Abstract
Purpose
The aim of this study was to identify the disease-causing mutations found in three infertile female patients who were diagnosed with abnormal zona pellucida (ZP) and empty follicle syndrome (EFS).
Methods
We performed whole-exome sequencing and Sanger sequencing to identify and verify the disease-causing mutations. Additionally, we performed Western blotting and mini-gene splicing assay to assess the effects of the mutations.
Results
We identified two novel compound heterozygous mutations in the ZP2 gene, a patient with an abnormal ZP carrying a novel compound heterozygous mutation (c.1695-2A>G and c.1831G>T, p.V611F) and a patient with EFS carrying a novel compound heterozygous mutation (c.1695-2A>G and c.1924 C>T, p.R642*). Furthermore, we identified a patient with typical abnormal ZP carrying a novel heterozygous mutation (c.400G>T, p.A134S) in the ZP3 gene. The splice site mutation (c.1695-2A>G) can cause abnormal pre-mRNA splicing that inserts an extra sequence of 61 bp in the mRNA of ZP2, and the missense mutation (c.1831G>T) can cause a decrease of ZP2 protein in HEK293 cells.
Conclusion
We identified three novel mutations in the ZP2 gene and the ZP3 gene in three Chinese female patients with infertility. Our study expands the spectrum of ZP gene mutations and phenotypes and thus is beneficial in the genetic diagnosis of infertility in females.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10815-022-02466-4.
Keywords: ZP2, ZP3, Mutation, Abnormal zona pellucida, Empty follicle syndrome
Introduction
Female infertility is a disease characterized by a woman who is not diagnosed with a clinical pregnancy after 12 months of regular unprotected sexual intercourse [1], and it is a global health and social problem with an estimated 48 million women suffering from infertility [2]. Unhealthy oocytes are one of the major causes of female infertility, manifested as abnormal zona pellucida, empty follicle syndrome, and oocyte degeneration.
The zone pellucida (ZP) is a thick extracellular coat of glycoproteins synthesized during follicular development and surrounds all mammalian eggs. The human ZP matrix is a highly organized, dynamic structure consisting of four glycoproteins designated as ZP1, ZP2, ZP3, and ZP4[3, 4]. The ZP is critical for oogenesis, fertilization, and early embryo development. During the final stages of oogenesis, ZP provides nutrition and supports the growth of oocytes; during fertilization, ZP regulates the species-restricted interaction between sperm and oocytes and participates in preventing polyspermy, and during the early stage of embryo development, ZP surrounds the embryo and serves as a protective barrier until implantation of the blastocyst occurs in the endometrium [4–9].
Mutations in ZP1, ZP2, ZP3, and ZP4 genes could cause abnormal ZP, empty follicle syndrome (EFS), and oocyte degeneration [3, 10]. Abnormal ZP indicates ZP dysmorphology such as dark ZP, thin ZP, lack of ZP, large perivitelline space, and oval-shaped or irregular-shaped ZP [11]. EFS is defined as a condition whereby no oocytes are obtained in assisted reproductive technology (ART) while follicle development and hormone levels appear normal [12]. And, thin ZP is defined as embryos that have zonae less than 13 microns [13].
In our study, we sampled three patients with female infertility, and through genetic analysis, we identified two novel compound heterozygous mutations in the ZP2 gene (c.1695-2A>G, c.1831G>T and c.1695-2A>G, c.1924 C>T). Additionally, we observed an infertile female with abnormal ZP who carried a novel heterozygous mutation in the ZP3 gene (c.400G>T, p.A134S). Our study serves to expand the spectrum of ZP gene mutations and phenotypes.
Materials and methods
Human subject
Seventy-four females were collected because of primary unexplained infertility and 21 of them were diagnosed with abnormal ZP and/or EFS in IVF at the Reproductive Medicine Center, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology. All patients in this study signed written informed consent for publication of the details of their medical case and any accompanying images.
Controlled ovarian hyperstimulation (COH) and oocytes retrieval
All infertile females received hormone measurement, Müllerian hormone (AMH), antral follicle count (AFC) to assess ovarian function. The ovarian stimulation protocols carried out in the three patients reported here were gonadotrophin-releasing hormone agonist (GnRH-agonist) protocol, a detailed description of it was presented as before [14]. Ten thousand IU recombinant HCG was administrated for the trigger when two or three dominant follicles with a diameter of more than 18 mm could be observed. Oocytes’ retrieval was performed by guided transvaginal ultrasound 36–38 h after HCG triggering.
