Abstract
Introduction
COVID-19 (coronavirus disease-2019) is an infectious disease caused by SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2). Immune dysregulation causes inflammation and massive production of inflammatory mediators that worsen the patients’ status. Here, regulatory immune cells may ameliorate inflammation and improve the severity of the disease.
Materials and methods
A total of 76 participants were enrolled in this study and divided into 3 groups as follows: patients with moderate/severe COVID-19 (n = 25), patients with critical COVID-19 (n = 26), and healthy controls (n = 25). After blood collection, peripheral blood mononuclear cells (PBMCs) were isolated and stained by FITC-conjugated anti-CD4 monoclonal antibodies (mABs), PE-conjugated anti-HLA-G mABs, PerCPCy5.5-conjugated anti-CD14 mABs, and APC-conjugated anti-CD8 mABs.
Results
Critical COVID-19 patients had a significantly lower frequency of CD4+ HLA-G+ T lymphocytes compared with moderate/severe COVID-19 patients (p value < 0.001; SMD, −1.27; 95% CI [-1.86, −0.66]) and healthy controls (p value < 0.05; SMD, −0.69; 95% CI [-1.25, −0.12]). Critical COVID-19 patients had a significantly lower frequency of CD14+ HLA-G+ monocytes compared with moderate/severe COVID-19 patients (p value < 0.001; SMD, −2.09; 95% CI [-2.77, −1.41]) and healthy controls (p value < 0.05; SMD, −0.83; 95% CI [-1.40, −0.25]). However, there was no difference between the groups regarding the frequency of CD8+ HLA-G+ T lymphocytes.
Conclusion
The increased amount of immunomodulatory HLA-G+ cells may reduce the severity of the disease in moderate/severe COVID-19 patients compared with critical COVID-19 patients.
Keywords: COVID-19, SARS-CoV-2, HLA-G+ T cells, HLA-G+ monocytes
1. Introduction
The outbreak of the COVID-19 pandemic has affected millions of people all over the world. COVID-19 is an infectious disease that leads to a dysregulated immune system. For example, in our previous articles, we showed that critical COVID-19 patients had a significantly higher frequency of both exhausted CD4+ and CD8+ T cells compared with non-critical COVID-19 patients [[1], [2], [3]]. A better understanding of the immune characteristics of COVID-19 patients may help clinicians to manage the disease more effectively. Since inflammatory responses are associated with the severity of COVID-19 [3,4], understanding the regulatory properties of patients’ immune systems is crucial.
HLA-G is a non-classical HLA molecule belonging to HLA class Ib. HLA-G has 7 isoforms from which HLA-G1 to HLA-G4 have a membrane-bound form, and HLA-G5 to HLA-G7 have a soluble form. HLA-G1 and soluble HLA-G5 (sHLA-G5) contain beta-2 microglobulin and therefore are important isoforms of HLA-Gs. Although HLA-G1 has a membrane-bound form, it can be shed or released from the surface of immune cells to the bloodstream. Both membrane-bound and soluble forms of HLA-G have immunomodulatory functions and modulate the inflammatory responses through inhibition of proliferation of CD4+ T cells. Among the immune cells, CD4+ and CD8+ T cells and CD14+ monocytes express HLA-G and exert immunomodulatory effects [[5], [6], [7]].
Both in vitro and in vivo evaluations showed that CD4+ HLA-G+ T cells could exert their suppressive capacity. In vitro CD4+ HLA-G+ T cells inhibit T cell responses via both cell-dependent and cell-independent mechanisms. CD4+ HLA-G+ T cells can secret anti-inflammatory mediators, such as sHLA-G, interleukin-10 (IL-10), IL-35, and transforming growth factor-β (TGF-β), to exert their immunomodulatory function [7]. Immune responses against the allograft and the semi-allograft (fetus) lead to adverse outcomes in the field of transplantation and reproductive immunology [[8], [9], [10], [11], [12]]. However, it was reported that increased frequency of HLA-G+ immune cells was associated with better allograft function and successful pregnancy [12,13]. Moreover, a study reported that increased sHLA-G was associated with improved COVID-19 outcomes [14]. Given the suppressive property of HLA-G+ T cells and monocytes, we aimed to evaluate the frequency of CD4+ and CD8+ HLA-G+ T cells and CD14+ HLA-G+ monocytes in our COVID-19 patients and compare them with healthy controls.
2. Materials and methods
2.1. Study subjects
A total of 76 participants were enrolled in this study and divided into 3 groups as follows: patients with moderate/severe COVID-19 (n = 25), patients with critical COVID-19 (n = 26), and healthy controls (n = 25). Written informed consent was obtained from all participants, and the study was approved by the Institutional Research Ethics Committee, Ayatollah Rouhani Hospital, Babol University of Medical Sciences.
