M (left ordinate axis) and cPHx3 (right ordinate axis) intensities following the photoactivation of SopBWT-464TAG. Data are the filtered baseline-corrected mean ± SEM from n=21 cells across 3 independent experiments.
(d) Comparison of 2xP4M responses to photoactivation of SopBWT-464TAG and SopBC460S-464TAG. Data are filtered baseline-corrected mean ± SEM 2xP4M intensity from n=21 cells (SopBWT-464TAG) and n=27 cells (SopBC460S-464TAG) across 3 independent experiments.
(e) AUC calculations of 2xP4M intensities from (d). Data are median, box (25th-75th) and whisker (10-90th) percentiles of n=21 cells (SopBWT-464TAG) and n=27 cells (SopBC460S-464TAG) across 3 independent experiments. P = 0.064.
(f) PtdIns(4,5)P2 depletion during PtdIns(3,4)P2 synthesis. Quantification of PH-PLCδ1 (left ordinate axis) and cPHx3 (right ordinate axis) PM intensities pre- and post-photoactivation of SopBWT-464TAG. Data are the filtered baseline-corrected mean ± SEM from n=40 cells across 7 independent experiments (unfiltered cPHx3 data presented in Fig. 2f).
(g) Data from (f) comparing the response of PH-PLCδ1 to photoactivation of SopBWT-464TAG and SopBC460S-464TAG. Data are filtered baseline-corrected mean ± SEM PH-PLCδ1 intensity from n=40 cells (SopBWT-464TAG) and n=10 cells (SopBC460S-464TAG) across 7 independent trials.
(h) AUC calculations of PH-PLCδ1 intensities from (g). Data are median, box (25th-75th) and whisker (10th-90th) percentiles of n=40 cells (SopBWT-464TAG) and n=10 cells (SopBC460S-464TAG) from 7 independent experiments. *P = 0.0297. Source numerical data are available in source data.
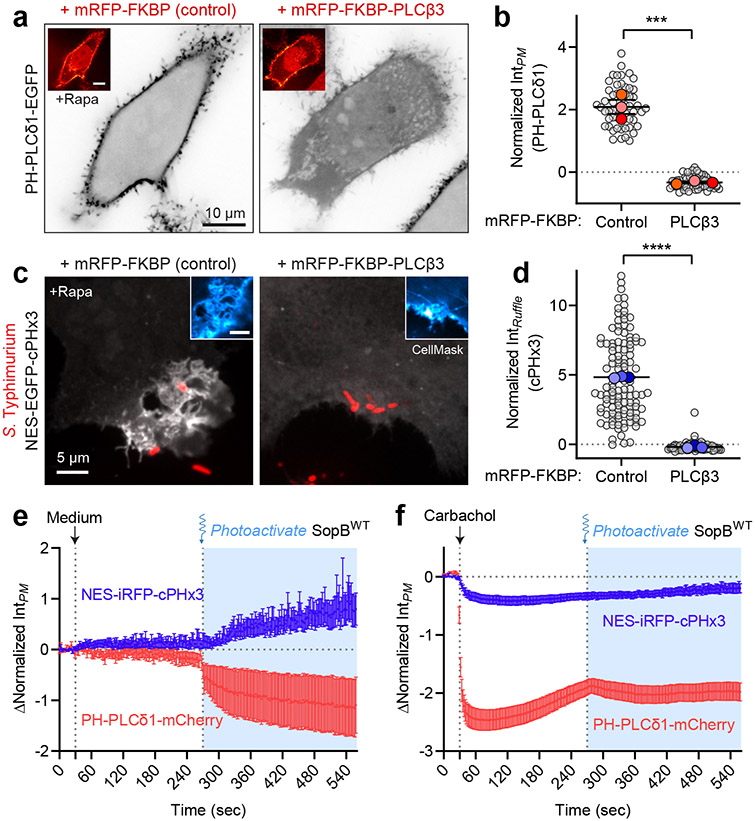
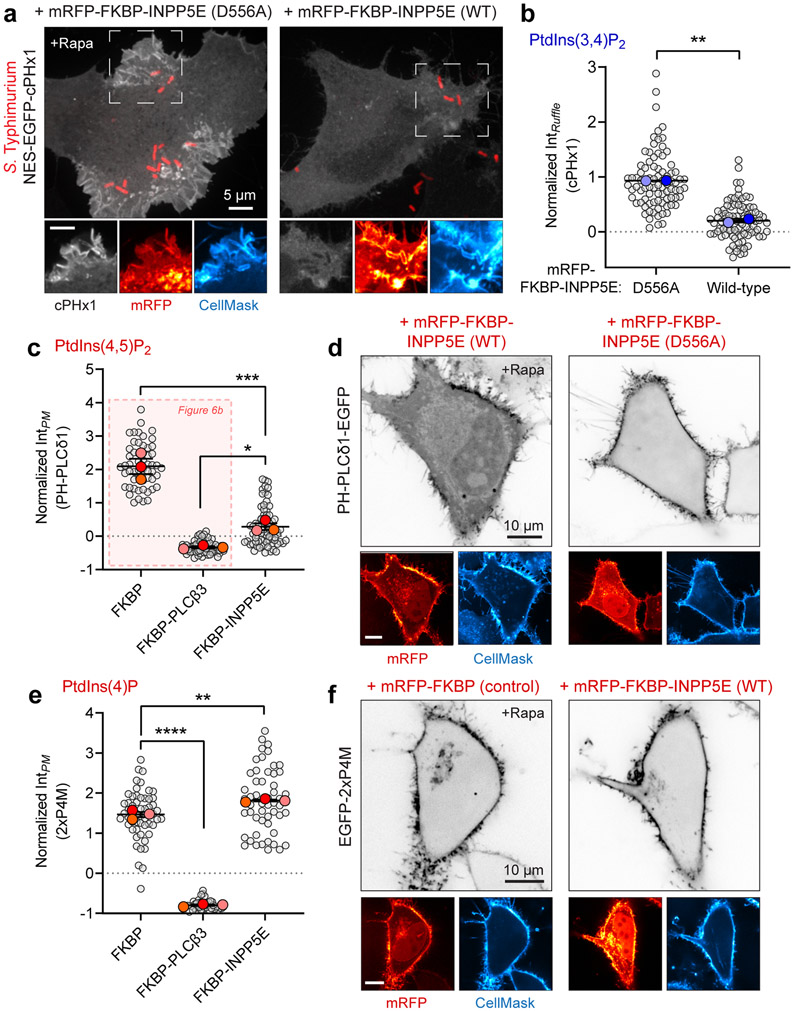
Fig. 6∣. SopB requires a phospholipase C-sensitive inositide to generate PtdIns(3,4)P2.
(a) Chemically induced recruitment of PLCβ3 to deplete PtdIns(4,5)P2 from the PM. HeLa cells expressing mRFP-FKBP (control) or mRFP-FKBP-PLCβ3 together with PM-targeted Lyn(11)-FRB and the biosensor PH-PLCδ1 (inverted gray scale) were treated with 1 μM rapamycin before imaging live. Insets show re-localization of FKBP-conjugates to the PM induced by rapamycin.
(b) Plasmalemmal PH-PLCδ1 intensity from experiments like (a) was quantified from n=3 independent experiments (control, 55 cells; PLCβ3, 56 cells). Data are mean ± SEM (foreground) overlaid on cell measurements (background). ***P = 0.0005
(c) Inhibition of PtdIns(3,4)P2 generated during invasion by pre-recruitment of PLCβ3. Cells transfected as in (a) but expressing cPHx3 (main panels, maximum intensity projections) were incubated with 1 μM rapamycin for 2 min before addition of wild-type BFP-expressing Salmonella for an additional 10 min. The PM was stained with CellMask (inset, confocal sections of invasion ruffle) before fixation and imaging.
(d) The resulting PM cPHx3 intensities from (c) were quantified in invasion ruffles from n=3 independent experiments (control, 115 cells; PLCβ3, 76 cells). Data are mean ± SEM (foreground) overlaid on individual cell measurements (background). ****P < 0.0001.
(e,f) Activation of endogenous PLCβ precludes SopB-mediated PtdIns(3,4)P2 synthesis. HeLa cells overexpressing SopBWT-464TAG and muscarinic M3 receptor were subjected to treatment with medium control (e) or 50 μM carbachol (f) at 30 sec to activate endogenous PLCβ, followed by 405-nm light illumination at 270 sec to optogenetically activate SopB. The decrease in PtdIns(4,5)P2 and response in PtdIns(3,4)P2 were monitored with PH-PLCδ1 and cPHx3, respectively. Baseline-corrected data are means ± SEM of individual cell measurements quantifying n=33 cells (medium control) and n=41 cells (carbachol) from 3 independent experiments. Note that a slight decrease in PM cPHx3 occurs following carbachol treatment likely due to inhibition of class I PI3K signaling constitutively stimulated by the presence of serum. Source numerical data are available in source data.
We turned our attention to the other major PPIns species of the PM, namely PtdIns(4,5)P2, using the PH domain of human PLCδ157,58. Photo-activation of SopB caused a sharp decrease in plasmalemmal PtdIns(4,5)P2 that coincided temporally with the appearance of PtdIns(3,4)P2 (Fig. 5f). Like the accompanying synthesis of PtdIns(3,4)P2, the decline in PtdIns(4,5)P2 was dependent on the integrity of the catalytic site of SopB (Fig. 5g,h). Similar results were obtained with IpgD (Extended Data Fig. 6b,c). These observations suggested that the decline in PtdIns(4,5)P2, previously noted to occur at the base of invasion ruffles55, might be required for the generation of PtdIns(3,4)P2.
To probe its requirement as a precursor for SopB-mediated PtdIns(3,4)P2 biosynthesis, we depleted PtdIns(4,5)P2 by recruiting PLCβ3 to the PM using a heterodimerization system59,60. PLCs are particularly useful in this context due to their low activity towards PtdIns(3,4,5)P3 and PtdIns(3,4)P2 61, while extensively depleting cellular PtdIns(4,5)P2 when recruited to the PM by a Lyn(11)-tagged FRB domain (Fig. 6a,b). Critically, the pre-recruitment of FKBP-tagged PLCβ3 to the PM led to a virtually complete inhibition of PtdIns(3,4)P2 formation in Salmonella-induced ruffles, as monitored by cPHx3 (Fig. 6c,d). Similarly, rapamycin-induced recruitment of the 5-phosphtase INPP5E –but not its catalytically inactive mutant– led to a marked reduction in PtdIns(3,4)P2 generation at invasion ruffles (Extended Data Fig. 7a,b). That INPP5E preserves PM PtdIns(4)P (Extended Data Fig. 7c-f) and does not generate additional second messengers (i.e. InsP3) argues for a direct role of PtdIns(4,5)P2 in the generation of PtdIns(3,4)P2 by SopB.
We also evaluated the role of PtdIns(4,5)P2 using photoactivatable SopB. We took advantage of endogenous PLCβ enzymes that were activated by addition of carbachol to cells over-expressing the M3 muscarinic receptor62. Thus, carbachol-mediated depletion of cellular PtdIns(4,5)P2 could be timed with the optogenetic activation of SopB (Fig. 6f). Pre-treatment with carbachol strongly depleted PtdIns(4,5)P2, as indicated by detachment of plasmalemmal PH-PLCδ1 (Fig. 7f), and blocked subsequent generation of PtdIns(3,4)P2 in response to photoactivated SopB. PtdIns(3,4)P2 was formed normally in these cells when carbachol pre-treatment was omitted (Fig. 7e). These results suggest that SopB consumes PtdIns(4,5)P2 as it generates PtdIns(3,4)P2. However, they do not rule out requirement for additional lipids or host factors.
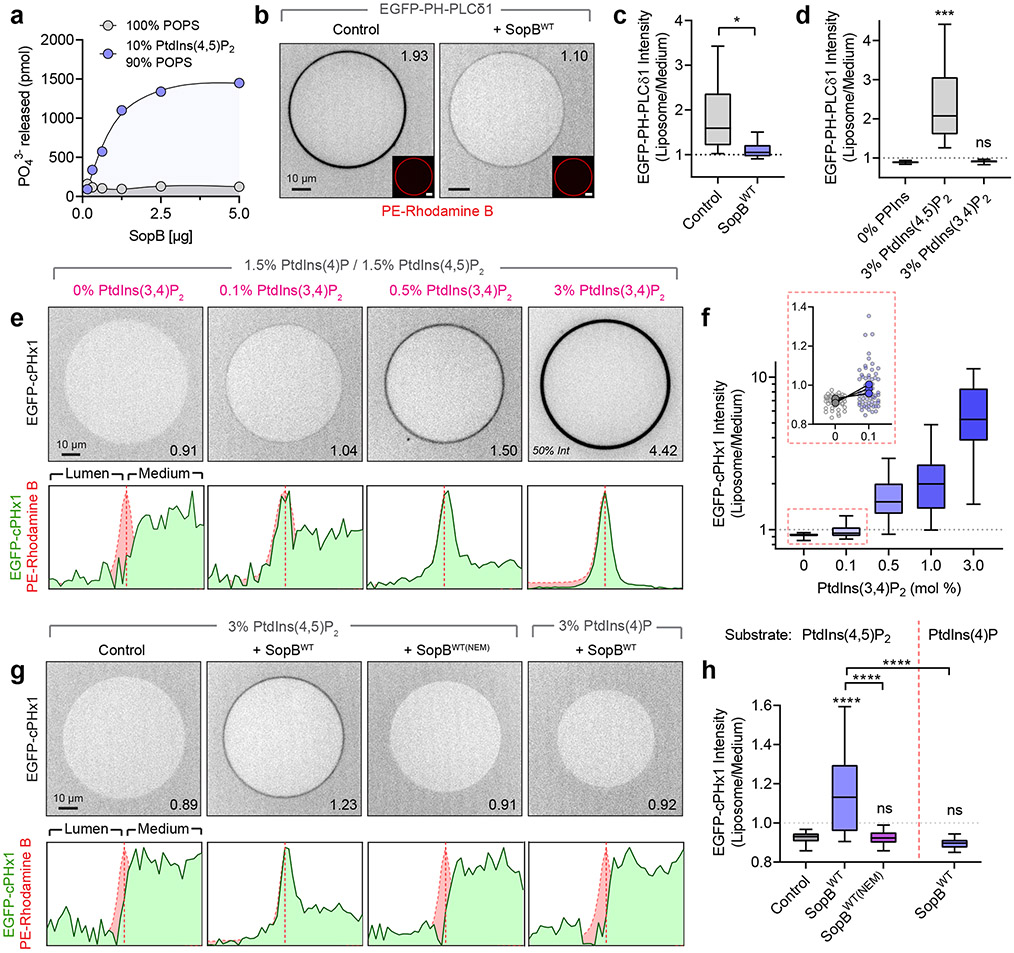
Fig. 7∣. In vitro reconstitution reveals SopB phosphotransferase activity.
