Abstract
Fentanyl is a fully synthetic opioid with analgesic and anesthetic properties. It has become a primary driver of the deadliest opioid crisis in the United States and elsewhere, consequently imposing devastating social, economic, and health burdens worldwide. However, the neural mechanisms that underlie the behavioral effects of fentanyl and its analogs are largely unknown, and approaches to prevent fentanyl abuse and fentanyl-related overdose deaths are scarce. This review presents the abuse potential and unique pharmacology of fentanyl and elucidates its potential mechanisms of action, including neural circuit dysfunction and neuroinflammation. We discuss recent progress in the development of pharmacological interventions, anti-fentanyl vaccines, anti-fentanyl/heroin conjugate vaccines, and monoclonal antibodies to attenuate fentanyl-seeking and prevent fentanyl-induced respiratory depression. However, translational studies and clinical trials are still lacking. Considering the present opioid crisis, the development of effective pharmacological and immunological strategies to prevent fentanyl abuse and overdose are urgently needed.
Keywords: Fentanyl abuse, Neural mechanisms, Pharmacological interventions, Fentanyl vaccines
Introduction
Fentanyl was initially designed and synthesized as a valuable therapeutic for anesthesia and severe pain management. It exerts analgesic and rewarding effects by binding to and activating μ-opioid receptors (MORs). Fentanyl has unique pharmacological properties, including a rapid onset of action, high potency at activating MOR-associated signaling in vivo (100-fold more potent than morphine), high lipophilicity, and the induction of muscle rigidity. Fentanyl abuse and overdose are becoming increasingly more common [1, 2]. Moreover, fentanyl is commonly mixed with cocaine, methamphetamine, heroin, and other illicit drugs among addicts. Over 40% of opioid-dependent people who inject drugs report lifetime purposeful fentanyl use, which is independently correlated with younger age, recent daily injection, and moderate/severe depression [3]. In the United States, urine drug test data has shown that positivity rates for non-prescribed fentanyl increased 1850% (from 0.9% in 2013 to 17.6% in 2018) among cocaine-positive samples and increased 798% (from 0.9% in 2013 to 7.9% in 2018) among methamphetamine-positive samples [4]. Positivity rates of co-occurring methamphetamine, cocaine, and heroin have also increased since 2013 among fentanyl-positive urine drug test results [5]. Fentanyl abuse is rarely found in China, and its manufacture, consumption, and storage are strictly controlled [6–9]. All fentanyl-related substances have been included in the supplementary list of controlled narcotic drugs and psychotropic substances with non-medical use in China since May 1, 2019 [10] (http://www.mps.gov.cn/n2254314/n2254487/c6473090/content.html).
Before and during the COVID-19 pandemic, the misuse and abuse of fentanyl appear to have persistently increased in the United States and elsewhere [11]. Overdose deaths and infections among people who use drugs, including fentanyl, increased during the COVID-19 crisis [12–14]. A study in Ontario, Canada, showed that fentanyl use increased by 108% among patients who were treated with opioid receptor agonists [15]. These patients have a high risk of COVID-19 infection, and their treatment can be problematic under conditions of quarantine and isolation and considering the scarcity of healthcare resources during the pandemic [16]. Prescription fentanyl demand has increased in critically ill COVID-19 patients who require mechanical ventilation, resulting in a shortage of intravenous fentanyl. The combined use of intravenous and transdermal fentanyl may be an option [17, 18]. However, intravenous fentanyl administration can disrupt brainstem respiratory systems, trigger respiratory depression, and impair the microcirculatory compensatory response to hemorrhage in the brainstem and lungs. Therefore, COVID-19 patients who use fentanyl should be closely monitored to avoid the exacerbation of clinical outcomes [19–21].
The present review introduces the receptor pharmacology and behavioral effects of fentanyl and elucidates the potential neural mechanisms that underlie fentanyl abuse from different perspectives, including neural circuit dysfunction and neuroinflammation. We also discuss the progress in the development of pharmacological interventions and active immunopharmacotherapies to attenuate fentanyl abuse and its side-effects.
Abuse Potential of Fentanyl
Despite its widespread use for chronic pain and severe cancer pain in the clinical setting, fentanyl and its analogs have been shown to produce hyperlocomotion and strong rewarding and reinforcing effects and have abuse potential.
Fentanyl Abuse and Withdrawal
Fentanyl induces a strong rewarding and reinforcing effects, which is the main contributor to its abuse and overdose deaths [22, 23]. Similar to morphine, fentanyl and its analogs, including isobutyrylfentanyl, crotonylfentanyl, para-fluorobutyrylfentanyl, β-methylfentanyl, paramethoxyfentanyl, fentanyl carbamate, and 3-furanylfentanyl, have MOR agonist-like hyperlocomotor effects in mice [24–26]. Self-administration models are commonly used to evaluate the reinforcing effects of drugs. Both male and female rats exhibit stable fentanyl self-administration, drug-primed reinstatement, and yohimbine (stress)- and cue-induced reinstatement [27]. When rats self-administer fentanyl with short access (ShA; 1 h are trained to duration daily) or long access (LgA; 6 h duration daily), both ShA and LgA rats exhibit an increase in craving during fentanyl-, yohimbine-, and cue-induced reinstatement [27], indicating strong fentanyl abuse potential. Monroe and Radke established an aversion-resistant fentanyl self-administration model in mice, and found that when quinine is added to the fentanyl solution, mice still robustly respond and consume the solution in both a home-cage two-bottle choice task and operant response task [28]. In addition, a fentanyl vapor self-administration model has been successfully established in mice. This model recapitulates key features of opioid addiction, including the escalation of fentanyl intake, somatic signs of withdrawal, punishment-resistant fentanyl intake, and relapse [29]. A study that combined fentanyl vapor self-administration and a behavioral economics approach found more inelastic demand for fentanyl and an increase in maximal response output in LgA (12 h) rats compared with ShA (1 h) rats [30]. These studies indicate that fentanyl is a powerful addictive drug with high abuse potential, which partially explain the sharp rise in fentanyl abuse in recent years.
Although chronic high-dose fentanyl administration reduces anxiety-like behavior, lowers sensitivity to painful stimuli, and decreases sensitivity to cocaine, fentanyl withdrawal increases anxiety-like behavior [31]. Spontaneous and naloxone-precipitated withdrawal from 2 weeks of intermittent fentanyl exposure also causes weight loss and increases signs of distress in both mice and rats [32]. States of opioid dependence and withdrawal can profoundly influence fentanyl reinforcement and the efficacy of therapeutic medications. The reinforcing effects of fentanyl increase in morphine-withdrawn rats, reflected by a rightward shift of the fentanyl dose-response curve with an increase in maximal effectiveness, whereas the reinforcing effects of fentanyl decrease in opioid-dependent rats [33]. However, no antinociceptive cross-tolerance occurs with repeated microinjections of morphine and fentanyl into the ventrolateral periaqueductal gray, which may be attributable to the different signaling mechanisms that mediate morphine- and fentanyl-induced antinociception [34, 35].
Influential Factors
The behavioral effects of fentanyl administration are influenced by sex, animal strain, and genetic and environmental factors. Fentanyl potently induces an acute locomotor response and behavioral sensitization in female rats but more potently induces contextual reward in a conditioned place preference paradigm in male rats [36]. Under a fixed-ratio 5 schedule of reinforcement and multi-day progressive-ratio schedule in a behavioral economics analysis, female rats exhibit greater reinforcing efficacy of fentanyl than male rats. In a fentanyl versus. food choice procedure, male rats are more sensitive to fentanyl than females [37]. Remifentanil self-administration-induced neuroadaptations have been shown to differ between males and females, indicating that sex-specific interventions that target opioid-induced adaptations should be developed [38]. Species differences have been found in the expression of fentanyl withdrawal-associated pain. Spontaneous and ongoing pain is preferentially expressed in rats, whereas withdrawal-associated thermal hyperalgesia is found only in mice [32]. Genetic and environmental factors influence the risk of opioid use disorder. Hnrnph1 mutant mice exhibit lower behavioral sensitivity to fentanyl, suggesting that the expression of this gene may be a biomarker that reflects addictive properties [39].
Unique Pharmacology of Fentanyl Treatment
Fentanyl is known to be a strong MOR agonist, similar to other opioids, such as morphine. The structural assessment of agonist efficacy at MORs has revealed different patterns between fentanyl and morphine [40, 41]. Principal component analysis and computational functional analysis have identified differences in ligand binding, intracellular signaling events, and receptor conformational changes between fentanyl and morphine, which may contribute to the greater efficacy of fentanyl over morphine [40–42]. Chevalier et al. exploited the X-ray structure of the MOR in complex with fentanyl and elucidated detailed fentanyl-MOR interaction profiles, thus providing a structural basis for the binding affinity, ligand recognition, and abuse potential of fentanyl and its analogs [43]. Using molecular dynamics techniques to identify binding events of the MOR with fentanyl and its analogs carfentanil and lofentanil, a previous study found that fentanyl induces the unique rotameric conformation of M153, which is required for inducing β-arrestin coupling [44]. These studies provide structural and molecular insights into the detailed mechanisms by which fentanyl binds to MORs, which is helpful for the further optimization of fentanyl-based analgesics with fewer side-effects and improved safety and efficacy.
Fentanyl and its analogs can have serious side-effects, such as increases in brain and kidney levels of malondialdehyde, neuroadaptive changes in the brain, and acceleration of the onset of acute postoperative pain [45, 46]. After exposure to fentanyl and its analogs in rats, the metabolite norfentanyl has been detected at high concentrations in urine and remains detectable for 3 days [47]. Fentanyl and its analogs have weak binding affinity for κ-opioid receptors, δ-opioid receptors, and the σ1 receptor, which could contribute to the pharmacological actions and side-effects of these drugs [22, 48]. Fentanyl also interacts with non-opioid recombinant human neurotransmitter receptors and transporters, such as the α1A and α1B adrenoceptor subtypes and the dopamine D1 and D4 receptor subtypes [49]. Human ether-a-go-go-related gene channels have been associated with fentanyl-induced sudden death, and this is exacerbated by hypoxia, hypokalemia, or alkalosis [50, 51]. The complex receptor pharmacology of fentanyl contributes to the different clinical manifestations of fentanyl use, including respiratory and cardiothoracic side-effects and overdose deaths.
Recent studies have reported that fentanyl inhibites the viability and invasion of non-small-cell lung cancer cells by reversing the expression of microRNA-331-3p and histone deacetylase 5 [52]. Fentanyl has been shown to have anti-leukemia activity via suppression of the STAT5 and Ras pathways [53]. Fentanyl inhibits the progression, invasion, and migration of gastric cancer cells by suppressing activity of the phosphoinositide 3-kinase (PI3K)/protein kinase B (Akt)/matrix metalloprotoneinase-9 signaling pathway [54]. Fentanyl also stimulates tumor angiogenesis and promotes the migration of some but not all tested human lung cancer cells; this may be related to the activation of multiple pro-angiogenic signaling pathways, including vascular endothelial growth factor receptor 2/focal adhesion kinase/PI3K/Akt and small guanosine triphosphatases [55]. This study indicates that fentanyl use in cancer patients may have potential adverse effects and should be administered with caution. The β-arrestin 2 pathway plays an important role in recovery from opioid overdose [56], and ketamine may help attenuate some of the adverse physiological consequences of fentanyl use [21]. Li et al. found that ketamine combined with fentanyl and dexmedetomidine induces safer and more efficient anesthesia than ketamine alone [57]. These studies provide insights into the molecular basis and signaling pathways that mediate the multiple pharmacological effects of fentanyl and its analogs.
Neural Circuit Dysfunction and Neuroinflammation Underlying Fentanyl Misuse and Abuse
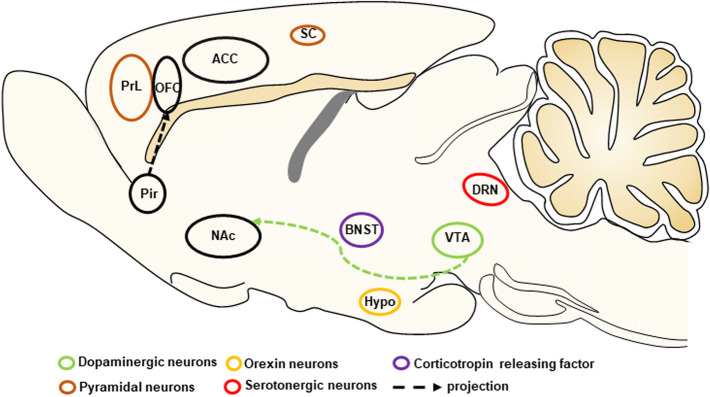
Perinatal fentanyl exposure in newborn male and female mice leads to signs of spontaneous somatic withdrawal-related behavior, an increase in anxiety-like behavior, and impaired sensory function that lasts at least to adolescence [58, 59]. Moreover, this exposure impaires synaptic transmission in the somatosensory cortex (SC) and anterior cingulate cortex (ACC), reduces ketamine-evoked cortical oscillations, and reduces the morphology of basal dendrites of pyramidal neurons in the SC [59]. Fentanyl binds to MORs in molecular-layer interneurons to reduce γ-aminobutyric acid (GABA)ergic neurotransmission to Purkinje cells, thus inhibiting sensory information processing [60]. In addition, fentanyl and morphine exposure during therapeutic hypothermia does not impair neurodevelopment and does not significantly affect cognition, language, or motor function in infants, but the long-term effects need further investigation [61]. Here, we describe the potential neural mechanisms that are involved in the pathophysiology of fentanyl abuse and fentanyl-related side-effects (Fig. 1).
