Abstract
Purpose
Utilizing SlowflowHD as a measurement of endometrial and sub-endometrial blood flow in women with infertility undergoing frozen embryo transfer (FET) cycles and correlation with pregnancy outcomes.
Methods
A prospective pilot study of 99 women undergoing hormone replacement FET cycles. Ultrasounds were performed with Voluson E8 at 3-time points: day 15, day of transfer, and 11 days post transfer (T + 11). SlowflowHD Doppler blood flow indices in the endometrium and sub-endometrium were compared in women who achieved pregnancy with those who did not.
Results
Using SlowflowHD, both pregnant and non-pregnant women had similar trends with decreased endometrial blood flow day of transfer compared with day 15. However, there was a borderline significantly lower mean percentage decrease of endometrial blood flow in women achieving a pregnancy (28.3% vs 42.9%). Significantly higher numbers of pregnant women had a 20% or less decrease in blood flow (21 vs 9) with increases in mean percentage blood flow on T + 11 (pregnant 39.59% vs non-pregnant 25.20%). The RI and S/D ratio in the spiral arteries was also significantly higher on transfer day in women who had a live birth RI (0.68 vs 0.65) and S/D (3.91 vs 3.17).
Conclusion
There are blood flow changes both in pregnant and non-pregnant patients with decreases in blood flow after progesterone replacement. Pregnancy and live births were associated with a lower mean percentage drop in blood flow from day 15 to the day of transfer and elevated RI and S/D ratio on transfer day.
Keywords: Frozen embryo transfer, Endometrial blood flow, Endometrial receptivity, SlowflowHD
Introduction
A receptive endometrium is essential for successful implantation. This consists of changes in the endometrium that are modulated by estradiol and progesterone which is used in hormonal prepared frozen embryo transfer (FET) cycles. The standard measurement of the endometrium in FET cycles is the endometrial thickness (EMT) and pattern. The optimal thickness and pattern in 2D for improved pregnancy is a minimum thickness of 7 mm, and a trilaminar pattern on the day of the progesterone start [1, 2].
Endometrial blood flow is necessary for implantation of the embryo. Endometrial and sub-endometrial blood flow examined by color and power Doppler measured by velocity index (VI) and flow index (FI) has been correlated with implantation and pregnancy rates during IVF treatment [3, 4]. 2D Doppler flow indices of spiral arteries such as resistance index (RI) pulse index (PI) and peak systolic velocity (PSV) are not predictive of pregnancy in some studies, although others have found significantly lower spiral artery PI in pregnant cycles than non-pregnant cycles [5, 6]. Absent endometrial and sub-endometrial blood flow is associated with significantly lower pregnancy rates [7, 8].
3D ultrasound and power Doppler angiography with the aid of the VOCAL © (Virtual Organ Computer-Aided Analysis) can be used to provide a fast means of measuring endometrial parameters, such as endometrial volume and angiography blood flow, to predict endometrial receptivity [4]. It was previously believed that uterine arterial resistance changes in 3D might reflect uterine receptivity [7]. Although pregnancy outcomes tended to be poor in patients with higher mean uterine arterial impedance indices, the predictive value of using a specific RI or PI in assessing endometrial receptivity seems to be limited [9].
SlowflowHD is a new technology that can be used to measure low-velocity vascular flow in the small arterioles that feed the endometrium and allows a comparison between the intensity of the color signals present in the endometrium and sub-endometrium. In comparison, conventional Doppler filters do not detect smaller blood vessels as it becomes more difficult to extract out of the clutter. SlowflowHD Doppler imaging uses a smart filter that allows it to differentiate between motion artifacts from the low-velocity signal within. It has been used to measure smaller vessels in the abdomen such as the liver, fetal tongue, and renal parenchyma [10–12].
The purpose of the study is to evaluate endometrial and sub-endometrial blood flow using SlowflowHD in women undergoing IVF freeze–thaw embryo transfers in an artificial hormonal prepared cycle. Due to the significant shift towards “freeze all cycles” in the USA, we chose to study this technology in FET cycles only [13]. The hypothesis is that the use of the newer technology may improve the predictive ability of the embryo to implant and predict outcomes.
Materials and methods
This is a prospective pilot study of 99 women undergoing autologous FET cycles between November 2019 and May 2020. Women were included if they or their partners were diagnosed with sub-fertility and were planning on a hormonal replacement FET cycle. Inclusion criteria were women under 40 years with a previous IVF stimulation, a normal uterine baseline ultrasound and endometrial cavity (by saline sonogram, hysteroscopy, or hysterosalpingogram within 6 months), a single embryo transfer of a good quality blastocyst (4BB or better), and minimal EMT on day 15 in 2D of 7 mm. Excluded women were those not meeting the above criteria, or with more than 2 miscarriages or 2 failed embryo transfers, and a duration of infertility greater than 2 years (defined from the initial day of initial consultation, until the day of transfer). None of the patients included in the study was smokers or consumed alcohol, and all patients had at least a high-school degree level of education. Other exclusions were severe adenomyosis, uterine fibroids, septate uterus, history of Ashermans or sub-mucosal fibroids, and uncontrolled endocrine abnormalities. IRB approval (IRB: 17–11-FB-0224) was obtained. All patients consented during their baseline ultrasound on day 15 of their cycle if they met the inclusion criteria.