(a) Inorganic phosphate release following 30 min treatment of liposomes of the indicated composition with recombinant SopBWT. Data are mean ± SEM of duplicate wells from a representative experiment.
(b) Confocal micrographs of GUVs treated for 30 min with SopBWT or an equal volume of dialysis buffer (control). Liposome composition was PtdCho:PtdSer:PtdIns(4,5)P2:PtdEth-Rhodamine B:DSPE-PEG-Biotin (76.8:20:3:0.1:0.1 mol %). Recombinant EGFP-PH-PLCδ1 (1 μM) was added before microscopy. Throughout the Figure, inset numbers are the Liposome/Medium EGFP intensity for the representative liposome.
(c) Normalized EGFP-PH-PLCδ1 liposome intensity from (b) across n=4 independent experiments (control, 142 GUVs; SopBWT, 155 GUVs). Data are median, box (25th-75th) and whisker (5th-95th) percentiles of individual liposome measurements. *P = 0.0269.
(d) Normalized EGFP-PH-PLCδ1 liposome intensity from n=3 independent experiments (0% PPIns, 86 GUVs; 3% PtdIns(4,5)P2, 84 GUVs; 3% PtdIns(3,4)P2, 83 GUVs). Data are median, box (25th-75th) and whisker (5th-95th) percentiles of individual liposome measurements. ***P = 0.0006.
(e) Representative micrographs of recombinant EGFP-cPHx1 (0.5 μM) incubated with increasing mol % PtdIns(3,4)P2. Background composition of liposomes was PtdCho:PtdIns(4,5)P2:PtdIns(4)P:PtdIns(3,4)P2:PE-Rhodamine B:DSPE-PEG-Biotin (77-X:1.5:1.5:X:0.1:0.1). Corresponding normalized intensity profiles of EGFP and Rhodamine B channels are plotted below.
(f) Corresponding normalized EGFP-cPHx1 liposome intensity from (e) across n=3 independent experiments (0%, 53 GUVs; 0.1%, 64 GUVs; 0.5%, 80 GUVs; 1.0%, 68 GUVs; 3.0%, 78 GUVs). Data are median, box (25th-75th) and whisker (5th-95th) percentiles of individual liposome measurements. Inset graph (red box) shows liposome measurements (background points) and paired trial averages (foreground points) from 0% and 0.1% PtdIns(3,4)P2-containing GUVs.
(g) GUVs treated for 30 min with dialysis buffer (control), SopBWT, or SopBWT(NEM) (enzyme pre-treated with a molar excess of NEM) were analyzed by confocal microscopy. Representative EGFP-cPHx1 localization is presented with normalized intensity profiles below. GUV compositions (mol %) were PtdCho:PtdSer:PtdIns(4,5)P2 or PtdIns(4)P:PtdEth-Rhodamine B:DSPE-PEG-Biotin (76.8:20:3:0.1:0.1).
(h) Quantification of (g) from n=5 independent experiments per condition (Control, 177 GUVs; SopBWT, 185 GUVs; SopBWT(NEM), 147 GUVs; SopB(WT)) or n=4 independent experiments (PtdIns(4)P substrate, 130 GUVs). Data are median, box (25th-75th) and whisker (5th-95th) percentiles of individual liposome measurements. ****P < 0.0001. Source numerical data are available in source data.
SopB functions as a phosphotransferase in vitro
We next considered the possibility that SopB could generate PtdIns(3,4)P2 directly through remodelling of pre-existing PPIns, likely PtdIns(4,5)P2. To this end we purified a recombinant construct consisting of residues 33-554 of SopB, that retains the putative catalytic site and has catalytic activity in vivo (Extended Data Fig. 3d). The phosphatase activity of this recombinant protein was initially validated measuring the release of inorganic phosphate from large unilamellar vesicles (LUVs) containing PtdIns(4,5)P2 and PtdSer; no phosphate was released from vesicles containing PtdSer only (Fig. 7a), confirming the specificity of the recombinant enzyme towards PPIns12.
The activity of the recombinant SopB was also validated using giant unilamellar vesicles (GUVs), which are amenable to analysis by confocal microscopy using fluorescent biosensors. GUVs were immobilized onto streptavidin-coated coverslips by incorporating biotinylated PtdEth (DSPE-PEG-Biotin) and detected by incorporating 0.1% rhodamine B-conjugated PtdEth. PPIns changes were monitored as the association/dissociation of purified PPIns-specific EGFP-tagged probes. As anticipated, purified recombinant EGFP-PH-PLCδ1 bound to the outer surface of GUVs containing PtdIns(4,5)P2, but not to those containing equimolar PtdIns(3,4)P2, confirming the specificity of the probe (Fig. 7d). Importantly, the binding of EGFP-PH-PLCδ1 diminished markedly when GUVs containing PtdIns(4,5)P2 were preincubated with SopB (Fig. 7b,c).
Considering these observations, it was conceivable that SopB was itself converting PtdIns(4,5)P2 or a product of its hydrolysis into PtdIns(3,4)P2. To address this possibility, we purified recombinant EGFP-cPHx1 to probe the formation of PtdIns(3,4)P2. EGFP-cPHx1 bound to GUVs in a manner proportional to the concentration of PtdIns(3,4)P2; no binding was detected in the absence of PtdIns(3,4)P2, even when other PPIns species were present (Fig. 7e,f). The lower limit of detection of PtdIns(3,4)P2 with this recombinant sensor was 0.1 mol% (Fig. 7f, inset). Using this system we tested whether SopB was capable of generating PtdIns(3,4)P2 in a recombinant system of defined composition. Liposomes containing PtdCho:PtdSer:PtdEth-Rhodamine:DSPE-PEG-Biotin (76.8:20:0.1:0.1) plus 3% of the PPIns of interest were incubated with SopB. EGFP-cPHx1 was added immediately before imaging to probe for the formation of PtdIns(3,4)P2. As shown in Fig. 7g and 7h, the addition of recombinant SopB to PtdIns(4,5)P2-containing liposomes caused translocation of EGFP-cPHx1 to the liposome surface. This effect was dependent on SopB activity, as it was abolished by pre-treating SopB with N-ethylmaleimide to modify cysteine residues63, including Cys460. The ability of SopB to produce PtdIns(3,4)P2 in this assay required PtdIns(4,5)P2; pre-incubation of SopB with liposomes containing an equimolar amount of PtdIns(4)P did not result in recruitment of EGFP-cPHx1 (Fig. 7g,h).
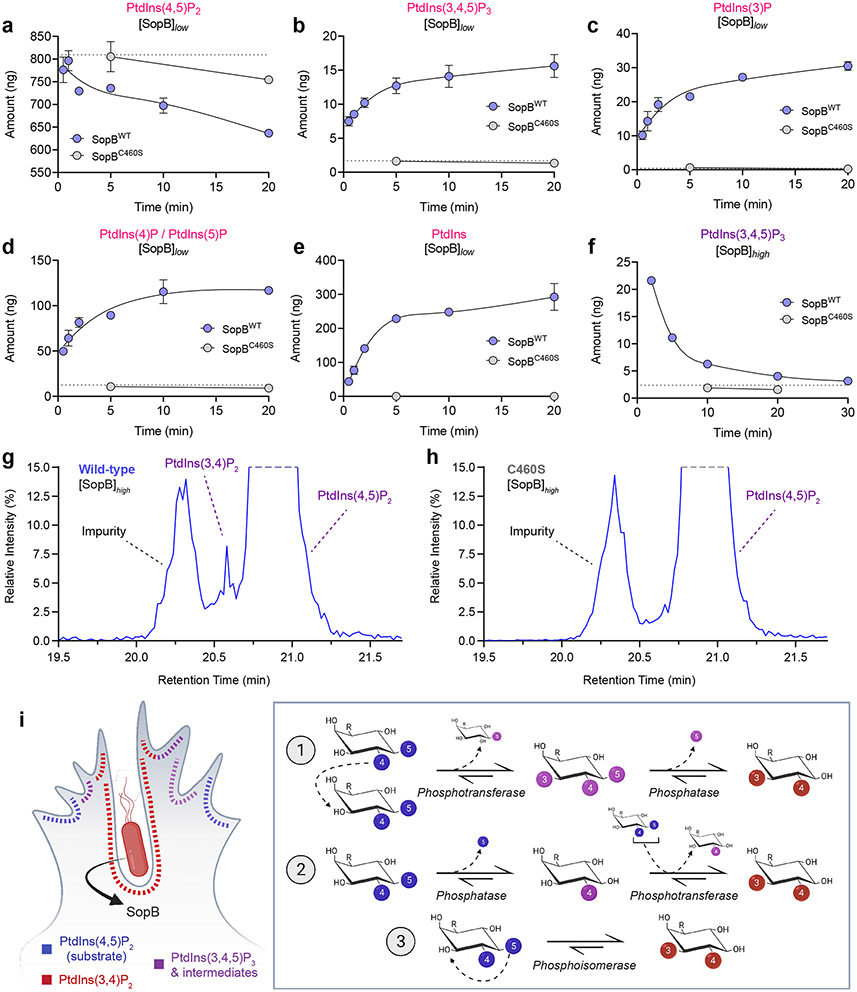
To gain quantitative insight into the flux of PPIns species under the influence of SopB, we analyzed enzyme-treated LUVs by high-performance liquid chromatography–mass spectrometry (HPLC-MS)64,65. Liposomes containing PtdCho:PtdSer:PtdIns(4,5)P2 (75:20:5) were incubated with wildtype or mutant enzyme. Treatment of liposomes with SopBC460S (grey, Fig. 8), which caused no detectable phosphate release (Extended Data Fig. 8a), served as negative control in these assays, as did LUVs incubated without enzyme (hatched lines). As expected, incubation of LUVs with SopBWT decreased PtdIns(4,5)P2 in a time-dependent manner (Fig. 8a). That SopB functions as a phosphatase capable of dephosphorylating the inositol ring at both 4- and 5-positions was confirmed by the progressive appearance of PtdIns(4)P/PtdIns(5)P (not differentiated by HPLC-MS; Fig. 8d) followed by PtdIns (Fig. 8e). Importantly, the HPLC-MS analyses also revealed that SopBWT acutely generates 3-phosphorylated PPIns including PtdIns(3,4,5)P3 (Fig. 8b,f), PtdIns(3,4)P2 (Fig. 8g and Extended Data Fig. 8b), and PtdIns(3)P (Fig. 8c). Gradual accumulation of PtdIns(3,4,5)P3 was observed when using a low (14 nM) concentration of SopB (Fig. 8b), while only a progressive decrease after rapid generation was seen with higher (72 nM) SopBWT (Fig. 8f). The peak corresponding to PtdIns(3,4)P2 was detected when using SopBWT (Fig. 8g; Extended Data Fig. 8b), but not when using SopBC460S (Fig. 8h). This peak could not be accurately quantified due to its closeness to two other, larger peaks.
Fig. 8. Time-resolved HPLC-MS analysis of SopB phosphotransferase-phosphatase activities.
(a-e) SopB generates multiple 3-phosphorylated PPIns species in vitro. Quantitative measurement of PPIns species by HPLC-MS. Initial LUV substrate composition (mol %) was PtdCho:PtdSer:PtdIns(4,5)P2 (75:20:5), with 0.5 nmol 18:0-20:4 PtdIns(4,5)P2 per reaction. [SopB]low-treated liposomes were incubated with either 0.1 μg (14.3 nM) recombinant SopBWT (blue) or SopBC460S (grey), or left untreated (no enzyme control, grey dotted line). At the indicated timepoints, samples were snap-frozen in liquid nitrogen. Samples were then quenched, internal standards added, and phosphate groups methylated by trimethylsilyl diazomethane during extraction. Following separation, PtdInsP3 levels (b) were quantified by infusing lipids into the mass spectrometer without ozonolysis, while the remainder (a,c,d,e) were analyzed post-ozonolysis. Data are presented as the mean amount of specified lipid (ng) per assay ± SEM from two biological replicates, corrected for relevant internal standards.
(f) PtdInsP3 was analyzed as in (b) but following the incubation of LUVs with 0.5 μg of enzyme per reaction (71.5 nM, [SopB]high). Data are plotted as the mean of duplicate HPLC-MS analyses from one biological reaction replicate.
