Abstract
Purpose of Review
The goal of this review is to provide an up to date understanding of the utility and limitations of the current tests utilized in the diagnosis of periprosthetic joint infection (PJI) in total knee and hip arthroplasty.
Recent Findings
Despite the growth in literature surrounding PJI diagnosis, there remains challenges in establishing a diagnosis of PJI. A combination of clinical, serum, and synovial tests and microbiologic and histologic examinations can yield a diagnosis in the majority of cases. Novel molecular and imaging studies may be beneficial for indeterminant cases. A number of emerging diagnostic tests have been proposed and may be incorporated into diagnostic algorithms in the future. Recently proposed stepwise diagnostic algorithms have shown high sensitivity and specificity.
Summary
The diagnosis of PJI remains challenging due to a lack of tests that can definitively rule out infection. Diagnosis and investigations should occur in a stepwise fashion. There has been a plethora of new diagnostic tests introduced in attempts to improve the accuracy of diagnostic algorithms. The definition and algorithms for the diagnoses of PJI will continue to evolve as new techniques and tests are introduced.
Keywords: Periprosthetic joint infection, Total joint arthroplasty, Diagnosis, Biomarkers, Complications
Introduction
Periprosthetic joint infection (PJI) is a devastating complication of total joint arthroplasty (TJA) surgery. Given that there is no direct blood supply to the implant, antibiotics are rarely sufficient to treat PJI [1]. The vast majority of cases require one or more surgeries, and in rare cases can eventually lead to amputation, disarticulation, and even death [2]. In addition to the obvious personal burden of PJI on patients, there is also a massive societal impact to be considered. Cost of revision TJA secondary to infection is up to five times higher than primary TJA [1, 3]. Infection is a leading cause of revision surgery, and the rate of revision surgery in the USA is expected to increase by 43–182% by 2030 [4]. Despite the fact that the rates of PJI are low (1–2%), the overall global burden is massive and continues to grow [5, 6].
A challenging aspect of PJI management is prompt and accurate diagnosis. Despite the growth in literature surrounding PJI diagnosis, there remains challenges in establishing a diagnosis of PJI. Firstly, there is a lack of a gold standard diagnostic test adopted by the literature [7]. The lack of a gold standard has created inconsistent thresholds for the growing lists of diagnostic tests [8]. Secondly, no single test exists in the literature that can reliably rule out infection. Finally, patients presenting with a potential PJI represent a heterogenous group with varying comorbidities, risk factors, and clinical presentations. Both patient and infection variables may alter the accuracy and reliability of diagnostic tests, making proposed diagnostic algorithms challenging to implement. Comorbidities such as inflammatory arthropathy, rheumatoid arthritis, lupus, inflammatory bowel disease, and malignancy are all known to have elevated C-reactive protein level, which can cloud the diagnostic accuracy of these tests.
The goal of this review is to provide an up to date understanding of the utility and limitations of the current tests utilized in the diagnosis of PJI in total knee and hip arthroplasty.
Clinical Presentation
Periprosthetic joint infection has a wide spectrum of clinical presentation from new onset pain to fulminant sepsis [1]. Patients with acute PJI may present with ongoing postoperative pain, continued surgical site drainage, or wound dehiscence. In the setting of chronic infection, patients often present with long-standing joint pain and reduced range of motion which is often indistinguishable from aseptic failures [1]. Although fever and surgical site erythema are highly specific for infection, they lack sensitivity and their absence should not be used to rule out PJI [9]. The only clinical signs widely utilized as a diagnostic criterion are the presence of a draining sinus communicating with the joint or exposed prosthesis [10••, 11•].
Diagnostic Tests
When a patient presents with signs and symptoms suggestive of PJI, orthopaedic surgeons are faced with the task of selecting and interpreting a growing list of diagnostic tests. Typically, diagnostic tests are performed in a stepwise fashion, starting with non-invasive testing with high negative predictive values [12]. If preliminary tests are suggestive of infection, more invasive testing in the form of joint aspiration is generally undertaken. If findings from the synovial fluid are suggestive or confirmatory for infection, patients generally undergo revision TJA for definitive management [13••]. Intraoperative tissue tests are utilized to confirm diagnosis, isolate pathogens, and guide postoperative management.
Serum
ESR and CRP
Serologic markers are routinely recommended as first-line testing as they are readily available and have reportedly high sensitivity in patients with suspected PJI [14, 15] (Table 1). C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR) constitute the most commonly used serum tests. For chronic PJI, the most widely accepted cutoff values for CRP and ESR are 10mg/L and 30mg/L, respectively [10••, 11•].
Table 1.
Serum markers
| Test | Acute (<90 days) cutoff | Chronic cutoff | Specificity/sensitivity (chronic) | Limitations |
|---|---|---|---|---|
| CRP | >100mg/L | >10mg/L | 0.813/0.845 | Nonspecific finding, affected by comorbidities |
| ESR | N/A | >30mm/h | 0.790/0.816 | Nonspecific finding, affected by comorbidities |
| D-dimer [26] | >860 ng/mL | >860 ng/mL | 0.76/0.88 | Nonspecific finding, affected by comorbidities |
| IL-6 | >6.6–12pg/mL | 0.870/0.875 | Lack of consistent cutoff values | |
| Procalcitonin | varies | 0.893/0.580 | Low sensitivity, lack of consistent cut off values |
CRP C reactive protein; ESR erythrocyte sedimentation rate; IL-6 interleukin 6
The utility of serum inflammatory markers in acute PJI is challenging, given the slow and variable return to normal values after surgery [16]. The current suggested cutoff for CRP levels in suspected acute PJI (<90 days) is 100mg/L. ESR levels demonstrate a variable course postoperatively, and thus, no values have been suggested when diagnosing PJI in the acute postoperative setting. Further studies are needed to validate the utility and diagnostic value of serum inflammatory markers in acute PJI.
Despite the popularity of ESR and CRP in the initial diagnosis of PJI, they pose a number of limitations and should not be used in isolation to diagnose or rule out a PJI [11•]. Firstly, both biomarkers are a reflection of general inflammation and can be elevated in a wide variety of non-infectious etiologies. Secondly, these inflammatory markers may be normal in PJI infected with low virulence organisms [17, 18].
IL-6
Interleukin-6 (IL-6) is a cytokine that is an immune response regulator and is stimulated by monocytes and macrophages. IL-6 may have utility in the diagnosis of PJI as serum levels return back to normal (<10 pg/mL) 2–3 days following TJA [19]. IL-6 has demonstrated excellent sensitivity and specificity (0.875, 0.870) in the diagnosis of chronic PJI and is able to differentiate between septic and aseptic loosening [8, 20].
However, there are limitations that may preclude the use of IL-6 in the current diagnostic algorithm of PJI. Firstly, there are differing cutoff values cited in the literature and lack of accepted threshold for PJI exists in the literature. Secondly, serum IL-6 is a relatively expensive test compared to other serum markers and is not as widely available. Given these limitations, IL-6 has not been included in the most widely recognized algorithms in the diagnosis of PJI [10••, 11•, 21].
