Abstract
Purpose:
We aimed to develop an accurate prognostic model to identify suitable candidates for definitive radiation therapy (DRT) in addition to palliative chemotherapy (PCT) among patients with de novo metastatic nasopharyngeal carcinoma (mNPC).
Methods and Materials:
Patients with de novo mNPC who received first-line PCT with or without DRT were included. Overall survival for patients who received PCT alone versus PCT plus DRT was estimated using inverse probability of treatment weighting–adjusted survival analyses. We developed and validated a prognostic model to predict survival and stratify risks in de novo mNPC. A model-based trees approach was applied to estimate stratified treatment effects using prognostic scores obtained from the prognostic model and to identify suitable DRT candidates. Dominance analysis was used to determine the relative importance of each predictor of receiving DRT.
Results:
A total of 460 patients were enrolled; 244 received PCT plus DRT and 216 received PCT alone. The 6-month conditional landmark, inverse probability of treatment weighting–adjusted Cox regression analysis showed that PCT plus DRT was associated with a significant survival benefit (hazard ratio: 0.516; 95% confidence interval, 0.403–0.660; P < .001). A prognostic model based on 5 independent prognostic factors, including serum lactate dehydrogenase, number of metastatic sites, presence of liver metastasis, posttreatment Epstein–Barr virus DNA level, and response of metastases to chemotherapy was developed and subsequently validated. Prognostic scores obtained from the prognostic model were used for risk stratification and efficacy estimation. High-risk patients identified using the proposed model would not benefit from additional DRT, whereas low-risk patients experienced significant survival benefits. Socioeconomic factors, including insurance status and education level, played an important role in receipt of DRT.
Conclusions:
Additional DRT after PCT was associated with increased overall survival in patients with de novo mNPC, especially low-risk patients identified with a newly developed prognostic model.
Introduction
Nearly 130,000 new cases of nasopharyngeal carcinoma (NPC) were diagnosed globally in 2018, and approximately 4% to 10% of these cases were de novo metastatic nasopharyngeal carcinomas (mNPCs).1–4 In recent years, there has been increasing interest in definitive radiation therapy (DRT) as a treatment option for patients with de novo mNPC. Growing evidence suggests that a combination of aggressive local treatment and systemic chemotherapy may be associated with increased survival or even cure in a subset of patients.5–16 National Comprehensive Cancer Network guidelines suggest DRT in a subset of patients with a low burden of distant metastases.17
Numerous studies have suggested a role for DRT in treating de novo mNPC; however, its potential benefit remains controversial. This is mainly attributed to the lack of robust prospective data examining the benefits of DRT combined with palliative chemotherapy (PCT) in comparison to PCT alone. Whether the benefits of DRT outweigh radiation therapy–related toxicities has been greatly debated.14–16 Furthermore, previous studies have shown heterogeneous survival rates in patients with de novo mNPC who received DRT after PCT.8–16 The prognosis of de novo mNPC may improve as a result of additional DRT; however, differences in clinical characteristics, access to DRT, and differential effects of DRT may result in inequalities in survival outcomes. Furthermore, because of the high cost of cancer treatments, socioeconomic disparities have been shown to affect treatment selection and survival outcomes for patients with cancer, including NPC.18–21 Therefore, there is an unmet need to develop an individualized and comprehensive treatment for patients with de novo mNPC.4,7,10
In this study, additional DRT after PCT is assumed to be beneficial in the survival of patients with de novo mNPC, but the benefits of DRT are not equally applied to all patients. Therefore, if the assumption is confirmed, controlling the underlying selection bias across treatment groups is crucial and developing a risk stratification tool to identify patients who could benefit more from additional DRT is of great necessity. The present study aimed to develop an easy-to-use prognostic model to identify suitable DRT candidates after comparing the survival outcomes between patients who received PCT alone versus PCT plus DRT. A useful prognostic model could help refine patient selection for DRT and avoid the administration of futile radiation therapy in patients with de novo mNPC.
Methods and Materials
Study population
This study was approved by the Research Ethics Committee of Sun Yat-Sen University Cancer Center (SYSUCC). Consecutive patients who received platinum-based first-line PCT for de novo mNPC at (SYSUCC) between January 2008 and December 2015 were screened. Patients who met the following inclusion criteria were enrolled: (1) untreated NPC with metastatic disease at initial diagnosis; (2) available TNM classifications; (3) no previous or synchronous malignant tumors; (4) receipt of platinum-contained chemotherapy as first-line treatment for a minimum of 2 cycles with or without DRT; (5) measurable metastatic lesions; and (6) available baseline or postchemotherapy radiologic evaluation data. The authenticity of this article has been validated by uploading the key raw data onto the Research Data Deposit public platform (www.researchdata.org.cn) with approval RDD number RDDA2020001582.
Baseline evaluation and treatment
The routine pretreatment evaluation included detailed medical history, physical examinations, hematologic and serum biochemical profiles, plasma Epstein–Barr virus (EBV) DNA detection, nasopharynx pathology, abdominal ultrasonography, chest radiography, whole-body bone scan, and magnetic resonance imaging (MRI) of the nasopharynx and neck. All patients received platinum-based first-line therapy. The PCT regimens used in this study included 5-fluorouracil and platinum; taxane and platinum and gemcitabine and platinum; and taxane, platinum, and 5-fluorouracil. A total of 244 patients underwent DRT after PCT. The radiation therapy techniques used in this study have been reported in previous studies.22,23 The details of treatment-related information are summarized in Table E1.
Follow-up and outcome
During the course of first-line PCT, radiologic evaluation, including computed tomography (CT), MRI, or positron emission tomography CT, was selectively performed to evaluate tumor response after every 2 cycles of chemotherapy. Tumor responses to therapy were evaluated by 2 independent investigators and classified using the revised Response Evaluation Criteria in Solid Tumors (rRECIST, version 1.1).24 The primary endpoint of the current study was overall survival (OS). OS was measured from the initiation of treatment to death from any cause. Patients were followed up at least once every 3 months after treatment completion. Patients lost to follow-up at the time of the last contact were censored.
Sample size and study variables
No prior sample size computation was performed due to limited evidence in building a risk stratification model to identify the best DRT candidates. However, total death events in this study reached 295. A ratio of 10 events per variable was exceeded, indicating sufficient power of estimation.25 Comorbidity was measured as the presence of ≥1 additional medical condition concomitant or concurrent with de novo mNPC, such as cerebrovascular disease, cardiovascular disease, chronic lung disease, chronic liver disease, chronic kidney disease, and diabetes. Plasma EBV DNA titers were measured using a quantitative polymerase chain reaction at baseline, every 2 cycles during first-line PCT, after first-line PCT, and every 3 months after completion of treatment. In the current study, both EBV DNA at baseline and after the first-line PCT in the 2 treatment arms were included in the analyses. The variables with missing data were C-reactive protein and pre- and posttreatment EBV DNA. Patients with missing data were excluded from univariate analyses. However, if a variable with missing data was identified as a significant risk factor in univariate analysis, multiple imputations of the missing value were performed using a multivariable imputation by chained equations algorithm aiming to evaluate the variable in the final Cox multivariable model.26
Statistical analysis
Statistical comparisons between groups were performed using χ2, continuity corrected χ2, or Fisher’s exact test, where appropriate. To account for the effect of selection bias in treatments, the observed differences in baseline characteristics between the PCT alone and PCT plus DRT groups were controlled using the inverse probability of treatment weighting (IPTW) approach.27 The propensity to receive DRT was calculated using a logistic regression model based on demographic features, clinical characteristics, and therapeutic response to first-line PCT, all of which might be related to treatment decision-making or the outcome of interest. The balance of covariates between treatment groups was assessed using the standardized mean difference. Kaplan-Meier curves were estimated, with or without IPTW adjustment, to compare OS between patients who received PCT alone and PCT plus DRT. Given the potential for immortal time bias, OS could artificially favor the PCT plus DRT group because these patients had to survive to exceed the initiation of DRT. Therefore, a 6-month conditional landmark survival analysis was performed. The corresponding hazard ratios (HRs) and 95% confidence intervals (CIs) of DRT were computed using a Cox proportional hazards regression model.