Procedures
Patients had a normal baseline ultrasound on day 1 or 2 of the cycle before estradiol started. The protocol for endometrial preparation was a hormone replacement cycle with 14 days of estradiol patches (2 × 100 mcg) changed every other day for 14 days starting on cycle day 1 or 2 of the cycle with a step-up to 4 patches cycle day 10. On cycle day 15, estradiol and progesterone levels were measured and if the progesterone level was < 1.5 ng/mL, estradiol patches were dropped from 4 to 2, and progesterone 50 mg IM was started that evening. The hormone levels were repeated 12 h after progesterone start with a 10 ng/mL considered adequate. Both estradiol and progesterone were continued daily until the pregnancy test. The patients had 3 ultrasound scans with a Voluson E10 (GE Healthcare, Zipf, Austria): morning of day 15 (D15) of their cycle (after 2 weeks of estradiol before initiation of progesterone), the morning of transfer (T-Day) prior to the embryo transfer (+ 5 days of IM progesterone exposure), and day of pregnancy test prior to the blood draw 11 days post-transfer. Women underwent a single embryo blastocyst transfer of good quality (4BB or better).
Blood flow to the endometrium was measured with the by a single trained physician to avoid inter-observer variations and with predetermined settings constant throughout the study. The results of the 3D data and Doppler studies were not known by the clinicians and did not affect decision-making. The true sagittal plane of the uterus was obtained, and 2D EMT was performed at the thickest level. Then a 3D sweep was taken with the sweep angle set at 90° and a complete uterine volume containing the entire endometrium and sub-endometrium was obtained. The volume was saved to the hard drive of the ultrasound machine for analysis and measurements.
Measurements were made for endometrial volume (EV), endometrial vascularization index (VI) endometrial flow index (FI), and endometrial vascularization flow index (VFI). The endometrial volume and various indices VI, FI, and VFI were measured using the virtual organ computer-aided analysis (VOCAL) imaging program for 3D power Doppler histogram analysis. Contour planes were analyzed with 15° rotation for the endometrium to cover 180°. Following the assessment of the sub-endometrial vascularity, shell-imaging was used to measure sub-endometrial blood flow in small vessels within the 2-mm shell of the endometrial/myometrial contour. VI represents the presence of blood vessels (vascularity) in the endometrium and is measured as the ratio of the number of color voxels to the total number of voxels and is expressed as a percentage (%) of the endometrial volume. FI is the mean power Doppler signal intensity inside the endometrium and represents the average intensity of flow. VFI is a combination of vascularity and flow intensity.
The SlowflowHD technology was then used. A 2D live image was then taken in the sagittal plane, using SlowflowHD. Three boxes were placed: one around the endometrium and one around the sub-endometrium anteriorly and posteriorly. A histogram analysis was performed representing the ratio of the number of color pixels to the total number of pixels as a percentage as 3D analysis was not possible. The power Doppler system was utilized under SlowflowHD. After at least 4 consecutive waveforms were obtained, the systolic/diastolic blood flow indices were measured in 3 different prominent spiral arteries showing flow into the endometrium and taking mean values. For the Slowflow HD, the default settings were Gain2.4, frequency low, wall motion filter mid1, quality high, artifact suppression on, and the pulse repetition frequency (PRF) was set at 0.2 kHz.
Serum pregnancy test was performed 11 days post-transfer. Pregnancy was defined as 2 positive rising β-HCG levels great than 10 mIU/mL on T + 11 and 5–7 days later. Clinical pregnancy was defined as a gestational sac in the uterine cavity and viable clinical pregnancy, presence of a positive fetal heart between 7- and 8-week gestation. Women who achieved a pregnancy were compared with those who did not. Pregnancies were followed for their entirety and live birth was recorded.
Statistical analysis was performed using SAS 9.4 and SPSS 23.0 (IBM Corp, 2015). Continuous data were expressed as mean ± SD, and p values < 0.05 were considered statistically significant. An independent T-test was used for continuous variables and chi-square analysis was used for categorical variables. A power analysis was performed to estimate a sample size needed and a sample size of 88 patients was required to have 80% power detect a statistically significant difference if there was at least a 10% change in endometrial blood flow (p < 0.05) in pregnant vs non-pregnant women undergoing ultrasound analysis. This power analysis was based on a previous study of 95 women undergoing IVF cycles, which resulted in 37 intrauterine pregnancies and a significantly different pulse Doppler between groups [3].