(g,h) Separation of PtdIns(3,4)P2 and PtdIns(4,5)P2 regio-isomers in samples treated with (g) SopBWT or (h) SopBC460S. An example HPLC-MS trace is presented derived from [SopB]high reaction following 2 min incubation with enzyme. Note the de novo appearance of PtdIns(3,4)P2 at the highlighted elution time of ≈20.6 min.
(i) Schematic illustration of possible pathways of PtdIns(4,5)P2 to PtdIns(3,4)P2 conversion by the phosphoinositide phosphotransferase activity of SopB. During invasion, PtdIns(3,4)P2 accumulates both in PM ruffles and the invaginating regions of the PM prior to fission of the vacuole neck and closure of the vacuole. PtdIns(4,5)P2 suffices to generate PtdIns(3,4)P2 via three possible phosphotransfer-based mechanisms: 1) intermolecular transfer giving rise to PtdIns(3,4,5)P3, followed by 5-phosphatase activity; 2) intermolecular transfer preceded by 5-phosphatase activity; and 3) intramolecular transfer (phosphoisomerase). The former pathways differ in the predicted intermediate species. Source numerical data are available in source data.
It is noteworthy that the cell-free systems we used were devoid of high-energy phosphates (e.g., ATP) and of the divalent cations generally required by kinases. Thus, we concluded that, in addition to functioning as a polyphosphoinositide phosphatase in host cells, SopB possesses phosphotransferase activity, relocating the phosphate groups of PtdIns(4,5)P2 to yield 3-phosphorylated PPIns such as PtdIns(3,4)P2 (Fig. 8i). Whether this enzyme possesses an intramolecular transferase (i.e., a phosphoisomerase) activity in addition to its intermolecular transferase function (Fig. 8i) remains unclear.
Discussion
Several Enterobacteriaceae species diverged from harmless symbionts to parasites. A key evolutionary driver of this feat was the acquisition of virulence factors that facilitate cellular adhesion, invasion, and manipulation of host signaling22,66. The activation of the host pro-survival kinase AKT by a Salmonella and Shigella effectors was described over 20 years ago11,12,14. By regulating the survival and proliferation of the infected host cells, AKT counters the pro-apoptotic nature of co-secreted effectors, thereby supporting intracellular bacterial growth14,67-69. In this context, AKT also modulates the inflammatory response36,70-72, drives the expansion of M cells35, and clinically promotes infection-associated carcinoma73. AKT was known to be activated by PtdIns(3,4,5)P3 or PtdIns(3,4)P2, but how accumulation of the responsible lipid is induced by the enteropathogens had not been delineated. A previous unbiased screen identified 52 kinases that, when silenced, partially reduced AKT phosphorylation during Salmonella invasion74. How these kinases impinge on AKT regulation is unclear and may reflect indirect pleiotropic effects.
Our data indicate that SopB and IpgD are sufficient to acutely stimulate the synthesis of 3-phosphorylated PPIns in host cells, particularly PtdIns(3,4)P2. They accomplish this without need to harness any of the previously recognized 3-PPIns biosynthetic pathways, namely class I, class II, or class III PI3Ks. Rather, the lipid arises by an effector-catalyzed phosphotransferase reaction.
Several lines of evidence indicate that PtdIns(4,5)P2 is the sole substrate required for the generation of PtdIns(3,4)P2. First, PtdIns(3,4)P2 production during infection is restricted to the PM (Fig. 1), the main PtdIns(4,5)P2 reservoir in host cells. Second, the invariable cysteine residue (Cys460 in SopB, Cys439 in IpgD) that is essential for the effector-induced disappearance of PtdIns(4,5)P2 is also essential for PtdIns(3,4)P2 production (Fig. 2, 5, and Extended Data Fig. 6). Third, depletion of PtdIns(4,5)P2 by PLCβ or INPP5E obliterated the generation of PtdIns(3,4)P2 by SopB (Fig. 7 and Extended Data Fig. 7). Fourth, and most important, the presence of PtdIns(4,5)P2 –but not of other phospholipids, including other PPIns– was absolutely required for SopB to generate PtdIns(3,4)P2 in a cell-free system (Fig. 7 and 8).
SopB had earlier been reported to function as a PPIns phosphatase12,33, an observation we confirmed (Fig. 7a and Extended Data Fig. 8a). This activity involves Cys460 which resides in a Cys-X5-Arg motif – similar motifs are prevalent throughout the protein tyrosine phosphatase superfamily75. It is noteworthy that Cys460 is also essential for the generation of PtdIns(3,4)P2 from PtdIns(4,5)P2; we therefore propose that a common intermediate step underlies the phosphatase and phosphotransferase activities of SopB (and presumably IpgD). In the case of simple phosphatases, cysteine-mediated nucleophilic attack of a scissile phosphate generates a cysteinyl-phosphate intermediate that is normally hydrolyzed by water to complete the phosphatase cycle75. We envisage three possible catalytic mechanisms to account for the observed phosphotransferase reaction (Fig. 8i): Cys460 could act as a nucleophile, attacking one of the phosphates (e.g. D-5) on PtdIns(4,5)P2, breaking the bond between the phosphate and inositol ring, and forming a high-energy phospho-cysteine intermediate. Rather than abstracting a proton from water, as would occur during an ordinary phosphatase reaction, proton abstraction would occur from the 3-position of the inositol ring. The neighbouring Asp465 of SopB could conceivably donate a proton to the leaving group (D-5) of the inositol ring. Thus, the D-3 hydroxyl group nucleophile would be prompted to attack the phospho-cysteine intermediate, generating PtdIns(3,4)P2 and regenerating the free cysteine. By catalyzing such an intramolecular rearrangement, SopB would operate as a phosphoisomerase (Fig. 8i, reaction 3).
Transfer of a phosphate from one inositide to another (i.e., intermolecular) by an analogous mechanism is also plausible (Fig. 8i, reactions 1–2) and could operate in combination with the phosphatase activity of SopB. By this putative mechanism, the phospho-cysteine intermediate would attach a second phosphate onto position D-3 of PtdIns(4)P, generated previously by dephosphorylation of PtdIns(4,5)P2. This would require sequential phosphatase and phosphotransferase reactions. Lastly, the reverse sequence can also be contemplated (Fig. 8i, reaction 1): the phospho-cysteine intermediate could generate PtdIns(3,4,5)P3 by inserting an additional phosphate into PtdIns(4,5)P2. That PtdIns(3,4,5)P3 was readily detected by our HPLC-MS analyses in vitro (Fig. 8b,f) and was earlier seen in HPLC analyses of infected cells76 argues in favor of intersubstrate phosphate transfer. This mechanism would also be consistent with the generation of PtdIns(5)P previously detected in host cells77. During infection, however, we hypothesize that PtdIns(3,4,5)P3 is a fleeting intermediate in the SopB-induced biogenesis of PtdIns(3,4)P2. The multiplicity of active host 5-phosphatases make it likely that PtdIns(3,4,5)P3 generated by SopB would be rapidly converted to PtdIns(3,4)P2 (Extended Data Fig. 8e). This may account for the minute amount of PtdIns(3,4,5)P3 detected (Fig. 1,2 and Extended Data Fig. 1,2) and would also explain why biochemical measurements of infected cells revealed PtdIns(3,4)P2 to be much more abundant (~7-fold) than PtdIns(3,4,5)P376.
Of note, deletion of residues 520 to 554 of SopB completely abrogated its phosphotransferase activity (Extended Data Fig. 3d). Multiple cationic residues encoded within this region could conceivably form an electrostatic counterpart to stabilize the negatively charged inositol head group during phosphate transfer. Mutation of two of these sites, Lys525 and Lys528, decreased AKT activation in response to SopB12 and suppressed the phosphotransferase activity of SopB (Extended Data Fig. 5a-d). Consistent with an accessory role, these residues are structurally predicted to neighbour the Cys-X5-Arg motif of SopB (Extended Data Fig. 5e,f)78.
In summary, this work identifies an unprecedented PtdIns(4,5)P2 to PtdIns(3,4)P2 conversion mechanism mediated by a phosphotransferase. Through a remarkable instance of convergent evolution, pathogenic organisms have acquired the ability to manipulate host signaling pathways that are central to endocytosis and survival signaling and can even provoke cellular transformation as illustrated by the development of gallbladder carcinomas during Salmonella infection73.
Methods
Plasmids and siRNA
Plasmids utilized in this study are summarized in Supplementary Table 1 and were verified by Sanger sequencing. Plasmids were constructed using In-Fusion HD EcoDry Cloning Kits (Takara 121416), NEB HiFi assembly (New England Biolabs E5520S), or traditional restriction enzyme methods. Site-directed mutagenesis was performed using targeted pairs of oligonucleotides that centrally housed the mutation. The codon corresponding to lysine 464 of wild-type and C460S SopB was substituted with the infrequently used Amber STOP codon (TAG) for photoactivation studies. The plasmid HAx3-AChR-M3 was kindly provided by J. Wes, SopBWT-EGFP by Daoguo Zhou, and cDNA to generate pUG34-GFP-2xFYVEVps27 by Scott D. Emr79. The plasmids Myc-p110α-CAAX80, Myc-PI3K-C2α-CAAX81, mRFP-FKBP-PLCβ382, Lyn11-FRB-HA83, and pSpCas9 (BB)-2A-Puro84 were previously generated.
Custom basic RNA oligos (Thermo Fisher 10620310) targeting PIK3C2A were 5'-GGAUCUUUUUAAACCUAUU-3' (sequence 1) and 5'-GCACAAACCCAGGCUAUUU-3' (sequence 2)23. 5’-phosphorylated oligonucleotides were suspended in water to 20 μM. An equimolar amount of ON-TARGETplus Non-targeting Control Pool (Dharmacon D-001810-10-20) was used as control siRNA.
Reagents, Lipids, and Antibodies
Commercially available inhibitors, stains, lipids, and other reagents are summarized in Supplementary Table 2. Commercially available primary and secondary antibodies and their dilutions are summarized in Supplementary Table 3. Unless otherwise stated, antibodies were incubated in 2.5% (w/v) milk powder in TBS-T during immunoblotting.
Commercially purchased diC16 PPIns were solubilized in 1 mL of 2:1:0.01 CHCl3:MeOH:1M HCl, vortexed, and dried under N2 stream before three resuspension and drying cycles in CHCl3. Finally, diC16 PPIns were resuspended in CHCl3 to 0.5 mg/mL. C18:0-20:4 PtdIns(4,5)P2 was synthesized by the Biological Chemistry Department at Babraham Institute (Babraham, Cambridge) and was dissolved in 20:9:1 CHCl3:MeOH:Water. Hydroxycoumarin lysine43 was synthesized by the Department of Chemistry at the University of Pittsburgh (Pittsburgh, Pennsylvania) and was dissolved at 100 mM in DMSO.
Cell culture and transfection
HeLa (CCL-2) and Henle 407 (CCL-6) of low passage number (<5) were obtained from the American Type Culture Collection (ATCC) and maintained in DMEM with 1.5 g/L sodium bicarbonate, sodium pyruvate (Wisent Bio Products 319-007-CL) and 10% heat-inactivated fetal bovine serum (Gibco 12483-020). Cells were incubated at 37 °C in a humidity-controlled atmosphere at 5% CO2 and were passaged 1:5-10 two to three times per week by detachment with 0.25% Trypsin-EDTA (Wisent Bio Products 325-043-EL). HeLa cells were mycoplasma-negative before freeze-down and were utilized between passage 5 and 25 from receipt. For photoactivation experiments, HeLa were cultured in low glucose DMEM (Life Technologies 10567022) containing 10% heat-inactivated fetal bovine serum (Life Technologies 10438-034), penicillin (100 units/mL), streptomycin (100 μg/mL; Life Technologies 15140122) and chemically-defined lipid supplement (1:1000; Life Technologies 11905031).
Cultures were enumerated (Z2 Beckman Coulter) and 8.5x104 cells were seeded two days before bacterial invasion on 18 mm Number 1.5 glass coverslips (Fisher Scientific 12545100). Per two coverslips transfected, 1.5 μg total DNA was combined with 4.5 μL FuGENE 6 (Promega E2691) pre-complexed in 100 μL serum-free. Cells were maintained in serum containing medium unless otherwise stated.
For photoactivation experiments, plasmids encoding SopB464TAG, the engineered pyrrolysyl-tRNA synthetase/tRNA pair, and various biosensors were transfected. Medium was supplemented with 250 μM of hydroxycoumarin lysine (HCK) in parallel or 4 h after transfection for unnatural amino acid incorporation. In response to the UAG (Amber) codon mutagenized in sopB, HCK is transacylated onto the tRNA and ribosomal incorporation of HCK in the growing polypeptide occurs in lieu of lysine 464 of SopB.
For RNA interference, cells were seeded in 12-well plates at 1.1x105 cells/well and transfected according to the manufacturer’s instructions on day 2 with 100 nM siRNA in Opti-MEM (Gibco 31985-070) using Lipofectamine RNAiMAX (Thermo Fisher 13778030). Media was changed on day 3 prior to a second round of siRNA transfection. Cells were lifted on day 4, counted, and seeded in parallel for immunoblotting (1.6x105 cells/well) and invasion experiments (1.3x105 cells/coverslip). Plasmids encoding biosensors were transfected 6-8 h after re-plating as described above. On day 5, lysates were collected for immunoblotting from the commonly derived population of cells utilized for bacterial invasion and microscopy.
