Abstract
BACKGROUND:
Massachusetts is a northeastern state with universally mandated health insurance since 2006. Although Black men have generally worse prostate cancer outcomes, emerging data suggest that they may experience equivalent outcomes within a fully insured system. In this setting, the authors analyzed treatments and outcomes of non-Hispanic White and Black men in Massachusetts.
METHODS:
White and Black men who were 20 years old or older and had been diagnosed with localized intermediate- or high-risk nonmetastatic prostate cancer in 2004–2015 were identified in the Massachusetts Cancer Registry. Adjusted logistic regression models were used to assess predictors of definitive therapy. Adjusted and unadjusted survival models compared cancer-specific mortality. Interaction terms were then used to assess whether the effect of race varied between counties.
RESULTS:
A total of 20,856 men were identified. Of these, 19,287 (92.5%) were White. There were significant county-level differences in the odds of receiving definitive therapy and survival. Survival was worse for those with high-risk cancer (adjusted hazard ratio [HR], 1.50; 95% CI, 1.4–1.60) and those with public insurance (adjusted HR for Medicaid, 1.69; 95% CI, 1.38–2.07; adjusted HR for Medicare, 1.2; 95% CI, 1.14–1.35). Black men were less likely to receive definitive therapy (adjusted odds ratio, 0.78; 95% CI, 0.74–0.83) but had a 17% lower cancer-specific mortality (adjusted HR, 0.83; 95% CI, 0.7–0.99).
CONCLUSIONS:
Despite lower odds of definitive treatment, Black men experience decreased cancer-specific mortality in comparison with White men in Massachusetts. These data support the growing body of research showing that Black men may achieve outcomes equivalent to or even better than those of White men within the context of a well-insured population.
Keywords: prostate cancer, racial disparities, surgical outcomes
LAY SUMMARY:
• There is a growing body of evidence showing that the excess risk of death among Black men with prostate cancer may be caused by disparities in access to care, with few or no disparities seen in universally insured health systems such as the Veterans Affairs and US Military Health System.
• Therefore, the authors sought to assess racial disparities in prostate cancer in Massachusetts, which was the earliest US state to mandate universal insurance coverage (in 2006).
• Despite lower odds of definitive treatment, Black men with prostate cancer experience reduced cancer-specific mortality in comparison with White men in Massachusetts. These data support the growing body of research showing that Black men may achieve outcomes equivalent to or even better than those of White men within the context of a well-insured population.
INTRODUCTION
There are well-documented race-based differences in prostate cancer outcomes. Black men with prostate cancer have higher incidence rates,1 a lower likelihood of receiving appropriate treatment, less access to high-quality care,2,3 and worse survival.4,5 To explain these differences, there is evidence for environmental,6 biological,7 social, demographic, and health-systems factors such as disparities in insurance and access to high-volume care.4,8,9,10,11 Their relative contributions remain a topic of ongoing study. Geographic analysis reveals where services are needed to reduce racial disparities.12
Geographic differences in outcomes and treatment are especially relevant for racial disparities because Black men tend to live within a specific subset of counties and receive care within a small subset of hospitals: among the more than 3000 counties in the United States, fewer than 100 have majority Black populations. In the South, these are predominantly rural areas, whereas Black men in the Midwest and Northeast tend to live in urban counties.13 This uneven distribution of Black patients has led some to hypothesize that race-based differences in health outcomes may be mediated by differences in where minorities receive care.14–16
Against this background, we sought to analyze race-based outcomes in Massachusetts. Unlike most of the United States, Massachusetts has had legally mandated insurance coverage since 2006 and extremely low rates of uninsured individuals. Black men typically reside in populous, urban counties such as Suffolk (including the city of Boston) and Hampden (composing the Massachusetts portion of the Hartford/Springfield metropolitan area). These counties have large numbers of qualified physicians and sophisticated networks of acute care hospitals and community health centers.17,18
Because there is evidence that racial disparities could be eliminated by the removal of access-related barriers such as insurance, we hypothesized that rates of definitive treatment would be similar for Black and White men throughout the state and that the odds of treatment would be highest in the populous eastern counties (which also happen to have high proportions of Black patients). We further hypothesized that there would be minimal survival differences between non-Hispanic Black men and non-Hispanic White men presenting with prostate cancer in Massachusetts.
MATERIALS AND METHODS
Setting
Massachusetts is a populous northeastern state consisting of 14 counties. The population is 6.9 million, of which 7.39% are Black or African American. The state has earned a high rank among its peers on metrics of economic development, income, and education.19–21 The state has been considered the birthplace of the Affordable Care Act; the Massachusetts 2006 health care reform law was a model for the 2010 Patient Protection and Affordable Care Act, a federal law designed to expand health insurance for all Americans. From the perspective of health care, Massachusetts performs well on a large number of metrics: The state has the highest number of physicians per capita,22 the lowest infant mortality rate,23 and the lowest uninsured rate of any US state.24
The state comprises 14 counties, which vary significantly in terms of their demographic composition. The eastern coastal counties of the state include Boston and its suburbs, with more than half (63.5%) of the state population. The central regions are composed of the rural region of central Massachusetts, the farmland and former industrial centers of the Connecticut River Valley, and the western mountains. The counties differ substantially in terms of their size, urban classification, and racial and age compositions. Although the urban center of Suffolk County (comprising Boston and 3 northern suburbs) has a high concentration of large academic medical centers and a robust community health system, many rural towns in western Massachusetts have few or no physicians.17
Data Source
The data source of this study is the Massachusetts Cancer Registry of the Massachusetts Department of Public Health (https://www.mass.gov/massachusetts-cancer-registry). This is a population-based surveillance system comprising all cases of newly diagnosed prostate cancer in the Commonwealth of Massachusetts. By law, all newly diagnosed cancer cases must be reported to the Massachusetts Cancer Registry. Deaths are reported to the Registry of Vital Records and Statistics of the Massachusetts Department of Public Health.