To provide clinicians with a quantitative tool to predict prognosis, a possible prognostic model was constructed and validated. The prognostic model was developed based on a training cohort of 296 patients who received PCT with or without DRT between 2008 and 2013. Univariate and multivariate Cox proportional hazards regression models were applied to the training data set to identify the prognostic factors. Variables with P < .1 in univariate analyses were entered into multivariate Cox regression analyses. Multicollinearity diagnostics in statistical modeling were conducted by evaluating the correlations, variance inflation factors, and eigenvalues. Model selection was based on a step-wise method using the Akaike information criterion as a stopping rule. The variables in the final model with P < .05 were selected to build the prognostic model, which was graphically presented as a nomogram. The discrimination, calibration, and clinical usefulness of the prognostic model were evaluated using Harrell’s C-index, calibration plot, and decision curve analyses, respectively.28,29 Internal validation of the prognostic model was performed using the bootstrap resampling method. An independent validation cohort consisting of 164 consecutive patients recruited between 2014 and 2015 at the same institution was subsequently applied to validate the prognostic model. A prognostic score for each patient was computed based on the nomogram. To identify suitable DRT candidates, model-based trees were used to estimate the personalized treatment effect based on prognostic scores.30 Model-based trees relax the assumption that the treatment effect is the same for all individuals and can estimate stratified treatment effects. Model-based trees can compute treatment effects for different strata of individuals by controlling the maximum depth of the tree. The strata are identified using a data-driven fashion and rely on features of the individuals.
Finally, to determine the factors involved in patients receiving and not receiving DRT, univariate and stepwise multivariate binary logistic regressions were performed to identify independent predictors. Identical modeling strategies, such as the methods used in the Cox regression models, were adapted. Dominance analysis was used to determine the relative importance of predictors in the logistic regression model.31
All statistical analyses in this study were performed using R (version 3.6.3). The threshold for statistical significance was set as a 2-tailed P < .05.
Results
Participant characteristics
A total of 460 patients with de novo mNPC who were treated with DRT (n = 244) or without DRT (n = 216) after first-line PCT between January 2008 and December 2015 met the eligibility criteria (Fig. 1). The patients included 391 men (85.0%) and 69 women (15.0%), with a median age of 46 years (interquartile range, 39–54 years). After a median follow-up time of 64.1 months (95% CI, 60.8–72.2 months), the median OS for the study population was 33.6 months (95% CI, 27.9–40.0 months). A comparison of patient characteristics stratified by treatment modality is presented in Table 1. Patients who received PCT alone presented with more unfavorable socioeconomic and clinical factors. After IPTW adjustment, an adequate balance was achieved for all variables. All standardized differences were <0.1, indicating that the treatment groups were subsequently comparable (Fig. E1).
Fig. 1.
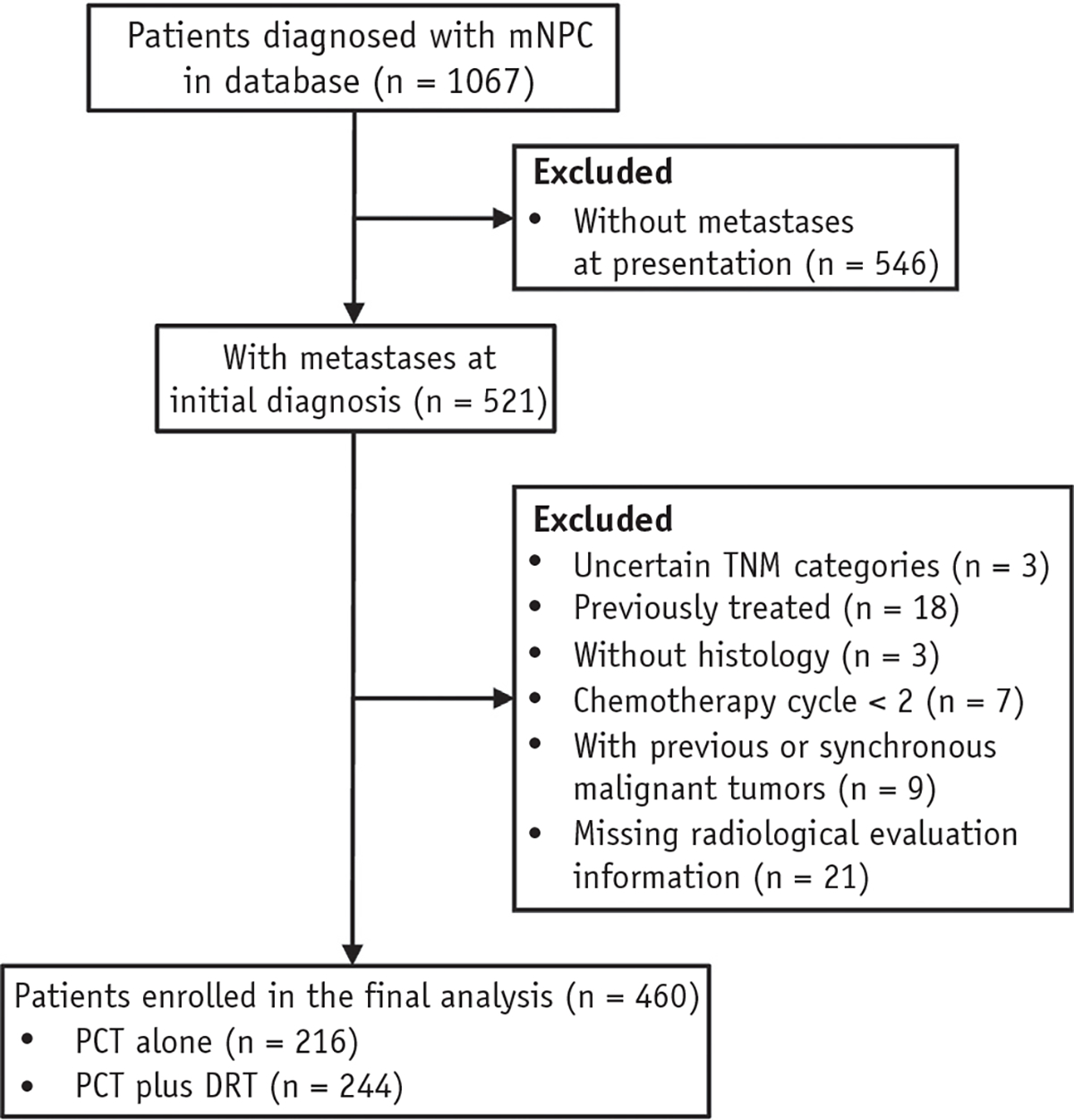
Patient selection diagram. Abbreviations: DRT = definitive radiation therapy; mNPC = metastatic nasopharyngeal carcinoma; PCT = palliative chemotherapy.