Fig. 1.
Schematic of potential neural mechanisms of fentanyl abuse. Specific types of neurons and neural circuits that are involved in fentanyl taking and seeking are shown in a sagittal cross-section of the brain. SC, somatosensory cortex; ACC, anterior cingulate cortex; VTA, ventral tegmental area; NAc, nucleus accumbens; Hypo, hypothalamus; BNST, bed nucleus of the stria terminalis; PrL, prelimbic cortex; OFC, orbitofrontal cortex; Pir, piriform cortex; DRN, dorsal raphe nucleus.
Reward and Stress Circuits
Drug addiction is viewed as a disorder of dopamine neurotransmission and the reward system in the brain, especially dopamine-releasing neurons in the ventral tegmental area (VTA) and its extensive projections [62, 63]. Previous studies have shown that fentanyl vapor self-administration causes long-lasting neuroadaptations of GABAB receptor-mediated currents in VTA dopamine neurons [29]. Both subcutaneous and intravenous fentanyl administration significantly increase dopamine release in the nucleus accumbens (NAc) and have reinforcing effects [64]. RNA sequencing has revealed that repeated fentanyl withdrawal elicits distinct transcriptional activity in the VTA and NAc in male and female rats [65]. In addition, fentanyl exhibits an affinity for, and effects on, recombinant human neurotransmitter transporters, including dopamine receptor subtypes [49]. These studies indicate that the dopamine system mediates the rewarding and reinforcing effects of fentanyl abuse.
The orexin system is involved in the pathophysiology of opioid addiction, and orexin receptor antagonism attenuates drug-seeking behavior and relapse [66, 67]. Intermittent fentanyl self-administration induces a multifaceted addiction-like state and increases the number of orexin neurons in both the dorsomedial/perifornical hypothalamus and lateral hypothalamus [68]. The orexin receptor 1 antagonist SB-334867 decreases fentanyl intake, decreases motivation for fentanyl, and attenuates the cue-induced reinstatement of fentanyl-seeking [68, 69]. Intravenous fentanyl also decreases glutamate release and increases GABA release in the anterior hypothalamus, measured by the push-pull superfusion technique, and this is reversed by intrahypothalamic administration of the opioid receptor antagonist naloxone [70]. In addition, corticotropin-releasing factor (CRF) in the bed nucleus of the stria terminalis (BNST) is a critical driver of stress-induced relapse to drug-seeking. Intra-BNST microinjections of the CRF1 receptor antagonist R121919 decreases cue-induced fentanyl-seeking and fentanyl-associated conditioned reinforcement in rats [71].
The SC and dorsal raphe nucleus (DRN) are critical regions involved in nociception and may also play a critical role in the process of fentanyl misuse. Fentanyl administration alteres the firing activity of neurons and serotonin (5-hydroxytryptamine, 5-HT) efflux in the DRN, which may involve both opioid and 5-HT1A receptors [72, 73]. Autoradiographic mapping has shown that sufentanil tolerance is associated with the large-scale downregulation of MOR binding sites in various brain regions, including the DRN and SC [74]. Fentanyl decreases synaptic excitation and inhibits nociceptive stimulus-induced cortical activity in both the ACC and SC [59, 75]. Synchronization between the right SC and right hippocampus is also impaired postoperatively after anesthesia with fentanyl and droperidol [76].
Chronic drug use is often accompanied by the loss of cognitive control, which is closely linked to the dysfunction of frontal cortex regions. Remifentanil self-administration causes a long-lasting hypoactive basal state of pyramidal neurons in the prelimbic cortex (PrL) in females, and this is driven by a reduction of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor-mediated excitatory synaptic transmission [38]. In addition, remifentanil self-administration has bidirectional effects on PrL layer 5/6 pyramidal neuron GABAB–G protein-coupled inwardly rectifying K+ channel signaling in males [38]. Moreover, the chemogenetic activation of PrL pyramidal neurons reverses the deficits in cognitive flexibility, the basal hypoactive state, and remifentanil-induced plasticity [38]. Reiner et al. developed a rat model of relapse to drug-seeking after food choice-induced voluntary abstinence and found that relapse to fentanyl-seeking is associated with an increase in activation in the orbitofrontal cortex (OFC) and its projection from the piriform cortex (Pir) but not in projections from the basolateral amygdala or thalamus. Using pharmacological inactivation and the anatomical disconnection of projections between the Pir and OFC, previous studies found that Pir–OFC projections play a critical role in relapse to fentanyl-seeking after voluntary abstinence [77, 78]. Future studies that use neural circuit-defined imaging and manipulation techniques may shed further light on the neurobiology of fentanyl relapse.
Neuroinflammation
Opioid misuse, tolerance, and withdrawal have been reported to induce innate and adaptive neuroimmune dysfunction, affect neuronal function, and modulate the activation and phenotyping of microglial cells, thus producing proinflammatory effects in the brain [79]. Fentanyl self-administration profoundly alters innate immune proteins in the NAc and hippocampus. Fentanyl administration increases the expression of cytokines (e.g., tumor necrosis factor α [TNFα], interleukin-1β [IL-1β], and IL5), chemokines (e.g., C-C motif chemokine 20), and interferon (IFN) proteins (e.g., IFNβ and IFNγ) in the NAc, decreases several interleukins (e.g., IL-4 and IL-7), chemokines (e.g., granulocyte-macrophage colony-stimulating factor, monocyte chemoattractant protein 1, and chemokine ligand 5), and interferons (e.g., IFNβ and IFNγ), and stimulates IFN gene protein expression in the hippocampus, which may initiate fentanyl misuse and perhaps the development of opioid use disorder [80, 81]. Fentanyl administration increases mechanical and thermal hyperalgesia, increases the expression of proinflammatory cytokines (e.g., IL-1β, IL-6, and TNF-α) in the spinal cord and dorsal root ganglia, and activates microglia in the spinal cord in rats [82]. Repeated fentanyl administration activates the NLRP3 inflammasome in astrocytes and serotonergic neurons and promotes NLRP3-dependent pyroptosis in the DRN. Furthermore, the antiinflammatory agent minocycline and the NLRP3 inhibitor MCC950 prevent fentanyl-induced antinociception and hyperalgesia [83]. Fentanyl has also been shown to reverse the lipopolysaccharide-induced release of TNF-α, IL-1β, and IL-10 by inhibiting Toll-like receptor-4 (TLR4) expression and glycogen synthase kinase-3β activation [84]. These studies indicate that neuroinflammation plays an important role in opioid-induced analgesia and fentanyl-induced hyperalgesia.
Gut microbiota and gut-brain communication also play a critical role in opioid use and opioid tolerance [85, 86]. Chronic opioid use alters fecal microbial diversity and composition and induces gut microbial dysbiosis in both rodents and humans, accompanied by increases in peripheral and central inflammation [87–89]. Moreover, microbial metabolites, such as short-chain fatty acids, are reduced, and intestinal and blood-brain barrier integrity is disrupted after opioid use, which could modulate the development, maturation, and function of microglia and exacerbate the neuroimmune responses [87, 89, 90]. Neuroinflammation that is induced by alterations of the microbiota can ultimately contribute to behaviors that are associated with opioid dependence [91, 92]. However, the effects of fentanyl use on the gut microbiota remain unknown. Future studies are needed to investigate the influence of alterations of the microbiota on brain immunity and function.
Pharmacological Interventions to Prevent Fentanyl Abuse and Side-effects
Pharmacotherapies that target brain opioid receptors, including agonists (e.g., methadone and buprenorphine) and antagonists (e.g., naltrexone and naloxone), have been approved by the Food and Drug Administration (FDA) for the treatment of opioid use disorder. A meta-analysis has shown that these treatments can reduce mortality among opioid users [93]. A long-acting injection of extended-release buprenorphine has been shown to be feasible for users of heroin-containing fentanyl in an open-label trial [94]. However, these pharmacotherapies are limited by high relapse rates, a high risk of precipitating withdrawal in individuals who are exposed to fentanyl, and weak efficacy in preventing fentanyl overdose [95]. Thus, new approaches and novel medications are still needed for the treatment of opioid use disorder.
Pharmacological Interventions to Prevent Fentanyl-Seeking
Here, we summarize the currently available pharmacological interventions to attenuate fentanyl taking and seeking in rodents and nonhuman primates (Table 1). These interventions mainly target opioid receptors and neurotransmitter and neuropeptide systems. Intravenous and subcutaneous administration of the long-lasting MOR antagonist methocinnamox reverses and prevents fentanyl-induced antinociception and respiratory depression in rats [96]. Methocinnamox attenuats heroin self-administration in rhesus monkeys, which continues to display efficacy even at very low plasma levels [97]. Contingent administration of the κ-opioid receptor agonists U50488 and nalfurafine decrease fentanyl choice, which is blocked by the selective κ-opioid receptor antagonist nor-binaltorphimine [98]. Levo-tetrahydropalmatine is an antagonist of dopamine receptors that suppresses the rewarding effects of fentanyl in the conditioned place preference paradigm by inhibiting the phosphorylation of extracellular signal-regulated kinase (ERK) and cyclic adenosine monophosphate response element binding protein (CREB) in the hippocampus, NAc, and prefrontal cortex in mice [99]. The acute stimulation of 5-HT2A receptors with the psychedelic 2,5-dimethoxy-4-iodoamphetamine decreases the economic demand for fentanyl and food after intermittent and continuous-access self-administration [100]. In a rat model of relapse, the glucagon-like peptide-1 receptor (GLP-1R) agonist exendin-4 reduces fentanyl self-administration in rats but causes malaise-like behavior. A novel dual agonist of GLP-1Rs and neuropeptide Y2 receptors, GEP44, reduces fentanyl self-administration with fewer adverse effects compared with exendin-4 [101, 102]. The growth hormone secretagogue receptor antagonist JMV2959 reduces fentanyl-induced conditioned place preference and fentanyl self-administration [64]. The glucocorticoid receptor antagonist PT150 reduces footshock-induced fentanyl-seeking [103]. Intra-BNST injections of the CRF1 receptor antagonist R121919 deceases cue-induced responding to fentanyl in both opioid-dependent and nondependent rats [71]. Compared with ShA and LgA to fentanyl, intermittent access increases the motivation for fentanyl and total intake, which is reversed by the orexin receptor-1 antagonist SB334867 [68].
Table 1.