2.2. Peripheral blood mononuclear cell isolation
The peripheral blood (5 mL) was collected into an EDTA anticoagulant tube. Ficoll-Hypaque gradient (Biowest, Nuaille, France) centrifugation was used to isolate peripheral blood mononuclear cells (PBMCs). In brief, the peripheral blood was directly added to the Ficoll-Hypaque gradient and centrifuged at 400×g for 25 min. The middle phase (i.e., PBMCc) was collected and washed with phosphate-buffered saline (PBS) at 300×g for 10 min.
2.3. Phenotypic analyses of PBMCs
To determine the frequency of CD4+ and CD8+ HLA-G+ T cells and CD14+ HLA-G+ monocytes, PBMCs (0.7 × 106) were stained for cell surface markers, i.e., CD4, CD8, CD14, and HLA-G. A fragment crystallizable (Fc) blocker (Biolegend, USA) was used for blocks of Fc receptors before staining. In brief, FITC-conjugated anti-CD4 monoclonal antibodies (mABs; Clone SK3, Biolegend, USA), PE-conjugated anti-HLA-G mABs (Clone 87G, Biolegend, USA), PerCPCy5.5-conjugated anti-CD14 mABs (Clone HCD14, Biolegend, USA), APC-conjugated anti-CD8 mABs (Clone RPA-T8, Biolegend, USA) were added to the cellular suspension and incubated at 4 °C for 20 min. The cells were read by a FACS Calibur Flow Cytometer (BD Biosciences, San Jose, CA, USA), and the data were analyzed by FlowJo version 7.6.1 (Tree Star Inc., Ashland, OR, USA).
2.4. Statistical analyses
Data were analyzed using STATA version 14.1 and GraphPad Prism version 6. Demographic and clinical characteristics are presented in Table 1 . The 1-way analysis of variance (ANOVA) test was used for crude data, and the 2-way ANOVA was used for adjusted ANOVA models. P values less than 0.05 were considered significant in all statistical tests. The data in Table 1 are presented as mean ± SD. For the main data, 3 effect sizes were reported, including mean difference (MD), standardized MD (SMD), and partial Eta2 with their 95% CIs. MD and SMD were used to assess pairwise comparison, and partial Eta2 was used to compare ANOVA models.
Table 1.
Demographic and clinical characteristics of study subjects.
| Variables | Healthy control [25] | Moderate/Severe [25] | Critical [26] |
|---|---|---|---|
| Age (years) | 52.6 ± 7.0 | 59.9 ± 15.6 | 65.1 ± 12.5 |
| Male (n; %) | 15 (60) | 11 (44) | 8 (31) |
| Female (n; %) | 10 (40) | 14 (56) | 18 (69) |
| O2 saturation | – | 90.1 ± 5.27 | 88.9 ± 5.91 |
| Respiratory rate | – | 18.7 ± 2.30 | 19.7 ± 3.15 |
| LDH | 227 ± 43 | 743 ± 310 | 1053 ± 639 |
| CRP | 2.6 ± 1.5 | 55 ± 32 | 112 ± 92 |
| ESR | 13 ± 5 | 34 ± 28 | 60 ± 29 |
| BUN | 15 ± 4 | 26 ± 13 | 26 ± 11 |
| Cr | 0.9 ± 0.2 | 1 ± 0.4 | 1 ± 0.5 |
| AST | 24 ± 8 | 53 ± 59 | 45 ± 29 |
| ALT | 25 ± 10 | 35 ± 21 | 40 ± 31 |
| ALP | 125 ± 81 | 161 ± 72 | 210 ± 121 |
| WBC | 6668 ± 1254 | 5883 ± 2708 | 10326 ± 4547 |
| Total bilirubin | 0.44 ± 0.08 | 0.43 ± 0.06 | 0.79 ± 0.64 |
| Direct bilirubin | 0.23 ± 0.06 | 0.20 ± 0.02 | 0.35 ± 0.35 |
| RBC | 6.15 ± 7.13 | 4.70 ± 0.65 | 4.14 ± 0.57 |
| Lymphocytes | 2504 ± 481 | 1634 ± 1719 | 1149 ± 381 |
| Neutrophil | 5184 ± 8341 | 4510 ± 2586 | 8936 ± 4324 |
| PLT | 234600 ± 65468 | 206083 ± 70078 | 192923 ± 75620 |
| NLR | 1.56 ± 0.63 | 4.41 ± 2.74 | 6.12 ± 5.40 |
| PLR | 91 ± 24 | 205 ± 122 | 183 ± 94 |
| Hb | 14 ± 1.69 | 13 ± 2.15 | 11 ± 2.05 |
3. Results
3.1. Demographic and clinical findings
COVID-19 patients showed lymphopenia and neutrophilia. Moreover, lactate dehydrogenase (LDH), erythrocyte sedimentation rate (ESR), and C-reactive protein (CRP) were higher in COVID-19 patients compared with healthy controls. Demographic and other clinical characteristics of the study subjects are shown in Table 1. Moreover, the mean ± SD for different HLA-G+ cells is shown in Table 2 .
