Abstract
Purpose
Anti-Müllerian hormone (AMH) and antral follicle count (AFC) are correlated with the ovarian response, but their reliability and reproducibility are questionable. This large multicenter study describes their distribution, inter-cycle and inter-center variability, and their correlation.
Methods
A total of 25,854 IVF cycles among 15,219 patients were selected in 12 ART centers. Statistical distribution of AMH and AFC was studied by using the Kolmogorov–Smirnov test and Shapiro goodness of fit test. The reproducibility of AFC and AMH was measured using a mixed model regressing the logarithmic transformation of AFC with age.
Results
The distribution of AMH and AFC was characterized by a wide dispersion of values, twice more important for AFC, and a logarithmic distribution. The faster decline in AMH than in AFC with age suggests that their correlation changes with age. AMH and AFC showed a very low proportion of concordance in the range of expected poor responders according to Bologna cutoffs. The heterogeneity for AMH and AFC across centers was small, but much larger across patients within each center. Concerning the patients with several successive cycles, the reproducibility for AMH seemed much better than for AFC. Comparing respective performances of AMH and AFC for the prediction of ovarian response depended on the local conditions for measuring these indicators and on the reproducibility of results improved over time.
Conclusion
Distribution of AMH and AFC was characterized by the wide dispersion of values, and a logarithmic distribution. Establishing cutoffs or a direct relationship AMH/AFC without considering age seems hazardous. Correlation between AMH and AFC was very poor in the range of poor responders.
Keywords: AMH, AFC, Ovarian reserve, Poor ovarian responders, Bologna criteria, IVF
Background
The main objective of treatment individualization in IVF is to offer tailored ovarian stimulation—namely choice of protocol and gonadotrophin starting dose—thus maximizing oocyte yield, preventing OHSS, and minimizing the frequency of cycle cancelation [1].
The prediction of the ovarian response also allows giving patients more accurate information on the likelihood of risks or cancelation, and on the probability of favorable outcome, and cumulative live birth rates (CLBR) steadily increasing with the number of retrieved oocytes [2].
The correct choice of stimulation protocols in IVF should be based on the most reliable markers of ovarian reserve. The clinical criteria used to select the appropriate controlled ovarian stimulation (COS) mainly include age, BMI, body shape and hairiness, periodicity of menstrual cycles, and, most importantly, the ovarian response of previous COS.
Although various biomarkers have been proposed as predictors of ovarian response, it is widely accepted that anti-Müllerian hormone (AMH) and antral follicle count (AFC) are appropriate markers of the functional ovarian reserve, namely the number of follicles at late stage of development and capable of responding to exogenous gonadotropins [3]. Several large systematic reviews and meta-analyses have demonstrated consistent positive association of AMH and AFC with the intensity of ovarian response, oocyte yield, and live birth in IVF cycles [1, 4–6]. AFC and AMH are used in several predictive models for identifying poor ovarian responders (Bologna criteria [7], Poseidon references [8], follicular output rate (FORT) [9]).
However, most of these predictive models have used AMH and AFC as fixed values without accounting for their reliability.
Such an extensive use in routine practice requires evidence of reliability (reproducibility) and feasibility in a practical environment. Indeed, AMH and AFC values reported in literature are very variable, thus creating difficulties for clinicians in selecting cutoff values based on evidence. AMH displays some variation within cycles, with a reduction in luteal phase [10], but cycle-independent measurement seems appropriate for clinical purposes. The age-related decline of AMH is constant, but, for a given age, AMH levels can vary substantially [11]. A recent meta-analysis suggests that BMI is negatively correlated with AMH in all study populations [12]. Concerning the duration of validity, a time interval up to 12 months between AMH serum sampling and initiation of ovarian stimulation does not appear to affect the correlation between AMH level and the number of oocytes retrieved [13]. AFC is also subject to intra-cyclic variation requiring its measurement during the early follicular phase. The inter-cycle variability of AFC is also consistent, but generally considered to be of minimal clinical relevance in the prediction of response in IVF [14]. The operator-dependent factor is also important to consider: for AFC, the variability of the results according to the operator and to the site, according to the assay used to determine AMH levels, and for both their evolution over time.
For all these reasons, before investigating the predictive value of AFC and AMH on the number of retrieved oocytes, we limited our objective to a better knowledge of these two markers, and more precisely, (a) their statistical distribution and the consequences on the calculation in practice on the necessity of transformations, (b) a comparison between their reproducibility/reliability and their sensitivity to age, and (c) the correlation between the two markers and its consequence on their complementarity or substitutability.
Material
Data sources and design
Data were extracted from a registry of ART cycles maintained by 12 French ART centers for collaborative research. The legal requirement of regular transfer of data to health authorities has facilitated our data collection task: The MediFirst-amp® (www.medifirst.fr) software is used by IVF centers for this purpose, and provides a standardized format of patient cycle characteristics without missing data, due to legal transfer. Data used for the analysis were processed in full conformity with the reference methodology (MR-003) of the French commission of privacy (Commission Nationale Informatique et Liberté (CNIL)). Patient privacy rights were fully protected. Only anonymous items were transferred from the source data. The encrypted raw data were provided to a third party academic statistician under constraint on non-disclosure of raw data or center-specific results.
