Abstract
The new dimethoxycatechol 4,5-dimethoxy-1,2-benzenediol (DMC) and the new dimethoxyhydroquinone 2,5-dimethoxy-1,4-benzenediol (DMH) were isolated from stationary cultures of the brown rot fungus Gloeophyllum trabeum growing on a glucose mineral medium protected from light. The structure was elucidated by gas chromatography-mass spectrometry through comparison to a synthetic standard. Further confirmation was obtained by forming a dimethoxyoxazole derivative by condensation of DMC with methylene chloride and through examination of methylated derivatives. DMC and DMH may serve as ferric chelators, oxygen-reducing agents, and redox-cycling molecules, which would include functioning as electron transport carriers to Fenton’s reactions. Thus, they appear to be important components of the brown rot decay system of the fungus.
The fundamental processes that mediate the degradation of lignocellulose and various anthropogenic chemicals by white rot fungi such as Phanerochaete chrysosporium have been known for several years (12, 17). New information refining the details of these processes in white rot fungi appears frequently. With regard to the degradation of lignin, a variety of extracellular peroxidases and oxidases, several systems that generate hydrogen peroxide, several small molecules that mediate free-radical reactions, and various metal ions together constitute what has been termed the lignolytic or ligninolytic system. Lignin degradation by white rot fungi is a free-radical-based process that has also been termed “biological combustion” (8, 16, 24, 25, 32).
Brown rot fungi, on the other hand, have been much less investigated than white rot fungi, and their mechanisms of wood decay remain more of a mystery. Several recent review articles have attempted to explain the biochemical mechanisms underlying brown rot decay (1, 14, 19, 28). An understanding of brown rot decay is needed to limit the damage to wood products by this type of fungal attack on lignocellulose, damage that costs billions of dollars annually in the United States. All present models of brown rot decay are only partially supported by experimental data. All of them assume that such decay involves a Fenton-type catalytic system that produces hydroxyl radicals that attack wood components.
A mechanism for the production of hydroxyl radicals by the brown rot fungus Coniophora puteana was described by Hyde and Wood (19), whose model suggested the reduction of Fe(III) by cellobiose dehydrogenase (CDH) within the cells, diffusion of the Fe(II) produced away from the hyphae, formation of an Fe(II)-oxalate complex, and, finally, Fenton-reaction-based hydroxyl radical formation at a “safe” distance from the hyphae. A weak point of this model is the very slow interaction of CDH with Fe(III). The production of CDH by brown rot fungi growing on natural substrates has been questioned (12), although Schmidthalter and Canevascini (27) did purify CDH from C. puteana. However, C. puteana may not be a typical brown rot fungus, if a typical “brown rotter” can indeed be described (1).
Roles for oxalic acid in both brown rot and white rot decay were suggested by Shimada et al. (28), who postulated that brown rot fungi may use oxalic acid as a proton donor for enzymatic and nonenzymatic hydrolysis of polysaccharides and as a chelator for an Fe(II)-H2O2 system generating hydroxyl radicals. A model of brown rot decay proposed by Enoki et al. (10, 11) requires the presence of extracellular NADH or ascorbate as a reductant of Fe(III), but no evidence was presented to suggest that NADH is excreted outside the cells of brown rot fungi. One unconfirmed report indicates that the white rot fungus P. chrysosporium secretes NAD+ and NADP+ (22).
The model of Goodell et al. (14) proposes that a low-molecular-weight metal chelator other than oxalate is involved in brown rot wood decay. The authors suggested that the natural chelators produced by brown rot fungi not only have a strong affinity for Fe(III) but also mediate redox cycling of iron at the low pHs associated with these fungal cultures. Fe(II) produced by the cultures could then react with H2O2 to produce active oxygen species involved in brown rot decay of wood. This model does not fully explain the electron source needed for the continuous reduction of Fe(III), a product of Fenton’s chemistry.
Here we provide evidence that the brown rot fungus G. trabeum growing on glucose in a mineral medium synthesizes de novo and secretes 4,5-dimethoxy-1,2-benzenediol (4,5-dimethoxycatechol [DMC]) and 2,5-dimethoxy-1,4-benzenediol (2,5-dimethoxyhydroquinone [DMH]). These compounds were detectable in 2-week-old cultures, reaching a maximum concentration of about 50 μM after 5 to 7 weeks.
MATERIALS AND METHODS
Reagents.