Insemination and embryo culture
Males received semen evaluation according to the World Health Organization (WHO) 2010 criteria [15] before they performed in vitro fertilization (IVF). For IVF patients, 30,000 motile spermatozoa were co-incubated with oocytes in IVF medium (Vitrolife, Sweden) 3–4 h after oocytes retrieval, and degranulation took place 4 h after fertilization to observe whether the second polar appear or not. Early rescue intracytoplasmic sperm injection (ICSI) would perform if the second polar was not observed within 6 h after fertilization. For patients who received intracytoplasmic sperm injection (ICSI), cumulus-oocyte complexes (COCs) were cultured in IVF medium (Vitrolife, Sweden) after oocytes retrieval for 2–3 h, afterwards transferred to 80 IU hyaluronidase (Vitrolife, Sweden) followed by mechanical pipetting to remove cumulus cells for denudation. Denuded oocytes were cultured in a G1-plus medium (Vitrolife, Sweden) for 1–2 h before sperm injection. After sperm to be injected was immobilized and fixed, adjusting the denuded oocyte so that the polar body of the oocyte is located in the position of 12 or 6 o’clock. The sperm was pushed by the injection needle at the position of 3 o’clock and entry into the endochylema. The presence of two pronuclei (2PN) 16–18 h after insemination was regarded as successful fertilization. The qualities of fertilized zygotes were assessed on day 2 and day 3, one or two embryos with the best quality were transferred. Redundant embryos with high quality were cryopreserved or extended culture to blastocyst for cryopreservation.
Clinical characteristics of the patients
In this study, the detailed data of hormone measurement, Müllerian hormone (AMH), and antral follicle count (AFC) from the patients were shown in Supplementary Table 1, and the detailed data of semen analyses of the patients’ husbands were shown in Supplementary Table 2.
Patient 1 from family 1 was a 34-year-old female who had experienced primary infertility for 9 years. Her three sisters did not have a history of infertility, and the semen examination of her husband did not indicate infertility. The chromosomal karyotype of the female was 46, XX, and that of her husband was 46, XY. Her first cycle of conventional IVF failed to lead to fertilization (December 17, 2020). Nineteen oocytes were obtained after denudation but all were found to be abnormal with a thin ZP matrix and enlarged perivitelline space (Fig. 1a). Subsequently, early rescue intracytoplasmic sperm injection (ICSI) was performed. Ten zygotes of 2 pronuclei (2PN) were formed, and one embryo was transferred on day 3 but failed to implant.
Fig. 1.
Morphology of oocytes from patients. a The oocytes and embryos development from patient 1. ZP was found to be abnormal with a thin ZP matrix and enlarged perivitelline space. b The oocytes of patient 2 in her second cycle of IVF showed a very thin ZP and exhibited abnormal structure. c Cumulus-oocyte complexes (COCs) from patient 2 exhibited obscure structures and turned out to be empty follicular after hyaluronidase digestion in her first cycle of IVF. d Oocytes of poor quality with thin ZP and abnormal morphology from patient 3 in her second cycle of IVF
Patient 2 from family 2 was a 25-year-old female who had experienced primary infertility for 3 years. Her chromosomal karyotype was 46, XX. She underwent two cycles of controlled ovarian hyperstimulation (COH) treatment. In the first cycle (November 23, 2018), 8 follicles with a diameter of more than 14 mm were aspirated while only 3 cumulus-oocyte complexes (COCs) with obscure structures were obtained, and after hyaluronidase digestion, they turned out to be empty follicular and no oocytes were obtained (Fig. 1c). In the second cycle (April 9, 2019), 13 follicles with a diameter of more than 14 mm were aspirated 37 h after HCG triggering, and 5 COCs were obtained but still exhibited obscure structures. After denudation, one had degenerated, and four oocytes were obtained with one exhibited lack of ZP and the rest of three exhibited abnormal morphologies. After ICSI, only two 2PN zygotes were obtained and developed poorly (Fig. 1b). At last, one zygote was cryopreserved 6 days later at the blastula stage. A month after ICSI, the patient received frozen-thawed embryo transfer (FET) but failed to become pregnant.
Patient 3 from family 3 was diagnosed with infertility due to diminished ovarian reserve; she was 32 years old and had experienced primary infertility for 6 years. She received two COH cycles. In the first cycle, 2 COCs were obtained, and after denudation, one had degenerated, and another one was situated at the germinal vesicle (GV) stage. In the second cycle, three oocytes were obtained, but all of them were of poor quality with thin ZP and abnormal oocyte morphology (Fig. 1d). Both the following round of IVF was canceled after her two CHO cycles because of the poor quality of oocytes.
Whole-exome sequencing
Whole-exome sequencing was used to identify the disease-causing mutations in these patients. Peripheral blood samples were obtained from the patients and their family members. Genomic DNA was extracted from peripheral blood using the potassium acetate (KAC) method and genomic DNA of affected individuals was processed and sequenced with 150-bp paired-end reads on a HiSeq2000 sequencer ((Illumina, USA). SNPs and variants within intergenic and UTR regions and synonymous mutations were detected and removed. Detailed methods for whole-exome sequencing and data analysis were described as before [16].
Sanger sequencing
All pathogenic mutations were confirmed by Sanger DNA sequencing. Polymerase chain reactions (PCR) were performed in 50 μl of standard PCR buffer containing 20 ng of human genomic DNA. Products were purified using the CWBio Gel Extraction Kit (CWBio, China). Sanger sequencing was then performed using the BigDye Terminator Cycle Sequencing v3.1 kit and an ABI 3500 Genetic Analyzer (Applied Biosystems, USA).
