Abstract
Objectives
We reviewed 594 consecutive patients with Coronavirus Disease 2019 supported with extracorporeal membrane oxygenation at 49 hospitals within 21 states and examined patient characteristics, treatments, and variation in outcomes over the course of the pandemic.
Methods
A multi-institutional database was used to assess all patients with Coronavirus Disease 2019 cannulated for extracorporeal membrane oxygenation between March 17, 2020, and December 20, 2021, inclusive, and separated from ECMO on or prior to January 14, 2022. Descriptive analysis was stratified by 4 time categories: group A = March 2020 to June 2020, group B = July 2020 to December 2020, group C = January 2021 to June 2021, group D = July 2021 to December 2021. A Bayesian mixed-effects logistic regression was used to assess continuous trends in survival where time was operationalized as the number of days between each patient's cannulation and that of the first patient in March 2020, controlling for multiple variables and risk factors.
Results
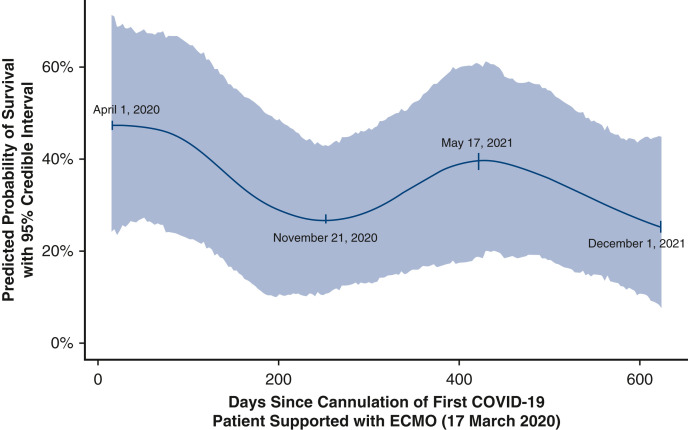
At hospital discharge, of 594 patients, 221 survived (37.2%) and 373 died. Throughout the study, median age [interquartile range] declined (group A = 51.0 [41.0-60.0] years, group D = 39.0 [32.0-48.0] years, P < .001); median days between Coronavirus Disease 2019 diagnosis and intubation increased (group A = 4.0 [1.0-8.5], group D = 9.0 [5.0-14.5], P < .001); and use of medications (glucocorticoids, interleukin-6 blockers, antivirals, antimalarials) and convalescent plasma fluctuated significantly (all P < .05). Estimated odds of survival varied over the study period with a decline between April 1, 2020, and November 21, 2020 (odds ratio, 0.39, 95% credible interval, 0.18-0.87, probability of reduction in survival = 95.7%), improvement between November 21, 2020, and May 17, 2021 (odds ratio, 1.85, 95% credible interval, 0.86-4.09, probability of improvement = 93.4%), and decline between May 17, 2021, and December 1, 2021 (odds ratio, 0.49, 95% credible interval, 0.19-1.44, probability of decrease = 92.1%).
Conclusions
Survival for patients with Coronavirus Disease 2019 supported with extracorporeal membrane oxygenation has fluctuated during the stages of the pandemic. Minimizing variability by adherence to best practices may refine the optimal use of extracorporeal membrane oxygenation in a pandemic response.
Key Words: acute respiratory distress syndrome, coronavirus, COVID-19, extracorporeal membrane oxygenation, pulmonary failure
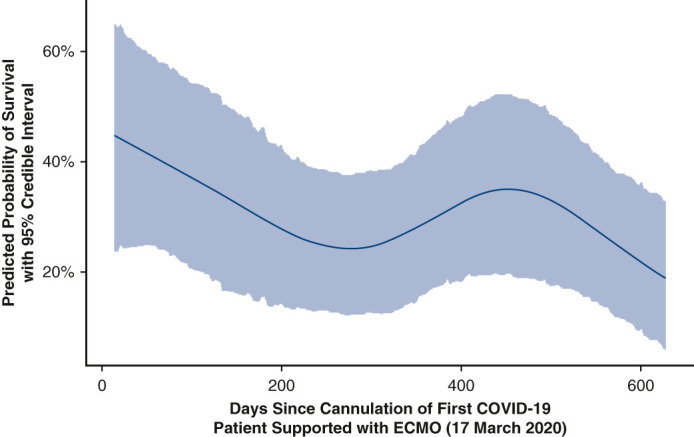
Estimated effect of time since pandemic start on survival, controlling for multiple factors.
Central Message.
Strategies of management and survival for patients with COVID-19 supported with ECMO have fluctuated over the course of the pandemic.
Perspective.
The evolution of management and outcomes of COVID-19 holds valuable lessons for the role of ECMO in a pandemic response. We reviewed our experience in 594 consecutive patients with COVID-19 who were supported with and separated from ECMO (cannulated between March 17, 2020, to December 20, 2021, inclusive, at 49 hospitals in 21 states) and examined variation in patient characteristics, treatment strategies, and outcomes over the course of the pandemic.
As of February 11, 2022, 406,809,841 patients around the world have been diagnosed with Coronavirus Disease 2019 (COVID-19), with 5,793,530 associated deaths (1.42% mortality worldwide).1 In the United States, as of February 11, 2022, 77,439,456 patients have been diagnosed with COVID-19, with 915,651 associated deaths (1.18% mortality in the United States).1 Most deaths in patients with COVID-19 are due to respiratory failure, with a small group dying of combined pulmonary and cardiac failure.2 , 3
The role of extracorporeal membrane oxygenation (ECMO) in the management of severely ill patients with COVID-19 continues to be defined.4, 5, 6, 7 We previously published analyses of our initial 32, 100, 200, and then 505 patients with COVID-19 with severe pulmonary compromise supported with ECMO.8, 9, 10, 11 These prior analyses documented the evolution of the use of ECMO to support patients with COVID-19 and supported the concept that “ECMO facilitates survival of select critically ill patients with COVID-19.”8, 9, 10, 11 Although substantial variation exists in drug treatment of COVID-19, ECMO offers a reasonable rescue strategy.9, 10, 11
Several analyses have described cohorts of patients with COVID-19 supported with ECMO.8, 9, 10, 11, 12, 13, 14, 15, 16, 17 Over the course of the pandemic, as knowledge evolved, multiple changes have occurred in both the strategies for management and the outcomes of treatment. Valuable lessons can be learned from this evolution of treatment and outcomes associated with COVID-19. The purpose of this study was (1) to review our clinical experience in 594 consecutive patients with COVID-19 who were cannulated for ECMO between March 17, 2020, and December 20, 2021, inclusive, and separated from ECMO on or prior to January 14, 2022, at 49 hospitals within 21 states, and (2) to examine the characteristics of patients, strategies of treatment, and variation in outcomes over the course of the pandemic.