Table 1.
Comparison of patient characteristics according to treatment modality
| Variable | Total, n (%) (n = 460) | PCT plus DRT, n (%) (n = 244) | PCT alone, n (%) (n = 216) | P-value |
|---|---|---|---|---|
|
| ||||
| Demographic features | ||||
| Age (y) | .667 | |||
| <45 | 204 (44.3) | 111 (45.5) | 93 (43.1) | |
| ≥45 | 256 (55.7) | 133 (54.5) | 123 (56.9) | |
| Sex | .417 | |||
| Female | 69 (15.0) | 33 (13.5) | 36 (16.7) | |
| Male | 391(85.0) | 211 (86.5) | 180 (83.3) | |
| Residence | .008 | |||
| Rural | 199 (43.3) | 91 (37.3) | 108 (50.0) | |
| Urban | 261 (56.7) | 153 (62.7) | 108 (50.0) | |
| Employment | .151 | |||
| Unemployed | 87 (18.9) | 38 (15.6) | 49 (22.7) | |
| Employed | 329 (71.5) | 182 (74.6) | 147 (68.1) | |
| Retired | 44 (9.6) | 24 (9.8) | 20 (9.26) | |
| Marital status | .103 | |||
| Unmarried | 12 (2.6) | 10 (4.1) | 2 (0.9) | |
| Married | 448 (97.4) | 234 (95.9) | 214 (99.1) | |
| Education | <.001 | |||
| Low | 74 (16.1) | 25 (10.2) | 49 (22.7) | |
| Medium | 248 (53.9) | 128 (52.5) | 120 (55.6) | |
| High | 138 (30.0) | 91 (37.3) | 47 (21.8) | |
| Insurance status | <.001 | |||
| Uninsured | 244 (53.0) | 99 (40.6) | 145 (67.1) | |
| Insured | 216 (47.0) | 145 (59.4) | 71 (32.9) | |
| Clinical characteristics | ||||
| Karnofsky performance score | .002 | |||
| ≥80 | 319 (69.3) | 185 (75.8) | 134 (62.0) | |
| <80 | 141 (30.7) | 59 (24.2) | 82 (38.0) | |
| Comorbidity | .381 | |||
| No | 322 (70.0) | 166 (68.0) | 156 (72.2) | |
| Yes | 138 (30.0) | 78 (32.0) | 60 (27.8) | |
| Smoking | .331 | |||
| No | 276 (60.0) | 152 (62.3) | 124 (57.4) | |
| Yes | 184 (40.0) | 92 (37.7) | 92 (42.6) | |
| Drinking | .554 | |||
| No | 414 (90.0) | 222 (91.0) | 192 (88.9) | |
| Yes | 46 (10.0) | 22 (9.0) | 24 (11.1) | |
| Body mass index (kg/m2) | .057 | |||
| <18.5 | 104 (22.6) | 45 (18.4) | 59 (27.3) | |
| 18.5–24 | 258 (56.1) | 141 (57.8) | 117 (54.2) | |
| >24 | 98 (21.3) | 58 (23.8) | 40 (18.5) | |
| Histology | .347 | |||
| II | 18 (3.9) | 12 (4.9) | 6 (2.8) | |
| III | 442 (96.1) | 232 (95.1) | 210 (97.2) | |
| Tumor category | .283 | |||
| T1 | 26 (5.65) | 14 (5.74) | 12 (5.56) | |
| T2 | 58 (12.6) | 27 (11.1) | 31 (14.4) | |
| T3 | 231 (50.2) | 117 (48.0) | 114 (52.8) | |
| T4 | 145(31.5) | 86 (35.2) | 59 (27.3) | |
| Node category | .261 | |||
| N0 | 13 (2.8) | 6 (2.5) | 7 (3.2) | |
| N1 | 74 (16.1) | 41 (16.8) | 33 (15.3) | |
| N2 | 191(41.5) | 110 (45.1) | 81 (37.5) | |
| N3 | 182 (39.6) | 87 (35.7) | 95 (44.0) | |
| Liver metastases | <.001 | |||
| No | 307 (66.7) | 197 (80.7) | 110 (50.9) | |
| Yes | 153 (33.3) | 47 (19.3) | 106 (49.1) | |
| Bone metastases | .184 | |||
| No | 155 (33.7) | 75 (30.7) | 80 (37.0) | |
| Yes | 305 (66.3) | 169 (69.3) | 136 (63.0) | |
| Lung metastases | .062 | |||
| No | 327 (71.1) | 183 (75.0) | 144 (66.7) | |
| Yes | 133 (28.9) | 61 (25.0) | 72 (33.3) | |
| No. of metastatic sites | <.001 | |||
| Single | 303 (65.9) | 189 (77.5) | 114 (52.8) | |
| Multiple | 157 (34.1) | 55 (22.5) | 102 (57.2) | |
| No. of metastatic lesions | <.001 | |||
| Single | 97 (21.1) | 71 (29.1) | 26 (12.0) | |
| Multiple | 363 (78.9) | 173 (70.9) | 190 (88.0) | |
| Alkaline phosphatase (U/L) | <.001 | |||
| <110 | 341 (74.1) | 202 (82.8) | 139 (64.4) | |
| ≥110 | 119 (25.9) | 42 (17.2) | 77 (35.6) | |
| C-reactive protein (g/mL) | .030 | |||
| <3 | 194 (42.2) | 111 (45.5) | 83 (38.4) | |
| ≥3 | 255 (55.4) | 124 (50.8) | 131 (60.6) | |
| Missing | 11 (2.39) | 9 (3.69) | 2 (0.93) | |
| Lactate dehydrogenase (U/L) | <.001 | |||
| <245 | 295 (64.1) | 178 (73.0) | 117 (54.2) | |
| ≥245 | 165 (35.9) | 66 (27.0) | 99 (45.8) | |
| Pretreatment EBV DNA | .005 | |||
| Undetectable | 67 (14.6) | 47 (19.3) | 20 (9.3) | |
| Detectable | 356 (77.4) | 182 (74.6) | 174 (80.5) | |
| Missing | 37 (8.0) | 15 (6.1) | 22 (10.2) | |
| Response to first-line PCT | ||||
| Posttreatment EBV DNA | <.001 | |||
| Undetectable | 240 (52.2) | 156 (63.9) | 84 (28.9) | |
| Detectable | 143 (31.1) | 61 (25.0) | 82 (38.0) | |
| Missing | 77 (16.7) | 27 (11.1) | 50 (23.1) | |
| Response of primary tumor | .770 | |||
| Complete response | 57 (12.4) | 33 (13.5) | 24 (11.1) | |
| Partial response | 376 (81.7) | 198 (81.1) | 178 (82.4) | |
| Stable disease | 18 (3.9) | 8 (3.3) | 10 (4.6) | |
| Progression disease | 9 (2.0) | 5 (2.0) | 4 (1.9) | |
| Response of metastasis | <.001 | |||
| Complete response | 45 (9.8) | 36 (14.8) | 9 (4.2) | |
| Partial response | 252 (54.8) | 138 (56.6) | 114 (52.8) | |
| Stable disease | 107 (23.3) | 57 (23.4) | 50 (23.1) | |
| Progression disease | 56 (12.2) | 13 (5.3) | 43 (19.9) | |
Abbreviations: DRT = definitive radiation therapy; EBV = Epstein—Barr virus; PCT = palliative chemotherapy.