Plasmid construct
Human ZP2 gene cDNA plasmid was provided by Lei Wang’s laboratory at Fudan University [9]. Full-length cDNA of the ZP2 gene was cloned into a pEGFP-C1 vector. Mutations (c.1831G>T, p.V611F and c.1924C>T, p.R642*) in ZP2 were synthesized by Mut Express II Fast Mutagenesis Kit V2 (Vazyme, China). Furthermore, splice site mutation (c.1695-2A>G) in ZP2 was constructed and inserted into the pcDNA 3.1(+) vector. All plasmids were confirmed by enzyme digestion and DNA sequencing.
RNA extracted and reverse transcription (RT)-PCR
To determine whether the ZP2 c.1695-2A>G mutation affects the splicing of mRNAs, pcDNA3.1-ZP2-WT (wild-type mini gene of ZP2 gene was cloned into the plasmid, the mini gene including genomic sequence from intron 11 to exon 17 of ZP2 gene), pcDNA3.1-ZP2-MUT (mutant-type mini gene of ZP2 gene was cloned into the plasmid), and the control pcDNA3.1 (+) plasmid were transfected into HEK293T cells using Lipofectamine 2000 (Vazyme, China) separately. After 48 h, total RNA was extracted using a Trizol kit (Takara, Japan), and cDNA was obtained using HiScript II 1st Strand cDNA Synthesis Kit (Vazyme, China). Primers for second-strand synthesis included a forward primer (5′-ggctgctaacccactttctctgaca-3′) and a reverse primer (5′-ctgggaggctgactgtcattttctc-3′).
Western blot
Forty-eight hours after transfection, total proteins were extracted from HEK293T cells and radioimmunoprecipitation (RIPA) lysis buffer (Beyotime, China) supernatants were collected by centrifugation at 12,000 g for 30 min, and 5 × loading buffer was added and heated for 10 min at 95 °C. Total proteins were separated in 10% SDS-polyacrylamide gel electrophoresis (SDS-PAGE) gels and transferred to polyvinylidene difluoride (PVDF) membranes. The primary antibodies used were against GFP (1:2000 dilution; ABclonal, China) and GAPDH (1:2000 dilution, ABclonal, China), and the secondary antibody was horseradish-peroxidase (HRP)-conjugated goat anti-mouse IgG (1:10,000 dilution; CWBio, China).
Results
Genetic analysis identified mutations in the ZP2 gene and in the ZP3 gene
Whole-exome sequencing was used to identify the mutations in disease-causing genes of female infertility, with the mutations confirmed via Sanger DNA sequencing. Additionally, we only identified the mutations in ZP2 or ZP3 but did not find any mutations in other disease-causing genes of female infertility. Two novel compound mutations (c.1695-2A>G, c.1831G>T, and c.1695-2A>G, c.1924C>T) in the ZP2 gene were identified in patient 1 (Fig. 2) and in patient 2 (Fig. 3), respectively. And, patient 3 carried a novel heterozygous mutation in the ZP3 gene (c.400G>T, p.A134S) (Fig. 4).
Fig. 2.
DNA sequencing of ZP2 gene in family 1 and amino acid sequence alignment of ZP2 protein. a Pedigree of family 1. Affected female is depicted with filled circles. Unaffected individuals are depicted with empty symbols. b DNA sequencing shows compound heterozygous mutation of the ZP2 gene. Patient 1, II-4, carries the compound heterozygous mutations and unaffected family members only carry a heterozygous mutation. c The amino acid sequence alignment of the ZP2 protein from different species shows that the V611 residue is highly conserved during evolution
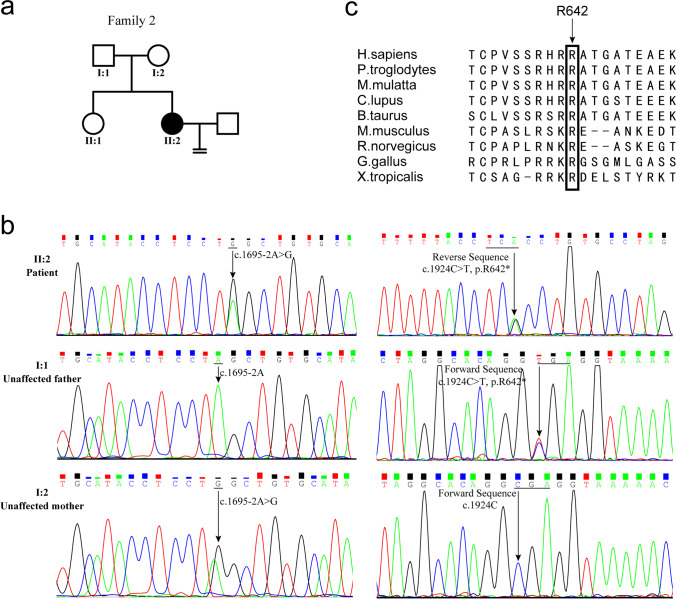
Fig. 3.