Imaging commenced 18-24 h post-transfection except for heterologous expression of SopB where 8-14 h yielded sufficient expression, and TagBFP2-INPP4B-CAAX which yielded optimal expression 40-48 h post-transfection. Where indicated, the PM of transfected cells was stained with CellMask. Coverslips were submerged in pre-chilled Tyrode’s buffer (140 mM NaCl, 5 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 10 mM D-Glucose, 10 mM HEPES pH 7.4) containing 0.5-1.0 μg/mL CellMask Deep Red, incubated for 4 min at 10 °C, and washed gently before fixing or imaging live in chilled Tyrode’s buffer to reduce endocytosis.
CRISPR-Cas9-mediated genome editing
Cdc42-specific single-guide RNAs (sgRNAs) were designed using the Graphical User Interface for DNA Editing Screens available at http://guides.sanjanalab.org/ and are summarized in Supplementary Table 4. Annealed oligonucleotides were ligated into pSpCas9 (BB)-2A-Puro84. A CACCG 5’ flanking sequence was added to each sense oligonucleotide and an AAAC 5’ flanking sequence to each anti-sense oligonucleotide for ligation into the BbsI site. A mixture of the ligated vectors was transfected and puromycin (2 μg/mL) was added to the culture medium 48 h post-transfection for 48 h. Single cell-dilutions were transferred into 96-well plates and allowed to recover to near confluency, at which time the knockout efficiency was determined by immunoblotting.
Salmonella strains, culture, and invasion
Salmonella strains used are derivatives of wild-type Salmonella enterica subspecies I serovar Typhimurium strain SL134485. Isogenic ΔsopB SL134411, ΔsopE/sopE2 SL134486, and ΔsopB SL1344 transformed with pACDE-SopB-myc were generated previously87. BFP- and mRFP1-expressing strains were generated by electroporating low copy plasmids that encoded each fluorophore (pFPV25.1 where EGFP was replaced by BFP, and pBR322 encoding mRFP1) under the control of the rpsM promoter88.
Epithelial cells were exposed to late-log phase Salmonella by a method optimized for bacterial invasion89. Several colonies were inoculated into 2 mL LB with antibiotic selection and grown shaking overnight at 37 °C. Stationary phase cultures were diluted 1:33 into fresh LB without antibiotics for 3.5 h at 37 °C and inocula were prepared by centrifugation at 10,000 x g for 2 min before resuspending in an equivalent volume of D-PBS. Each inoculum was diluted 1:50 (BFP- and mRFP-expressing strains) to 1:100 in D-PBS and 1 mL was added per well for 10 min at 37 °C. During time course studies, extracellular bacteria were removed by extensively washing and 100 μg/mL gentamycin was added to the cell culture medium 30 min post-infection. The concentration of gentamycin was reduced to 10 μg/mL 2 h post-infection for the remainder of the experiment.
To monitor biosensors statically during invasion, coverslips were submerged for 5 min in pre-chilled D-PBS containing CellMask (1 μg/mL), followed by two gentle washes, and PFA (2% w/v) fixation. Fixed cells were imaged immediately following PBS washes. To label the PM with a fluorescently labeled lectin, infected cells were incubated for 10 min in pre-chilled Tyrode’s buffer containing 10 μg/mL WGA-Texas Red before washing and fixing.
Yeast strains and culture
SEY6210 wild-type S. cerevisiae (MATα leu2-3,112 ura3-52 his3-Δ200 trpl-Δ901 lys2-801 suc2-Δ9 GAL) 90 and SEY6210 vps34Δ1::TRP1 (PHY102) 53 transformed with a low-copy plasmid (CEN URA3) encoding a temperature-sensitive mutant of the vps34 allele were generated previously 54. Plasmids were transformed by the lithium-acetate method and strains maintained on appropriate minimal medium containing 1.7 g/L yeast nitrogen base without amino acids, 5 g/L ammonium sulphate, amino acids, 2% (w/v) D-Glucose or 2% (w/v) D-Galactose, and 2% (w/v) bacto-agar. Yeast strains grown overnight in appropriate media at 28-30 °C were sub-cultured into fresh glucose-containing medium at 0.25 OD600 units and allowed to grow for 3 h. Logarithmically growing cells were transferred into galactose-containing medium for 2 h to induce SopB or IpgD before imaging. Vps34p was inactivated in the vps34ts strain by shifting cultures to 38 °C (non-permissive temperature) for 90 min prior to inducing SopB with galactose for an additional 60-90 min. Cells were collected by centrifugation and were resuspended in medium containing 0.1% Trypan Blue prior to mounting on 2% agarose (w/v) pads overlaid with Number 1.5 1.8-cm glass coverslips. Where indicated, Concanavalin A (5 μg/mL) was added for 10 min prior to centrifugation to demarcate the cell wall.
Immunostaining
Paraformaldehyde (2% w/v)-fixed cells were permeabilized in 0.1 % (v/v) Triton X-100 in PBS for 5 min, blocked in 2% (w/v) BSA in PBS for 20 min, and overlaid consecutively for 1 h with primary and secondary antibodies in 1% BSA, separated by PBS and BSA washes. To stain epitope tagged SopB, cells were permeabilized for 30 min with 0.2% saponin in PBS supplemented with 10% goat serum (v/v). Primary and secondary antibodies were overlaid in permeabilizing solution for 60 min, separated by PBS washes. Invasion efficiency was assessed by differential inside-outside staining: coverslips were sequentially blocked and stained in PBS containing 10% goat serum, prior to proceeding with saponin permeabilization and staining internalized bacteria.
Recombinant protein production
Recombinant proteins were purified from BL21 (DE3) E. coli (NEB C2527) using Isopropyl β-D-1-thiogalactopyranoside (IPTG)-inducible expression systems and amino-terminal hexa-histidine tags. Colonies were inoculated into Terrific Broth containing 4 mL/L glycerol (Multicell Cat No. 800-067-LG: total volume 5 mL), 50 ug/mL Kanamycin, and 0.8 % (w/v) D-Glucose. Starter cultures were incubated shaking overnight at 37 °C before 1:50 dilution into 300 mL pre-warmed Terrific Broth with antibiotic selection. At OD600 0.6-0.8, cultures were equilibrated (23 °C) and induced with 0.05-0.1 mM IPTG for 20 h at 23 °C (210 rpm) before harvesting by centrifugation (5,000 x g for 15 min, 4 °C) and freezing for downstream purification.
Pellets containing His6-EGFP-cPHx1 were resuspended in 20 mL of 150 mM NaCl, 20 mM Tris, pH 7.4 (10 °C), 1x EDTA-free protease inhibitors (Thermo Fisher A32955), and DNase I (Sigma-Aldrich DN25). Pellets containing His6-EGFP-PH-PLCδ1 or His6-SMT3-SopB33-554 were resuspended in 25 mL of 300 mM NaCl, 40 mM Tris, 15 mM Imidazole, 5% (v/v) glycerol, pH 7.4 (10 °C), 5 mM β-mercaptoethanol, protease inhibitors, and DNase. Bacteria were disrupted by French press (SLM AMINCO Spectronic Instruments) before centrifugation (15,000 x g for 20 min, 4 °C). Clarified supernatants were incubated with TALON® Metal Affinity Resin (Takara 635502) and rotated at 4 °C for 30 min (cPHx1) to overnight (PH-PLCδ1, SUMO-SopB). Resin was washed twice with 10-15 mM Imidazole in lysis buffer, before loading into a TALON® 2 ml Gravity Column (Takara 635606). Columns were washed once before elution in a single step (150 mM Imidazole, cPHx1) or a stepwise gradient (15, 30, 40, 50, 60, 180, 180, 240 mM Imidazole; PH-PLCδ1, SUMO-SopB). Peak fractions analyzed by SDS-PAGE were pooled.
cPHx1 fractions were dialyzed several hundred-fold into imidazole-free buffer (150 mM NaCl, 20 mM Tris, pH 7.4) during concentration by an Amicon® Ultra-15 Centrifugal Filter Unit (Millipore UFC903008). PH-PLCδ1 fractions were dialyzed (ThermoFisher 66380; Slide-A-Lyzer™ Dialysis Cassettes) into imidazole-free buffer (150 mM NaCl, 20 mM Tris, 5% (v/v) glycerol, 0.5 mM β-mercaptoethanol, pH 7.4). SMT3-SopB33-554 fractions were diluted with additional buffer before dialysis. Products were aliquoted and snap frozen in N2(l) for storage.
Unilamellar Vesicle Assays
Giant unilamellar vesicles (GUVs) were generated on a film of agarose91. Glass slides overlaid with 1% (w/v) ultra-low gelling temperature agarose were gently heated until translucent. Liposome compositions were prepared by adding chloroform-suspended lipids to glass vials, drying under N2(g), and resuspending in a small volume of chloroform (generally ≈1.33 μmol total lipid in 35 μL chloroform). Lipids were spread over the agarose, chloroform was thoroughly evaporated under N2(g), and slides were submerged in assay buffer (200 mM NaCl, 20 mM Tris, pH 7.4). GUVs harvested 3-20 h later were used directly or centrifuged (4,000 x g, 5 min) to concentrate. Preparations were used within 7 days by storing at 4 °C.
To stabilize GUVs for fluorescence microscopy, acetone-washed coverslips were passivated with a 3:1 mixture of PLL(20)-g[3.5]- PEG(5) to PLL(20)-g[3.5]- PEG(2)/PEG(3.4)-biotin(20%) at 100 μg/mL. After washing, 25 μg/mL streptavidin was overlaid to allow coupling to the biotin conjugate. GUVs containing DSPE-PEG(2000)-Biotin could thus adhere to the glass surface via sequential biotin-streptavidin-biotin interactions. During enzymatic reactions, 6 μg of SopB (428.9 nM) was incubated at 37 °C for 30 min in assay buffer containing liposomes (≈666 μM) with freshly thawed dithiothreitol (5 mM). Fluorescent biosensors (1 μM His6-EGFP-PH-PLCδ1; 0.5 μM His6-EGFP-cPHx1) were added immediately before microscopy.
Large unilamellar vesicles (LUVs) were generated by sonication and extrusion92. The required amount of each lipid was dried under N2(g) stream prior to resuspending in assay buffer (200 mM NaCl, 20 mM Tris, pH 7.4 at room temperature). Resuspended lipids were moved to 37 °C and vortexed every 10 min for 30 min to generate multilamellar vesicles, prior to bath sonicating for 10 min. Lipid suspensions were extruded 18 times through a single polycarbonate 1000 nm-pore filter in a LiposoFast (Avestin), according to the manufacturer’s recommended setup, to generate unilamellar vesicles.
Phosphate release from LUVs was assessed using malachite green. A 50 μL mixture of assay buffer containing DTT, enzyme, and LUVs (1 nmol lipid/μL) was utilized per well of a 96-well plate. This provided a theoretical maximum of 1250 pmol free PO43- assuming complete hydrolysis of the 4- and 5-positions of the inositide and equal bilayer distribution of lipids. Reactions were terminated with molar excess N-ethylmaleimide, or by directly adding 100 μL malachite green solution. Data were plotted after calibrating absorbance measurements of KH2PO4(aq) in 100% POPS or by directly plotting 620 nm absorbance without calibration.
For HPLC-MS assays, LUVs contained stearoyl-arachidonoyl (18:0-20:4) PtdIns(4,5)P2. A 100 μL mixture of pre-warmed assay buffer with DTT and LUVs (10 nmol lipid) was utilized, providing 0.5 nmol (≈523 ng) PtdIns(4,5)P2 per reaction. 0.5 μg (71.5 nM; [SopB]high) or 0.1 μg (14.3 nM; [SopB]low) of total enzyme was added before incubating at 37 °C with 350 rpm rotation. At the indicated timepoints, reaction tubes were snap frozen in N2(l) and stored at −80 °C for HPLC-MS analysis.
HPLC-MS
To each 100 μL reaction volume, 750 μL quench solution containing MeOH:CHCl3:1 M HCl (484:242:23.55) and 70 μL water was added. Before extraction, 10 μL of the following internal standards suspended in CHCl3:MeOH:H2O (20:9:1) were added, except PtdIns which utilized 40 μL: d6-18:0-20:4 PtdIns(4)P (9.25 ng/μL), d6-18:0-20:4 PtdIns(4,5)P2/PtdIns(3,4)P2 (1:1 mix at 10 ng/μL), and d6-18:0-20:4 PtdIns (1.87 ng/μL). For PtdInsP3 analysis, the internal standard utilized was 10 ng of 1-heptadecanoyl-2-hexadecanoyl-sn-glycero-3-(phosphoinositol-3,4,5-trisphosphate) (C17:0-16:0 PtdIns(3,4,5)P3), as a hepta-sodium salt suspended to 1 ng/μL in MeOH:H2O (1:4). All internal standard lipids were synthesized by the Biological Chemistry Department at Babraham Institute (Babraham, Cambridge).
PPIns were extracted and quantified by mass spectrometry64. Prior to ozonolysis, 20 μl (11%) of the extract was removed and made up to 50 μl final in 4:1 (v/v) MeOH:H2O for PtdInsP3 analysis as optimized previously65.
Data are presented as the amount of specified lipid per sample corrected for relevant internal standards and are averages of duplicate samples. The PtdIns(4)P internal standard was used to correct both 18:0-20:4 PtdIns(4)P and 18:0-20:4 PtdIns(3)P. The 1:1 mixture of PtdIns(4,5)P2/PtdIns(3,4)P2 allowed assessment of regio-isomer separation and correction for endogenous (substrate) 18:0-20:4 PtdIns(4,5)P2.