D-Dimer [22]
D-dimer is a specific degradation product of fibrin monomer and is a specific marker of the fibrinolysis process [23]. D-dimer was traditionally used as a screening test for the detection of venous thromboembolism [23]. Both systemic and local infections result in fibrinolytic activity resulting in elevated d-dimer levels. Given this, there has been considerable interest in the utility of D-dimer as a diagnostic test in patients with potential PJI [10••, 22]. Recent literature has demonstrated that D-dimer is significantly elevated in patients with PJI compared to patients undergoing revision for aseptic failures [22]. D-dimer was added to the 2018 updated algorithm by Parvizi et al. as a minor criterion for diagnosis [10••]. Potential advantages to D-dimer as a diagnostic tool is relatively rapid normalization postoperatively [16]. When compared to serum CRP and ESR, some studies have demonstrated improved accuracy of D-dimer whereas others showed no difference [22, 24].
However, the utility of D-dimer in the acute setting remains untested and should be utilized with caution. Furthermore, serum D-dimer is not a specific finding and can indicate the presence of an unrelated inflammatory process.
Procalcitonin [25]
Procalcitonin is a protein produced by parafollicular cells of the thyroid. Levels of serum procalcitonin increase with bacterial infections and have been found to be useful in the diagnosis of systemic infections [25]. In recent years, procalcitonin has been investigated as a potential diagnostic marker for PJI. Unfortunately, recent analysis has suggested that serum procalcitonin has relatively poor sensitivity and cannot be used to reliably rule out PJI [8].
Serum Ratios
There has been recent interest in evaluating the utility of alterations in the ratios of routinely collected laboratory values. Neutrophil to lymphocyte (NLR), platelet to mean platelet volume (PVR), and platelet to lymphocyte (PLR) ratios all have prognostic value in other infectious and inflammatory conditions. The potential benefit of serum ratios is that they utilize readily available laboratory markers obtained from a complete blood count [27].
Serum NLR has recently been investigated as a potential biomarker for predicting both acute and chronic PJI. Zhao et al. demonstrated that NLR was a better predictor of early PJI compared to ESR and CRP [28]. Golge et al. reported on the predictive value of NLR in the setting of chronic PJI and found it to be a useful marker for infection [29]. Similarly, both PVR and PLR have been shown to be of diagnostic value in predicting PJI. Tirumala et al. demonstrated that PLR and PVR, when used in combination with commonly available serum and synovial markers, increase the sensitivity and specificity for PJI to greater than 97% [27]. Currently, the literature regarding the predictive value of serum ratios for PJI is in its infancy and more studies across different patient populations are required to understand their utility.
Novel Serum Markers
There has been a growing interest in evaluating the oxidative stress secondary to the inflammation created in the setting of PJI [30]. Markers of oxidative stress called advanced glycation end products have been investigated as diagnostic tests for PJI. In particular, plasmastic soluble receptor for advanced glycation end products, thiobarbituric acid reactive substance, and toll-like receptor 2 are serum markers that have been evaluated [30, 31]. However, the current literature is experimental in nature and further clinical research is required before these tests are adopted into practice.
Synovial Fluid
Obtaining synovial fluid is generally the next step in the diagnosis of PJI when clinical findings and serum markers are suggestive of a potential infection. Synovial fluid analysis remains a cornerstone of the diagnostic algorithm and historically included evaluation of synovial fluid leukocyte count, polymorphonuclear leukocyte percentage (PMN %), gram stain, and culture (Table 2). In addition to synovial fluid cytology and microbiology evaluation, several biomarkers have been developed to aid in the diagnosis of PJI [32].
Table 2.
Synovial markers
| Test | Acute (<90 days) cutoff | Chronic cutoff | Specificity/sensitivity (chronic) | Limitations |
|---|---|---|---|---|
| WBC count (cells | >10,000 | >3,000 | 0.897/0.416 | Altered with antibiotic use, medical comorbidities |
| % PMNs | 90% | 80% | 0.878/0.907 | Variable cutoff values used. Affected by antibiotics |
|
Alpha-defensin, ELISA (signal to-cutoff ratio) |
1.0 | 1.0 | 0.968/0.967 | False positives in metallosis, inflammatory conditions. Expensive |
| Alpha-defensin, lateral flow | + | + | 0.955/0.821 | Qualitative analysis |
| LER strips | 2+ | 0.971/0.930 | Poor performance with bloody synovial fluid | |
| CRP (mg/L) | >6.9 | >6.9 | 0.933/0.888 | |
| D-lactate (mmol/L) | >1.3 | 0.808/0.864 | Lack of available data | |
| CRP C-reactive protein; PMNs polymorphonuclear; LER leukocyte esterase, WBC white blood cell | ||||
CRP C-reactive protein; PMNs polymorphonuclear; LER leukocyte esterase; WBC white blood cell
WBC and PMN%
Synovial white blood cell counts collected from synovial fluid cytology are included in all widely major definitions of PJI [10••, 11•, 33]. The suggested cutoffs for chronic PJI in the literature range from >1500 to >4000, with the most commonly utilized cutoff being >3000 and 65–80% PMNs [10••, 11•]. For acute PJI occurring within 90 days of the index surgery, cell counts of 10,000 with >90% PMNs have been suggested [10••].
It is important to note that although single cutoff values have been created for simplicity, there are several variables that may influence the synovial fluid cytology. The use of antibiotics prior to joint aspiration is common and is associated with lower WBC counts and PMN% [34]. Other patient-specific factors including frank puss, metal on metal arthroplasty, and small volume aspirations can alter the accuracy of synovial cytology results [35].
Alpha-Defensin
Alpha-defensin is a relatively new synovial fluid biomarker and has been added to the 2018 revised MSIS diagnostic algorithm [10••]. Alpha-defensin is an antimicrobial peptide released from neutrophils and disrupts pathogen cell membranes [36]. Alpha-defensin can be measured quantitatively utilizing enzyme-linked immunosorbent assay (ELISA) or qualitatively with a lateral flow cassette. Laboratory-based ELISA testing has demonstrated excellent diagnostic accuracy with calculated sensitivities and specificities of 96.8% and 96.7%, respectively [8]. Importantly, the qualitative lateral flow analysis of alpha-defensin has significantly lower performance compared to laboratory testing [36].
Alpha-defensin testing has some unique advantages over other synovial fluid markers of infection. Firstly, alpha-defensin appears to maintain its diagnostic accuracy even with antibiotic treatment [37]. Unlike other synovial test, it remains accurate even with blood contamination [38•]. Finally, it remains sensitive in the setting of a wide spectrum of pathogens including organisms with low virulence [39].
However, quantitative laboratory testing of alpha-defensin is still not available universally, and lateral flow kits remain expensive [40]. Additionally, the use of alpha-defensin may not be warranted in the routine workup of PJI as it has not been shown to improve the diagnostic accuracy when added to readily available serum and synovial tests [40]. There are some limitations to the utility of alpha-defensin, as non-infectious conditions including inflammatory disorders and metallosis can cause false-positive results [41, 42].
Leukocyte Esterase Reagent
Leukocyte esterase is an enzyme that is found in activated PMNs and is found in a variety of infected bodily fluids and is commonly used in the diagnosis of urinary tract infections [43]. Leukocyte esterase reagent (LER) strips are point of care testing diagnostic tests that have been utilized in the diagnosis of PJI [44]. When the reagent strip comes into contact with neutrophils in the synovial fluid, they lyse and subsequently release leukocyte esterase which causes a color change on the strip. The more neutrophils present, the more intense colour change that occurs, making this a semi-quantitative test [43]. Advocates of LER testing cite the low cost and universal availability and the rapid, point of care results [44, 45].