Table 2.
Frequency of different HLA-G+ cells.
3.2. The lower frequency of CD4+ HLA-G+ T lymphocytes in critical COVID-19 patients
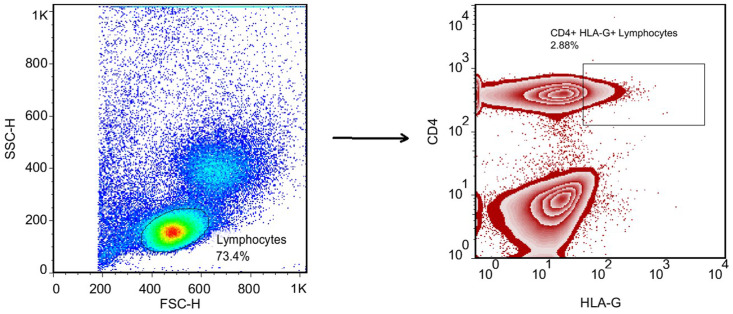
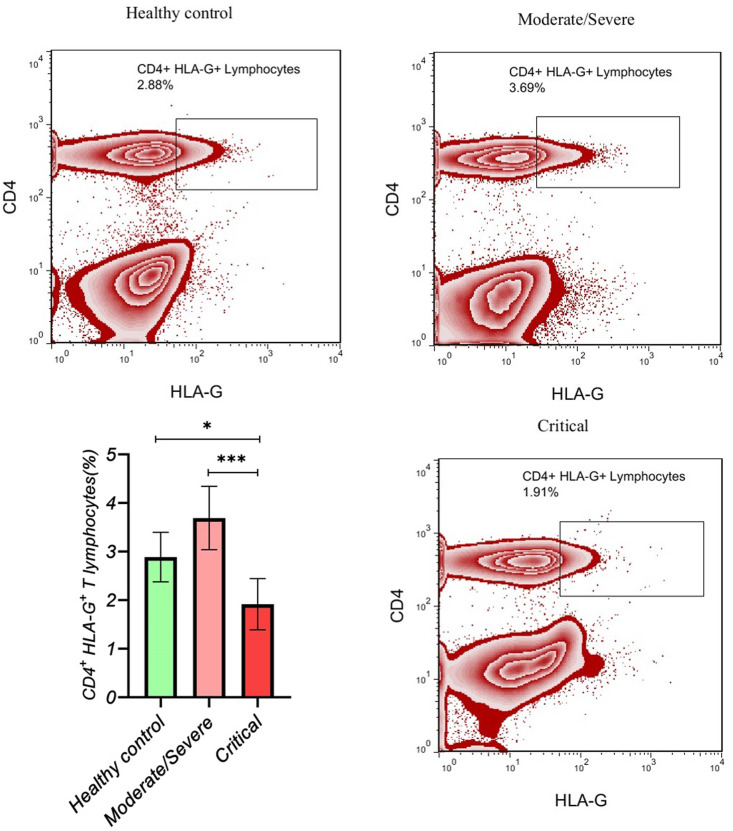
The gating strategy for the determination of CD4+ HLA-G+ T lymphocytes is shown in Fig. 1 . Critical COVID-19 patients had a significantly lower frequency of CD4+ HLA-G+ T lymphocytes compared with moderate/severe COVID-19 patients (p value < 0.001; SMD, −1.27; 95% CI [−1.86, −0.66]) and healthy controls (p value < 0.05; SMD, −0.69; 95% CI [−1.25, −0.12]). Moreover, adjusted analyses for age or gender showed the same results (Table 3 , Fig. 2 ). Although the difference was not significant between moderate/severe patients and healthy controls, moderate/severe patients had a higher frequency of CD4+ HLA-G+ T lymphocytes compared with healthy controls (p value > 0.05; SMD, 0.57; 95% CI [0.01, 1.13]). Moreover, adjusted analyses for age or gender showed the same results, and Eta2 was not different between the 3 ANOVA models (Table 3).
Fig. 1.
The gating strategy for determination of the CD4+ HLA-G+ T lymphocytes.
Table 3.