Each record documents an IVF/ICSI cycle defined as either controlled ovarian stimulation (COS) or a thawed embryo transfer from a previous COS. For each cycle, baseline variables, treatment-related data, and reproductive outcomes were reported up to live birth.
Each sample of each center was merged by one of the investigators, and the final database provided to an academic statistician.
Selection
Only cycles including a COS with simultaneous determination of AMH and AFC on both ovaries were included, between 2005 and 2016. Canceled cycles were included when their cause was related to ovarian response; other causes of cancelation (treatment error, intercurrent disease, spontaneous pregnancy) were removed from the database.
An individualized COS protocol was attributed to each patient according to specific characteristics, such as ovarian reserve tests, BMI, age, and ovarian response to previous COS. Sonographic assessment of AFC was performed for all patients on cycle days 1–3, before the onset of gonadotropin stimulation. AMH serum level was first assessed before the first COS, and then generally re-evaluated on an annual basis.
Time schedule and Review Board
A Scientific Review Board was composed by the three authors and three colleagues to develop the protocol (locked Oct. 20, 2019), examine descriptive data when the database was available, writing the statistical analysis plan (finalized Dec. 17, 2020), and discussing results. The statistical analysis was conducted on February 8, 2020. The results of this report were discussed on March 15, 2020.
Used variables
For each COS, the main baseline variables recorded were female age, AMH, AFC, BMI, smoking habits, and number and outcome of previous COS. We justify this choice as these variables were identified in previous studies as predictors of ovarian response.
Statistical analysis
Our study population was constituted by all the COS recorded on the 12 participating centers from 2005 until 2016 included irrespective of whether or not oocyte retrieval was done (intent to treat selection). No alternative selection was used.
We assessed the parent distribution family by using the Shapiro–Wilk tests. The assessment on reliability was conducted through a longitudinal approach analyzing the change in time on the same patients: We used mixed linear models assessing AFC and AMH as dependent variables, age as a fixed covariate, and center and patient as random nested factors. Due to the expected log-normal distribution of these variables, log-transformed values were used; thus, the standard error of measurement (SEM) estimates the relative error defined as the ratio between the residual variability and the mean. Bologna criteria were used as follows: AMH cutoffs were in the range [0.5, 1, 1] ng/mL and AFC of 5 and 7 [7]. We finally used a test–retest based on two successive measures of the AFC in the same COS to provide a supplementary estimate of AFC reliability. These two measurements, separated by 3 to 7 days, were carried out in 1912 COS, in case of incomplete ovarian suppression prior to gonadotropin stimulation.
The registry of each center is transferred to the French health authorities. To increase the quality and integrity of the data, MediFirst software provides interactive facilities for the data entry including on-line quality control and consistency checks. Due to this legal context, missing data are seldom. AMH was not always documented and was a condition of selection. A possible bias of this selection is discussed further. A sensitivity analysis compared the results based on multiple imputation over the whole population and the results based on cycles without missing data.
A linear model with a mean number of 2 measures per patients, and a variation inflation of the correlation in time (number of replications in time = 2, intraclass correlation = 0.5), needs a minimum of 3259 patients to provide a power of 0.95, at a 95% two-sided significance test.
Results
Sample description
A total of 25,854 COS undertaken by 15,283 women were found according to the selection of the 12 centers. Patient characteristics (Table 1) were similar to the general profile provided by large national surveys [15, 16].
Table 1.
Sample description
| Sample description | Arithmetic mean ± SD or proportion % (count) |
|---|---|
| Age | 34.77 ± 4.55 |
| BMI | 21.92 ± 7.19 |
| Infertility duration (years) | 5.05 ± 2.74 |
| Number of attempts | 2.07 ± 1.30 |
| Previous live birth | 24.4% (6300) |
| Smoking habits | 21.3% (5007) |
| Secondary infertility | 47.2% (12,072) |
| Infertility cause | |
| Male | 30.6% (6566) |
| Tubal | 8.0% (1724) |
| Endometriosis | 7.9% (1693) |
| Unexplained | 11.0% (2360) |
| Ovulatory | 4.9% (1045) |
| Other | 0.7% (154) |
| Mixed | 36.9% (7926) |
Outcome distribution
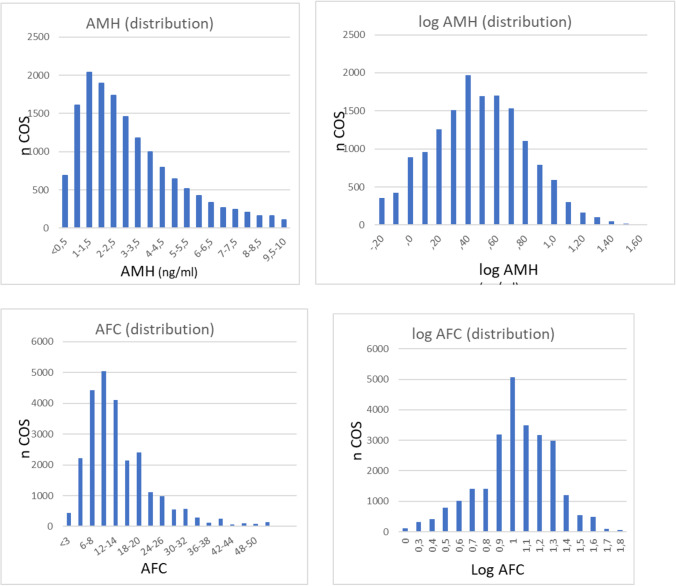
AMH and AFC were characterized by a marked positive skewness (Fig. 1), and were found distributed according logarithmic-normal distribution (Kolmogorov–Smirnov test). The mean and median values of AMH were 3.4 and 2.5 ng/mL, of AFC 14.1 and 12.