Results
Patient demographics and cycle characteristics
There were 53 patients in the pregnant group and 46 in the non-pregnant group. Of the 53 pregnant women, 50 continued to have a viable clinical pregnancy at 7–8-week gestation with positive fetal heart activity and 45 continued to have a live birth. The analysis did not change by excluding these 3 patients from the pregnancy group. There were no differences among groups (pregnant vs non-pregnant) in age, BMI, race, gravidity, parity, infertility diagnosis, duration of infertility, maximum FSH dose used in their stimulation, number of eggs retrieved, number of two pronuclei (2PN), number of embryo’s frozen, trigger medication used, whether pre-implantation genetic testing (PGT-A) was utilized, number of prior transfers, estradiol levels, progesterone levels, EMT, endometrial volume (EV) on D15, and sub-endometrial volume at on day 15 of a FET cycle. The only significant difference with the above parameters was on the day of transfer; the EMT was lower in pregnant women (10.79 vs 11.96, p = 0.01) (Table 1). This may not be clinically significant as there was no difference in EMT on day 15.
Table 1.
Characteristics of women in the pregnant group compared with the non-pregnant group. 2pn, two pronuclei; DOR, diminished ovarian reserve; EMT, endometrial thickness; EV, endometrial volume; FSH, follicle-stimulating hormone; GnRH, gonadotropin-releasing hormone; HCG, human chorionic gonadotropin; PGT-A, preimplantation genetic testing-aneuploidy
| Characteristics | Pregnant | N | Mean | Std. error mean | p-value |
|---|---|---|---|---|---|
| Age | Yes | 53 | 34.67 | 0.67 | 0.97 |
| No | 46 | 34.64 | 0.76 | ||
| BMI | Yes | 53 | 26.87 | 0.83 | 0.34 |
| No | 46 | 27.52 | 1.21 | ||
| Race | Total | Pregnant | Non-pregnant | ||
| White | 74 (75%) | 40 (75%) | 34 (74%) | χ2 (4) = 1.6, p = 0.8 | |
| Black | 13 (13%) | 8 (15%) | 5 (11%) | ||
| Hispanic | 4 (4%) | 2 (4%) | 2 (4%) | ||
| Asian | 7 (7%) | 3 (6%) | 4 (9%) | ||
| Other | 1 (1%) | 0 (0%) | 1 (2%) | ||
| Diagnosis | |||||
| DOR | 13 (13%) | 8 (15%) | 5 (11%) | χ2 (8) = 7.7, p = 0.56 | |
| Endometriosis | 2 (2%) | 1 (2%) | 1 (2%) | ||
| Ovulator dysfunction | 11 (11%) | 5 (10%) | 6 (13%) | ||
| Multiple female factors | 3 (3%) | 2 (4%) | 1 (2%) | ||
| Tubal factor | 16 (16%) | 9 (17%) | 7 (15%) | ||
| Male factor | 18 (18%) | 10 (19%) | 8 (17%) | ||
| Male and female factor | 9 (7%) | 7 (13%) | 2 (4%) | ||
| Unexplained | 18 (18%) | 9 (17%) | 9 (20%) | ||
| Other | 9 (9%) | 2 (4%) | 7 (15%) | ||
| Gravidity | Yes | 53 | 1.2 | 0.22 | 0.73 |
| No | 46 | 1.2 | 0.19 | ||
| Parity | Yes | 53 | 0.4 | 0.12 | 0.66 |
| No | 46 | 0.4 | 0.11 | ||
| Duration of infertility (years) | Yes | 53 | 0.49 | 0.67 | 0.79 |
| No | 46 | 0.52 | 0.65 | ||
| Number of prior transfers | Yes | 53 | 1.67 | 0.06 | 0.1 |
| No | 46 | 1.52 | 0.07 | ||
| MAX FSH DOSAGE | Yes | 53 | 2642 | 166 | 0.88 |
| No | 46 | 2609 | 157 | ||
| Eggs RETRIEVED | Yes | 53 | 18.3 | 1.3 | 0.36 |
| No | 46 | 16.6 | 1.3 | ||
| Embryos FROZEN | Yes | 53 | 5.7 | 0.5 | 0.48 |
| No | 46 | 6.4 | 0.8 | ||
| 2PN | Yes | 53 | 9.4 | 0.7 | 0.65 |
| No | 46 | 9.9 | 0.9 | ||
| PGT-A | Yes | 53 | 0.4 | 0.07 | 0.63 |
| No | 46 | 0.4 | 0.07 | ||
| Trigger SHOT HCG | Yes | 39 (74%) | χ2 (3) = 3.5, p = 0.18 | ||
| No | 35 (76%) | ||||
| Trigger SHOT GNRH AGONIST | Yes | 10 (19%) | |||
| No | 13 (28%) | ||||
| Trigger SHOT BOTH | Yes | 3 (6%) | |||
| No | 1 (2%) | ||||
| Day 15 ESTROGEN | Yes | 53 | 943.98 | 75.74 | 0.91 |
| No | 46 | 955.13 | 74.26 | ||
| Day 15 PROGESTERONE | Yes | 53 | 0.27 | 0.018 | 0.26 |
| No | 46 | 0.58 | 0.26 | ||