Image Acquisition and Photoactivation
Spinning disk confocal microscopy was performed on a Quorum WaveFX spinning disk system (Quorum Technologies Inc.) consisting of an Axiovert 200M microscope (Carl Zeiss), equipped with a 63x/1.4 NA oil objective and 25x/0.8 NA multi-immersion objective multiplied by a 1.53x magnifying lens. Scanning is performed by the CSU10 confocal multi-beam scanner (Yokagawa). Fluorophores were excited consecutively by a four-line laser module (405, 491, 561, 640 nm; Spectral Applied Research) and filtered by a corresponding emission filter wheel (447/40, 515/40, 515 LP, 594/40, 624/40, 670/40). Signal was detected by a back-thinned EM-CCD camera (Hamamatsu ImageEM C9100-13). This system is driven by a motorized XY stage (Applied Scientific Instrumentation) and Piezo Focus Drive (Ludl Instruments). Acquisition settings and capture were controlled by Volocity v6.2.1 (PerkinElmer). For live cell imaging, coverslips were mounted within a Chamlide™ magnetic chamber (Live Cell Instrument Inc.) overlaid with pre-warmed phenol red-free Tyrode’s buffer and maintained at 37 °C using an environmental chamber (Live Cell Instruments Inc.). Up to 10 fields were acquired consecutively with 0.3 μm Z-steps to capture entire bacterial invasion ruffles.
Airyscan microscopy was performed on the LSM880 Airyscan system (Carl Zeiss) which is equipped with a 63x/1.4 NA oil objective and three detectors: two PMT and one 32-channel spectral Airyscan PMT. Laser lines were 488 nm (Argon) 552 nm, and 642 nm under Airyscan mode. Acquisitions are driven by motorized XY stage and Z-Piezo Drive (Carl Zeiss), and settings and capture controlled by Zen Black software v2.3 (Carl Zeiss).
Optogenetic experiments were imaged on a Nikon TiE inverted stand confocal microscope with an A1R resonant scan head and fiber-coupled four-line excitation (Ex) LU-NV laser combiner (405, 488, 561, 640 nm). 8 or 16 frame averaging was used to improve signal-to-noise. A 100× 1.45 NA plan-apochromatic oil immersion objective was used. To minimize crosstalk, blue (emission 425-475 nm) and yellow/orange (emission 570-620 nm) fluorescence were acquired on a separate excitation scan to the green (emission 500-550 nm) and far red (emission 663-737 nm) channels. Optogenetic activation of SopB was performed after acquiring ≈30 s of data with 405 nm illumination set to zero power, at which time transmission was increased to 20% of the maximum available power from the LU-NV unit. Acquisition settings and capture were controlled by Nikon Elements AR-5.21.02. Cells were imaged in FluoroBrite DMEM (Life Technologies A1896702) supplemented with 25 mM Hepes (pH 7.4), chemically defined lipid supplement (1:1000; Life Technologies 11905031), and 10% FBS.
Western blots were imaged digitally on an Odyssey FC System (LI-COR) equipped with 685 nm and 785 nm lasers and acquisition-controlled by Image Studio v4.0 software (LI-COR).
Image Analysis
Nikon (.nd2) and Volocity (.mvd2) files were exported and analyzed in FiJi v1.53f51. To quantify biosensor intensity in the bulk PM or invasion ruffles, an index was produced by normalizing the fluorescence intensity in the membrane to the cytosol after background-correcting. Data are graphically presented as the IntPM/IntCytosol for photoactivation data and IntPM - IntCytosol/IntCytosol for remaining cell culture experiments. Baseline correction of optogenetic datasets was computed by subtracting the average ratio of frames before photoactivation from the ratio along the time lapse. The PM was defined by the signal of CellMask staining (see below) or by manually selecting a region of interest (ROI) based on lipid biosensors enriched at the PM prior to photoactivation. Except for the direct comparison of cPHx3 in cells expressing SopBWT-464TAG-EGFP and SopBC460S-464TAG-EGFP (Fig. 2f), optogenetic activation data were filtered for optimal SopB uncaging by monitoring cells with baseline (t = 0 s) cPHx3 IntPM/IntCytosol <1 (t = 0 s) and/or IntPM/IntCytosol >1 at t = 300 s.
To estimate fluorescence intensity in the bulk PM, a binary mask of the compartment was generated by à trous wavelet decomposition93,94 using CellMask fluorescence as a PM marker. The resulting wavelet product was thresholded to include pixel intensities several fold-times the standard deviation of the wavelet product (empirically determined per dataset and ranged from 0.8-1.5 x StdDev). After manually producing ROIs that encompassed single transfected cells and restricting measurement to within that region, the resulting binary mask served to quantify the fluorescence intensity of a given biosensor in the PM compartment.
To capture biosensor recruitment to bacterial invasion ruffles, individual circular ROIs (pixelradius = 100 or ≈13.2 μm) encompassing single invasion sites were manually annotated. As above, fluorescence intensity of biosensors was quantified within a binary mask generated from parallel acquisition of CellMask. Binary masks of ruffles were generated by first smoothing CellMask images with a 3x3 neighbourhood pixel averaging. After converting to an 8-bit gray scale, a local threshold was then applied to images using Bernsen’s method computed for a sliding circular window with pixelradius = 3. Biosensor intensity was then analyzed within the circular binary mask of the invasion membrane.
To enumerate the number of SopB-EGFP puncta per cell, the wavelet product of maximum intensity projections was first calculated by à trous wavelet decomposition. This product was thresholded by Bernsen’s method (pixelradius = 3) and particles meeting the following criteria were annotated: size 0-200 pixel2, circularity 0.5-1.0, and mean particle intensity ≥ 2-fold the total cellular median intensity. Transfected cells reaching mean EGFP intensity ≥ 1.25-fold that of the background intensity were included in the analysis and identified puncta were reported per transfected cell.
To quantify biosensor recruitment to liposomes, an annulus of the liposome surface was generated and compared to an annulus of the surrounding medium. Otsu’s method was applied to the PE-Rhodamine channel to threshold pixel intensities of individual liposomes, and the binary mask was filled and iteratively eroded. The resulting binary shape conforming to the shape of the liposome was outlined and dilated to create a 4-pixel wide annulus of the liposome surface. A corresponding ‘medium’ measurement was generated by dilating this annulus 10 pixels beyond the liposome surface. Data are presented as the resulting liposome/medium intensity ratio per liposome.
Immunoblot densitometric calculations were performed in Image Studio Lite v5.2 (LI-COR).
Statistics and Reproducibility
Data were imported into GraphPad Prism 9 for statistical analysis and presentation. Superplots95 were generated to communicate cellular and invasion-level variability (background plots) and trial-to-trial variability (overlayed in the foreground). The number of cells analyzed, number of independent experiments (separate days), and statistical results are indicated within individual Figure Legends. No statistical method was used to predetermine sample size and no data were excluded from analyses. The experiments were not randomized. The Investigators were not blinded to allocation during experiments and outcome assessment. Cell culture experiments were repeated on separate passages of cells. Except for photoactivation assays, trial averages or independent experimental values, rather than individual cell measurements, were subjected to statistical analyses. Shapiro-Wilk normality tests (n ≥ 3) were consistent with a normal distribution, so parametric tests were applied. Phosphate release and HPLC-MS data are plotted directly and fit to a curve by the smoothing spline (4 knots) feature. To compare photoactivation datasets, baseline-corrected data were sorted into individual groups of curves each comprising measurements of a normalized ROI across an entire time lapse. The area under the curve (AUC) was calculated for each time lapse, and the resulting net AUCs were sorted by condition to compare groups statistically. Shapiro-Wilk normality tests were applied to each grouping, and parametric or non-parametric t-tests were applied accordingly as described below.
Significance was assessed by an ordinary one-way ANOVA with Bonferroni’s multiple comparisons test in Figure 1b, 1e, 3b, 3e, 3j, 3l, 4b, 7d, 7h and Extended Data Figure 1d, 4c, 5c, 6e, 6g, 7c, 7e. Significance was assessed by an ordinary two-way ANOVA of trial averages with Bonferroni’s multiple comparisons test in Figure 4e. Significance was assessed by an unpaired two-tailed t-test in Figure 2b, 6b, 6d and Extended Data Figure 2c, 2e, 3g, 5d, 6c, 7b. Significance was assessed by an unpaired one-tailed t-test in Extended Data Figure 4a, by an unpaired one-tailed t-test with Welch’s correction in Figure 5e, and by a two-tailed ratio paired t-test of trial means in Figure 7c. A non-parametric one-tailed Mann-Whitney test of ranks was utilized to assess significance in Figure 3g and 5h. Exact P values are described within individual Figure Legends. P > 0.05 was considered not significant (ns).
Representative images were chosen based on being phenotypical, possessing good signal-to-noise ratio, and being quantitatively representative of the dataset. Merging and cropping fluorescent channels was performed in FiJi. To aid visualization, linear adjustments were made to brightness and contrast across the entire image or alternative lookup tables were applied. Exported RGB format .tiff images were sized once in Adobe Photoshop v21.1.1 to the final publication format prior to assembling in Adobe Illustrator CS6 v16.0.0.
Extended Data
Extended Data Fig. 1. Rapid and sustained PtdIns(3,4)P2 synthesis during Salmonella entry and maturation.
(a) Model of PtdIns(3,4)P2 biosensors based on single, double-, or triple-tandem carboxy-terminal PH domains from TAPP1 (PLEKHA1). NES, nuclear export signal. Right, gel electrophoresis of PCR amplicons generated by primers that house the open reading frames.
(b) Cells expressing cPHx1, cPHx2, or cPHx3 were infected for 10 min with wild-type Salmonella prior to staining the PM with CellMask. Representative maximum intensity projections (main) and a corresponding confocal section of the invasion ruffle (bottom panels) are presented.
(c) Confocal imaging of cells (three examples in I, II, and III) expressing cPHx3 during invasion by wild-type Salmonella. Bottom vertical panels are expanded from the white box region and correspond to the minute-by-minute time series. cPHx3 is also presented in a gray inverted lookup table (RGB intensity 0=white, 255=black).
(d) As in (c), three examples (I, II, and III) of cells expressing the PtdIns(3,4,5)P3 sensor aPHx2 during invasion by wild-type Salmonella.
(e) Cells serum-starved for 3 h were infected by Salmonella. Extracellular bacteria were removed, and cells returned to serum-free medium containing gentamycin. PM cPHx3 intensities were quantified in the following number of cells: 59 (control), 60 (WT, 30 min), 42 (ΔsopB, 30 min), 58 (WT, 60 min), 53 (ΔsopB, 60 min), 75 (WT, 120 min), 48 (ΔsopB, 120 min), 66 (WT, 240 min), and 36 (ΔsopB, 240 min) across n=3 independent experiments. Data are trial means ± SEM (foreground) overlaid on cell measurements (background). Data from Fig. 1c are presented with ΔsopB infections. ****P < 0.0001; **P = 0.0054 (UI-vs-WT60), **P = 0.0044 (WT120-vs-ΔsopB120); ns, not significant.
(f) Cells serum-starved for 3 hours, were exposed to Salmonella (wild-type or ΔsopB) for 10 min. Extracellular bacteria were removed, and cells were returned to serum-free medium with gentamycin. Lysates were collected at the indicated time-points and analyzed on parallel membranes for pAKT (S473) or pAKT (T308) prior to stripping and re-probing for total AKT or GAPDH (loading control). Representative immunoblots are presented from n=2 independent experiments. UI, uninfected. Source numerical data and unprocessed blots are available in source data.
Extended Data Fig. 2. PtdIns(3,4,5)P3 analysis during Salmonella invasion or during optogenetic activation of SopB.
(a) Model of the PtdIns(3,4,5)P3 biosensor NES-EGFP-aPHx2 derived from tandem ARNO PH domains (2G splice variant, I303E mutation). NES, nuclear export signal.
(b) Cells were exposed to invasive RFP-expressing wild-type or isogenic ΔsopB Salmonella and the PM was stained with CellMask prior to imaging. Maximum intensity projections (main) and single confocal sections of the invasion ruffle are presented at right for each.
(c) Quantification from (b). Normalized intensity of aPHx2 in the PM of invasion sites from n=3 independent experiments analyzing 71 (WT) and 63 (ΔsopB) invasion sites. *P = 0.0294.
(d) Normalized intensity of aPHx2 in the PM during optogenetic activation of SopBWT-464TAG or SopBC460S-464TAG. Filtered baseline-corrected time lapse data are mean ± SEM of individual cell measurements from 3 independent experiments quantifying n=29 cells (WT) and n=13 cells (C460S).
(e) AUC calculations of aPHx2 intensities from (d). Data are median, box (25th-75th) and whisker (10th-90th) percentiles of n=29 cells (WT) and n=13 cells (C460S) from 3 independent experiments. P = 0.4415, ns.
(f,g) SopB-mediated synthesis of PtdIns(3,4)P2 is not accompanied by a robust PtdIns(3,4,5)P3 response. Representative confocal sections of aPHx2 and cPHx3 localization during optogenetic activation of WT or C460S SopB. Left, corresponding linear RGB intensity scales are presented. The indicated times are prior to (t - 30 s) or after illumination with 405 nm light to photolyze hydroxycoumarin lysine in SopB. See corresponding quantification of (f) presented in Fig. 2g. Source numerical data are available in source data.
Extended Data Fig. 3. Sequence determinants of SopB-mediated PtdIns(3,4)P2 generation.