Inclusion and Exclusion Criteria
Data were extracted from the Massachusetts Registry for all men aged 20 years or older of any race with newly diagnosed intermediate-or high-risk prostate cancer from 2004 to 2015 in any of the 14 counties in Massachusetts. Prostate cancer cases were extracted on the basis of National Comprehensive Cancer Network–defined intermediate-(prostate-specific antigen level of 10–20 ng/mL or Gleason score of 7 [Grade group 2 or 3], cT2b or cT2c) and high-risk groups (prostate-specific antigen level > 20 ng/mL or Gleason score ≥ (Grade group 4) or c≥T3a). For the purpose of this study, we focused on men of Black and White (non-Hispanic) race. This was done because the gap between Blacks and Whites is the largest racial gap in mortality among men with prostate cancer.5 We excluded men with regional and distant metastatic disease, which is generally incurable with definitive local treatment. This was done to be able to assess differences in access to care and definitive treatments. Men for whom the grade, stage, and Gleason score were missing were excluded. We excluded those with low-risk disease because these men commonly have good outcomes, regardless of treatment.25 We then excluded those with missing socioeconomic variables and survival times. The same cohorts were used for the analyses of both survival and definitive therapy.
Predictor Variable
The primary predictor variable for this analysis was patient-reported race. The secondary predictor variable was the county of diagnosis. This was used because it was felt to be a large enough geographic area to capture variability in the receipt of prostate cancer treatment and had been used in prior studies to assess the degree of variability within states.26,27
Outcome Variables
The coprimary outcomes of this study were receipt of definitive therapy (defined as prostatectomy or radiation therapy within 90 days of diagnosis) and cancer-specific survival based on the time from diagnosis to cancer-specific mortality as reported to the Massachusetts Cancer Registry. The 90-day period from diagnosis to treatment is a commonly used interval to assess treatment delays in studies of racial disparities.28 A separate sensitivity analysis was also performed with a cutoff of 180 days from treatment.
Covariates
Patient-level covariates, including age, patient insurance type, and risk group (intermediate vs high), were extracted from the state cancer registry. Insurance coverage was categorized as private insurance (generally employer-sponsored or purchased insurance), Medicare (the US fee-for-service insurance system for men and women older than 65 years), Medicaid (public insurance for low-income adults, which is subsidized by the US federal government), or no insurance. Contextual neighborhood sociodemographic variables were obtained from the year 2000 census at the block group level. We linked the percentage of patients under the poverty line, median income, median home value, education (based on the proportion of adults older than 25 years completing high school), and population density to participants’ residential addresses. The race-income index of patients’ residences was used to estimate segregation by race.29 Residential addresses were statistically perturbed within 250 m of the residence to preserve patient confidentiality.
Statistical Analysis
Means and standard deviations or medians and interquartile ranges (IQRs) were reported for normally or nonnormally distributed continuous variables, respectively. Categorical variables were presented as frequencies and proportions.
We modeled the receipt of definitive therapy via multivariable logistic regression adjusted for patient race, county of residence, and the aforementioned patient-level covariates. This was done to determine the racial inequality that would remain after differences in socioeconomic status across racial groups had been addressed.30 A multiplicative interaction term between race and country was used to test whether odds of definitive treatment varied between counties. We generated predicted probabilities by using the county-level coefficients and setting the covariates at the population mean.
To compare cancer-specific survival between Black and White men, we first used an unadjusted Kaplan-Meier survival analysis. An adjusted Cox proportional hazards model was then used to identify independent predictors of cancer-specific survival.31 We stratified by year at diagnosis to meet the proportional hazards assumption. A variable for each county was included to assess for survival in each country. Testing for the significance of an interaction term between county and race was performed to assess whether the effect of race on overall survival varied in a significant fashion between counties.
Because Black men in Massachusetts are more likely to have Medicaid insurance and more likely to be uninsured, a stratified analysis was performed to assess for race-based differences in definitive treatment and survival among the 4 categories of insurance status (uninsured, Medicare, Medicaid, and private insurance).
Statistical analyses were performed with R version 1.2.1335. The study was performed under a general study protocol using state administrative data sets for the study of cancer trends. This was approved by the Brigham and Women’s Hospital institutional review board.
RESULTS
Cohort selection and exclusion criteria are outlined in Figure 1. After the aforementioned exclusion criteria had been applied, there were a total of 20,856 men of Black or White race who were diagnosed with intermediate-or high-risk prostate cancer from 2004 to 2015 in the 14 Massachusetts counties. Of these, 19,287 (92.5%) were White, and 1569 (7.5%) were Black. Among Massachusetts men with prostate cancer, Black men had a younger median age of 63 years (IQR, 57–69 years) versus 66 years (IQR, 60–72 years) for White men. Compared with White men, Black men were also more likely to be uninsured (2.8% vs 0.4% of White men) and more likely to have Medicaid insurance (17.3% vs 2.5%; P < .0001 for both). The remaining baseline characteristics of the Black and White patients included in our population are summarized in Table 1.
Figure 1.
