Abstract
Biomaterial based strategies have been widely explored to preserve and restore the juvenile phenotype of cells of the nucleus pulposus (NP) in degenerated intervertebral discs (IVD). With aging and maturation, NP cells lose their ability to produce necessary extracellular matrix and proteoglycans, accelerating disc degeneration. Previous studies have shown that integrin or syndecan binding peptide motifs from laminin can induce NP cells from degenerative human discs to re-express juvenile NP-specific cell phenotype and biosynthetic activity. Here, we engineered alginate hydrogels to present integrin- and syndecan-binding peptides alone or in combination (cyclic RGD and AG73, respectively) to introduce bioactive features into the alginate gels. We demonstrated human NP cells cultured upon and within alginate hydrogels presented with cRGD and AG73 peptides exhibited higher cell viability, biosynthetic activity, and NP-specific protein expression over alginate alone. Moreover, the combination of the two peptide motifs elicited markers of the NP-specific cell phenotype, including N-Cadherin, despite differences in cell morphology and multicellular cluster formation between 2D and 3D cultures. These results represent a promising step toward understanding how distinct adhesive peptides can be combined to guide NP cell fate. In the future, these insights may be useful to rationally design hydrogels for NP cell-transplantation based therapies for IVD degeneration.
Keywords: intervertebral disc, disc degeneration, microenvironment, laminin-mimetic peptides, ligand presentation, cell therapy
1. Introduction
Age related degeneration of the intervertebral disc (IVD) is a major contributor to disability and health related economic burden of musculoskeletal disease in industrialized societies. Annual costs associated with IVD degeneration exceed $100 billion in the United States alone [1-4]. Disc degeneration is a multifactorial process characterized by alteration of biomechanical and biochemical properties in disc tissue that lead to structural failure associated with lower back pain [5]. The IVD is composed of an inner nucleus pulposus (NP) layer, a central anulus fibrosus layer, and an outer cartilage endplate. Each of these structures is important to the overall mechanical function of the disc [6]. Changes to NP cell phenotype and density are the earliest observed changes in IVD degeneration [7-9]. These early changes in NP cells result in changes to the NP extracellular matrix, leading to a loss of hydration and concordant stiffening of the tissue [10]. In turn, this creates a local milieu that causes the NP cells to become fibrotic, senescent and/or apoptotic, further accelerating IVD degeneration [11-13].
Given the importance of NP cells in disc maintenance, NP cell replacement therapies have been widely explored for treatment of IVD degeneration [14]. NP cells transplanted without a carrier show minimal capacity for engraftment and survival, making NP cell delivery within biomaterials an attractive option [15-17]. Synthetic, biocompatible materials with mechanical properties and hydration matched to that of the native NP tissue (e.g. hydrogels) are particularly well-suited to support NP cell survival and matrix biosynthesis [18-23]. Studies incorporating native extracellular matrix constituents in a hydrogel scaffold, such as collagen, laminin, or glycosaminoglycans have demonstrated favorable phenotypically appropriate cell signaling and matrix synthesis in NP cells [24-28]. In particular, alginate hydrogels are especially promising for NP tissue repair because these materials have similar elasticity and viscoelasticity to healthy NP tissue [29-33]. Encapsulation of NP cells and chondrocytes in alginate hydrogels is the most widely-used method for in vitro cell culture [30]. It has been documented the encapsulation of NP cells in alginate support rounded NP cell morphologies and production of proteoglycans and collagen both in vitro and in vivo [34-40].
In healthy NP tissue, resident NP cells interact with elastin, fibronectin, collagens, and laminins through integrin and other adhesive receptors, including syndecans [41, 42]. These interactions regulate cell fate, phenotypes, morphology, and metabolic activity [43-47]. For example, in vitro, adhesion to laminin can partially reverse the fibroblast-like phenotype of NP cells isolated from degenerated human IVD tissues, allowing these cells to exhibit phenotypes (e.g. elevated proteoglycan synthesis) commonly seen in juvenile NP cells [42, 43]. NP cell interactions with laminin are mediated by integrins (α3, α5, α6, β1 and β4) and syndecans (1 and 4) [43-47]. The binding of AG73 (RKRLQVQLSIRT) peptide to syndecan domains has shown to promote cell adhesion in NP cells and other cell types [47, 48]. Additionally, the binding of the Arg-Gly-Asp (RGD) peptide motif to α5β1, has been identified to be a particularly important modulator of NP cell mechanotransduction [49, 50].