Fig. 1.
Statistical distribution of AFC and AMH
Reliability of AFC measurement
Through a longitudinal analysis on the same women, we compared the reproducibility of AMH and AFC in estimating the relative error ϵ (ϵ = ratio of the residual uncontrolled variability on the mean) for the two markers (Table 2).We found a mean AFC of 16.3 [14.5, 18.4] for 25 years aged women, a linear decrease of 3.9% per year ([3.7, 4.1], p < 0.001), and a relative error ϵ = 0.37 [0.34, 0.40] (estimated by the SEM on log-transformed values), without difference between centers.
Table 2.
Mixed model for AFC and AMH. We used log-transformed values (enclosed within parentheses). Effects and 95%CI are provided as anti-logs for easier interpretation
| Effect | 95% CI | p value | ϵ (SEM) | ||
|---|---|---|---|---|---|
| AFC | Mean (25 years) | 16.3 (2.8) | 14.5, 18.4 | < 0.001 | 0.37 |
| Age | 0.961 (− 0.04) | 0.959, 0.963 | < 0.001 | ||
| AMH | Mean (25 years) | 3.9 (1.4) | 3.6, 4.2 | < 0.001 | 0.18 |
| Age | 0.95 (− 0.05) | 0.948, 0.952 | < 0.001 |
We completed this result in conducting a test–retest on two successive measurements of the AFC. We found an intraclass correlation ICC = 0.773 95%CI [0.725, 0.823], and a relative error ϵ = 0.39 95%CI 0.36–0.42.
We did the same exercise for AMH, and found a mean of 3.9 [3.6, 4.2] for women aged 25 years with a decrease of 5% per year ([4.8, 5.2], p < 0.001), and a relative error of ϵ = 0.18 [0.13, 0.24] without noticeable difference among centers.
Table 2 provides evidence on a better reproducibility of AMH (ϵ = 0.18) compared with AFC (ϵ = 0.37) and shows that AMH and AFC are both impacted by age. The constancy of the relative implies that the absolute error increases with the magnitude of the variable, as shown in Table 3.
Table 3.
Absolute error (AE) estimated for AMH and AFC depending on the magnitude of each variable. For AMH of magnitude 0.5, a small absolute error AE = 0.08 was found, contrasting with a much higher AE = 1.23 for an AMH of magnitude 7.4. The estimated AE is even bigger for AFC, with for instance RA = 20.2 associated with a AFC magnitude of 55
| AMH magnitude | AR | AFC magnitude | AR |
|---|---|---|---|
| 0.5 | 0.08 | 4 | 1.5 |
| 1 | 0.17 | 7 | 2.6 |
| 1.5 | 0.25 | 12 | 4.4 |
| 2.7 | 0.45 | 20 | 7.4 |
| 4.5 | 0.76 | 30 | 11.1 |
| 7.4 | 1.23 | 55 | 20.2 |
Concordance/discordance between AFC and AMH
We estimated the concordance between AFC and AMH in dichotomizing AMH and AFC according to Bologna criteria of POR [7], namely AMH with cutoff points of 0.5 and 1.1 ng/mL and AFC with 5 and 7 (Table 4). The concordance between AFC and AMH was poor among predicted POR, as for instance only 29.9% of COS with low AFC (< 5) within the category of low AMH (AMH < 0.5), with a small value of the Kendall coefficient of association (0.296, 95%CI [0.289, 0.305]).
Table 4.
AFC vs AMH
| Concordance AMH/AFC | |||||||
|---|---|---|---|---|---|---|---|
| AMH < 0.5 | 0.5 < AMH < 1.1 | AMH > 1.1 | Total | ||||
| n COS (%) | % concordance | n COS (%) | % concordance | n COS (%) | % concordance | n COS | |
| AFC < 5 | 346 (1.3%) | 29.9% | 470 (1.8%) | 14.2% | 867 (3.4%) | 4.1% | 1683 (6.5%) |
| 5 < AFC < 7 | 317 (1.2%) | 27.4% | 707 (2.7%) | 21.4% | 1421 (5.5%) | 6.6% | 2445 (9.5%) |
| AFC > 7 | 493 (1.9%) | 42.6% | 2132 (8.2%) | 64.4% | 19,095 (73.9%) | 89.3% | 21,720 (84.0%) |
| Total | 1156 (4.5%) | 3309 (12.8%) | 21,383 (82.7%) | 25,848 (100%) | |||
Discussion
AMH and AFC were both distributed according to a log-normal distribution. This is an important metric property not pointed out so far for AFC, but already commented for particular purposes of AMH: defining cutoff points [17], age-dependent decrease in AMH level [18], or predictive model for oocyte retrieval [19].