| Day 16 ESTROGEN | Yes | 53 | 618.19 | 67.77 | 0.73 |
| No | 46 | 588.05 | 52.26 | ||
| Day 16 PROGESTERONE | Yes | 53 | 17.63 | 0.96 | 0.74 |
| No | 46 | 18.16 | 1.24 | ||
| Day 15 EMT | Yes | 53 | 10.23 | 0.31 | 0.24 |
| No | 46 | 10.73 | 0.29 | ||
| Transfer EMT | Yes | 53 | 10.79 | 0.28 | 0.01 |
| No | 46 | 11.96 | 0.37 | ||
| Transfer + 11 EMT | Yes | 53 | 13.62 | 0.39 | 0.47 |
| No | 46 | 13.07 | 0.64 | ||
| Day 15 EV | Yes | 53 | 3.92 | 0.30 | 0.66 |
| No | 46 | 4.12 | 0.36 | ||
| Transfer EV | Yes | 53 | 3.99 | 0.27 | 0.49 |
| No | 46 | 4.31 | 0.37 | ||
| Transfer + 11 EV | Yes | 53 | 5.83 | 0.55 | 0.45 |
| No | 46 | 6.53 | 0.72 | ||
3D Doppler outcomes
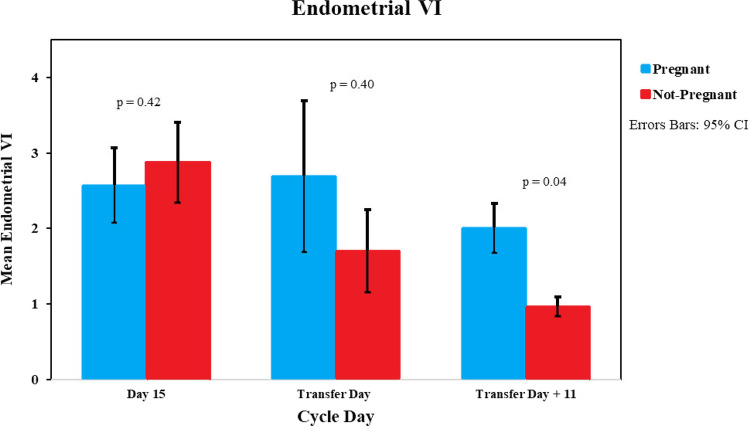
Endometrial VI showed no change from day 15 to transfer day in pregnant patients (Fig. 1). In non-pregnant patients, there was a decreasing trend in VI from day 15 to transfer day and a continued decrease on T + 11. The VI was borderline significantly higher in pregnant patients compared with non-pregnant patients on the day of pregnancy test (T + 11 VI 2.0 vs 0.96, p = 0.04). FI was similar on day 15 and day of transfer for both pregnant and non-pregnant at the endometrial and sub-endometrial levels (D15 FI pregnant 19.6 vs non-pregnant 18.6, p = 0.48, T-Day FI 18.6 vs 17.7, p = 0.58). The VFI of the endometrium on transfer + 11 trended lower but non-significant in non-pregnant patients (pregnant 0.37 vs non-pregnant 0.22, p = 0.05).
Fig. 1.
3D power Doppler VI of the endometrium bar graph
Using the SlowflowHD live, cineclips were saved and compared for the intensity of flow and the flow was quantitated using the histogram analysis of boxes placed in the endometrium and sub-endometrium. Examples of the SlowflowHD on day 15, day of transfer, and transfer + 11 are shown in Fig. 2a–h. In pregnant and non-pregnant women, there was higher intensity blood flow on day 15 and then a decrease in blood flow on transfer day. In review of the clips from transfer day, there appeared to be more visible flow through the small vessels in the endometrium in women who achieved pregnancy with a live birth. On transfer + 11, differences were more striking. To quantitate differences in SlowflowHD in 2D, a histogram analysis of the endometrium and the sub-endometrium flow was presented as a percentage. Both pregnant and non-pregnant women had a lower mean percentage of blood vessels on the day of transfer compared with estradiol-only exposure D15 (Fig. 3) and there was an overlap between pregnant and non-pregnant women. The mean percentage difference in the endometrial blood flow from day 15 to transfer day (P + 5) was lower in pregnant compared with non-pregnant patients with borderline significance (27% vs 41%, p = 0.04). Significantly higher numbers of pregnant women had a 20% or less decrease in blood flow from day 15 to transfer day (21 vs 9, p = 0.03). Nine women in the non-pregnant group had an absence of vessels in the endometrium on transfer day compared with 2 women in the pregnant group. The trend in both groups showed an increase in the percentage of blood vessels on T + 11 compared with transfer day, and T + 11 showed a significant increase in the percentage of blood vessels in pregnant women compared to non-pregnant (pregnant 39.59% vs non-pregnant 25.20%, p = 0.01). Sub-endometrial changes showed a decrease in flow from day 15 to transfer day but no differences between pregnant and non-pregnant women (Fig. 4). An example of the placement of the boxes to calculate the percentage of flow is seen in Fig. 2c–d and the calculation of the power Doppler in Fig. 5.