Summary of recent findings on pharmacological interventions to attenuate fentanyl taking and seeking.
| Drug | Route of administration | Pharmacological target | Subjects | Paradigm (fentanyl dose, model) | Main findings | References |
|---|---|---|---|---|---|---|
| SB-334867 | IP | Orexin-1 receptor antagonist | Male Sprague-Dawley rats | Self-administration and behavioral economics procedure | SB-334867 decreases motivation for fentanyl without affecting drug consumption at null cost | Fragale et al. [69] |
| R121919 | Intra-BNST injections | Corticotropin-releasing factor-1 receptor antagonist | Male Sprague-Dawley rats | 2.5 mg/kg, infusion, self-administration | These patterns of responding with R121919 treatment result in less fentanyl-associated conditioned reinforcement during test | Gyawali et al. [71] |
| JMV2959 | IP | Growth hormone secretagogue receptor antagonist | Male adult Wistar rats | 20 or 30 μg/kg, SC; 10 μg/kg, IV | Pretreatment with JMV2959 significantly reduces the number of active lever presses and reduces fentanyl seeking/relapse-like behavior in rats on day 12 of forced abstinence | Sustkova-Fiserova et al. [64] |
| Methocinnamox | SC | μ-Opioid receptor antagonist | Male and female rhesus monkeys | 0.00032 mg/kg, infusion, self-administration | Methocinnamox selectively reduces opioid self-administration and remains effective at times when its plasma levels are very low | Maguire et al. [97] |
| U50488 and nalfurafine | IV | κ-Opioid receptor agonists | Male and female Sprague-Dawley rats | 0, 0.32–10 μg/kg, infusion, self-administration | Both U50488 and nalfurafine decrease fentanyl choice when administered contingently | Townsend [98] |
| PT150 | IP | Glucocorticoid receptor antagonist | Male and female Sprague-Dawley rats | 2.5 μg/kg, infusion, self-administration | Both footshock and yohimbine reinstate fentanyl-seeking; only footshock-induced reinstatement is decreased by PT150 (50 and 100 mg/kg) | Hammerslag et al. [103] |
| Levo-tetrahydropalmatine | IV | Dopamine receptor antagonist | Male C57BL/6 mice | 0.05 mg/kg, conditioned place preference | Levo-tetrahydropalmatine suppresses the rewarding properties of fentanyl-induced conditioned place preference; the inhibitory effect may be related to the suppression of ERK and CREB phosphorylation in the hippocampus, nucleus accumbens, and prefrontal cortex in mice | Du et al. [99] |
| GEP44 | SC | Novel dual agonist of glucagon-like peptide-1 receptors and neuropeptide Y Y2 receptors | Male Sprague-Dawley rats | 2.5 μg/kg, self-administration | GEP44 attenuates opioid taking and seeking at a dose that does not suppress food intake or produce adverse malaise-like effects in fentanyl-experienced rats | Zhang et al. [101] |
| 2,5-Dimethoxy-4-iodoamphetamine (DOI) | IP | Psychedelic 5-HT2A receptor agonist | Male Sprague-Dawley rats | 2.5 μg/kg, injection, self-administration, intermittent and continuous schedules | DOI acts through 5-HT2A receptors to alter economic demand for fentanyl; in economic food demand experiments, DOI (0.4 mg/kg) increases demand elasticity and reduces food consumption | Martin et al. [100] |
| SB334867 | IP | Orexin-1 receptor antagonist | Male Sprague-Dawley male rats | Intermittent self-administration | Addiction-like behaviors induced by intermittent access to fentanyl are reversed by SB-334867 | Fragale et al. [68] |
IP, intraperitoneal; SC, subcutaneous; IV, intravenous; BNST, bed nucleus of the stria terminalis.
Pharmacological Interventions to Attenuate Fentanyl-Induced Respiratory Depression
Fentanyl overdose fatalities and the opioid crisis are mainly attributable to drug-induced respiratory depression. Fentanyl overdose induces hippocampal ischemia, accompanied by delayed leukoencephalopathy [104]. Fentanyl but not morphine alters mitochondrial morphology, depending on the dosage and cell type, and this may be associated with neuronal apoptosis [105, 106]. Fentanyl-induced respiratory depression is associated with electrocortical changes, underscoring the importance of considering the activity of cortical and subcortical areas when evaluating fentanyl-induced respiratory depression [107]. Fentanyl and its analogs can rapidly produce airway closure and rigidity in the upper airway and diaphragm chest wall, which may involve α-adrenergic and cholinergic receptor-mediated mechanical failure of the respiratory and cardiovascular systems [49, 108].
We summarize currently available pharmacological interventions to prevent fentanyl-induced respiratory depression in Table 2. D-amphetamine hastens the recovery of arterial pH and partial pressure of CO2, O2, and sO2 in rats, promotes the alleviation of respiratory depression, and escalates the return of consciousness following high-dose fentanyl [109]. The G protein-biased cannabinoid CB2 receptor agonist LY2828360 decreases fentanyl-induced respiratory depression in wildtype mice but not in G protein-biased CB2 receptor knockout mice [110]. The acyclic cucurbit[n]uril molecular container calabadion 1 reverses fentanyl-induced respiratory depression in rats [111]. The selective α4β2 nicotinic receptor agonist A85380 confers robust and rapid reversal of fentanyl-induced respiratory depression, without any obvious side effects. A85380 also significantly decreases respiratory depression and apnea when co-administered with fentanyl and remifentanil [112]. Brackley et al. found that the peptide oxytocin receptor agonist oxytocin acetate and the non-peptide oxytocin receptor agonist WAY-267464 without vasopressin-1A receptor cross-activation rescues fentanyl-induced respiratory depression [113]. The clinically approved superoxide dismutase mimetic 4-hydroxy-2,2,6,6-tetramethylpiperidine-N-oxyl (Tempol) has also been reported to attenuate the cardiorespiratory depressant effects of fentanyl without affecting its analgesic effects [114].
Table 2.
Summary of recent findings on pharmacological interventions to prevent fentanyl-induced respiratory depression.
| Drug | Route of administration | Pharmacological target | Subjects | Paradigm (fentanyl dose, model) | Main findings | References |
|---|---|---|---|---|---|---|
| A85380 | SC | α4β2 nicotinic acetylcholine receptor | Male and female Sprague-Dawley rats | 20 μg/kg, IV | Co-administration of A85380 (0.06 mg/kg) and fentanyl or remifentanil markedly reduces respiratory depression and apnea and enhances fentanyl-induced analgesia | Ren et al. [112] |
| D-amphetamine | IV | Dopamine D1 receptor | Male and female Sprague-Dawley rats | 55 μg/kg, IV | D-amphetamine attenuates respiratory acidosis, increases arterial oxygenation, and accelerates the return of consciousness in the setting of fentanyl intoxication | Moody et al. [109] |
| LY2828360 | IP | G protein-biased cannabinoid CB2 receptor | Wildtype and CB2 knockout mice | 0.2 mg/kg, IP | Combination of CB2 agonist and fentanyl may represent a safer adjunctive therapeutic strategy compared with a narcotic analgesic alone by attenuating the development of opioid-induced respiratory depression | Zavala et al. [110] |
| Calabadion 1 | IV | Acyclic cucurbit[n]uril molecular container | Male Sprague-Dawley rats | 12.5 or 25 μg/kg, IV | Calabadion 1 selectively and dose-dependently reverses the respiratory and central nervous system side-effects of fentanyl | Thevathasan et al. [111] |
| Oxytocin and WAY-267464 | IP | Oxytocin receptor | Male Sprague-Dawley rats | 60 nmol/kg, IV | Without vasopressin 1A receptor cross-activation, peptide and non-peptide agonist activation of oxytocin receptors (oxytocin and WAY-267464) rescue fentanyl-induced respiratory depression | Brackley et al. [113] |
| Methocinnamox | IV and SC | μ-Opioid receptor | Male Sprague-Dawley rats | 0.0032–0.178 mg/kg, IV | Methocinnamox reverses and prevents fentanyl-induced antinociception and respiratory depression | Jimenez et al. [96] |
IP, intraperitoneal; IV, intravenous; SC, subcutaneous.
Altogether, mounting preclinical data provide proof-of-concept of the efficacy of potential pharmacological interventions to attenuate fentanyl-seeking and prevent fentanyl-induced respiratory depression, demonstrating their potential for the treatment of fentanyl abuse and overdose. However, translational studies and clinical trials still need to be conducted.
Progress in the Development of Anti-Fentanyl Conjugate Vaccines as Candidate Medications
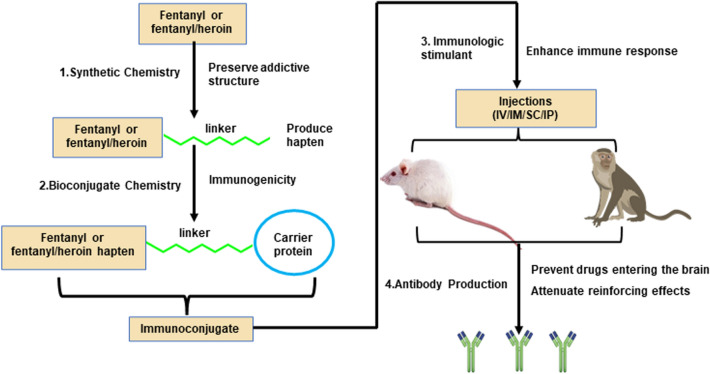
The management and prevention of fentanyl-related overdoses are notable challenges. Anti-opioid vaccines may be a promising therapeutic approach to prevent opioid overdose and reduce opioid-induced behavior, including drug self-administration in mice, rats, and nonhuman primates [14, 115]. Vaccines produce fentanyl-specific antibodies and sequester large amounts of these drugs in blood, thus reducing biodistribution in the brain and preventing binding to targeted receptors in the brain [116, 117]. Compared with FDA-approved small-molecule pharmacotherapies (e.g., methadone, buprenorphine, and naltrexone), fentanyl vaccines offer several potential clinical benefits, including prolonged protection because of the relatively long serum half-life, fewer side-effects by directly binding and sequestering drugs in the periphery, high selectivity, and no abuse liability. Vaccines that target opioids (e.g., heroin, morphine, oxycodone, and hydrocodone) and psychostimulants (e.g., cocaine and methamphetamine) have shown promising results in animal models and are currently in various stages of preclinical development [118–121]. Anti-fentanyl vaccines are still in the early stages of development. Currently, several fentanyl and fentanyl/heroin vaccines have been developed with different designs (Fig. 2), including the optimization of fentanyl-based haptens and linker chemistry, the choice of carrier proteins and adjuvants, delivery strategies, and the development of novel carriers and delivery platforms (Table 3).
Fig. 2.
Design and production of fentanyl and fentanyl/heroin conjugate vaccines. Using synthetic chemistry, a specific linker is added to the fentanyl or fentanyl/heroin without affecting biological functionality to create a hapten. Linkage of the fentanyl or fentanyl/heroin hapten to the carrier protein occurs through bioconjugate chemistry, and subsequent adjuvating of the immunologic stimulant generates complete vaccines. These vaccines have been tested in mice, rats, and nonhuman primates, where they have been shown to enhance innate and adaptive immunity, produce fentanyl-specific antibodies, sequester opioid drugs in the periphery, and prevent them from entering the brain, thus attenuating the reinforcing effects of fentanyl or fentanyl-contaminated heroin. IV, intravenous; IM, intramuscular; SC, subcutaneous; IP, intraperitoneal.
Table 3.
Summary of active immunopharmacotherapies developed for fentanyl abuse.