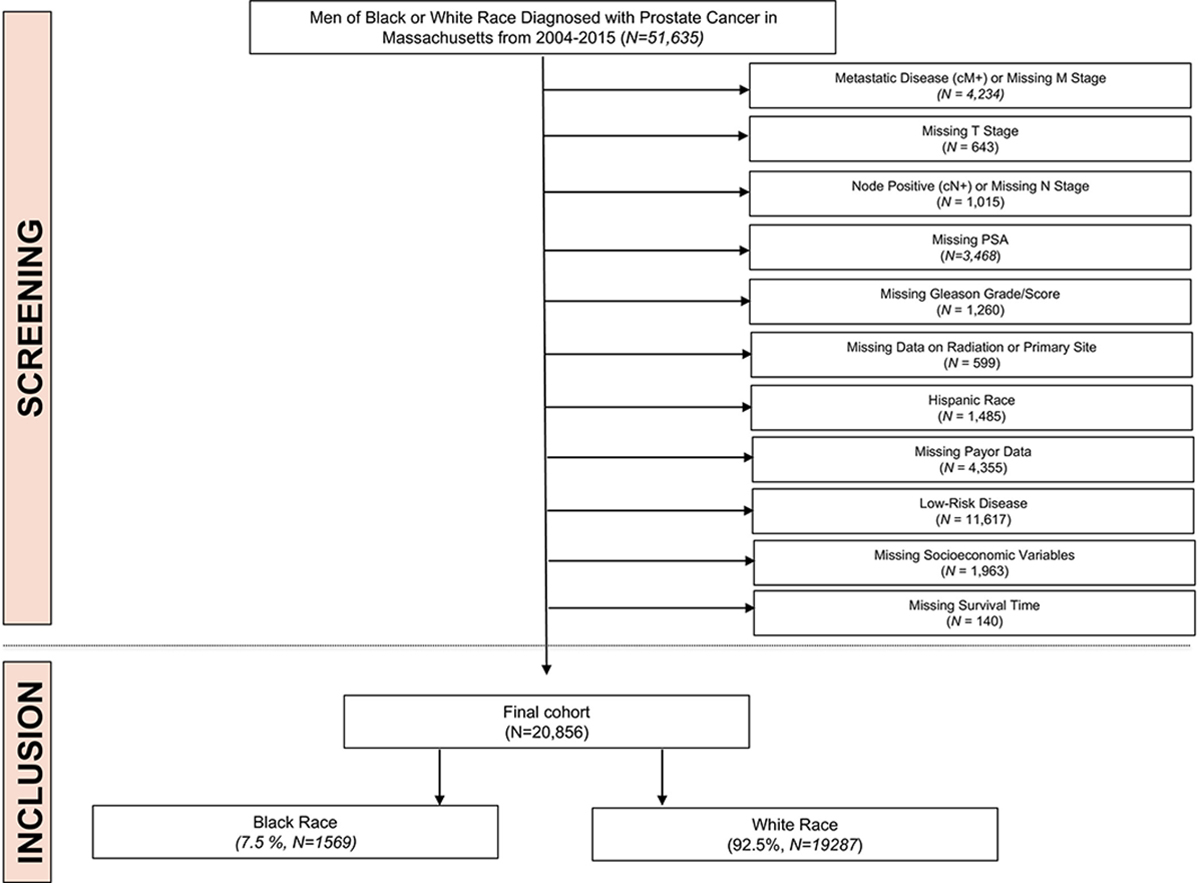
Study cohort selection. PSA indicates prostate-specific antigen
TABLE 1.
Characteristics of 20,856 Men in Massachusetts Diagnosed With Intermediate- and High-Risk Localized Prostate Cancer, 2004–2015
| Total (n = 20,856), No. (%) | Black (n = 1569 [7.5%]), No. (%) | White (n = 19,287 [92.5%]), No. (%) | P | |
|---|---|---|---|---|
|
| ||||
| Risk group | <.0001 | |||
| Intermediate | 12,843 (61.6) | 933 (59.5) | 11,910 (61.8) | |
| High | 8013 (38.4) | 636 (40.5) | 7377 (38.3) | |
| Insurance | <.0001 | |||
| Private | 10,387 (49.8) | 700 (44.6) | 9687 (50.2) | |
| Uninsured | 124 (0.6) | 44 (2.8) | 80 (0.4) | |
| Medicare | 9588 (46.0) | 553 (35.3) | 9035 (46.9) | |
| Medicaid | 757 (3.6) | 272 (17.3) | 485 (2.5) | |
| Year of diagnosis | <.0001 | |||
| 2004–2007 | 7907 (37.9) | 496 (31.6) | 7411 (38.4) | |
| 2008–2011 | 7425 (35.6) | 553 (35.3) | 6872 (35.6) | |
| 2012–2015 | 5524 (26.5) | 520 (33.1) | 5004 (25.9) | |
| County | <.0001 | |||
| Suffolk | 1467 (7.0) | 615 (39.2) | 852 (4.4) | |
| Barnstable | 1304 (6.3) | 13 (0.8) | 1291 (6.7) | |
| Berkshire | 596 (2.9) | –a | 589 (3.1) | |
| Bristol | 1859 (8.9) | 67 (4.3) | 1792 (9.3) | |
| Dukes | 56 (0.3) | –a | 52 (0.3) | |
| Essex | 2570 (12.3) | 75 (4.8) | 2495 (12.9) | |
| Franklin | 199 (1.0) | –a | 198 (1.0) | |
| Hampden | 1288 (6.2) | 143 (9.1) | 1145 (5.9) | |
| Hampshire | 421 (2.0) | –a | 414 (2.2) | |
| Middlesex | 4649 (22.3) | 283 (18.0) | 4366 (22.6) | |
| Nantucket | 30 (0.1) | –a | 28 (0.2) | |
| Norfolk | 2214 (10.6) | 119 (7.6) | 2095 (10.9) | |
| Plymouth | 1734 (8.3) | 138 (8.8) | 1596 (8.3) | |
| Worcester | 2469 (11.8) | 95 (6.1) | 2374 (12.3) | |
| Treatment typeb | <.0001 | |||
| Radiation | 2978 (14.3) | 158 (10.7) | 2820 (14.3) | |
| Surgery | 6332 (30.4) | 368 (23.5) | 5964 (30.4) | |
| No definitive treatment | 11,994 (57.5) | 1062 (67.7) | 10,932 (57.5) | |
Cells with <10 individuals were censored for privacy.
Because of the small number of men who received both surgery and radiation within our study timeframe, these values sum to >100%.
The independent predictors of definitive treatment are summarized in Table 2. After adjusting for county, risk category, age, insurance type, and demographic variables, including income, education, density, race-income index, and percentage living under the poverty line, we found that Black men were significantly less likely to receive definitive therapy than White men with clinically localized high- or intermediate-risk prostate cancer (adjusted odds ratio [OR], OR 0.67; 95% CI, 0.59–0.77). Other independent predictors of lower odds of treatment included public insurance, including Medicaid (adjusted OR vs private insurance, 0.7; 95% CI, 0.60–0.82) and Medicare (adjusted OR vs private insurance, 0.92; 95% CI, 0.85–0.99). The results of a sensitivity analysis using the 180-day cutoff are shown in Supporting Table 3. There was a similar pattern of lower odds of definitive treatment in Black men with a slightly smaller but still significant effect (adjusted OR, 0.719; 95% CI, 0.66–0.81; P < .0001). One notable difference was that the odds of definitive treatment shifted to be higher in high-risk disease with the longer cutoff.