Fig. 2.
Endometrial SlowflowHD in pregnant and non-pregnant women across the 3 timepoints: a D15 pregnant; b D15 non-pregnant; c, d T-Day pregnant; e, f T-Day non-pregnant day; g T + 11 pregnant; h T + 11 non-pregnant
Fig. 3.
Endometrial SlowflowHD histogram bar graph
Fig. 4.
Sub-endometrial SlowflowHD histogram bar graph
Fig. 5.
Endometrial SlowflowHD power Doppler
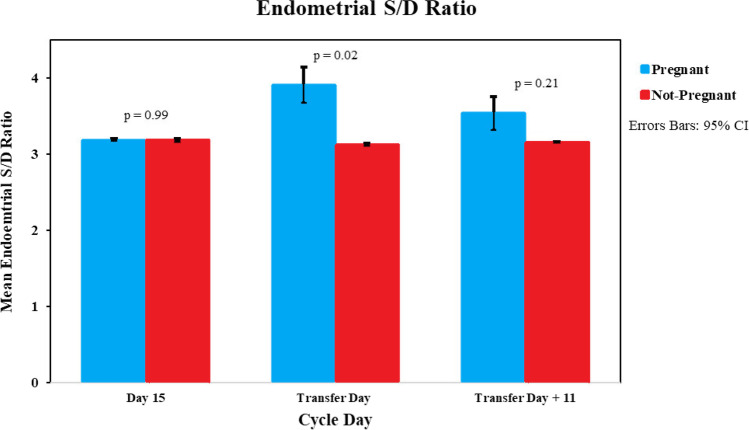
When the power Doppler system was used in SlowflowHD, there was no significant difference in the RI on day 15 between pregnant and non-pregnant women. However, the RI increased in pregnant women on transfer day compared to non-pregnant (0.68 vs 0.65, p = 0.03), and no change was seen again on T + 11 (Fig. 6). The S/D ratio also followed a similar trend with similar values on D15; however, pregnant women had a significantly higher S/D ratio on T-Day (3.91 vs 3.17, p = 0.02). The S/D ratio then decreased in pregnant women T + 11) (Fig. 7).
Fig. 6.
Endometrial SlowflowHD power Doppler RI bar graph
Fig. 7.
Endometrial SlowflowHD power Doppler S/D bar graph
A receiver operating characteristic (ROC) curve was performed and when using power Doppler and SlowflowHD on transfer day, an S/D ratio cut-off of 3.31 can be used to identify an endometrium that is likely to result in implantation. The area under the curve (AUC) was 0.613, p = 0.065 (Fig. 8).
Fig. 8.

ROC curve using S/D on T-Day to identify a receptive endometrium
Discussion
Using SlowflowHD, a higher pregnancy and live birth rate was associated with a lower mean percentage difference in the endometrial blood flow from day 15 to transfer day (P + 5) and a significantly higher RI and S/D ratio on the day of transfer with low predictability by the ROC curve. However, there is a potential for SlowflowHD to be used as a screening tool alongside endometrial thickness and shape.
This advanced technology has the advantage of extreme and improved sensitivity to low and slow flow in the small vessels in the endometrium and feeding the endometrium as small as 1 mm. Both the 3D power Doppler ultrasound and the 2D Slowflow were used. Unfortunately, the E10-19 used in this study had only the 2D format. The 2 technologies showed different results. In both groups and using both Doppler applications, estradiol increased the neovascularization and endometrial angiogenesis. The effect of progesterone showed decreased endometrial blood flow in both groups with a more noticeable decrease in the non-pregnant group. Surprisingly on the day of transfer was also an increase in the mean resistance to flow seen by higher RI and S/D ratios in transfer day, using SlowflowHD. There have been variable results with changes in endometrial and sub-endometrial blood flow in IVF. Ng et al. found no differences in blood flow on HCG day or day of transfer in fresh IVF cycles in pregnancy cycles [14]. Schild et al. showed sub-endometrial vascularization index (VI), flow index (FI), and vascularization flow index (VFI) were lower in pregnant than non-pregnant cycles [15]. Kupesic showed the day of transfer sub-endometrial FI was significantly higher in pregnant cycles with VI and VFI similar [6]. In FET cycles, results varied as well. Merce et al. found that the endometrial 3D power Doppler flow indices were statistically significantly higher in the pregnant group yet two other studies showed no significant difference in Doppler blood flow in pregnant and non-pregnant women [9, 16, 17].