| Vaccine | Hapten structure | Carrier protein | Adjuvant | Route of administration | Vaccinations/overall time | Subjects | Main findings | References |
|---|---|---|---|---|---|---|---|---|
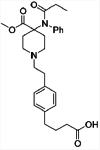
| 1 |
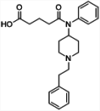
|
TT | Alum and CpG | SC | 3 injections (0, 2, 4 weeks) | Male Swiss-Webster mice | Single conjugate vaccine elicits high levels of antibodies with cross-reactivity for a wide panel of fentanyl analogs and protects against lethal fentanyl doses | Bremer et al. [123] |
| 2 |
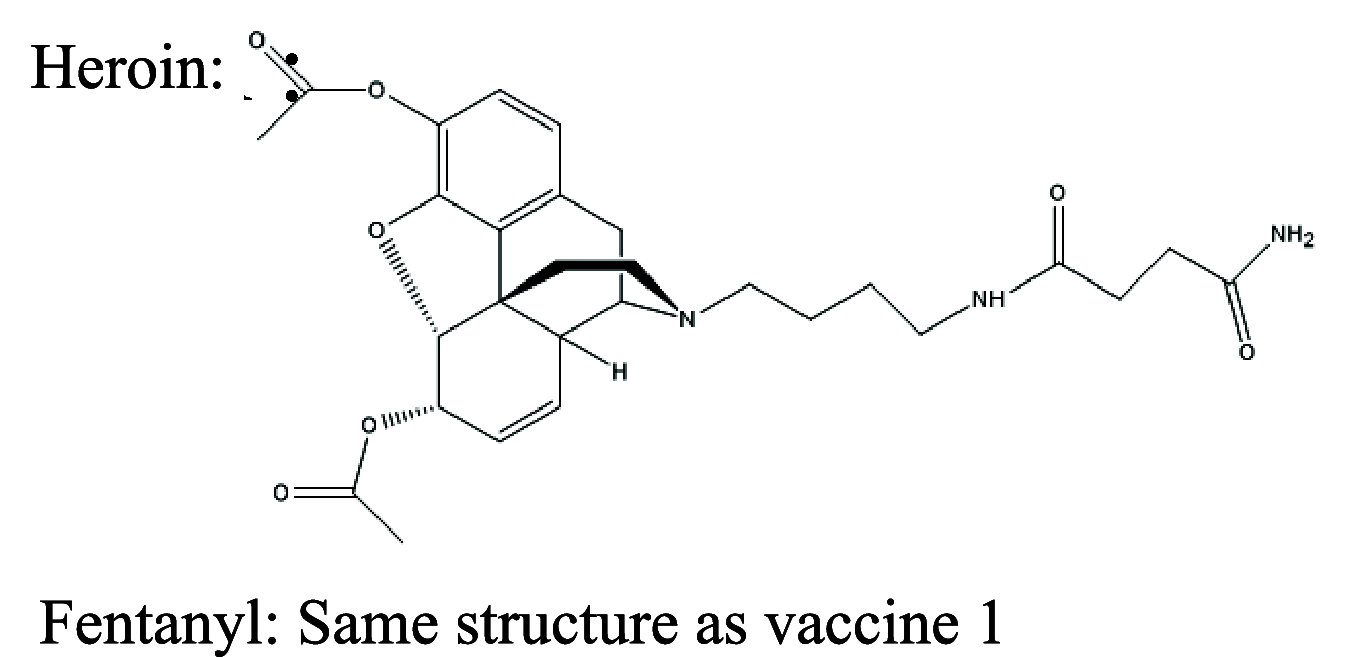
|
TT or KLH | Alum and CpG | SC | 3 injections (0, 2, 4 weeks) | Female BALB/c mice | Admixture vaccines sequester opioid drugs in blood and reduce fentanyl-induced antinociception | Hwang et al. [142, 143] |
| 3 | Same structure as vaccine 1 | TT | CpG and alhydrogel | IV | 3 injections (0, 3, 17 weeks) | Male and female Sprague-Dawley rats | Vaccination is very effective in preventing the binding of fentanyl to μ-opioid receptors in the central nervous system and reduces fentanyl-induced antinociception and its reinforcing effects | Townsend et al. [124] |
| 4 | Same structure as vaccine 1 | TT | Alhydrogel | IM | 6 injections (0, 2, 4, 9, 19, 44 weeks) | Adult male rhesus monkeys | The vaccine increases plasma fentanyl levels and reduces fentanyl-induced antinociception | Tenney et al. [133] |
| 5 |
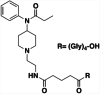
|
KLH | Alum | SC | 3 injections (0, 2, 4 weeks) | Male BALB/c mice and male Holtzman rats | The vaccine reduces fentanyl-induced antinociception in hot-plate test, respiratory depression, and bradycardia over a range of cumulative subcutaneous fentanyl doses | Raleigh et al. [122] |
| 6 |
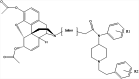
|
KLH | Alum and CpG ODN 1826 | IP | 4 injections (0, 2, 4, 7 weeks) | Female BALB/c mice | New vaccines developed from chemically contiguous haptens composed of both heroin- and fentanyl-like domains; they confer robust protection against heroin but attenuate protection against fentanyl | Natori et al. [141] |
| 7 | Same structure as vaccine 5 | KLH | Alum | IP | 3 injections (0, 3, 8 weeks) | Female A/J mice | Monoclonal antibodies generated in hybridomas from mice vaccinated with a fentanyl conjugate vaccine reverse fentanyl/carfentanil-induced antinociception | Smith et al. [135] |
| 8 |
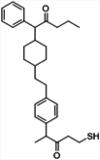
|
TT | ALF43A | IM | 4 injections (0, 3, 6, 14 weeks) | Female BALB/c mice | The vaccine induces high-affinity antibodies against fentanyl and its highly potent analogs and protects mice against fentanyl-induced antinociceptive effects | Barrientos et al. [129] |
| 9 |
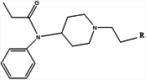
|
KLH or CRM | Alum | IM | 4 injections (0, 14, 28, 29 weeks) | Male BALB/c mice and male and female Sprague-Dawley rats | Prophylactic vaccination reduces fentanyl- and sufentanil-induced respiratory depression, antinociception, and bradycardia; therapeutic vaccination reduces intravenous fentanyl self-administration | Robinson et al. [131] |
| 10 |
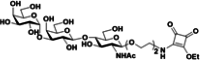
|
OVA | Alum | IP | 3 injections (2 weeks) | α1,3GalT knockout mice | The vaccine reduces psychoactive effects of fentanyl without addition of the immunostimulant CpG oligodeoxynucleotide | Wang et al. [132] |
| 11 |
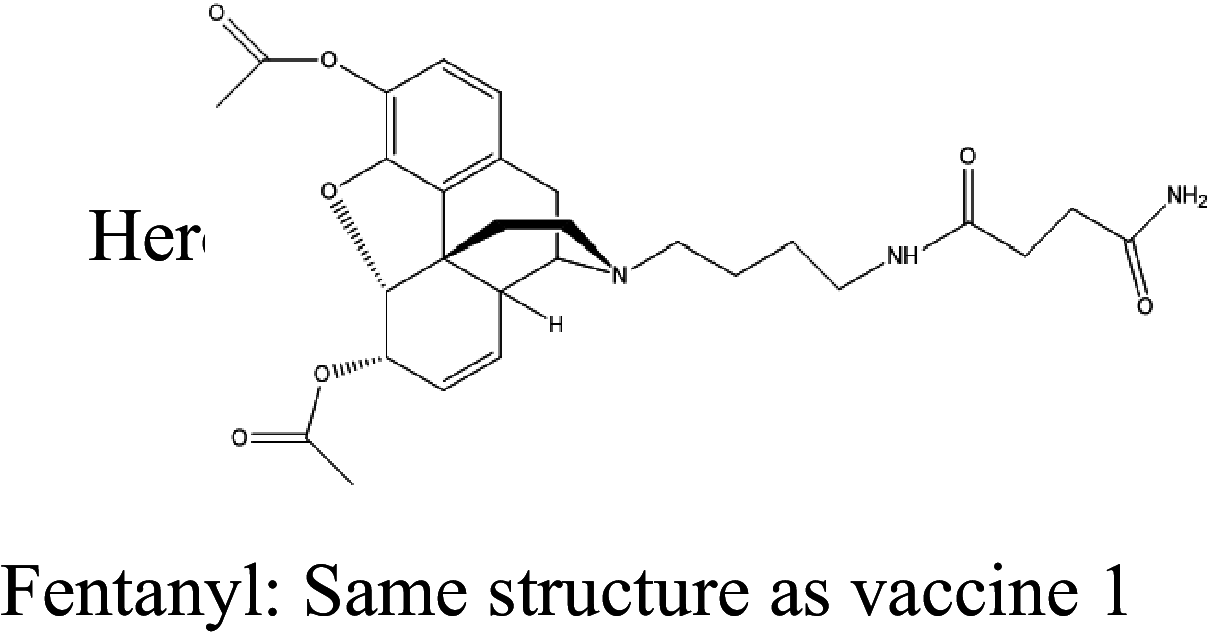
|
CRM or TT | Alhydrogel | IV | 4 injections (0, 14, 42, 70 days) | Male and female Sprague-Dawley rats | The vaccine attenuates thermal antinociceptive effects of fentanyl/heroin but has no effects on fentanyl/heroin mixture self-administration | Townsend et al. [139] |
| 12 | Same structure as vaccine 5 | KLH | Alum | IP | 3 injections (0, 2, 4 weeks) | Male and female BALB/c mice and male Sprague-Dawley rats | α-Fentanyl monoclonal antibodies generated from hybridomas via magnetic enrichment; passive immunization reduces fentanyl-induced antinociception, respiratory depression, and bradycardia | Baehr et al. [136] |
| 13 | Same structure as vaccine 1 | CRM | δ-Inulin | SC |
3 injections (2 weeks) in mice 4 injections (0, 1, 2, 4 months) in monkeys |
Male BALB/c mice and cynomolgus monkeys | A novel vaccine against heroin and fentanyl developed through the optimization of adjuvants and enhancing stability via lyophilization; it produces high-affinity antibodies and reduces fentanyl/heroin-induced antinociception | Blake et al. [137] |
| 14 |
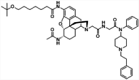
|
CRM | Alum | IP | 4 injections (0, 2, 4, 7 weeks) | Female BALB/c mice | The vaccine with an optimized dual hapten produces antibodies with nanomolar affinities and blocks the analgesic effects of fentanyl-contaminated heroin | Park et al. [138] |
| 15 | Same structure as vaccine 1 | TT | Liposomal | IM | 4 injections (0, 3, 6, 14 weeks) | Female BALB/c mice | Combining TT-6-AmHap and TT-para-AmFenHap yielded an effective bivalent vaccine that ablates the effects of heroin and fentanyl | Barrientos et al. [140] |
| 16 | Same structure as vaccine 7 | CRM | dmLT or LTA1 | IM | 2 injections (0, 4 weeks) | Female BALB/c mice | dmLT or LTA1 adjuvants and mucosal delivery may be attractive strategies for improving the efficacy of vaccines against fentanyl use disorder | Stone et al. [128] |
| 17 | Same structure as vaccine 1 | CRM | Alum | IM | 4 injections (0, 3, 8, 15 weeks) | Rhesus monkeys | Fentanyl vaccine effectively reduces fentanyl vs food choice in rhesus monkeys | Townsend et al. [133] |
| 18 |
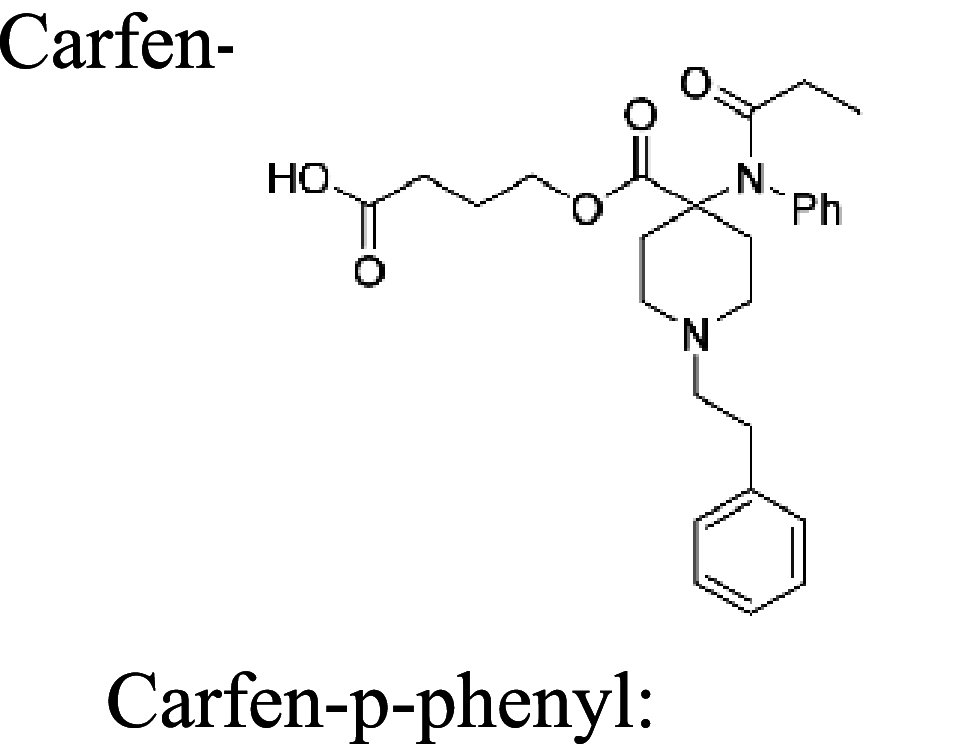
|
TT | alum and CpG | IP | 4 injections (0, 2, 4, 9 weeks) | Female Swiss-Webster mice | The two synthetic vaccines reduce carfentanil-induced antinociception and respiratory depression | Eubanks et al. [127] |
|
TT, tetanus toxoid; CpG, cytosine-phosphodiester-guanine oligodeoxynucleotide 1826; KLH, keyhole limpet hemocyanin; CRM, diphtheria toxin; OVA, ovalbumin; IV, intravenous; IM, intramuscular; SC, subcutaneous; IP, intraperitoneal.