Material and Methods
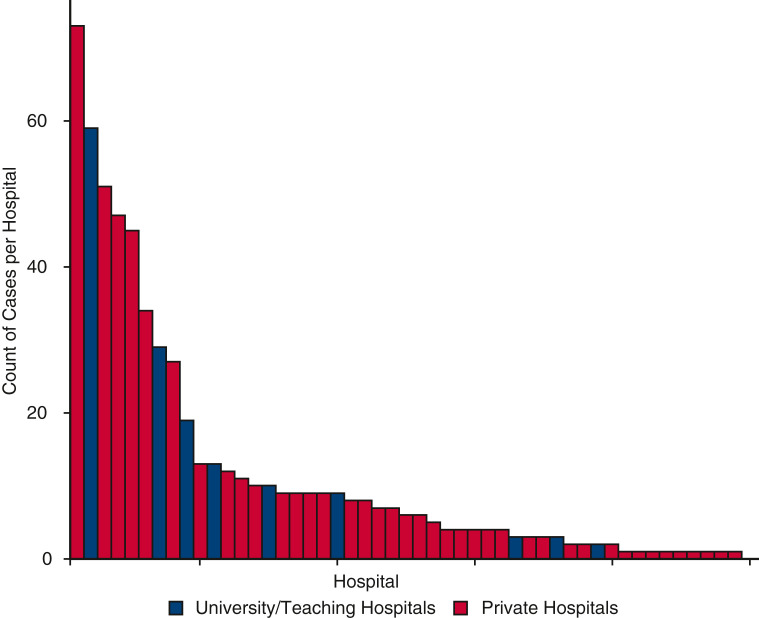
A multi-institutional database was used to assess all patients with COVID-19 who were supported with ECMO at 49 hospitals located in 21 states in the United States. This database is prospectively maintained on all patients supported with ECMO and has been used for data collection and analysis for quality improvement. This database is a component of the SpecialtyCare Operative Procedural rEgistry (SCOPE) (https://specialtycareus.com/). SpecialtyCare is a US provider of Allied Health services, and SCOPE contains data from more than 1 million perfusion procedures in more than 40 states at more than 300 hospitals. Although SCOPE contains data from more than 300 hospitals, only 49 of these hospitals cared for patients eligible for this study. This article describes the ECMO experience at these 49 hospitals that have supported patients with COVID-19 with ECMO. Of the 49 hospitals enrolling patients in this study, 40 were private hospitals and 9 were university/teaching hospitals. The mean number of COVID-19 ECMO cases at each of the 49 hospitals was 12.1 (median, 6, range, 1-73, interquartile range [IQR], 2-11).
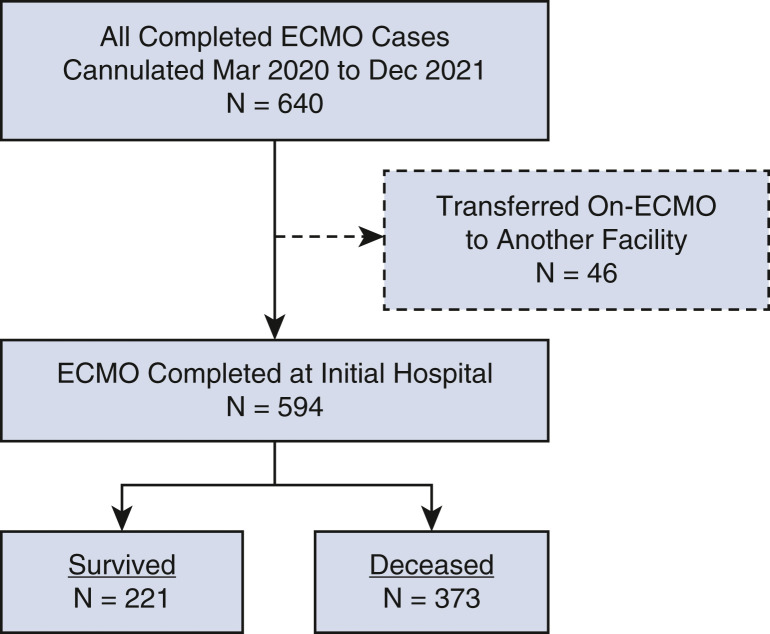
This analysis includes 594 consecutive patients aged more than 18 years at the time of cannulation for ECMO with confirmed COVID-19 who were both (1) cannulated for ECMO between March 17, 2020 (when our first patient with COVID-19 was placed on ECMO) and December 20, 2021 (when our last patient in this series was cannulated), inclusive, and (2) separated from ECMO on or prior to January 14, 2022. Patients with COVID-19 who were cannulated for ECMO and remained on ECMO support on January 15, 2022, were not included in this study. Forty-six patients who were cannulated but transferred to other hospitals on ECMO were not included in this analysis (Figure 1 ). Data analyzed included patient characteristics, pre-COVID-19 risk factors and comorbidities, confirmation of COVID-19 diagnosis, features of ECMO support, specific medications used in an attempt to treat COVID-19, and short-term outcomes through hospital discharge.
Figure 1.
CONSORT flow diagram depicting the distribution of all 640 patients by category of outcome. ECMO, Extracorporeal membrane oxygenation.
Criteria for placement on ECMO were determined by the individual patient care team(s) at each of the contributing 49 hospitals; all patients who were placed on ECMO had the diagnosis of COVID-19 with severe respiratory failure deemed to be refractory to conventional management. The decision to initiate ECMO, the mode of therapy (ie, venovenous, venoarterial), and the cannulation strategy were each determined by the individual ECMO teams, in keeping with their respective individual institutional protocols and guidelines. This analysis includes all patients with COVID-19 placed on ECMO at the 49 hospitals participating in this study during the period of this analysis. None of these 594 patients were placed on ECMO during cardiopulmonary resuscitation. Extracorporeal cardiopulmonary resuscitation was not used for patients with COVID-19 at these 49 hospitals.