Efficacy of additional DRT
In the crude Kaplan-Meier curves (Fig. 2A), the median OS in the PCT plus DRT group was 60.9 months (95% CI, 44.5–83.8 months), which was significantly longer than in the PCT group (20.9 months; 95% CI, 17.9–24.6 months; P < .001). IPTW-adjusted Kaplan-Meier curves with a 6-month conditional landmark (Fig. 2B) showed that the median OS was significantly longer for patients who received PCT plus DRT (60.9 months; 95% CI, 46.5–86.3 months; P < .001) than for those who received PCT alone (29.7 months; 95% CI, 27.1–40.2 months). In a 6-month conditional landmark IPTW-adjusted Cox regression analysis, PCT followed by DRT was associated with a significant OS benefit (HR: 0.516; 95% CI, 0.403–0.660; P < .001).
Fig. 2.
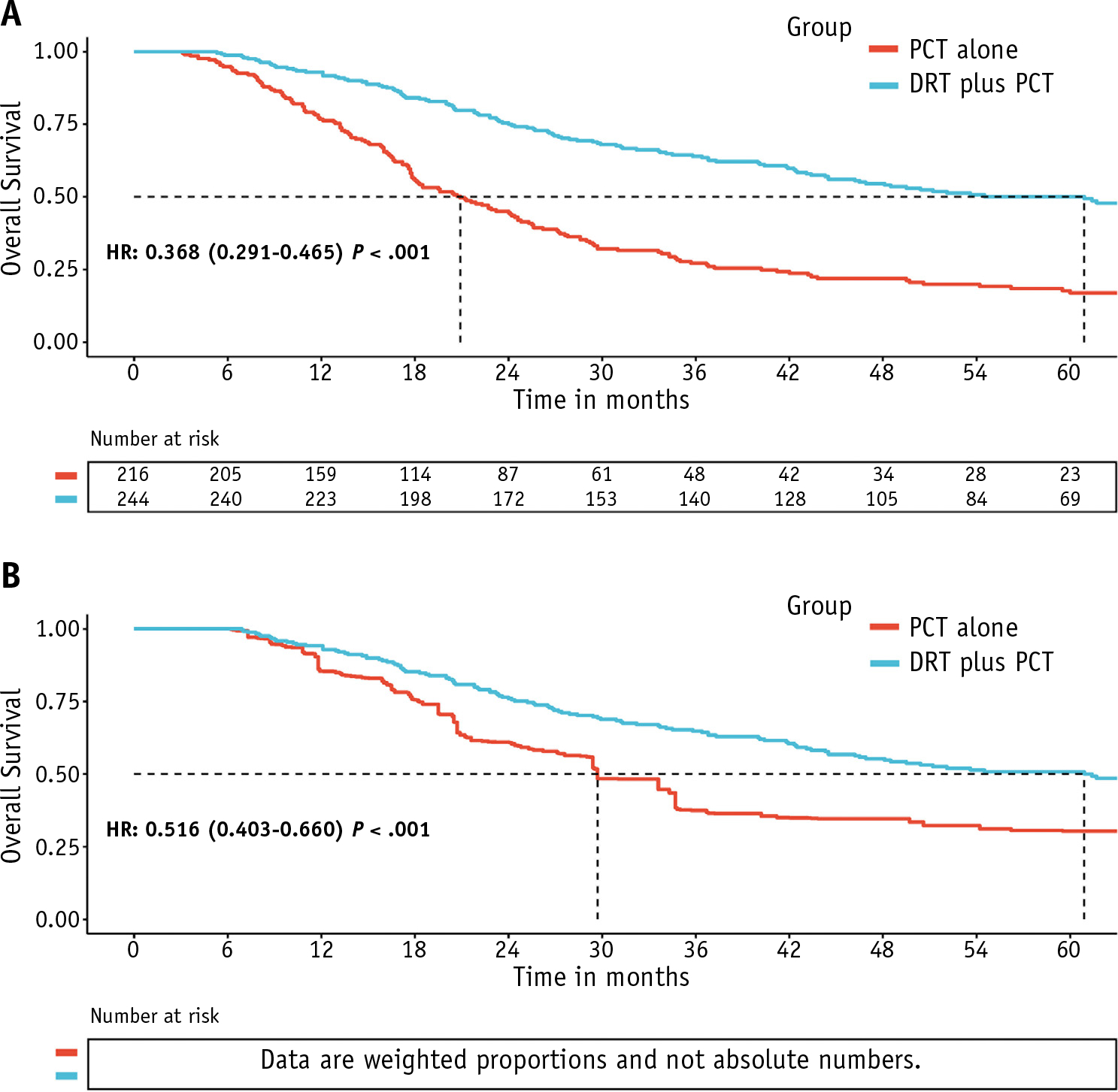
(A) Unadjusted and (B) 6-month conditional landmark inverse probability of treatment weighting-adjusted Kaplan-Meier analyses of overall survival for patients receiving definitive radiation therapy after palliative chemotherapy versus palliative chemotherapy alone for patients with de novo metastatic nasopharyngeal carcinoma.
Development and validation of a possible prognostic model
The comparisons of patient characteristics in training and validation cohorts are shown in Table E2. The results of the univariate and multivariate Cox regression models are summarized in Table E3 and Table 2, respectively. Eleven variables that had P < .1 in univariate analyses were entered into a stepwise multivariate Cox regression analysis. Multicollinearity diagnostic tests, including pairwise correlations, variance inflation factors plot, and eigenvalues plot, indicated that severe multicollinearity issues would not exist in the multivariate model (Figs. E2 and E3). Using the retrospective training cohort with 296 patients treated between January 2008 and December 2013, 5 independent prognostic factors, including serum lactate dehydrogenase level, number of metastatic sites, presence of liver metastasis, posttreatment EBV DNA level, and response of metastases to chemotherapy were identified. Based on these prognostic factors, a prognostic model for the prediction of 1-, 3-, and 5-year OS was developed and graphically presented as a nomogram (Fig. 3A).
Table 2.