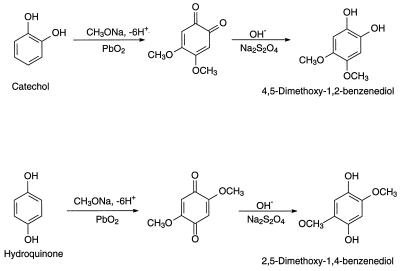
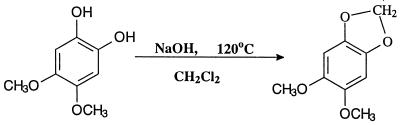
All chemicals, unless otherwise specified, were purchased from Aldrich (Milwaukee, Wis.) or Sigma Chemical Co. (St. Louis, Mo.), at the highest obtainable purity. Coenzyme Q0 (2,3-dimethoxy-5-methyl-1,4-benzoquinone) was purchased from Fluka (Buchs, Switzerland). 1,2,3,4-Tetramethoxybenzene, 1,2,4,5-tetramethoxybenzene, and 1,2,3,5-tetramethoxybenzene were obtained from Philip Kersten (USDA Forest Products Laboratory, Madison, Wis.) and were prepared as described earlier (21). 4,5-Dimethoxy-1,2-benzoquinone was prepared from catechol according to the method of Wounzlick and Jahnke (30), slightly modified. Sulfuric acid replaced oxalic and acetic acids for the removal of lead from the product. Prior to the removal of lead, the quinone was reduced to its hydroquinone form with Na-dithionite. 2,5-Dimethoxy-1,4-benzoquinone was prepared by the same procedure, substituting hydroquinone for catechol. Briefly, a solution containing 2 g of NaOH in 100 ml of pure methanol and 20 g of PbO2 was stirred at 20°C, and a solution of 2.2 g of catechol or hydroquinone was added dropwise. The mixture was kept under nitrogen at 20 to 24°C for 15 min after the organic reactant was completely added. The resulting solution was filtered under vacuum to remove PbO. The soluble lead was precipitated by addition of 50% H2SO4 to the filtrate until the pH reached 5. The suspension was filtered again, and the clear solution was twice extracted with methylene chloride. The combined solvent extracts were reduced by evaporation to 1/10 of the original volume. The sample was then acidified with acetic acid, saturated with nitrogen, and cooled to 5°C, where the products crystallized. To obtain the corresponding hydroquinones (DMC and DMH), the mixture was reduced with Na-dithionite (4 g in 10 ml of water) after the first filtration (13). After reduction, the pH was adjusted to 4 with H2SO4, and the procedure was continued as described above for the benzoquinones. Benzo-1,3-dioxole (methylenedioxybenzene) was synthesized from catechol, and 5,6-dimethoxybenzo-1,3-dioxole was synthesized from 4,5-dimethoxy-1,2-benzenediol as described previously (5).
Culture conditions.
Gloeophyllum trabeum (ATCC 11539) was obtained from the University of Maine collection. It was grown at room temperature in a defined liquid medium originally developed for P. chrysosporium (4) and containing 1% glucose, 0.5 mM NH4NO3, 0.5 mM asparagine, and 0.1 mM MnCl2 (20). The medium was buffered with 10 mM 2,2′-dimethylsuccinic acid sodium salt at a pH of 4.5. Stationary cultures were incubated in Roux flasks containing 150 ml of medium at 24°C. The flasks were inoculated with about 2 cm2 of mycelial mat from a 6-week-old liquid culture. After this material was added, it was broken into small pieces by shaking the flasks for about 20 s. Since DMC and related compounds are light sensitive, the cultures were incubated in the dark. In cultures exposed to laboratory light, DMC was not detected.
Extraction and analysis of culture broths.
G. trabeum cultures grown for 2 to 12 weeks were analyzed for production of organic compounds. Noninoculated media were always prepared alongside experimental flasks and used as controls for all extractions and analyses. In no cases were the organic metabolites found in uninoculated cultures. All observations were repeated multiple times to confirm their reproducibility (data not presented). In a typical procedure, a culture was filtered through glass wool to remove mycelia, and the filtrate was extracted twice with half volumes of methylene chloride. Prior to the second extraction, 0.3 ml of 50% H2SO4 was added to the filtrate. Methylene chloride extracts were combined, dried over Na2SO4, and filtered, and the solvent was removed by evaporation under a gentle stream of nitrogen. During the concentration step, the solution was protected from moisture. The dried extract was dissolved in a small volume of methylene chloride or acetonitrile and analyzed. For some analyses, the dry extract was first methylated with diazomethane added in methyltertbutyl ether. Diazomethane was generated from Diazald (N-methyl-N-nitroso-p-toluenesulfonamide) with a diazomethane-generating glassware kit (Aldrich) according to the previously described directions (3, 9). Samples were analyzed on the day of preparation. DMC was unstable in concentrated extracts and was detectable only in fresh samples.