Statistics and Institutional Review Board Approval
Descriptive data tabulations were performed by grouping cases into 4 time categories representing the first and second halves of 2020 and 2021:
-
•
Group A = March 2020 to June 2020
-
•
Group B = July 2020 to December 2020
-
•
Group C = January 2021 to June 2021
-
•
Group D = July 2021 to December 2021
Descriptive summaries of the data were tabulated according to time category using mean and standard deviation with Welch's analysis of variance and/or median and IQR with Kruskal–Wallis rank sum test for continuous variables, and count and percent with chi-square test for categorical variables. The primary outcome of interest was survival during the index hospitalization.
Inferential analysis centered on a Bayesian mixed-effects logistic regression model (with relatively uninformative normal priors for beta parameters [beta standard deviation = 100]). To assess possible trends in survival, time was operationalized as the number of days between each patient's cannulation and that of the first patient in March 2020. Controls included multiple variables and potential risk factors: age, body mass index (BMI), gender, the presence or absence of one or more pre-ECMO comorbidities (ie, obesity, asthma, hypertension, heart disease, diabetes, chronic renal failure, cancer), days from diagnosis of COVID-19 to intubation, days from intubation to cannulation for ECMO, prone positioning, and whether or not a circuit change-out was required. A random intercept term was included to account for variation in survival by hospital.
Data were missing for less than 10% of cases for all modeled variables except for BMI (22% missing), days between diagnosis and intubation (19% missing), and days from intubation to cannulation (17% missing). To limit bias introduced by missing data, regression analysis was performed on 25 multiply-imputed data sets generated using the chained equations method, as implemented by Harrell and colleagues.18 Results reported are the blended outcomes of these 25 regressions by “stacking” of the posterior distributions of each into a single posterior distribution. All analyses were conducted using the R statistical computing environment (version 4.0.3)19 using the “Hmisc,” “compareGroups,” and “rmsb” packages.18 , 20 , 21
Regression results are reported using contrasts between the 75th percentile and 25th percentile for continuous variables such as age, BMI, days between diagnosis and intubation, and days between intubation and cannulation. Several contrasts are reported to demonstrate the magnitude of changes in survival between key inflection points in the estimated time trend; these have been translated from their original form (days between March 17, 2020, and cannulation date) into their corresponding calendar dates to aid in interpretation.
Institutional Review Board approval and waiver of the need for consent were obtained. The human subjects research protocol for this study was reviewed and approved by an independent Institutional Review Board. Institutional ethics review board approval was obtained for the use of data from the SCOPE Registry (Protocol #012017, originally approved January 20, 2017, renewed annually, and most recently approved January 6, 2022, ADVARRA Center for IRB Intelligence). This study involved a retrospective review of data contained within the SCOPE Registry; the reviewed data documented the individualized ECMO care provided at the direction of each patient's medical team. Consent for ECMO treatment was managed according to local hospital protocols. ECMO care was not altered for purposes of this study. ECMO records were archived in the SCOPE Registry for quality review purposes. A full waiver of the need for patient consent for retrospective research through SCOPE was approved by the ADVARRA Institutional Review Board (Protocol #012017).
Results
During the 22 months of this study, 640 consecutive patients with COVID-19 were supported with and separated from ECMO at 49 different hospitals. Forty-six patients who were cannulated but transferred to other hospitals on ECMO were not included in this analysis. Of 594 remaining consecutive patients included in this study (nontransferred and separated from ECMO before the completion of this study), 221 patients survived (37.2% survival; 95% Wilson confidence interval [CI], 33.4-41.2) and 373 patients died before discharge from the hospital (62.8%). Figure 1 is a CONSORT flow diagram that depicts the distribution of all 640 patients by category of outcome. Figure 2 depicts the number of COVID-19 ECMO cases at each of the 49 hospitals.
Figure 2.
The number of COVID-19 ECMO cases at each of the 49 hospitals (mean number of COVID-19 ECMO cases at each of the 49 hospitals was 12.1 [median, 6, range, 1-73, IQR, 2-11]).
Table 1 provides detailed data about the characteristics and outcomes of all 594 consecutive nontransferred patients with COVID-19 supported with and separated from ECMO. Of note, of 594 patients, 371 (63.2%) were obese, 236 (43.7%) had hypertension, 191 (35.5%) had diabetes, 70 (13.0%) had asthma, 47 (8.7%) had heart disease, 35 (6.6%) had chronic renal failure, and 10 (1.9%) had cancer. The median time on ECMO was 18 days (IQR, 10-30 days). Survival with venovenous ECMO was 211 of 547 patients (38.6%), and survival with venoarterial ECMO was 10 of 47 patients (21.3%). The median per-hospital survival for venovenous ECMO was 30.0% (range, 0%-100%, IQR, 0%-42.9%). Survivors had a lower median age (43 vs 49 years, P < .001) and shorter median time interval from diagnosis to intubation (7 days vs 10 days, P < .001). Female sex was positively associated with survival.
Table 1.