Significant prognostic factors for overall survival in the training set
| Characteristics | HR (95% CI) | P-value |
|---|---|---|
|
| ||
| Lactate dehydrogenase (U/L) | .015 | |
| <245 | Reference | |
| ≥245 | 1.491 (1.081–2.057) | |
| No. of metastatic sites | .050 | |
| Single | Reference | |
| Multiple | 1.429 (1.000–2.047) | |
| Liver metastasis | <.001 | |
| No | Reference | |
| Yes | 2.319 (1.254–4.291) | |
| Posttreatment EBV DNA | <.001 | |
| Undetectable | Reference | |
| Detectable | 1.923 (1.406–2.630) | |
| Response of metastasis | ||
| Complete response | Reference | |
| Partial response | 1.668 (0.839–3.316) | .144 |
| Stable disease | 2.813 (1.373–5.762) | .005 |
| Progression disease | 6.607 (3.025–14.429) | <.001 |
Abbreviations: CI = confidence interval; EBV = Epstein—Barr virus; HR = hazard ratio.
Variables with a P < .1 in the univariate analyses were entered into a stepwise multivariate Cox regression analysis. Model selection was made based on a stepwise method using the Akaike information criterion as a stop rule. The Akaike information criterion of the final model was 1838.2.
Fig. 3.
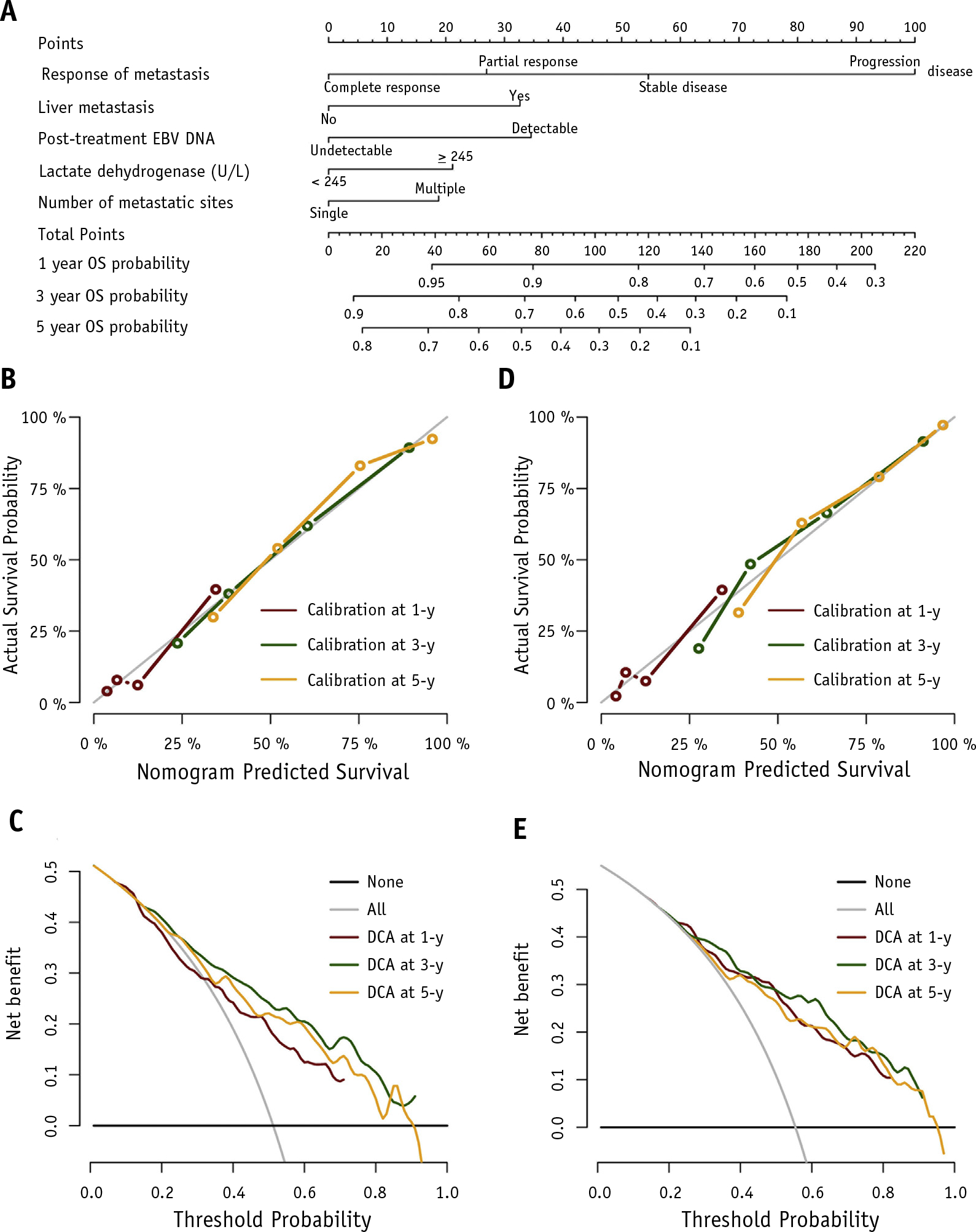
(A) Nomogram-based prognostic model for the prediction of 1-, 3-, and 5-year overall survival. To use the nomogram, draw an upward vertical line from a variable value to the points bar to determine points that correspond to that variable value. Then sum up the points from each variable value to get the prognostic score. Based on the sum, draw a downward vertical line from the total points line to calculate overall survival probability. The calibration plots at 1, 3, and 5 years in the (B) training and (D) validation cohorts. The decision curve analyses at 1, 3, and 5 years in the (C) training and (E) validation cohorts.
The developed prognostic model showed good discrimination for OS prediction in the training cohort, with a C-index of 0.738 (95% CI, 0.702–0.775). Internal validation of the prognostic model using 1000 bootstrap resampling analyses provided a bias-corrected C-index of 0.730, indicating the robustness of the proposed model. The calibration plots at 1-, 3-, and 5-year survival illustrated favorable calibration between the predicted and observed OS in the training cohort (Fig. 3B). The decision curve analyses for 1-, 3-, and 5-year survival prediction of the prognostic model in the training cohort is shown in Figure 3C. The plot showed that the proposed model conferred more net benefits compared with both the treat-all-patients scheme and the treat-none scheme.
The prognostic model was further validated using an independent validation cohort of 164 patients treated between January 2014 and December 2015. The C-index in the validation cohort was 0.746 (95% CI, 0.696–0.795). The calibration curves in the validation cohort showed good agreement between the predicted and observed OS (Fig. 3D). The decision curve analyses in the validation cohort yielded similar clinical usefulness (Fig. 3E).
Identification of best-fit DRT candidates
The stratified model-based trees (Fig. E4) were used to classify patients into different treatment effect subgroups based on the prognostic score derived from the nomogram (Fig. 3A) by controlling the maximum depth of the tree. In this study, a maximum depth of 3 was found to be ideal. Patients with a prognostic score of <102 exhibited more survival benefit when comparing the 2 treatment modalities. Based on this optimal cutoff value, patients were classified as low- or high-risk patients. The 6-month conditional landmark Kaplan-Meier curves revealed that low-risk patients receiving DRT after PCT in the training cohort had a significant OS benefit (median OS: 87.6 vs 31.0 months; P < .001; Fig. 4A). However, this benefit disappeared in the high-risk patients (median OS: 17.2 vs 13.3 months; P = .520; Fig. 4A). Similar results were observed when further validation of the stratified treatment effects was performed in an independent validation cohort recruited in the following 2 years (Fig. 4B).