This has important implications for practice: the log-normal distribution implies that the relative error is constant, whereas the absolute error increases with the magnitude.
Because AMH measurement has no per se clinical interpretation, and due to its log-normal distribution, the log-transformed value of AMH should be substituted in practice to its untransformed value. This is different for AFC having an obvious clinical meaning and relevance.
Contrary to the existing approaches, our analysis was based on a longitudinal mixed model assessing the within-patient reliability and between-patient covariates.
For both AMH and AFC, we identified a decrease with age. This decrease was manifestly linear for the two markers. We failed to find a non-linear trend as Dewailly et al. [3].
Serum AMH levels declined by 5% per year and AFC by 4.0% per year. The observed slope of this decline was similar to that in [20] also describing serum AMH levels peaked before 25 years of age, with mean AMH levels halving by 36 and falling to a quarter of their peak by 40 years of age. The slightly faster decline in AMH than in AFC with age is in line with published data: 5.6%/year for AMH and 4.4%/year for AFC [21]; in another study among a normal population of 2055 women, the median AMH levels were 6.2 for age < 25, 5.6 for age range 25–30 years, 4.6 for 30–33 years, 3.7 for 33–37 years, 2.8 for 37–40 years, and 1.09 for age > 40 years, also corresponding to an average decrease of 5% per year, while the simultaneous decline of AFC was only 4.1% [22]; average rates of loss per year for AFC and AMH were, respectively, − 0.57 and − 1.09 at age 30, and − 1.33 and − 3.06 at age 40 [21]. This gap suggests that the correlation between AMH and AFC changes with age. One possible explanation is a shift toward larger follicles (8–10 mm) with increasing age, whereas it is the 5–8-mm follicles that are the most productive of AMH [23] and the larger follicles (> 6 mm) seem to be less responsive to the exogenous FSH [24]. Accordingly, establishing a direct relationship between AMH and AFC, or cutoffs for oocyte retrieval, without considering age, seems hazardous. In our data, following the range of expected poor responders according to Bologna cutoffs (AMH < 1.1 ng/mL and AFC ≤ 7), the direct correlation between AMH and AFC shows a very low coefficient of concordance; for instance, almost half of patients with low AMH (48.6%) exhibit an AFC > 7. This discordance between AFC and AMH levels—i.e., normal AFC and low AMH or low AFC and normal AMH—was confirmed by recent studies and seems to concern approximately one in five patients in clinical practice [25].
Limitations of AMH concentration assessment are the absence of international standardization and the high cost; standardization combined with a stable automated assay is likely to enhance its performance in the prediction of ovarian response [6]. In our database, we had no information on the dosing technique used for AMH. The first fully automated immunoassay for AMH was evaluated in 2014 [26]. Another important issue is the comparison between manual versus fully automated AMH assays: as compared with manual AMH Gen II assay results, 1- serum AMH concentrations were − 16% and − 20% lower with Access AMH and Elecsys AMH, respectively; and 2- automated assays were more strongly correlated to AFC in the subset of patients with reduced follicle count [27]. The other limitation to the use of AMH in France was the cost not covered by health insurance. As a result, the dosage was not systematically requested, and not regularly repeated before each attempt.
The reliability of AMH and AFC measurement was evaluated by a longitudinal analysis, thus according to inter-cycle variations for the same patient, and inter-center variations. In our database, we found a small heterogeneity across centers for AMH and AFC compared with the heterogeneity across patients within each center for AMH. AFC reliability was assessed though two different techniques: the mixed model and the test–retest calculation provided similar estimate of the relative error ϵ of 0.37 [0.34, 0.40] and 0.39 [0.36, 0.42], respectively. We found that the estimation of reliability based on longitudinal data provides evidence of a better reproducibility of AMH (relative error ϵ = 0.17) compared with AFC. This lower inter-cycle variability of the AMH compared to that of AFC or basal FSH had already been published [28, 29]. However, despite the variability in the same patient, a low AMH and AFC remain in the lower zone, and similarly a high AMH and AFC remain in the higher zone. Furthermore, AMH is not affected by oral contraceptive pretreatment used for scheduling purposes [30]. Concerning the between-center comparison of AFC and AMH, there was a statistically significant variance in AFC and a significant between-center variation in the relationship between AFC and age, but not in fully automated AMH [29]. However, despite the use of a fully automated assay, AMH showed significant intra- and inter-cycle variations, which are not caused by analytical variability [31].
Our estimate of reproducibility indistinctly accounts for the lack of reliability of the AMH assays or the skill of AFC and the physiological fluctuation in ovarian function in time. Because the reliability is measured though inter-cycle standard deviation for the same women and AFC was measured more often than AMH before each COS onset, the observed relative precision of the AMH is better than AFC.
