Abstract
Purpose
The study aims to evaluate whether frozen embryo transfer can restore optimal receptivity leading to better assisted reproductive technology outcomes in women with endometriosis.
Methods
This systematic review and meta-analysis, conducted from January 10, 2021 to July 1, 2021, searched the Cochrane Library, PubMed, Embase, Web of Science, OVID, and Clinicaltrials.gov databases from inception to January 10, 2021. The search strategy combined search terms as follows: (“endometriosis” OR “deep endometriosis” OR “endometrioma”) AND (“frozen-thawed embryo transfer” OR “frozen embryo transfer” OR “freeze-all strategy”) AND (“pregnancy outcome” OR “live birth rate” OR “clinical pregnancy rate” OR “miscarriage rate”). No publication time or language limits were set during the searches. In addition, references of the related articles were searched by hand. Patients were included if they had a history of endometriosis and had received fresh or frozen embryo transfer. Only the first transfer cycle was included. Odds ratios (ORs) and 95% confidence intervals (CIs) were used to express outcomes, and data synthesis was conducted using RevMan, version 5.4 software.
Results
A total of six studies with moderate methodologic quality were retrieved in the meta-analysis. The studies included 3010 women with endometriosis who wanted to conceive; 1777 (59.0%) had frozen embryo transfer, and 1233 (41.0%) had fresh embryo transfer. There was a significantly higher frequency of live births in the frozen embryo group than in the fresh embryo group (OR, 1.53; 95% CI, 1.13–2.08; P = .007). Despite a similar clinical pregnancy rate in the two groups (OR, 1.26; 95% CI, 0.95–1.69; P = .11), the difference in miscarriage rate was significant (OR, 0.70; 95% CI, 0.50–0.97; P = .03). Evidence quality was considered moderate.
Conclusion
Cryopreserved embryo transfer has resulted in preferable reproduction outcomes when compared with fresh embryo transfer in patients with endometriosis, but the evidence is not yet abundant. More strictly designed research is needed to evaluate whether frozen embryo transfer leads to better reproductive outcomes in women with endometriosis compared with those receiving fresh embryo transfer.
Registration number
PROSPERO CRD42021248313.
Keywords: Endometriosis, Fertility, Fresh embryo transfer, Frozen-thawed embryo transfer, Meta-analysis
Introduction
Endometriosis is a common condition found in 10 to 15% of reproductive-age women. A hallmark of the disease is the presence of endometrial glands and stroma outside the uterus. Symptoms include ovarian mass, pelvic pain, and infertility. Studies have shown that 30 to 50% of women with infertility have endometriosis [1] and that women with endometriosis have less successful pregnancy outcomes than those who are not diagnosed with the condition [2, 3]. The negative effect of endometriosis on a woman’s fertility is in part caused by distortion of pelvic anatomy and pelvic organ adhesions found in the more advanced severe stages of the disease. However, because patients with a normal pelvis in minimal, mild, and moderate stages of endometriosis may also have adverse fertility outcomes, the pathogenesis remains unclear [4]. Recent research has suggested that infertility in endometriosis may be related to both oocyte development and embryo implantation. The endometrium must be receptive for successful embryo implantation to take place [5, 6].
Currently, more women with endometriosis are achieving pregnancy through assisted reproductive technology (ART) [7], and studies have suggested similar success rates in women with an endometriosis diagnosis compared with those with other diagnoses [8]. However, some other studies showed that compared with women with other causes of infertility (i.e., tubal factors), women with endometriosis have pregnancy rates that are almost 50% less [9]. Researchers have hypothesized that the endometriosis potentially causes some detrimental effects on reproduction [10]. In addition, despite great advancements in ART, particularly the improvement in embryo quality, implantation rate remains low [11]. Studies have shown that controlled ovarian stimulation may alter endometrial receptivity through the expansion of the implantation window and higher levels of estrogen [12, 13]. As endometriosis is an estrogen-dependent condition with dysregulated steroid hormone pathways in the eutopic endometrium [14], it is reasonable to hypothesize that a supraphysiological concentration of estrogen may alter endometrial receptivity and then contribute to a lower rate of pregnancy.
Improvements in cryopreservation have made feasible the deferment of frozen embryo transfer as an alternative to the transfer of fresh embryos. Cryopreservation was developed initially to prevent ovarian hyperstimulation syndrome; however, its use has evolved to include the improvement of endometrial implantation. One study [15] found that frozen embryo transfer improved infertility outcomes in women with endometriosis. Endometriosis causes fluctuations in serum progesterone and estradiol levels, which result in asynchrony between the endometrium and embryo; use of frozen-thawed embryo transfer may alter this asynchrony [16]. Administration of both progesterone and estradiol creates a natural endometrial environment for the embryo. The use of frozen embryo transfer is currently considered controversial by many scholars and as such, is not accepted as an alternative procedure to fresh embryo transfer in infertile women with endometriosis [17].