The LER strip is a qualitative analysis and is generally considered positive for infection if the strip is grade + or ++. A positive LER graded ++ is more sensitive and specific and is the accepted cutoff in most diagnostic algorithms [8, 10••]. Limitations of LER testing include poor performance with bloody aspirations and the diagnostic utility in the setting of indeterminant results (1+) [45].
Synovial CRP
Synovial CRP represents another novel synovial fluid biomarker that has shown promise in the diagnosis of PJI [46]. Given that CRP activates the complement system to dispose of dying cells, it would be reasonable to expect that levels are higher in the area of the inflammatory/infectious process (i.e., the joint). Synovial CRP has been successfully utilized to differentiate between inflammatory and non-inflammatory causes of arthritis in native knees [47]. Several independent studies have demonstrated the high sensitivity and specificity in diagnosing PJI in both knee and hip arthroplasty [46, 48–50]. Given these findings, elevated synovial CRP (>6.9mg/L) has been included in the recent MSIS diagnostic algorithm [10••]. However, a number of questions still remain including the impact of prior antibiotics, medical comorbidities, and optimal cutoff values.
Calprotectin
Calprotectin is a protein that is released from activated granulocytes and macrophages during inflammation [51]. It has been utilized in the diagnosis of other inflammatory and infectious etiologies including inflammatory bowel disease and spontaneous bacterial peritonitis [52, 53]. Recently, calprotectin has been a synovial biomarker of interest in the diagnosis of PJI [54]. Early results have demonstrated high sensitivity and specificity and is not affected by prior antibiotic use [51, 54]. Larger studies examining its efficacy across a range of organisms and patients are needed before synovial calprotectin can be considered as a first line diagnostic test [11•].
D-lactate
D-lactate is a form of lactate produced by bacteria and has been used as a marker to diagnose various bacterial infections elsewhere in the body [55]. Recently, synovial d-lactate has been investigated as a potential marker and diagnostic tool in evaluating for PJI [56, 57]. The potential utility of d-lactate is that, unlike other markers, it is a pathogen-specific marker that is only elevated in the presence of bacteria. The current literature suggests that d-lactate has a comparable diagnostic efficacy to synovial leukocyte counts [57].
Microbiology and Histology
The identification of a causative microorganism in the management if PJI is critical to tailor appropriate antibiotics, long-term treatment, and prognosis of these patients [58]. Historically, this was done through synovial fluid culture preoperatively and intraoperative tissue samples. These methods have shown poor sensitivity and new diagnostic methods have been developed to improve the identification of microorganisms in the setting of PJI (Table 3).
Table 3.
Tissue and molecular tests
| Test | Diagnostic criteria | Specificity/sensitivity (chronic) | Limitations |
|---|---|---|---|
| Histologic analysis | >5 PMNs/HPF | 0.956/0.766 | Altered with antibiotic use, medical comorbidities |
| Synovial fluid culture | Organism identification | 0.964/0.686 | Reduced utility with antibiotics |
| Intraop tissue culture | Organism identification | 0.975/0.729 | Reduced utility with antibiotics |
| Sonication (colony-forming units/mL) | >5–100 | 0.950/0.80 | Variable cutoffs |
| PCR | Organism identification | 0.953/0.665 | Detects a limited number of organisms |
| NGS | Organism identification | Unclear cost-effectiveness |
PMNs polymorphonuclear; HPF high-powered field; PCR polymerase chain reaction; NGS next-generation sequencing
Synovial Fluid Culture
The utilization of synovial fluid aerobic and anaerobic bacterial cultures are included in the most widely available diagnostic algorithms [10••, 11•, 33]. As mentioned, the sensitivity of preoperative synovial fluid culture in chronic infections is low and cannot be relied upon to rule out PJI [8]. The use of antibiotics prior to joint aspiration is a risk factor culture-negative PJI and antibiotics should ideally be held until joint aspiration is performed [33]. In the setting of chronic PJI in otherwise stable patients, antibiotics should ideally be held for 2 weeks prior to aspiration [59]. Despite these limitations, synovial fluid cultures should be included in the preoperative workup for PJI as positive cultures provide invaluable information.
Intraoperative Tissue Cultures
Obtaining periprosthetic tissue cultures remain the cornerstone of definitively diagnosing and managing PJI [10••, 11•, 33]. Periprosthetic tissue cultures have superior diagnostic sensitivity and specificity compared to those taken from synovial fluid aspirations. However, the rates of culture-negative PJI ranges from 7 to 12% [58]. Preoperative antibiotic use, culture techniques, and use of bacteriostatic compounds have all been implicated in culture-negative PJI [58]. A number of clinical recommendations have been made to maximize the utility of intraoperative cultures.
Similar to synovial aspiration culture, it has been recommended to refrain from antibiotic therapy for two weeks prior to obtaining culture [59]. Multiple independent cultures should be obtained from deep regions of the joint [58]. The intramedullary canal and prosthesis-bone interface are considered high-yield areas. Samples should be obtained using separate instruments and transferred immediately to sterile transport bottles without coming into contact with drapes or gloves [11•]. Tissue samples should be obtained prior to irrigation of the wound. The optimal number of required samples and the incubation periods depends on the isolated organisms. Recent evidence suggests that at least 5 distinct intraoperative tissue samples should be obtained to maximize the yield for positive cultures [60]. Organisms should be cultured for 14 days to account for slow-growing organisms such as Propionibacterium acnes [60].
A single positive culture remains a diagnostic dilemma and should be taken into consideration alongside other findings when making a diagnosis [10••]. When virulent or uncommon contaminants are isolated, it is more suggestive of an underlying infection. When common contaminants are isolated, infection cannot be confirmed but further investigations should be performed [11•].
Histology
Histologic examination of tissue obtained preoperative or intraoperatively is a useful tool to diagnose PJI. The presence of acute inflammatory cells (neutrophils), within the tissue, is specific for PJI [8]. As with obtaining tissue for culture, it is important to obtain several deep tissue samples as the inflammatory process is not uniform throughout the joint. The samples should be evaluated for the presence of neutrophils under high-powered fields. The most common diagnostic criteria are the presence of five or more neutrophils in five different high-powered (×400 magnification) fields [10••, 11•]. As with cultures, histology has a lower sensitivity than specificity and should not be used to rule out infection as a stand-alone test [8].
Sonication
In chronic PJI, microorganisms often exist within a biofilm on the surface of the implant, making organism isolation challenging in traditional tissue cultures [61]. Sonication in the setting of PJI refers to the application of an ultrasound bath in attempts to remove the biofilm from the explanted prosthetic implants [59]. Sonication has been shown to disrupt the biofilm but preserve microbial viability in PJI [62]. The sonication fluid is then cultured in a similar fashion to tissue cultures taken at the time of surgery. There is a growing body of evidence that sonication increases the likelihood of isolating a causative organism compared to traditional intraoperative tissue cultures [59, 63]. Although any positive cultures should be suspicious for infection, 20–100 colony-forming units/mL (CFU/mL) is considered diagnostic [59, 64•]. Sonication is limited by its lack of widespread availability.
Bead Mill Processing [65]
Bead mill processing of intraoperative bone samples is a relatively simple technique utilized to improve the sensitivity of sample cultures [66]. Intraoperative bone fragments are added to a solution of fluid and beads and then “agitated” before being cultured. No comparative studies have been performed but a prospective cohort study reported 90% sensitivity for intraoperative cultures after bead mill processing [65].
