Abstract
Tendon injuries positively correlate with patient age, as aging has significant effects on tendon homeostatic maintenance and healing potential after injury. Vascularity is also influenced by age, with both clinical and animal studies demonstrating reduced blood flow in aged tissues. However, it is unknown how aging effects vascularity following tendon injury, and if this vascular response can be modulated through the delivery of angiogenic factors. Therefore, the objective of this study is to evaluate the vascular response following Achilles tendon injury in adult and aged rats, and to define the alterations to tendon healing in an aged model following injection of angiogenic factors. It was determined that aged rat Achilles tendons have a reduced angiogenesis following injury. Further, the delivery of vascular endothelial growth factor, VEGF, caused an increase in vascular response to tendon injury and improved mechanical outcome in this aged population. This work suggests that reduced angiogenic potential with aging may be contributing to impaired tendon healing response and that the delivery of angiogenic factors can rescue this impaired response. This study was also the first to relate changes in vascular response in an aged model using in vivo measures of blood perfusion to alterations in healing properties.
Keywords: Tendon, Aging, Ultrasound, Vascularity, Mechanics
INTRODUCTION
The frequency of musculoskeletal injury is expected to increase greatly in the coming decades with both increased life expectancy and a sustained higher level of activity with aging.12,46 There are many challenges associated with advanced age, namely the development of multiple impairments, alterations in physiological functions, decline in functional capacity, and ultimately loss of independence.12 Many of the contributors to these challenges and disabilities are due to structural and functional changes to the musculoskeletal system with aging.12
Tendon injuries positively correlate with patient age, as aging has significant effects on both tendon homeostatic maintenance as well as tendon healing potential after injury.25 Mechanical evaluations have demonstrated impaired structural and material properties with aging, including alterations in elastic modulus, viscoelasticity, and stiffness.20,30,43 Additionally, this decline in mechanical properties correlates with reduced collagen content.9,10 On a cellular level, aged tenocytes are less viable with a lower proliferation rate, motility, density, and organization within the tissue.1,3,42,47 This could indicate a reduced potential for cellular maintenance or repair, which may lead to tendon degeneration. Protein and mRNA expression is significantly altered, with decreased extracellular matrix proteins such as proteoglycans, elastin, aggrecan, and collagens I, III, and V, as well as increased matrix metallopeptidase (MMP)-2 and -9, indicating increased matrix degradation.18,23,42,47 Following tendon injury, aging causes significant reductions in max load and stiffness, along with less organized fiber structure and reduced matrix production at the injury site.1,29 This data suggests that aging has major effects on the tendon’s ability to maintain homeostasis, increasing susceptibility to rupture, as well as impairing the ability to recover from an injury.
Tendon vascularity is also altered with aging. Clinical ultrasound studies have demonstrated reduced blood flow,13,21,37 and histological studies show decreased vessel density in uninjured tendons with aging.23,24 Cellular studies have demonstrated reduced vascular endothelial cell expansion and differentiation potential in tendon cell populations harvested from older age groups.44 This suggests that aged tendons are less capable of forming or maintaining necessary vascular structure, which could alter their cellular responses, contributing to reduced healing capacity in the aged population.
While aging affects tendon maintenance, vascular structure, and healing potential, it is unknown how aging affects the vascular response following injury in the tendon. Additionally, we have shown that local delivery of VEGF and anti-VEGF antibody to tendons can increase and decrease the vascular response after injury in young animals, respectively.32,34 However, the effect of altering the vascular response after injury in the aged population is unknown. Therefore, the objective of this study is to evaluate the vascular response following Achilles tendon injury in adult and aged rats (Study 1), and to define the alterations to tendon healing in an aged model following injection of angiogenic factors (Study 2). We hypothesize that when compared to adult rats, aged rats will demonstrate a decrease in blood flow parameters, as well as a decrease in vascular density following injury. Additionally, we hypothesize that increasing the vascular response through the administration of a pro-angiogenic treatment will improve healing capacity as shown by increased mechanical properties.
MATERIALS AND METHODS
All animal work was approved by and performed in accordance with guidelines of the University of Pennsylvania IACUC.
Study Design
Study 1: Vascular Response to Injury with Aging
We investigated 5 adult (4 months) and 5 aged (16 months) Sprague Dawley rats. All animals underwent a bilateral Achilles incisional injury, followed by bilateral color Doppler ultrasound imaging and sacrifice for histological evaluation on day 7 post-injury (n = 8–9 tendons/group).
Study 2: Vascular Modulation with Aging
Thirty-six Fischer 344 rats (19–20 months old) underwent a bilateral Achilles incisional injury, followed by local injections of vascular endothelial growth factor (VEGF) (Peprotech, Rocky Hill, NJ), anti-VEGF antibody (B20.4–1-1, Genentech, San Francisco, CA), or saline (SAL). In vivo ultrasound imaging (b-mode, Doppler, photoacoustics, and contrast-enhanced ultrasound) was performed and animals were sacrificed 14 days after injury for histological and mechanical evaluation. Bilateral samples were always used in separate assays (i.e. one limb for mechanical testing and one limb for histology) and so each replicate within an assay is from an independent animal.
Surgical Approach
Animals were anesthetized with isoflurane inhalation and using aseptic technique a skin incision was made on the medial side of the ankle to isolate the Achilles tendon. Using a 1.5mm flat scalpel blade (#61, MYCO Medical, Apex, NC), a partial-width, full-thickness incisional injury was made in the center of the tendon in the mid-substance region (Fig. S1A). The tendon was left unrepaired and the skin was sutured closed.
Angiogenic Injections
For Study 2 only, each animal received either 5 μg VEGF or 250 μg anti-VEGF antibody (B20) in 20 μL saline, or 20 μL saline only, injected bilaterally intratendinously on days 4–6 after surgical injury. These dosages were chosen based on literature values4,9,16,22,28,48 and our previous work.32,34 The injections were administrated percutaneously in the coronal plane from the medial side of the tendon, with 10 μL of the solution injected above and 10 μL injected below the injury site. The saline injection control group served as a sham surgical group to account for the effect of the needle puncture.
Ultrasound Imaging
Imaging was performed on day 7 (Study 1 and 2) and 14 (Study 2 only) using a Vevo LAZR ultrasound system (VisualSonics, Toronto, ON) with a 40 MHz center frequency transducer (LZ550). Following anesthetization using inhalation of isoflurane, all hair was removed from the hind limb by shaving and hair removal cream to allow for ultrasound visualization. The animal was placed on a heated imaging table with the ankle secured at 90° flexion. The transducer was placed to image the sagittal plane, ensuring that the tendon was parallel to the surface of the transducer, and the tendon length was in plane with the transducer length. The tendon was centered at a focal zone at 7 mm image depth. All ultrasound quantification analyses were conducted within two regions of interest (ROIs): (1) the entire tendon (tendon ROI) and (2) a 3 mm2 rectangular area over the injury region (injury ROI). Within each imaging modality, image acquisition settings were held constant for all specimens and measures for all image segments in a specimen were averaged to obtain a representative value for the entire tendon.
Color Doppler Ultrasound
Spatially sequential color Doppler ultrasound images were acquired every 0.1mm across the tendon over 3.5 mm as described.31,32,34 Imaging persistence was used to remove motion artifact from the scanner. The central 8–10 tendon images were analyzed using a custom IDL program (Harris Geospatial Solutions, Herndon, VA). The mean color level (average blood flow velocity), the fractional area (% area of Doppler signal), and the color weighted fractional area (weighted average of blood flow velocity/unit area) were quantified.39
Photoacoustic Imaging
As described,32,34 photoacoustic images were taken at two wavelengths (750 and 850 nm) based on the absorption spectrum of oxygenated (HbO2) and deoxygenated hemoglobin (Hb), respectively.27 Three images were acquired within the center of the tendon. Image acquisition settings were held constant for all specimens. Blood oxygenation (sO2 Avg), average hemoglobin (HbT Avg), and relative tissue oxygenation (sO2 Tot) were quantified.
Contrast-Enhanced Ultrasound
Contrast-enhanced imaging was performed as previously described.32 Following anesthetization, a tail vein catheter was inserted and secured. The Achilles tendon was visualized in non-linear contrast mode using the MS250 transducer (18MHz center frequency). An ultrasound video was initiated at start of the bolus injection of 100 μL of Definity (Lantheus Medical Imaging, Billerica, MA) microbubble contrast agent, followed immediately by a bolus injection of 200 μL of saline. The video clip was taken for 200 seconds to capture the wash-in and wash-out of the contrast in the tissue. The ultrasound clip was analyzed using a contrast analysis program, VevoCQ (VisualSonics, Toronto, ON).26 Amplitude- and time-based perfusion parameters were derived from this analysis.26
B-mode Ultrasound Alignment Analysis
Images were taken at a center frequency of 40 MHz (LZ550 transducer) with an effective resolution of 40 μm for collagen alignment analysis.33 Spatially sequential b-mode ultrasound images were acquired every 0.1 mm across the tendon over 3.5 mm. The central 5 images were analyzed using a custom Matlab program (Mathworks, Natick, MA). Briefly, collagen fascicles appear hyperechoic, where the noncollagenous matrix between the fascicles appears hypoechoic, giving rise to the appearance of bands in the images.14 These bands are analyzed to determine a quantitative measure of tendon organization. The circular standard deviation (CSD), a measure of the distribution of collagen alignment, and echogenicity measures were calculated as previously described.33
Tendon Histology
Study 1: Vascular Response to Injury with Aging
Bilateral tendon samples from 7 days post-injury (n = 8–9 tendons/group) were dissected, paraffin processed, and sectioned at 5 μm in the sagittal plane. Sections were stained with hematoxylin-eosin (H&E) and immunohistochemical (IHC) staining for vascular endothelial cell marker (CD34). Histology images were taken in the injury region of the tendon at × 50 magnification for CD34 (in order to better view vascular structure) and × 100 magnification for H&E, and images were semi-quantitatively graded by three blinded investigators.