TABLE 2.
Multivariable Analysis of Predictors of Receiving Definitive Treatment Among 20856 Black and White Men in Massachusetts with Intermediate- and High-Risk Prostate Cancer
| Effect | Odds Ratio | 95% Confidence Intervals | P | |
|---|---|---|---|---|
|
| ||||
| Race | ||||
| White Race | ref | |||
| Black Race | 0.67 | 0.59 | 0.77 | <.0001 |
| Risk Group | ||||
| Intermediate Risk | ref | |||
| High Risk | 0.97 | 0.92 | 1.03 | .30 |
| Insurance | ||||
| Private Insurance | ref | |||
| Medicaid | 0.70 | 0.60 | 0.82 | <.0001 |
| Medicare | 0.92 | 0.85 | 0.99 | .02 |
| Uninsured | 0.72 | 0.49 | 1.05 | .09 |
| Age at Diagnosis (per year of additional age) | 0.97 | 0.96 | 0.97 | <.0001 |
| County | ||||
| Suffolk | ref | |||
| Barnstable | 1.19 | 0.99 | 1.43 | .06 |
| Berkshire | 2.39 | 1.92 | 2.97 | <.0001 |
| Bristol | 1.32 | 1.12 | 1.57 | .0014 |
| Dukes | 0.82 | 0.45 | 1.49 | .52 |
| Essex | 1.14 | 0.98 | 1.34 | .10 |
| Franklin | 0.97 | 0.70 | 1.34 | .83 |
| Hampden | 1.25 | 1.05 | 1.49 | .01 |
| Hampshire | 1.53 | 1.20 | 1.95 | .0005 |
| Middlesex | 1.144 | 0.99 | 1.32 | .07 |
| Nantucket | 0.18 | 0.05 | 0.67 | .01 |
| Norfolk | 1.22 | 1.04 | 1.43 | .016 |
| Plymouth | 1.33 | 1.13 | 1.58 | .0008 |
| Worcester | 1.28 | 1.09 | 1.51 | .002 |
Census level variables including percentage of residents living under the poverty line, median home value, percentage with no HS diploma population density, and race-income index were not associated with receipt of definitive treatment (P > 0.05) and are left out for clarity.
As for geographic variability, the odds of definitive treatment differed greatly between counties, as shown in Figure 2. The interaction between county and race was not statistically significant, and this suggested that the impact of race on receipt of definitive therapy did not vary significantly between counties.
Figure 2.

Predicted probabilities of receiving definitive therapy by county in Massachusetts, 2004–2015.
A Kaplan-Meier analysis of cancer-specific survival over a median follow-up of 5.9 years is shown in Figure 3. In this unadjusted analysis, Black race was associated with a small but significant trend toward improved cancer-specific mortality. The proportions of men surviving prostate cancer at 5 and 10 years were 92.1% and 75.%, respectively, for Black men and 89.7% and 72.2%, respectively, for White men (P < .01 by a log-rank test).
Figure 3.
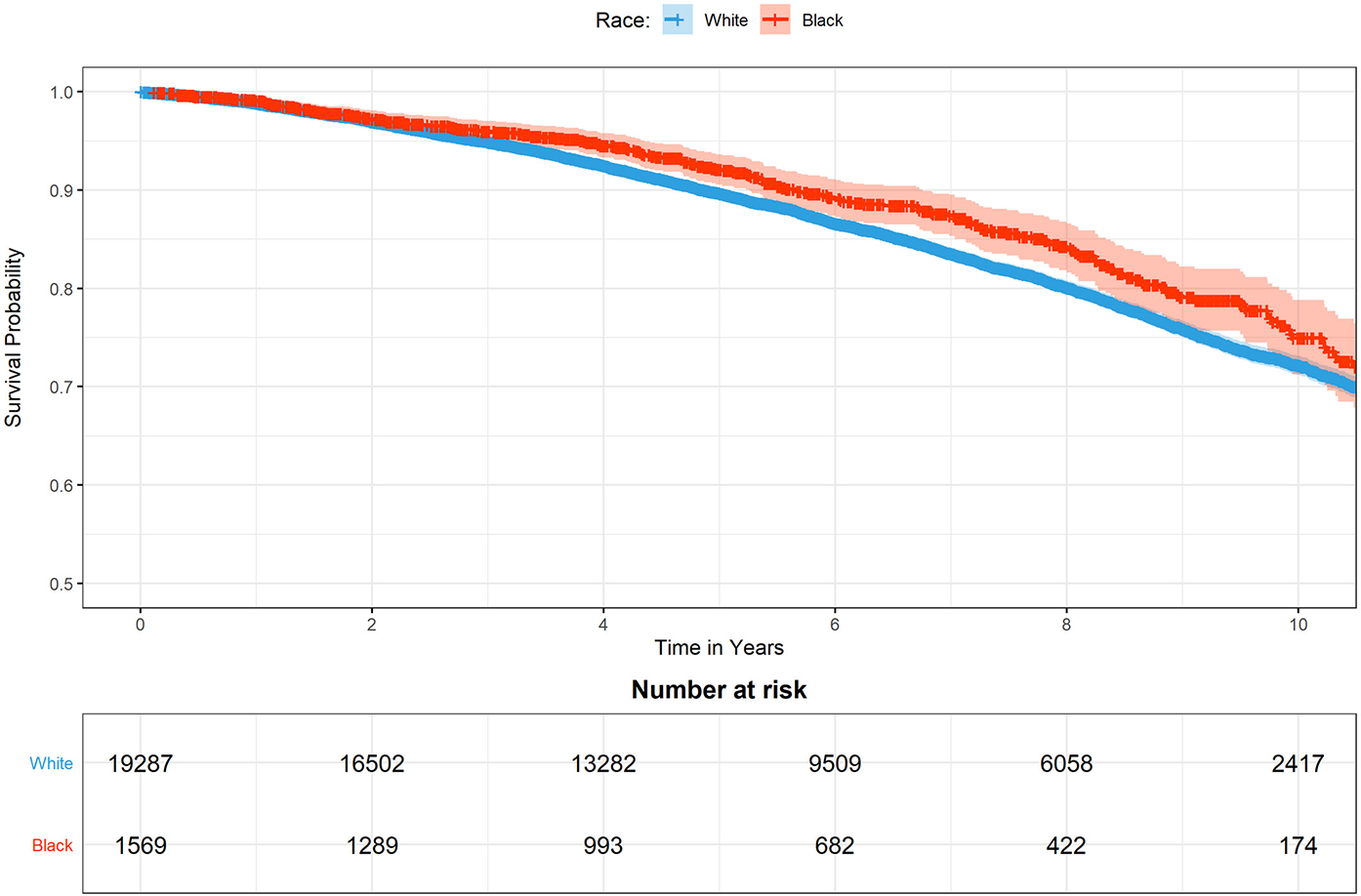
Unadjusted Kaplan-Meier curves showing cancer-specific survival among Black and White men with prostate cancer in Massachusetts.