Chemical Dislodgement [67]
An alternative to mechanical disruption of biofilms is chemical dislodgement utilizing DL-dithiothreitol (DTT) [67]. DTT has the ability to change the extracellular biofilm matrix allowing for release of bacteria [68]. Unlike sonication and bead mill processing, DTT does not require specialized laboratory equipment. Recent literature has demonstrated high sensitivity of intraoperative cultures after chemical dislodgement in previously culture-negative PJI [67].
Molecular Testing
Polymerase Chain Reactions [69]
Given the aforementioned limitations associated with culture diagnosis of PJI, there has been significant interest in culture-independent molecular technologies to identify microorganisms. Traditional molecular testing consisted of polymerase chain which demonstrated improved sensitivity in culture-negative PJI [70]. However, PCR is limited in that it utilizes a specific primer and can only detect a single microorganism [71]. Although multiplex PCR assays that utilize several primers, such as 16S rRNA, have been developed, they have demonstrated a low sensitivity and lack advantages over tissue cultures [72].
Next-Generation Sequencing
Next-generation sequencing (NGS) allows for sequencing of all DNA present within a sample, avoiding the limitations of previous molecular techniques such as PCR. Next-generation sequencing can be performed rapidly, which allows for tailored treatment and mitigates the need for prolonged cultures. It has been shown to identify causative organisms in otherwise culture-negative PJI [73]. However, the literature is contradictory on the value of NGS and the cost-effectiveness when compared to traditional methods is unknown [74].
Nuclear Medicine
Bone Scintigraphy
The role of nuclear medicine in the diagnosis of PJI is evolving. Three-phase bone scintigraphy the classic nuclear medicine scan is used in the skeletal system and is highly sensitive to bone remodeling [75]. Due to normal remodeling that occurs postoperatively, it has been recommended that three-bone scintigraphy becomes more reliable 2 years after THA or 5 years after TKA [11•]. The benefit of bone scintigraphy is its high specificity and low false-positive rates [76]. In the setting of a positive three-phase bone scan, the addition of a WBC scintigraphy increases the sensitivity and specificity for PJI [77]. Given the false-positives associated with three-bone scintigraphy, it has been recommended to proceed directly to WBC scintigraphy within the first 2 years of the index surgery. Finally, the combination of WBC scintigraphy and bone marrow scintigraphy has been advocated for as it increases the detection of PJI by reducing false-positive rates [77].
FDG PET [78]
Fluorodeoxyglucose (FDG) positron emission tomography PET is a nuclear medicine scan that provides high-resolution images. FDG PET has advantages over bone scintigraphy as it is non-invasive and can be performed quickly. Currently, there are conflicting criteria and published diagnostic accuracy for diagnosing PJI in the literature [76]. Given the sensitive nature of PET scans, they have a propensity for false positives, particularly in the setting of aseptic loosening [78]. Given this sensitive nature of the scan, it has a high negative predictive value and may effectively rule out PJI [76].
Definitions of Periprosthetic Joint Infection
One of the challenges in diagnosing PJI is the lack of standardized diagnostic criteria. Although many attempts at a universal diagnostic algorithm have been made, there is no consensus as to the optimal definition. The advent of new diagnostic tests has led to new proposed definitions and algorithms.
Musculoskeletal Infection Society
The most widely utilized contemporary definition both clinically and in the literature is the Musculoskeletal Infection Society (MSIS) definition first introduced in 2011 (Table 4) [79]. The MSIS criteria consist of 2 major criteria and 6 minor criteria with patients requiring 1 major criterion or 4 minor criteria for a diagnosis of PJI. The criteria have been validated on an external cohort of patients and demonstrated a sensitivity of 79.3% [10••].
Table 4.
2011 MSIS criteria for periprosthetic joint infection
| Major criteria (at least 1) | |
| 1. Sinus tract communicating with prosthesis | |
| 2. Pathogen is isolated by culture from 2 separate tissue or fluid samples obtained from affected joint | |
| OR | |
| Minor criteria (4 of the following 6) | |
| 1. Elevated ESR or CRP | |
| 2. Elevated synovial fluid WBC count | |
| 3. Elevated synovial neutrophil percentage | |
| 4. Presence of purulence in the affected joint | |
| 5. Isolation of a microorganism in one culture of periprosthetic tissue or fluid | |
| 6. Greater than five neutrophils per high-power field in five high-power fields observed from histologic analysis of periprosthetic tissue at x400 magnification |
Adapted from Parvizi et al. (2011)
MSIS Musculoskeletal Infection Society; ESR erythrocyte sedimentation rate; WBC white blood cell
In 2018, with the advent of newly available diagnostic tests, Parzivi et al. proposed modifications of the MSIS criteria (Table 5) [10••]. The group utilized a cohort of patients and applied a range of diagnostic tests, applying a multivariable stepwise regression analysis to evaluate the relative importance and weight of each diagnostic test. Based on these results, tests were given a score and cutoffs were created to diagnose infection. Notably, serum D-dimer, synovial CRP, and synovial alpha-defensin were added to the updated criteria [79]. Additionally, a scoring system was created for intraoperative diagnosis of PJI in the setting of an inconclusive preoperative score or dry aspiration preoperatively. This revised definition was validated on a cohort of patients and was found to have a sensitivity and specificity of 97.7% and 99.5%, respectively [10••].
Table 5.
Revised 2018 MSIS criteria for periprosthetic joint infection by Parvizi et al.
| Major criteria | Decision | |
|---|---|---|
|
1. Sinus tract communicating with the joint or visualization of prosthesis 2. Two positive cultures of the same organism |
Infected | |
| Minor criteria | Score | |
| 1. Elevated serum CRP or D-Dimer | 2 | |
| 2. Elevated serum ESR | 1 | ≥6 = infected |
| 3. Elevated synovial WBC count or LE | 3 | 2–5 = possibly infected |
| 4. + Alpha-defensin | 3 | 0–1 = not infected |
| 5. Elevated synovial PMN (%) | 2 | |
| 6. Elevated synovial CRP | 1 | |
| Inconclusive pre-op score or dry tap | Score | |
| 1. Preoperative score | - | ≥6 = infected |
| 2. Positive Histology | 3 | 4–5 = Inconclusive* |
| 3. Positive purulence | 3 | ≤3 = not infected |
| 4. Single positive culture | 2 | |
Adapted from Parvizi et al. (2018)
MSIS Musculoskeletal Infection Society; ESR erythrocyte sedimentation rate; WBC white blood cell; LE leukocyte esterase; PMN polymorphonuclear
The European Bone and Joint Infection Society (EBJIS) recently developed a newly proposed definition of PJI [11•]. They note that it is not practical to have a binary definition, infected or not, given that no current test can definitively exclude infection. Given this, a three-step definition was developed; infection unlikely, infection likely, and infection confirmed. They utilize clinical, serum, synovial fluid, and microbiology diagnostic tests at each step to determine the likelihood of infection. Patients who fall into the infection likely category should undergo further comprehensive testing to assess for infection. The EBJIS 2021 definition has yet to be validated on a cohort of patients.