Study 2: Vascular Modulation with Aging
Tendon samples from 7 and 14 days post-injury (n = 6/group) were dissected, paraffin processed, and sectioned at 5 μm in the sagittal plane. Sections were stained with hematoxylin–eosin (H&E) and immunohistochemical (IHC) staining for vascular endothelial cell marker (CD34), vascular endothelial growth factor (VEGF), angiopoietin-1 (Ang-1), collagen type III (Col III), matrix metallopeptidase-13 (MMP-13), and tumor necrosis factor alpha (TNFα). Processing protocol details are outlined in Table 1.
TABLE 1.
Immunohistochemistry protocols.
| Primary antibody | Antigen retrieval | Secondary Ab/amplification |
|---|---|---|
|
| ||
| Rabbit Anti-CD34 (Abcam, ab81289) | Heat Induced at 75 °C for 20 min in 1 mM EDTA, pH 8.0 | Vectastain Elite ABC HRP Kit (Vector Laboratories, PK-6200) |
| Rabbit Anti-Ang1 (Abcam, ab102015) | Heat induced at 95 °C for 10 min in 10 mM sodium citrate, pH 6.0 | Vectastain Elite ABC HRP Kit (Vector Laboratories, PK-6200) |
| Rabbit Anti-VEGF (Abcam, ab46154) | Digestion in 0.5 mg/mL hyaluronidase for 60 min at 37 °C | EnVision + HRP labelled polymer solution (Dako, K4002) |
| Mouse Anti-Col III (Sigma-Aldrich, C7805) | Digestion in 0.4 mg/mL protease K in 30 mM Tris HCl for 4 min at RT, 0.5mg/mL Hyaluronidase for 60 min at 37 °C, and 0.5 N acetic acid for 4 h at 4 °C | Secondary (BD Biosciences, 550331), ABC amplification (Vector Laboratories, PK-6200) |
| Rabbit Anti-TNFα (Novus Biologics, NBP1-19532) | Digestion in 0.5 mg/mL Pepsin in 0.1 N HCl for 20 min at RT | Secondary (Jackson Co. 111-035-003) |
| Rabbit Anti-MMP13 (Abcam, ab39012) | Digestion in 0.5 mg/mL Hyaluronidase for 60 min at 37 °C | Secondary (BD Sciences, 550338), ABC amplification (vector laboratories, PK-6200). |
Histology images were taken in the injury region of the tendon at × 50 magnification for CD34 (in order to better view vascular structure) and × 100 magnification for all other stains. H&E and CD34 were semi-quantitatively graded by three blinded investigators. H&E was graded for cell shape (1 = spindle to 3 = round shape) and cellularity (1 = less cells to 3 = more cells), and CD34 was graded for vessel density (1 = less to 4 = more dense) and vessel size (1 = small to 4 = large diameter). All other IHC stains were quantitatively analyzed for percent area of positive stain using a custom MATLAB program (Mathworks, Natick, MA).
Tendon Mechanics
Tendons from 14 days after injury (n = 12/group) were prepared for tensile testing. The Achilles tendon was removed with the muscle and foot attached. The tendon was fine dissected to remove all connective tissue, leaving the calcaneus insertion and foot intact. Verhoeff stain was applied to the tendon for optical strain measurements of the full tendon and the injury region (Fig. S1B). Tendon cross-sectional area was measured using a custom laser-based device.11 The proximal side of the tendon was fixed between two layers of sandpaper using cyanoacrylate adhesive at the 12 mm stain line. The entire foot was secured in polymethylmethacrylate. The specimen was positioned so the foot and the tendon were oriented perpendicular and submerged in a 37 °C phosphate-buffered saline bath. The tendon was tested in tension using an ElectroPuls E3000 (Instron, Norwood, MA) with a 250 N load cell. The mechanical protocol consisted of (1) preloading (0.15 N), (2) preconditioning (0.5% to 1.5% strain at 0.25 Hz for 30 cycles), (3) stress-relaxation (6% strain for 10 min), (4) a dynamic frequency sweep (0.125% strain amplitude at 0.1, 1, 5, and 10 Hz, for 10 cycles each), and (5) ramp to failure (0.1% strain/s) (Fig. S1C). Images for optical strain measures were captured. Tendon viscoelastic and quasi-static properties were computed. Full tendon and injury site modulus calculations were performed by using the optical strain and cross-sectional area measurements of either the full tendon or injury regions.
Statistics
Normally distributed data (ultrasound, mechanics, and quantitative histology) was analyzed using a 1-way ANOVA followed by Bonferroni multiple comparisons post-hoc tests with all comparisons made to saline control within a time point. Non-normally distributed data (semi-quantitative histology grading for H&E and CD34) was analyzed using Kruskal-Wallis 1-way ANOVA followed by Dunn’s multiple comparison post-hoc tests with comparisons made to saline control within a time point. Significance was set at p ≤ 0.05 (indicated by solid bars) and trends at p ≤ 0.1 (indicated by dashed bars). Bar plots are displayed as mean and standard deviation and box plots represent median and interquartile range.
RESULTS
Study 1: Vascular Response to Injury with Aging
Color Doppler Ultrasound
For the analysis of color Doppler ultrasound (Fig. 1), there was a significant decrease in mean color level (MCL), fractional area (FA), and color weighted fractional (CWFA) area following injury in the aged group at 7 days post-injury.
FIGURE 1.
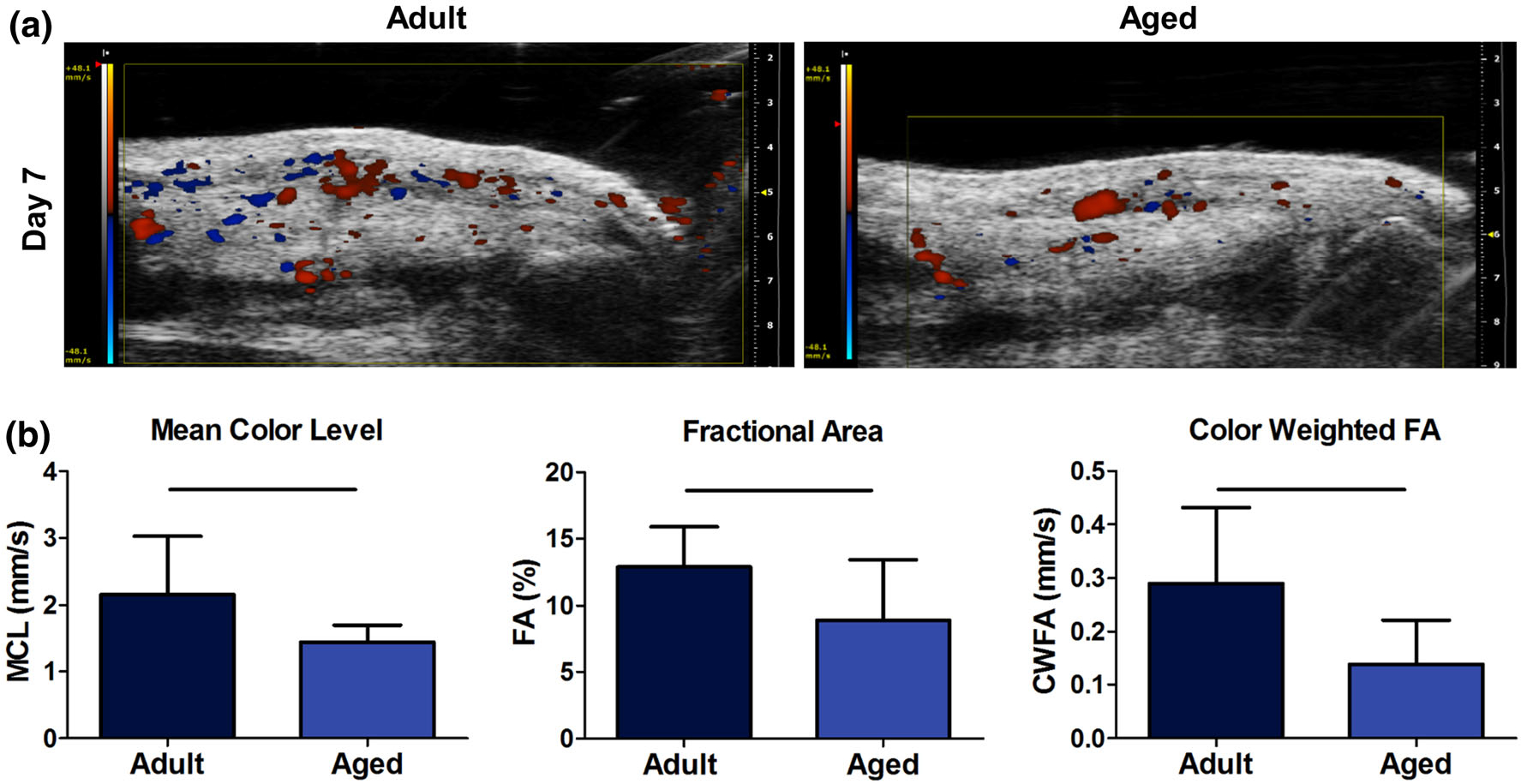
(a) Representative images for color Doppler ultrasound analysis. (b) Quantification of mean color level (MCL), fractional area (FA), and color weighted fractional area (CWFA). Solid bars indicate p < 0.05.