Therefore, this study was conducted with the goal of assessing the relationship between frozen embryo transfer and fertility in women with endometriosis.
Materials and methods
This study was a systematic review and meta-analysis; it was conducted from January 10, 2021. This study followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines (PROSPERO: CRD42021248313).
Search strategy
A literature search was conducted by two authors (Y.C., M.S.) using OVID, the Cochrane Library, Web of Science, PubMed, Embase, and Clinicaltrials.gov databases from inception to January 10, 2021. Search terms were combined as follows: (“endometriosis” OR “deep endometriosis” OR “endometrioma”) AND (“frozen-thawed embryo transfer” OR “frozen embryo transfer” OR “freeze-all strategy”) AND (“pregnancy outcome” OR “live birth rate” OR “clinical pregnancy rate” OR “miscarriage rate”). The related articles were searched by hand. During the searches, no limits were set for publication time or language.
Study selection
Studies were included in this meta-analysis if they (1) enrolled patients with endometriosis; (2) were randomized clinical trials, case–control studies, or cohort trials; (3) included patients who received transfer of frozen or fresh embryos (and this was their first transfer cycle during the time period); and (4) their outcomes included rates of miscarriage, live births, or clinical pregnancy. Studies were excluded if they (1) were case reports; (2) did not include the first transfer cycle; or (3) included animal subjects.
Studies were independently selected by two authors (Y.C., S.W.). From the searches, first the abstracts and titles were scanned; next, the full text of the chosen articles was analyzed. In any disagreements arose, they were resolved via discussion or consensus with a third author (H.D.).
Risk of bias
The risk of bias was independently assessed by two authors (S.W., M.S.) using the Newcastle–Ottawa Scale (http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp). Three items in the Newcastle–Ottawa Scale totaled 9 points: case and control selection, case and control comparability, and exposure ascertainment.
Sensitivity analysis was conducted for the primary outcome (live birth rate) to test the stability of the meta-analysis result.
In a table of findings summary, the evidence quality was shown using the Grading of Recommendations, Assessment, Development, and Evaluations (GRADE) criteria (GRADEpro GDT software [McMaster University, Hamilton, Ontario, Canada]). Two review authors (S.W., M.S.) independently performed the evaluation. A resolution was found for any disagreements through discussion or evaluation by a third author (H.D.).
Data extraction and synthesis
Data were independently extracted by two authors (Y.C., M.S.) using a data extraction form containing patient characteristics and outcome data. Patients were included in the frozen embryo group if they received transfer of frozen embryos, and patients were included in the control group if they received transfer of fresh embryos. Reproductive outcomes (i.e., rates for miscarriage, live births, and clinical pregnancy) were compared between the groups. Data synthesis was performed using RevMan software, version 5.4 (The Cochrane Collaboration). Data were synthesized and analyzed from March 10, 2021 to May 1, 2021, using RevMan software, version 5.4 (The Cochrane Collaboration).
For dichotomous data, the I2 statistic was used to examine odds ratio (OR) heterogeneity; I2 statistic analysis (where an I2 value < 50% suggested no substantial heterogeneity) was conducted using random-effect or fixed-effect models. Significance level for all two-sided P values was set at less than 0.05.
Result
Study selection
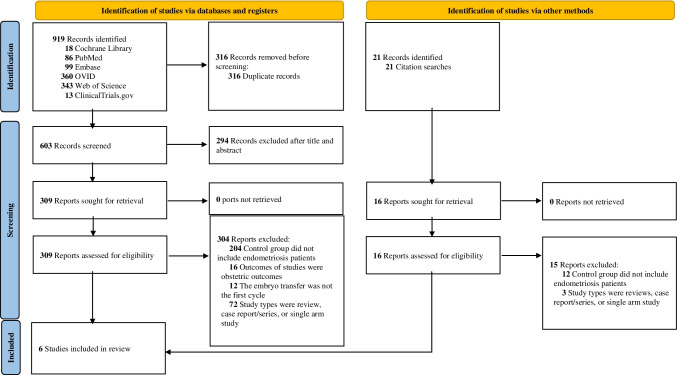
Using standard searching techniques, a total of 919 studies were found. The selection procedure is illustrated in the flow diagram (Fig. 1). Of the 919 studies, 316 were duplicates, 294 were excluded after review of the abstract and title, and 304 were excluded because their control groups included patients who did not have endometriosis. In addition, one article [15] was included from a manual search of the references.
Fig. 1.