Proposed Algorithms
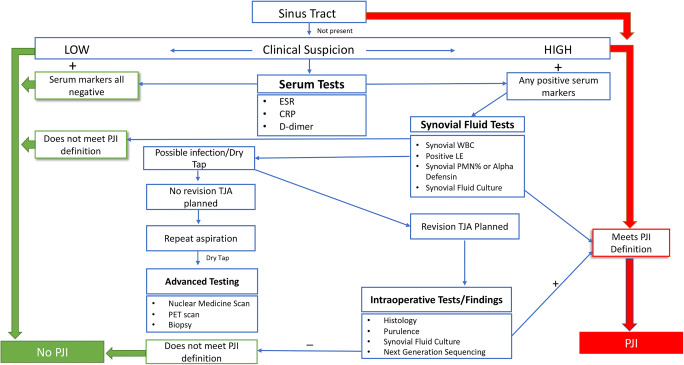
Although the aforementioned definitions are important, a stepwise diagnostic algorithm is useful to apply to patients in the clinical setting. There have been several proposed algorithms with varying degrees of diagnostic accuracy [12, 13••]. Shohat et al. [13••] developed a stepwise clinical algorithm based on the revised 2018 MSIS definition (Fig. 1). They applied the algorithm to a multi-centered cohort of patients and demonstrated high sensitivity (96.6%) and specificity (99.5%). The cohort of patients included low virulent infections and patients who were culture negative, improving the clinical applicability. This algorithm demonstrated that the relatively expensive alpha-defensin test did not provide added diagnostic benefit in routine diagnostic tests. The easy to apply stepwise approach, reliance on commonly available laboratory tests, and the high diagnostic accuracy of this algorithm make it widely clinical applicable.
Fig. 1.
Stepwise clinical algorithm adapted from Shohat et al. (2019) based on the revised 2018 MSIS criteria proposed by Parvizi et al. (2018). Shohat N, Tan TL, Della Valle CJ, Calkins TE, George J, Higuera C, et al. Development and validation of an evidence-based algorithm for diagnosing periprosthetic joint infection. The Journal of Arthroplasty. 2019;34:2730-2736.e1
Conclusions
This review provides an up to date synopsis of the currently available diagnostic tests utilized in the diagnosis of PJI. The diagnosis of PJI remains challenging due to a lack of tests that can definitively rule out infection. Diagnosis and investigations should occur in a stepwise fashion. There has been a plethora of new diagnostic tests introduced in attempts to improve the accuracy of diagnostic algorithms. The definition and algorithms for the diagnoses of PJI will continue to evolve as new techniques and tests are introduced.
Author Contribution
All authors (AG, TJW, DT, and KB) contributed substantially to the conceptualization, drafting or writing of the article, and approval of the final manuscript.
Declarations
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Footnotes
This article is part of the Topical Collection on Outcomes in Research in Orthopedics
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.Kapadia BH, Berg RA, Daley JA, Fritz J, Bhave A, Mont MA. Periprosthetic joint infection. Lancet. 2016;387:386–394. doi: 10.1016/S0140-6736(14)61798-0. [DOI] [PubMed] [Google Scholar]
- 2.Tande AJ, Patel R. Prosthetic joint infection. Clinical Microbiology Reviews. 2014 [DOI] [PMC free article] [PubMed]
- 3.Kapadia BH, McElroy MJ, Issa K, Johnson AJ, Bozic KJ, Mont MA. The economic impact of periprosthetic infections following total knee arthroplasty at a specialized tertiary-care center. J Arthroplast. 2014;29:929–932. doi: 10.1016/j.arth.2013.09.017. [DOI] [PubMed] [Google Scholar]
- 4.Schwartz AM, Farley KX, Guild GN, Bradbury TL., Jr Projections and epidemiology of revision hip and knee arthroplasty in the United States to 2030. J Arthroplast. 2020;35:S79–S85. doi: 10.1016/j.arth.2020.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bozzo A, Madden K, Pond G, Ghert M, Winemaker M, Adili A, et al. Risk factors for prosthetic joint infection following primary total hip arthroplasty: a 15-year population-based cohort study. Journal of Bone & Joint Surgery. 2019. [DOI] [PubMed]
- 6.Hinarejos P, Guirro P, Leal J, Montserrat F, Pelfort X, Sorli ML, Horcajada JP, Puig L. The use of erythromycin and colistin-loaded cement in total knee arthroplasty does not reduce the incidence of infection : a prospective randomized study in 3000 knees. J Bone Joint Surg- Ser A. 2013;95:769–774. doi: 10.2106/JBJS.L.00901. [DOI] [PubMed] [Google Scholar]
- 7.Parvizi MD, Gehrke MD. Definition of periprosthetic joint infection. 2014. [DOI] [PubMed]
- 8.Carli AV, Abdelbary H, Ahmadzai N, Cheng W, Shea B, Hutton B, Sniderman J, Philip Sanders BS, Esmaeilisaraji L, Skidmore B, Gauthier-Kwan OY, Bunting AC, Gauthier P, Crnic A, Logishetty K, Moher D, Fergusson D, Beaulé PE. Diagnostic accuracy of serum, synovial, and tissue testing for chronic periprosthetic joint infection after hip and knee replacements: a systematic review. JBJS. 2019;101:635–649. doi: 10.2106/JBJS.18.00632. [DOI] [PubMed] [Google Scholar]
- 9.Shohat N, Goswami K, Tan TL, Henstenburg B, Makar G, Rondon AJ, et al. Fever and erythema are specific findings in detecting infection following total knee arthroplasty. Journal of bone and joint infection. Copernicus GmbH. 2019;4:92–98. doi: 10.7150/jbji.30088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.•• Parvizi J, Tan TL, Goswami K, Higuera C, Della Valle C, Chen AF, et al. The 2018 definition of periprosthetic hip and knee infection: an evidence-based and validated criteria. J Arthroplast 2018;33:1309-1314.e2. This updated version of the MSIS offers an evidence based definition of diagnosing PJI and was validated on an external cohort [DOI] [PubMed]
- 11.• McNally M, Sousa R, Wouthuyzen-Bakker M, Chen AF, Soriano A, Vogely HC, et al. The EBJIS definition of periprosthetic joint infection: a practical guide for clinicians. Bone Joint J. 2021;103:18–25 This paper represents a project of the European Bone and Joint Infection Society (EBJIS) and represents a novel treatment algorithm that was formed at the annual EBJIS meeting in 2018 with over 450 delegates.