Histological Analysis
H&E analysis of cell number and cell shape did not demonstrate differences between groups (Figs. 2a, 2b). Immunohistochemical evaluation of CD34 (Figs. 2c, 2d), a marker of vascular endothelial cells, showed a significant decrease in vessel density, but no change in vessel size following injury in the aged group.
FIGURE 2.
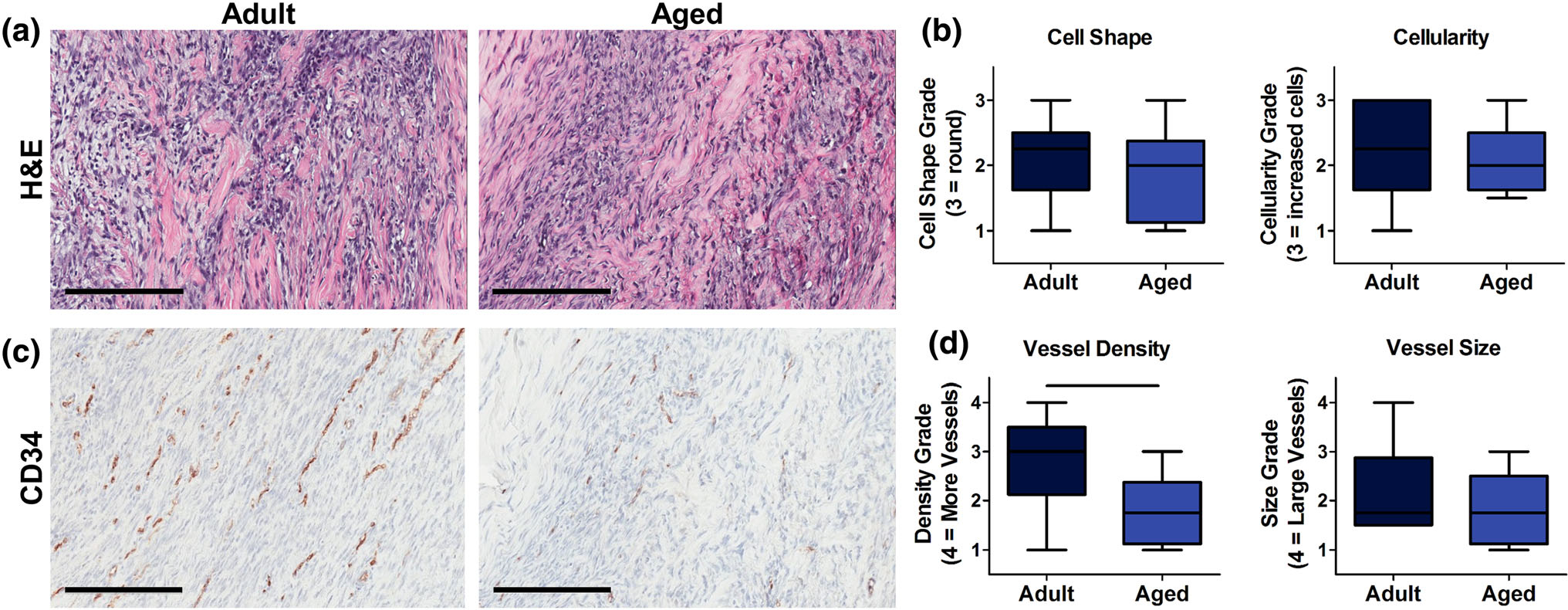
(a) H&E representative images (scale bar 200 μm) and (b) semi-quantitative analysis of cell shape and cellularity. Images taken at × 100 magnification. (c) CD34 representative images (scale bar 200 μm) and (b) semi-quantitative analysis of vessel density and vessel size. Aged animals had a significantly reduced vessel density compared to adult animals. Solid bars indicate p < 0.05.
Study 2: Vascular Modulation with Aging
Color Doppler Ultrasound
There was a significant increase in MCL, representing increased blood flow velocity, in the VEGF group at day 7 after injury in the tendon ROI (Fig. 3b). The B20 group had a trending decrease in MCL and significant decreases in FA and CWFA at days 7 and 14 (Fig. 3) in the tendon ROI. Similar changes were observed when evaluating the injury site ROI (Fig. S2).
FIGURE 3.
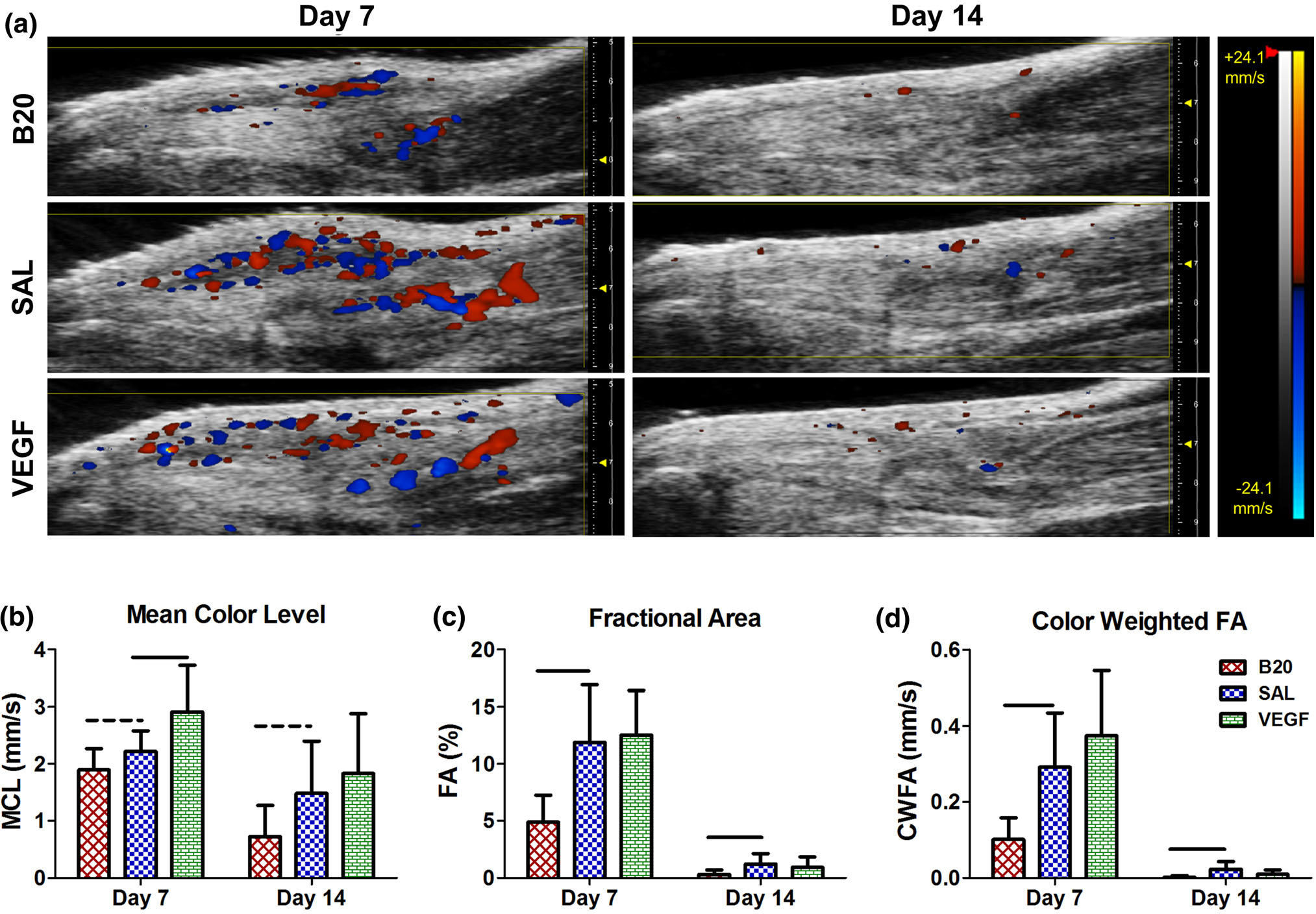
(a) Representative images of color Doppler ultrasound, where the red and blue color scale represents blood flow velocity towards and away from the transducer, respectively. Quantification of (b) mean color level (MCL), (c) fractional area (FA), and (d) color weighted fractional area (CWFA) in the full tendon ROI. The vascular endothelial growth factor group (VEGF) caused a significant increase in MCL at day 7, where the anti-VEGF antibody group (B20) caused trending or significant decreases in all three properties at multiple time points compared to the saline control (SAL). Solid bars indicate p < 0.05 and dashed bars indicated p < 0.1.
Photoacoustic Imaging
Representative images of photoacoustic imaging are shown in Fig. 4a. In the tendon ROI, the VEGF group had a trending increase in blood oxygenation at day 14 (Fig. 4b). Additionally, this group had a significant increase in average hemoglobin (Fig. 4c) and tissue oxygenation (Fig. 4d) on day 7. The B20 group average hemoglobin and tissue oxygenation was significantly decreased on day 7 (Figs. 4c, 4d). Similar changes were observed for the VEGF group when evaluating the injury ROI, but there were no significant differences in the B20 group (Fig. S3).
FIGURE 4.