The results of the adjusted survival analysis are shown in Table 3. Black race was an independent predictor of lower cancer-specific mortality (adjusted hazard ratio [HR], 0.83; 95% CI, 0.7–0.99). This corresponded to predicted 5- and 10-year survival rates of 95.4% and 89.8% for Black men and 94.5% and 87.6% for White men. Other predictors of worse cancer-specific mortality included high-risk cancer (adjusted HR, 1.50; 95% CI, 1.40–1.60) and public insurance, including Medicaid (adjusted HR, 1.69; 95% CI, 1.38–2.07) and Medicare (adjusted HR, 1.24; 95% CI, 1.14–1.35). Unlike the case with definitive treatment, there were no significant county-level differences in adjusted cancer-specific survival. Similarly, the interaction of race and county was not significantly associated with cause specific mortality, suggesting no differential effect of race in the different Massashusetts counties.
TABLE 3.
Predictors of Cancer Specific Mortality Among 20856 Black and White Men in Massachusetts with Intermediate- and High-Risk Prostate Cancer
| Hazard Ratio | 95% Confidence Intervals | P | ||
|---|---|---|---|---|
|
| ||||
| Race | ||||
| White Men | ref | |||
| Black Men | 0.83 | 0.70 | 0.99 | .03 |
| Risk Category | ||||
| Intermediate | ref | |||
| High Risk | 1.50 | 1.40 | 1.60 | <.0001 |
| Insurance Category | ||||
| Private | ref | |||
| Medicaid | 1.69 | 1.38 | 2.07 | <.0001 |
| Medicare | 1.24 | 1.14 | 1.35 | <.0001 |
| Uninsured | 1.27 | 0.81 | 2.01 | .30 |
| Age | ||||
| (per year of additional age) | 1.09 | 1.08 | 1.09 | <.0001 |
| County | ||||
| Suffolk | ref | |||
| Barnstable | 1.08 | 0.88 | 1.34 | .47 |
| Berkshire | 1.09 | 0.86 | 1.40 | .47 |
| Bristol | 1.07 | 0.87 | 1.316 | .52 |
| Dukes | 1.15 | 0.65 | 2.04 | .64 |
| Essex | 1.12 | 0.92 | 1.36 | .25 |
| Franklin | 0.94 | 0.64 | 1.38 | .74 |
| Hampden | 1.08 | 0.88 | 1.34 | .47 |
| Hampshire | 1.02 | 0.76 | 1.37 | .90 |
| Middlesex | 1.18 | 0.99 | 1.40 | .067 |
| Nantucket | 1.1 | 0.44 | 2.77 | .84 |
| Norfolk | 1.16 | 0.95 | 1.42 | .14 |
| Plymouth | 1.24 | 1.01 | 1.52 | .04 |
| Worcester | 1.22 | 1 | 1.48 | .05 |
Census level variables including percentage of residents living under the poverty line, median home value, percentage with no HS diploma, population density, and race-income index were not associated with receipt of definitive treatment (P > 0.05) and are left out for clarity.
Stratified Analysis by Insurance Status
Because Black men in Massachusetts are more likely to have Medicaid insurance and more likely to be uninsured (Table 1), a stratified analysis was performed to assess for race-based differences in definitive treatment and survival among the 4 categories of insurance status (Supporting Table 2). The results globally mirrored those of the overall cohort. Among those men with private insurance, no insurance, and Medicare, there were no differences in cancer-specific survival, and among those with Medicaid, there was a lower hazard of cancer-specific mortality among Black men (adjusted HR, 0.54; 95% CI, 0.32–0.93).
DISCUSSION
In this study, we describe outcomes and treatment of Black and White men with prostate cancer in Massachusetts. Although there were significant geographic differences in the receipt of definitive therapy across the state, with Black men being overall less likely to receive definitive therapy in comparison with White men, there was no worse survival among Black men. In fact, Black men with localized high- or intermediate-risk prostate cancer had significantly higher overall survival than White men. This was true in both adjusted and unadjusted analyses.
These findings contrast with much prior observational research showing that Black men experience worse cancer-specific survival than men of all other races.32–34 Some investigators advocate for a biological explanation for these differences: data exist suggesting that even among men within the same risk category, Black men may have worse biological phenotypes suggesting a distinct tumor biology.35,36 On the other hand, a large number of studies have shown that Black men have worse access to prostate cancer screening,37 appropriate follow-up,38 and receipt of definitive therapy.2 This naturally raises the question of whether differences in outcomes may be related to differences in access to care.
Our group recently published an analysis of national cancer registry data for the overall survival of Black and White men with prostate cancer.4 When we analyzed the relative contributions of treatment, tumor characteristics, and access to care, we found that adjusting for access-related variables greatly attenuated the association between race and overall mortality. After generating simulated cohorts with equal treatment and equivalent tumor stages (eg, to account for a tendency of delayed diagnosis in Black men), we found that Black men had better survival than Whites (HR, 0.9; 95% CI, 0.86–1.01; HR, 0.92; 95% CI, 0.84–1.00).