- 12.Abdelbary H, Cheng W, Ahmadzai N, Carli AV, Shea BJ, Hutton B, Fergusson DA, Beaulé PE. Combination tests in the diagnosis of chronic periprosthetic joint infection: systematic review and development of a stepwise clinical decision-making tool. JBJS. 2020;102:114–124. doi: 10.2106/JBJS.20.00097. [DOI] [PubMed] [Google Scholar]
- 13.•• Shohat N, Tan TL, Della Valle CJ, Calkins TE, George J, Higuera C, et al. Development and validation of an evidence-based algorithm for diagnosing periprosthetic joint infection. J Arthroplast. 2019;34:2730–2736.e1 Shohat et al. developed a stepwise clinical algorithm based on the revised 2018 MSIS definition. They applied the algorithm to a multi-centered cohort of patients and demonstrated high sensitivity (96.6%) and specificity (99.5%). [DOI] [PubMed]
- 14.Valle CD, Parvizi J, Bauer TW, DiCesare PE, Evans RP, Segreti J, et al. Diagnosis of periprosthetic joint infections of the hip and knee. JAAOS – J Am Acad Orthop Surg. 2010;18:760–770. doi: 10.5435/00124635-201012000-00006. [DOI] [PubMed] [Google Scholar]
- 15.Zmistowski B, Della Valle C, Bauer TW, Malizos KN, Alavi A, Bedair H, Booth RE, Choong P, Deirmengian C, Ehrlich GD, Gambir A, Huang R, Kissin Y, Kobayashi H, Kobayashi N, Krenn V, Lorenzo D, Marston SB, Meermans G, et al. Diagnosis of periprosthetic joint infection. J Arthroplast Elsevier. 2014;29:77–83. [DOI] [PubMed]
- 16.Azboy I, Çatal B, Başarır K, Mutlu M, Bilgen ÖF, Parvizi J. The natural course of serum D-dimer, C-reactive protein and erythrocyte sedimentation rate levels after uneventful primary total joint arthroplasty. The Journal of Arthroplasty [Internet]. 2021 [cited 2021 May 18]; Available from: https://www.sciencedirect.com/science/article/pii/S0883540321004034. Accessed 1 Jul 2021. [DOI] [PubMed]
- 17.Akgün D, Müller M, Perka C, Winkler T. The serum level of C-reactive protein alone cannot be used for the diagnosis of prosthetic joint infections, especially in those caused by organisms of low virulence. Bone Joint J. 2018;100-B:1482–1486. doi: 10.1302/0301-620X.100B11.BJJ-2018-0514.R1. [DOI] [PubMed] [Google Scholar]
- 18.Kheir MM, Tan TL, Shohat N, Foltz C, Parvizi J. Routine diagnostic tests for periprosthetic joint infection demonstrate a high false-negative rate and are influenced by the infecting organism. JBJS. 2018;100:2057–2065. doi: 10.2106/JBJS.17.01429. [DOI] [PubMed] [Google Scholar]
- 19.Wirtz DC, Heller K-D, Miltner O, Zilkens K-W, Wolff JM. Interleukin-6: a potential inflammatory marker after total joint replacement. Int Orthop. 2000;24:194–196. doi: 10.1007/s002640000136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Randau TM, Friedrich MJ, Wimmer MD, Reichert B, Kuberra D, Stoffel-Wagner B, et al. Interleukin-6 in serum and in synovial fluid enhances the differentiation between periprosthetic joint infection and aseptic loosening. PLoS One. 2014;9:e89045. doi: 10.1371/journal.pone.0089045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Austin MS, Ghanem E, Joshi A, Lindsay A, Parvizi J. A Simple, Cost-effective screening protocol to rule out periprosthetic infection. J Arthroplast. 2008;23:65–68. doi: 10.1016/j.arth.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 22.Shahi A, Kheir MM, Tarabichi M, Hosseinzadeh HRS, Tan TL, Parvizi J. Serum D-dimer test is promising for the diagnosis of periprosthetic joint infection and timing of reimplantation. JBJS. 2017;99:1419–1427. doi: 10.2106/JBJS.16.01395. [DOI] [PubMed] [Google Scholar]
- 23.Bounameaux H, de Moerloose P, Perrier A, Reber G. Plasma measurement of D-dimer as diagnostic aid in suspected venous thromboembolism: an overview. Thromb Haemost. 1994;71:1–6. doi: 10.1055/s-0038-1642375. [DOI] [PubMed] [Google Scholar]
- 24.Xiong L, Li S, Dai M. Comparison of D-dimer with CRP and ESR for diagnosis of periprosthetic joint infection. J Orthop Surg Res. 2019;14:240. doi: 10.1186/s13018-019-1282-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xie K, Qu X, Yan M. Procalcitonin and α-defensin for diagnosis of periprosthetic joint infections. J Arthroplast. 2017;32:1387–1394. doi: 10.1016/j.arth.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 26.Lu G, Li T, Ye H, Liu S, Zhang P, Wang W. D-dimer in the diagnosis of periprosthetic joint infection: a systematic review and meta-analysis. J Orthop Surg Res. 2020;15:265. doi: 10.1186/s13018-020-01761-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tirumala V, Klemt C, Xiong L, Chen W, van den Kieboom J, Kwon Y-M. Diagnostic utility of platelet count/lymphocyte count ratio and platelet count/mean platelet volume ratio in periprosthetic joint infection following total knee arthroplasty. J Arthroplast. 2021;36:291–297. doi: 10.1016/j.arth.2020.07.038. [DOI] [PubMed] [Google Scholar]
- 28.Zhao G, Chen J, Wang J, Wang S, Xia J, Wei Y, et al. Predictive values of the postoperative neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, and lymphocyte-to-monocyte ratio for the diagnosis of early periprosthetic joint infections: a preliminary study. Journal of Orthopaedic Surgery and Research. BioMed Central. 2020;15:1–7. doi: 10.1186/s13018-020-02107-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Golge U, Kaymaz B, PAZARCI Ö, Kilinc S, ÖZTEMÜR Z, Bulut O. Neutrophil to lymphocyte ratio may be a diagnostic marker for prosthetic joint infection. 2016
- 30.Massaccesi L, Bonomelli B, Marazzi MG, Drago L, Romanelli MMC, Erba D, Papini N, Barassi A, Goi G, Galliera E. Plasmatic soluble receptor for advanced glycation end products as a new oxidative stress biomarker in patients with prosthetic-joint-associated infections? Dis Markers. 2017;2017:1–7. doi: 10.1155/2017/6140896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Galliera E, Drago L, Vassena C, Romanò C, Marazzi MG, Salcito L, et al. Toll-like receptor 2 in serum: a potential diagnostic marker of prosthetic joint infection? J Clin Microbiol. 2014;52:620. doi: 10.1128/JCM.02727-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee YS, Koo K-H, Kim HJ, Tian S, Kim T-Y, Maltenfort MG, Chen AF. Synovial fluid biomarkers for the diagnosis of periprosthetic joint infection: a systematic review and meta-analysis. J Bone Joint Surg Am. 2017;99:2077–2084. doi: 10.2106/JBJS.17.00123. [DOI] [PubMed] [Google Scholar]
- 33.Tubb CC, Polkowksi GG, Krause B. Diagnosis and prevention of periprosthetic joint infections. JAAOS-J Am Acad Orthop Surg. 2020;28:e340–e348. doi: 10.5435/JAAOS-D-19-00405. [DOI] [PubMed] [Google Scholar]