Fig. 4.
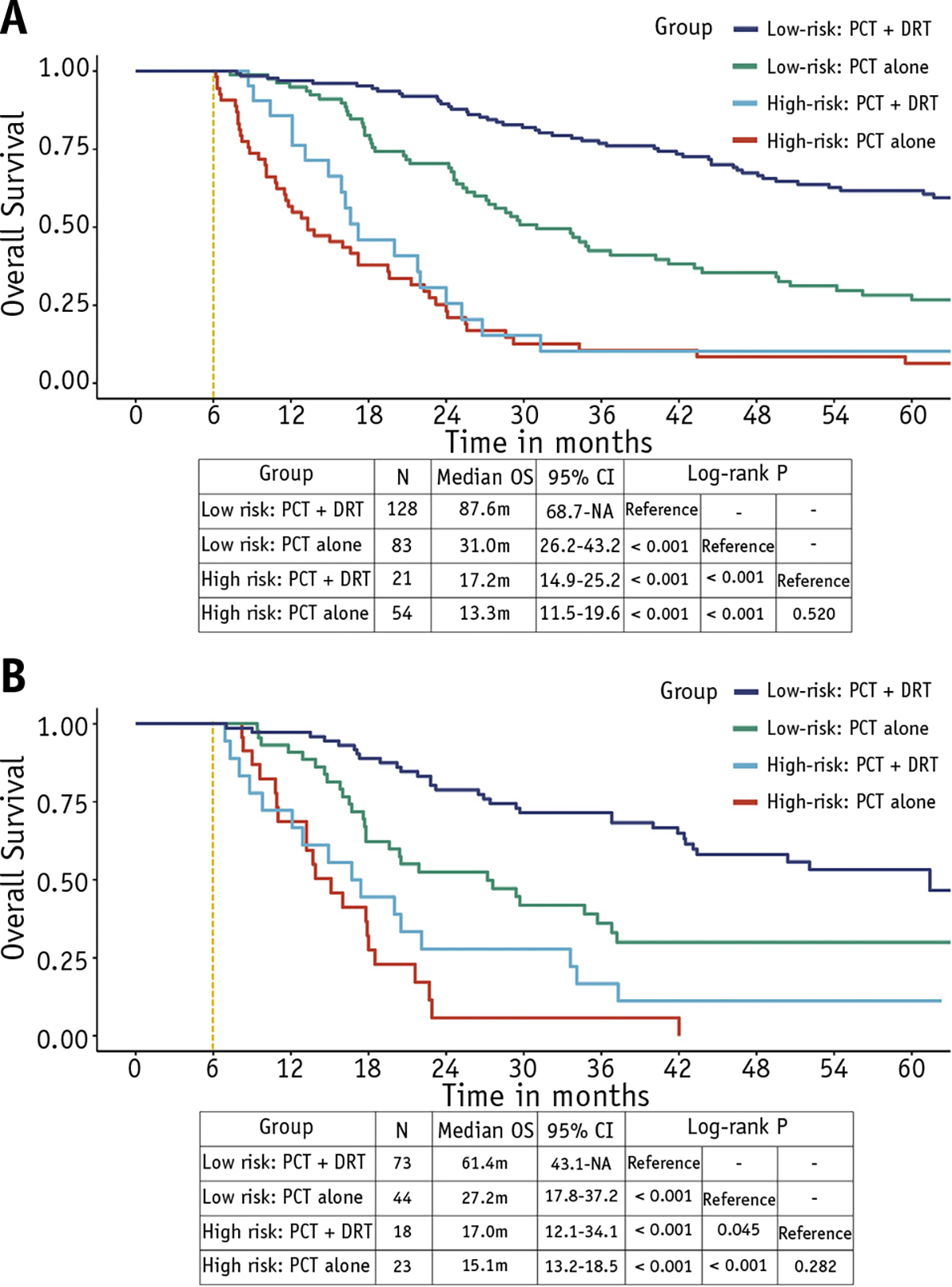
Six-month conditional landmark Kaplan-Meier analyses of overall survival for low-risk (score ≤102) and high-risk (score >102) patients receiving definitive radiation therapy after palliative chemotherapy or palliative chemotherapy alone in the (A) training and (B) validation cohorts.
Determinants of receiving DRT after PCT
The results of the multivariate logistic regression analysis determining the administration of DRT after PCT are summarized in Table E4. In the entire cohort, insurance status, education, Karnofsky performance score, liver metastasis, number of metastatic sites, number of metastatic lesions, pretreatment EBV DNA, and response of metastasis were found to be significant independent predictors for receiving DRT. In the low-risk cohort, insurance status, education level, T category, liver metastasis, and response of metastasis were found to be significant independent predictors for receiving DRT. However, in the high-risk cohort, only alkaline phosphatase was a significant independent predictor.
Dominance analysis was used to determine the relative importance of each independent predictor in the logistic regression model in the low-risk cohort. The results revealed that individual insurance status was the most important predictor of receiving DRT (0.059), followed by liver metastasis (0.047), response of metastasis (0.034), education level (0.023), T category (0.011), and pretreatment EBV DNA (0.010) (Fig. E5).
Discussion
NPC is relatively sensitive to chemoradiation therapy, and platinum-containing regimens have been the mainstay of treatment for mNPC, with a response rate of approximately 70% to 80%.4,10,32 PCT alone in de novo mNPC has a median OS of 10 to 15 months. However, for patients receiving combined therapy, such as DRT and local treatment of metastatic lesions, the median survival is significantly improved.5–16 Rusthoven et al reported that the addition of DRT after PCT in patients with de novo mNPC was associated with improved survival in both univariate analysis (3-year OS: 37% vs 20%; P < .001) and multivariate analysis (HR: 0.61; 95% CI, 0.51–0.74; P < .001).14 Retrospective studies conducted in endemic regions have reported similar findings.11–13 In the current study, the results were consistent with those reported in previous studies. Patients in the PCT plus DRT group were associated with a significant OS benefit (HR: 0.516; 95% CI, 0.403–0.660; P < .001) after controlling for the observed difference between treatment groups using the IPTW method.
The National Comprehensive Cancer Network guidelines include DRT as a treatment option for patients with de novo mNPC; however, there is currently no consensus regarding who should receive DRT after first-line PCT. Therefore, the identification of patients with de novo mNPC suitable to receive DRT requires urgent research.4 Currently, no prospective phase 3 clinical trials have tried to identify ideal DRT candidates. Accurate risk stratification and the estimation of treatment effect can be used to identify suitable patient candidates for DRT. Furthermore, the use of prognostic models can prevent use of potentially futile local aggressive therapy. Prognostic models based on baseline clinical features, therapeutic response, and biomarkers have been used to stratify patients with NPC into different prognostic subgroups.33–37 This strategy can also be used to tailor therapy for patients and to refine future clinical trial designs.