Descriptive summary of all 594 patients and the patients stratified by time category
| All patients |
Group A March 2020 to June 2020 |
Group B July 2020 to December 2020 |
Group C January 2021 to June 2021 |
Group D July 2021 to December 2021 |
P value | |
|---|---|---|---|---|---|---|
| N = 594 | N = 116 | N = 183 | N = 143 | N = 152 | ||
| Alive at last follow-up∗ | .208 | |||||
| Nonsurvivors | 373 (62.8%) | 63 (54.3%) | 120 (65.6%) | 93 (65.0%) | 97 (63.8%) | |
| Survivors | 221 (37.2%) | 53 (45.7%) | 63 (34.4%) | 50 (35.0%) | 55 (36.2%) | |
| Days from COVID diagnosis to intubation† | 9.7 (7.1) | 5.2 (4.8) | 10.6 (7.5) | 12.6 (7.2) | 10.0 (6.0) | <.001 |
| Days from COVID diagnosis to intubation‡ | 9.0 [4.0-14.0] | 4.0 [1.0-8.5] | 10.0 [4.0-15.0] | 12.0 [8.0-16.0] | 9.0 [5.0-14.5] | <.001 |
| Days from intubation to cannulation† | 4.6 (4.9) | 4.5 (3.8) | 5.2 (4.9) | 4.4 (6.5) | 4.2 (3.5) | .378 |
| Days from intubation to cannulation‡ | 4.0 [1.0-6.0] | 4.0 [1.0-6.0] | 4.0 [1.0-7.0] | 3.0 [1.0-6.0] | 3.0 [2.0-6.0] | .442 |
| Days from COVID diagnosis to cannulation† | 13.4 (8.4) | 9.2 (6.2) | 14.3 (8.9) | 16.2 (9.6) | 13.2 (5.9) | <.001 |
| Days from COVID diagnosis to cannulation‡ | 13.0 [7.0-18.0] | 8.0 [5.0-13.0] | 14.0 [7.0-19.5] | 15.0 [10.0-20.0] | 13.0 [8.8-18.0] | <.001 |
| Days on ECMO† | 23.8 (20.3) | 19.2 (16.8) | 24.8 (18.9) | 24.2 (21.3) | 25.7 (23.1) | .048 |
| Days on ECMO‡ | 18.0 [10.0-30.0] | 13.5 [8.0-26.2] | 21.0 [12.0-32.0] | 19.0 [10.0-29.5] | 17.5 [10.0-33.0] | .004 |
| Hours on ECMO† | 560.1 (487.8) | 450.1 (403.0) | 583.3 (453.4) | 570.1 (508.7) | 606.8 (554.5) | .051 |
| Hours on ECMO‡ | 426.5 [228.5-715.8] | 310.0 [190.8-628.8] | 489.0 [286.5-761.0] | 433.0 [234.5-699.5] | 416.0 [238.5-784.2] | .005 |
| Age† | 46.2 (12.3) | 50.1 (12.5) | 49.5 (11.8) | 45.4 (12.0) | 40.1 (10.5) | <.001 |
| Age‡ | 47.0 [36.0-56.0] | 51.0 [41.0-60.0] | 51.0 [41.5-58.0] | 47.0 [35.0-54.0] | 39.0 [32.0-48.0] | <.001 |
| Gender∗ | .735 | |||||
| Female | 186 (31.3%) | 36 (31.0%) | 54 (29.5%) | 43 (30.1%) | 53 (34.9%) | |
| Male | 408 (68.7%) | 80 (69.0%) | 129 (70.5%) | 100 (69.9%) | 99 (65.1%) | |
| BMI (kg/m2)† | 34.5 (8.1) | 33.5 (7.8) | 34.2 (8.5) | 34.9 (9.3) | 35.1 (6.9) | .471 |
| Asthma∗ | 70 (13.0%) | 22 (19.0%) | 21 (11.7%) | 14 (10.5%) | 13 (12.0%) | .193 |
| Cancer∗ | 10 (1.9%) | 5 (4.3%) | 3 (1.6%) | 1 (0.8%) | 1 (0.9%) | .228 |
| Chronic renal failure∗ | 35 (6.6%) | 3 (2.6%) | 24 (13.3%) | 8 (6.1%) | 0 (0.0%) | <.001 |
| Diabetes∗ | 191 (35.5%) | 41 (35.3%) | 72 (39.8%) | 50 (37.6%) | 28 (25.9%) | .11 |
| Heart disease∗ | 47 (8.7%) | 13 (11.2%) | 21 (11.6%) | 10 (7.5%) | 3 (2.8%) | .05 |
| Hypertension∗ | 236 (43.7%) | 43 (37.4%) | 106 (58.2%) | 56 (41.8%) | 31 (28.4%) | <.001 |
| Obesity∗ | 371 (63.2%) | 66 (56.9%) | 121 (66.5%) | 90 (63.4%) | 94 (63.9%) | .413 |
| ≥1 comorbid conditions∗ | 475 (80.9%) | 93 (80.2%) | 160 (87.9%) | 115 (81.0%) | 107 (72.8%) | .007 |
| Proned before ECMO∗ | 346 (67.1%) | 76 (67.3%) | 114 (65.9%) | 91 (69.5%) | 65 (65.7%) | .91 |
| Tracheostomy performed∗ | 249 (41.9%) | 26 (22.4%) | 94 (51.4%) | 76 (53.1%) | 53 (34.9%) | <.001 |
| No. of circuit changes‡ | 0.0 [0.0-1.0] | 0.0 [0.0-1.0] | 0.0 [0.0-1.0] | 0.0 [0.0-1.0] | 0.0 [0.0-1.0] | .064 |
| ≥1 circuit changes∗ | 223 (38.8%) | 33 (30.6%) | 71 (39.9%) | 51 (35.9%) | 68 (46.3%) | .068 |
| CVVH or CRRT used∗ | 145 (27.6%) | 34 (29.6%) | 61 (34.5%) | 33 (25.0%) | 17 (16.8%) | .013 |
| ECMO type∗ | .788 | |||||
| VA | 47 (7.9%) | 8 (6.9%) | 17 (9.3%) | 12 (8.4%) | 10 (6.6%) | |
| VV | 547 (92.1%) | 108 (93.1%) | 166 (90.7%) | 131 (91.6%) | 142 (93.4%) | |
| Anticoagulation type∗ | <.001 | |||||
| Argatroban | 39 (6.6%) | 8 (6.9%) | 5 (2.7%) | 8 (5.6%) | 18 (12.1%) | |
| Bivalirudin | 156 (26.4%) | 16 (13.8%) | 53 (29.1%) | 50 (35.0%) | 37 (24.8%) | |
| Heparin | 394 (66.8%) | 92 (79.3%) | 124 (68.1%) | 85 (59.4%) | 93 (62.4%) | |
| None | 1 (0.2%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (0.7%) | |
| Antiviral medication∗ | 392 (74.7%) | 41 (36.0%) | 152 (84.4%) | 114 (87.0%) | 85 (85.0%) | <.001 |
| Convalescent plasma∗ | 227 (44.8%) | 48 (47.1%) | 113 (63.8%) | 52 (40.3%) | 14 (14.1%) | <.001 |
| Hydroxychloroquine∗ | 58 (11.1%) | 42 (36.8%) | 10 (5.6%) | 5 (3.8%) | 1 (1.0%) | <.001 |
| Interleukin-6 blocker∗ | 180 (34.5%) | 47 (42.0%) | 46 (25.6%) | 47 (35.6%) | 40 (40.8%) | .012 |
| Prostaglandin∗ | 165 (32.1%) | 39 (36.4%) | 73 (41.0%) | 38 (29.2%) | 15 (15.2%) | <.001 |
| Steroids∗ | 452 (87.3%) | 60 (55.0%) | 167 (92.8%) | 127 (97.7%) | 98 (99.0%) | <.001 |
COVID, Coronavirus Disease; ECMO, extracorporeal membrane oxygenation; BMI, body mass index; CVVH, continuous venovenous hemofiltration; CRRT, continuous renal replacement therapy; VA, venoarterial; VV, venovenous.