(a) Bacterially injected SopB localizes to the vacuolar membrane and PM invaginations. HeLa cells were exposed to ΔsopB (control) or ΔsopB + SopB-c-myc Salmonella for 10 min prior to labeling the PM (WGA) and immunostaining against c-myc and Salmonella. Inset panels are expanded from the hashed box region. Representative maximum intensity projections are from n=3 independent experiments.
(b) Anti-myc fluorescence is not contaminated by non-secreted SopB or anti-Salmonella immunostaining. ΔsopB + SopB-c-myc Salmonella were centrifuged onto poly-L-lysine coated coverslips. Bacteria were processed as in (a) and imaged with equivalent acquisition settings. The representative maximum intensity projection is from n=3 independent experiments.
(c) Heterologous SopB coalesces within puncta that abut cortical membranes. Full-length SopB constructs were co-transfected with cPHx3 and imaged live. Amino-terminally tagged SopB was enriched along cytosolic reticular structures; amino- and carboxy-terminal EGFP fusions also enriched on the basal footprint of cells. Representative confocal sections are presented for each construct, from more than three similar experiments. CellMask served as a PM marker.
(d) SopB requires amino acids 68-172 and 520-554 for PtdIns(3,4)P2 generation. The indicated SopB plasmids (top panels) were co-transfected with cPHx3 (bottom panels) and imaged live in HeLa cells. Representative confocal sections are presented. Note that membrane-targeting of SopB is disrupted following the deletion of amino acids 68-172 while PM-targeting is preserved following the deletion of amino acids 520-554.
(e) CRISPR-Cas9-mediated deletion of Cdc42. Total lysates from control sgRNA- or Cdc42-specific sgRNA-treated Henle 407 cells were immunoblotted against Cdc42. Alpha tubulin served as a loading control.
(f) Cdc42 regulates SopB targeting but is not strictly required for PtdIns(3,4)P2 generation. Representative maximum intensity projections are presented of SopB (1-561)-EGFP localization in parental wild-type Henle (top) or Cdc42 KO Henle cells (bottom). Inset images depict localization of cPHx3 (confocal section). cPHx3 was markedly enriched in the PM with a concomitant decrease in cytosolic fluorescence in (mean ± SEM) 94.1 ± 2.92% of parental WT and 88.5 ± 7.28% of Cdc42 KO cells across n=3 independent experiments.
(g) Quantification of SopB-EGFP puncta per cell from n=3 independent experiments analyzing the following number of Henle 407 cells: 91 (WT) and 59 (Cdc42 KO). Data are trial means ± SEM (foreground) overlaid on cell measurements (gray, background). **P = 0.0014. Source numerical data and unprocessed blots are available in source data.
Extended Data Fig. 4. 3-phosphorylated phosphoinositides promote bacterial invasion of host cells.
(a) Cells were exposed to wild-type or ΔsopB Salmonella for 10 min and extracellular bacteria were removed by extensive washing before returning to growth medium for an additional 20 min. Extracellular and intracellular bacteria were differentially stained. Data are mean ± SEM from n=3 independent experiments quantifying ≥300 infected cells per strain. *P = 0.0333.
(b,c) PtdIns(3,4)P2 and/or PtdIns(3,4,5)P2 promote bacterial invasion. (b) Model of PTEN-catalyzed reactions: membrane-associated PTEN dephosphorylates both PtdIns(3,4,5)P3 and PtdIns(3,4)P2 at the 3-position of the inositol ring. (c) Cells transfected with EGFP (control) or the A4 mutant of PTEN were left untreated or pre-treated with LY294002 (10 μM, 30 min) prior to invasion. Two-fold excess of ΔsopE/sopE2 bacteria was used to obtain sufficient infected cells. Data are mean ± SEM (normalized to respective control) from n=3 independent experiments quantifying ≥300 infected cells per strain. WT: **P = 0.0022, ***P = 0.0007; ΔsopE/E2: *P = 0.0125, **P = 0.0039.
(d) Cells were co-transfected with cPHx3 and PM-targeted catalytic subunit of class IA PI3K (p110α-CAAX). Images were acquired immediately before (left) or 3 min after addition of DMSO (vehicle, right).
(e) HeLa cells expressing vector control or p110α-CAAX were treated for 20 min with DMSO, wortmannin (100 nM), PI-103 (500 nM), or GDC-0941 (500 nM) prior to collection of cell lysates and immunoblotting using phospho-AKT (S473) and pan AKT antibodies. The immunoblot presented is representative of n=3 independent experiments. Corresponding quantification, Fig. 3c.
(f) Confirmation of anti-PI3K-C2α polyclonal antibody labelling of heterologously-expressed PI3K-C2α. HeLa cells transfected with PM-targeted myc-PI3K-C2α were co-stained for the myc epitope tag and PI3K-C2α. Panels right depict enlargement of hashed box regions (I, II), where the non-transfected cells (I) were overexposed to visualize endogenous staining.
(g) Class II PI3K-C2α is not enriched at the site of Salmonella invasion. Confocal sections of uninfected and wild-type Salmonella infected HeLa cells stained for endogenous PI3K-C2α. Source numerical data and unprocessed blots are available in source data.
Extended Data Fig. 5. Lysine residues within the carboxyl-terminus of SopB support optimal phosphotransferase activity.
(a) Alignment of SopB amino acids 504-554 with other PtdIns(4,5)P2 and PtdIns(3,4,5)P3, 5-phosphatases. Basic residues in SopB are highlighted red along with conserved basic residues in the other phosphatases; additional conserved residues are highlighted purple, and regions of highest similarity are boxed (grey). Notably, residues that share sequence similarity with Mus musculus Synaptojanin-1 (SYNJ1) 534-584 fall outside of the SYNJ1 5-phosphatase domain and have not been implicated in its catalytic activity. Local homology between SopB and INPP5B or OCRL failed to be identified by NCBI BLAST.
(b) Mutation of lysines 525 and 528 blunt PtdIns(3,4)P2 generation by SopB. Wild-type, K525A, K528A, and C460S SopB amino-terminally tagged with EGFP (bottom panels) were transiently expressed with cPHx3 (inverted grey, main panels). The PM was stained with CellMask before imaging live. Representative confocal sections are presented for each. Note that expression of the K528A mutant led to notable perturbation of cellular morphology including rounding, cortical membrane ‘crumpling’, and blebbing.
(c) Normalized intensity of PM cPHx3 from (b) was quantified across n=3 independent experiments (WT, 78 cells; K525A, 81 cells; K528A, 86 cells; C460S, 57 cells). Data are trial means ± SEM (foreground) overlaid on cell measurements (gray, background). ****P < 0.0001.
(d) cPHx3 intensities from K528A- and C460S-SopB-transfected cells (red box, panel c) plotted on an expanded axis. Subtle PM-enrichment of cPHx3 was evident in K528A-transfected cells relative to the C460S-transfected mutant. Data are trial means ± SEM (foreground) overlaid on cell measurements (gray, background). **P = 0.0017.
(e,f) Lysine 525 and 528 of SopB are structurally predicted to neighbour the C(X)5R motif. The primary sequence of SopB (residues 1-561) was analyzed by RoseTTAFold and the resulting highest ranked model is presented in the surface filling view (grey). The location of lysine 525 and 528 (green) and the C(X)5R motif of SopB (blue) are annotated. Source numerical data are available in source data.
Extended Data Fig. 6. Generation of PtdIns(3,4)P2 by the Shigella flexneri effector IpgD does not require class I or class II PI3Ks.
(a) Sequence alignment of the phosphate-binding (P-loop) sequence from SopB (Salmonella enterica) and IpgD (Shigella flexneri). Residue 439 of IpgD encodes the cysteine of the C(X)5R motif.
(b) IpgD reduces PM PtdIns(4,5)P2 levels in a C(X)5R-dependent manner. Heterologous expression of EGFP-IpgD (WT or C439S) and PH-PLCδ1 (inverted gray, main panels). The PM was stained with CellMask prior to imaging live.
(c) Quantification of PH-PLCδ1 intensity in the PM from (b) across n=3 independent experiments quantifying 82 cells (IpgDWT) and 74 cells (IpgDC439S. Data are trial means ± SEM (foreground) overlaid on cell measurements (gray, background). ****P < 0.0001.
(d) Live cell imaging of heterologously-expressed EGFP-IpgD (WT or C439S) and NES-mCherry-cPHx3 following PM staining with CellMask. Cells were treated with the indicated inhibitors (PI-103, 500 nM; wortmannin, 100 nM) for 20 min prior to and throughout imaging.
(e) PM cPHx3 intensity was quantified from (d) across 3 independent trials (IpgDWT, 70 cells; IpgDC439S, 62 cells; IpgDWT(PI-103), 68 cells; IpgDWT(Wortmannin), 76 cells). Data are trial means ± SEM (foreground) overlaid on cell measurements (gray, background). ****P < 0.0001.
(f) PM PtdIns(3,4)P2 synthesis in S. cerevisiae. Galactose-inducible empty vector (control), IpgDWT, or IpgDC439S were induced for 2 hours in yeast that expressed cPHx3. Trypan Blue staining demarcates the cell wall and non-viable yeast.
(g) Yeast from (f) were scored for plasmalemmal cPHx3 localization across n=4 independent experiments analyzing the following number of yeast: control, 881 cells; IpgDWT, 1165 cells; IpgDC439S, 938 cells. Data are mean ± SEM. ****P < 0.0001. Source numerical data are available in source data.
Extended Data Fig. 7. SopB requires an INPP5E-sensitive plasmalemmal inositide for the generation of PtdIns(3,4)P2.
(a) Inhibition of PtdIns(3,4)P2 generated during invasion by pre-recruitment of INPP5E. HeLa cells expressing mRFP-FKBP-INPP5E (WT or D556A) together with PM-targeted Lyn(11)-FRB and the biosensor cPHx1 (gray) were treated with 1 μM rapamycin for 2 min before addition of wild-type Salmonella (BFP, red) for an additional 10 min. The PM was stained (CellMask) and cells fixed before imaging. Maximum intensity projections (main panels) and confocal sections of the boxed region (bottom panels) are presented. Re-localization of mRFP-FKBP-conjugates to the PM is depicted in the bottom panels.
(b) Normalized PM intensity of cPHx1 was quantified from part (a) across n=2 independent trials (INPP5EWT, 90 cells; INPP5ED556A, 85 cells). Data are trial means ± SEM (foreground) overlaid on cell measurements (gray, background). **P = 0.0019.
(c) Comparison of PtdIns(4,5)P2 depletion by chemically-induced recruitment of PLCβ3 and INPP5E. Normalized PM intensity of PH-PLCδ1 was quantified from n=3 independent experiments (control, 55 cells; PLCβ3, 56 cells; INPP5E, 74 cells). Data from Fig. 6a,b are re-plotted with INPP5E (acquired in parallel) and support that INPP5E results in a modest PtdIns(4,5)P2-depletion relative to PLCβ3. Data are trial means ± SEM (foreground) overlaid on cell measurements (gray, background). ***P = 0.0002, *P = 0.0477.
(d) PtdIns(4,5)P2-depletion by INPP5E recruitment depends on its catalytic activity. Comparison of HeLa cells co-transfected as in (a) but expressing the biosensor PH-PLCδ1-EGFP. Representative confocal sections are presented following 5-min rapamycin treatment (1 μM) and PM staining with CellMask.
(e,f) Chemically-induced INPP5E recruitment modestly increases PM PtdIns(4)P. HeLa cells expressing mRFP-FKBP-(control), -PLCβ3, or -INPP5E (wild-type) together with PM-targeted Lyn(11)-FRB and EGFP-2xP4M were imaged live 5 min after treatment with 1 μM rapamycin and staining the PM (CellMask). (e) The normalized PM intensity of 2xP4M was quantified from n=3 independent experiments (control, 55 cells; PLCβ3, 41 cells; INPP5E, 53 cells). Data are trial means ± SEM (foreground) overlaid on cell measurements (gray, background). **P = 0.0020. (f) Representative confocal sections of each channel are presented. Source numerical data are available in source data.
Extended Data Fig. 8. Time-resolved HPLC-MS analysis of SopB phosphotransferase-phosphatase activities.
(a) Inorganic phosphate release assessed following treatment of liposomes with 2.5 μg recombinant SopBWT or SopBC460S. Liposome composition was (mol%) POPS:PtdIns(4,5)P2 (90:10) and 2.5 nmol PtdIns(4,5)P2 was provided per reaction. At the indicated timepoints, reactions were terminated by addition of 50 mM NEM. Data are mean ± SEM of duplicate wells. Time 0 min corresponds to a no enzyme control.
(b) Separation of PtdIns(3,4)P2 and PtdIns(4,5)P2 regio-isomers in a sample treated with SopBWT. An example HPLC-MS trace derived from LUVs treated with 0.5 μg (71.5 nM) SopBWT for 5 min. Note the appearance of PtdIns(3,4)P2 at ≈20.6 min.
(c) Model of the PtdIns(3,4,5)P3 biosensor designed with tandem Bruton’s Tyrosine Kinase (BTK) PH domains (bPHx2). Each component was separated by flexible, serine- and glycine-rich linker sequences. NES, nuclear export signal.
(d) Heterologous expression of wild-type SopB induces PM-translocation of bPHx2. Representative confocal micrographs of mCherry-tagged bPHx2 (inverted gray) co-transfected with EGFP-SopBC460S or EGFP-SopBWT.