- 34.Shahi A, Deirmengian C, Higuera C, Chen A, Restrepo C, Zmistowski B, et al. Premature therapeutic antimicrobial treatments can compromise the diagnosis of late periprosthetic joint infection. Clin Orthop Relat Res. 2015;473:2244–2249. doi: 10.1007/s11999-015-4142-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ottink KD, Strahm C, Muller-Kobold A, Sendi P, Wouthuyzen-Bakker M. Factors to consider when assessing the diagnostic accuracy of synovial leukocyte count in periprosthetic joint infection. Journal of bone and joint infection. Copernicus GmbH. 2019;4:167–173. doi: 10.7150/jbji.34854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marson BA, Deshmukh SR, Grindlay DJC, Scammell BE. Alpha-defensin and the Synovasure lateral flow device for the diagnosis of prosthetic joint infection: a systematic review and meta-analysis. Bone Joint J. 2018;100-B:703–711. doi: 10.1302/0301-620X.100B6.BJJ-2017-1563.R1. [DOI] [PubMed] [Google Scholar]
- 37.Shahi A, Parvizi J, Kazarian GS, Higuera C, Frangiamore S, Bingham J, et al. The alpha-defensin test for periprosthetic joint infections is not affected by prior antibiotic administration. Clin Orthop Relat Res. 2016;474:1610–1615. doi: 10.1007/s11999-016-4726-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.• Renz N, Yermak K, Perka C, Trampuz A. Alpha defensin lateral flow test for diagnosis of periprosthetic joint infection: not a screening but a confirmatory test. JBJS. 2018;100:742–50 Alpha-defensin represents a novel diagnostic test for PJI. Alpha defensin was tested on an external cohort and demonstrated high specificity but low sensitivity suggesting that it should be used as a confirmatory test for PJI. [DOI] [PubMed]
- 39.Deirmengian C, Kardos K, Kilmartin P, Gulati S, Citrano P, Booth RE. The alpha-defensin test for periprosthetic joint infection responds to a wide spectrum of organisms. Clin Orthop Relat Res. 2015;473:2229–2235. doi: 10.1007/s11999-015-4152-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Amanatullah DF, Cheng RZ, Huddleston Iii JI, Maloney WJ, Finlay AK, Kappagoda S, et al. The routine use of synovial alpha-defensin is not necessary. Bone Joint J. 2020;102:593–599. doi: 10.1302/0301-620X.102B5.BJJ-2019-0473.R3. [DOI] [PubMed] [Google Scholar]
- 41.Plate A, Stadler L, Sutter R, Anagnostopoulos A, Frustaci D, Zbinden R, Fucentese SF, Zinkernagel AS, Zingg PO, Achermann Y. Inflammatory disorders mimicking periprosthetic joint infections may result in false-positive α-defensin. Clin Microbiol Infect. 2018;24:1212–12e1. doi: 10.1016/j.cmi.2018.02.019. [DOI] [PubMed] [Google Scholar]
- 42.Bonanzinga T, Zahar A, Dütsch M, Lausmann C, Kendoff D, Gehrke T. How reliable is the alpha-defensin immunoassay test for diagnosing periprosthetic joint infection? A prospective study. Clin Orthop Relat Res. 2017;475:408–415. doi: 10.1007/s11999-016-4906-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kusumi RK, Grover PJ, Kunin CM. Rapid detection of pyuria by leukocyte esterase activity. JAMA. 1981;245:1653–1655. doi: 10.1001/jama.1981.03310410031022. [DOI] [PubMed] [Google Scholar]
- 44.Wetters NG, Berend KR, Lombardi AV, Morris MJ, Tucker TL, Della Valle CJ. Leukocyte esterase reagent strips for the rapid diagnosis of periprosthetic joint infection. J Arthroplast. 2012;27:8–11. doi: 10.1016/j.arth.2012.03.037. [DOI] [PubMed] [Google Scholar]
- 45.Parvizi J, Jacovides C, Antoci V, Ghanem E. Diagnosis of periprosthetic joint infection: the utility of a simple yet unappreciated enzyme. JBJS. 2011;93:2242–2248. doi: 10.2106/JBJS.J.01413. [DOI] [PubMed] [Google Scholar]
- 46.Omar M, Ettinger M, Reichling M, Petri M, Guenther D, Gehrke T, Krettek C, Mommsen P. Synovial C-reactive protein as a marker for chronic periprosthetic infection in total hip arthroplasty. Bone Joint J. 2015;97-B:173–176. doi: 10.1302/0301-620X.97B2.34550. [DOI] [PubMed] [Google Scholar]
- 47.Zamani B, Jamali R, Ehteram H. Synovial fluid adenosine deaminase and high-sensitivity C-reactive protein activity in differentiating monoarthritis. Rheumatol Int. 2012;32:183–188. doi: 10.1007/s00296-010-1602-3. [DOI] [PubMed] [Google Scholar]
- 48.Buttaro MA, Martorell G, Quinteros M, Comba F, Zanotti G, Piccaluga F. Intraoperative synovial C-reactive protein is as useful as frozen section to detect periprosthetic hip infection. Clin Orthop Relat Res. 2015;473:3876–3881. doi: 10.1007/s11999-015-4340-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Parvizi J, McKenzie JC, Cashman JP. Diagnosis of periprosthetic joint infection using synovial C-reactive protein. J Arthroplast. 2012;27:12–16. doi: 10.1016/j.arth.2012.03.018. [DOI] [PubMed] [Google Scholar]
- 50.Parvizi J, Jacovides C, Adeli B, Am Jung K, Hozack WJ, Mark B. Coventry Award: synovial C-reactive protein: a prospective evaluation of a molecular marker for periprosthetic knee joint infection. Clin Orthop Relat Res. 2012;470:54–60. doi: 10.1007/s11999-011-1991-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Salari P, Grassi M, Cinti B, Onori N, Gigante A. Synovial fluid calprotectin for the preoperative diagnosis of chronic periprosthetic joint infection. J Arthroplast. 2020;35:534–537. doi: 10.1016/j.arth.2019.08.052. [DOI] [PubMed] [Google Scholar]
- 52.Fernandes SR, Santos P, Fatela N, Baldaia C, Tato Marinho R, Proença H, et al. Ascitic calprotectin is a novel and accurate marker for spontaneous bacterial peritonitis. J Clin Lab Anal. 2016;30:1139–1145. doi: 10.1002/jcla.21994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kopi TA, Shahrokh S, Mirzaei S, Aghdaei HA, Kadijani AA. The role of serum calprotectin as a novel biomarker in inflammatory bowel diseases: a review study. Gastroenterology and hepatology from bed to bench. Shahid Beheshti University of Medical Sciences; 2019;12:183. [PMC free article] [PubMed]
- 54.Zhang Z, Cai Y, Bai G, Zhang C, Li W, Yang B, et al. The value of calprotectin in synovial fluid for the diagnosis of chronic prosthetic joint infection. Bone Joint Res. 2020;9:450–456. doi: 10.1302/2046-3758.98.BJR-2019-0329.R2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen Z, Wang Y, Zeng A, Chen L, Wu R, Chen B, et al. The clinical diagnostic significance of cerebrospinal fluid d-lactate for bacterial meningitis. Clin Chim Acta. 2012;413:1512–1515. doi: 10.1016/j.cca.2012.06.018. [DOI] [PubMed] [Google Scholar]
- 56.Yermak K, Karbysheva S, Perka C, Trampuz A, Renz N. Performance of synovial fluid D-lactate for the diagnosis of periprosthetic joint infection: a prospective observational study. J Inf Secur. 2019;79:123–129. doi: 10.1016/j.jinf.2019.05.015. [DOI] [PubMed] [Google Scholar]
- 57.Karbysheva S, Yermak K, Grigoricheva L, Renz N, Perka C, Trampuz A. Synovial fluid D-lactate—a novel pathogen-specific biomarker for the diagnosis of periprosthetic joint infection. J Arthroplast. 2020;35:2223–2229. doi: 10.1016/j.arth.2020.03.016. [DOI] [PubMed] [Google Scholar]