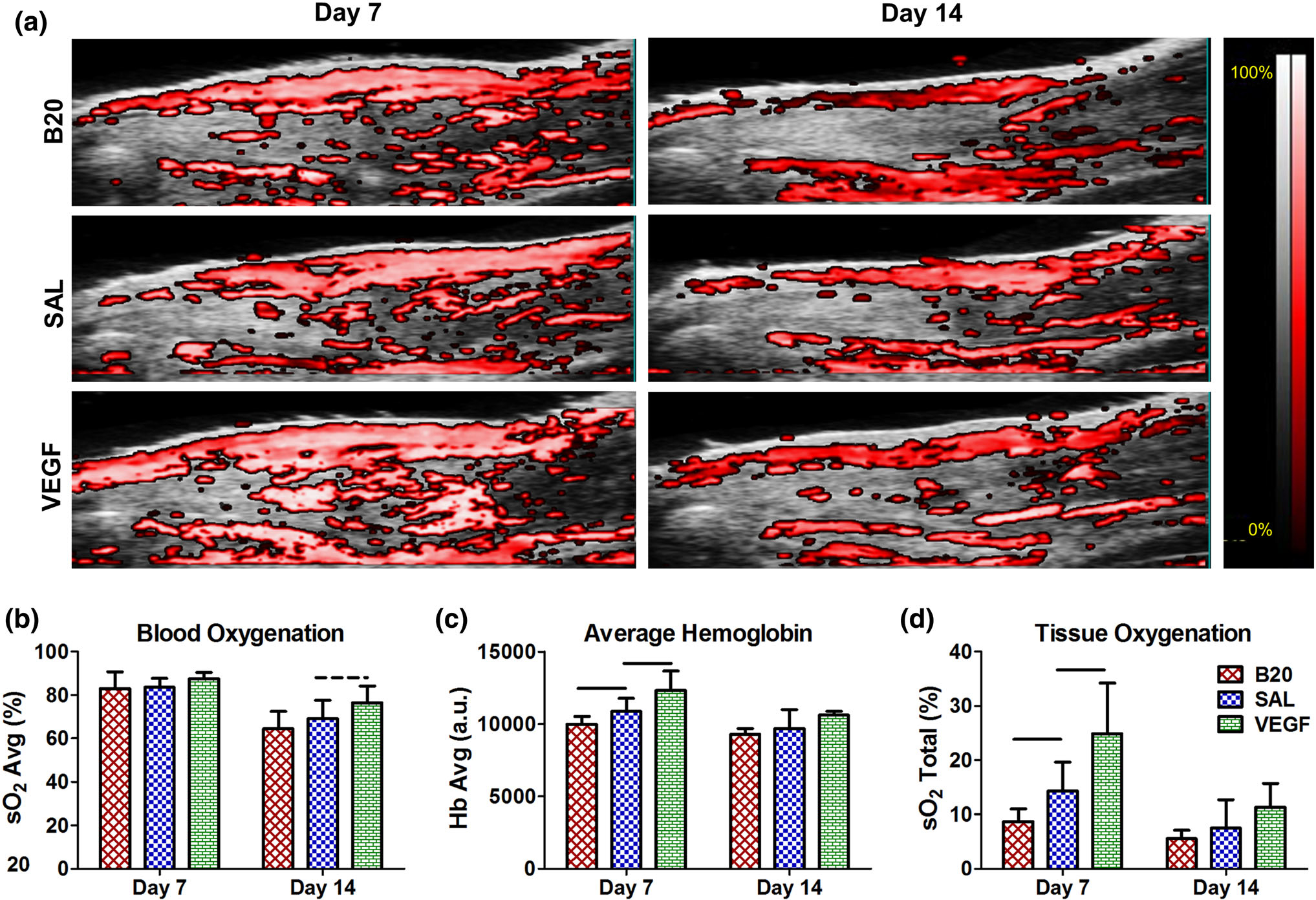
(a) Representative images of photoacoustic ultrasound, where the overlaid color scale indicates oxygen saturation of the detected hemoglobin. Quantification of (b) blood oxygenation (sO2 Avg), (c) average hemoglobin (Hb Avg), and (d) tissue oxygenation (sO2 Total) for the full tendon ROI. The vascular endothelial growth factor group (VEGF) caused significant or trending increases in all three parameters at multiple time points, where the anti-VEGF antibody group (B20) caused significant decreases in Hb Avg and sO2 Total at day 7 compared to the saline control (SAL). Solid bars indicate p < 0.05 and dashed bars indicate p < 0.1.
Contrast-Enhanced Ultrasound
Averaged echo-power vs time curves for the contrast wash-in and wash-out of the tissue are displayed in Fig. 5a. For the injury site analysis, the B20 group had significant or trending decreases in all parameters at both time points (Figs. 5b, 5d–g) except for the injury site rise time (Fig. 5C). Similar changes were observed in the tendon ROI analysis, but to a lesser extent (Fig. S4). The VEGF group did not have any changes in any parameter (Fig. 5, Fig. S4).
FIGURE 5.
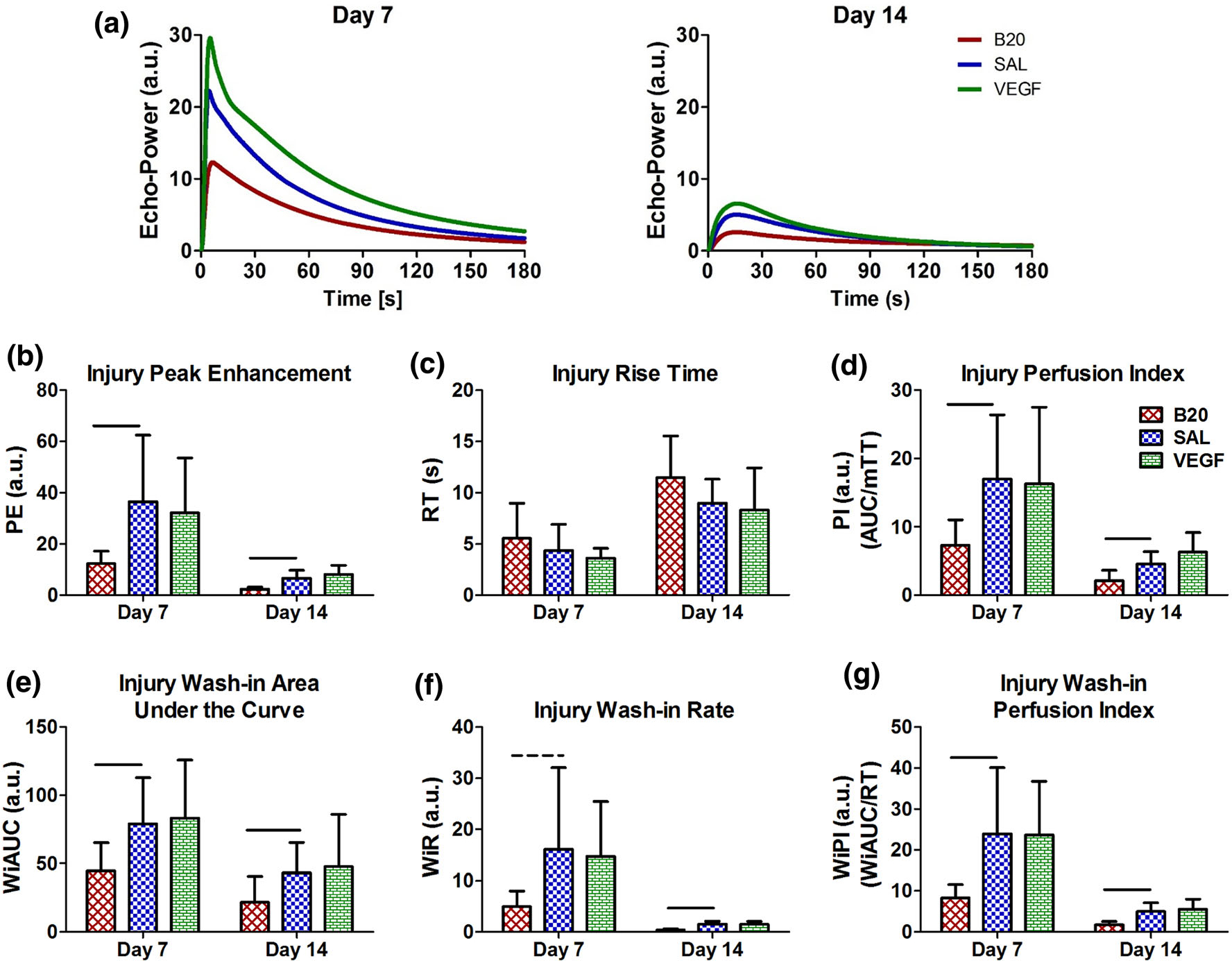
(a) Average echo-power vs. time curves for each treatment on days 7 and 14. Quantification of parameters within the injury site ROI including (b) peak enhancement (PE), (c) rise time (RT), (d) perfusion index (PI), (e) wash-in area under the curve (WiAUC), (f) wash-in rate (WiR), and (g) wash-in perfusion index (WiPI). The anti-VEGF antibody group (B20) was decreased in multiple parameters at multiple time points, where the vascular endothelial growth factor group (VEGF) caused no change compared to saline (SAL). There were no changes in rise time. Solid bars indicate p < 0.05 and dashed bars indicate p < 0.1.
B-Mode Ultrasound Alignment Analysis
There was no significant change in either circular standard deviation (CSD) or echogenicity in either group when evaluating the full tendon ROI (Figs. 6b, 6c). However, there was a significant decrease in CSD, indicating an increase in collagen alignment, on day 14 in the B20 group injury site (Fig. 6d). Additionally, the VEGF group had a trending increase in echogenicity on day 7 in the injury region (Fig. 6e).
FIGURE 6.