Our study agreed with Nandi et al. who evaluated 45 women undergoing a FET on days 5, 10, and 15 of estradiol administration and the day of the transfer in FET cycles using the three-dimensional power Doppler. They saw similar trends in blood flow as our findings in SlowflowHD, showing a decrease in blood flow after 5 days of progesterone. In their study, the peak blood flow was on day 15 of estradiol with a decrease on the day of transfer. The endometrial and sub-endometrial blood flow showed a similar pattern of change over time in women who conceived with those that did not, and the authors found no statistically significant differences in endometrial vascularization index (VI), flow index (FI), and vascularization flow index (VFI) between the pregnant and non-pregnant groups [16], which further questions the utility of VOCAL 3D Doppler as a marker of adequate endometrial preparation. Kim et al. saw higher VI, on the day of transfer in pregnant women in fresh IVF transfer cycles but did not look in frozen cycles.
Using SlowflowHD, in both pregnant and non-pregnant women, there was the same decrease in blood flow on transfer day compared to day 15. This drop was more severe in the non-pregnant group compared to the pregnant group and coincides with the initiation of progesterone, a known endometrial vasodilator [18]. Compared to transfer day, increases in blood flow were seen on transfer day 11 with a significantly higher result in pregnant women, most likely is the result of successful implantation.
It is well documented in the literature that increased endometrial vascularity is associated with higher levels of estradiol which can be due to pregnancy, endometrial polyps, or increased infertility [19]. SlowflowHD can potentially be used to screen women with increased endometrial vascularity on day 15 that may be associated with polypoid endometrium or even low vascularity associated with adhesions that might not be suitable for transfer. However, further studies need to be performed to assess its utilization to identify endometrial polyps and adhesions.
Our biggest limitation was that the E10 Model BT19 could only assess the endometrial cavity in 2D planes, and this creates difficultly when measuring the blood flow for a rotated uterus. Slowflow3D technology was not available at the time of study initiation; however, with our feedback, GE healthcare was able to improve their technology in their most current model allowing SlowflowHD to be performed in a 3D fashion. We also understand that there is potential bias due to multiple factors affecting pregnancy rates besides endometrial blood flow, even though patient demographics and cycle characteristics were similar in both groups. We tried to limit bias by performing ultrasound measurements and analysis by one person and by consistant machine settings. Another important limitation is the absence of genetic testing for the majority of our patients; however, it is known that PGT-A does not necessarily improve pregnancy outcomes but can be used as another screening tool [20].
The main strength of the study was that measurements were completed at 3 different time points in each patient’s FET cycle. This allowed us to compare within the same patient the effect of the hormones on vascularity in an FET cycle and the difference between pregnant and non-pregnant women at each stage. Even though differences detected on 11 days post-transfer likely reflect vascular changes of early pregnancy, it was still important to note the trend in blood flow during the process of FET, rather than one instance in time. Using Slowflow technology, both pregnant and non-pregnant groups showed a drop in the endometrial vascularization from day 15 to the day of embryo transfer in the endometrium and sub-endometrium. This was also seen in the study by Nandi et al. [16]. In our study, there was a higher percentage decrease in the non-pregnant group which may contribute to the lower implantation. The decrease in endometrial blood flow on transfer day may be hormonally related. The serum concentrations of estradiol were the same in each group and doses of estradiol were decreased with the start of progesterone. Most likely, the high estradiol increases vascularization and the effect of the decrease in estradiol and addition of progesterone leads to the decreases seen.
The S/D ratio and RI measured in our study are indices of arterial blood flow at the endometrial-sub-endometrial transition, allowing us to identify the amount of resistance in spiral arteries. Previous studies that used a combination of fresh and frozen transfers attempted to assess endometrial blood flow without the availability of SlowflowHD [5, 21, 22], and none of these studies showed a correlation between S/D ratio and RI, and pregnancy or implantation rates. The surprising finding in our study was the visible blood flow on the day of transfer in women who achieved a pregnancy was associated with significantly increased resistance to blood flow as measured by these parameters. One possible explanation for the difference in observations is that a decreased resistance may be associated with vascular dysfunction or the opposite, and a transient increase in resistance during the transfer window may aid in implantation. This may also reflect the balance between the estradiol and progesterone effects on endometrial blood flow. These results differ from previous studies showing increased resistance in non-pregnant women, but they were done in a combination of fresh cycles and frozen cycles variations in the exact day of the blood flow measurement (HCG trigger day, transfer day) and the arteries chosen to measure blood flow (uterine artery, ovarian artery, spiral arteries) using the traditional power Doppler technique [5, 6, 23]. Our study used SlowflowHD which may more accurately measure the resistance of blood flow at the endometrial and sub-endometrial transition. Based on our results, an S/D ratio of 3.31 on transfer day can be used to identify a more favorable endometrium (specificity of 65%) and 2.89 as non-favorable (sensitivity of 70.8%) leading to a potentially a higher pregnancy and live birth rate. However, the ability to predict outcomes from this ROC curve is too low and this alone is not a strong marker of endometrial receptivity and should be used concurrently with other screening techniques such as EMT and pattern [24].