Fentanyl Vaccines in Mice and Rats
Considering the devastating opioid crisis worldwide, different carrier proteins, adjuvants, delivery strategies, and fentanyl-based haptens are being developed to improve fentanyl vaccine efficacy, shelf life, and safety. A vaccine that consists of a fentanyl-based hapten that is conjugated to the keyhole limpet hemocyanin (KLH) carrier protein or GMP-grade subunit KLH reduces fentanyl distribution in the brain and reduces fentanyl-induced antinociception and respiratory depression in mice and rats [122]. Fentanyl-tetanus toxoid (TT) conjugate vaccines elicit high anti-fentanyl antibody titers and ablate lethal doses of fentanyl and its analogs, including 3-methylfentanyl and α-methylfentanyl [123]. A fentanyl-TT conjugate vaccine has also beenreported to decrease fentanyl reinforcement, increase food reinforcement in a fentanyl versus. food choice procedure, and prevent the expression of opioid withdrawal-associated increases in fentanyl choice in male and female rats [124, 125]. Carfentanil is ~100-fold more potent than fentanyl and can be lethal at extremely low doses. Pretreatment with the opioid receptor antagonist naltrexone decreases the seeking of fentanyl and heroin but not carfentanil [126]. Recently, two synthetic vaccines, Carfen-ester-TT and Carfen-p-phenyl-TT, have been shown to reduce carfentanil biodistribution in the brain and blunt carfentanil-induced antinociception and respiratory depression [127].
A recent study found that a fentanyl vaccine that is adjuvanted with a heat-labile toxin of E. coli, including dmLT and LTA1, elicits higher levels of anti-fentanyl antibodies than the traditional adjuvant alum. Vaccination with sublingual dmLT or intranasal LTA1 confers the robust blockade of fentanyl-induced analgesia and strong central nervous system penetration of anti-fentanyl antibodies [128]. This study also found that dmLT and LTA1 adjuvants and mucosal delivery enhance the immunogenicity and efficacy of fentanyl conjugate vaccines. A novel fentanyl vaccine, composed of a novel fentanyl hapten (para-AmFenHap), a safe and immunogenic carrier protein TT, and a potent liposomal adjuvant (ALF43A) that contains monophosphoryl lipid A, has also been synthesized and shown to prevent the side-effects of fentanyl [129]. The stimulation of TLR5 may also be an option to improve antibody production and enhance innate and adaptive immunity during active vaccination against fentanyl and its analogs [130]. Robinson et al. developed prophylactic vaccination and therapeutic vaccination that consists of novel fentanyl-based haptens that are conjugated to carrier proteins. Prophylactic vaccination reduces fentanyl- and sufentanil-induced respiratory depression, antinociception, and bradycardia in mice and rats, and therapeutic vaccination reduces fentanyl self-administration in rats [131]. Wang et al. developed a new fentanyl conjugate vaccine that is conjugated to the Galα1-3Gal epitope, which is based on preformed antibody-assisted antigen presentation, and this vaccine reduces fentanyl-associated side-effects [132].
Fentanyl Vaccines in Nonhuman Primates and Monoclonal Antibodies against Fentanyl
In non-opioid-dependent rats and rhesus monkeys, fentanyl vaccine administration blunts fentanyl reinforcement and increases food reinforcement for a prolonged period [124, 133]. A fentanyl-CRM197 conjugate vaccine produces anti-fentanyl antibodies and attenuates fentanyl versus. food choice in male and female rhesus monkeys [133]. A fentanyl-TT conjugate vaccine increases plasma fentanyl levels ~6-fold and shifts fentanyl potency at least 10-fold in both schedule-controlled responding and thermal nociception assays in male rhesus monkeys [134]. Monoclonal antibodies (mAbs), especially 6A4, have been generated in hybridomas derived from mice vaccinated with a fentanyl conjugate vaccine. These mAbs have been reported to sequester large amounts of these drugs in blood, reverse fentanyl/carfentanil-induced antinociception, and prevent fentanyl-induced lethality, which could be a promising approach to treat opioid overdose and opioid use disorder [135]. α-Fentanyl mAbs have been generated from mice immunized with a fentanyl-sKLH conjugate vaccine. Pretreatment with these mAbs reduces fentanyl-induced antinociception, respiratory depression, and bradycardia in mice and rats [136]. These studies indicate that mAbs against fentanyl and its analogs have potential applications for the treatment of opioid use disorder and the prevention of overdose and toxicity.
Fentanyl/Heroin Vaccines
Overdose deaths caused by opioids have increased substantially in recent years, attributable to the adulteration of these drugs with fentanyl. Many immunopharmacotherapeutic studies have focused on developing new vaccines that can effectively target dual haptens to produce antibodies that are able to sequester fentanyl and contaminated opioids in the periphery. Blake et al. developed a heroin/fentanyl combination vaccine that consists of inulin-based formulations (Advax) that contain a CpG oligodeoxynucleotide and act as effective adjuvants when combined with a heroin conjugate. This vaccine produces high-opioid-affinity serum antibodies and reduces antinociception in both mice and cynomolgus monkeys [137]. By implementing an optimized dual hapten, a vaccine has been developed to produce antibodies that are able to bind fentanyl-contaminated heroin in the periphery, thus effectively blocking their analgesic effects [138]. A dual fentanyl/heroin vaccine generates high-affinity anti-fentanyl and anti-heroin antibodies and decreases the antinociceptive potency of a fentanyl/heroin mixture but does not attenuate combined fentanyl/heroin self-administration [139]. By combining TT-6-AmHap (a heroin monovalent vaccine) and TT-para-AmFenHap (a fentanyl monovalent vaccine) to formulate a bivalent vaccine that is adjuvanted with liposomes that contain monophosphoryl lipid A adsorbed on aluminum hydroxide, a study found that this bivalent conjugate vaccine induces dual immunogenic responses that ablate the effects of both heroin and fentanyl in mice [140]. A chemically contiguous heroin-fentanyl vaccine that uses a hapten with one epitope that has domains for both fentanyl and heroin has also been developed. This vaccine confers protection against heroin and fentanyl in an antinociception analysis [141]. Admixture vaccination strategies have also been applied to target two different drug species and combat fentanyl-adulterated heroin [142, 143]. These new fentanyl/heroin vaccines appear promising but need further testing for possible translation to clinical use.
Conclusions and Perspectives
In conclusion, fentanyl and its analogs have complex receptor pharmacology and produce multifaceted behavioral effects and clinical characteristics, including analgesia, anesthesia, sedation, and respiratory and cardiothoracic side-effects. Fentanyl transdermal microneedles have a rapid onset of antinociceptive action, providing an effective mode of opioid delivery for immediate pain relief with limited side-effects [144]. Microneedles that are loaded with low doses of fentanyl may reduce the risk of overdose fatalities. Developing novel technologies to reduce the clinical dose of fentanyl may be useful for mitigating the opioid crisis. Mechanistically, the behavioral effects of fentanyl likely result from a combination of its chemistry, receptor pharmacology, receptor signaling, rapid distribution to the central nervous system, neural circuit dysfunction, and neuroinflammation. However, the detailed neurobiological mechanisms that contribute to fentanyl abuse and overdose remain largely unknown. Novel effective pharmacological and psychosocial treatments and vaccines are urgently needed.
Drug self-administration procedures and investigations of the choice between opioids and non-drug reinforcers are recommended to evaluate the effectiveness of candidate interventions in preclinical studies. States of opioid dependence and withdrawal should also be considered because such states can profoundly influence the results. The currently available small-molecule pharmacotherapies that mainly target MORs have limited efficacy to combat fentanyl abuse and overdose. Further elucidation of the molecular and structural bases of biased signaling and interactions between fentanyl and MORs is promising for optimizing fentanyl-based analgesics with fewer side-effects, a better safety profile, and higher efficacy. Future studies are needed to improve the effectiveness of opioid medications, such as G-protein biased MOR agonists, and develop novel non-opioid medications that target other systems, such as neurotransmitter and neuropeptide systems. Traditional Chinese medicine, including herbal therapy and acupuncture, has few side-effects and could also be considered for the treatment of fentanyl addition [145–147].
Anti-fentanyl vaccines produce antibodies that sequester opioid drugs in the periphery and prevent them from entering the brain and activating reward circuits. They have high selectivity and long-lasting efficacy and do not interfere with endogenous opioids, thus providing safe and cost-effective interventions for the management of fentanyl abuse and overdose. However, although several fentanyl and fentanyl/heroin vaccines have been shown to be effective in rodents and nonhuman primates, these vaccines are still in preclinical stages and need to be translated to the clinical treatment of opioid use disorder and fentanyl overdose. To facilitate preclinical-to-clinical translation and increase the success of drug discovery, it is critical to develop clinically viable formulations and optimize rational vaccine designs to maximize efficacy, understand the immunological mechanisms that underlie drug-motivated behavior and vaccine-evoked immune responses, and identify biomarkers that are predictive of individual variability and support patient stratification.
Acknowledgements
This review was supported by the National Key Research and Development Program of China (2019YFC0118604) and the National Natural Science Foundation of China (32071058).
Conflict of Interest
The authors declare that they do not have any conflicts of interest.
Contributor Information
Wei Yan, Email: weiyan@bjmu.edu.cn.
Lin Lu, Email: linlu@bjmu.edu.cn.
References
- 1.Volkow ND. The epidemic of fentanyl misuse and overdoses: Challenges and strategies. World Psychiatry. 2021;20:195–196. doi: 10.1002/wps.20846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Di Trana A, Del Rio A. Fentanyl analogues potency: What should be known. Clin Ter. 2020;171:e412–e413. doi: 10.7417/CT.2020.2250. [DOI] [PubMed] [Google Scholar]
- 3.Chandra DK, Altice FL, Copenhaver MM, Zhou X, Didomizio E, Shrestha R. Purposeful fentanyl use and associated factors among opioid-dependent people who inject drugs. Subst Use Misuse. 2021;56:979–987. doi: 10.1080/10826084.2021.1901931. [DOI] [PubMed] [Google Scholar]
- 4.LaRue L, Twillman RK, Dawson E, Whitley P, Frasco MA, Huskey A, et al. Rate of fentanyl positivity among urine drug test results positive for cocaine or methamphetamine. JAMA Netw Open. 2019;2:e192851. doi: 10.1001/jamanetworkopen.2019.2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Twillman RK, Dawson E, LaRue L, Guevara MG, Whitley P, Huskey A. Evaluation of trends of near-real-time urine drug test results for methamphetamine, cocaine, heroin, and fentanyl. JAMA Netw Open. 2020;3:e1918514. doi: 10.1001/jamanetworkopen.2019.18514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fang YX, Wang YB, Shi J, Liu ZM, Lu L. Recent trends in drug abuse in China. Acta Pharmacol Sin. 2006;27:140–144. doi: 10.1111/j.1745-7254.2006.00270.x. [DOI] [PubMed] [Google Scholar]
- 7.Lu L, Fang YX, Wang X. Drug abuse in China: Past, present and future. Cell Mol Neurobiol. 2008;28:479–490. doi: 10.1007/s10571-007-9225-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun HQ, Bao YP, Zhou SJ, Meng SQ, Lu L. The new pattern of drug abuse in China. Curr Opin Psychiatry. 2014;27:251–255. doi: 10.1097/YCO.0000000000000073. [DOI] [PubMed] [Google Scholar]