These model-based results have also been confirmed in observational studies restricted to equal-opportunity environments. For example, Cullen et al39 analyzed men treated within the Department of Defense health system and found comparable use of active surveillance and comparable overall survival among Black and White men. A recent study also using Department of Defense data showed better prostate cancer screening among Black men within the Department of Defense Health System in comparison with White men.40 Freedland et al, in an equal-access US Veterans Affairs data set, also showed similar risks of metastasis and prostate cancer–specific death among Black and White men undergoing radical prostatectomy.41 Similar results have been shown in universally insured civilian populations. Among men served by Medicare (the US universal health insurance system for the elderly), our group has published work showing no race-based differences in prostate cancer–specific or overall mortality.28
The idea that Black men may do as well as or better than White men with prostate cancer if they receive appropriate treatment is also supported by clinical trial data. Men enrolled in clinical trials typically receive uniformly close follow-up, a high standard of care and often have access to promising modern medications. Two recent meta-analyses of clinical trials also found no worse survival in Black men with prostate cancer who received specific chemotherapy regimens. The first, by Spratt et al,42 found no race-based differences in progression-free or overall survival in 5 contemporary randomized trials of men with castrate-resistant prostate cancer.42 Subsequently, Halabi et al43 found that Black men who received docetaxel for metastatic castrate-resistant prostate cancer had better overall survival than White men. Finally, the recently presented Abi-Race trial by George et al44 found that Black patients had a greater and more durable prostate-specific antigen response to abiraterone in comparison with White patients. Similar to our findings, these studies support the concept that Black men with prostate cancer can do as well or better than White men with prostate cancer if they receive high quality care.
Our findings of better outcomes for Black men in Massachusetts with prostate cancer compare favorably with 2 studies of men in Georgia and South Carolina that also explored geospatial differences in prostate cancer mortality.26,27 These studies assessed mortality-to-incidence ratios for White and Black men across the states. They showed significantly worse mortality-to-incidence ratios among Black men: 0.26 (Black men) versus 0.16 (White men) in South Carolina and 0.58 (Black men) versus 0.24 (White men) in Georgia. These states tend to have more rural minority populations and did not have universally mandated insurance coverage at the time of publication (2009 and 2012, respectively). Furthermore, some have argued for adverse health outcomes as a consequence of historically racist social policies; for example, work in breast cancer has shown worse outcomes in Jim Crow states compared with non–Jim Crow states.45,46 It stands to reason that such policies may have had a greater impact on Black patients with prostate cancer in Georgia and South Carolina in comparison with Massachusetts.
Curiously, in our analysis of definitive treatment, we found both more geographic variability and lower utilization of definitive therapy among Black men. This is puzzling: Why would there be both lower utilization of definitive therapy and better survival among Black men? One explanation is simply that prompt definitive therapy is less important for long-term cancer outcomes. Data from Vickers et al,47 in their institutional series from Memorial Sloan Kettering, would seem to support this hypothesis: they found no greater risk of biochemical recurrence among even high-risk men who delayed surgery for greater than 6 months versus 12 months. Data from Johns Hopkins also suggest that a moderate delay may not matter in the long run, even for men with high-risk disease.48 In contrast, some registry data suggest that delays of 3 months or more may be associated with worsened survival in intermediate prostate cancer.49 We explored this by increasing the definition of definitive treatment to 180 days in our sensitivity analysis. There was a reduction in the disparity in the receipt of definitive treatment, and this suggests that some Black men who are not treated within 90 days do receive treatment within 180 days. However, there remained a large disparity in the number of men Black men who were still not treated even with the longer cutoff.
Regarding our finding that definitive treatment of high risk disease became more likely when using the longer cutoff: This may be related to a variety of factors including a more time required to complete staging workups and may also be a result of our definition of definitive treatment as surgery or radiation. Some high-risk men may have undergone a period of ADT prior to starting radiation or surgery would have prolonged the duration of time to treatment among high-risk men.
This study is not without limitations. First, although observational studies like ours may yield insights into the ways in which policies and health systems influence health outcomes, they cannot definitively show that policies themselves cause these outcomes.50 In particular, we could not control for behavioral and lifestyle risk factors that could have led to a confounding bias in this study. Second, there were small numbers of cases in some counties (eg, both Nantucket County and Dukes County had fewer than 100 cases each), and very few of these were Black men. Thus, although the effect of race on survival did not vary significantly across counties, there is limited power to draw statistical inferences about specific counties. Prostate cancer tends to have a long latency period between treatment and overall survival.51 Lastly and importantly, we assessed only men with localized high- or intermediate-risk prostate cancer. This was done because these cancers are generally curable but do require treatment and can progress if left untreated. Thus, we hypothesized that they may be particularly sensitive to differences in treatment and access to care. Because we focused on this population, we would not capture disparities related to diagnostic delays among Black men. For example, there may be unmeasured behavioral risk factors that could be associated with stage at diagnosis and mortality. To address the possibility of a selection bias, we did perform an analysis of baseline characteristics of the overall cohort before applying exclusion criteria (Supporting Table 1), and the overall baseline characteristics were similar.
In summary, the data herein describe cancer-specific survival for Black and White men with prostate cancer in the Commonwealth of Massachusetts from 2004 to 2015—a period when the state had nearly universal insurance and a highly developed health system in the urban areas with large proportions of minority patients. Despite a lower likelihood of definitive treatment, Black men had better cancer-specific survival than White men in both adjusted and unadjusted survival analyses. Although prior studies have shown that Black men can have outcomes as good as or better than those of White men in universally insured Medicare and Veterans Affairs populations or within the context of a clinical trial, this is the first study of which we are aware to show that this is also possible in a real-world setting in a US state with robust policies to mandate insurance coverage and health care access.