With the advent of SlowflowHD, ultrasound definition has significantly improved allowing us to easily identify smaller vessels in the radial and spiral arteries that may be more clinically relevant. We believe given this tool, results can be easily reproduced in comparison to past studies. With the 3D technology on the newer machines and increased patient numbers, the use of this technology in the endometrium can be further explored.
Conclusion/summary
This is the first prospective study to look at the utilization of the SlowflowHD technology and allows blood flow measurement with higher sensitivity in the smaller spiral artery vessels that surround the endometrium. There are blood flow changes both in pregnant and non-pregnant patients before and after progesterone replacement and between transfer day and day 11 after the transfer, with decreases in blood flow after progesterone replacement and increases in endometrial blood flow between transfer and transfer + 11. A higher pregnancy and live birth rate are associated with an elevated RI and S/D ratio on the day of transfer, in comparison to non-pregnant women. The potential for SlowflowHD to be used as a screening tool for favorable endometrium is present, but a second trial utilizing the newly available 3D technology is needed to confirm our results.
Funding
No external funds were used in this work. However, GE Healthcare provided the loaner Voluson E10 Ultrasound machine used for this study.
Data availability
Data provided upon request.
Declarations
Ethics approval
EVMS IRB approval number 17–11-FB-0224.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zhang T, Li Z, Ren X, Huang B, Zhu G, Yang W, et al. Endometrial thickness as a predictor of the reproductive outcomes in fresh and frozen embryo transfer cycles: a retrospective cohort study of 1512 IVF cycles with morphologically good-quality blastocyst. Medicine (Baltimore) 2018;97:e9689. doi: 10.1097/MD.0000000000009689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Craciunas L, Gallos I, Chu J, Bourne T, Quenby S, Brosens JJ, et al. Conventional and modern markers of endometrial receptivity: a systematic review and meta-analysis. Hum Reprod Update. 2019;25:202–223. doi: 10.1093/humupd/dmy044. [DOI] [PubMed] [Google Scholar]
- 3.Yang JH, Wu MY, Chen CD, Jiang MC, Ho HN, Yang YS. Association of endometrial blood flow as determined by a modified colour Doppler technique with subsequent outcome of in-vitro fertilization. Hum Reprod. 1999;14:1606–1610. doi: 10.1093/humrep/14.6.1606. [DOI] [PubMed] [Google Scholar]
- 4.Zhang T, He Y, Wang Y, Zhu Q, Yang J, Zhao X, et al. The role of three-dimensional power Doppler ultrasound parameters measured on hCG day in the prediction of pregnancy during in vitro fertilization treatment. Eur J Obstet Gynecol Reprod Biol. 2016;203:66–71. doi: 10.1016/j.ejogrb.2016.05.016. [DOI] [PubMed] [Google Scholar]
- 5.Yuval Y, Lipitz S, Dor J, Achiron R. The relationships between endometrial thickness, and blood flow and pregnancy rates in in-vitro fertilization. Hum Reprod. 1999;14:1067–1071. doi: 10.1093/humrep/14.4.1067. [DOI] [PubMed] [Google Scholar]
- 6.Kupesic S, Bekavac I, Bjelos D, Kurjak A. Assessment of endometrial receptivity by transvaginal color Doppler and three-dimensional power Doppler ultrasonography in patients undergoing in vitro fertilization procedures. J ultrasound Med Off J Am Inst Ultrasound Med. 2001;20:125–34. doi: 10.7863/jum.2001.20.2.125. [DOI] [PubMed] [Google Scholar]
- 7.Chien L-W, Au H-K, Chen P-L, Xiao J, Tzeng C-R. Assessment of uterine receptivity by the endometrial-subendometrial blood flow distribution pattern in women undergoing in vitro fertilization-embryo transfer. Fertil Steril. 2002;78:245–251. doi: 10.1016/S0015-0282(02)03223-5. [DOI] [PubMed] [Google Scholar]
- 8.Maugey-Laulom B, Commenges-Ducos M, Jullien V, Papaxanthos-Roche A, Scotet V, Commenges D. Endometrial vascularity and ongoing pregnancy after IVF. Eur J Obstet Gynecol Reprod Biol. 2002;104:137–43. doi: 10.1016/S0301-2115(02)00102-1. [DOI] [PubMed] [Google Scholar]
- 9.Ng EHY, Chan CCW, Tang OS, Yeung WSB, Ho PC. The role of endometrial and subendometrial blood flows measured by three-dimensional power Doppler ultrasound in the prediction of pregnancy during IVF treatment. Hum Reprod. 2006;21:164–170. doi: 10.1093/humrep/dei277. [DOI] [PubMed] [Google Scholar]