Gas chromatography-mass spectrometry (GC-MS) was performed with a Hewlett-Packard (HP) series II 5890 gas chromatograph equipped with a capillary fused-silica column (30 m by 0.25 mm) coated with CP-SIL 8CB MS (Chrompack, Middelburg, The Netherlands). The injector temperature was set at 250°C, and the GC-MS interface was set at 280°C. The analyses were run under an oven linear temperature gradient from 100 to 200°C at a rate of 5°C/min and then from 200 to 300°C at 20°C/min. Samples (2 μl) were introduced to the GC by an automatic injector (HP-7673). A HP quadrupole MS (5989A) controlled by HP MS Chemstation software (PC version) was used for MS analyses under the following standard conditions: repeller, 7 V; emission, 300 V; and electron energy, 70 eV. The source temperature was 250°C, and the quadrupole temperature was 125°C. The scan parameters were 30 to 350 or 30 to 750 m/z. Perfluorotributylamine was used as the calibration standard for the MS engine. Interpretation of the MS spectrum was aided by the Wiley and National Institute of Standards and Technology library of mass spectra stored in the Chemstation database (approximately 200,000 spectra).
RESULTS
G. trabeum produced a number of aromatic metabolites while growing in a defined mineral medium with glucose as the carbon and energy source. The medium was similar to that used in many studies of the growth of P. chrysosporium, except that it contained a small additional amount of asparagine to enhance the yield of this slow-growing brown rot fungus. We identified benzoic acid (compound XIV), benzene ethanol (XIII), and the methyl ester of α-hydroxybenzeneacetic acid (XV). MS spectra of these metabolites (Table 1) gave high-quality matches with spectra of identical compounds stored in the Wiley HP Chemstation database (spectra not shown here). These minor metabolites have not been previously observed in cultures of G. trabeum and thus increase the number of its known metabolites beyond those reported in an earlier investigation of the growth of the fungus on cellulose (14).
TABLE 1.
Mass spectra and GC retention times of aromatic compounds used in this research
| Compound (no.) | GC retention timea | Mass spectrum m/z [relative intensity (%)] |
|---|---|---|
| DMC isolated from fungal culture (I) | 13.63 | 170 (79), 155 (100), 127 (36.6), 112 (19.2), 109 (6.3) |
| DMC (synthetic) (II) | 13.70 | 170 (71.2), 155 (100), 127 (31.5), 112 (15.3), 109 (5.3) |
| DMH (III) | 16.46 | 170 (60.7), 155 (100), 127 (56.1), 112 (27), 109 (9.0) |
| DMH (synthetic) | 13.40 | 170 (62.3), 155 (100), 127 (67.5), 112 (32), 109 (8.2) |
| 2,5-Dimethoxy-1,4-benzoquinone (IV) | 15.73 | 170 (28.8), 168 (100), 155 (14.8), 140 (29.1), 138 (39.2), 127 (11.5), 125 (32.6), 112 (47.3), 110 (23.3), 97 (21.4), 82 (11.5), 80 (76.5), 53 (46.2), 41 (18.4) |
| 2,4,5-Trimethoxyphenol (V) | 14.19 | 184 (75.8), 169 (100), 141 (11.9), 126 (26.6), 123 (7.8), 109 (43.8), 95 (7.1), 81 (8.2) |