Flow diagram
Study characteristics
All six articles retrieved from the search were retrospective cohort studies. There were no available randomized clinical trials on this topic. A total of six studies with moderate methodologic quality were retrieved in the meta-analysis. The studies included 3010 women with endometriosis who wanted to conceive; 1777 (59.0%) had frozen embryo transfer, and 1233 (41.0%) had fresh embryo transfer. Five studies [15, 18–21] reported live birth rate and miscarriage, and all six studies [15, 18–22] reported clinical pregnancy rate. Two studies [19, 21] clarified the endometriosis phenotypes, and three [15, 18, 20] clarified stages. Five studies [18–22] described the stimulation protocol and the frozen embryo transfer protocol. Three studies [19, 21, 22] clarified prior endometriosis surgery. Four studies [15, 18, 19, 21] described concomitant infertility factors. Only one study [18] used a subgroup analysis according to the age of the participants; another study [15] performed a subgroup analysis by the number of oocytes retrieved, and one study [18] performed a subgroup analysis by preimplantation genetic testing (PGT-A). Characteristics of the retrieved articles are displayed in Table 1.
Table 1.
Study characteristics
| Study | Design | No. of patients | Patient age, mean ± SD, years | Phenotype and stage | Duration of infertility | Diagnostic testing | Concomitant infertility factors | No. of previous attempts | Prior endometriosis surgery | Embryo transfer option | Stimulation protocol | No. of retrieved oocytes | FET protocol | High-quality embryo rate (transferred) | No. of transfers (mean ± SD) | Live birth | Clinical pregnancy | Miscarriage |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Asoglu (2020) | Retrospective cohort |
315 ± 180 (OG) 135 (CG) |
33.1 ± 4.2 (OG) 33.3 ± 3.4 (CG) |
Endometrioma |
3.2 ± 2 (OG) 2.9 ± 2 (CG) |
Ultrasonography | Tubal factor, male factor (severe male factor or ovulatory dysfunction was excluded) |
0.8 ± 1.2 (OG) 0.9 ± 1.5 (CG) |
0 (OG) 0 (CG) |
Based on a joint decision by the patient and doctor, but strongly recommend FET in those with premature progesterone elevation or ovarian hyperstimulation syndrome risk | GnRH-antagonist protocol, long GnRH-agonist protocol |
8.5 ± 3.5 (OG) 8.5 ± 3.2 (CG) |
Artificial cycle |
113 (OG) 82 (CG) |
1.4 ± 0.5 (OG) 1.3 ± 0.5 (CG) |
101 (OG) 55 (CG) |
113 (OG) 65 (CG) |
15(OG) 12 (CG) |
| Bourdon (2018) | Retrospective cohort |
270 ± 135 (OG) 135 (CG) |
34.3 ± 4.1 (OG) 34.3 ± 3.9 (CG) |
SUP, OMA, DIE |
4.7 ± 2.7 (OG) 4.4 ± 2.3 (CG) |
Imaging criteria using TVUS, MRI, or transrectal ultrasonography or surgery and histologic proof | Male infertility, tubal factor, adenomyosis |
2.0 ± 1.1 (OG) 1.9 ± 1.0 (CG) |
43 (OG) 100 (CG) |
Based on a joint decision by the patient and doctor | Long GnRH-agonist protocol, antagonist protocol, short agonist protocol |
9.9 ± 7.0 (OG) 7.4 ± 4.3 (CG) |
Artificial cycle | NOS |
1.7 ± 0.9 (OG) 2.1 ± 0.9 (CG) |
41 (OG) 21 (CG) |
58 (OG) 40(CG) |
11 (OG) 16 (CG) |