Categorical data summarized as N (%).
Data summarized as mean (standard deviation).
Data summarized as median [25th quartile, 75th quartile].
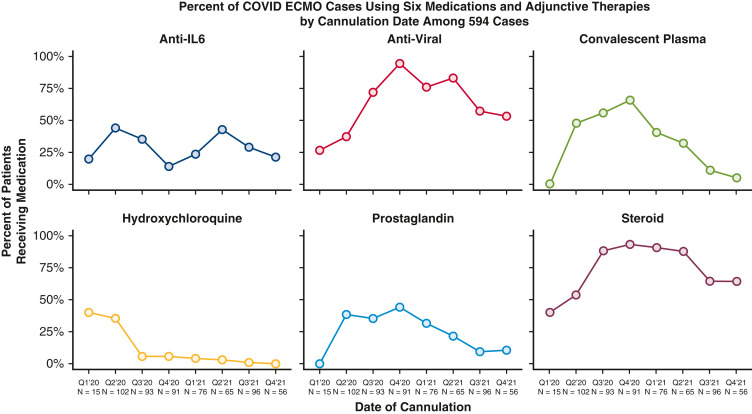
Table 1 also provides detailed data comparing the characteristics and outcomes of all 594 patients, stratified by era. Throughout the study, median age [IQR] declined (group A = 51.0 [41.0-60.0], group D = 39.0 [32.0-48.0], P < .001); median days between COVID-19 diagnosis and intubation increased (group A = 4.0 [1.0-8.5], group D = 9.0 [5.0-14.5], P < .001); and use of medications (glucocorticoids, interleukin-6 blockers, antivirals, antimalarials) and convalescent plasma fluctuated significantly (all P < .05) (Figure 3 ). Figure 3 depicts quarterly trends over time in the use of 6 adjunctive therapies in patients with COVID-19 while supported with ECMO.
Figure 3.
Quarterly trends over time in the use of 6 adjunctive therapies in patients with COVID-19 while supported with ECMO during the 22 months of analysis: anti-interleukin-6–receptor monoclonal antibodies (tocilizumab or sarilumab [blue line]), antiviral medications (remdesivir [red line]), convalescent plasma (green line), hydroxychloroquine (yellow line), Flolan (prostaglandin [cyan line]), and intravenous steroids (mauve line). COVID, Coronavirus Disease; ECMO, extracorporeal membrane oxygenation.
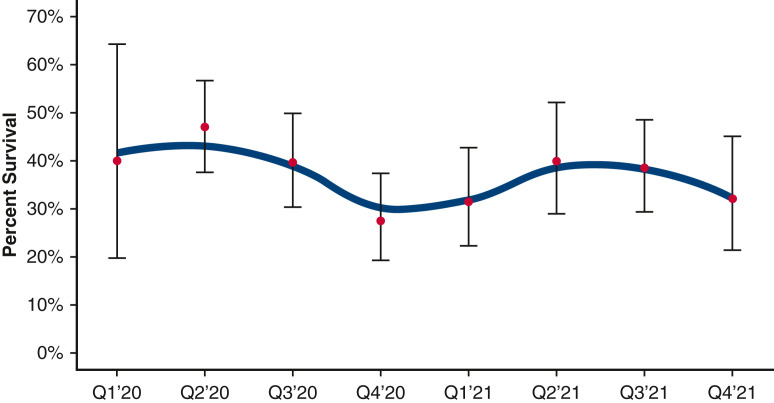
Figure 4 depicts unadjusted quarterly percent of survival for all patients over the study period (with 95% Wilson CI), accompanied by a Locally Estimated Scatterplot Smoother curve to indicate unadjusted trends. Table 2 and Figure 5 summarize results of the Bayesian mixed-effects logistic regression model. Estimated odds of survival varied over the study period with a decline between April 1, 2020, and November 21, 2020 (odds ratio [OR], 0.39, 95% credible interval [CrI], 0.18-0.87, probability of reduction in survival = 95.7%), improvement between November 21, 2020, and May 17, 2021 (OR, 1.85, 95% CrI, 0.86-4.09, probability of improvement = 93.4%), and decline between May 17, 2021, and December 1, 2021 (OR, 0.49, 95% CrI, 0.19-1.44, probability of decrease = 92.1%). Time trends accounted for 18.4% of relative explained variation in survival outcome, age accounted for 58.5%, days from diagnosis to intubation 8.1%, and circuit change-out 5.5%, with the remaining fixed-effect variables in the model accounting for less than 2% of relative explained variation each. The inclusion of hospital as a random effect accounted for 2.7% of overall variation in survival. The overall model predictive performance was relatively modest with a C-statistic of 0.655.
Figure 4.
Unadjusted quarterly percent of survival for all patients over the study period (with 95% Wilson CI), accompanied by a Locally Estimated Scatterplot Smoother curve to indicate unadjusted trends.
Table 2.
Bayesian mixed-effects logistic regression results for survival
| Variable | Survival OR for contrast and 95% CrI | Posterior probability of |
|---|---|---|
| Age (56 vs 36 y) | 0.29 [0.16-0.51] | Decreased survival: 94.65% |
| Cannulation date | ||
| November 21, 2020, vs April 1, 2020 | 0.39 [0.18-0.87] | Decreased survival: 95.70% |
| May 17, 2021, vs November 21, 2020 | 1.85 [0.86-4.09] | Increased survival: 93.40% |
| December 1, 2021, vs May 17, 2021 | 0.49 [0.19-1.44] | Decreased survival: 92.10% |
| Days from diagnosis to intubation (14 vs 4) | 0.72 [0.52-0.98] | Decreased survival: 98.15% |
| Circuit change-out (yes vs no) | 0.70 [0.46-1.04] | Decreased survival: 94.65% |
| BMI (39 vs 29 kg/m2) | 1.22 [0.70-2.06] | Increased survival: 78.03% |
| Female vs male | 1.19 [0.76-1.84] | Increased survival: 58.78% |
| Any comorbidity (no vs yes) | 1.06 [0.62-1.78] | Increased survival: 57.98% |
| Prone positioning before ECMO (no vs yes) | 1.04 [0.67-1.57] | Increased survival: 95.85% |
| Days from intubation to cannulation (6 vs 1) | 1.01 [0.83-1.25] | Increased survival: 52.13% |
Contrasts for age, BMI, days from diagnosis to intubation, and days from intubation to cannulation are 75th percentile versus 25th percentile. Contrasts for cannulation date were chosen to assess the magnitude of differences in estimated survival early and late in the study period, and at major inflection points of the estimated survival trend. OR, Odds ratio; CrI, credible interval; BMI, body mass index; ECMO, extracorporeal membrane oxygenation.