| 1,2,4,5-Tetramethoxybenzene (VI) (after methylation of the culture extract) | 14.65 | 198 (100), 183 (86.3), 155 (37.0), 140 (27.1), 125 (20.8), 123 (28.7), 111 (3.7), 95 (11.7) |
| 1,2,3,4-Tetramethoxybenzene (VII) | 12.68 | 198 (100), 183 (66.3), 168 (14.1), 155 (10.8), 140 (32.9), 123 (15.7), 97 (10.6), 95 (10.6) |
| 1,2,3,5-Tetramethoxybenzene (VIII) | 14.88 | 198 (66.5), 183 (100), 168 (1.6), 155 (31.4), 140 (18.31), 125 (16.0), 123 (11.7), 109 (5.9), 95 (4.8) |
| 2,3-Dimethoxy-5-methyl-1,4-benzenediol (IX) | 11.57 | 184 (100), 169 (24.4), 154 (10.3), 151 (6.4), 141 (11.3), 126 (25.50), 123 (24.4), 95 (5.4) |
| 2,3-Dimethoxy-5-methyl-1,4-benzoquinone (X) | 10.75 | 182 (80.1), 157 (30.2), 153 (18.2), 139 (27.2), 137 (100), 136 (29.0), 111 (21.7), 96 (12.0), 83 (56.7), 69 (43.4) |
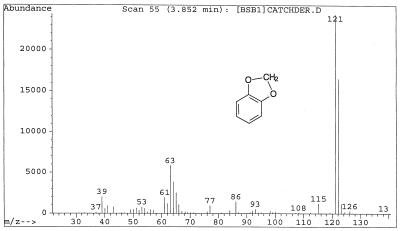
| 1,3-Benzodioxole (XI) | 3.85 | 122 (67.7), 121 (100), 93 (2.2), 86 (5.9), 65 (10.3), 64 (15.9), 65 (24.0) |
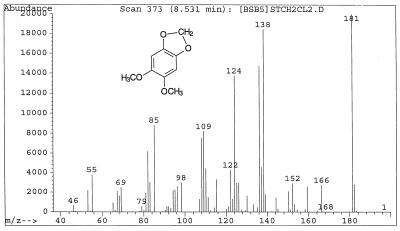
| 5,6-Dimethoxybenzo-1,3-dioxole (XII) | 8.55 | 182 (14.1), 181 (100), 166 (13.7), 138 (92.2), 136 (73.8), 126 (14.8), 124 (68.7), 122 (21.4), 110 (22.0), 109 (41.0), 108 (37.5), 85 (44.1), 82 (30.9) |
| Benzenethanol (XIII) | 5.48 | 122 (34.7), 92 (66.7), 91 (100), 77 (5.4), 65 (22.5), 63 (4.6), 51 (6.9) |
| Benzoic acid (XIV) | 6.85 | 122 (89.4), 105 (100), 77 (71.6), 74 (9.4), 51 (27.4), 50 (14.0) |
| α-Hydroxybenzeneacetic acid methyl ester (XV) | 9.01 | 166 (13.7), 107 (100), 108 (8,5), 79 (60.5), 77 (34.34), 51 (102), 39 (3.0) |
Underlined retention times represent compounds isolated from G. trabeum cultures.
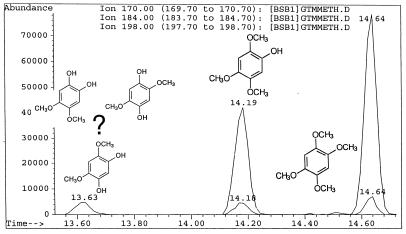
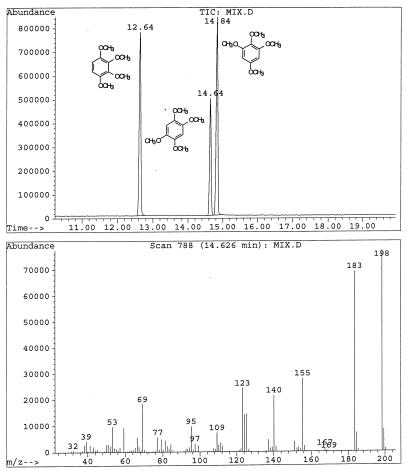
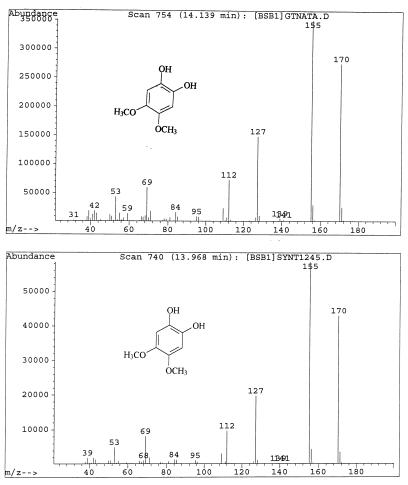
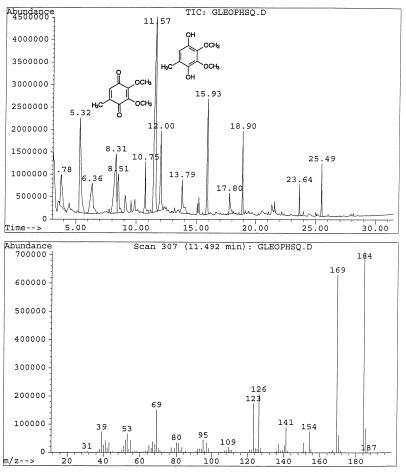
G. trabeum cultures also produced, but in significantly higher concentrations, dimethoxycatechols (i.e., DMC [compound I]) and dimethoxybenzoquinone (DMH [compound III]), both showing a characteristic molecular weight of 170. A methylene chloride extract of the culture methylated with diazomethane showed the expected conversion of the DMC to 2,4,5-trimethoxyphenol (V) and 1,2,4,5-tetramethoxybenzene (VI) (Fig. 1). Thus, the derivatization confirmed that the metabolite contained two free hydroxyl substituents. We observed that by increasing the reaction time of methylation, the proportion of DMC converted to tetramethoxybenzene increased. Three synthetic isomeric forms of tetramethoxybenzene were used as standards (Table 1) to confirm the structure of the fungal DMC metabolite. The GC retention time and the mass spectrum of the methylated fungal product matched those of synthetic 1,2,4,5-tetramethoxybenzene (VI) (Fig. 2). Finally, to distinguish unequivocally among the isomeric possibilities—1,2-, 1,3-, or 1,4-benzenediol—we prepared synthetic DMC (II) and DMH (III) from catechol and hydroquinone, respectively (Fig. 3). Compound II might