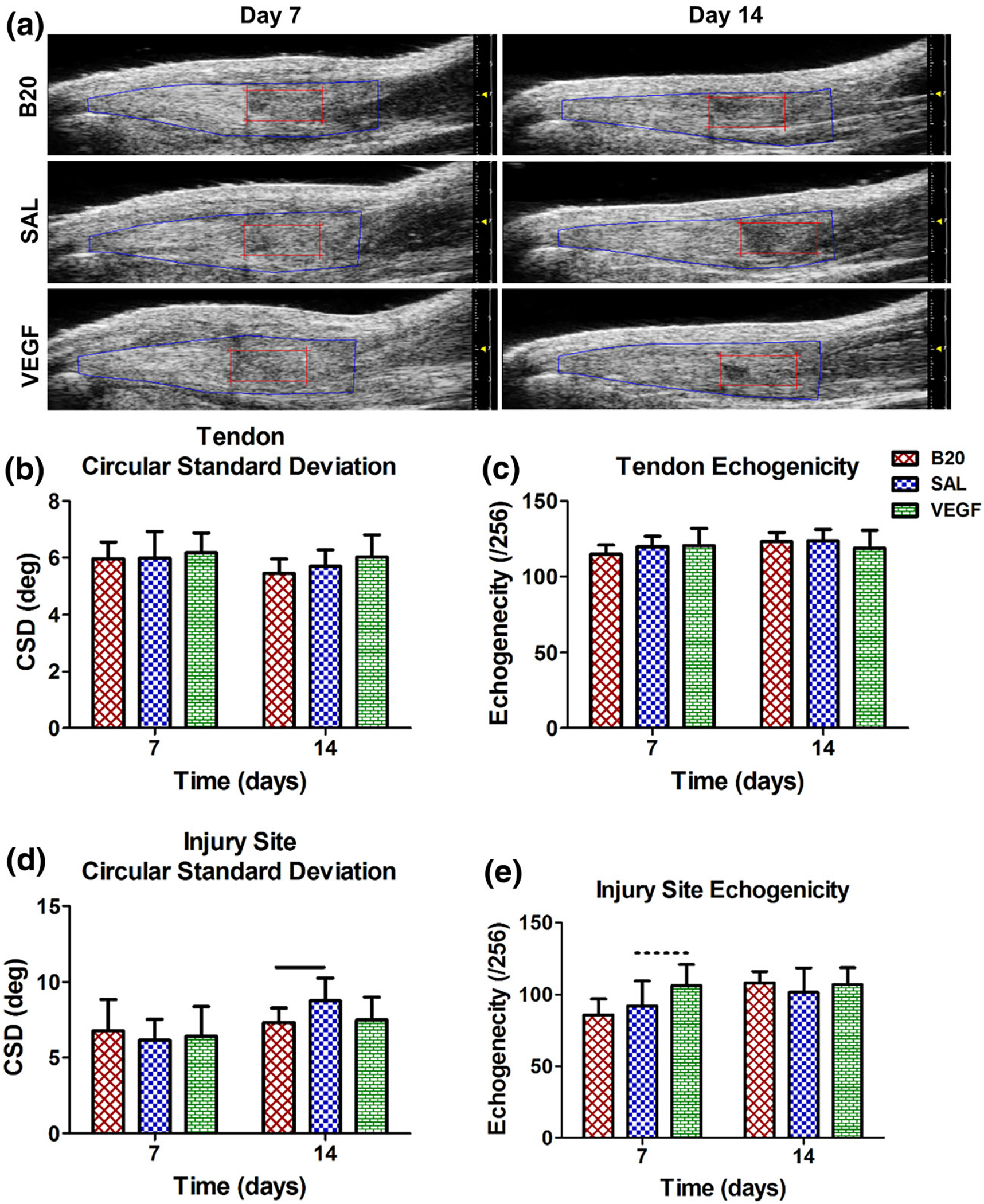
(a) Representative b-mode ultrasound images showing the full tendon (blue) and injury (red) ROIs. Quantification of (b, d) circular standard deviation (CSD) and (c, e) echogenicity in the (b, c) full tendon and (d, e) injury site ROIs. There were no significant changes in the full tendon ROI, but the anti-VEGF antibody group (B20) significantly decreased circular standard deviation, indicating increased collagen alignment, in the injury site. Solid bars indicate p < 0.05 and dashed bars indicate p < 0.1.
Histological Analysis
H&E histological analysis showed no changes in cell shape or cellularity in either group (Fig. S5a, b). CD34 immunohistochemical staining also showed no changes in vessel density or vessel size in either group (Fig. S5a, c).
Representative images for immunohistochemistry are shown in Fig. S6. Quantification of IHC staining showed that the delivery of B20 caused a significant increase in angiopoietin-1 (Fig. S6). No other stains showed significant differences between groups.
Mechanical Analysis
For the structural and geometric parameters, there was no change in full tendon or injury cross-sectional area, max displacement, or stiffness for either group (Fig. 7). However, the VEGF group had a significant decrease in percent relaxation (Fig. 7c) and a significant increase in max load (Fig. 7f). The B20 group also had a trending increase in max load (Fig. 7f). The material mechanical property analysis showed no change in the max stress or full tendon modulus (Figs. 7g, 7h), but there was a significant increase in injury site modulus in the VEGF group, and a trending increase in the B20 group (Fig. 7i). Additionally, the dynamic mechanical analysis showed no changes in tangent of delta, but a trending increase in dynamic modulus in the VEGF group at all 4 frequencies (Figs. 7j, 7k).
FIGURE 7.
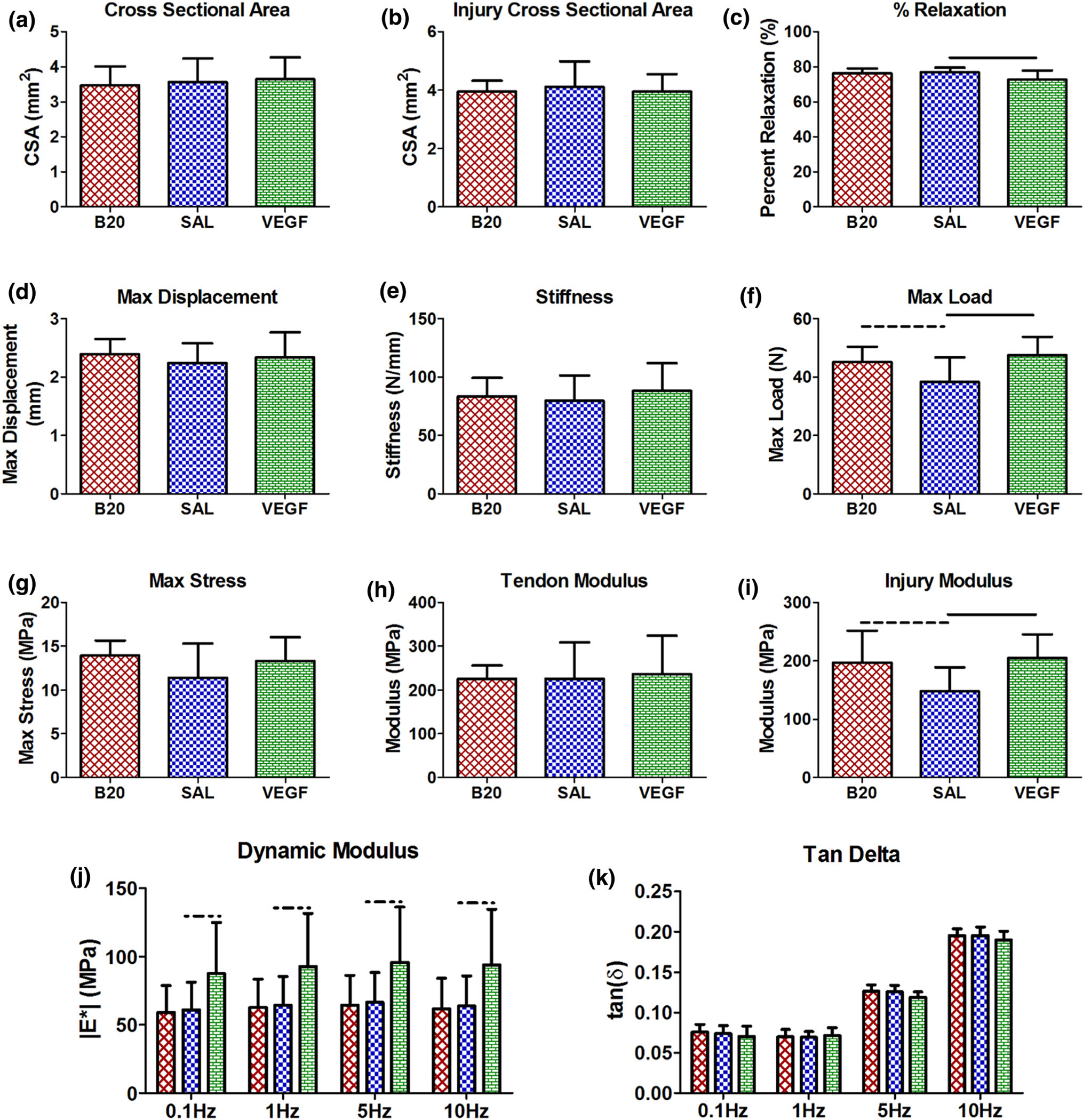
Mechanical properties. (a) Full tendon and (b) injury site cross-sectional area, (c) percent relaxation, (d) max displacement, (e) stiffness, and (f) max load, (g) max stress, (h) tendon and (i) injury site modulus, (j) dynamic modulus, and (k) tan delta. The vascular endothelial growth factor (VEGF) group percent relaxation was decreased and the max load and injury site modulus were increased compared to the saline control (SAL). Solid bars indicate p < 0.05 and dashed bars indicated p < 0.1.