ANOVA analyses of CD4+ HLA-G+ T lymphocytes.
| Model | Groups | Mean difference (95% CI) | SMD (95% CI) | Partial Eta2 | P value$ |
|---|---|---|---|---|---|
| Crude | Critical vs Moderate/Severe | −1.77 (−2.72; −0.82) | −1.27 (−1.86; −0.66) | 0.222 | 0.0001 |
| Critical vs Healthy control | −0.97 (−1.92; −0.01) | −0.69 (−1.25; −0.12) | – | – | |
| Moderate/Severe vs Healthy control | 0.80 (−0.15; 1.76) | 0.57 (0.01; 1.13) | – | – | |
| Adjusted for age | Critical vs Moderate/Severe | −1.81 (−2.79; −0.83) | −1.25 (−1.84; −0.65) | 0.222 | 0.0003 |
| Critical vs Healthy control | −0.92 (−1.97; 0.11) | −0.63 (−1.19; −0.06) | – | – | |
| Moderate/Severe vs Healthy control | 0.88 (−0.11; 1.88) | 0.60 (0.04; 1.16) | – | – | |
| Adjusted for gender | Critical vs Moderate/Severe | −1.89 (−2.85; −0.93) | −1.34 (−1.94; −0.73) | 0.248 | 0.0001 |
| Critical vs Healthy control | −1.11 (−2.09; −0.13) | −0.79 (−1.35; −0.21) | – | – | |
| Moderate/Severe vs Healthy control | 0.78 (−0.18; 1.75) | 0.55 (−0.006; 1.11) | – | – |
Fig. 2.
The gating of CD4+ HLA-G+ T lymphocytes is presented in each group.
3.3. The lower frequency of CD14+ HLA-G+ monocytes in critical COVID-19 patients
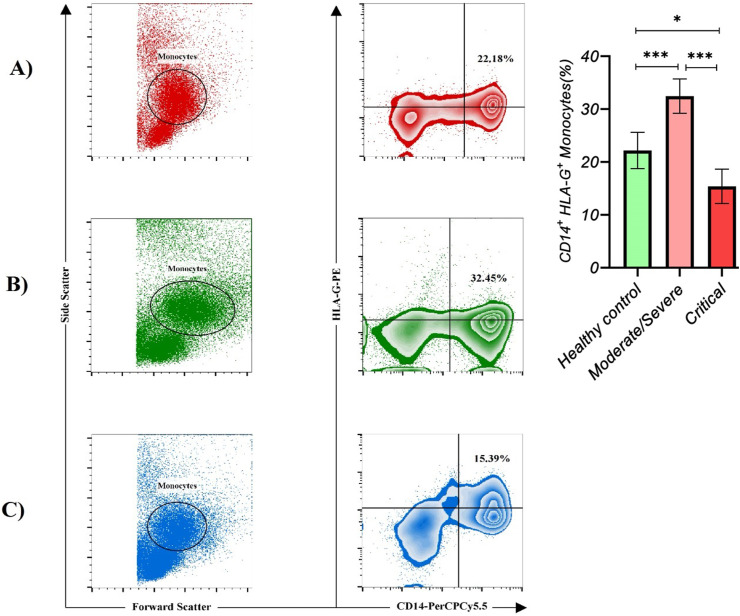
Critical COVID-19 patients had a significantly lower frequency of CD14+ HLA-G+ monocytes compared with moderate/severe COVID-19 patients (p value < 0.001; SMD, −2.09; 95% CI [−2.77, −1.41]) and healthy controls (p value < 0.05; SMD, −0.83; 95% CI [−1.40, −0.25]). Moreover, adjusted analyses for age or gender showed the same results (Table 4 , Fig. 3 ). On the other hand, moderate/severe COVID-19 patients had a significantly higher frequency of CD14+ HLA-G+ monocytes compared with healthy controls (p value < 0.001; SMD, 1.26; 95% CI [0.65, 1.86]). Moreover, adjusted analyses for age or gender showed the same results, and Eta2 was not different between the 3 ANOVA models (Table 4). The gating strategy for the determination of CD14+ HLA-G+ monocytes is shown in Fig. 3.
Table 4.
ANOVA analyses of CD14+ HLA-G+ monocytes.
| Model | Groups | Mean difference (95% CI) | SMD (95% CI) | Partial Eta2 | P value$ |
|---|---|---|---|---|---|
| Crude | Critical vs Moderate/Severe | −17.06 (−22.59; −11.54) | −2.09 (−2.77; −1.41) | 0.439 | 0.0000 |
| Critical vs Healthy control | −6.79 (−12.37; −1.20) | −0.83 (−1.40; −0.25) | – | – | |
| Moderate/Severe vs Healthy control | 10.27 (4.69; 15.85) | 1.26 (0.65; 1.86) | – | – | |
| Adjusted for age | Critical vs Moderate/Severe | −17.22 (−22.94; −11.51) | −2.04 (−2.70; −1.35) | 0.439 | 0.0000 |
| Critical vs Healthy control | −6.51 (−12.63; −0.38) | −0.76 (−1.32; −0.18) | – | – | |
| Moderate/Severe vs Healthy control | 10.71 (4.86; 16.57) | 1.26 (0.65; 1.86) | – | – | |
| Adjusted for gender | Critical vs Moderate/Severe | −17.53 (−23.18; −11.88) | −2.10 (−2.78; −1.42) | 0.449 | 0.0000 |
| Critical vs Healthy control | −7.20 (−12.98; −1.42) | −0.86 (−1.43; −0.28) | – | – | |
| Moderate/Severe vs Healthy control | 10.32 (4.61; 16.04) | 1.24 (0.63; 1.83) | – | – |
Fig. 3.