| Mohamed (2011) | Retrospective cohort |
415 ± 148 (OG) 267 (CG) |
34 (OG) 34 (CG) |
NOS | NOS | Laparoscopy | NOS | NOS |
148 (OG) 267 (CG) |
NOS | Long GnRH-agonist protocol | NOS | Down-regulated hormonally controlled cycle | NOS | NOS |
25 (OG) 52 (CG) |
27 (OG) 54 (CG) |
2 (OG) 2 (CG) |
| Tan (2021) | Retrospective cohort |
728 ± 389 (OG) 339 (CG) |
35.9 ± 0.3 (OG) 35.5 ± 0.2 (CG) |
Stage I-IV (rASRM) |
2.9 ± 0.3 (OG) 2.8 ± 0.3 (CG) |
Surgery and histologic proof | Male factor (severe male factor, adenomyosis, or tubal factor was excluded) | NOS | NOS | Based on a joint decision by the patient and doctor | GnRH-antagonist protocol |
8.2 ± 0.8 (OG) 7.4 ± 4.3 (CG) |
Artificial cycle | NOS | NOS | NOS |
159 (OG) 139 (CG) |
34 (OG) 29 (CG) |
| Wang (2018) | Retrospective cohort |
521 ± 419 (OG) 102 (CG) |
30.4 ± 3.9 (OG) 31.2 ± 3.8 (CG) |
Stage I-II (rASRM) |
4.0 ± 2.2 (OG) 4.1 ± 3.0 (CG) |
Laparoscopy | NOS | NOS | NOS | FET was conducted in those with other uterine factors (such as endometrial polyps, submucosal fibroids), premature progesterone elevation, or ovarian hyperstimulation syndrome risk | Ultra-long protocol, modified ultra-long protocol, long GnRH-agonist protocol |
15.1 ± 8.9 (OG) 13.2 ± 8.0 (CG) |
Down-regulated hormonally controlled cycle |
65.5% (OG) 69.8% (CG) |
NOS |
141 (OG) 29 (CG) |
180 (OG) 48 (CG) |
12 (OG) 7 (CG) |
| Wu (2019) | Retrospective cohort |
315 ± 180 (OG) 135 (CG) |
33.1 ± 4.2 (OG) 33.3 ± 3.4 (CG) |
Stage III-IV (rASRM) |
3.2 ± 2 (OG) 2.9 ± 2 (CG) |
Laparoscopy | Tubal factor, male factor | NOS | NOS | Based on a joint decision by the patient and doctor, but strongly recommend FET in those with premature progesterone elevation or ovarian hyperstimulation syndrome risk | NOS |
8.5 ± 3.5 (OG) 8.5 ± 3.2 (CG) |
NOS | NOS |
2 (OG) 2 (CG) |
101 (OG) 55 (CG) |
113 (OG) 65 (CG) |
15 (OG) 12 (CG) |
ASRM American Society of Reproductive Medicine, CG control group, DIE deeply infiltrating endometriosis, FET frozen embryo transfer, MRI magnetic resonance imaging, NOS not otherwise specified, OG observation group, OMA ovarian endometrioma, SUP superficial peritoneal endometriosis, TVUS transvaginal ultrasonography
Meta-analysis
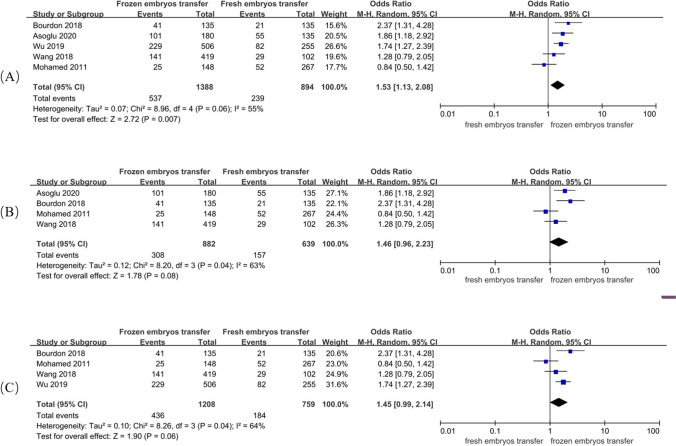
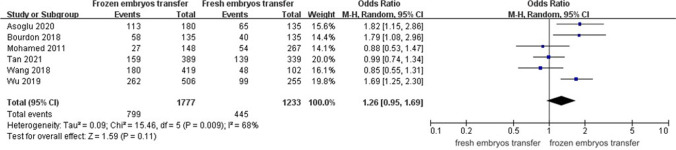
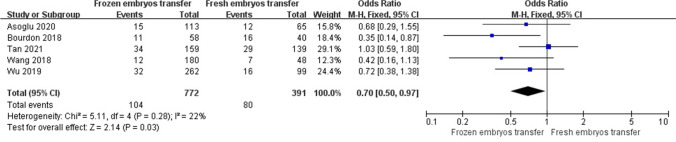
A random-effect model revealed an I2 statistic of 55% for live birth rate and 68% for clinical pregnancy rate, which was interpreted as obvious heterogeneity. A fixed-effect model revealed no significant heterogeneity for miscarriage rate (I2 = 22%). Analysis of ORs was conducted via the Mantel–Haenszel technique. The frozen embryo transfer group had better reproductive outcomes in patients with endometriosis than in the control group (OR, 1.53; 95% CI, 1.13–2.08; P = 0.007) (Fig. 2a). The two groups had a similar clinical pregnancy rate (OR, 1.26; 95% CI, 0.95–1.69; P = 0.11) (Fig. 3), but the difference in the rate of miscarriage between the two groups was significant (OR, 0.70; 95% CI, 0.50–0.97; P = 0.03) (Fig. 4).