DNA sequencing of ZP2 gene in family 2 and the amino acid sequence alignment of ZP2 protein. a Pedigree of family 2. b DNA sequencing shows compound heterozygous mutation of the ZP2 gene. Patient 2, II-2, carries the compound heterozygous mutations and unaffected family members only carry a heterozygous mutation. c The amino acid sequence alignment of the ZP2 protein from different species shows that the R642 residue is highly conserved during evolution
Fig. 4.
DNA sequencing of ZP3 gene in family 3 and the amino acid sequence alignment of ZP3 protein. a Pedigree of family 3. b DNA sequencing shows a heterozygous mutation in patient 3 of the ZP3 gene. c The amino acid sequence alignment of the ZP3 protein from different species shows that the A134 residue is highly conserved during evolution
Expression of wild-type and mutant-type ZP2 proteins in HEK293T cells
As indicated by Western blot analysis, the expression of mutant ZP2 proteins (c.1831G>T, p.V611F) was significantly decreased (p < 0.01), and the mutant ZP2 gene that carries the missense mutation (c.1924C>T, p.R642*) generated truncated proteins (Fig. 5a).
Fig. 5.
Mutation affects the expression and splicing of the ZP2 pre-mRNA. a Left = Western blot of wild-type and mutant ZP2 in HEK293T cells; right = statistical analysis showed that the V611F mutation in ZP2 significantly decreased its protein expression level (n = 5; p < 0.05 was considered to be significantly different, p < 0.01 was highly significantly different, **represents p < 0.01.). b Diagram of the influence of splice site mutation of c.1695-2A>G. c Sanger sequencing of the PCR products of RT-PCR showed an extra sequence of 61 bp in the mRNA of ZP2 caused by the splice site mutation
Transcription analysis of wild-type and mutant-type ZP2 mini gene
RT-PCR analysis of ZP2 mRNA from total RNA obtained from cells transfected with pcDNA3.1-WT ZP2 mini gene and transfected with pcDNA3.1-MUT ZP2 mini gene. Sanger sequencing showed that the PCR products from cells transfected with the mutant-type ZP2 gene were 61-bp longer than cells transfected with the wild-type ZP2 gene, as shown in Fig. 5b. No PCR products were detected in the cells transfected with the control vector. Further sequencing analysis showed that the 61-bp unspliced sequence contained the sequence in intron 14 of the ZP2 gene (Fig. 5c). The full-length sequence of the PCR products from the cells transfected with the mutant-type ZP2 mini-gene is shown in Supplementary Figure 1. Thus, the splice site mutation of c.1695-2A>G in ZP2 could lead to abnormal pre-mRNA splicing and insert an extra sequence of 61 bp in the mRNA of ZP2 and may lead to the production of premature stop codons, which would further affect the function of the ZP2 protein.
Discussion
In our study, we sampled three independent patients suffering from primary infertility. Patient 1 from family 1 exhibited an abnormal ZP and carried a novel compound heterozygous mutation (c.1695-2A>G and c.1831G>T, p.V611F) in the ZP2 gene. Patient 2 from family 2 exhibited EFS and carried a novel compound heterozygous mutation (c.1695-2A>G and c.1924C>T, p.R642*) in the ZP2 gene. Patient 3, who suffered from typical thin ZP, carried a novel heterozygous mutation (c.400G>T, p.A134S) in the ZP3 gene. Both patients from family 1 and family 2 carried the same splice site mutation (c.1695-2A>G), which led to the insertion of a 61-bp intron sequence that may cause premature termination of the ZP2 protein. Furthermore, the missense mutation (c.1831G>T, p.V611F) of the ZP2 gene could decrease the expression level of the ZP2 protein. The p.R642* mutation of the ZP2 gene was also previously researched by our laboratory, which was observed to cause premature termination of the protein and to produce a truncated protein, and was further confirmed in this study [17]. The patient in family 3 carried the heterozygous mutation in the ZP3 gene (c.400G>T, p.A134S) and exhibited the typical ZP abnormality characterized by oocyte degeneration. The position of ZP3 was also previously reported in a sterile female with abnormal ZP and oocyte degeneration who carried the mutation of c.400G>A, p.A134T [10].
We also verified the effect of protein expression of variant p.V611F and p.R642* in the ZP2 gene in Chinese hamster ovary (CHO) cells by Western blotting, which corresponded with HEK293T cells. Furthermore, using a confocal microscope, we evaluated the location of ZP2 proteins in CHO cells, with both the wild-type and mutant-type ZP2 proteins observed to be located in the cytoplasm (data not shown).