- 9.Zhu WL, Zhang W, Li JL, Ding ZB, Huang YJ, Lu L. The abuse of anesthetic propofol: Associated with cognitive impairment. Sci China Life Sci. 2018;61:1428–1431. doi: 10.1007/s11427-018-9401-9. [DOI] [PubMed] [Google Scholar]
- 10.Bao YP, Meng SQ, Shi J, Lu L. Control of fentanyl-related substances in China. Lancet Psychiatry. 2019;6:e15. doi: 10.1016/S2215-0366(19)30218-4. [DOI] [PubMed] [Google Scholar]
- 11.Ciccarone D. The rise of illicit fentanyls, stimulants and the fourth wave of the opioid overdose crisis. Curr Opin Psychiatry. 2021;34:344–350. doi: 10.1097/YCO.0000000000000717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Striley CW, Hoeflich CC. Converging public health crises: Substance use during the coronavirus disease 2019 pandemic. Curr Opin Psychiatry. 2021;34:325–331. doi: 10.1097/YCO.0000000000000722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DiGennaro C, Garcia GGP, Stringfellow EJ, Wakeman S, Jalali MS. Changes in characteristics of drug overdose death trends during the COVID-19 pandemic. Int J Drug Policy. 2021;98:103392. doi: 10.1016/j.drugpo.2021.103392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kosten TR, Petrakis IL. The hidden epidemic of opioid overdoses during the coronavirus disease 2019 pandemic. JAMA Psychiatry. 2021;78:585–586. doi: 10.1001/jamapsychiatry.2020.4148. [DOI] [PubMed] [Google Scholar]
- 15.Morin KA, Acharya S, Eibl JK, Marsh DC. Evidence of increased fentanyl use during the COVID-19 pandemic among opioid agonist treatment patients in Ontario. Canada. Int J Drug Policy. 2021;90:103088. doi: 10.1016/j.drugpo.2020.103088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun YK, Bao YP, Kosten T, Strang J, Shi J, Lu L. Editorial: Challenges to opioid use disorders during COVID-19. Am J Addict. 2020;29:174–175. doi: 10.1111/ajad.13031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herndon KT, Claussen KS, Braithwaite JJ. A novel clinical consideration to conserve parenteral fentanyl during the COVID-19 pandemic. Anesth Analg. 2020;131:1355–1357. doi: 10.1213/ANE.0000000000005168. [DOI] [PubMed] [Google Scholar]
- 18.Kang N, Alrashed MA, Place EM, Nguyen PT, Perona SJ, Erstad BL. Clinical outcomes of concomitant use of enteral and intravenous sedatives and analgesics in mechanically ventilated patients with COVID-19. Am J Health Syst Pharm. 2022;79:S21–S26. doi: 10.1093/ajhp/zxab385. [DOI] [PubMed] [Google Scholar]
- 19.Blackwood CA, Cadet JL. COVID-19 pandemic and fentanyl use disorder in African Americans. Front Neurosci. 2021;15:707386. doi: 10.3389/fnins.2021.707386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tabatabai M, Kitahata LM, Collins JG. Disruption of the rhythmic activity of the medullary inspiratory neurons and phrenic nerve by fentanyl and reversal with nalbuphine. Anesthesiology. 1989;70:489–495. doi: 10.1097/00000542-198903000-00020. [DOI] [PubMed] [Google Scholar]
- 21.Xiang LS, Calderon AS, Klemcke HG, Scott LL, Hinojosa-Laborde C, Ryan KL. Fentanyl impairs but ketamine preserves the microcirculatory response to hemorrhage. J Trauma Acute Care Surg. 2020;89:S93–S99. doi: 10.1097/TA.0000000000002604. [DOI] [PubMed] [Google Scholar]
- 22.Comer SD, Cahill CM. Fentanyl: Receptor pharmacology, abuse potential, and implications for treatment. Neurosci Biobehav Rev. 2019;106:49–57. doi: 10.1016/j.neubiorev.2018.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Han Y, Yan W, Zheng YB, Khan MZ, Yuan K, Lu L. The rising crisis of illicit fentanyl use, overdose, and potential therapeutic strategies. Transl Psychiatry. 2019;9:282. doi: 10.1038/s41398-019-0625-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Varshneya NB, Walentiny DM, Moisa LT, Walker TD, Akinfiresoye LR, Beardsley PM. Fentanyl-related substances elicit antinociception and hyperlocomotion in mice via opioid receptors. Pharmacol Biochem Behav 2021: 173242. [DOI] [PMC free article] [PubMed]
- 25.Varshneya NB, Walentiny DM, Moisa LT, Walker TD, Akinfiresoye LR, Beardsley PM. Opioid-like antinociceptive and locomotor effects of emerging fentanyl-related substances. Neuropharmacology. 2019;151:171–179. doi: 10.1016/j.neuropharm.2019.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Varshneya NB, Hassanien SH, Holt MC, Stevens DL, Layle NK, Bassman JR, et al. Respiratory depressant effects of fentanyl analogs are opioid receptor-mediated. Biochem Pharmacol. 2022;195:114805. doi: 10.1016/j.bcp.2021.114805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Malone SG, Keller PS, Hammerslag LR, Bardo MT. Escalation and reinstatement of fentanyl self-administration in male and female rats. Psychopharmacology (Berl) 2021;238:2261–2273. doi: 10.1007/s00213-021-05850-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Monroe SC, Radke AK. Aversion-resistant fentanyl self-administration in mice. Psychopharmacology (Berl) 2021;238:699–710. doi: 10.1007/s00213-020-05722-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moussawi K, Ortiz MM, Gantz SC, Tunstall BJ, Marchette RCN, Bonci A, et al. Fentanyl vapor self-administration model in mice to study opioid addiction. Sci Adv 2020, 6: eabc0413. [DOI] [PMC free article] [PubMed]
- 30.McConnell SA, Brandner AJ, Blank BA, Kearns DN, Koob GF, Vendruscolo LF, et al. Demand for fentanyl becomes inelastic following extended access to fentanyl vapor self-administration. Neuropharmacology. 2021;182:108355. doi: 10.1016/j.neuropharm.2020.108355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fujii K, Koshidaka Y, Adachi M, Takao K. Effects of chronic fentanyl administration on behavioral characteristics of mice. Neuropsychopharmacol Rep. 2019;39:17–35. doi: 10.1002/npr2.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Uddin O, Jenne C, Fox ME, Arakawa K, Keller A, Cramer N. Divergent profiles of fentanyl withdrawal and associated pain in mice and rats. Pharmacol Biochem Behav. 2021;200:173077. doi: 10.1016/j.pbb.2020.173077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seaman RW, Jr, Collins GT. Impact of morphine dependence and withdrawal on the reinforcing effectiveness of fentanyl, cocaine, and methamphetamine in rats. Front Pharmacol. 2021;12:691700. doi: 10.3389/fphar.2021.691700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morgan MM, Tran A, Wescom RL, Bobeck EN. Differences in antinociceptive signalling mechanisms following morphine and fentanyl microinjections into the rat periaqueductal gray. Eur J Pain. 2020;24:617–624. doi: 10.1002/ejp.1513. [DOI] [PubMed] [Google Scholar]
- 35.Bobeck EN, Schoo SM, Ingram SL, Morgan MM. Lack of antinociceptive cross-tolerance with co-administration of morphine and fentanyl into the periaqueductal gray of male Sprague-Dawley rats. J Pain. 2019;20:1040–1047. doi: 10.1016/j.jpain.2019.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gaulden AD, Burson N, Sadik N, Ghosh I, Khan SJ, Brummelte S, et al. Effects of fentanyl on acute locomotor activity, behavioral sensitization, and contextual reward in female and male rats. Drug Alcohol Depend. 2021;229:109101. doi: 10.1016/j.drugalcdep.2021.109101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Townsend EA, Negus SS, Caine SB, Thomsen M, Banks ML. Sex differences in opioid reinforcement under a fentanyl vs. food choice procedure in rats. Neuropsychopharmacology 2019, 44: 2022–2029. [DOI] [PMC free article] [PubMed]
- 38.Anderson EM, Engelhardt A, Demis S, Porath E, Hearing MC. Remifentanil self-administration in mice promotes sex-specific prefrontal cortex dysfunction underlying deficits in cognitive flexibility. Neuropsychopharmacology. 2021;46:1734–1745. doi: 10.1038/s41386-021-01028-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bryant CD, Healy AF, Ruan QT, Coehlo MA, Lustig E, Yazdani N, et al. Sex-dependent effects of an Hnrnph1 mutation on fentanyl addiction-relevant behaviors but not antinociception in mice. Genes Brain Behav. 2021;20:e12711. doi: 10.1111/gbb.12711. [DOI] [PubMed] [Google Scholar]
- 40.Ricarte A, Dalton JAR, Giraldo J. Structural assessment of agonist efficacy in the μ-opioid receptor: morphine and fentanyl elicit different activation patterns. J Chem Inf Model. 2021;61:1251–1274. doi: 10.1021/acs.jcim.0c00890. [DOI] [PubMed] [Google Scholar]
- 41.Lipiński PFJ, Jarończyk M, Dobrowolski JC, Sadlej J. Molecular dynamics of fentanyl bound to μ-opioid receptor. J Mol Modeling. 2019;25:1–17. doi: 10.1007/s00894-019-3999-2. [DOI] [PubMed] [Google Scholar]
- 42.Lipinski PFJ, Kosson P, Matalinska J, Roszkowski P, Czarnocki Z, Jaronczyk M, et al. Fentanyl family at the mu-opioid receptor: uniform assessment of binding and computational analysis. Molecules 2019, 24. [DOI] [PMC free article] [PubMed]
- 43.Vo QN, Mahinthichaichan P, Shen J, Ellis CR. How μ-opioid receptor recognizes fentanyl. Nat Commun. 2021;12:984. doi: 10.1038/s41467-021-21262-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.de Waal PW, Shi JJ, You EL, Wang XX, Melcher K, Jiang Y, et al. Molecular mechanisms of fentanyl mediated β-arrestin biased signaling. PLoS Comput Biol. 2020;16:e1007394. doi: 10.1371/journal.pcbi.1007394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yadav SK, Nagar DP, Bhattacharya R. Effect of fentanyl and its three novel analogues on biochemical, oxidative, histological, and neuroadaptive markers after sub-acute exposure in mice. Life Sci. 2020;246:117400. doi: 10.1016/j.lfs.2020.117400. [DOI] [PubMed] [Google Scholar]
- 46.Rupniewska-Ladyko A, Malec-Milewska M. A high dose of fentanyl may accelerate the onset of acute postoperative pain. Anesth Pain Med. 2019;9:e94498. doi: 10.5812/aapm.94498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li L, Yu X, Lyu L, Duan H, Chen Y, Bian J, et al. Determination of fentanyl, alpha-methylfentanyl, beta-hydroxyfentanyl, and the metabolite norfentanyl in rat urine by LC-MS/MS. J Anal Toxicol. 2021 doi: 10.1093/jat/bkab021. [DOI] [PubMed] [Google Scholar]
- 48.Lipinski PFJ, Szucs E, Jaronczyk M, Kosson P, Benyhe S, Misicka A, et al. Affinity of fentanyl and its derivatives for the σ1-receptor. Med Chem Comm. 2019;10:1187–1191. doi: 10.1039/c9md00222g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Torralva R, Eshleman AJ, Swanson TL, Schmachtenberg JL, Schutzer WE, Bloom SH, et al. Fentanyl but not morphine interacts with nonopioid recombinant human neurotransmitter receptors and transporters. J Pharmacol Exp Ther. 2020;374:376–391. doi: 10.1124/jpet.120.265561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tschirhart JN, Li WT, Guo J, Zhang ST. Blockade of the human ether A-go-go-related gene (hERG) potassium channel by fentanyl. Mol Pharmacol. 2019;95:386–397. doi: 10.1124/mol.118.114751. [DOI] [PubMed] [Google Scholar]
- 51.Tschirhart JN, Zhang ST. Fentanyl-induced block of hERG channels is exacerbated by hypoxia, hypokalemia, alkalosis, and the presence of hERG1b. Mol Pharmacol. 2020;98:508–517. doi: 10.1124/mol.119.119271. [DOI] [PubMed] [Google Scholar]
- 52.Gong SK, Ying L, Fan YN, Sun ZT. Fentanyl inhibits lung cancer viability and invasion via upregulation of miR-331-3p and repression of HDAC5. Onco Targets Ther. 2020;13:13131–13141. doi: 10.2147/OTT.S281095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dai SB, Zhang XQ, Zhang P, Zheng XS, Pang QY. Fentanyl inhibits acute myeloid leukemia differentiated cells and committed progenitors via opioid receptor-independent suppression of Ras and STAT5 pathways. Fundam Clin Pharmacol. 2021;35:174–183. doi: 10.1111/fcp.12581. [DOI] [PubMed] [Google Scholar]
- 54.Li CL, Qin Y, Zhong Y, Qin YY, Wei Y, Li L, et al. Fentanyl inhibits the progression of gastric cancer through the suppression of MMP-9 via the PI3K/Akt signaling pathway. Ann Transl Med. 2020;8:118. doi: 10.21037/atm.2019.12.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu W, Chen Y, Xu W, Wang W, Tang L, Xia R, et al. Fentanyl stimulates tumor angiogenesis via activating multiple pro-angiogenic signaling pathways. Biochem Biophys Res Commun. 2020;532:225–230. doi: 10.1016/j.bbrc.2020.08.038. [DOI] [PubMed] [Google Scholar]