Fig. 2.
Forest plot of live birth rate
Fig. 3.
Forest plot of clinical pregnancy rate
Fig. 4.
Forest plot of miscarriage rate
We did not test for funnel plot asymmetry because of the possibility of achieving a false-positive result (< 10 included studies according to Cochrane Handbook for Systematic Reviews of Interventions) (https://training.cochrane.org/handbook). Because there were only six studies included in this analysis, we chose not to assess for publication bias. Quality assessment scores were in the range of 8 to 9 scores (Table 2).
Table 2.
Study quality assessment
| Authors | Year | Selection | comparability | outcome assessment | Score | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |||
| Asoglu | 2020 | ☆ | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | ☆ | 9 |
| Bourdon | 2018 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 8 |
| Mohamed | 2011 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 8 |
| Tan | 2021 | ☆ | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | ☆ | 9 |
| Wang | 2018 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 8 |
| Wu | 2019 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 8 |
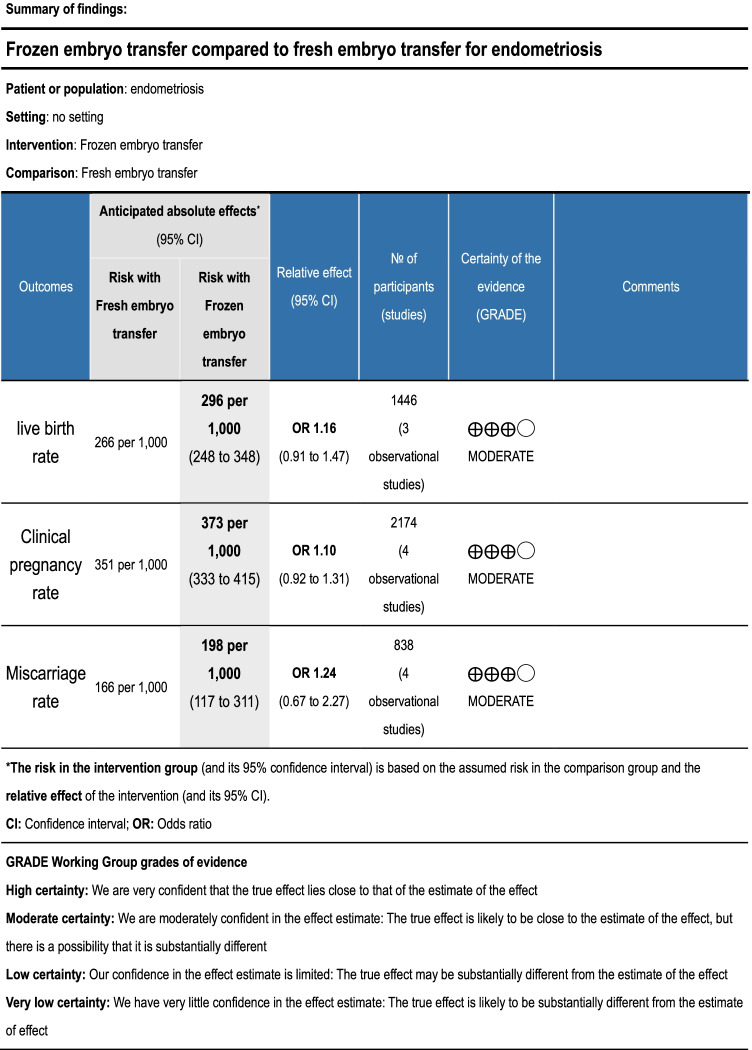
Using the GRADE approach, evidence quality was classified as moderate (Table 3). There were not enough data to conduct a subgroup analysis according to endometriosis phenotypes, stages, age of participants, stimulation protocol, or frozen embryo transfer protocol.
Table 3.
Summary of findings (GRADE)
Sensitivity analysis
A sensitivity analysis was conducted, and it suggested that the results were not stable. Even with removal of the study by Wu et al. [15] or Asoglu et al. [21], there was no significant difference in the live birth rate (OR, 1.46; 95% CI, 0.96–2.23; P = 0.08 and OR, 1.45; 95% CI, 0.99–2.14; P = 0.06, respectively) (Fig. 2b and c).
Discussion
Main finding
This study aimed to evaluate the relationship between the transfer of frozen embryos and fertility in patients with endometriosis.
Results suggest that the transfer of frozen embryos was associated with improved rates of live birth and fertility among women with endometriosis, whereas the rate of clinical pregnancy between both groups was similar. Further, there was a significantly lower rate of miscarriage in the group with frozen embryo transfer. However, results of a sensitivity analysis suggested that our results were unstable. This may have been the result of the small number of retrieved studies. All of these studies were retrospective cohort trials, and their allocation was not entirely random. Whether the study participants received frozen or fresh embryo transfer was based on discussion rather than randomization.