(e) Hypothesized PPIns conversions catalyzed by SopB and potential modulation by host phosphatases. In the presence of PtdIns(4,5)P2 in vitro, SopB generates the species PtdIns(3,4,5)P3, PtdIns(3,4)P2, and PtdIns(3)P. The latter species are hypothesized to arise, at least in part, by sequential dephosphorylation of PtdIns(3,4,5)P3. In vivo, Salmonella infection favours the accumulation of PtdIns(3,4)P2 likely due to the high basal activity of host 5-phosphatases (INPP5B, SYNJ1/2, SHIP1/2, and others) that rapidly convert PtdIns(3,4,5)P3 to PtdIns(3,4)P2. It remains unclear if the rapid clearance of PtdIns(3,4)P2 following fission of the Salmonella-containing vacuole from the PM is due to the intrinsic activity of SopB or to the activity of host 4-phosphatases (INPP4A/B). Nonetheless, SopB is sufficient to give rise to PtdIns(3)P in vitro from PtdIns(4,5)P2, arguing for a second –likely minor–pathway to generate this inositide in addition to Vps34-mediated synthesis on the bacterial vacuole (Mallo et. al., 2008). Finally, phosphatidylinositol may arise by direct dephosphorylation of the 4- and 5-positions of PtdIns(4,5)P2 by SopB, or indirectly by dephosphorylation of products of the phosphotransferase reaction. Source numerical data are available in source data.
Supplementary Material
Supplementary Video 1 Live cell imaging of cPHx3 (gray, left) expressed in HeLa cells during invasion by RFP-expressing Salmonella (red on left). Frames were acquired at 30 sec intervals and are presented as maximum intensity projections of stacks of serial confocal images with 12 fps playback. cPHx3 was pseudo-coloured (right) to ease visualization, according to the inset calibration bar. See corresponding insets in Fig. 1a.
Supplementary Video 2 Time-lapse of fluorescence imaging of cPHx3 (gray, left) expressed during invasion by RFP-expressing Salmonella (red, left) delivered at high (>10) multiplicity of infection. Frames were acquired at 1 min intervals and single confocal sections are presented at 6 fps playback. cPHx3 was pseudo-coloured (right) to ease visualization according to the inset calibration bar. See additional examples in Extended Data Fig. 1c.
Supplementary Video 3 Time-lapse of fluorescence imaging of aPHx2 (gray) expressed during invasion by wild-type RFP-expressing Salmonella (red on left). Frames were acquired at 1 min intervals, and playback is 6 fps. Merged confocal sections are presented left, and aPHx2 is pseudo-coloured right as indicated by the inset calibration bar. Additional examples are provided in Extended Data Fig. 1d.
Supplementary Video 4 Confocal time-lapse of NES-iRFP-cPHx3 (gray) during optogenetic activation of EGFP-SopBWT-464-TAG. 405-nm illumination begins at frame 15 (30 s) and remains for the duration of the video (5:29). Captures were made at 2.3 sec intervals and playback is 60 fps. See corresponding panels and quantification in Fig. 2.
Table 1∣ Plasmids used in this study.
Table 2∣ Reagents used in this study.
Table 3∣ Antibodies used in this study.
Table 4∣ sgRNA oligonucleotides used in this study.
Acknowledgments
We thank Dr. Scott Emr (Department of Molecular Biology and Genetics, Cornell University) for kindly sharing plasmids for this study. We thank Dr. Kimberley Lau and Paul Paroutis (The Imaging Facility, The Hospital for Sick Children) for technical training and analyses discussions. Models were generated with BioRender.com and exported under a paid subscription. G.F.W.W. is gratefully supported by a Vanier Canada Graduate Scholarship from the Canadian Institutes of Health Research (CIHR) and by an MD/PhD Studentship from the University of Toronto. J.H.B. is supported by the CIHR grant FDN-154329. A.D. is funded by grant R01GM132565 from the National Institutes of Health. G.R.V.H. is funded by grant 1R35GM119412-01 from the National Institutes of Health. S.G. is supported by the CIHR grant FDN-143202, and G.D.F. is supported by CIHR project grant PJT165968 and an Natural Sciences and Engineering Research Council (NSERC) of Canada.
Footnotes
Competing Interests Statement
The authors declare no competing interests.
Code Availability
Custom macros used in this study have been deposited on GitHub and are available for download at the manuscript repository (https://github.com/walpoleg/Walpole-et-al-Nature-Cell-Biology-2022).
Reporting Summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data Availability
Data supporting the findings of this study are available from the corresponding authors upon reasonable request. Source data are provided with this paper.
References
- 1.Balla T Phosphoinositides: tiny lipids with giant impact on cell regulation. Physiological reviews 93, 1019–1137 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hakim S, Bertucci MC, Conduit SE, Vuong DL & Mitchell CA Inositol polyphosphate phosphatases in human disease. Current topics in microbiology and immunology 362, 247–314 (2012). [DOI] [PubMed] [Google Scholar]
- 3.Sasaki T et al. Mammalian phosphoinositide kinases and phosphatases. Progress in Lipid Research 48, 307–343 (2009). [DOI] [PubMed] [Google Scholar]
- 4.Stephens LR, Jackson TR & Hawkins PT Agonist-stimulated synthesis of phosphatidylinositol(3,4,5)-trisphosphate: a new intracellular signalling system? Biochimica et biophysica acta 1179, 27–75 (1993). [DOI] [PubMed] [Google Scholar]
- 5.Bilanges B, Posor Y & Vanhaesebroeck B PI3K isoforms in cell signalling and vesicle trafficking. Nature Reviews Molecular Cell Biology 20, 515–534 (2019). [DOI] [PubMed] [Google Scholar]
- 6.Hawkins PT & Stephens LR Emerging evidence of signalling roles for PI(3,4)P2 in Class I and II PI3K-regulated pathways. Biochemical Society Transactions 44, 307–314 (2016). [DOI] [PubMed] [Google Scholar]
- 7.Fruman DA & Rommel C PI3K and cancer: Lessons, challenges and opportunities. Nature Reviews Drug Discovery 13, 140–156 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lien EC, Dibble CC & Toker A PI3K signaling in cancer: beyond AKT. Current Opinion in Cell Biology 45, 62–71 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walpole GFW & Grinstein S Endocytosis and the internalization of pathogenic organisms: focus on phosphoinositides. F1000Research 9, 368 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pizarro-Cerdá J, Kühbacher A & Cossart P Phosphoinositides and host-pathogen interactions. Biochimica et biophysica acta 1851, 911–8 (2015). [DOI] [PubMed] [Google Scholar]
- 11.Steele-Mortimer O et al. Activation of Akt/protein kinase B in epithelial cells by the Salmonella typhimurium effector sigD. The Journal of biological chemistry 275, 37718–24 (2000). [DOI] [PubMed] [Google Scholar]
- 12.Marcus SL, Wenk MR, Steele-Mortimer O & Finlay BB A synaptojanin-homologous region of Salmonella typhimurium SigD is essential for inositol phosphatase activity and Akt activation. FEBS Letters 494, 201–207 (2001). [DOI] [PubMed] [Google Scholar]
- 13.Niebuhr K et al. Conversion of PtdIns(4,5)P(2) into PtdIns(5)P by the S.flexneri effector IpgD reorganizes host cell morphology. The EMBO journal 21, 5069–78 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pendaries C et al. PtdIns5P activates the host cell PI3-kinase/Akt pathway during Shigella flexneri infection. The EMBO journal 25, 1024–34 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ebner M, Lučić I, Leonard TA & Yudushkin I PI(3,4,5)P3 Engagement Restricts Akt Activity to Cellular Membranes. Molecular cell 65, 416–431.e6 (2017). [DOI] [PubMed] [Google Scholar]
- 16.Manna D, Albanese A, Park WS & Cho W Mechanistic basis of differential cellular responses of phosphatidylinositol 3,4-bisphosphate- and phosphatidylinositol 3,4,5-trisphosphate-binding pleckstrin homology domains. The Journal of biological chemistry 282, 32093–105 (2007). [DOI] [PubMed] [Google Scholar]
- 17.Goulden BD et al. A high-avidity biosensor reveals plasma membrane PI(3,4)P2 is predominantly a class I PI3K signaling product. The Journal of cell biology 218, 1066–1079 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu S-L et al. Quantitative Lipid Imaging Reveals a New Signaling Function of Phosphatidylinositol-3,4-Bisphophate: Isoform- and Site-Specific Activation of Akt. Molecular cell 71, 1092–1104.e5 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dowler S et al. Identification of pleckstrin-homology-domain-containing proteins with novel phosphoinositide-binding specificities. Biochemical Journal 351, 19–31 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thomas CC, Dowler S, Deak M, Alessi DR & van Aalten DM Crystal structure of the phosphatidylinositol 3,4-bisphosphate-binding pleckstrin homology (PH) domain of tandem PH-domain-containing protein 1 (TAPP1): molecular basis of lipid specificity. The Biochemical journal 358, 287–94 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Branchu P, Bawn M & Kingsley RA Genome variation and molecular epidemiology of Salmonella enterica serovar Typhimurium pathovariants. Infection and Immunity 86, 1–17 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.LaRock DL, Chaudhary A & Miller SI Salmonellae interactions with host processes. Nature Reviews Microbiology 13, 191–205 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Posor Y et al. Spatiotemporal control of endocytosis by phosphatidylinositol-3,4-bisphosphate. Nature 499, 233–237 (2013). [DOI] [PubMed] [Google Scholar]
- 24.Gewinner C et al. Evidence that inositol polyphosphate 4-phosphatase type II is a tumor suppressor that inhibits PI3K signaling. Cancer cell 16, 115–25 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Norris FA & Majerus PW Hydrolysis of phosphatidylinositol 3,4-bisphosphate by inositol polyphosphate 4-phosphatase isolated by affinity elution chromatography. The Journal of biological chemistry 269, 8716–20 (1994). [PubMed] [Google Scholar]
- 26.Klarlund JK, Tsiaras W, Holik JJ, Chawla A & Czech MP Distinct polyphosphoinositide binding selectivities for pleckstrin homology domains of GRP1-like proteins based on diglycine versus triglycine motifs. The Journal of biological chemistry 275, 32816–21 (2000). [DOI] [PubMed] [Google Scholar]
- 27.Cronin TC, DiNitto JP, Czech MP & Lambright DG Structural determinants of phosphoinositide selectivity in splice variants of Grp1 family PH domains. The EMBO journal 23, 3711–20 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Venkateswarlu K, Oatey PB, Tavaré JM & Cullen PJ Insulin-dependent translocation of ARNO to the plasma membrane of adipocytes requires phosphatidylinositol 3-kinase. Current biology : CB 8, 463–6 (1998). [DOI] [PubMed] [Google Scholar]
- 29.Galán JE, Lara-Tejero M, Marlovits TC & Wagner S Bacterial type III secretion systems: specialized nanomachines for protein delivery into target cells. Annual review of microbiology 68, 415–38 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rodríguez-Escudero I, Ferrer NL, Rotger R, Cid VJ & Molina M Interaction of the Salmonella Typhimurium effector protein SopB with host cell Cdc42 is involved in intracellular replication. Molecular microbiology 80, 1220–40 (2011). [DOI] [PubMed] [Google Scholar]
- 31.Marcus SL, Knodler LA & Finlay BB Salmonella enterica serovar Typhimurium effector SigD/SopB is membrane-associated and ubiquitinated inside host cells. Cellular Microbiology 4, 435–446 (2002). [DOI] [PubMed] [Google Scholar]
- 32.Galyov EE et al. A secreted effector protein of Salmonella dublin is translocated into eukaryotic cells and mediates inflammation and fluid secretion in infected ileal mucosa. Molecular microbiology 25, 903–12 (1997). [DOI] [PubMed] [Google Scholar]
- 33.Anderson Norris F, Wilson MP, Wallis TS, Galyov EE & Majerus PW SopB, a protein required for virulence of Salmonella dublin, is an inositol phosphate phosphatase. Proceedings of the National Academy of Sciences 95, 14057–14059 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang S et al. The Salmonella enterica serotype typhimurium effector proteins SipA, SopA, SopB, SopD, and SopE2 act in concert to induce diarrhea in calves. Infection and immunity 70, 3843–55 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tahoun A et al. Salmonella transforms follicle-associated epithelial cells into M cells to promote intestinal invasion. Cell Host and Microbe 12, 645–656 (2012). [DOI] [PubMed] [Google Scholar]
- 36.Kum WWS, Lo BC, Yu HB & Finlay BB Protective role of Akt2 in Salmonella enterica serovar Typhimurium-induced gastroenterocolitis. Infection and Immunity 79, 2554–2566 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rogers LD, Brown NF, Fang Y, Pelech S & Foster LJ Phosphoproteomic analysis of Salmonella-infected cells identifies key kinase regulators and SopB-dependent host phosphorylation events. Science Signaling 4, 1–14 (2011). [DOI] [PubMed] [Google Scholar]
- 38.Feng Y, Wente SR & Majerus PW Overexpression of the inositol phosphatase SopB in human 293 cells stimulates cellular chloride influx and inhibits nuclear mRNA export. Proceedings of the National Academy of Sciences of the United States of America 98, 875–879 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cooper KG et al. Activation of Akt by the bacterial inositol phosphatase, SopB, is wortmannin insensitive. PloS one 6, e22260 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou D, Chen LM, Hernandez L, Shears SB & Galan JE A Salmonella inositol polyphosphatase acts in conjunction with other bacterial effectors to promote host cell actin cytoskeleton rearrangements and bacterial internalization. Molecular microbiology 39, 248–259 (2001). [DOI] [PubMed] [Google Scholar]