- 56.Haouzi P, McCann M, Tubbs N. Respiratory effects of low and high doses of fentanyl in control and β-arrestin 2-deficient mice. J Neurophysiol. 2021;125:1396–1407. doi: 10.1152/jn.00711.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li CZ, Peng JL, Hu R, Yan J, Sun Y, Zhang L, et al. Safety and efficacy of ketamine versus ketamine-fentanyl-dexmedetomidine combination for anesthesia and analgesia in rats. Dose Response. 2019;17:1559325819825902. doi: 10.1177/1559325819825902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Alipio JB, Brockett AT, Fox ME, Tennyson SS, deBettencourt CA, El-Metwally D, et al. Enduring consequences of perinatal fentanyl exposure in mice. Addict Biol. 2021;26:e12895. doi: 10.1111/adb.12895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Alipio JB, Haga C, Fox ME, Arakawa K, Balaji R, Cramer N, et al. Perinatal fentanyl exposure leads to long-lasting impairments in somatosensory circuit function and behavior. J Neurosci. 2021;41:3400–3417. doi: 10.1523/JNEUROSCI.2470-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yang HM, Zhan LJ, Lin XQ, Chu CP, Qiu DL, Lan Y. Fentanyl inhibits air puff-evoked sensory information processing in mouse cerebellar neurons recorded in vivo. Front Syst Neurosci. 2020;14:51. doi: 10.3389/fnsys.2020.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gundersen JK, Chakkarapani E, Jary S, Menassa DA, Scull-Brown E, Frymoyer A, et al. Morphine and fentanyl exposure during therapeutic hypothermia does not impair neurodevelopment. EClinicalMedicine. 2021;36:100892. doi: 10.1016/j.eclinm.2021.100892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Morales M, Margolis EB. Ventral tegmental area: Cellular heterogeneity, connectivity and behaviour. Nat Rev Neurosci. 2017;18:73–85. doi: 10.1038/nrn.2016.165. [DOI] [PubMed] [Google Scholar]
- 63.Nutt DJ, Lingford-Hughes A, Erritzoe D, Stokes PRA. The dopamine theory of addiction: 40 years of highs and lows. Nat Rev Neurosci. 2015;16:305–312. doi: 10.1038/nrn3939. [DOI] [PubMed] [Google Scholar]
- 64.Sustkova-Fiserova M, Puskina N, Havlickova T, Lapka M, Syslova K, Pohorala V, et al. Ghrelin receptor antagonism of fentanyl-induced conditioned place preference, intravenous self-administration, and dopamine release in the nucleus accumbens in rats. Addict Biol. 2020;25:e12845. doi: 10.1111/adb.12845. [DOI] [PubMed] [Google Scholar]
- 65.Townsend EA, Kim RK, Robinson HL, Marsh SA, Banks ML, Hamilton PJ. Opioid withdrawal produces sex-specific effects on fentanyl-vs.-food choice and mesolimbic transcription. Biol Psychiatry Glob Open Sci 2021, 1: 112–122. [DOI] [PMC free article] [PubMed]
- 66.Han Y, Yuan K, Zheng YB, Lu L. Orexin receptor antagonists as emerging treatments for psychiatric disorders. Neurosci Bull. 2020;36:432–448. doi: 10.1007/s12264-019-00447-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Matzeu A, Martin-Fardon R. Targeting the orexin system for prescription opioid use disorder. Brain Sci. 2020;10:226. doi: 10.3390/brainsci10040226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fragale JE, James MH, Aston-Jones G. Intermittent self-administration of fentanyl induces a multifaceted addiction state associated with persistent changes in the orexin system. Addict Biol. 2021;26:e12946. doi: 10.1111/adb.12946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fragale JE, Pantazis CB, James MH, Aston-Jones G. The role of orexin-1 receptor signaling in demand for the opioid fentanyl. Neuropsychopharmacology. 2019;44:1690–1697. doi: 10.1038/s41386-019-0420-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pourzitaki C, Tsaousi G, Papazisis G, Kyrgidis A, Zacharis C, Kritis A, et al. Fentanyl and naloxone effects on glutamate and GABA release rates from anterior hypothalamus in freely moving rats. Eur J Pharmacol. 2018;834:169–175. doi: 10.1016/j.ejphar.2018.07.029. [DOI] [PubMed] [Google Scholar]
- 71.Gyawali U, Martin DA, Sulima A, Rice KC, Calu DJ. Role of BNST CRFR1 receptors in incubation of fentanyl seeking. Front Behav Neurosci. 2020;14:153. doi: 10.3389/fnbeh.2020.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Alojado ME, Ohta Y, Yamamura T, Kemmotsu O. The effect of fentanyl and morphine on neurons in the dorsal raphe nucleus in the rat: An in vitro study. Anesth Analg. 1994;78:726–732. doi: 10.1213/00000539-199404000-00019. [DOI] [PubMed] [Google Scholar]
- 73.Tao R, Karnik M, Ma ZY, Auerbach SB. Effect of fentanyl on 5-HT efflux involves both opioid and 5-HT1A receptors. Br J Pharmacol. 2003;139:1498–1504. doi: 10.1038/sj.bjp.0705378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Díaz A, Pazos A, Flórez J, Hurlé MA. Autoradiographic mapping of µ-opioid receptors during opiate tolerance and supersensitivity in the rat central nervous system. Naunyn Schmiedeberg's Arch Pharmacol. 2000;362:101–109. doi: 10.1007/s002100000258. [DOI] [PubMed] [Google Scholar]
- 75.Peng YZ, Li XX, Wang YW. Effects of parecoxib and fentanyl on nociception-induced cortical activity. Mol Pain. 2010;6:3. doi: 10.1186/1744-8069-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Xie P, Yu T, Fu XY, Tu Y, Zou Y, Lui S, et al. Altered functional connectivity in an aged rat model of postoperative cognitive dysfunction: A study using resting-state functional MRI. PLoS One. 2013;8:e64820. doi: 10.1371/journal.pone.0064820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Reiner DJ, Lofaro OM, Applebey SV, Korah H, Venniro M, Cifani C, et al. Role of projections between piriform cortex and orbitofrontal cortex in relapse to fentanyl seeking after palatable food choice-induced voluntary abstinence. J Neurosci. 2020;40:2485–2497. doi: 10.1523/JNEUROSCI.2693-19.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Everett NA, Baracz SJ. A piriform-orbitofrontal cortex pathway drives relapse to fentanyl-seeking after voluntary abstinence. J Neurosci. 2020;40:8208–8210. doi: 10.1523/JNEUROSCI.1295-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dominguini D, Steckert AV, Michels M, Spies MB, Ritter C, Barichello T, et al. The effects of anaesthetics and sedatives on brain inflammation. Neurosci Biobehav Rev. 2021;127:504–513. doi: 10.1016/j.neubiorev.2021.05.009. [DOI] [PubMed] [Google Scholar]
- 80.Cisneros IE, Cunningham KA. Self-administered fentanyl profoundly impacts rat brain innate immune targets. Neuropsychopharmacology. 2021;46:247. doi: 10.1038/s41386-020-00853-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ezeomah C, Cunningham KA, Stutz SJ, Fox RG, Bukreyeva N, Dineley KT, et al. Fentanyl self-administration impacts brain immune responses in male Sprague-Dawley rats. Brain Behav Immun. 2020;87:725–738. doi: 10.1016/j.bbi.2020.03.003. [DOI] [PubMed] [Google Scholar]
- 82.Chang L, Ye F, Luo QH, Tao YX, Shu HH. Increased hyperalgesia and proinflammatory cytokines in the spinal cord and dorsal root ganglion after surgery and/or fentanyl administration in rats. Anesth Analg. 2018;126:289–297. doi: 10.1213/ANE.0000000000002601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Carranza-Aguilar CJ, Hernández-Mendoza A, Mejias-Aponte C, Rice KC, Morales M, González-Espinosa C, et al. Morphine and fentanyl repeated administration induces different levels of NLRP3-dependent pyroptosis in the dorsal raphe nucleus of male rats via cell-specific activation of TLR4 and opioid receptors. Cell Mol Neurobiol. 2022;42:677–694. doi: 10.1007/s10571-020-00957-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang J, Jin YJ, Li JC. Protective role of fentanyl in lipopolysaccharide-induced neuroinflammation in BV-2 cells. Exp Ther Med. 2018;16:3740–3744. doi: 10.3892/etm.2018.6590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Akbarali HI, Dewey WL. The gut-brain interaction in opioid tolerance. Curr Opin Pharmacol. 2017;37:126–130. doi: 10.1016/j.coph.2017.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ren M, Lotfipour S. The role of the gut microbiome in opioid use. Behav Pharmacol. 2020;31:113–121. doi: 10.1097/FBP.0000000000000538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cruz-Lebrón A, Johnson R, Mazahery C, Troyer Z, Joussef-Piña S, Quiñones-Mateu ME, et al. Chronic opioid use modulates human enteric microbiota and intestinal barrier integrity. Gut Microbes. 2021;13:1946368. doi: 10.1080/19490976.2021.1946368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang FY, Meng JJ, Zhang L, Johnson T, Chen C, Roy S. Morphine induces changes in the gut microbiome and metabolome in a morphine dependence model. Sci Rep. 2018;8:3596. doi: 10.1038/s41598-018-21915-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lee K, Vuong HE, Nusbaum DJ, Hsiao EY, Evans CJ, Taylor AMW. The gut microbiota mediates reward and sensory responses associated with regimen-selective morphine dependence. Neuropsychopharmacology. 2018;43:2606–2614. doi: 10.1038/s41386-018-0211-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Agirman G, Hsiao EY. SnapShot: The microbiota-gut-brain axis. Cell. 2021;184:2524–2524. doi: 10.1016/j.cell.2021.03.022. [DOI] [PubMed] [Google Scholar]
- 91.Hofford RS, Russo SJ, Kiraly DD. Neuroimmune mechanisms of psychostimulant and opioid use disorders. Eur J Neurosci. 2019;50:2562–2573. doi: 10.1111/ejn.14143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang Z, Chen WH, Li SX, He ZM, Zhu WL, Ji YB, et al. Gut microbiota modulates the inflammatory response and cognitive impairment induced by sleep deprivation. Mol Psychiatry. 2021;26:6277–6292. doi: 10.1038/s41380-021-01113-1. [DOI] [PubMed] [Google Scholar]
- 93.Ma J, Bao YP, Wang RJ, Su MF, Liu MX, Li JQ, et al. Effects of medication-assisted treatment on mortality among opioids users: A systematic review and meta-analysis. Mol Psychiatry. 2019;24:1868–1883. doi: 10.1038/s41380-018-0094-5. [DOI] [PubMed] [Google Scholar]
- 94.Mariani JJ, Mahony AL, Podell SC, Brooks DJ, Brezing C, Luo SX, et al. Open-label trial of a single-day induction onto buprenorphine extended-release injection for users of heroin and fentanyl. Am J Addict. 2021;30:470–476. doi: 10.1111/ajad.13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Varshneya NB, Thakrar AP, Hobelmann JG, Dunn KE, Huhn AS. Evidence of buprenorphine-precipitated withdrawal in persons who use fentanyl. J Addict Med. 2021 doi: 10.1097/ADM.0000000000000922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jimenez VM, Jr, Castaneda G, France CP. Methocinnamox reverses and prevents fentanyl-induced ventilatory depression in rats. J Pharmacol Exp Ther. 2021;377:29–38. doi: 10.1124/jpet.120.000387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Maguire DR, Gerak LR, Sanchez JJ, Javors MA, Disney A, Husbands SM, et al. Effects of acute and repeated treatment with methocinnamox, a mu opioid receptor antagonist, on fentanyl self-administration in rhesus monkeys. Neuropsychopharmacology. 2020;45:1986–1993. doi: 10.1038/s41386-020-0698-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Townsend EA. Effects of kappa opioid receptor agonists on fentanyl vs. food choice in male and female rats: Contingent vs. non-contingent administration. Psychopharmacology (Berl) 2021, 238: 1017–1028. [DOI] [PubMed]
- 99.Du KL, Wang ZY, Zhang HM, Zhang YF, Su HL, Wei ZW, et al. Levo-tetrahydropalmatine attenuates the acquisition of fentanyl-induced conditioned place preference and the changes in ERK and CREB phosphorylation expression in mice. Neurosci Lett. 2021;756:135984. doi: 10.1016/j.neulet.2021.135984. [DOI] [PubMed] [Google Scholar]
- 100.Martin DA, Gyawali U, Calu DJ. Effects of 5-HT 2A receptor stimulation on economic demand for fentanyl after intermittent and continuous access self-administration in male rats. Addict Biol. 2021;26:e12926. doi: 10.1111/adb.12926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhang YF, Rahematpura S, Ragnini KH, Moreno A, Stecyk KS, Kahng MW, et al. A novel dual agonist of glucagon-like peptide-1 receptors and neuropeptide Y2 receptors attenuates fentanyl taking and seeking in male rats. Neuropharmacology. 2021;192:108599. doi: 10.1016/j.neuropharm.2021.108599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Merkel R, Moreno A, Zhang YF, Herman R, Ben Nathan J, Zeb S, et al. A novel approach to treating opioid use disorders: Dual agonists of glucagon-like peptide-1 receptors and neuropeptide Y2 receptors. Neurosci Biobehav Rev. 2021;131:1169–1179. doi: 10.1016/j.neubiorev.2021.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hammerslag LR, Denehy ED, Carper B, Nolen TL, Prendergast MA, Bardo MT. Effects of the glucocorticoid receptor antagonist PT150 on stress-induced fentanyl seeking in male and female rats. Psychopharmacology (Berl) 2021;238:2439–2447. doi: 10.1007/s00213-021-05865-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Switzer AR, Beland B, Sarna JR, Walzak A, Pfeffer G. Fentanyl overdose causing hippocampal ischaemia followed by delayed leukoencephalopathy. Can J Neurol Sci. 2020;47:398–399. doi: 10.1017/cjn.2020.33. [DOI] [PubMed] [Google Scholar]