ZP is critical during oogenesis, fertilization, and early embryo development. Using high-resolution scanning electron microscopy, it was revealed that, the ZP is a meshwork of thin interconnected filaments, in which ZP2 and ZP3 form heterodimers and polymerized into long fibrils, with ZP1 and ZP4 interconnected in [8, 18, 19]. It was reported that the ZP matrix of all eutherian mammals has both ZP2 and ZP3 [18]. In mice, female mice lacking either ZP2 or ZP3 are unable to form a ZP and are infertile [20, 21]. But several studies indicated that mutations in the ZP1 gene and the ZP4 gene could also cause abnormal ZP and are related to female infertility in humans. Mutations in the ZP1 gene could cause EFS[17, 22–28], lack of ZP[9, 29–31], enlarged ZP[31], and oocyte degeneration [32]. And, mutations in the ZP4 gene could cause thin and irregular ZP [33].
Mutations in the ZP2 gene and the ZP3 gene could also affect the zona pellucida and cause infertility, not only indicated by this study but also demonstrated by a set of reports. In 2017, Chen et al. identified a patient with EFS due to the heterozygous mutation (c.400G>A, p.Ala134Thr) in the ZP3 gene, which the mutation affected the interaction between ZP2 and ZP3. Thus, oocyte degeneration and empty COCs resulted [10]. Cao et al. also reported the same heterozygous mutation in the ZP3 (c.400G>A, p.Ala134Thr) gene in a primary infertility patient with ZP-free oocyte and degeneration. The corresponding in vivo study revealed that the A134T mutation can also reduce the interaction between ZP2 and ZP3 and affect the secretion of ZP proteins [30]. Yang et al. reported two novel heterozygous mutations in the ZP2 gene (c.1599G>T, p.R533S and c.1696T>C, p.C566R) in patients suffering from oocyte morphological defects [32]. In their further studies, Liu et al. indicated that ZP variants in different ZP genes might have dosage effects on ZP formation, as their study showed that a patient with abnormal ZP carried a heterozygous mutation in ZP2 (c.2092C>T) and a heterozygous mutation in ZP3 (c.1045_1046insT). The patient’s mother, who carried the heterozygous mutation in ZP3 but no mutations in ZP2, was asymptomatic. Their conclusion was confirmed via a mouse model [34–36]. Dai et al. reported two homozygous mutations in ZP2 (c.1695-2A>G, and c.1691_1694dup, respectively), with both mutations being able to cause a frameshift and introduce a premature termination codon at the same site in mRNA (p.C566Wfs*5) and produce a truncated ZP2 protein. In their study, both patients exhibited thin ZP of oocytes, which led to a deficiency of sperm-binding as well as IVF failure [2].
Additional studies have reported novel mutations of the ZP2 and/or ZP3 gene, validating mutation impacts by CHO cells. Zhou et al. reported that a homozygous mutation (c.1115G>C, p.Cys372Ser) in the ZP2 gene caused thin ZP and a heterozygous mutation (c.763C>G, p.Arg255Gly) in the ZP3 gene caused ZP free. In both cases, the patients suffered from recurrent IVF failure. Further studies in CHO cells demonstrate that mutant ZP2 protein cannot be secreted, while the interaction between ZP2 and the other three types of ZP proteins was not affected. Moreover, the R255G mutation in the ZP3 gene seems to not affect protein secretion but could enhance binding to the other three wild-type ZP proteins [9]. Chen et al. reported a novel heterozygous deletion mutation in the ZP3 gene (c.565_579del, p.Thr189_Gly193del) of an EFS patient, which could decrease the expression of the ZP3 protein in CHO cells [37]. Furthermore, a study by Yang et al. identified two novel heterozygous variants of the ZP2 gene (c. 1925G>A, p.R642Q and c. 1856T>A, p.I619N) in two patients suffering from EFS caused by oocyte degeneration. By studying CHO cells, they speculated that the p.R642Q mutation could increase the molecular weight of uncleaved ZP2 protein and that the p.I619N mutation may affect protein secretion; the ZP2 protein level was increased in the cell lysate but decreased in the medium [27]. Sun et al. identified a homozygous frameshift mutation in the ZP2 gene (c.1235_1236del, p.Q412Rfs*17) in patients with thin ZP. Studies in CHO cells indicate that the frameshift variation could produce a truncated ZP2 protein with a low expression level, impeding the interaction between ZP2 and ZP3 [38].
There were two additional studies in our laboratory regarding ZP mutations. Luo et al. reported that a compound mutation in the ZP2 gene (c.860_861delTG, p.Val287fs, and c.1924C>T, p.Arg642Ter) led to the production of a truncated ZP2 protein in a female who was diagnosed with primary infertility and exhibited thin ZP [17]. Furthermore, Zhang et al. sampled a patient with EFS due to a heterozygous mutation (c.518C>G, p.Ser173Cys) of the ZP3 gene; the S173C variation did not affect the expression in the cell lysates but impeded the interaction between ZP2 and ZP3 [39]. Taken together, it is clear that variants in the ZP2 or ZP3 gene could affect its protein expression, secretion, or interaction with wild-type ZP proteins, influencing the normal function of ZP and causing infertility in females.