also be prepared by the method of Prati and Rossi (26). The mass spectrum and GC retention time of the dimethoxycatechol extracted from the fungal culture matched synthetic 4,5-dimethoxy-1,3-benzenediol (Fig. 4 and Table 1). Similarly, the mass spectrum and GC retention time of synthetic 2,5-dimethoxy-1,4-benzenediol matched the synthetic standard. As further proof of the structure, the DMC produced by G. trabeum reacted with methylene chloride to form 5,6-dimethoxybenzo-1,3-dioxole (XII) (Fig. 5), in a manner similar to that of catechol, which formed 1,3-benzodioxole (methylenedioxybenzedine [XI]) (Fig. 6) (5). This reaction (Fig. 7) is characteristic of ortho hydroquinones only. Both dioxoles produced very characteristic mass spectra, with the most intensive signal from the parent ions minus a proton (Table 1 and Fig. 5 and 6).
FIG. 1.
GC-MS three-ion chromatogram of methylated metabolites extracted from a 4-week-old G. trabeum culture. The ions extracted from the total ion chromatogram (50 to 650 mass units) were 170, 184, and 198. Single-hydroxyl methylation increased the mass by 14; two-hydroxyl methylation increased the mass by 28. Three possible isomeric structures of dihydroxydimethoxybenzene resulting from methylation are shown.
FIG. 2.
GC-MS three-ion chromatogram (TIC) of reference tetramethoxy-benzenes. The retention time and mass spectrum of synthetic 1,2,4,5-tetramethoxybenzene matched the metabolite in the methylated extract shown in Fig. 1.
FIG. 3.
Synthesis of DMC and DMH.
FIG. 4.
(Upper panel) Mass spectrum of natural dimethoxycatechol (DMC) occurring as a fungal metabolite. (Lower panel) Mass spectrum of synthetic DMC.
FIG. 5.
Mass spectrum of 5,6-dimethoxybenzo-1,3-dioxole, a reaction product of DMC with methylene chloride, under alkaline conditions at a temperature of 120°C.
FIG. 6.
Mass spectrum of benzo-1,3-dioxole, a reaction product of catechol and methylene chloride.
FIG. 7.
Reaction of DMC with methylene chloride.
DISCUSSION
The newly described, de novo-synthesized G. trabeum metabolites DMC and DMH were produced during most of the fungal growth cycle at levels as high as 0.05 mM at 5 weeks. The concentration then slowly decreased as the culture aged but remained detectable for up to 12 weeks of culture. DMC and DMH are interesting compounds in that the methoxyl groups increase redox reactivity of the hydroxyls, which can readily reduce oxygen. The methoxy ortho quinone in the two-electron oxidized form is a good metal chelator. Since DMC was found both extracellularly and within the fungal cells, it is a good candidate for an electron carrier between hyphae and Fenton reaction centers outside the cell. Also, DMC and DMH in their quinone forms could be directly involved in a variety of redox reactions during brown rot decay of lignocellulose. DMC and DMH thus appear to fit well into the Fenton chemistry model of brown rot decay of wood proposed by Goodell et al. (14).