In this study, baseline clinical characteristics, including the number of metastatic sites, liver metastasis, and lactate dehydrogenase level, were significantly associated with OS. These results were consistent with the results from previous reports examining patients with asynchronous mNPC.4–6,9–14,34,35 Previous studies have also suggested that clearance of plasma EBV DNA after PCT and tumor response to therapy have predictive value for survival outcomes in patients with metastatic or recurrent NPC.38–40 These findings were confirmed in the current study. Plasma EBV DNA level after PCT and the response of metastases to therapy were not only associated with survival outcomes but were also shown to be among the most important prognostic factors. We developed a prognostic model based on these prognostic factors. The proposed model exhibited adequate accuracy with individual prediction and could identify suitable DRT candidates. Risk stratification based on the prognostic scores showed that the median OS for low-risk patients receiving DRT after PCT was significantly longer compared with low-risk patients receiving PCT alone. However, this benefit was not observed in high-risk patients. These findings suggest that patients with de novo mNPC should be treated based on their risk because high-risk patients are likely not to benefit from DRT.
Intervention-generated inequalities are usually described as unintended variations in an outcome that results from the organization and delivery of health interventions.41 Although tumor characteristics represent the dominant factor in treatment decision-making, socioeconomic status has also been shown to affect treatment selections in various cancers. Patients with low socioeconomic status are more likely to receive treatment that deviates from standard care and guideline therapy.18–21 The findings from the current study were consistent with those reported in previous studies: Both insurance status and education level were critical socioeconomic factors affecting treatment selection.42–45 In our series, we observed that uninsured or low-educated patients were less likely to receive DRT, especially low-risk patients. The inaccessibility of DRT among these patients might ultimately lead to their worse survival outcomes.
This study has some limitations. First, as an observational study in real-world settings, potential selection bias is unavoidable. However, this was minimized by recruiting all consecutive eligible patients and using a large cohort of patients with de novo NPC with a broad spectrum of baseline features adjusted. Second, because patients were enrolled from a single institution, no external validation of our proposed model was performed. External validation would strengthen our findings and generalize our proposed model into clinical practice. Third, information regarding complications during and after DRT was not analyzed, warranting further investigation. Fourth, NPC from the endemic region is mainly EBV-related and might present different tumor features from those in patients from low-risk areas.4 Given these limitations, the application of our proposed model as a stratification tool in other study settings needs further validation. Finally, we admit that model-based trees only generalize the treatment effect for a subgroup of patients but not individuals; thus, they can only be considered a step in the direction toward personalized medicine.46 Future studies should make efforts to provide more methods to estimate the individual treatment effect, which allows the determination of whether a specific treatment for a new patient is appropriate.
Conclusions
The application of DRT after PCT was found to be associated with prolonged survival in patients with de novo mNPC after controlling for potential selection bias. However, the survival benefit of DRT could be heterogeneous. A novel prognostic model we established in this study was able to stratify patients with de novo mNPC into low- or high-risk groups and identify suitable DRT candidates. High-risk patients did not benefit from DRT, but low-risk patients experienced significant survival benefits. A prospective clinical trial is expected to confirm these results.
Supplementary Material
Acknowledgments
This study is supported by the National Natural Science Foundation of China (No. 81672680 and 81802712), Guangdong Medical Research Foundation (No. A2017492), and Young Teacher Training Project of Sun Yat-Sen University (No. 19ykpy188).
Footnotes
Disclosures: The authors have no conflicts of interest to declare.
Data sharing statement: The authenticity of this article has been validated by uploading the key raw data onto the Research Data Deposit public platform (www.researchdata.org.cn) with approval RDD number RDDA2020001582. All data will be shared upon request to the corresponding author.
Supplementary material for this article can be found at https://doi.org/10.1016/j.ijrobp.2020.08.045.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- 2.Lee AWM, Ng WT, Chan LK, et al. The strength/weakness of the AJCC/UICC staging system (7th edition) for nasopharyngeal cancer and suggestions for future improvement. Oral Oncol 2012;48:1007–1013. [DOI] [PubMed] [Google Scholar]
- 3.Pan JJ, Ng WT, Zong JF, et al. Proposal for the 8th edition of the AJCC/UICC staging system for nasopharyngeal cancer in the era of intensity-modulated radiotherapy. Cancer 2016;122:546–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen YP, Chan ATC, Le QT, Blanchard P, Sun Y, Ma J. Nasopharyngeal carcinoma. Lancet 2019;394:64–80. [DOI] [PubMed] [Google Scholar]
- 5.Fandi A, Bachouchi M, Azli N, et al. Long-term disease-free survivors in metastatic undifferentiated carcinoma of nasopharyngeal type. J Clin Oncol 2000;18:1324–1330. [DOI] [PubMed] [Google Scholar]
- 6.Hui EP, Leung SF, Au JS, et al. Lung metastasis alone in nasopharyngeal carcinoma: A relatively favorable prognostic group. A study by the Hong Kong Nasopharyngeal Carcinoma Study Group. Cancer 2004;101:300–306. [DOI] [PubMed] [Google Scholar]
- 7.Cao X, Han Y, He L, Xiang J, Wen Z. Risk subset of the survival for nasopharyngeal carcinoma patients with bone metastases: who will benefit from combined treatment? Oral Oncol 2011;47:747–752. [DOI] [PubMed] [Google Scholar]
- 8.Lin S, Tham IWK, Pan J, Han L, Chen Q, Lu JJ. Combined high-dose radiation therapy and systemic chemotherapy improves survival in patients with newly diagnosed metastatic nasopharyngeal cancer. Am J Clin Oncol 2012;35:474–479. [DOI] [PubMed] [Google Scholar]
- 9.Chen MY, Jiang R, Guo L, et al. Locoregional radiotherapy in patients with distant metastases of nasopharyngeal carcinoma at diagnosis. Chin J Cancer 2013;32:604–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chan OS, Ngan RK. Individualized treatment in stage IVC nasopharyngeal carcinoma. Oral Oncol 2014;50:791–797. [DOI] [PubMed] [Google Scholar]
- 11.Zeng L, Tian YM, Huang Y, et al. Retrospective analysis of 234 nasopharyngeal carcinoma patients with distant metastasis at initial diagnosis: Therapeutic approaches and prognostic factors. PloS One 2014;9:e108070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu SX, He XH, Dong M, et al. Systemic chemotherapy followed by locoregional definitive intensity-modulated radiation therapy yields prolonged survival in nasopharyngeal carcinoma patients with distant metastasis at initial diagnosis. Med Oncol 2015;32:224. [DOI] [PubMed] [Google Scholar]
- 13.Sun XS, Liu LT, Liu SL, et al. Identifying optimal candidates for local treatment of the primary tumor among patients with de novo metastatic nasopharyngeal carcinoma: A retrospective cohort study based on Epstein–Barr virus DNA level and tumor response to palliative chemotherapy. BMC Cancer 2019;19:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rusthoven CG, Lanning RM, Jones BL, et al. Metastatic nasopharyngeal carcinoma: Patterns of care and survival for patients receiving chemotherapy with and without local radiotherapy. Radiother Oncol 2017;124:139–146. [DOI] [PubMed] [Google Scholar]
- 15.Yeh SA, Tang Y, Lui CC, Huang EY. Treatment outcomes of patients with AJCC stage IVC nasopharyngeal carcinoma: Benefits of primary radiotherapy. Jpn J Clin Oncol 2006;36:132–136. [DOI] [PubMed] [Google Scholar]
- 16.Setton J, Wolden S, Caria N, Lee N. Definitive treatment of metastatic nasopharyngeal carcinoma: Report of 5 cases with review of literature. Head Neck 2012;34:753–757. [DOI] [PubMed] [Google Scholar]
- 17.National Comprehensive Cancer Network. Head and neck cancers, version 2.2020. Available at: https://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdf. Accessed September 12, 2020.