The versatile nature of alginate is an ideal biomaterial for NP cell culture given its beneficial mechanics and bio-inductive activity. However, despite the broad use of alginate as a culture vehicle for NP cells, and the importance of receptor-mediated adhesion in control over NP cell fate, there exist no studies assessing how modifying alginate gels with ligands for these critical adhesion receptors influences NP cell fate. In the context of repairing other musculoskeletal tissues, enhanced cell adhesion and differentiation of preosteoblasts and mesenchymal stem cells (MSCs) were observed when these cells were cultured in alginate gels grafted with cell-adhesive peptides, as compared to culture in unmodified alginate [51, 52]. There is some evidence that NP cells are not able to assemble a functional extracellular matrix in ‘naked’ alginate, making the initial modification of this material with adhesive ligands particularly desirable [20, 53]. Prior work with alternative hydrogel materials, including poly(ethylene glycol) (PEG) based gels, has shown that a density of 100 μM laminin mimetic peptides was capable of promoting elevated glycosaminoglycan synthesis and other markers of healthy, juvenile NP cells in cells isolated from human degenerative discs [46, 47]. In these studies, which were mostly conducted in 2D culture, engineered biomaterial promoted higher number of adherent cells and the typical rounded morphology of NP cells. Additionally, higher cell attachment is linked to their ability to form multicellular clusters. However, biomaterial-assisted NP cell transplantation therapy is likely to involve cells encapsulated inside gels (3D) rather than atop these materials (2D). Considering the strong likelihood that both cell shape and multicellular clustering are altered in 2D versus 3D culture due to differential cell confinement between these culture formats [54-56], this makes it essential to study the relationship between biomaterial composition and NP cell fate in 3D.
In this study, we hypothesized that integrin and syndecan binding peptides (cyclic RGD and AG73, respectively) presented from alginate hydrogels would provide a combinatorial benefit in degenerative human nucleus pulposus cells to re-express a more biosynthetically active, juvenile NP phenotype as compared to unmodified alginate and hydrogel presenting with equimolar of either peptide. Our results demonstrate the value of incorporating cell adhesive domains in combination with syndecan binding domains for NP cell culture and suggest the potential to design bioactive features into the alginate hydrogel system that are capable of supporting NP specific cell phenotypes in vitro and possibly in vivo.
2. Materials and methods
2.1. Preparation of maleimide-modified alginate and BCN-amine-modified alginate
Preparation of unmodified alginate
High-molecular-weight, high G-block-containing alginate (Manugel, Dupont, Wilmington, De, USA) was dissolved at 1% (w/v) solution in Dulbecco’s PBS (dPBS). Alginate polymer solution was purified by dialyzing against deionized water. The unmodified alginate was sterilized by filtration through a 0.22 μm membrane under aseptic conditions. Sterile polymer solution was lyophilized and stored at −80 °C for further use.
Peptide-modified Alginate
AG73-alginate modification
Alginate was modified with N-terminal cysteine presenting AG73 peptides using maleimide-thiol click chemistry previously optimized for alginate by Madl et al. [57]. Briefly, for AG73 grafting via maleimide-thiol chemistry: high-molecular-weight, high G-block-containing alginate (Manugel) was dissolved at 1% w/v in 0.5 M 2-(N-morpholino)ethane sulfonic acid (MES) buffer (pH 5) by stirring for 2 h. A 2-fold molar excess of N-β-maleimidoproprionic acid hydrazide (BMPH; Sigma-Alrich, St.Louis, MO, USA) and a 8-fold molar excess of four- and 1-Ethyl-3-[3-dimethylaminopropyl]carbodiimide hydrochloride (EDC, Thermo Fisher Scientific, Waltham, MA, USA) were added to the stirring alginate solution. After 4 h at room temperature, the maleimide-modified alginate was precipitated with five-fold excess of methanol twice and vacuum dried overnight. The final maleimide-modified alginate polymer was then re-dissolved in ultrapure water and lyophilized and stored at −80 °C for further use. To graft the cysteine-terminated AG73 peptide (CGGRKRLQVQLSIRT; GenScript, Piscataway, NJ), the maleimide-modified alginate was dissolved at 1% w/v in ultrapure water for 2 h. The sulfhydryl-containing peptide was dissolved in ultrapure water and added dropwise to the maleimide-modified alginate solution at an equivalent to twice the molar ratio of BMPH conjugated to the alginate polymers [e.g., when the measured molar output for maleimide was 5 maleimide-polymer, a molar input of 10 AG73-polymer was used]. The reaction was incubated at room temperature for 24 h with constant stirring (Fig. 1A). Following dialysis (3400 Da cutoff) for 72 h, polymers were sterile filtered (0.45μm) and freeze-dried polymers were maintained under aseptic conditions and stored at −80 °C for further use.
Figure 1. Schematic of bioconjugation reaction for grafting cysteine terminated peptide.