DMC has several important implications for fungal brown rot decay mechanisms. This compound first of all is similar in structure to catecholate-type ferric-chelating agents previously described in microorganisms (31). Such catechol-containing siderophores include, for example, chrysobactin, enterobactin, and vibriobactin (31). Thus, DMC may serve to increase the availability and solubility of Fe(III) outside the fungal cells. Fe(III) and DMC should form a classic octahedral, hexacoordinate complex involving three molecules of DMC and one of Fe(III). It is well known that the solubility of Fe(III) oxyhydroxy polymers in natural aquatic environments is very low. The concentration of Fe(III) in water in the presence of air is estimated to be about 10−18 M at pH 7.4 (23). For Fenton’s reactions to occur at an appreciable rate, the Fe(III) must be solubilized before it can be reduced to the Fe(II) state, whose solubility is on the order of 100 mM. Thus, DMC may well facilitate the stabilization in solution of Fe(III) to make it available for reduction to Fe(II) and participation in extracellular Fenton’s reactions.
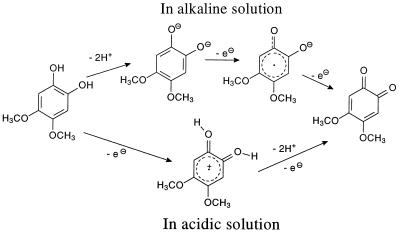
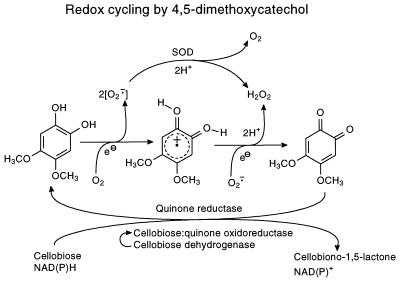
Another important role for DMC and DMH may be as the major participants in an extracellular redox cycle. DMC is probably involved in the reduction of other compounds as part of a hydroquinone-quinone redox cycle in which the hydroquinone loses electrons to form the corresponding benzoquinone (Fig. 8). We suggest that a quinone cation radical might be involved in fungal cultures, since their acidic conditions favor that route (Fig. 8) (29), rather than the semiquinone radical as postulated by Guillen et al. (15).
FIG. 8.
Redox reaction of DMC under alkaline and acidic conditions. The formation of semiquinone radicals is more probable under alkaline conditions (29), but cation radicals will be more probable under acidic conditions of G. trabeum culture.
DMC and DMH can also participate in the reduction of oxygen to a superoxide anion and hydrogen peroxide (Fig. 9) (15). Superoxide dismutase, found universally in aerobic microorganisms, will convert superoxide anions to hydrogen peroxide, an activity reported in saprophytic fungi (18). Its interaction with lignin peroxidase as a source of hydrogen peroxide has been suggested (2).
FIG. 9.
Oxygen reduction by DMC. Superoxide ions and hydrogen peroxide will be formed as a result of this reaction. Superoxide dismutase (SOD) could catalyze the removal of oxygen free radicals, increasing the pool of available hydrogen peroxide (15). The resulting quinone could be reduced to hydroquinone by quinone-specific reductases.
We observed only the hydroquinone forms of DMC and DMH in culture filtrates or cells of G. trabeum, which implies the presence of quinone-reducing enzymes in the fungus. Several quinone-reducing enzymes have been isolated from various fungi, and their properties and functions were discussed in a recent review (1). These specialized enzymes, which convert quinones to hydroquinones both within and outside the fungal hyphae, include cellobiose-quinone oxidoreductase, cellobiose dehydrogenase, and intracellular NAD(P)H-dependent quinone reductases (6, 7). G. trabeum was able to reduce Q0 (compound X). After 1 day of incubation in a 2-week-old stationary culture, 50% of a 10 mg/liter addition of Q0 was reduced to the hydroquinone form (IX). After 4 days, the conversion was 90%, and very little degradation of the IX formed was observed over the course of the experiment (Fig. 10).
FIG. 10.
Reduction of coenzyme Q0 by a culture of G. trabeum. The reduced Q0 retention time was 11.57 min; the original Q0 retention time was 10.75 min. The GC-MS chromatogram shows Q0(reduced) (upper panel) and Q0(oxidized) (lower panel) in a culture extract after 4 days of incubation with 20 ppm of Q0 added at time zero. DMC eluted at 12 min. The mass spectra of reduced and oxidized coenzyme Q0 are shown in Table 1. TIC, total-ion chromatogram.