DISCUSSION
This work demonstrated that while aging caused a significant reduction in vascular response to injury, the delivery of a pro-angiogenic factor, VEGF, can restore some of the lost angiogenic potential occurring with age and improve healing outcomes. Interestingly, while the delivery of anti-angiogenic factor, B20, reduced vascularity during healing, it also demonstrated improvements with healing, such as increased collagen organization.
In the first study, we evaluated the effect of aging on vascular response to injury. We demonstrated that aging causes significant decreases in both blood flow, as shown by the decreased mean color level and color weighted fractional area measures, as well as vascular density, as shown by the ultrasound fractional area and histological vessel density measures. This data aligns with previous human ultrasound studies,21,37 showing decreased blood flow in elderly patients in both the uninjured Achilles and supraspinatus tendons. This study supports the use of the aged rat as a model of naturally reduced vascular response following injury. As different rat strains were used for Study 1 and Study 2, direct comparisons cannot be made between the studies due to strain related differences including relative age and size. Different strains were used due to the availability of aged rats at the time of the two studies [aged in the lab vs. acquired through the National Institute of Aging (NIA)].
In the second study, we evaluated how the delivery of both pro- and anti-angiogenic factors altered vascular response and healing outcomes in aged animals. There were significant changes to the vascular response after injury in the aged animals, similar to the response seen in previous work in adult animals.32,34 However, there were larger changes with the VEGF delivery in the aged animals compared to the adult response (Fig. 8).32 We previously found that in adult animals, decreasing the vascularity following injury impaired healing, but increasing vascularity did not alter healing outcome.32 In the aged animals, increasing the vascular response following injury caused an improvement in mechanical properties. Additionally, decreasing the vascular response did not impair healing outcome, but surprisingly resulted in a trending improvement (Fig. 8). Since the aged animals naturally exhibit a reduced vascular response following injury, it is possible that stimulating an increase in angiogenesis had more effect than in adult animals, which already have a robust natural vascular response. The VEGF treatment could be counter-acting the inherent reduction of vascularity with aging, and therefore restoring some of the lost healing potential. Since the improved mechanical function with VEGF treatment did not coincide with measurable improvements in collagen organization, we speculate that other factors such as collagen content or changes in composition of other extracellular matrix components could be contributing to the improved mechanical outcome. Additionally, we find that with VEGF delivery in particular, color Doppler and photoacoustic vascular measures are as good, if not better, at detecting differences in vascular parameters as contrast-enhanced ultrasound. This is surprising given that contrast-enhanced ultrasound can detect perfusion of much smaller vessels. However, it suggests that changes in the density of capillaries may not be as impactful, and larger vessels may be undergoing structural changes that induce increased blood flow and oxygenation, which are more easily detected using these lower resolution ultrasound imaging techniques. This is promising given that color Doppler imaging is already used clinically in the evaluation of tendinopathy.
FIGURE 8.
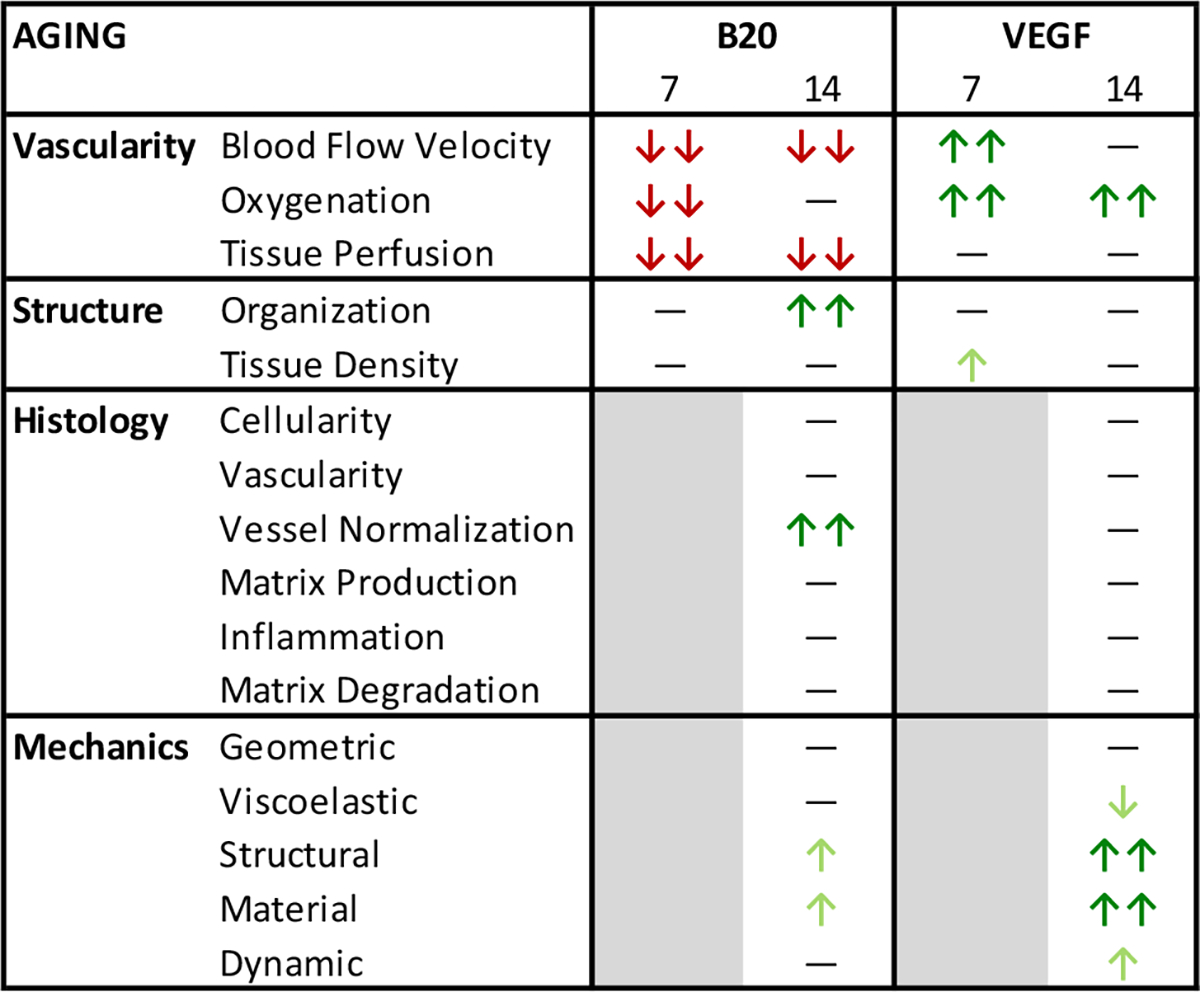
Overview of results for Study 2: The effect of vascular modulation on aged Achilles tendons. The findings for each analysis category are displayed with double arrows for a large (overall significant) change, and single arrows for a small (overall trending) change. Arrows are pointing up for increasing values and down for decreasing values. Arrows are colored green to indicate either increased vascularity or improved properties, or colored red to indicate either decreased vascularity or worsened properties. While both B20 and VEGF induced significant decreases and increases in vascular properties after injury, respectively, only the VEGF group caused significant changes in mechanical properties, demonstrating improvements in tendon healing.
Similarly, since the aged animals already have a reduced vascular and healing response, a further reduction in vascularity does not cause a significant change in healing outcome as observed previously in the adult animals. Additionally, in both the adult and aged animals the reduction in vascularity through the delivery of B20 causes the tendons to have improved collagen alignment properties. While the reduction in vascularity may hinder healing, the resulting improvements in collagen organization could explain the improved mechanics observed 4 weeks following injury in the adults and the improvement seen at 2 weeks in the aged animals. This is consistent with previous work demonstrating Bevacizumab improves Achilles tendon repair in a rat model associated with reduced cross-sectional area and matrix organization.41 Finally, histologically there was a significant increase in Angiopoietin 1 (Ang1) in the B20 group. While VEGF and Angiopoietin 2 have roles in vessel destabilization, sprouting, and growth, the role of Ang1 is to initiate vascular stabilization and endothelial protection.6,10 It is possible that with lowered VEGF signaling in a model with already reduced vascular response with aging, increased Ang1 is normalizing the vessels and allowing them to be more efficient than the leaky vasculature normally formed at the start of angiogenesis. This could be a mechanism for the trending improvements in mechanical properties in this group as well.
Aging has been shown to have significant impacts on tendon compositional, mechanical, and vascular properties. Aging causes reduced collagen and elastin production, as well as increased MMP and decreased TIMP activity.18,38,47 Given these compositional changes, it is no surprise that aged tendons exhibit inferior mechanical properties.20,30,43 Additionally, clinical studies have reported alterations in vascularity with aging, including reduced blood flow in and around tendons,13,21 as well as a reduced vascular endothelial cell population, decreased cell expansion, and diminished angiogenic-lineage differentiation potential in ligament-based cell populations from aged patients.44 This data supports the findings from our first study, where aging decreased vessel density and blood flow velocity. However, there is no previous data reporting changes to tendon vascularity following tendon injury, so this is the first study to report this finding in an Achilles tendon injury model.