- 41.Raffatellu M et al. SipA, SopA, SopB, SopD, and SopE2 contribute to Salmonella enterica serotype typhimurium invasion of epithelial cells. Infection and Immunity 73, 146–154 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rahdar M et al. A phosphorylation-dependent intramolecular interaction regulates the membrane association and activity of the tumor suppressor PTEN. Proceedings of the National Academy of Sciences of the United States of America 106, 480–485 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Luo J et al. Genetically encoded optochemical probes for simultaneous fluorescence reporting and light activation of protein function with two-photon excitation. Journal of the American Chemical Society 136, 15551–15558 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Courtney TM & Deiters A Optical control of protein phosphatase function. Nature Communications 10, 1–10 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Arcaro A & Wymann MP Wortmannin is a potent phosphatidylinositol 3-kinase inhibitor: the role of phosphatidylinositol 3,4,5-trisphosphate in neutrophil responses. The Biochemical journal 296 (Pt 2, 297–301 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Knight ZA et al. A pharmacological map of the PI3-K family defines a role for p110alpha in insulin signaling. Cell 125, 733–47 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Folkes AJ et al. The identification of 2-(1H-indazol-4-yl)-6-(4-methanesulfonyl-piperazin-1-ylmethyl)-4-morpholin-4-yl-thieno[3,2-d]pyrimidine (GDC-0941) as a potent, selective, orally bioavailable inhibitor of class I PI3 kinase for the treatment of cancer . Journal of medicinal chemistry 51, 5522–32 (2008). [DOI] [PubMed] [Google Scholar]
- 48.Domin J et al. Cloning of a human phosphoinositide 3-kinase with a C2 domain that displays reduced sensitivity to the inhibitor wortmannin. The Biochemical journal 326 (Pt 1, 139–47 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Virbasius JV, Guilherme A & Czech MP Mouse p170 is a novel phosphatidylinositol 3-kinase containing a C2 domain. The Journal of biological chemistry 271, 13304–7 (1996). [DOI] [PubMed] [Google Scholar]
- 50.Schu PV et al. Phosphatidylinositol 3-kinase encoded by yeast VPS34 gene essential for protein sorting. Science (New York, N.Y.) 260, 88–91 (1993). [DOI] [PubMed] [Google Scholar]
- 51.Vanhaesebroeck B, Guillermet-Guibert J, Graupera M & Bilanges B The emerging mechanisms of isoform-specific PI3K signalling. Nature reviews. Molecular cell biology 11, 329–41 (2010). [DOI] [PubMed] [Google Scholar]
- 52.Alemán A et al. The amino-terminal non-catalytic region of Salmonella typhimurium SigD affects actin organization in yeast and mammalian cells. Cellular Microbiology 7, 1432–1446 (2005). [DOI] [PubMed] [Google Scholar]
- 53.Herman PK & Emr SD Characterization of VPS34, a gene required for vacuolar protein sorting and vacuole segregation in Saccharomyces cerevisiae. Molecular and cellular biology 10, 6742–54 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stack JH, DeWald DB, Takegawa K & Emr SD Vesicle-mediated protein transport: regulatory interactions between the Vps15 protein kinase and the Vps34 PtdIns 3-kinase essential for protein sorting to the vacuole in yeast. The Journal of cell biology 129, 321–34 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Terebiznik MR et al. Elimination of host cell PtdIns(4,5)P(2) by bacterial SigD promotes membrane fission during invasion by Salmonella. Nature cell biology 4, 766–73 (2002). [DOI] [PubMed] [Google Scholar]
- 56.Hammond GRV, Machner MP & Balla T A novel probe for phosphatidylinositol 4-phosphate reveals multiple pools beyond the Golgi. Journal of Cell Biology 205, 113–126 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Várnai P & Balla T Visualization of phosphoinositides that bind pleckstrin homology domains: Calcium- and agonist-induced dynamic changes and relationship to myo-[3H]inositol-labeled phosphoinositide pools. Journal of Cell Biology 143, 501–510 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stauffer TP, Ahn S & Meyer T Receptor-induced transient reduction in plasma membrane PtdIns(4,5)P2 concentration monitored in living cells. Current biology : CB 8, 343–6 (1998). [DOI] [PubMed] [Google Scholar]
- 59.Won DH et al. PI(3,4,5)P3 and PI(4,5)P2 lipids target proteins with polybasic clusters to the plasma membrane. Science 314, 1458–1461 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Varnai P, Thyagarajan B, Rohacs T & Balla T Rapidly inducible changes in phosphatidylinositol 4,5-bisphosphate levels influence multiple regulatory functions of the lipid in intact living cells. The Journal of cell biology 175, 377–82 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Serunian LA et al. Polyphosphoinositides produced by phosphatidylinositol 3-kinase are poor substrates for phospholipases C from rat liver and bovine brain. Journal of Biological Chemistry 264, 17809–17815 (1989). [PubMed] [Google Scholar]
- 62.Willars GB, Nahorski SR & Challiss RAJ Differential regulation of muscarinic acetylcholine receptor-sensitive polyphosphoinositide pools and consequences for signaling in human neuroblastoma cells. Journal of Biological Chemistry 273, 5037–5046 (1998). [DOI] [PubMed] [Google Scholar]
- 63.Gregory JD The Stability of N-Ethylmaleimide and its Reaction with Sulfhydryl Groups. Journal of the American Chemical Society 77, 3922–3923 (1955). [Google Scholar]
- 64.Malek M et al. PTEN Regulates PI(3,4)P2 Signaling Downstream of Class I PI3K. Molecular cell 68, 566–580.e10 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Clark J et al. Quantification of PtdInsP3 molecular species in cells and tissues by mass spectrometry. Nature methods 8, 267–72 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schroeder GN & Hilbi H Molecular pathogenesis of Shigella spp.: controlling host cell signaling, invasion, and death by type III secretion. Clinical microbiology reviews 21, 134–156 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Finn CE, Chong A, Cooper KG, Starr T & Steele-Mortimer O A second wave of Salmonella T3SS1 activity prolongs the lifespan of infected epithelial cells. PLoS Pathogens 13, 1–28 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Knodler LA, Finlay BB & Steele-Mortimer O The Salmonella effector protein SopB protects epithelial cells from apoptosis by sustained activation of Akt. The Journal of biological chemistry 280, 9058–64 (2005). [DOI] [PubMed] [Google Scholar]
- 69.Hu GQ et al. Salmonella outer protein B suppresses colitis development via protecting cell from necroptosis. Frontiers in Cellular and Infection Microbiology 9, 1–10 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hu GQ et al. Cirtical role for Salmonella effector SopB in regulating inflammasome activation. Molecular Immunology 90, 280–286 (2017). [DOI] [PubMed] [Google Scholar]
- 71.Zhang K et al. Minimal SPI1-T3SS effector requirement for Salmonella enterocyte invasion and intracellular proliferation in vivo. PLoS pathogens 14, e1006925 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bruno VM et al. Salmonella typhimurium type III secretion effectors stimulate innate immune responses in cultured epithelial cells. PLoS Pathogens 5, (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Scanu T et al. Salmonella Manipulation of Host Signaling Pathways Provokes Cellular Transformation Associated with Gallbladder Carcinoma. Cell Host and Microbe 17, 763–774 (2015). [DOI] [PubMed] [Google Scholar]
- 74.Roppenser B et al. Multiple Host Kinases Contribute to Akt Activation during Salmonella Infection. PLoS ONE 8, 1–10 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hsu F & Mao Y The structure of phosphoinositide phosphatases: Insights into substrate specificity and catalysis. Biochimica et biophysica acta 1851, 698–710 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mallo G. v. et al. SopB promotes phosphatidylinositol 3-phosphate formation on Salmonella vacuoles by recruiting Rab5 and Vps34. Journal of Cell Biology 182, 741–752 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mason D et al. Alteration of epithelial structure and function associated with PtdIns(4,5)P2 degradation by a bacterial phosphatase. The Journal of general physiology 129, 267–283 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Baek M et al. Accurate prediction of protein structures and interactions using a three-track neural network. Science (New York, N.Y.) 373, 871–876 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
Methods References
- 79.Zhu L, Jorgensen JR, Li M, Chuang YS & Emr SD ESCRTS function directly on the lysosome membrane to downregulate ubiquitinated lysosomal membrane proteins. eLife 6, 1–20 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wennström S & Downward J Role of Phosphoinositide 3-Kinase in Activation of Ras and Mitogen-Activated Protein Kinase by Epidermal Growth Factor. Molecular and Cellular Biology 19, 4279–4288 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Leibiger B et al. Insulin-feedback via PI3K-C2alpha activated PKBalpha/Akt1 is required for glucose-stimulated insulin secretion. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 24, 1824–37 (2010). [DOI] [PubMed] [Google Scholar]
- 82.Levin R et al. Multiphasic dynamics of phosphatidylinositol 4-phosphate during phagocytosis. Molecular biology of the cell 28, 128–140 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Inoue T, do Heo W, Grimley JS, Wandless TJ & Meyer T An inducible translocation strategy to rapidly activate and inhibit small GTPase signaling pathways. Nature Methods 2, 415–418 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ran FA et al. Genome engineering using the CRISPR-Cas9 system. Nature Protocols 8, 2281–2308 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hoiseth SK & Stocker BA Aromatic-dependent Salmonella typhimurium are non-virulent and effective as live vaccines. Nature 291, 238–9 (1981). [DOI] [PubMed] [Google Scholar]
- 86.Stender S et al. Identification of SopE2 from Salmonella typhimurium, a conserved guanine nucleotide exchange factor for Cdc42 of the host cell. Molecular microbiology 36, 1206–21 (2000). [DOI] [PubMed] [Google Scholar]
- 87.Knodler LA et al. Salmonella effectors within a single pathogenicity island are differentially expressed and translocated by separate type III secretion systems. Molecular Microbiology 43, 1089–1103 (2002). [DOI] [PubMed] [Google Scholar]
- 88.Valdivia RH, Hromockyj AE, Monack D, Ramakrishnan L & Falkow S Applications for green fluorescent protein (GFP) in the study of host-pathogen interactions. Gene 173, 47–52 (1996). [DOI] [PubMed] [Google Scholar]
- 89.Steele-Mortimer O, Meresse S, Gorvel JP, Toh BH & Finlay BB Biogenesis of Salmonella typhimurium-containing vacuoles in epithelial cells involves interactions with the early endocytic pathway. Cell. Microbiol 1, 33–49. (1999). [DOI] [PubMed] [Google Scholar]
- 90.Robinson JS, Klionsky DJ, Banta LM & Emr SD Protein sorting in Saccharomyces cerevisiae: isolation of mutants defective in the delivery and processing of multiple vacuolar hydrolases. Molecular and Cellular Biology 8, 4936–4948 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Horger KS, Estes DJ, Capone R & Mayer M Films of agarose enable rapid formation of giant liposomes in solutions of physiologic ionic strength. Journal of the American Chemical Society 131, 1810–9 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.MacDonald RC et al. Small-volume extrusion apparatus for preparation of large, unilamellar vesicles. BBA - Biomembranes 1061, 297–303 (1991). [DOI] [PubMed] [Google Scholar]
- 93.Smal I, Loog M, Niessen W & Meijering E Quantitative comparison of spot detection methods in fluorescence microscopy. IEEE Transactions on Medical Imaging 29, 282–301 (2010). [DOI] [PubMed] [Google Scholar]
- 94.Olivo-Marin J-C Extraction of spots in biological images using multiscale products. Pattern Recognition 35, 1989–1996 (2002). [Google Scholar]
- 95.Lord SJ, Velle KB, Mullins RD & Fritz-Laylin LK SuperPlots: Communicating reproducibility and variability in cell biology. The Journal of cell biology 219, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Video 1 Live cell imaging of cPHx3 (gray, left) expressed in HeLa cells during invasion by RFP-expressing Salmonella (red on left). Frames were acquired at 30 sec intervals and are presented as maximum intensity projections of stacks of serial confocal images with 12 fps playback. cPHx3 was pseudo-coloured (right) to ease visualization, according to the inset calibration bar. See corresponding insets in Fig. 1a.
Supplementary Video 2 Time-lapse of fluorescence imaging of cPHx3 (gray, left) expressed during invasion by RFP-expressing Salmonella (red, left) delivered at high (>10) multiplicity of infection. Frames were acquired at 1 min intervals and single confocal sections are presented at 6 fps playback. cPHx3 was pseudo-coloured (right) to ease visualization according to the inset calibration bar. See additional examples in Extended Data Fig. 1c.
Supplementary Video 3 Time-lapse of fluorescence imaging of aPHx2 (gray) expressed during invasion by wild-type RFP-expressing Salmonella (red on left). Frames were acquired at 1 min intervals, and playback is 6 fps. Merged confocal sections are presented left, and aPHx2 is pseudo-coloured right as indicated by the inset calibration bar. Additional examples are provided in Extended Data Fig. 1d.
Supplementary Video 4 Confocal time-lapse of NES-iRFP-cPHx3 (gray) during optogenetic activation of EGFP-SopBWT-464-TAG. 405-nm illumination begins at frame 15 (30 s) and remains for the duration of the video (5:29). Captures were made at 2.3 sec intervals and playback is 60 fps. See corresponding panels and quantification in Fig. 2.
Table 1∣ Plasmids used in this study.
Table 2∣ Reagents used in this study.
Table 3∣ Antibodies used in this study.
Table 4∣ sgRNA oligonucleotides used in this study.
Data Availability Statement
Data supporting the findings of this study are available from the corresponding authors upon reasonable request. Source data are provided with this paper.