Patients with ZP mutations may overcome infertility by ART. In a consanguineous family reported by Dai et al., two infertility females carried the homozygous splicing mutation in the ZP2 gene (c.1695-2A>G). The elder sister was 30 years old and had 10 years history of primary infertility and the younger sister was 28 years old and had a 3-year history of infertility. Only the younger sister received ARF treatment and in the first cycle of IVF, there were no oocytes fertilized, and 8-cell embryos were obtained through ICSI but failed in implantation in the initial attempt, and in the following FET cycle, two vitrified blastocysts were used and eventually resulted in a successful pregnancy [2]. And, in a family with two sisters carrying a homozygous mutation in the ZP2 gene (c.1115G > C, p.Cys372Ser), the elder sister was 34-year-old and had a 6 years history of primary infertility; she received four IVF/ICSI cycles (specific information of this patient was not available) and only obtained a poor-quality embryo but led to a successful pregnancy. The younger sister was 33-year-old and had a 10 years history of primary infertility; she received three IVF attempts but failed pregnancy [9]. There was also a patient with heterozygous mutation (c.326G>A, p.Arg109His) in the ZP1 gene who exhibited a lack of ZP. The patient was 29-year-old and had a 4 years history of primary infertility but eventually successfully delivered a baby after undergoing ICSI [30].
In fact, the universally established protocol of the IVF-ICSI cycle may not solve rare events of primary infertility [40]. For example, during the IVF cycle, oocytes may present empty zona pellucida (EZP) caused by operations during oocyte retrieval or the ZP1-4 genes mutations. Thus, genetic analysis of EZP patients is important, and these patients should consider alternative IVF protocols, such as mild stimulation protocols or natural IVF cycles [40]. There was a case that could certify the importance of a proper ICSI treatment cycle. A 35-year-old female patient carried the homozygous mutation (c.706T>C, p.Cys236Arg) in the ZP1 gene and suffered from infertility over 10 years. She was successfully pregnant using “diagnostic ICSI (D-ICSI)”, an ICSI cycle in her natural menstrual cycle and without COH treatment to get development information of ZP-free oocytes after sperm injection. Briefly, there were no oocytes nor ZPs were observed after removal of the COCs in her first IVF-ICSI cycle. Then, she further received “diagnostic ICSI (D-ICSI).” A transvaginal ultrasound scan was performed to measure the diameter of follicles and when the largest follicle come up to 20 mm, the patient received HCG treatment and oocyte pick-up was carried out 36 h later. Two COCs were obtained and denudation by strippers, and eventually, one oocyte was obtained, although no polar bodies or ZP was observed. ICSI was performed on this oocyte and successfully fertilization and developed into the blastocyst stage. Embryo transfer was not performed because of the thin endometrium of the patient. And 2 months later, the patient received therapeutic according to the results of the D-ICSI; eight ZP-free oocytes were obtained and six 2PN zygotes were observed after sperm injection; one embryo was selected and administrated embryo transfer. The patient was successfully pregnant and delivered a healthy baby [41].
Thus, the integrality of the ZP structure is critical for fertilization. Excessive confirmation analysis and diagnosis for unexplained primary female infertility with ZP abnormality should be performed, and patients with female infertility who carry ZP mutations may cause fertilization failure in conventional IVF, and it is recommended to choose the proper ICSI treatment cycle to improve the probability of pregnancy.
In conclusion, we identified mutations in the ZP2 gene and the ZP3 gene in three females with infertility. Our findings expand the spectrum of ZP genes mutations and may enhance the genetic diagnosis of infertility in females.
Supplementary information
Sanger sequencing of the PCR products from the cells transfected with mutant-type ZP2 mini gene (PNG 771 kb)
(DOCX 17 kb)
(DOCX 17 kb)
Acknowledgements
We would like to thank Sang Qing and Lei Wang from Fudan University for kindly providing the gift of ZP2 and ZP3 plasmids. And, we thank the family members for their enthusiastic participation in this study.
Funding
This work was supported by The National Natural Science Foundation of China (81000079, 81170165 and 81870959 to X.Z.), supported by The Program for HUST Academic Frontier Youth Team (2016QYTD02), and supported by The Fundamental Research Funds for the Central Universities (HUST: 2019JYCXJJ035).
Data availability
Research data are not shared.
Declarations
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the ethics committee on human subject research at Huazhong University of Science and Technology (2019S1160) and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Consent to participate
Written informed consent was obtained from the parents.
Consent for publication
The participant has consented to the submission of the case report to the journal.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Weimin Jia and Qingsong Xi contributed equally to this work.
Contributor Information
Juan Hu, Email: hujuan310@126.com.
Xianqin Zhang, Email: xqzhang04@hust.edu.cn.