While there is limited data regarding tendon vascularity with aging, it is well established that there are aging-related impairments in angiogenesis studied in other tissues. An arterial injury model demonstrated that aging impaired the ability for re-endothelialization.15 Additionally, vascular ingrowth models show decreased endothelial cell proliferation and function with aging.36,45 There are numerous accounts of a decline in production of pro-angiogenic growth factors and cytokines, including VEGF, basic fibroblast growth factor (bFGF), and transforming growth factor beta 1 (TGF-β1), with the induction of angiogenesis in aged tissues.2,15,35,36,38,45 Specifically, there is a decrease in VEGF upregulation in response to hypoxia35 as well as a reduction in hypoxia induced factor-1α (HIF-1α) DNA binding and downregulation of importin-α expression, both of which help regulate VEGF transcription.2 Additionally, thrombospondin-2 (TSP-2), an angiogenic inhibitor, increases with aging.38 These alterations in angiogenic factors caused a reduction in angiogenic invasion and capillary density in aged tissues.2,36,38,45 This phenomenon was not only observed in these other tissue vascular models, but was also observed in our tendon studies as a clear reduction in vascularity in our aged model.
The effect of angiogenic factor delivery on vascular response and healing outcome has been described in multiple injury models, as well as our previous work in adult animals.32 In particular, VEGF delivery increased vascular response after injury and caused improvements in healing potential in some studies.5,7,8,16,17,19,40,48 However, there is very little research related to aiding tissue healing in aged specimens through the delivery of angiogenic factors. In this work, the delivery of VEGF not only increased angiogenic response to injury, but also improved the healing outcome in the aged Achilles tendon. While there is no previous work assessing angiogenic therapies in aging tendon models, rescuing an impaired angiogenic response through the delivery of VEGF in other aged tissues has been evaluated. The local delivery of adenoviral VEGF to injured iliac arteries in aged rabbits significantly improved re-endothelialization of the damaged arteries.15 Additionally, VEGF delivery following femoral artery resection in aged rats improved blood pressure ratio, angiographic score, and capillary density.36 Finally, VEGF 121 gene transfer enhanced the blood vessel, fibrovascular tissue ingrowth, and endothelial cell proliferation in a subcutaneous vascular ingrowth model in aged rats.45 Interestingly, this same study also found that their VEGF treatment failed to further stimulate the already more robust angiogenesis in young animals.45 This body of work demonstrates the ability to rescue the impaired angiogenic response in aged tissue, and supports the improved healing outcomes that we observed in our studies. Additionally, it supports that improvements to angiogenic response are frequently only possible in models where there is a natural vascular impairment, such as with aging, but not in models of robust vascular response, such as with adult or young populations.
This study is not without limitations. First, histological and mechanical properties were only evaluated at day 14. This was primarily due to the limited availability of aged animals and so this time point was chosen based on when the most significant mechanical changes were observed in previous adult studies. However, it would be beneficial to have histological samples at earlier time points to evaluate early extracellular matrix and inflammatory changes. We also only evaluated one histological blood vessel marker, CD34. While future work could investigate other vascular markers to provide additional information about the forming vasculature, in this study CD34 was shown to be a consistent marker of vascular endothelial cells in the tendon for the purpose of semi-quantitative evaluation of vascular density and size. Additionally, future work could evaluate mechanical properties at later time points to see if improvements due to VEGF or B20 administration is transient or persists over time. In this evaluation, it would be beneficial to compare to uninjured tendon to understand how significant the change in mechanical outcome is relative to healthy tendon. Another limitation is that this injury model does not have a direct clinical parallel since it is a central partial rupture. Also, tendon ruptures in the older population are often preceded by tendinopathy. While studying vascularity in tendinopathy would be extremely valuable, especially in the aged population, there are unfortunately no reliable and repeatable rat models of chronic Achilles tendinopathy. The use of the Achilles tendon provided the ability to develop and utilize the ultrasound imaging techniques to study vascularity. Additionally, this controlled injury model does not require surgical repair of the tendon or immobilization of the limb, which is also important for the use of ultrasound imaging. Therefore, while acute rupture is not as common as degeneration prior to rupture in the aged population, aging is still a relevant and interesting model of naturally reduced vascularity that can help us to better understand the role of vascular modulation in tendon healing.
In this work, aging caused a significant reduction in vascular response to injury. This is most likely due to the impaired capacity for angiogenesis and reduced VEGF expression in aged tissues, as described in previous work. This reduction in angiogenic potential could alter cellular responses and healing capacity after injury and could help explain the reduced tendon healing potential of the aged population. While VEGF treatment did not cause any significant improvements in healing in our previous work in adult animals where there is a relatively normal vascular response, it did cause an improvement in healing outcome in this aged population study. Additionally, while anti-angiogenic treatments decreased healing properties in adult animals, it caused trending improvements in the aged animals. This demonstrates that an aged population not only has inherently different vascular properties, but also responds differently to vascular modulation after injury. Additionally, the delivery of VEGF can restore some of the lost angiogenic potential occurring with age. While angiogenesis in aging tissues has been studied before, to our knowledge there have been no studies demonstrating a recovery of vascularity and consequently an improvement in healing in an aged tendon injury model. This study was also the first to relate changes in vascular response in an aged model using in vivo measures of blood perfusion to alterations in healing properties.
Supplementary Material
ACKNOWLEDGMENTS
This study was funded by an NIH/NIAMS (Grant No. P30AR069619) supported Penn Center for Musculoskeletal Disorders Imaging Seed Grant, an NIH T32 Rheumatology Training Grant (Grant No. 4T32AR007442–29) and an NSF Graduate Research Fellowship. The aged Fischer 344 rats were provided by the NIH National Institute on Aging. There are no other conflicts of interest.
Footnotes
SUPPLEMENTARY INFORMATION
The online version contains supplementary material available at https://doi.org/10.1007/s10439-022-02948-7.
REFERENCES
- 1.Ackerman JE, Bah I, Jonason JH, Buckley MR, and Loiselle AE Aging does not alter tendon mechanical properties during homeostasis, but does impair flexor tendon healing. J. Orthop. Res. 35(12):2716–2724, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahluwalia A, Narula J, Jones MK, Deng X, and Tarnawski AS Impaired angiogenesis in aging myocardial microvascular endothelial cells is associated with reduced importin alpha and decreased nuclear transport of HIF1 alpha: mechanistic implications. J. Physiol. Pharmacol. 61:133–139, 2010. [PubMed] [Google Scholar]
- 3.Arnesen SM, and Lawson MA Age-related changes in focal adhesions lead to altered cell behavior in tendon fibroblasts. Mech. Ageing Dev. 127:726–732, 2006. [DOI] [PubMed] [Google Scholar]
- 4.Barros LF, and Belfort R Jr. The effects of the subconjunctival injection of bevacizumab (Avastin) on angiogenesis in the rat cornea. Anais da Academia Brasileira de Ciencias. 79:389–394, 2007. [DOI] [PubMed] [Google Scholar]
- 5.Basu G, Downey H, Guo S, Israel A, Asmar A, Hargrave B, and Heller R Prevention of distal flap necrosis in a rat random skin flap model by gene electro transfer delivering VEGF(165) plasmid. J. Gene Med. 16:55–65, 2014. [DOI] [PubMed] [Google Scholar]
- 6.Bouis D, Kusumanto Y, Meijer C, Mulder NH, and Hospers GA A review on pro- and anti-angiogenic factors as targets of clinical intervention. Pharmacol. Res. 53:89–103, 2006. [DOI] [PubMed] [Google Scholar]
- 7.Chereddy KK, Lopes A, Koussoroplis S, Payen V, Moia C, Zhu H, Sonveaux P, Carmeliet P, Des Rieux A, Vandermeulen G, and Preat V Combined effects of PLGA and vascular endothelial growth factor promote the healing of non-diabetic and diabetic wounds. Nanomedicine. 11:1975–1984, 2015. [DOI] [PubMed] [Google Scholar]
- 8.Corral CJ, Siddiqui A, Wu L, Farrell CL, Lyons D, and Mustoe TA Vascular endothelial growth factor is more important than basic fibroblastic growth factor during ischemic wound healing. Arch. Surg. 134:200–205, 1999. [DOI] [PubMed] [Google Scholar]
- 9.Emami MJ, Jaberi FM, Azarpira N, Vosoughi AR, and Tanideh N Prevention of arthrofibrosis by monoclonal antibody against vascular endothelial growth factor: a novel use of bevacizumab in rabbits. Orthop. Traumatol. Surg. Res. 98:759–764, 2012. [DOI] [PubMed] [Google Scholar]