The gating of the CD14+ HLA-G+ monocytes is presented in each group.
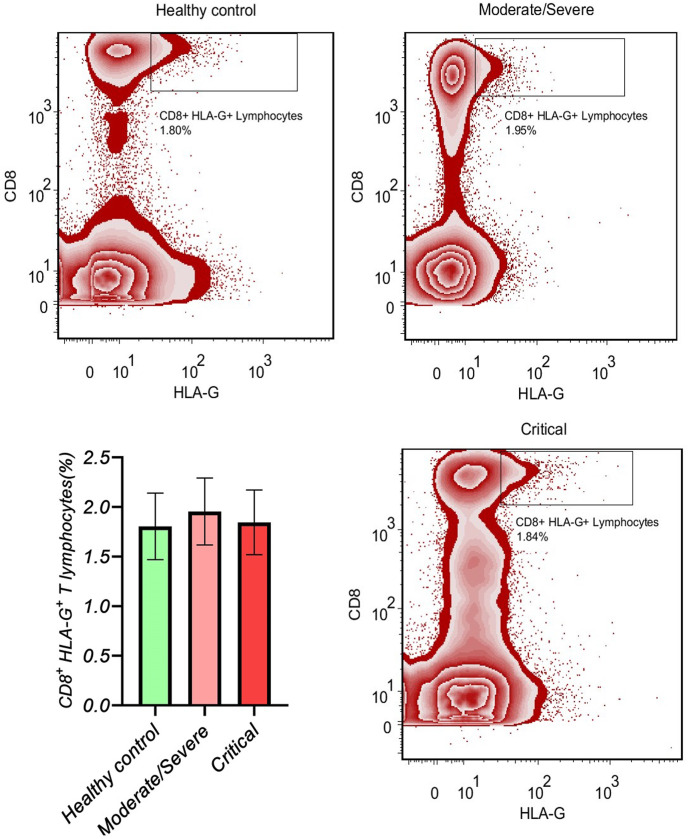
3.4. The same frequency of CD8+ HLA-G+ T lymphocytes between the study groups
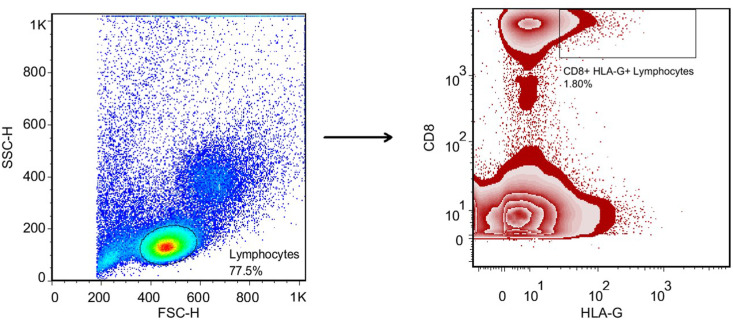
The gating strategy for the determination of CD8+ HLA-G + T lymphocytes is shown in Fig. 4 . There was no difference between the study groups regarding the frequency of CD8+ HLA-G+ T lymphocytes: critical COVID-19 patients vs. moderate/severe COVID-19 patients (p value > 0.05; SMD, −0.13; 95% CI [−0.67, 0.41]), critical COVID-19 patients vs. healthy controls (p value > 0.05; SMD, 0.04; 95% CI [−0.49, 0.59]), and moderate/severe COVID-19 patients vs. healthy controls (p value > 0.05; SMD, 0.18; 95% CI [−0.36, 0.73]; Table 5 , Fig. 5 ). Moreover, the adjusted analyses showed the same results, and Eta2 was not different between the 3 ANOVA models (Table 5).
Fig. 4.
The gating strategy for determination of the CD8+ HLA-G+ T lymphocytes.
Table 5.
ANOVA analyses of CD8+ HLA-G+ T lymphocytes.