- 10.Tierney J, Walsh K, Griffith H, Baker J, Brown DB, Byram B. Combining slow flow techniques with adaptive demodulation for improved perfusion ultrasound imaging without contrast. IEEE Trans Ultrason Ferroelectr Freq Control. 2019;66:834–48. doi: 10.1109/TUFFC.2019.2898127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hernandez-Andrade E, Romero R. Visualization of fetal tongue circulation using Doppler ultrasound. Ultrasound Obstet Gynecol Off J Int Soc Ultrasound Obstet Gynecol. 2020;55:550–60. doi: 10.1002/uog.20393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hata T, Koyanagi A, Yamanishi T, Bouno S, Takayoshi R, Miyake T. Fetal abdominal blood vessels and organ microvasculature detected by Slowflow HD. Ultrasound Obstet Gynecol Off J Int Soc Ultrasound Obstet Gynecol. 2020;56:955–7. doi: 10.1002/uog.22043. [DOI] [PubMed] [Google Scholar]
- 13.Jain T, Missmer SA, Hornstein MD. Trends in embryo-transfer practice and in outcomes of the use of assisted reproductive technology in the United States. N Engl J Med. 2004;350:1639–1645. doi: 10.1056/NEJMsa032073. [DOI] [PubMed] [Google Scholar]
- 14.Ng EHY, Chan CCW, Tang OS, Yeung WSB, Ho PC. Changes in endometrial and subendometrial blood flow in IVF. Reprod Biomed Online. 2009;18:269–275. doi: 10.1016/S1472-6483(10)60265-9. [DOI] [PubMed] [Google Scholar]
- 15.Schild RL, Holthaus S, d’Alquen J, Fimmers R, Dorn C, van Der Ven H, et al. Quantitative assessment of subendometrial blood flow by three-dimensional-ultrasound is an important predictive factor of implantation in an in-vitro fertilization programme. Hum Reprod. 2000;15:89–94. doi: 10.1093/humrep/15.1.89. [DOI] [PubMed] [Google Scholar]
- 16.Nandi A, Martins WP, Jayaprakasan K, Clewes JS, Campbell BK, Raine-Fenning NJ. Assessment of endometrial and subendometrial blood flow in women undergoing frozen embryo transfer cycles. Reprod Biomed Online. 2014;28:343–351. doi: 10.1016/j.rbmo.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 17.Merce LT, Barco MJ, Bau S, Troyano J. Are endometrial parameters by three-dimensional ultrasound and power Doppler angiography related to in vitro fertilization/embryo transfer outcome? Fertil Steril. 2008;89:111–117. doi: 10.1016/j.fertnstert.2007.02.029. [DOI] [PubMed] [Google Scholar]
- 18.de Ziegler D, Bessis R, Frydman R. Vascular resistance of uterine arteries: physiological effects of estradiol and progesterone. Fertil Steril. 1991;55:775–779. doi: 10.1016/S0015-0282(16)54247-2. [DOI] [PubMed] [Google Scholar]
- 19.Ni J, Han B, Liang J, Wang F. Three-dimensional 3D ultrasound combined with power Doppler for the differential diagnosis of endometrial lesions among infertile women. Int J Gynaecol Obstet Off organ Int Fed Gynaecol Obstet. 2019;145:212–8. doi: 10.1002/ijgo.12787. [DOI] [PubMed] [Google Scholar]
- 20.Practice Committee of the American Society for Reproductive Medicine and the Society for Assisted Reproductive Technology The use of preimplantation genetic testing for aneuploidy (PGT-A): a committee opinion. Fertil Steril. 2018;109:429–36. doi: 10.1016/j.fertnstert.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 21.Wang L, Qiao J, Li R, Zhen X, Liu Z. Role of endometrial blood flow assessment with color Doppler energy in predicting pregnancy outcome of IVF-ET cycles. Reprod Biol Endocrinol. 2010;8:122. doi: 10.1186/1477-7827-8-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim A, Jung H, Choi WJ, Hong SN, Kim HY. Detection of endometrial and subendometrial vasculature on the day of embryo transfer and prediction of pregnancy during fresh in vitro fertilization cycles. Taiwan J Obstet Gynecol. China. 2014;53:360–5. doi: 10.1016/j.tjog.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 23.Sardana D, Upadhyay AJ, Deepika K, Pranesh GT, Rao KA. Correlation of subendometrial-endometrial blood flow assessment by two-dimensional power Doppler with pregnancy outcome in frozen-thawed embryo transfer cycles. J Hum Reprod Sci India. 2014;7:130–135. doi: 10.4103/0974-1208.138872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holden EC, Dodge LE, Sneeringer R, Moragianni VA, Penzias AS, Hacker MR. Thicker endometrial linings are associated with better IVF outcomes: a cohort of 6331 women. Hum Fertil England. 2018;21:288–293. doi: 10.1080/14647273.2017.1334130. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data provided upon request.