An important information would be to determine whether the physiological fluctuation in ovarian function is important and has a significant influence on oocyte retrieval, especially in poor responders in whom even an additional oocyte changes the prognosis. The practical implication would be to start a COS only with an optimal AFC. The individual subject variation in ovarian response between repeated cycles with the same ovarian stimulation protocol has been reported in 25% of the successive cycles [32]. In this study involving a limited number of cycles, AFC did not significantly predict changes in ovarian response: due to the importance of this question, further studies on a larger database would be helpful.
The major advantage of AFC over AMH is that the pelvic ultrasound is always part of the initial assessment before ART, insofar as it is able to provide other essential information, as the position and structure of the ovaries, the presence of hydrosalpinx or pelvic endometriosis, and the assessment of uterine cavity, endometrium, and myometrium. Difficulties in performing AFC arise from the presence of cysts, particularly endometriomas, and after pelvic surgery, as local inflammation, fibrosis, and adhesions are likely to increase the distance between the ovarian tissue and the US probe, resulting in impaired visualization of the small follicles. Morbid obesity, or when a transvaginal approach causes pain, can also limit the lateral positioning of the vaginal probe and interfere with the visualization of the ovaries. In our database, the reliability of very low AFC ≤ 2 seemed questionable, correlating very weakly with previous or further measure for the same patient and with egg collection, and raised the hypothesis of a technical problem, either ultrasound visibility of the ovaries or data input error.
To maximize reliability, the AFC should be performed only by a competent operator and with US equipment with a minimum frequency of 7 MHz [33]. Considering the studies evaluating AFC over time, there is a clear increase in the median AFC values, consistent with notion that the increasing transducer frequency facilitates the detection of more small follicles [3].
Comparing respective performances of AMH and AFC for the prediction of ovarian response in published studies suggests that the results are highly dependent on the local conditions for measuring these indicators. Large-scale multicenter RCTs, with centralized assays for AMH, have demonstrated that AMH seems the more accurate and robust biomarker, probably reflecting heterogeneity of AFC determination [6]. All these data on AMH and AFC assume that the reproducibility of results improves over time, as ultrasound scanner technology improves and fully automated AMH assays become more widespread.
The problems of reproducibility of the AFC seem less related to a physiological inter-cycle variability than to a problem of reliability of the examination itself, related to the expertise of the operators and/or the quality of the ultrasound equipment. With trained well-equipped operators, the AFC determination was shown much more reliable, improving with the number of cycles [34], but declining with higher follicle counts [35]. Despite these limitations, in our database, the test–retest provided an acceptable correlation.
Limitations
Disentangling the variability inherent to the physiological fluctuation of the ovarian function [36] with the lack of precision of one surrogate measurement is difficult, in particular in studies where surrogate markers are used without the knowledge of their reproducibility. However, measurement heterogeneity appears to improve over time, suggesting that it is likely related to the skills of the sonographer and to the quality of the equipment used.
Our data were collected on a routine practice basis, avoiding quality control of the measurements of each marker and concurrent measures on other measurement devices.
AFC is measured at each cycle, whereas AMH is generally limited to a yearly measure. Thus, compared with AMH, AFC will capture more fluctuation of the physiological ovarian function, which might indirectly be assimilated to a lack of reproducibility.
The poor correlation between AFC and AMH in their lower range may hamper the precision of their predictive value on oocyte collection, as this prediction is especially important in POR where the number of oocytes is low.
The database does not provide information on the technical quality of the measurements performed (expertise of the operator, difficulty in visualizing the ovaries, quality of the ultrasound machine, AMH dosage kit).
The selection of COS with both AMH and AFC values potentially biased the selection of population, as AMH dosing is expensive and was not covered by health insurance in France.
For long agonist protocols, AFC was performed during pituitary suppression. As the correlation between AFC and ovarian response is not compromised during GnRH agonist use, this will probably not have influenced classification of response.
Finally, our sample was recruited from a convenience sample of centers. The overall estimates at a population might be biased compared with a randomized one-stage cluster sampling. However, our main results aiming at assessing the reliability of AMH and AFC and their association showed a very little center effect. Furthermore, the comparison of the patient baseline profile with general retrospective studies in various countries did not show marked differences, thus confers a reasonable external validity to our results.
Conclusions
Due to the log-normal distribution of AMH and AFC, their standard deviation and thus their precision were proportional to their magnitude: large values will be associated with large confidence area. AMH should be systematically used by its log-transformed value.
The longitudinal analysis of both markers in time showed homogeneous mean values of AMH and AFC among centers and a large variability across patients within each center. A better reproducibility of AMH was found compared with AFC and a linear decreasing effect with age was observed on the two markers, with a more pronounced decrease for AFC, suggesting that the inter-correlation AFC-AMH varies with age. A weak concordance was found between the two markers. This research was concerned with the reliability of the two markers; their use to predict ovarian reserve constitutes the objective of a forthcoming research.