Figure 5.
Estimated time trends in probability of survival, controlling for the following variables and potential risk factors: age, gender, BMI, the presence or absence of one or more pre-ECMO comorbidities (ie, obesity, asthma, hypertension, heart disease, diabetes, chronic renal failure, cancer), days from diagnosis of COVID-19 to intubation, days from intubation to cannulation for ECMO, prone positioning, and whether or not a circuit change-out was required, with a random intercept term for variations by hospital. This figure exhibits variation in predicted probability of survival over time. Annotated dates have been added to key inflection points in the estimated trend to aid interpretation in concert with Table 2. COVID, Coronavirus Disease; ECMO, extracorporeal membrane oxygenation.
Discussion
Survival for patients with COVID-19 supported with ECMO has fluctuated during the stages of the pandemic. Our multi-institutional analysis of 594 consecutive patients with COVID-19 who were supported with ECMO and subsequently decannulated provides clear evidence that ECMO facilitates salvage and survival of select critically ill patients with COVID-19. Our analysis also provides clear evidence of changes over time in several domains, including selection of patients with COVID-19 for ECMO, adjuvant therapeutic strategies, management of ECMO, and outcomes. Survivors had lower median age (43 vs 49 years, P < .001) and shorter median time interval from diagnosis to intubation (7 days vs 10 days, P < .001). Female sex was positively associated with survival. Survival with venovenous ECMO was 211 of 547 patients (38.6%), and survival with venoarterial ECMO was 10 of 47 patients (21.3%). Substantial variation existed in the use of adjunctive drugs and therapies in the treatment of COVID-19, but these findings support the selective use of venovenous ECMO as a reasonable rescue strategy.
It is not surprising that we found the selection of patients with COVID-19 for ECMO, adjuvant therapeutic strategies, and management of ECMO all evolved during the course of the pandemic, as we learned more about the virus and its response to medications and ECMO. The initial decrease in survival of patients with COVID-19 supported with ECMO is somewhat surprising but may be potentially explained by a broadening of the selection criteria after some initial successes. The eventual improvement in survival is encouraging and hopefully represents an increased understanding of COVID-19 in our community. The subsequent decrease in survival may be related to expansion of the use of ECMO across more centers over time in an effort to salvage critically ill patients with COVID-19.
Our analysis reveals that survival for patients with COVID-19 supported with ECMO has fluctuated during the stages of the pandemic. Our study includes patients with the diagnosis of COVID-19 who were cannulated for ECMO between March 17, 2020, when our first patient with COVID-19 was placed on ECMO, and December 20, 2021, inclusive. The COVID-19 vaccine became available in December 2020, so only the last 12 months of the 21 months in this study could include vaccinated patients. Unfortunately, our database does not contain useful data about vaccination status of our patients. Many hospitals would not permit collection of data about vaccination status. Nevertheless, it is clear that zero patients were vaccinated in group A and few if any were vaccinated in group B, whereas some were likely vaccinated in groups C and D. In our study, because of the lack of complete data about vaccination status, no clear relationship can be described between vaccination availability and survival for patients with COVID-19 supported with ECMO.
We previously reported that “Days from COVID Diagnosis to ECMO Cannulation” is inversely related to survival after ECMO for COVID-19,11 and in this same publication, we reported that “Days from COVID Diagnosis to Intubation” is a more important predictor of outcome than “Days from Intubation to ECMO Cannulation.”11 In this prior publication,11 median “Days from COVID Diagnosis to Intubation” was 7 days in survivors versus 11 days in nonsurvivors (P = .001), whereas median “Days from Intubation to ECMO Cannulation” was 3.5 days in survivors versus 4 days in nonsurvivors (P = .001). Furthermore, in this prior publication, we documented that “Adjusting for several confounding factors, we estimated that an ECMO patient intubated on day 14 post COVID-19 diagnosis versus day 4 had a relative odds of survival of 0.65 (95% credible interval [CrI], 0.44-0.96, posterior probability of negative effect: 98.5%).” In this current analysis, survivors also had a shorter median time interval from diagnosis to intubation (7 days vs 10 days, P < .001). Although it might be expected that the reason that “Days from COVID Diagnosis to ECMO Cannulation” is inversely related to survival after ECMO for COVID-19 is primarily based on the length of time on the ventilator before ECMO cannulation (because this time period may be associated with ventilator-related lung injury), it is not really surprising that the length of time from diagnosis to institution of mechanical ventilation is perhaps even a greater risk factor. Brochard and colleagues22 argued that “application of a lung-protective ventilation, today best applied with sedation and endotracheal intubation, might be considered a prophylactic therapy, rather than just a supportive therapy, to minimize the progression of lung injury from a form of patient self-inflicted lung injury.”22 These authors stated: “A major concern in mechanically ventilated patients is the risk of ventilator-induced lung injury, which is partially prevented by lung-protective ventilation. Spontaneously breathing, nonintubated patients with acute respiratory failure may have a high respiratory drive and breathe with large tidal volumes and potentially injurious transpulmonary pressure swings. In patients with existing lung injury, regional forces generated by the respiratory muscles may lead to injurious effects on a regional level. In addition, the increase in transmural pulmonary vascular pressure swings caused by inspiratory effort may worsen vascular leakage. Recent data suggest that these patients may develop lung injury that is similar to the ventilator-induced lung injury observed in mechanically ventilated patients.”22 This logic potentially explains our finding that “Days from COVID Diagnosis to ECMO Cannulation” is inversely related to survival after ECMO for COVID-19, and that “Days from COVID Diagnosis to Intubation” is a more important predictor of outcome than “Days from Intubation to ECMO Cannulation.”