Strengths and limitations
This was the first systematic review and meta-analysis, to our knowledge, of frozen embryo transfer in women with endometriosis. We screened the references manually and performed a systematic search of the literature. The inclusion and exclusion criteria were strict, and the methodology was rigorous. Live birth rate, the ideal outcome variable, was the primary outcome for this study. In addition, all the included participants underwent their first transfer cycle.
This study had several limitations. First, this analysis lacked the inclusion of randomized clinical trials, as there were none available on this subject. Moreover, there were some factors contributing to the heterogeneity of the meta-analysis, including maternal age, phenotype, and stage of endometriosis, and concomitant infertility factors including adenomyosis, prior endometriosis surgery, stimulation protocol, number of retrieved oocytes, frozen embryo transfer protocol, and number of embryo transfers, which were inconsistent across the included studies. Meanwhile, there were not enough data to conduct a subgroup analysis according to these factors.
Implications for clinical practice
Endometriosis is the etiology of infertility in millions of women worldwide. Assisted reproductive technology has allowed for the improved management of endometriosis-related infertility. After controlled ovarian stimulation, the transfer of fresh embryos is typically performed; this process generates high levels of estrogen. Because endometriosis is considered to be an estrogen-dependent disease, the high levels of estrogen generated from fresh embryo transfer are speculated to interfere with endometrial receptivity. This results in fewer occurrences of pregnancy. Several studies have shown that ovarian stimulation can cause a decrease in endometrial and subendometrial blood flow and can induce histopathological changes in the endometrium [23–25]. In addition, researchers have found that during fresh embryo transfer cycles, the gene transcription involved in endometrial receptivity is disrupted [26–29]. Deferred frozen-thawed embryo transfer permits the embryo transfer to be carried out in a subsequent, separate cycle, which results in several potential advantages compared with fresh embryo transfer. These advantages include (1) the restoration of synchrony between endometrium and embryo and (2) the ability to reset the natural physiologic milieu of the uterus for optimal implantation. Several studies have shown a higher rate of live births and pregnancy with the use of frozen embryo transfer [15, 30]. The main question was whether frozen embryo transfer could restore optimal receptivity and improve fertility in women with endometriosis, which would lead to an increased frequency of pregnancy.
Because of the dysfunction of eutopic endometrium of endometriosis, including progesterone-resistance and decidualization defects, endometrial receptivity is thought to be the key factor in a successful pregnancy [10]. Meanwhile, numerous biomarkers have been proposed to identify the optimal endometrial receptivity [31, 32]. However, the known evidence of endometrial receptivity defects in endometriosis was based on the physiologic mechanism; none of the markers were used in clinical practice because of poor pregnancy prediction accuracy. Studies should focus on the improvement of in vitro fertilization, including the protocols of stimulation and transfer, timing of transplantation, and fertilization mode.
Our study results suggest that the transfer of cryopreserved embryos was associated with better reproduction outcomes than the transfer of fresh embryos in patients with endometriosis. However, evidence for this is not yet abundant. In addition, a cryopreservation strategy may result in the following disadvantages: increased time to pregnancy, unnecessary interventions, increased patient expense, and high concentrations of cryoprotectants, which may be toxic to embryos. Therefore, proceeding with frozen embryo transfer as a routine management strategy for endometriosis should be done cautiously. Individualized treatment of patients is necessary owing to the high level of heterogeneity with endometriosis. It should note that cryopreserved embryo transfers have been shown to provide better outcomes than fresh embryo transfers in a general population of ovulatory women [33]. Thus, the advantages of cryopreservation strategies may not be limited to endometriosis.
Implications for further research
All of the study objectives were not addressed owing to the limited evidence retrieved from the six retrospective cohort trials. In particular, we were unable to perform subgroup analysis based on concomitant infertility factors, prior endometriosis surgery, stimulation protocol, number of retrieved oocytes, transfer of frozen embryo protocol, and embryo transfer number. In the future, more research in the form of randomized clinical trials should be performed to establish the efficacy of frozen embryo transfer in the reproduction prognosis of endometriosis.
Conclusions
The results suggest that the transfer of frozen embryos may improve the rate of live births and decrease the rate of miscarriages in patients with endometriosis. However, the benefit of frozen embryo transfer in the improvement of fertility outcomes remains uncertain. Evidence quality was considered moderate. More strictly designed research is needed to evaluate whether frozen embryo transfer leads to better outcomes in women with endometriosis compared with fresh embryo transfer using assisted reproductive technology. Using frozen embryo transfer as a routine management strategy for endometriosis should be performed cautiously.