Abbreviations
- AFC
Antral follicle count
- AMH
Anti-Müllerian hormone
- ART
Assisted reproductive technologies
- BMI
Body mass index
- CLBR
Cumulative live birth rate
- COS
Controlled ovarian stimulation
Author contribution
PA, PL, and CR wrote the manuscript. PA was responsible for data collection, PL was in charge of statistical analysis. All authors revised and approved the final version of the manuscript.
Data Availability
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval
Data used for the analysis were processed in full conformity with the reference methodology (MR-003) of the French Commission of Privacy (Commission Nationale Informatique et Liberté (CNIL)).
Consent for publication
Patient privacy rights were fully protected. Only anonymous items were transferred from the source data. The encrypted raw data were provided to a third party academic statistician under constraint on non-disclosure of raw data or center-specific results.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.La Marca A, Sunkara SK. Individualization of controlled ovarian stimulation in IVF using ovarian reserve markers: from theory to practice. Hum Reprod Update. 2014;20(1):124–40. [DOI] [PubMed]
- 2.Polyzos NP, Drakopoulos P, Parra J, Pellicer A, Santos-Ribeiro S, Tournaye H, Bosch E, Garcia-Velasco J. Cumulative live birth rates according to the number of oocytes retrieved after the first ovarian stimulation for in vitro fertilization/intracytoplasmic sperm injection: a multicentre multinational analysis including ∼15,000 women. Fertil Steril. 2018;110(4):661–670. doi: 10.1016/j.fertnstert.2018.04.039. [DOI] [PubMed] [Google Scholar]
- 3.Dewailly D, Lujan ME, Carmina E, Cedars MI, Laven J, Norman RJ, Escobar Morreale HF. Definition and significance of polycystic ovarian morphology: a task force report from the Androgen Excess and Polycystic Ovary Syndrome Society. Hum Reprod Update. 2014;20:334–352. doi: 10.1093/humupd/dmt061. [DOI] [PubMed] [Google Scholar]
- 4.La Marca A, Sighinolfi G, Radi D, Argento C, Baraldi E, Artenisio AC, Stabile G, Volpe A. Anti-Mullerian hormone (AMH) as a predictive marker in assisted reproductive technology (ART) Hum Reprod Update. 2010;16(2):113–30. doi: 10.1093/humupd/dmp036. [DOI] [PubMed] [Google Scholar]
- 5.Broer SL, van Disseldorp J, Broeze KA, Dolleman M, Opmeer BC, Bossuyt P, Eijkemans MJ, Mol BW, Broekmans FJ. IMPORT study group Added value of ovarian reserve testing on patient characteristics in the prediction of ovarian response and ongoing pregnancy an individual patient data approach. Hum Reprod Update. 2013;19(1):26–36. doi: 10.1093/humupd/dms041. [DOI] [PubMed] [Google Scholar]
- 6.Iliodromiti S, Anderson RA, Nelson SM. Technical and performance characteristics of anti-Müllerian hormone and antral follicle count as biomarkers of ovarian response. Hum Reprod Update. 2015;21(6):698–710. doi: 10.1093/humupd/dmu062. [DOI] [PubMed] [Google Scholar]
- 7.Ferraretti AP, La Marca A, Fauser BC, Tarlatzis B, Nargund G, Gianaroli L. ESHRE working group on Poor Ovarian Response Definition. ESHRE consensus on the definition of ‘poor response’ to ovarian stimulation for in vitro fertilization the Bologna criteria. Hum Reprod. 2011;26(7):1616–24. doi: 10.1093/humrep/der092. [DOI] [PubMed] [Google Scholar]
- 8.Humaidan P, Alviggi C, Fischer R, Esteves SC. The novel POSEIDON stratification of ‘low prognosis patients in assisted reproductive technology’ and its proposed marker of successful outcome. F1000Res. 2016;5:2911. doi: 10.12688/f1000research.10382.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gallot V, da BerwangerSilva AL, Genro V, Grynberg M, Frydman N, Fanchin R. Antral follicle responsiveness to follicle-stimulating hormone administration assessed by the follicular output rate (FORT) may predict in vitro fertilization-embryo transfer outcome. Hum Reprod. 2012;27:1066–72. doi: 10.1093/humrep/der479. [DOI] [PubMed] [Google Scholar]
- 10.Hadlow N, Longhurst K, McClements A, Natalwala J, Brown SJ, Matson PL. Variation in antimullerian hormone concentration during the menstrual cycle may change the clinical classification of the ovarian response. Fertil Steril. 2013;99:1791–1797. doi: 10.1016/j.fertnstert.2013.01.132. [DOI] [PubMed] [Google Scholar]