- 58.Parvizi J, Erkocak OF, Della Valle CJ. Culture-negative periprosthetic joint infection. JBJS. 2014;96:430–436. doi: 10.2106/JBJS.L.01793. [DOI] [PubMed] [Google Scholar]
- 59.Trampuz A, Piper KE, Jacobson MJ, Hanssen AD, Unni KK, Osmon DR, et al. Sonication of removed hip and knee prostheses for diagnosis of infection. N Engl J Med. 2007;357:654–663. doi: 10.1056/NEJMoa061588. [DOI] [PubMed] [Google Scholar]
- 60.Kheir MM, Tan TL, Ackerman CT, Modi R, Foltz C, Parvizi J. Culturing periprosthetic joint infection: number of samples, growth duration, and organisms. J Arthroplast. 2018;33:3531–3536. doi: 10.1016/j.arth.2018.06.018. [DOI] [PubMed] [Google Scholar]
- 61.Gomez E, Cazanave C, Cunningham SA, Greenwood-Quaintance KE, Steckelberg JM, Uhl JR, et al. Prosthetic joint infection diagnosis using broad-range PCR of biofilms dislodged from knee and hip arthroplasty surfaces using sonication. J Clin Microbiol. 2012;50:3501–3508. doi: 10.1128/JCM.00834-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tunney MM, Patrick S, Gorman SP, Nixon JR, Anderson N, Davis RI, Hanna D, Ramage G. Improved detection of infection in hip replacements: a currently underestimated problem. J Bone J Surg Br Vol. 1998;80:568–572. doi: 10.1302/0301-620X.80B4.0800568. [DOI] [PubMed] [Google Scholar]
- 63.Rothenberg AC, Wilson AE, Hayes JP, O’Malley MJ, Klatt BA. Sonication of arthroplasty implants improves accuracy of periprosthetic joint infection cultures. Clin Orthop Relat Res. 2017;475:1827–1836. doi: 10.1007/s11999-017-5315-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.• Sebastian S, Malhotra R, Sreenivas V, Kapil A, Chaudhry R, Dhawan B. Sonication of orthopaedic implants: a valuable technique for diagnosis of prosthetic joint infections. J Microbiol Methods. 2018;146:51–4 In a significant proportion of patients with PJI, a causative organism is never recovered. This is in part due to the biofilm formed by bacteria on the prosthesis. Sonication represents a novel technique that disrupts the biofilm layer and increases the ability to detect pathogens. [DOI] [PubMed]
- 65.Roux A-L, Sivadon-Tardy V, Bauer T, Lortat-Jacob A, Herrmann J-L, Gaillard J-L, et al. Diagnosis of prosthetic joint infection by beadmill processing of a periprosthetic specimen. Clin Microbiol Infect. 2011;17:447–450. doi: 10.1111/j.1469-0691.2010.03359.x. [DOI] [PubMed] [Google Scholar]
- 66.Arvieux C, Common H. New diagnostic tools for prosthetic joint infection. Orthop Traumatol Surg Res. 2019;105:S23–S30. doi: 10.1016/j.otsr.2018.04.029. [DOI] [PubMed] [Google Scholar]
- 67.Kolenda C, Josse J, Batailler C, Faure A, Monteix A, Lustig S, et al. Experience with the use of the MicroDTTect device for the diagnosis of low-grade chronic prosthetic joint infections in a routine setting. Front Med (Lausanne) [Internet]. 2021 [cited 2021 Jun 1];8. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8007775/. Accessed 1 Jul 2021. [DOI] [PMC free article] [PubMed]
- 68.Drago L, Romanò CL, Mattina R, Signori V, De Vecchi E. Does dithiothreitol improve bacterial detection from infected prostheses? A pilot study. Clin Orthop Relat Res. 2012;470:2915–2925. doi: 10.1007/s11999-012-2415-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bémer P, Plouzeau C, Tande D, Léger J, Giraudeau B, Valentin AS, et al. Evaluation of 16S rRNA gene PCR sensitivity and specificity for diagnosis of prosthetic joint infection: a prospective multicenter cross-sectional study. J Clin Microbiol. 2014;52:3583. doi: 10.1128/JCM.01459-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Goldberg B, Sichtig H, Geyer C, Ledeboer N, Weinstock GM. Making the leap from research laboratory to clinic: challenges and opportunities for next-generation sequencing in infectious disease diagnostics. mBio [Internet]. American Society for Microbiology; 2015 [cited 2021 May 23];6. Available from: https://mbio.asm.org/content/6/6/e01888-15. Accessed 1 Jul 2021. [DOI] [PMC free article] [PubMed]
- 71.Wasterlain AS, Goswami K, Ghasemi SA, Parvizi J. Diagnosis of periprosthetic infection: recent developments. JBJS. 2020;102:1366–1375. doi: 10.2106/JBJS.19.00598. [DOI] [PubMed] [Google Scholar]
- 72.Marín M, Garcia-Lechuz JM, Alonso P, Villanueva M, Alcala L, Gimeno M, et al. Role of universal 16S rRNA gene PCR and sequencing in diagnosis of prosthetic joint infection. J Clin Microbiol. 2012;50:583–589. doi: 10.1128/JCM.00170-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tarabichi M, Shohat N, Goswami K, Alvand A, Silibovsky R, Belden K, Parvizi J. Diagnosis of periprosthetic joint infection: the potential of next-generation sequencing. JBJS. 2018;100:147–154. doi: 10.2106/JBJS.17.00434. [DOI] [PubMed] [Google Scholar]
- 74.Kildow BJ, Ryan SP, Danilkowicz R, Lazarides AL, Penrose C, Bolognesi MP, Jiranek W, Seyler TM. Next-generation sequencing not superior to culture in periprosthetic joint infection diagnosis. Bone Joint J. 2021;103:26–31. doi: 10.1302/0301-620X.103B1.BJJ-2020-0017.R3. [DOI] [PubMed] [Google Scholar]
- 75.Diagnosis of peripheral bone and prosthetic joint infections: overview on the consensus documents by the EANM, EBJIS, and ESR (with ESCMID endorsement) | SpringerLink [Internet]. [cited 2021 May 23]. Available from: 10.1007/s00330-019-06326-1 [DOI] [PubMed]
- 76.Romanò CL, Petrosillo N, Argento G, Sconfienza LM, Treglia G, Alavi A, et al. The role of imaging techniques to define a peri-prosthetic hip and knee joint infection: multidisciplinary consensus statements. J Clin Med. 2020;9:2548. doi: 10.3390/jcm9082548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Signore A, Jamar F, Israel O, Buscombe J, Martin-Comin J, Lazzeri E. Clinical indications, image acquisition and data interpretation for white blood cells and anti-granulocyte monoclonal antibody scintigraphy: an EANM procedural guideline. Eur J Nucl Med Mol Imaging. 2018;45:1816–1831. doi: 10.1007/s00259-018-4052-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kiran M, Donnelly TD, Armstrong C, Kapoor B, Kumar G, Peter V. Diagnostic utility of fluorodeoxyglucose positron emission tomography in prosthetic joint infection based on MSIS criteria. Bone Joint J. 2019;101-B:910–914. doi: 10.1302/0301-620X.101B8.BJJ-2018-0929.R2. [DOI] [PubMed] [Google Scholar]
- 79.Parvizi J, Zmistowski B, Berbari EF, Bauer TW, Springer BD, Della Valle CJ, et al. New definition for periprosthetic joint infection: from the Workgroup of the Musculoskeletal Infection Society. Clin Orthop Relat Res. 2011;469:2992–2994. doi: 10.1007/s11999-011-2102-9. [DOI] [PMC free article] [PubMed] [Google Scholar]