- 105.Nylander E, Zelleroth S, Nyberg F, Grönbladh A, Hallberg M. The effects of morphine, methadone, and fentanyl on mitochondria: A live cell imaging study. Brain Res Bull. 2021;171:126–134. doi: 10.1016/j.brainresbull.2021.03.009. [DOI] [PubMed] [Google Scholar]
- 106.Finsterer J, Zarrouk-Mahjoub S. Fentanyl can be mitochondrion-toxic depending on dosage and cell type. J Anaesthesiol Clin Pharmacol. 2019;35:570–571. doi: 10.4103/joacp.JOACP_262_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Montandon G, Horner RL. Electrocortical changes associating sedation and respiratory depression by the opioid analgesic fentanyl. Sci Rep. 2019;9:14122. doi: 10.1038/s41598-019-50613-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Torralva R, Janowsky A. Noradrenergic mechanisms in fentanyl-mediated rapid death explain failure of naloxone in the opioid crisis. J Pharmacol Exp Ther. 2019;371:453–475. doi: 10.1124/jpet.119.258566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Moody OA, Zhang ER, Arora V, Kato R, Cotten JF, Solt K. D-amphetamine accelerates recovery of consciousness and respiratory drive after high-dose fentanyl in rats. Front Pharmacol. 2020;11:585356. doi: 10.3389/fphar.2020.585356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zavala CA, Thomaz AC, Iyer V, Mackie K, Hohmann AG. Cannabinoid CB 2 receptor activation attenuates fentanyl-induced respiratory depression. Cannabis Cannabinoid Res. 2021;6:389–400. doi: 10.1089/can.2020.0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Thevathasan T, Grabitz SD, Santer P, Rostin P, Akeju O, Boghosian JD, et al. Calabadion 1 selectively reverses respiratory and central nervous system effects of fentanyl in a rat model. Br J Anaesth. 2020;125:e140–e147. doi: 10.1016/j.bja.2020.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ren J, Ding XQ, Greer JJ. Activating α4β2 nicotinic acetylcholine receptors alleviates fentanyl-induced respiratory depression in rats. Anesthesiology. 2019;130:1017–1031. doi: 10.1097/ALN.0000000000002676. [DOI] [PubMed] [Google Scholar]
- 113.Brackley AD, Toney GM. Oxytocin receptor activation rescues opioid-induced respiratory depression by systemic fentanyl in the rat. J Pharmacol Exp Ther. 2021;378:96–107. doi: 10.1124/jpet.121.000535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Baby S, Gruber R, Discala J, Puskovic V, Jose N, Cheng FX, et al. Systemic administration of tempol attenuates the cardiorespiratory depressant effects of fentanyl. Front Pharmacol. 2021;12:690407. doi: 10.3389/fphar.2021.690407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Baehr C, Pravetoni M. Vaccines to treat opioid use disorders and to reduce opioid overdoses. Neuropsychopharmacology. 2019;44:217–218. doi: 10.1038/s41386-018-0197-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Pravetoni M, Comer SD. Development of vaccines to treat opioid use disorders and reduce incidence of overdose. Neuropharmacology. 2019;158:107662. doi: 10.1016/j.neuropharm.2019.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Olson ME, Janda KD. Vaccines to combat the opioid crisis: Vaccines that prevent opioids and other substances of abuse from entering the brain could effectively treat addiction and abuse. EMBO Rep. 2018;19:5–9. doi: 10.15252/embr.201745322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Banks ML, Olson ME, Janda KD. Immunopharmacotherapies for treating opioid use disorder. Trends Pharmacol Sci. 2018;39:908–911. doi: 10.1016/j.tips.2018.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Li QQ, Luo YX, Sun CY, Xue YX, Zhu WL, Shi HS, et al. A morphine/heroin vaccine with new hapten design attenuates behavioral effects in rats. J Neurochem. 2011;119:1271–1281. doi: 10.1111/j.1471-4159.2011.07502.x. [DOI] [PubMed] [Google Scholar]
- 120.Liao F, Wang HX, Dao YK, Yuan K, Lu JZ, Shi J, et al. Synthesis and biological evaluation of a lipopeptide-based methamphetamine vaccine. Chin Chem Lett. 2021;32:1575–1579. [Google Scholar]
- 121.Townsend EA, Banks ML. Preclinical evaluation of vaccines to treat opioid use disorders: How close are we to a clinically viable therapeutic? CNS Drugs. 2020;34:449–461. doi: 10.1007/s40263-020-00722-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Raleigh MD, Baruffaldi F, Peterson SJ, Le Naour M, Harmon TM, Vigliaturo JR, et al. A fentanyl vaccine alters fentanyl distribution and protects against fentanyl-induced effects in mice and rats. J Pharmacol Exp Ther. 2019;368:282–291. doi: 10.1124/jpet.118.253674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bremer PT, Kimishima A, Schlosburg JE, Zhou B, Collins KC, Janda KD. Combatting synthetic designer opioids: A conjugate vaccine ablates lethal doses of fentanyl class drugs. Angew Chem Int Ed Engl. 2016;55:3772–3775. doi: 10.1002/anie.201511654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Townsend EA, Blake S, Faunce KE, Hwang CS, Natori Y, Zhou B, et al. Conjugate vaccine produces long-lasting attenuation of fentanyl vs. food choice and blocks expression of opioid withdrawal-induced increases in fentanyl choice in rats. Neuropsychopharmacology 2019, 44: 1681–1689. [DOI] [PMC free article] [PubMed]
- 125.Tunstall BJ, Vendruscolo LF. Utility of fentanyl vaccines: Unique challenges posed by preventing opioid overdose and treating opioid use disorder. Neuropsychopharmacology. 2019;44:1675–1676. doi: 10.1038/s41386-019-0418-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Flynn SM, France CP. Discriminative stimulus effects of carfentanil in rats discriminating fentanyl: Differential antagonism by naltrexone. Drug Alcohol Depend. 2021;221:108599. doi: 10.1016/j.drugalcdep.2021.108599. [DOI] [PubMed] [Google Scholar]
- 127.Eubanks LM, Blake S, Natori Y, Ellis B, Bremer PT, Janda KD. A highly efficacious carfentanil vaccine that blunts opioid-induced antinociception and respiratory depression. ACS Chem Biol. 2021;16:277–282. doi: 10.1021/acschembio.1c00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Stone AE, Scheuermann SE, Haile CN, Cuny GD, Velasquez ML, Linhuber JP, et al. Fentanyl conjugate vaccine by injected or mucosal delivery with dmLT or LTA1 adjuvants implicates IgA in protection from drug challenge. NPJ Vaccines. 2021;6:69. doi: 10.1038/s41541-021-00329-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Barrientos RC, Bow EW, Whalen C, Torres OB, Sulima A, Beck Z, et al. Novel vaccine that blunts fentanyl effects and sequesters ultrapotent fentanyl analogues. Mol Pharm. 2020;17:3447–3460. doi: 10.1021/acs.molpharmaceut.0c00497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Yang F, Kosten TR. Psychopharmacology: neuroimmune signaling in psychiatric disease-developing vaccines against abused drugs using toll-like receptor agonists. Psychopharmacology. 2019;236:2899–2907. doi: 10.1007/s00213-019-5176-9. [DOI] [PubMed] [Google Scholar]
- 131.Robinson C, Gradinati V, Hamid F, Baehr C, Crouse B, Averick S, et al. Therapeutic and prophylactic vaccines to counteract fentanyl use disorders and toxicity. J Med Chem. 2020;63:14647–14667. doi: 10.1021/acs.jmedchem.0c01042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wang JX, Ellis B, Zhou B, Eubanks LM, Blake S, Janda KD. A fentanyl vaccine constructed upon opsonizing antibodies specific for the Galα1-3Gal epitope. Chem Commun (Camb) 2020;56:6551–6554. doi: 10.1039/d0cc02107e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Townsend EA, Bremer PT, Jacob NT, Negus SS, Janda KD, Banks ML. A synthetic opioid vaccine attenuates fentanyl-vs-food choice in male and female rhesus monkeys. Drug Alcohol Depend. 2021;218:108348. doi: 10.1016/j.drugalcdep.2020.108348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Tenney RD, Blake S, Bremer PT, Zhou B, Hwang CS, Poklis JL, et al. Vaccine blunts fentanyl potency in male rhesus monkeys. Neuropharmacology. 2019;158:107730. doi: 10.1016/j.neuropharm.2019.107730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Smith LC, Bremer PT, Hwang CS, Zhou B, Ellis B, Hixon MS, et al. Monoclonal antibodies for combating synthetic opioid intoxication. J Am Chem Soc. 2019;141:10489–10503. doi: 10.1021/jacs.9b04872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Baehr C, Kelcher AH, Khaimraj A, Reed DE, Pandit SG, AuCoin D, et al. Monoclonal antibodies counteract opioid-induced behavioral and toxic effects in mice and rats. J Pharmacol Exp Ther. 2020;375:469–477. doi: 10.1124/jpet.120.000124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Blake S, Bremer PT, Zhou B, Petrovsky N, Smith LC, Hwang CS, et al. Developing translational vaccines against heroin and fentanyl through investigation of adjuvants and stability. Mol Pharm. 2021;18:228–235. doi: 10.1021/acs.molpharmaceut.0c00837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Park H, Lee JC, Eubanks LM, Ellis B, Zhou B, Janda KD. Improvements on a chemically contiguous hapten for a vaccine to address fentanyl-contaminated heroin. Bioorg Med Chem. 2021;41:116225. doi: 10.1016/j.bmc.2021.116225. [DOI] [PubMed] [Google Scholar]
- 139.Townsend EA, Bremer PT, Faunce KE, Negus SS, Jaster AM, Robinson HL, et al. Evaluation of a dual fentanyl/heroin vaccine on the antinociceptive and reinforcing effects of a fentanyl/heroin mixture in male and female rats. ACS Chem Neurosci. 2020;11:1300–1310. doi: 10.1021/acschemneuro.0c00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Barrientos RC, Whalen C, Torres OB, Sulima A, Bow EW, Komla E, et al. Bivalent conjugate vaccine induces dual immunogenic response that attenuates heroin and fentanyl effects in mice. Bioconjug Chem. 2021;32:2295–2306. doi: 10.1021/acs.bioconjchem.1c00179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Natori Y, Hwang CS, Lin L, Smith LC, Zhou B, Janda KD. A chemically contiguous hapten approach for a heroin-fentanyl vaccine. Beilstein J Org Chem. 2019;15:1020–1031. doi: 10.3762/bjoc.15.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hwang CS, Smith LC, Natori Y, Ellis B, Zhou B, Janda KD. Improved admixture vaccine of fentanyl and heroin hapten immunoconjugates: Antinociceptive evaluation of fentanyl-contaminated heroin. ACS Omega. 2018;3:11537–11543. doi: 10.1021/acsomega.8b01478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hwang CS, Smith LC, Natori Y, Ellis B, Zhou B, Janda KD. Efficacious vaccine against heroin contaminated with fentanyl. ACS Chem Neurosci. 2018;9:1269–1275. doi: 10.1021/acschemneuro.8b00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Maurya A, Rangappa S, Bae J, Dhawan T, Ajjarapu SS, Murthy SN. Evaluation of soluble fentanyl microneedles for loco-regional anti-nociceptive activity. Int J Pharm. 2019;564:485–491. doi: 10.1016/j.ijpharm.2019.04.066. [DOI] [PubMed] [Google Scholar]
- 145.Liu TT, Shi J, Epstein DH, Bao YP, Lu L. A meta-analysis of acupuncture combined with opioid receptor agonists for treatment of opiate-withdrawal symptoms. Cell Mol Neurobiol. 2009;29:449–454. doi: 10.1007/s10571-008-9336-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Shi J, Liu YL, Fang YX, Xu GZ, Zhai HF, Lu L. Traditional Chinese medicine in treatment of opiate addiction. Acta Pharmacol Sin. 2006;27:1303–1308. doi: 10.1111/j.1745-7254.2006.00431.x. [DOI] [PubMed] [Google Scholar]
- 147.Sun HQ, Chen HM, Yang FD, Lu L, Kosten TR. Epidemiological trends and the advances of treatments of amphetamine-type stimulants (ATS) in China. Am J Addict. 2014;23:313–317. doi: 10.1111/j.1521-0391.2014.12116.x. [DOI] [PubMed] [Google Scholar]