- 11.Lee JY, Ahn S, Lee JR, Jee BC, Kim CH, Seo S, Suh CS, Kim SH. Reference values for the revised anti-Müllerian hormone generation II assay: infertile population-based study. Korean Med Sci. 2017;32(5):825–829. doi: 10.3346/jkms.2017.32.5.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moslehi N, Shab-Bidar S, Ramezani Tehrani F, Mirmiran P, Azizi F. Is ovarian reserve associated with body mass index and obesity in reproductive aged women? A meta-analysis Menopause. 2018;25(9):1046–1055. doi: 10.1097/GME.0000000000001116. [DOI] [PubMed] [Google Scholar]
- 13.Polyzos NP, Nelson SM, Stoop D, Nwoye M, Humaidan P, Anckaert E, Devroey P, Tournaye H. Does the time interval between antimüllerian hormone serum sampling and initiation of ovarian stimulation affect its predictive ability in in vitro fertilization-intracytoplasmic sperm injection cycles with a gonadotropin-releasing hormone antagonist? A retrospective single-center study. Fertil Steril. 2013;100(2):438–44. doi: 10.1016/j.fertnstert.2013.03.031. [DOI] [PubMed] [Google Scholar]
- 14.Broekmans FJ, de Ziegler D, Howles CM, Gougeon A, Trew G, Olivennes F. The antral follicle count: practical recommendations for better standardization. Fertil Steril. 2010;94:1044–1051. doi: 10.1016/j.fertnstert.2009.04.040. [DOI] [PubMed] [Google Scholar]
- 15.Baker VL, Luke B, Brown MB, Alvero R, Frattarelli JL, Usadi R, Grainger DA, Armstrong AY. Multivariate analysis of factors affecting probability of pregnancy and live birth with in vitro fertilization: an analysis of the Society for Assisted Reproductive Technology Clinic Outcomes Reporting System. Fertil Steril. 2010;94(4):1410–1416. doi: 10.1016/j.fertnstert.2009.07.986. [DOI] [PubMed] [Google Scholar]
- 16.Dhillon RK, McLernon DJ, Smith PP, Fishel S, Dowell K, Deeks JJ, Bhattacharya S, Coomarasamy A. Predicting the chance of live birth for women undergoing IVF: a novel pretreatment counselling tool. Hum Reprod. 2016;31(1):84–92. doi: 10.1093/humrep/dev268. [DOI] [PubMed] [Google Scholar]
- 17.Heidar Z, Bakhtiyari M, Mirzamoradi M, Zadehmodarres S, Sarfjoo FS, Mansournia MA. Prediction of different ovarian responses using anti-Müllerian hormone following a long agonist treatment protocol for IVF. J Endocrinol Invest. 2015;38(9):1007–1015. doi: 10.1007/s40618-015-0297-4. [DOI] [PubMed] [Google Scholar]
- 18.Li R, Gong F, Zhu Y, Fang W, Yang J, Liu J, Hu L, Yang D, Liang X, Qiao J. Anti-Müllerian hormone for prediction of ovarian response in Chinese infertile women undergoing IVF/ICSI cycles: a prospective, multi-centre, observational study. Reprod Biomed Online. 2016;33(4):506–512. doi: 10.1016/j.rbmo.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 19.Scheinhardt MO, Lerman T, König IR, Griesinger G. Performance of prognostic modelling of high and low ovarian response to ovarian stimulation for IVF. Hum Reprod. 2018;33(8):1499–1505. doi: 10.1093/humrep/dey236. [DOI] [PubMed] [Google Scholar]
- 20.Tremellen K, Zander-Fox D. Serum anti-Mullerian hormone assessment of ovarian reserve and polycystic ovary syndrome status over the reproductive lifespan. Aust N Z J Obstet Gynaecol. 2015;55(4):384–389. doi: 10.1111/ajo.12366. [DOI] [PubMed] [Google Scholar]
- 21.Bentzen JG, Forman JL, Johannsen TH, Pinborg A, Larsen EC, Nyboe AA. Ovarian antral follicle subclasses and anti-Mullerian hormone during normal reproductive aging - J Clin Endocrinol Metab. 2013;98:1602–1611. doi: 10.1210/jc.2012-1829. [DOI] [PubMed] [Google Scholar]
- 22.Du X, Ding T, Zhang H, Zhang C, Ma W, Zhong Y, Qu W, Zheng J, Liu Y, Li Z, Huang K, Deng S, Ma L, Yang J, Jiang J, Yang S, Huang J, Wu M, Fang L, Lu Y, Luo A, Wang S. Age-specific normal reference range for serum anti-Müllerian hormone in healthy Chinese Han women: a nationwide population-based study. Reprod Sci. 2016;23(8):1019–1027. doi: 10.1177/1933719115625843. [DOI] [PubMed] [Google Scholar]
- 23.Jeppesen JV, Anderson RA, Kelsey TW, Christiansen SL, Kristensen SG, Jayaprakasan K, Raine-Fenning N, Campbell BK. Yding Andersen C- Which follicles make the most anti-Mullerian hormone in humans? Evidence for an abrupt decline in AMH production at the time of follicle selection. Mol Hum Reprod. 2013;19(8):519–527. doi: 10.1093/molehr/gat024. [DOI] [PubMed] [Google Scholar]