- 10.Falcon BL, Hashizume H, Koumoutsakos P, Chou J, Bready JV, Coxon A, Oliner JD, and McDonald DM Contrasting actions of selective inhibitors of angiopoietin-1 and angiopoietin-2 on the normalization of tumor blood vessels. Am. J. Pathol. 175:2159–2170, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fevata M Scarless Healing in the Fetus: Implications and Strategies for Postnatal Tendon Repair. Philadelphia: University of Pennsylvania, 2006. [Google Scholar]
- 12.Frontera WR Physiologic changes of the musculoskeletal system with aging: a brief review. Phys. Med. Rehabil. Clin. N. Am. 28:705–711, 2017. [DOI] [PubMed] [Google Scholar]
- 13.Funakoshi T, Iwasaki N, Kamishima T, Nishida M, Ito Y, Kondo M, and Minami A In vivo visualization of vascular patterns of rotator cuff tears using contrast-enhanced ultrasound. Am. J. Sports Med. 38:2464–2471, 2010. [DOI] [PubMed] [Google Scholar]
- 14.Garcia T, Hornof WJ, and Insana MF On the ultrasonic properties of tendon. Ultrasound Med. Biol. 29:1787–1797, 2003. [DOI] [PubMed] [Google Scholar]
- 15.Gennaro G, Menard C, Michaud SE, and Rivard A Age-dependent impairment of reendothelialization after arterial injury: role of vascular endothelial growth factor. Circulation. 107:230–233, 2003. [DOI] [PubMed] [Google Scholar]
- 16.Ju YJ, Tohyama H, Kondo E, Yoshikawa T, Muneta T, Shinomiya K, and Yasuda K Effects of local administration of vascular endothelial growth factor on properties of the in situ frozen-thawed anterior cruciate ligament in rabbits. Am. J. Sports Med. 34:84–91, 2006. [DOI] [PubMed] [Google Scholar]
- 17.Kaux JF, Janssen L, Drion P, Nusgens B, Libertiaux V, Pascon F, Heyeres A, Hoffmann A, Lambert C, Le Goff C, Denoel V, Defraigne JO, Rickert M, Crielaard JM, and Colige A Vascular endothelial growth factor-111 (VEGF-111) and tendon healing: preliminary results in a rat model of tendon injury. Muscles Ligaments Tendons J. 4:24–28, 2014. [PMC free article] [PubMed] [Google Scholar]
- 18.Kostrominova TY, and Brooks SV Age-related changes in structure and extracellular matrix protein expression levels in rat tendons. Age (Dordr). 35:2203–2214, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kryger Z, Zhang F, Dogan T, Cheng C, Lineaweaver WC, and Buncke HJ The effects of VEGF on survival of a random flap in the rat: examination of various routes of administration. Br. J. Plast. Surg. 53:234–239, 2000. [DOI] [PubMed] [Google Scholar]
- 20.LaCroix AS, Duenwald-Kuehl SE, Brickson S, Akins TL, Diffee G, Aiken J, Vanderby R Jr., and Lakes RS Effect of age and exercise on the viscoelastic properties of rat tail tendon. Ann. Biomed. Eng. 41:1120–1128, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Langberg H, Olesen J, Skovgaard D, and Kjaer M Age related blood flow around the Achilles tendon during exercise in humans. Eur. J. Appl. Physiol. 84:246–248, 2001. [DOI] [PubMed] [Google Scholar]
- 22.Manzano RP, Peyman GA, Khan P, and Kivilcim M Testing intravitreal toxicity of bevacizumab (Avastin). Retina. 26:257–261, 2006. [DOI] [PubMed] [Google Scholar]
- 23.Marqueti RC, Durigan JLQ, Oliveira AJS, Mekaro MS, Guzzoni V, Aro AA, Pimentel ER, and Selistre-de-Araujo HS Effects of aging and resistance training in rat tendon remodeling. FASEB J. 32(1):353–368, 2017. [DOI] [PubMed] [Google Scholar]
- 24.Marquez-Arabia WH, Gomez-Hoyos J, Gomez M, Florez I, Gallo JA, Monsalve F, Arias LF, and Martin HD Influence of aging on microvascular supply of the gluteus medius tendon: a cadaveric and histologic study. Arthroscopy. 33:1354–1360, 2017. [DOI] [PubMed] [Google Scholar]
- 25.McCarthy MM, and Hannafin JA The mature athlete: aging tendon and ligament. Sports Health. 6:41–48, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Needles A, Arditi M, Rognin NG, Mehi J, Coulthard T, Bilan-Tracey C, Gaud E, Frinking P, Hirson D, and Foster FS Nonlinear contrast imaging with an array-based micro-ultrasound system. Ultrasound Med. Biol. 36:2097–2106, 2010. [DOI] [PubMed] [Google Scholar]
- 27.Needles A, Heinmiller A, Sun J, Theodoropoulos C, Bates D, Hirson D, Yin M, and Foster FS Development and initial application of a fully integrated photoacoustic micro-ultrasound system. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 60:888–897, 2013. [DOI] [PubMed] [Google Scholar]
- 28.Okada Y, Akisue T, Hara H, Kishimoto K, Kawamoto T, Imabori M, Kishimoto S, Fukase N, Onishi Y, and Kurosaka M The effect of bevacizumab on tumour growth of malignant fibrous histiocytoma in an animal model. Anticancer Res. 30:3391–3395, 2010. [PubMed] [Google Scholar]
- 29.Plate JF, Brown PJ, Walters J, Clark JA, Smith TL, Freehill MT, Tuohy CJ, Stitzel JD, and Mannava S Advanced age diminishes tendon-to-bone healing in a rat model of rotator cuff repair. Am. J. Sports Med. 42:859–868, 2014. [DOI] [PubMed] [Google Scholar]
- 30.Plate JF, Wiggins WF, Haubruck P, Scott AT, Smith TL, Saul KR, and Mannava S Normal aging alters in vivo passive biomechanical response of the rat gastrocnemius-Achilles muscle-tendon unit. J. Biomech. 46:450–455, 2013. [DOI] [PubMed] [Google Scholar]
- 31.Riggin CN, Chen M, Gordon JA, Schultz SM, Soslowsky LJ, and Khoury V Ultrasound-guided dry needling of the healthy rat supraspinatus tendon elicits early healing without causing permanent damage. J. Orthop. Res. 37:2035–2042, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Riggin CN, Rodriguez AB, Weiss SN, Raja HA, Chen M, Schultz SM, Sehgal CM, and Soslowsky LJ Modulation of vascular response after injury in the rat Achilles tendon alters healing capacity. J. Orthop. Res. 39:2000–2016, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Riggin CN, Sarver JJ, Freedman BR, Thomas SJ, and Soslowsky LJ Analysis of collagen organization in mouse achilles tendon using high-frequency ultrasound imaging. J. Biomech. Eng. 136:021029, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Riggin CN, Schultz SM, Sehgal CM, and Soslowsky LJ Ultrasound evaluation of anti-vascular endothelial growth factor-induced changes in vascular response following tendon injury. Ultrasound Med. Biol. 45:1841–1849, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rivard A, Berthou-Soulie L, Principe N, Kearney M, Curry C, Branellec D, Semenza GL, and Isner JM Age-dependent defect in vascular endothelial growth factor expression is associated with reduced hypoxia-inducible factor 1 activity. J. Biol. Chem. 275:29643–29647, 2000. [DOI] [PubMed] [Google Scholar]
- 36.Rivard A, Fabre JE, Silver M, Chen D, Murohara T, Kearney M, Magner M, Asahara T, and Isner JM Age-dependent impairment of angiogenesis. Circulation. 99:111–120, 1999. [DOI] [PubMed] [Google Scholar]
- 37.Rudzki JR, Adler RS, Warren RF, Kadrmas WR, Verma N, Pearle AD, Lyman S, and Fealy S Contrast-enhanced ultrasound characterization of the vascularity of the rotator cuff tendon: age- and activity-related changes in the intact asymptomatic rotator cuff. J. Shoulder Elbow Surg. 17:96S–100S, 2008. [DOI] [PubMed] [Google Scholar]
- 38.Sadoun E, and Reed MJ Impaired angiogenesis in aging is associated with alterations in vessel density, matrix composition, inflammatory response, and growth factor expression. J. Histochem. Cytochem. 51:1119–1130, 2003. [DOI] [PubMed] [Google Scholar]
- 39.Sehgal CM, Arger PH, Silver AC, Patton JA, Saunders HM, Bhattacharyya A, and Bell CP Renal blood flow changes induced with endothelin-1 and fenoldopam mesylate at quantitative Doppler US: initial results in a canine study. Radiology. 219:419–426, 2001. [DOI] [PubMed] [Google Scholar]
- 40.Takayama K, Kawakami Y, Mifune Y, Matsumoto T, Tang Y, Cummins JH, Greco N, Kuroda R, Kurosaka M, Wang B, Fu FH, and Huard J The effect of blocking angiogenesis on anterior cruciate ligament healing following stem cell transplantation. Biomaterials. 60:9–19, 2015. [DOI] [PubMed] [Google Scholar]
- 41.Tempfer H, Kaser-Eichberger A, Lehner C, Gehwolf R, Korntner S, Kunkel N, Wagner A, Gruetz M, Heindl LM, Schroedl F, and Traweger A Bevacizumab improves achilles tendon repair in a rat model. Cell Physiol. Biochem. 46:1148–1158, 2018. [DOI] [PubMed] [Google Scholar]
- 42.Torricelli P, Veronesi F, Pagani S, Maffulli N, Masiero S, Frizziero A, and Fini M In vitro tenocyte metabolism in aging and oestrogen deficiency. Age (Dordr). 35:2125–2136, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Turan A, Teber MA, Yakut ZI, Unlu HA, and Hekimoglu B Sonoelastographic assessment of the age-related changes of the Achilles tendon. Med. Ultrason. 17:58–61, 2015. [DOI] [PubMed] [Google Scholar]
- 44.Uefuji A, Matsumoto T, Matsushita T, Ueha T, Zhang S, Kurosaka M, and Kuroda R Age-related differences in anterior cruciate ligament remnant vascular-derived cells. Am. J. Sports Med. 42:1478–1486, 2014. [DOI] [PubMed] [Google Scholar]
- 45.Wang H, Keiser JA, Olszewski B, Rosebury W, Robertson A, Kovesdi I, and Gordon D Delayed angiogenesis in aging rats and therapeutic effect of adenoviral gene transfer of VEGF. Int. J. Mol. Med. 13:581–587, 2004. [PubMed] [Google Scholar]
- 46.World Health Organization. World Report on Ageing and Health. Geneva: World Health Organization, 2015. [Google Scholar]
- 47.Yu TY, Pang JH, Wu KP, Chen MJ, Chen CH, and Tsai WC Aging is associated with increased activities of matrix metalloproteinase-2 and -9 in tenocytes. BMC Musculoskelet. Disord 14:2, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang F, Liu H, Stile F, Lei MP, Pang Y, Oswald TM, Beck J, Dorsett-Martin W, and Lineaweaver WC Effect of vascular endothelial growth factor on rat Achilles tendon healing. Plast. Reconstr. Surg. 112:1613–1619, 2003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