| Model | Groups | Mean difference (95% CI) | SMD (95% CI) | Partial Eta2 | P value$ |
|---|---|---|---|---|---|
| Crude | Critical vs Moderate/Severe | −0.11 (−0.66; 0.44) | −0.13 (−0.67; 0.41) | 0.006 | 0.7928 |
| Critical vs Healthy control | 0.04 (−0.52; 0.60) | 0.04 (−0.49; 0.59) | – | – | |
| Moderate/Severe vs Healthy control | 0.15 (−0.41; 0.71) | 0.18 (−0.36; 0.73) | – | – | |
| Adjusted for age | Critical vs Moderate/Severe | −0.13 (−0.70; 0.43) | −0.16 (−0.70; 0.38) | 0.004 | 0.5837 |
| Critical vs Healthy control | −0.08 (−0.69; 0.52) | −0.09 (−0.64; 0.45) | – | – | |
| Moderate/Severe vs Healthy control | 0.05 (−0.52; 0.63) | 0.06 (−0.48; 0.61) | – | – | |
| Adjusted for gender | Critical vs Moderate/Severe | −0.10 (−0.67; 0.46) | −0.12 (−0.67; 0.41) | 0.003 | 0.8293 |
| Critical vs Healthy control | −0.002 (−0.58; 0.57) | −0.003 (−0.55; 0.54) | – | – | |
| Moderate/Severe vs Healthy control | 0.10 (−0.47; 0.67) | 0.12 (−0.42; 0.67) | – | – |
Fig. 5.
The gating of CD8+ HLA-G+ T lymphocytes is presented in each group.
4. Discussion
COVID-19 is an infectious disease that leads to a dysregulated immune system in critical patients. Understanding the immunological features of COVID-19 patients may help clinicians to manage the disease more effectively. In our previous articles, we assessed some immunological features of moderate/severe and critical COVID-19 patients with interesting results. We showed that non-intensive care unit (ICU) patients had a significantly higher amount of interferon λ1 (IFN-λ1) (836.7 ± 284.6 vs. 81.57 ± 34.25) and INF-λ2 (798.8 ± 301.5 vs. 48.32 ± 28.13) compared with ICU patients [15]. The INF-λ family or type III interferon is the most recently discovered interferon and divide into 4 members, including IFN-λ1/IL-29, IFN-λ2/IL-28 A, IFN-λ3/IL-28 B, and IFN-λ4. INF-λ receptor 1 (INF-λR1) and IL-10R2 are the heterodimeric receptors of INF-λ [15]. Since the expression of INF-λR1 is primarily limited to the respiratory tract epithelial cells, INF-λ has a major role in mucosal immunity and viral respiratory tract infection [[15], [16], [17]]. These results indicate an immune dysregulation in which enough amount of type III interferons are not secreted.
Cellular immunity is a crucial component of the immune system in which CD4+ T cells and CD8+ T cells play an important function against foreign antigens. We and others showed that in addition to lymphopenia, exhaustion of both CD4+ T cells and CD8+ T cells are associated with critical COVID-19 [1,2,18,19]. These results indicate another immune dysregulation in which cellular immunity is dysregulated.
Inflammation and cytokine storm are other kinds of immune dysregulation observed in critical COVID-19 patients [20]. Inflammation and cytokine storm are characterized by great production of IL-1, IL-6, IL8, tumor necrosis factor α (TNF-α), etc. [3]. Moreover, other inflammatory factors, such as CRP and pentraxin 3 (PTX3), are linked to critical COVID-19 [4]. As a result, corticosteroid therapy is used to reduce inflammation and improve the disease severity, which is useful [21]. In this regard, the role of regulatory cells to alleviate inflammation and control excessive immune responses is crucial in the inflammatory phase of COVID-19.
Several studies have evaluated the frequency of regulatory T cells (Tregs) in COVID-19 patients. CD4+ FOXP3+ Tregs can ameliorate the immunopathology of respiratory syncytial virus infection [22] and, thereby, reduce disease severity. In the setting of COVID-19, studies have shown inconsistent results, which may be due to the different strategies used for the determination of Tregs. Some studies have reported that severe COVID-19 patients had a lower frequency of Tregs compared with healthy controls [23]; in other words, the higher the severity of COVID-19, the lower the frequency of Tregs [24,25]. On the other hand, some studies have found no difference between different stages of COVID-19 [26] or found that severe COVID-19 patients had a higher frequency of Tregs compared with mild COVID-19 patients [27].
HLA-G+ cells are defined as cells with immunomodulatory properties. There is little information about the role of these cells and their possible immunomodulatory function in the setting of COVID-19. In this study, we evaluated the frequency of these cells and found that critical COVID-19 patients had a significantly lower frequency of both CD4+ HLA-G+ T lymphocytes and CD14+ HLA-G+ monocytes compared with moderate/severe COVID-19 patients and healthy controls. On the other hand, the frequency of the mentioned cells was significantly higher in moderate/severe COVID-19 patients compared with healthy controls. These results indicate that moderate/severe COVID-19 patients have more immunomodulatory properties than critical COVID-19 patients, and such a feature may help moderate/severe COVID-19 patients modulate inflammatory conditions. Both crude and adjusted analyses showed the same results. In line with our study, Bortolotti et al. showed that increased levels of sHLA-G were associated with improved COVID-19 status [14]. However, more studies are required to further evaluate the role of HLA-G+ cells and their association with COVID-19 improvement. Regarding CD8+ HLA-G+ T cells, we did not find any significant and considerable difference between our study groups in both crude and adjusted analyses.