References
- 1.Zegers-Hochschild F, et al. The international glossary on infertility and fertility care, 2017. Fertil Steril. 2017;108(3):393–406. doi: 10.1016/j.fertnstert.2017.06.005. [DOI] [PubMed] [Google Scholar]
- 2.Dai C, et al. ZP2 pathogenic variants cause in vitro fertilization failure and female infertility. Genet Med. 2019;21(2):431–440. doi: 10.1038/s41436-018-0064-y. [DOI] [PubMed] [Google Scholar]
- 3.Moros-Nicolás C, et al. New insights into the Mammalian Egg Zona Pellucida. Int J Mol Sci. 2021:22(6). [DOI] [PMC free article] [PubMed]
- 4.Lefièvre L, et al. Four zona pellucida glycoproteins are expressed in the human. Hum Reprod. 2004;19(7):1580–1586. doi: 10.1093/humrep/deh301. [DOI] [PubMed] [Google Scholar]
- 5.Gupta SK. The human egg’s zona pellucida. Curr Top Dev Biol. 2018;130:379–411. doi: 10.1016/bs.ctdb.2018.01.001. [DOI] [PubMed] [Google Scholar]
- 6.Conner SJ, et al. Cracking the egg: increased complexity in the zona pellucida. Hum Reprod. 2005;20(5):1148–1152. doi: 10.1093/humrep/deh835. [DOI] [PubMed] [Google Scholar]
- 7.Ganguly A, et al. ‘ZP domain’ of human zona pellucida glycoprotein-1 binds to human spermatozoa and induces acrosomal exocytosis. Reprod Biol Endocrinol. 2010;8:110. doi: 10.1186/1477-7827-8-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Litscher ES, Wassarman PM. Zona Pellucida Proteins, Fibrils, and Matrix. Annu Rev Biochem. 2020;89:695–715. doi: 10.1146/annurev-biochem-011520-105310. [DOI] [PubMed] [Google Scholar]
- 9.Zhou Z, et al. Novel mutations in ZP1, ZP2, and ZP3 cause female infertility due to abnormal zona pellucida formation. Hum Genet. 2019;138(4):327–337. doi: 10.1007/s00439-019-01990-1. [DOI] [PubMed] [Google Scholar]
- 10.Chen T, et al. A recurrent missense mutation in ZP3 causes empty follicle syndrome and female infertility. Am J Hum Genet. 2017;101(3):459–465. doi: 10.1016/j.ajhg.2017.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sauerbrun-Cutler MT, et al. Oocyte zona pellucida dysmorphology is associated with diminished in-vitro fertilization success. J Ovarian Res. 2015;8:5. doi: 10.1186/s13048-014-0111-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coulam CB, Bustillo M, Schulman JD. Empty follicle syndrome. Fertil Steril. 1986;46(6):1153–1155. doi: 10.1016/S0015-0282(16)49898-5. [DOI] [PubMed] [Google Scholar]
- 13.Cohen J, et al. Implantation enhancement by selective assisted hatching using zona drilling of human embryos with poor prognosis. Hum Reprod. 1992;7(5):685–691. doi: 10.1093/oxfordjournals.humrep.a137720. [DOI] [PubMed] [Google Scholar]
- 14.Wang M, et al. The relationship between a novel evaluation parameter of premature luteinization and IVF outcomes. Reprod Biomed Online. 2021;42(2):323–331. doi: 10.1016/j.rbmo.2020.10.009. [DOI] [PubMed] [Google Scholar]
- 15.Cooper TG, et al. World Health Organization reference values for human semen characteristics. Hum Reprod Update. 2010;16(3):231–245. doi: 10.1093/humupd/dmp048. [DOI] [PubMed] [Google Scholar]
- 16.Jia W, et al. A novel UBE2A mutation in a Chinese family with X-linked intellectual disability. J Gene Med. 2020;22(8):e3191. doi: 10.1002/jgm.3191. [DOI] [PubMed] [Google Scholar]
- 17.Luo G, et al. Novel mutations in ZP1 and ZP2 cause primary infertility due to empty follicle syndrome and abnormal zona pellucida. J Assist Reprod Genet. 2020;37(11):2853–2860. doi: 10.1007/s10815-020-01926-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gupta SK. Human zona pellucida glycoproteins: binding characteristics with human spermatozoa and induction of acrosome reaction. Front Cell Dev Biol. 2021;9:619868. doi: 10.3389/fcell.2021.619868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Monné M, Jovine L. A structural view of egg coat architecture and function in fertilization. Biol Reprod. 2011;85(4):661–669. doi: 10.1095/biolreprod.111.092098. [DOI] [PubMed] [Google Scholar]
- 20.Rankin TL, et al. Defective zonae pellucidae in Zp2-null mice disrupt folliculogenesis, fertility and development. Development. 2001;128(7):1119–1126. doi: 10.1242/dev.128.7.1119. [DOI] [PubMed] [Google Scholar]