- 18.Forrest LF, Adams J, Wareham H, et al. Socioeconomic inequalities in lung cancer treatment: Systematic review and meta-analysis. PLoS Med 2013;10:e1001376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Long B, Chang J, Ziogas A, et al. Impact of race, socioeconomic status, and the health care system on the treatment of advanced-stage ovarian cancer in California. Am J Obstet Gynecol 2015;212. 468.e1–9. [DOI] [PubMed] [Google Scholar]
- 20.Kuijer A, Verloop J, Visser O, et al. The influence of socioeconomic status and ethnicity on adjuvant systemic treatment guideline adherence for early-stage breast cancer in the Netherlands. Ann Oncol 2017; 28:1970–1978. [DOI] [PubMed] [Google Scholar]
- 21.Xu C, Chen YP, Liu X, et al. Socioeconomic factors and survival in patients with non-metastatic head and neck squamous cell carcinoma. Cancer Sci 2017;108:1253–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu Z, Luo W, Zhou GC, et al. Impact of changing gross tumor volume delineation of intensity-modulated radiotherapy on the dose distribution and clinical outcome after induction for the primary locoregionally advanced nasopharyngeal carcinoma. Ai Zheng 2009;28:1132–1137. [DOI] [PubMed] [Google Scholar]
- 23.Sun X, Liu S, Luo M, et al. The association between the development of radiation therapy, image technology, and chemotherapy, and the survival of patients with nasopharyngeal carcinoma: A cohort study from 1990 to 2012. Int J Radiat Oncol Biol Phys 2019;105:581–590. [DOI] [PubMed] [Google Scholar]
- 24.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–247. [DOI] [PubMed] [Google Scholar]
- 25.Peduzzi P, Concato J, Feinstein AR, Holford TR. Importance of events per independent variable in proportional hazards regression analysis. II. Accuracy and precision of regression estimates. J Clin Epidemiol 1995;48:1503–1510. [DOI] [PubMed] [Google Scholar]
- 26.Li P, Stuart EA, Allison DB. Multiple imputation: A flexible tool for handling missing data. JAMA 2015;314:1966–1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res 2011;46:399–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Crowson CS, Atkinson EJ, Therneau TM. Assessing calibration of prognostic risk scores. Stat Methods Med Res 2016;25:1692–1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vickers AJ, Elkin EB. Decision curve analysis: A novel method for evaluating prediction models. Med Decis Making 2006;26:565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seibold H, Zeileis A, Hothorn T. model4you: An R package for personalised treatment effect estimation. J Open Res Softw 2019;7. [Google Scholar]
- 31.Azen R, Traxel N. Using dominance analysis to determine predictor importance in logistic regression. J Educ Behav Stat 2009;34:319–347. [Google Scholar]
- 32.Lee AWM, Ng WT, Chan YH, Sze H, Chan C, Lam TH. The battle against nasopharyngeal cancer. Radiother Oncol 2012;104:272–278. [DOI] [PubMed] [Google Scholar]
- 33.Wang HY, Sun BY, Zhu ZH, et al. Eight-signature classifier for prediction of nasopharyngeal [corrected] carcinoma survival. J Clin Oncol 2011;29:4516–4525. [DOI] [PubMed] [Google Scholar]
- 34.Liu N, Chen NY, Cui RX, et al. Prognostic value of a microRNA signature in nasopharyngeal carcinoma: A microRNA expression analysis. Lancet Oncol 2012;13:633–641. [DOI] [PubMed] [Google Scholar]
- 35.Jin Y, Ye X, Shao L, et al. Serum lactic dehydrogenase strongly predicts survival in metastatic nasopharyngeal carcinoma treated with palliative chemotherapy. Eur J Cancer 2013;49:1619–1626. [DOI] [PubMed] [Google Scholar]
- 36.Jin Y, Cai XY, Cai YC, et al. To build a prognostic score model containing indispensible tumour markers for metastatic nasopharyngeal carcinoma in an epidemic area. Eur J Cancer 2012;48:882–888. [DOI] [PubMed] [Google Scholar]
- 37.Toh CK, Heng D, Ong YK, Leong SS, Wee J, Tan EH. Validation of a new prognostic index score for disseminated nasopharyngeal carcinoma. Br J Cancer 2005;92:1382–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang WY, Twu CW, Chen HH, et al. Plasma EBV DNA clearance rate as a novel prognostic marker for metastatic/recurrent nasopharyngeal carcinoma. Clin Cancer Res 2010;16:1016–1024. [DOI] [PubMed] [Google Scholar]
- 39.Hsu CL, Chang KP, Lin CY, et al. Plasma Epstein-Barr virus DNA concentration and clearance rate as novel prognostic factors for metastatic nasopharyngeal carcinoma. Head Neck 2012;34:1064–1070. [DOI] [PubMed] [Google Scholar]
- 40.An X, Wang FH, Ding PR, et al. Plasma Epstein-Barr virus DNA level strongly predicts survival in metastatic/recurrent nasopharyngeal carcinoma treated with palliative chemotherapy. Cancer 2011;117:3750–3757. [DOI] [PubMed] [Google Scholar]
- 41.White M, Adams J, Heywood P. How and why do interventions that increase health overall widen inequalities within populations? In: Babones S, editor. Health, inequality and society. Bristol, UK: Policy Press; 2009. p. 65–83. [Google Scholar]
- 42.Harlan LC, Greene AL, Clegg Limin X, et al. Insurance status and the use of guideline therapy in the treatment of selected cancers. J Clin Oncol 2005;23:9079–9088. [DOI] [PubMed] [Google Scholar]
- 43.Grant SR, Walker GV, Koshy M, et al. Impact of insurance status on radiation treatment modality selection among potential candidates for prostate, breast, or gynecologic brachytherapy. Int J Radiat Oncol Biol Phys 2015;93:968–975. [DOI] [PubMed] [Google Scholar]
- 44.Fossati N, Nguyen DP, Trinh Q, et al. The impact of insurance status on tumor characteristics and treatment selection in contemporary patients with prostate cancer. J Natl Compr Canc Netw 2015;13:1351–1358. [DOI] [PubMed] [Google Scholar]
- 45.Agarwal P, Jones EA, Devaiah AK. Education and insurance status: Impact on treatment and survival of sinonasal cancer patients. Laryngoscope 2020;130:649–658. [DOI] [PubMed] [Google Scholar]
- 46.Seibold H, Zeileis A, Hothorn T. Individual treatment effect prediction for amyotrophic lateral sclerosis patients. Stat Methods Med Res 2018; 27:3104–3125. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