Acknowledgements
We thank the authors of the retrieved studies for their research contributions to the field of reproductive fertility in the setting of endometriosis. We thank BioMed Proofreading® LLC for their copyediting services.
Author contribution
H.D.: conception and design, data collection, analytic strategy, manuscript revision, and final article approval. Y.C.: conception and design, search and collection of data, analysis and synthesis, writing, and final article approval. M.S.: search and collection of data, analysis, manuscript revision, and final article approval. S.W.: collection of data, manuscript revision, and final article approval. X.L.: conception and design, manuscript revision, and final approval.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Saunders P, Horne AW. Endometriosis: etiology, pathobiology, and therapeutic prospects. Cell. 2021;184(11):2807–2824. doi: 10.1016/j.cell.2021.04.041. [DOI] [PubMed] [Google Scholar]
- 2.Cimadomo D, et al. Definition, diagnostic and therapeutic options in recurrent implantation failure: an international survey of clinicians and embryologists. Hum Reprod. 2021;36(2):305–317. doi: 10.1093/humrep/deaa317. [DOI] [PubMed] [Google Scholar]
- 3.Kong H, et al. A multi-center, randomized controlled clinical trial of the application of a shortened protocol of long-acting Triptorelin down-regulated prior to IVF/ICSI among patients with endometriosis: a protocol. Reprod Health. 2018; 15(1). [DOI] [PMC free article] [PubMed]
- 4.de Ziegler D, Borghese B, Chapron C. Endometriosis and infertility: pathophysiology and management. Lancet. 2010;376(9742):730–738. doi: 10.1016/S0140-6736(10)60490-4. [DOI] [PubMed] [Google Scholar]
- 5.Donnez J, et al. Oxidative stress in the pelvic cavity and its role in the pathogenesis of endometriosis. Fertil Steril. 2016;106(5):1011–1017. doi: 10.1016/j.fertnstert.2016.07.1075. [DOI] [PubMed] [Google Scholar]
- 6.Máté G, Bernstein LR, Török AL. Endometriosis is a cause of infertility. does reactive oxygen damage to gametes and embryos play a key role in the pathogenesis of infertility caused by endometriosis? Front Endocrinol. 2018; 9. [DOI] [PMC free article] [PubMed]
- 7.Chapron C, et al. Rethinking mechanisms, diagnosis and management of endometriosis. Nat Rev Endocrinol. 2019;15(11):666–682. doi: 10.1038/s41574-019-0245-z. [DOI] [PubMed] [Google Scholar]
- 8.Gonzalez-Comadran M, et al. The impact of endometriosis on the outcome of Assisted Reproductive Technology. Reprod Biol Endocrinol. 2017;15(1):8. doi: 10.1186/s12958-016-0217-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barnhart K, Dunsmoor-Su R, Coutifaris C. Effect of endometriosis on in vitro fertilization. Fertil Steril. 2002;77(6):1148–1155. doi: 10.1016/S0015-0282(02)03112-6. [DOI] [PubMed] [Google Scholar]
- 10.Corachan A, et al. Novel therapeutic targets to improve IVF outcomes in endometriosis patients: a review and future prospects. Hum Reprod Update. 2021;27(5):923–972. doi: 10.1093/humupd/dmab014. [DOI] [PubMed] [Google Scholar]
- 11.Leone RMU, et al. A systematic review on endometriosis during pregnancy: diagnosis, misdiagnosis, complications and outcomes. Hum Reprod Update. 2016;22(1):70–103. doi: 10.1093/humupd/dmv045. [DOI] [PubMed] [Google Scholar]
- 12.Andersen CY. Grand challenges in reproductive endocrinology. Front Endocrinol. 2017; 7. [DOI] [PMC free article] [PubMed]
- 13.Valbuena D, Martin J, de Pablo JL, Remohí J, Pellicer A, Simón C. Increasing levels of estradiol are deleterious to embryonic implantation because they directly affect the embryo. Fertil Steril. 2001;76(5):962–968. doi: 10.1016/S0015-0282(01)02018-0. [DOI] [PubMed] [Google Scholar]
- 14.Lee JH, et al. Machine learning approach to find the relation between endometriosis, benign breast disease, cystitis and non-toxic goiter. Sci Reports. 2019. 9(1). [DOI] [PMC free article] [PubMed]
- 15.Wu J, et al. Fertility and neonatal outcomes of freeze-all vs. fresh embryo transfer in women with advanced endometriosis. Front Endocrinol. 2019. 10. [DOI] [PMC free article] [PubMed]
- 16.Erşahin AA, et al. Frozen embryo transfer prevents the detrimental effect of high estrogen on endometrium receptivity. J Turk. 2017;18(1):38–42. doi: 10.4274/jtgga.2016.0186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tan J, Cerrillo M, Cruz M, Cecchino GN, Garcia-Velasco JA. Early pregnancy outcomes in fresh versus deferred embryo transfer cycles for endometriosis-associated infertility: a retrospective cohort study. J Clin Med. 2021;10(2):1–9. doi: 10.3390/jcm10020344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tan Q, et al. MicroRNAs in small extracellular vesicles indicate successful embryo implantation during early pregnancy. Cells. 2020;9(3):645. doi: 10.3390/cells9030645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bourdon M, et al. The deferred embryo transfer strategy improves cumulative pregnancy rates in endometriosis-related infertility: a retrospective matched cohort study. PLOS ONE. 2018;13(4):e0194800. doi: 10.1371/journal.pone.0194800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fang Wang, X.K.M.L., Clinical outcome of first frozen-thawed embryo transfer cycle after whole embryo cryopreservation in patients with mild endometriosis. Journal of Reproductive Medicine, 2018. 27(3): p. 248–253.