- 21.Liu C, et al. Targeted disruption of the mZP3 gene results in production of eggs lacking a zona pellucida and infertility in female mice. Proc Natl Acad Sci U S A. 1996;93(11):5431–5436. doi: 10.1073/pnas.93.11.5431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yuan P, et al. Novel mutation in the ZP1 gene and clinical implications. J Assist Reprod Genet. 2019;36(4):741–747. doi: 10.1007/s10815-019-01404-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dai C, et al. ZP1 mutations are associated with empty follicle syndrome: evidence for the existence of an intact oocyte and a zona pellucida in follicles up to the early antral stage. A case report. Hum Reprod. 2019;34(11):2201–2207. doi: 10.1093/humrep/dez174. [DOI] [PubMed] [Google Scholar]
- 24.Sun L, et al. Compound heterozygous ZP1 mutations cause empty follicle syndrome in infertile sisters. Hum Mutat. 2019;40(11):2001–2006. doi: 10.1002/humu.23864. [DOI] [PubMed] [Google Scholar]
- 25.Liu M, et al. Novel biallelic loss-of-function variants in ZP1 identified in an infertile female with empty follicle syndrome. J Assist Reprod Genet. 2020;37(9):2151–2157. doi: 10.1007/s10815-020-01855-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu Q, et al. A novel homozygous nonsense ZP1 variant causes human female infertility associated with empty follicle syndrome (EFS) Mol Genet Genomic Med. 2020;8(7):e1269. doi: 10.1002/mgg3.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang P, et al. The critical role of ZP genes in female infertility characterized by empty follicle syndrome and oocyte degeneration. Fertil Steril. 2021;115(5):1259–1269. doi: 10.1016/j.fertnstert.2020.11.003. [DOI] [PubMed] [Google Scholar]
- 28.Wang J, et al. A novel homozygous nonsense mutation in zona pellucida 1 (ZP1) causes human female empty follicle syndrome. J Assist Reprod Genet. 2021;38(6):1459–1468. doi: 10.1007/s10815-021-02136-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang HL, et al. Mutant ZP1 in familial infertility. N Engl J Med. 2014;370(13):1220–1226. doi: 10.1056/NEJMoa1308851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cao Q, et al. Heterozygous mutations in ZP1 and ZP3 cause formation disorder of ZP and female infertility in human. J Cell Mol Med. 2020;24(15):8557–8566. doi: 10.1111/jcmm.15482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Okutman Ö, et al. Homozygous splice site mutation in ZP1 causes familial oocyte maturation defect. Genes (Basel). 2020:11(4). [DOI] [PMC free article] [PubMed]
- 32.Yang P, et al. Novel zona pellucida gene variants identified in patients with oocyte anomalies. Fertil Steril. 2017;107(6):1364–1369. doi: 10.1016/j.fertnstert.2017.03.029. [DOI] [PubMed] [Google Scholar]
- 33.Wei X, et al. Mutations in ZP4 are associated with abnormal zona pellucida and female infertility. J Clin Pathol. 2021. [DOI] [PubMed]
- 34.Liu W, et al. Dosage effects of ZP2 and ZP3 heterozygous mutations cause human infertility. Hum Genet. 2017;136(8):975–985. doi: 10.1007/s00439-017-1822-7. [DOI] [PubMed] [Google Scholar]
- 35.Barbaux S, El Khattabi L, Ziyyat A. ZP2 heterozygous mutation in an infertile woman. Hum Genet. 2017;136(11-12):1489–1491. doi: 10.1007/s00439-017-1844-1. [DOI] [PubMed] [Google Scholar]
- 36.Liu W, et al. Additive-effect pattern of both ZP2 and ZP3 in human and mouse. Hum Genet. 2017;136(11-12):1493–1495. doi: 10.1007/s00439-017-1848-x. [DOI] [PubMed] [Google Scholar]
- 37.Chen Y, et al. Case report: a novel heterozygous ZP3 deletion associated with empty follicle syndrome and abnormal follicular development. Front Genet. 2021;12:690070. doi: 10.3389/fgene.2021.690070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sun Y, et al. A novel homozygous variant in ZP2 causes abnormal zona pellucida formation and female infertility. J Assist Reprod Genet. 2021;38(5):1239–1245. doi: 10.1007/s10815-021-02107-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang D, et al. A novel mutation in ZP3 causes empty follicle syndrome and abnormal zona pellucida formation. J Assist Reprod Genet. 2021;38(1):251–259. doi: 10.1007/s10815-020-01995-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Siristatidis, C., et al., Empty zona pellucida only case: a critical review of the literature. Int J Environ Res Public Health, 2021. 18(17). [DOI] [PMC free article] [PubMed]
- 41.Metwalley A, et al. Role of diagnostic intracytoplasmic sperm injection (ICSI) in the management of genetically determined zona pellucida-free oocytes during in vitro fertilization: a case report. Zygote. 2020;28(6):519–523. doi: 10.1017/S0967199420000441. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Sanger sequencing of the PCR products from the cells transfected with mutant-type ZP2 mini gene (PNG 771 kb)
(DOCX 17 kb)
(DOCX 17 kb)
Data Availability Statement
Research data are not shared.