Supplementary Material
FUNDING SUPPORT
Alexander P. Cole and Quoc-Dien Trinh are supported by American Cancer Society and Pfizer Global Medical Grants (grant 63354905). Quoc-Dien Trinh is supported by the Brigham Research Institute Fund to Sustain Research Excellence, the Beal Surgical Fellowship, the Genentech Bio-Oncology Career Development Award from the Conquer Cancer Foundation of the American Society of Clinical Oncology (grant 10202), a Health Services Research pilot test grant from the Defense Health Agency, and the Clay Hamlin Young Investigator Award from the Prostate Cancer Foundation (grant 16YOUN20). Hari Iyer received salary support from the National Institutes of Health (T32 CA 009001). Brandon A. Mahal reports grants from the American Society for Radiation Oncology/Prostate Cancer Foundation, the US Department of Defense, and the National Institutes of Health.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
Speaker fees from Myovant; and stock options in Novavax. Quoc-Dien Trinh reports honoraria from Bayer, Janssen, and Astellas and research funding from Intuitive Surgical. The other authors made no disclosures.
REFERENCES
- 1.Brawley OW. Prostate cancer epidemiology in the United States. World J Urol. 2012;30:195–200. [DOI] [PubMed] [Google Scholar]
- 2.Friedlander DF, Trinh QD, Krasnova A, et al. Racial disparity in delivering definitive therapy for intermediate/high-risk localized prostate cancer: the impact of facility features and socioeconomic characteristics. Eur Urol. 2018;73:445–451. [DOI] [PubMed] [Google Scholar]
- 3.Trinh QD, Sun M, Sammon J, et al. Disparities in access to care at high-volume institutions for uro-oncologic procedures. Cancer. 2012;118:4421–4426. [DOI] [PubMed] [Google Scholar]
- 4.Krimphove MJ, Cole AP, Fletcher SA, et al. Evaluation of the contribution of demographics, access to health care, treatment, and tumor characteristics to racial differences in survival of advanced prostate cancer. Prostate Cancer Prostatic Dis. 2019;22:125–136. [DOI] [PubMed] [Google Scholar]
- 5.Aizer AA, Wilhite TJ, Chen MH, et al. Lack of reduction in racial disparities in cancer-specific mortality over a 20-year period. Cancer. 2014;120:1532–1539. [DOI] [PubMed] [Google Scholar]
- 6.Iyer HS, Valeri L, James P, et al. The contribution of residential greenness to mortality among men with prostate cancer: a registry-based cohort study of Black and White men. Environ Epidemiol. 2020;4:e087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sundi D, Kryvenko ON, Carter HB, Ross AE, Epstein JI, Schaeffer EM. Pathological examination of radical prostatectomy specimens in men with very low risk disease at biopsy reveals distinct zonal distribution of cancer in Black American men. J Urol. 2014;191:60–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mahal BA, Ziehr DR, Aizer AA, et al. Getting back to equal: the influence of insurance status on racial disparities in the treatment of African American men with high-risk prostate cancer. Urol Oncol. 2014;32:1285–1291. [DOI] [PubMed] [Google Scholar]
- 9.Dess RT, Hartman HE, Mahal BA, et al. Association of Black race with prostate cancer–specific and other-cause mortality. JAMA Oncol. 2019;5:975–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nabi J, Tully KH, Cole AP, et al. Access denied: the relationship between patient insurance status and access to high-volume hospitals. Cancer. 2021;127:577–585. [DOI] [PubMed] [Google Scholar]
- 11.Cole AP, Lu C, Krimphove MJ, et al. Comparing the association between insurance and mortality in ovarian, pancreatic, lung, colorectal, prostate, and breast cancers. J Natl Compr Canc Netw. 2019;17:1049–1058. [DOI] [PubMed] [Google Scholar]
- 12.Oliver A, Mossialos E. Equity of access to health care: outlining the foundations for action. J Epidemiol Community Health. 2004;58:655–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McKinnon J The Black Population: 2000. US Census Bureau. Published August 2001. Accessed September 9, 2020. https://www.census.gov/prod/2001pubs/c2kbr01-5.pdf [Google Scholar]
- 14.Cole AP, Nguyen DD, Meirkhanov A, et al. Association of care at minority-serving vs non–minority-serving hospitals with use of palliative care among racial/ethnic minorities with metastatic cancer in the United States. JAMA Netw Open. 2019;2:e187633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krimphove MJ, Fletcher SA, Cole AP, et al. Quality of care in the treatment of localized intermediate and high risk prostate cancer at minority serving hospitals. J Urol. 2018;27:4300. [DOI] [PubMed] [Google Scholar]
- 16.Hasnain-Wynia R, Kang R, Landrum MB, Vogeli C, Baker DW, Weissman JS. Racial and ethnic disparities within and between hospitals for inpatient quality of care: an examination of patient-level Hospital Quality Alliance measures. J Health Care Poor Underserved. 2010;21:629–648. [DOI] [PubMed] [Google Scholar]
- 17.2017 Massachusetts State Health Assessment. Massachusetts Department of Public Health. Published October 2017. Accessed September 9, 2020. https://www.mass.gov/service-details/2017-state-health-assessment, https://www.mass.gov/doc/2017-massachusetts-state-health-assessment/download [Google Scholar]
- 18.Summary of U.S. Census Bureau’s 2018 County Characteristics Estimates for Massachusetts Counties. UMass Donahue Institute. Published June 20, 2019. Accessed July 13, 2019. https://donahue.umass.edu/documents/UMDI_Summary_of_Census_County_Characteristics_V2018.pdf [Google Scholar]
- 19.The 10 Best U.S. States for Education: 1. Massachusetts. U.S. News and World Report.