We recognize that DMC and DMH may not be the only hydroquinones produced by G. trabeum under all growth conditions (14). However, the isolated hydroquinones are clearly important metabolites of this fungus under the growth conditions we employed. Thus, we have observed significant amounts of the new metabolites DMC and DMH in glucose-fed cultures of G. trabeum an mineral medium. We have also observed strong reductive capacity of the fungal cultures toward quinone structures. These data imply multiple roles of DMC in fungal brown rot, including stabilizing Fe(III) for reduction to Fe(II) and thereby increasing the availability of iron for extracellular Fenton’s reactions. DMC and DMH may also function directly in redox cycling with iron and other molecules, as they appear to remain in their reduced hydroquinone form throughout the fungal growth cycle.
ACKNOWLEDGMENTS
We thank Lisa Allenbach for assistance with fungal extractions and GC-MS analyses, Connie Bollinger for editorial assistance, and Stefan Goszczynski for help with chemical synthesis. We also appreciate Kenneth E. Hammel’s helpful review of this contribution.
Footnotes
Publication no. 98502 of the Idaho Agricultural Experiment Station.
REFERENCES
- 1.Ander P, Marzullo L. Sugar oxidoreductases and veratryl alcohol oxidase as related to lignin degradation. J Biotechnol. 1997;53:115–131. doi: 10.1016/s0168-1656(97)01680-5. [DOI] [PubMed] [Google Scholar]
- 2.Barr D P, Aust S D. Effect of superoxide and superoxide dismutase on lignin peroxidase-catalyzed veratryl alcohol oxidation. Arch Biochem Biophys. 1994;311:378–382. doi: 10.1006/abbi.1994.1251. [DOI] [PubMed] [Google Scholar]
- 3.Black T H. The preparation and reactions of diazomethane. Aldrichimica Acta. 1983;16:3–10. [Google Scholar]
- 4.Bonnarme P, Perez J, Jefferies T M. Regulation of ligninase production in white-rot fungi. In: Leatham G, Himmel M, editors. Enzymes in biomass conversion. Washington, D.C: American Chemical Society; 1991. pp. 200–206. [Google Scholar]
- 5.Bonthrone W, Cornforth J W. The methylation of catechol. J Chem Soc (C) 1969;1969:1202–1204. [Google Scholar]
- 6.Brock B J, Gold M H. 1,4-Benzoquinone reductase from the basidiomycete Phanerochaete chrysosporium: spectral and kinetic analysis. Arch Biochem Biophys. 1996;331:31–40. doi: 10.1006/abbi.1996.0279. [DOI] [PubMed] [Google Scholar]
- 7.Brock B J, Rieble S, Gold M H. Purification and characterization of a 1,4-benzoquinone reductase from the basidiomycete Phanerochaete chrysosporium. Appl Environ Microbiol. 1995;61:3076–3081. doi: 10.1128/aem.61.8.3076-3081.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bumpus J A. Perspectives on the use of white rot fungi in bioremediation technologies. In: Sikdar S K, Irvine R L, editors. Biodegradation technology developments. Bioremediation, principles and practice. Vol. 2. Lancaster, Pa: Technomic Publishing Co.; 1998. pp. 67–109. [Google Scholar]
- 9.de Boer T J, Backer H J. Diazomethane. In: Rabjohn N, editor. Organic synthesis. Vol. 4. New York, N.Y: John Wiley & Sons; 1963. pp. 250–253. [Google Scholar]
- 10.Enoki A, Hirano T, Tanaka H. Extracellular substance from brown-rot basidiomycete G. trabeum that produces and reduces hydrogen peroxide. Mater Org. 1992;27:247–261. [Google Scholar]
- 11.Enoki A, Itakura S, Tanaka H. The involvement of extracellular substances for reducing molecular oxygen to hydroxyl radical and ferric iron to ferrous iron in wood degradation by wood decay fungi. J Biotechnol. 1997;53:265–272. [Google Scholar]
- 12.Eriksson K-E L, Blanchette R A, Ander P. Microbial and enzymatic degradation of wood and wood components. New York, N.Y: Springer-Verlag; 1990. [Google Scholar]
- 13.Furniss B S, Hannaford A J, Smith P W, Tatchell A R. Quinones. In: Vogel A I, editor. Vogel’s textbook of practical organic chemistry. 5th ed. London, England: Longman; 1989. p. 1261. [Google Scholar]