Others have also reported an initial time-related decrease in survival over time of patients with COVID-19 supported with ECMO.23 In October 2021, Barbaro and colleagues23 reported a retrospective analysis of the Extracorporeal Life Support Organization Registry and COVID-19 Addendum that compared 3 groups of patients with COVID-19 (aged ≥16 years) supported with ECMO:
-
•
Group A1 was composed of patients with COVID-19 supported with ECMO in whom ECMO was initiated on or before May 1, 2020, at “early-adopting centers,” which were defined as centers using ECMO support for patients with COVID-19 throughout 2020.
-
•
Group A2 was composed of patients with COVID-19 supported with ECMO in whom ECMO was initiated between May 2, 2020, and December 31, 2020, at “early-adopting centers,” which were defined as centers using ECMO support for patients with COVID-19 throughout 2020.
-
•
Group B was composed of patients with COVID-19 supported with ECMO in whom ECMO was initiated between May 2, 2020, and December 31, 2020, at “late-adopting centers,” which were defined as centers using ECMO support for patients with COVID-19 only after May 1, 2020.
In the Extracorporeal Life Support Organization Registry, in 2020, 4812 patients with COVID-19 were supported with ECMO at 349 centers within 41 countries. At early-adopting centers, the cumulative incidence of in-hospital mortality 90 days after ECMO initiation was 36.9% (95% CI, 34.1-39.7) in patients who started ECMO on or before May 1 (group A1) versus 51.9% (95% CI, 50.0-53.8) in patients who started ECMO after May 1 (group A2). At late-adopting centers (group B), the cumulative incidence of in-hospital mortality 90 days after ECMO initiation was 58.9% (95% CI, 55.4-62.3). Relative to patients in group A2, group A1 patients had a lower adjusted relative risk of in-hospital mortality 90 days after ECMO (hazard ratio, 0.82; 95% CI, 0.70-0.96), whereas group B patients had a higher adjusted relative risk (hazard ratio, 1.42; 95% CI, 1.17-1.73). The authors reported the following conclusion and interpretation: “Mortality after ECMO for patients with COVID-19 worsened during 2020. These findings inform the role of ECMO in COVID-19 for patients, clinicians, and policy makers.”
Value of This Analysis
Our study adds to the body of knowledge and the literature by providing more granular multi-institutional data about our cohort of 594 patients with COVID-19 supported with ECMO at 49 hospitals. As previously described, several published analyses have studied the outcomes of ECMO in patients with COVID-19, and these outcomes have been heterogenous.8, 9, 10, 11, 12, 13, 14, 15, 16, 17 Our analysis of the SCOPE Registry adds another dataset of multi-institutional data to the growing body of literature about the use of ECMO in patients with COVID-19 and demonstrates that support with ECMO facilitates salvage and survival of select critically ill patients with COVID-19. Survivors had lower median age (43 vs 49 years, P < .001) and shorter median time interval from diagnosis to intubation (7 days vs 10 days, P < .001). Most importantly, this analysis provides a unique picture of the evolution of the COVID-19 pandemic and the associated evolution of multiple related domains, including the selection of patients with COVID-19 for ECMO, adjuvant therapeutic strategies, management of ECMO, and outcomes. The lessons learned from this analysis can inform both the care of patients with and without COVID-19 supported with ECMO and the overall and ECMO-specific approaches to future pandemics.
Future Directions
Much remains to be learned about the role of ECMO in these patients. From our analysis, no specific demographic, clinical, or laboratory data, to date, are predictive of outcome with ECMO in patients with COVID-19, with the exception of younger age and shorter time from diagnosis to intubation. Survivors tend to be younger and to have a shorter duration from diagnosis to intubation. Meanwhile, the role of multiple medications in the treatment of COVID-19 remains unclear: None of the adjunct therapies are associated with increased survival in patients with COVID-19 supported with ECMO. More information is needed to better determine which patients with COVID-19 will benefit from ECMO and which patients with COVID-19 will benefit from lung transplantation. Lessons learned from the use of ECMO to support patients with COVID-19 will inform the management of other patients with different forms of severe respiratory failure.
Study Limitations
This analysis is based on the available data in our database. Potential limitations include patient selection bias, institutional bias, confounding bias, and possible underpowering of the analysis. Additional follow-up is required on all surviving patients. Further patient accrual will enhance continued analysis of outcomes. We plan to continue gathering data to provide additional insight as to guideposts for patient selection and predictors of outcomes. It is our hope that by sharing our experience, other hospitals and patients may benefit.
Conclusions
Survival for patients with COVID-19 supported with ECMO has fluctuated during the stages of the pandemic. Our experience and analysis of 594 consecutive patients at 49 hospitals reveal that ECMO facilitates salvage and survival of select critically ill patients with COVID-19. Highest survival occurred when venovenous only ECMO was applied with a shorter median time interval from the diagnosis of COVID-19 to ECMO cannulation, driven mostly by the observation that survivors also had a shorter median time interval from COVID-19 diagnosis to intubation for mechanical ventilation. Substantial variation exists in drug treatment of COVID-19, but ECMO offers a reasonable rescue strategy. Additional gathering and analysis of data will inform appropriate selection of patients and provide guidance as to the best use of ECMO in terms of timing, implementation, duration of support, and best criteria for discontinuation. Minimizing variability on how ECMO is applied as a rescue strategy by adherence to best practices may lead to improved survival and may aid in guiding the role of ECMO in a future pandemic response.
Webcast
You can watch a Webcast of this AATS meeting presentation by going to: https://www.aats.org/resources/1975.

Conflict of Interest Statement
J.P.J. is a Professor of Surgery and Pediatrics at University of Florida and a Consultant for SpecialtyCare. All other authors reported no conflicts of interest.
The Journal policy requires editors and reviewers to disclose conflicts of interest and to decline handling or reviewing manuscripts for which they may have a conflict of interest. The editors and reviewers of this article have no conflicts of interest.
References
- 1.Coronavirus COVID-19 global cases by the Center for Systems Science and Engineering (CSSE) https://coronavirus.jhu.edu/map.html Johns Hopkins University & Medicine.