- 20.2018. American Community Survey. US Census Bureau. Accessed September 9, 2020. https://www.census.gov/programs-surveys/acs/news/data-releases.2017.html [Google Scholar]
- 21.2020. United States Department of Commerce, Gross Domestic Product by State, 4th Quarter and Annual 2019 (April 7, 2020). Accessed June 16, 2020. https://www.bea.gov/data/by-place-us
- 22.2017 State Physician Workforce Data Report. Published 2017. Updated November 2017. Accessed 16 June 2020. https://store.aamc.org/downloadable/download/sample/sample_id/30/
- 23.The 2017 Massachusetts State Health Assessment: Chapter 2: Maternal, Infant, and Child Health. Massachusetts Department of Public Health. Published October 2017. https://www.mass.gov/doc/chapter-2-maternal-infant-and-child-health/download [Google Scholar]
- 24.Keith K Two New Federal Surveys Show Stable Uninsured Rate. Health Affairs Blog. Health Affairs. Published September 13, 2018. Accessed September 9, 2020. 10.1377/hblog20180913.896261/full/ [DOI]
- 25.Bruinsma SM, Bangma CH, Carroll PR, et al. Active surveillance for prostate cancer: a narrative review of clinical guidelines. Nat Rev Urol. 2016;13:151–167. [DOI] [PubMed] [Google Scholar]
- 26.Hebert JR, Daguise VG, Hurley DM, et al. Mapping cancer mortality-to-incidence ratios to illustrate racial and sex disparities in a high-risk population. Cancer. 2009;115:2539–2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wagner SE, Hurley DM, Hebert JR, McNamara C, Bayakly AR, Vena JE. Cancer mortality-to-incidence ratios in Georgia: describing racial cancer disparities and potential geographic determinants. Cancer. 2012;118:4032–4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schmid M, Meyer CP, Reznor G, et al. Racial differences in the surgical care of Medicare beneficiaries with localized prostate cancer. JAMA Oncol. 2016;2:85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krieger N, Waterman PD, Spasojevic J, Li W, Maduro G, Van Wye G. Public health monitoring of privilege and deprivation with the index of concentration at the extremes. Am J Public Health. 2016;106:256–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.VanderWeele TJ, Robinson WR. On the causal interpretation of race in regressions adjusting for confounding and mediating variables. Epidemiology. 2014;25:473–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cole AP, Trinh QD. Secondary data analysis: techniques for comparing interventions and their limitations. Curr Opin Urol. 2017;27:354–359. [DOI] [PubMed] [Google Scholar]
- 32.Mukerji B, Baptiste C, Chen L, et al. Racial disparities in young women with endometrial cancer. Gynecol Oncol. 2018;148:527–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miller JW, Smith JL, Ryerson AB, Tucker TC, Allemani C. Disparities in breast cancer survival in the United States (2001–2009): findings from the CONCORD-2 study. Cancer. 2017;123(suppl 24):5100–5118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.White A, Joseph D, Rim SH, Johnson CJ, Coleman MP, Allemani C. Colon cancer survival in the United States by race and stage (2001–2009): findings from the CONCORD-2 study. Cancer. 2017;123(suppl 24):5014–5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Powell IJ, Dyson G, Land S, et al. Genes associated with prostate cancer are differentially expressed in African American and European American men. Cancer Epidemiol Biomarkers Prev. 2013;22:891–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taioli E, Sears V, Watson A, et al. Polymorphisms in CYP17 and CYP3A4 and prostate cancer in men of African descent. Prostate. 2013;73:668–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mahal BA, Chen YW, Muralidhar V, et al. Racial disparities in prostate cancer outcome among prostate-specific antigen screening eligible populations in the United States. Ann Oncol. 2017;28:1098–1104. [DOI] [PubMed] [Google Scholar]
- 38.Barocas DA, Grubb R III, Black A, et al. Association between race and follow-up diagnostic care after a positive prostate cancer screening test in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial. Cancer. 2013;119:2223–2229. [DOI] [PubMed] [Google Scholar]
- 39.Cullen J, Brassell SA, Chen Y, et al. Racial/ethnic patterns in prostate cancer outcomes in an active surveillance cohort. Prostate Cancer. 2011;2011:234519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gild P, Von Landenberg N, Cole A, et al. The use of prostate-specific antigen screening in purchased versus direct care settings: data from the TRICARE military database. Eur Urol Suppl. 2017;16:e414–e415. [DOI] [PubMed] [Google Scholar]
- 41.Freedland SJ, Vidal AC, Howard LE, et al. Race and risk of metastases and survival after radical prostatectomy: results from the SEARCH database. Cancer. 2017;123:4199–4206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Spratt DE, Chen YW, Mahal BA, et al. Individual patient data analysis of randomized clinical trials: impact of Black race on castration-resistant prostate cancer outcomes. Eur Urol Focus. 2016;2:532–539. [DOI] [PubMed] [Google Scholar]
- 43.Halabi S, Dutta S, Tangen CM, et al. Overall survival of Black and White men with metastatic castration-resistant prostate cancer treated with docetaxel. J Clin Oncol. 2019;37:403–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.George DJ, Heath EI, Sartor AO, et al. Abi Race: a prospective, multicenter study of Black (B) and White (W) patients (pts) with metastatic castrate resistant prostate cancer (mCRPC) treated with abiraterone acetate and prednisone (AAP). J Clin Oncol. 2018;36(suppl):LBA5009. [Google Scholar]
- 45.Krieger N, Jahn JL, Waterman PD, Chen JT. Breast cancer estrogen receptor status according to biological generation: US Black and White women born 1915–1979. Am J Epidemiol. 2018;187:960–970. [DOI] [PubMed] [Google Scholar]
- 46.Krieger N, Jahn JL, Waterman PD. Jim Crow and estrogen-receptor–negative breast cancer: US-born Black and White non-Hispanic women, 1992–2012. Cancer Causes Control. 2017;28:49–59. [DOI] [PubMed] [Google Scholar]
- 47.Vickers AJ, Bianco FJ Jr, Boorjian S, Scardino PT, Eastham JA. Does a delay between diagnosis and radical prostatectomy increase the risk of disease recurrence? Cancer. 2006;106:576–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gupta N, Bivalacqua TJ, Han M, et al. Evaluating the impact of length of time from diagnosis to surgery in patients with unfavourable intermediate-risk to very-high-risk clinically localised prostate cancer. BJU Int. 2019;124:268–274. [DOI] [PubMed] [Google Scholar]
- 49.Cone EB, Marchese M, Paciotti M, et al. Assessment of time-to-treatment initiation and survival in a cohort of patients with common cancers. JAMA Netw Open. 2020;3:e2030072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sedgwick P Understanding the ecological fallacy. BMJ. 2015;351:h4773. [DOI] [PubMed] [Google Scholar]
- 51.Bill-Axelson A, Holmberg L, Garmo H, et al. Radical prostatectomy or watchful waiting in early prostate cancer. N Engl J Med. 2014;370:932–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