- 14.Goodell B, Jellison J, Liu J, Daniel G, Paszczynski A, Fekete F, Krishnamurthy S, Jun L, Xu G. Low molecular weight chelators and phenolic compounds isolated from wood decay fungi and their role in the fungal biodegradation of wood. J Biotechnol. 1997;53:133–162. [Google Scholar]
- 15.Guillen F, Martinez M J, Munoz C, Martinez A T. Quinone redox cycling in the ligninolytic fungus Pleurotus eryngii leading to extracellular production of superoxide anion radical. Arch Biochem Biophys. 1997;339:190–199. doi: 10.1006/abbi.1996.9834. [DOI] [PubMed] [Google Scholar]
- 16.Hammel K E, Jensen K A, Mozuch M D, Landucci L L, Tien M, Pease E A. Ligninolysis by a purified lignin peroxidase. J Biol Chem. 1993;268:12274–12281. [PubMed] [Google Scholar]
- 17.Higuchi T. Biosynthesis and biodegradation of wood components. London, England: Academic Press, Inc.; 1985. [Google Scholar]
- 18.Holdom M D, Hay R J, Hamilton A J. The Cu,Zn superoxide dismutases of Aspergillus flavus, Aspergillus niger, Aspergillus terreus: purification and biochemical comparison with the Aspergillus fumigatus Cu,Zn superoxide dismutase. Infect Immun. 1996;64:3326–3332. doi: 10.1128/iai.64.8.3326-3332.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hyde S M, Wood P M. A mechanism for production of hydroxyl radicals by the brown-rot fungus Coniophora puteana: Fe(III) reduction by cellobiose dehydrogenase and Fe(II) oxidation at a distance from the hyphae. Microbiology. 1997;143:259–266. doi: 10.1099/00221287-143-1-259. [DOI] [PubMed] [Google Scholar]
- 20.Kawai S, Jensen K A, Bao W, Hammel K E. New polymeric model substrates for the study of microbial ligninolysis. Appl Environ Microbiol. 1995;61:3407–3414. doi: 10.1128/aem.61.9.3407-3414.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kersten P, Tien M, Kalyanaraman B, Kirk K. The ligninase of Phanerochaete chrysosporium generates cation radicals from methoxybenzenes. Biol Chem. 1985;260:2609–2612. [PubMed] [Google Scholar]
- 22.Kuwahara M, Ishida T, Miyagawa Y, Kawakami C. Production of extracellular NAD and NADP by a lignin degrading fungus Phanerochaete chrysosporium. J Ferment Technol. 1984;62:237–242. [Google Scholar]
- 23.Neilands J, Nakamura K. Detection, determination, isolation, characterisation and regulation of microbial iron chelates. In: Winkelmann G, editor. Handbook of microbial iron chelators. Boca Raton, Fla: CRC Press, Inc.; 1991. pp. 1–14. [Google Scholar]
- 24.Paszczynski A, Crawford R L. Potential for bioremediation of xenobiotic compounds by the white-rot fungus Phanerochaete chrysosporium. Biotechnol Prog. 1995;11:368–379. [Google Scholar]
- 25.Poulos T L. Peroxidases. Curr Biol. 1993;4:484–489. doi: 10.1016/0958-1669(93)90016-p. [DOI] [PubMed] [Google Scholar]
- 26.Prati L, Rossi M A. A simple route to 4,5-dialkoxy-o-quinones by catalytic oxidation of phenol. Gaz Chim Ital. 1995;125:83–86. [Google Scholar]
- 27.Schmidthalter D R, Canevascini G. Isolation and characterization of cellobiose dehydrogenase from the brown-rot fungus Coniophora puteana (Schum ex Fr) Karst. Arch Biochem Biophys. 1993;300:559–563. doi: 10.1006/abbi.1993.1077. [DOI] [PubMed] [Google Scholar]
- 28.Shimada M, Akamtsu Y, Tokimatsu T, Mii K, Hattori T. Possible biochemical roles of oxalic acid as a low molecular weight compound involved in brown-rot and white-rot wood decays. J Biotechnol. 1997;53:103–113. [Google Scholar]
- 29.Varagant J. Kirk-Othmer encyclopedia of chemical technology. 3rd ed. Vol. 13. New York, N.Y: John Wiley & Sons; 1981. Hydroquinone, resorcinol, and catechol; pp. 39–69. [Google Scholar]
- 30.Wanzlick H-W, Jahnke U. Basenkatalysierte Alkoholaddition an in situ erzeugte o-Chinone. Chem Ber. 1968;101:3744–3752. [Google Scholar]
- 31.Winkelmann G. CRC handbook of microbial iron chelators. Boca Raton, Fla: CRC Press, Inc.; 1991. [Google Scholar]
- 32.Zapanta L S, Tien M. The roles of veratryl alcohol and oxalate in fungal lignin degradation. J Biotechnol. 1997;53:93–102. [Google Scholar]