5. Conclusion
We showed that critical COVID-19 patients had a lower frequency of HLA-G+ cells, and given the modulatory role of HLA-G+ cells, it can be implied that such lower immunomodulatory properties may lead to the critical condition in these patients.
Declarations
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
The dataset analyzed in this article is not publicly available. Requests to access the datasets should be directed to the corresponding author's email address.
Conflict interest statement
The authors declare no conflict of interest.
Funding
Not applicable.
CRediT authorship contribution statement
Somayeh Ramzannezhad: Writing – original draft, Methodology, Investigation. Mona Tarighi: Writing – original draft, Methodology, Investigation. Mousa Mohammadnia-Afrouzi: Writing – original draft, Software, Methodology, Investigation. Soudabeh Aghapour: Investigation, Formal analysis. Mojgan Bagherzadeh: Writing – original draft, Investigation. Zahra Ahmadnia: Methodology, Investigation. Akramossadat Hosseini: Writing – review & editing, Formal analysis. Mostafa Javanian: Visualization, Methodology. Housein Ghorbani: Writing – review & editing, Supervision. Mehdi Shahbazi: Writing – review & editing, Supervision, Conceptualization.
Declaration of competing interest
The authors declare no competing financial interests. The authors alone are responsible for the content and writing of the paper.
Acknowledgments
None.
Data availability
Data will be made available on request.
References
- 1.Modabber Z., Shahbazi M., Akbari R., Bagherzadeh M., Firouzjahi A., Mohammadnia‐Afrouzi M. TIM‐3 as a potential exhaustion marker in CD4+ T cells of COVID‐19 patients. Immun. Inflam. Dis. 2021;9(4):1707–1715. doi: 10.1002/iid3.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shahbazi M., Moulana Z., Sepidarkish M., Bagherzadeh M., Rezanejad M., Mirzakhani M., et al. Pronounce expression of Tim-3 and CD39 but not PD1 defines CD8 T cells in critical Covid-19 patients. Microb. Pathog. 2021;153 doi: 10.1016/j.micpath.2021.104779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mrityunjaya M., Pavithra V., Neelam R., Janhavi P., Halami P., Ravindra P. Immune-boosting, antioxidant and anti-inflammatory food supplements targeting pathogenesis of COVID-19. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.570122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moulana Z., Bagherzadeh M., Mirzakhani M., Rostami A., Mohammadnia-Afrouzi M., Shahbazi M. Increased levels of serum pentraxin 3 in critical coronavirus disease-2019 patients. Environ. Sci. Pollut. Control Ser. 2021:1–5. doi: 10.1007/s11356-021-15183-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amodio G., Gregori S. Hla-g genotype/expression/disease association studies: success, hurdles, and perspectives. Front. Immunol. 2020;11:1178. doi: 10.3389/fimmu.2020.01178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zidi I. Puzzling out the COVID-19: therapy targeting HLA-G and HLA-E. Hum. Immunol. 2020;81(12):697–701. doi: 10.1016/j.humimm.2020.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Contini P., Murdaca G., Puppo F., Negrini S. HLA-G expressing immune cells in immune mediated diseases. Front. Immunol. 2020;11:1613. doi: 10.3389/fimmu.2020.01613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mirzakhani M., Shahbazi M., Shamdani S., Naserian S., Mohammadnia-Afrouzi M. Innate immunity: trained immunity and innate allorecognition against the allograft. Int. Rev. Immunol. 2021:1–8. doi: 10.1080/08830185.2021.1921175. [DOI] [PubMed] [Google Scholar]
- 9.Mirzakhani M., Shahbazi M., Akbari R., Oliaei F., Asgharpour M., Nikoueinejad H., et al. Reduced CD4+ CD25++ CD45RA− Foxp3hi activated regulatory T cells and its association with acute rejection in patients with kidney transplantation. Transpl. Immunol. 2020;60 doi: 10.1016/j.trim.2020.101290. [DOI] [PubMed] [Google Scholar]
- 10.Mirzakhani M., Shahbazi M., Oliaei F., Mohammadnia‐Afrouzi M. Immunological biomarkers of tolerance in human kidney transplantation: an updated literature review. J. Cell. Physiol. 2019;234(5):5762–5774. doi: 10.1002/jcp.27480. [DOI] [PubMed] [Google Scholar]
- 11.Ehsani M., Mohammadnia-Afrouzi M., Mirzakhani M., Esmaeilzadeh S., Shahbazi M. Female unexplained infertility: a disease with imbalanced adaptive immunity. J. Hum. Reprod. Sci. 2019;12(4):274. doi: 10.4103/jhrs.JHRS_30_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ehsani M., Mohammadnia-Afrouzi M., Esmaeilzadeh S., Tajalli Z., Jafari M., Shahbazi M. Decreased frequency of CD8+ HLA-G+ T cell in the peripheral blood of primary unexplained infertile females. Reprod. Sci. 2021:1–6. doi: 10.1007/s43032-020-00431-z. [DOI] [PubMed] [Google Scholar]