- 24.Bessow C, Donato R, de Souza T, Chapon R, Genro V, Cunha-Filho JS. Antral follicle responsiveness assessed by follicular output rate (FORT) correlates with follicles diameter. J Ovarian Res. 2019;12(1):48. doi: 10.1186/s13048-019-0522-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang Y, Xu Y, Xue Q, Shang J, Yang X, Shan X, Kuai Y, Wang S, Zeng C. Discordance between antral follicle counts and anti-Müllerian hormone levels in women undergoing in vitro fertilization. Reprod Biol Endocrinol. 2019;17(1):51. doi: 10.1186/s12958-019-0497-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gassner D, Jung R. First fully automated immunoassay for anti-Müllerian hormone. Clin Chem Lab Med. 2014;52(8):1143–1152. doi: 10.1515/cclm-2014-0022. [DOI] [PubMed] [Google Scholar]
- 27.Tadros T, Tarasconi B, Nassar J, Benhaim JL, Taieb J, Fanchin R. New automated antimüllerian hormone assays are more reliable than the manual assay in patients with reduced antral follicle count. Fertil Steril. 2016;106(7):1800–1806. doi: 10.1016/j.fertnstert.2016.08.045. [DOI] [PubMed] [Google Scholar]
- 28.van Disseldorp J, Lambalk CB, Kwee J, Looman CW, Eijkemans MJ, Fauser BC, Broekmans FJ. Comparison of inter- and intra-cycle variability of anti-Mullerian hormone and antral follicle counts. Hum Reprod. 2010;25(1):221–227. doi: 10.1093/humrep/dep366. [DOI] [PubMed] [Google Scholar]
- 29.Anderson RA, Anckaert E, Bosch E, Dewailly D, Dunlop CE, Fehr D, Nardo L, Smitz J, Tremellen K, Denk B, Geistanger A, Hund M. Prospective study into the value of the automated Elecsys antimüllerian hormone assay for the assessment of the ovarian growing follicle pool. Fertil Steril. 2015;103(4):1074–1080. doi: 10.1016/j.fertnstert.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 30.Andersen AN, Witjes H, Gordon K, Mannaerts B. Xpect investigators. Predictive factors of ovarian response and clinical outcome after IVF/ICSI following a rFSH/GnRH Hum Reprod. 2011;26(12):3413–3423. doi: 10.1093/humrep/der318. [DOI] [PubMed] [Google Scholar]
- 31.Melado L, Lawrenz B, Sibal J, Abu E, Coughlan C, Navarro AT. Fatemi HM - Anti-Müllerian hormone during natural cycle presents significant intra and intercycle variations when measured with fully automated assay. Front Endocrinol (Lausanne) 2018;27(9):686. doi: 10.3389/fendo.2018.00686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rombauts L, Lambalk CB, Schultze-Mosgau A, van Kuijk J, Verweij P, Gates D, Gordon K, Griesinger G. Intercycle variability of the ovarian response in patients undergoing repeated stimulation with corifollitropin alfa in a gonadotropin-releasing hormone antagonist protocol. Fertil Steril. 2015;104(4):884–890. doi: 10.1016/j.fertnstert.2015.06.027. [DOI] [PubMed] [Google Scholar]
- 33.Coelho Neto MA, Ludwin A, Borrell A, Benacerraf B, Dewailly D, da Silva CF, Condous G, Alcazar JL, Jokubkiene L, Guerriero S, Van den Bosch T, Martins WP. Counting ovarian antral follicles by ultrasound: a practical guide. Ultrasound Obstet Gynecol. 2018;51(1):10–20. doi: 10.1002/uog.18945. [DOI] [PubMed] [Google Scholar]
- 34.Subirá J, Alberola-Rubio J, Núñez MJ, Escrivá AM, Pellicer A, Montañana V, Díaz-García C. Inter-cycle and inter-observer variability of the antral follicle count in routine clinical practice. Gynecol Endocrinol. 2017;33(7):515–518. doi: 10.1080/09513590.2017.1291614. [DOI] [PubMed] [Google Scholar]
- 35.Scheffer GJ, Broekmans FJ, Bancsi LF, Habbema JD, Looman CW, Te Velde ER. Quantitative transvaginal two- and three-dimensional sonography of the ovaries: reproducibility of antral follicle counts. Ultrasound Obstet Gynecol. 2002;20(3):270–275. doi: 10.1046/j.1469-0705.2002.00787.x. [DOI] [PubMed] [Google Scholar]
- 36.Rustamov O, Wilkinson J, La Marca A, Fitzgerald C, Roberts SA. How much variation in oocyte yield after controlled ovarian stimulation can be explained? A multilevel modelling study. Hum Reprod Open. 2017;13(3):1–10. [DOI] [PMC free article] [PubMed]
- 37.Hsieh FY, Bloch DA, Larsen MD. A simple method of sample size calculation for linear and logistic regression. Stat Med. 1998;17(14):1623–1634. doi: 10.1002/(SICI)1097-0258(19980730)17:14<1623::AID-SIM871>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 38.Rosen MP, Johnstone E, McCulloch CE, Schuh-Huerta SM, Sternfeld B, Reijo-Pera RA, Cedars MI. A characterization of the relationship of ovarian reserve markers with age. Fertil Steril. 2012;97(1):238–243. doi: 10.1016/j.fertnstert.2011.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets analyzed during the current study are available from the corresponding author on reasonable request.