- 21.Asoglu MR, Celik C, Bahceci M. Frozen blastocyst transfer improves the chance of live birth in women with endometrioma. Gynecol Endocrinol. 2020;36(10):902–906. doi: 10.1080/09513590.2020.1781082. [DOI] [PubMed] [Google Scholar]
- 22.Mohamed AMF, et al. Live birth rate in fresh and frozen embryo transfer cycles in women with endometriosis. Eur J Obstet Gynecol Reprod Biol. 2011;156(2):177–180. doi: 10.1016/j.ejogrb.2011.01.020. [DOI] [PubMed] [Google Scholar]
- 23.R. Silva Martins, A.H.O.D., Subendometrial resistence and pulsatility index assessment of endometrial receptivity in assisted reproductive technology cycles. Reproductive Biology and Endocrinology, 2019(17): p. 1–7. [DOI] [PMC free article] [PubMed]
- 24.Weinerman R, Mainigi M. Why we should transfer frozen instead of fresh embryos: the translational rationale. Fertil Steril. 2014;102(1):10–18. doi: 10.1016/j.fertnstert.2014.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ng EH, et al. Comparison of endometrial and subendometrial blood flow measured by three-dimensional power Doppler ultrasound between stimulated and natural cycles in the same patients. Hum Reprod. 2004;19(10):2385–2390. doi: 10.1093/humrep/deh384. [DOI] [PubMed] [Google Scholar]
- 26.Horcajadas JA, et al. Effect of controlled ovarian hyperstimulation in IVF on endometrial gene expression profiles. MHR Basic Sci Reprod Med. 2005;11(3):195–205. doi: 10.1093/molehr/gah150. [DOI] [PubMed] [Google Scholar]
- 27.Haouzi D, et al. Gene expression profile of human endometrial receptivity: comparison between natural and stimulated cycles for the same patients. Human Reprod (Oxford) 2009;24(6):1436–1445. doi: 10.1093/humrep/dep039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhu X, et al. Endometrium cytokine profiles are altered following ovarian stimulation but almost not in subsequent hormone replacement cycles. Cytokine. 2019;114:6–10. doi: 10.1016/j.cyto.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 29.Mirkin S, et al. Gene expression profiles and structural/functional features of the peri-implantation endometrium in natural and gonadotropin-stimulated cycles. J Clin Endocrinol Metab. 2004;89(11):5742–5752. doi: 10.1210/jc.2004-0605. [DOI] [PubMed] [Google Scholar]
- 30.Du Y, et al. Effect of human chorionic gonadotropin injection before frozen embryo transfer on pregnancy outcomes in endometriosis-associated infertility. Front Med. 2020 7. [DOI] [PMC free article] [PubMed]
- 31.Lessey BA, Young SL. What exactly is endometrial receptivity? Fertil Steril. 2019;111(4):611–617. doi: 10.1016/j.fertnstert.2019.02.009. [DOI] [PubMed] [Google Scholar]
- 32.Wang X, Yu Q. Endometriosis-related ceRNA network to identify predictive biomarkers of endometrial receptivity. Epigenomics. 2019;11(2):147–167. doi: 10.2217/epi-2018-0190. [DOI] [PubMed] [Google Scholar]
- 33.Wei D, et al. Frozen versus fresh single blastocyst transfer in ovulatory women: a multicentre, randomised controlled trial. Lancet. 2019;393(10178):1310–1318. doi: 10.1016/S0140-6736(18)32843-5. [DOI] [PubMed] [Google Scholar]