- 13.Rebmann V., da Silva Nardi F., Wagner B., Horn P.A. HLA-G as a tolerogenic molecule in transplantation and pregnancy. J. immunology. res. 2014;2014:297073–297089. doi: 10.1155/2014/297073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bortolotti D., Gentili V., Rizzo S., Schiuma G., Beltrami S., Spadaro S., et al. Increased sHLA-G is associated with improved COVID-19 outcome and reduced neutrophil adhesion. Viruses. 2021;13(9):1855. doi: 10.3390/v13091855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shahbazi M., Amri Maleh P., Bagherzadeh M., Moulana Z., Sepidarkish M., Rezanejad M., et al. Linkage of lambda interferons in protection against severe COVID-19. J. Interferon Cytokine Res. 2021;41(4):149–152. doi: 10.1089/jir.2020.0187. [DOI] [PubMed] [Google Scholar]
- 16.Davidson S., McCabe T.M., Crotta S., Gad H.H., Hessel E.M., Beinke S., et al. IFN λ is a potent anti‐influenza therapeutic without the inflammatory side effects of IFN α treatment. EMBO Mol. Med. 2016;8(9):1099–1112. doi: 10.15252/emmm.201606413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Galani I.E., Triantafyllia V., Eleminiadou E.-E., Koltsida O., Stavropoulos A., Manioudaki M., et al. Interferon-λ mediates non-redundant front-line antiviral protection against influenza virus infection without compromising host fitness. Immunity. 2017;46(5):875–890. doi: 10.1016/j.immuni.2017.04.025. e6. [DOI] [PubMed] [Google Scholar]
- 18.De Biasi S., Meschiari M., Gibellini L., Bellinazzi C., Borella R., Fidanza L., et al. Marked T cell activation, senescence, exhaustion and skewing towards TH17 in patients with COVID-19 pneumonia. Nat. Commun. 2020;11(1):1–17. doi: 10.1038/s41467-020-17292-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zheng M., Gao Y., Wang G., Song G., Liu S., Sun D., et al. Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cell. Mol. Immunol. 2020;17(5):533–535. doi: 10.1038/s41423-020-0402-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Magro G. COVID-19: review on latest available drugs and therapies against SARS-CoV-2. Coagulation and inflammation cross-talking. Virus Res. 2020;286 doi: 10.1016/j.virusres.2020.198070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Paassen J., Vos J.S., Hoekstra E.M., Neumann K.M., Boot P.C., Arbous S.M. Corticosteroid use in COVID-19 patients: a systematic review and meta-analysis on clinical outcomes. Crit. Care. 2020;24(1):1–22. doi: 10.1186/s13054-020-03400-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu Y., Qi G., Bellanti J.A., Moser R., Ryffel B., Zheng S.G. Regulatory T cells: a potential weapon to combat COVID‐19? MedComm. 2020;1(2):157–164. doi: 10.1002/mco2.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sadeghi A., Tahmasebi S., Mahmood A., Kuznetsova M., Valizadeh H., Taghizadieh A., et al. Th17 and Treg cells function in SARS‐CoV2 patients compared with healthy controls. J. Cell. Physiol. 2021;236(4):2829–2839. doi: 10.1002/jcp.30047. [DOI] [PubMed] [Google Scholar]
- 24.Wang H., Wang Z., Cao W., Wu Q., Yuan Y., Zhang X. Regulatory T cells in COVID-19. Aging and disease. 2021;12(7):1545–1553. doi: 10.14336/AD.2021.0709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meckiff B.J., Ramírez-Suástegui C., Fajardo V., Chee S.J., Kusnadi A., Simon H., et al. Imbalance of regulatory and cytotoxic SARS-CoV-2-reactive CD4+ T cells in COVID-19. Cell. 2020;183(5):1340–1353. doi: 10.1016/j.cell.2020.10.001. e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shahbazi M., Jafari M., Moulana Z., Sepidarkish M., Bagherzadeh M., Rezanejad M., et al. Reduced frequency of T helper 17 and T helper 1 cells and their association with critical coronavirus disease 2019. Apmis. 2021;129(5):271–279. doi: 10.1111/apm.13129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Galván-Peña S., Leon J., Chowdhary K., Michelson D.A., Vijaykumar B., Yang L., et al. Profound Treg perturbations correlate with COVID-19 severity. Proc. Natl. Acad. Sci. Unit. States Am. 2021;118(37) doi: 10.1073/pnas.2111315118. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The dataset analyzed in this article is not publicly available. Requests to access the datasets should be directed to the corresponding author's email address.
Data will be made available on request.