- 2.Clerkin K.J., Fried J.A., Raikhelkar J., Sayer G., Griffin J.M., Masoumi A., et al. COVID-19 and cardiovascular disease. Circulation. 2019;141:1648–1655. doi: 10.1161/CIRCULATIONAHA.120.046941. [DOI] [PubMed] [Google Scholar]
- 3.Madjid M., Safavi-Naeini P., Solomon S.D., Vardeny O. Potential effects of coronaviruses on the cardiovascular system: a review. JAMA Cardiol. 2020;5:831–840. doi: 10.1001/jamacardio.2020.1286. [DOI] [PubMed] [Google Scholar]
- 4.Rajagopal K., Keller S.P., Akkanti B., Bime C., Loyalka P., Cheema F.H., et al. Advanced pulmonary and cardiac support of COVID-19 patients: emerging recommendations from ASAIO-a "Living Working Document". ASAIO J. 2020;66:588–598. doi: 10.1097/MAT.0000000000001180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rajagopal K., Keller S.P., Akkanti B., Bime C., Loyalka P., Cheema F.H., et al. Advanced pulmonary and cardiac support of COVID-19 patients: emerging recommendations from ASAIO-a Living Working Document. Circ Heart Fail. 2020;13 doi: 10.1161/CIRCHEARTFAILURE.120.007175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bartlett R.H., Ogino M.T., Brodie D., McMullan D.M., Lorusso R., MacLaren G., et al. Initial ELSO guidance document: ECMO for COVID-19 patients with severe cardiopulmonary failure. ASAIO J. 2020;66:472–474. doi: 10.1097/MAT.0000000000001173. Erratum in: ASAIO J. 2020;66:e113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Badulak J., Antonini M.V., Stead C.M., Shekerdemian L., Raman L., Paden M.L., et al. Extracorporeal membrane oxygenation for COVID-19: updated 2021 guidelines from the Extracorporeal Life Support Organization. ASAIO J. 2021;67:485–495. doi: 10.1097/MAT.0000000000001422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jacobs J.P., Stammers A.H., St Louis J., Hayanga J.W.A., Firstenberg M.S., Mongero L.B., et al. Extracorporeal membrane oxygenation in the treatment of severe pulmonary and cardiac compromise in Coronavirus Disease 2019: experience with 32 patients. ASAIO J. 2020;66:722–730. doi: 10.1097/MAT.0000000000001185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jacobs J.P., Stammers A.H., St Louis J.D., Hayanga J.W.A., Firstenberg M.S., Mongero L.B., et al. Multi-institutional analysis of 100 consecutive patients with COVID-19 and severe pulmonary compromise treated with extracorporeal membrane oxygenation: outcomes and trends over time. ASAIO J. 2021;67:496–502. doi: 10.1097/MAT.0000000000001434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jacobs J.P., Stammers A.H., St Louis J.D., Hayanga J.W.A., Firstenberg M.S., Mongero L.B., et al. Multi-institutional analysis of 200 COVID-19 patients treated with ECMO: outcomes and trends. Ann Thorac Surg. 2021;113(5):1452–1460. doi: 10.1016/j.athoracsur.2021.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hall C.A., Jacobs J.P., Stammers A.H., St Louis J.D., Hayanga J.W.A., Firstenberg M.S., et al. Multi-institutional analysis of 505 COVID-19 patients supported with ECMO: predictors of survival. Ann Thorac Surg. 2022;114(1):61–68. doi: 10.1016/j.athoracsur.2022.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang X., Yu Y., Xu J., Shu H., Xia J., Liu H., et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Henry B.M. COVID-19, ECMO, and lymphopenia: a word of caution. Lancet Respir Med. 2020;8:e24. doi: 10.1016/S2213-2600(20)30119-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kon Z.N., Smith D.E., Chang S.H., Goldenberg R.M., Angel L.F., Carillo J.A., et al. Extracorporeal membrane oxygenation support in severe COVID-19. Ann Thorac Surg. 2021;111:537–543. doi: 10.1016/j.athoracsur.2020.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barbaro R.P., MacLaren G., Boonstra P.S., Iwashyna T.J., Slutsky A.S., Fan E., et al. Extracorporeal membrane oxygenation support in COVID-19: an international cohort study of the Extracorporeal Life Support Organization registry. Lancet. 2020;396:1071–1078. doi: 10.1016/S0140-6736(20)32008-0. Erratum in: Lancet. 2020;396:1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shih E., DiMaio J.M., Squiers J.J., Banwait J.K., Meyer D.M., George T.J., et al. Venovenous extracorporeal membrane oxygenation for patients with refractory coronavirus disease 2019 (COVID-19): multicenter experience of referral hospitals in a large health care system. J Thorac Cardiovasc Surg. 2020 doi: 10.1016/j.jtcvs.2020.11.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shih E., Squiers J.J., DiMaio J.M., George T., Banwait J., Monday K., et al. Outcomes of extracorporeal membrane oxygenation in patients with severe acute respiratory distress syndrome caused by COVID-19 versus influenza. Ann Thorac Surg. 2021;113:1445–1451. doi: 10.1016/j.athoracsur.2021.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harrell F.E., Jr., with contributions from Charles Dupont and many others ‘Hmisc’: Harrell Miscellaneous. R package version 4.4-2. 2020. https://CRAN.R-project.org/package=Hmisc Accessed April 21, 2022.
- 19.R: a language and environment for statistical computing. version 4.0.3. R Foundation for Statistical Computing. 2020 [Google Scholar]
- 20.Subirana I., Sanz H., Vila J. Building bivariate tables: the compareGroups package for R. J Stat Software. 2014;5712:1–16. [Google Scholar]
- 21.Harrell F.E., Jr. rmsb: Bayesian regression modeling strategies. R package version 0.0.2. https://CRAN.R-project.org/package=rmsb Accessed April 21, 2022.
- 22.Brochard L., Slutsky A., Pesenti A. Mechanical ventilation to minimize progression of lung injury in acute respiratory failure. Am J Respir Crit Care Med. 2017;195:438–442. doi: 10.1164/rccm.201605-1081CP. [DOI] [PubMed] [Google Scholar]
- 23.Barbaro R.P., MacLaren G., Boonstra P.S., Combes A., Agerstrand C., Annich G., et al. Extracorporeal Life Support Organization. Extracorporeal membrane oxygenation for COVID-19: evolving outcomes from the international Extracorporeal Life Support Organization Registry. Lancet. 2021;398:1230–1238. doi: 10.1016/S0140-6736(21)01960-7. [DOI] [PMC free article] [PubMed] [Google Scholar]