A) Maleimide-thiol Michael-type addition reaction was employed for peptide conjugation. In the first step, maleimide groups are grafted onto the backbone of alginate polymer chain. In the second step, cysteine terminated (AG73) peptide reacts with the grafted maleimide group through thiol reaction to obtain maleimide–AG73–alginate. B) Maleimide–AG73–alginate mixed with BCN–Az-cRGD–alginate at equimolar peptide concentration was ionically crosslinked using divalent calcium ions to create combination peptide (cRGD/AG73) alginate hydrogel.
cRGD-alginate modification
Alginate was modified with cRGD peptides coupled through a heterobifunctional spacer using Strain Promoted Azide Alkyne Cycloaddition (SPAAC) click chemistry described in our prior study (Fig. S1, [58]). Bicyclo[6.1.0]non-4-yn-9-ylmethyloxycarbonyl]-1,8-diamino-3,6-dioxaoctane (BCN-amine; Sigma-Aldrich, St. Louis, MO, USA) was coupled to alginate following our previously established method (Fig. S1, [58]). Briefly, alginate was dissolved at 1% w/v in 0.1 M MES buffer (pH 6) by stirring for 2 h. A 36-fold molar excess of EDC (Thermo Scientific) and 8-fold molar excess of N-hydroxysuccinimide (NHS, Fisher Scientific) were first dissolved in 0.5 ml of 0.1M MES buffer and added to the alginate mix. Next, a twofold molar excess of BCN-amine was added. The reaction mix was incubated at room temperature for 24 h. The BCN-amine-modified alginate was then precipitated with 5-fold excess of methanol twice and vacuum dried overnight. The final BCN conjugated alginate was re-dissolved in ultrapure water and freeze dried. Next, BCN-alginates were dissolved at 1%w/v in dPBS for 2 h. Azide cRGD (cyclo[Arg-Gly-Asp-D-Phe-Lys-(azide)]; Peptides International; Louisville, KE, USA) was then added dropwise to BCN-alginate solution at an equivalent to twice the molar ratio of BCN amine conjugated to the alginate polymer and allowed to react at room temperature with constant stirring for 24 h (Fig. S1). The final products of Az-cRGD-alginate and AG73-alginate were maintained under aseptic conditions and stored at −80 °C for further use.
Unmodified alginate, AG73-presenting alginate, cRGD-presenting alginate, and combination of cRGD-alginate and AG73-alginate at equimolar peptide density were ionically crosslinked using divalent calcium ions and used for subsequent studies (Fig. 1B).
2.2. Quantification of maleimide-thiol reaction efficiency
Peptide coupling efficiency to maleimide-modified alginate was predicted using a fluorogenic probe, 5-fluorescein-thiol (FAM-thiol; BioActs, Incheon, Korea), as a surrogate for thiol-containing peptides. The reaction conditions were same as used for peptide coupling as described above. The final product was obtained post dialysis (3400 DA cutoff dialysis membrane for 72 h) and freeze-dried. Different molar inputs of FAM-thiol coupled to alginate were determined by preparing serial dilutions of the FAM-thiol-alginate and comparing the fluorescence to standards of the respective free dyes (ex/em of 488/520 nm). Moles of FAM-thiol (as a surrogate for AG73) coupled to alginate was expressed as the measured molar ratio of FAM-thiol to alginate. Coupling efficiency was calculated as a ratio of the measured FAM-thiol molar concentration to the input molar concentration. Quantification was performed for n=3 independently prepared batches of maleimide-alginate polymer.
Maleimide-alginate coupling was also verified using 1H NMR (Agilent DD2 NMR, 500 MHz, Santa Clara, CA) and analyzed using Bruker NMR software (4.0.7). Alginate and maleimide-alginate were dissolved at 1% w/v in deuterium oxide with 0.75% 2,2,3,3-d4-3-trimethylsilylpropionate added as an internal standard.
2.3. Mechanical testing
Unmodified alginate, BCN-modified, and maleimide-alginate were synthesized as described above. Freeze-dried alginate polymers were dissolved at 1% w/v in 100 mM HEPES buffer (pH 7.2). Alginate solutions were quickly mixed with a sterile 1.34 M calcium sulfate slurry at 8% (v/v) of total solution; final calcium concentration in gels: 100 mM. The solutions were quickly poured between two glass plates separated by 1 mm spacers and incubated at room temperature for an hour to complete crosslinking of gels. The hydrogels were then punched into discs 5 mm in diameter. Compressive and shear properties of the hydrogel samples were measured at one hour after crosslinking and at three days and seven days of pre-incubation in media in an unconfined compression configuration via use of a displacement-controlled axial compression protocol (ElectroForce 3200 TA Instruments, New Castle, DE). In brief, samples were subjected to unconfined compressive loading at 0.5 mm/min and axial load recorded to calculate a compressive stress from the cross-sectional area (5 mm diameter). Linear regression of compressive stress within the first 10-20% of strain was performed for n=4 samples for each condition to determine a compressive modulus, E.
2.4. Primary human nucleus pulposus cell culture
Human NP cells (n≥3, male and female) were isolated from the NP region of to-be-discarded surgical waste IVD tissues; patients were de-identified and only sex, race and age of the patient were recorded (non-human subjects research with approval by Washington University IRB) [45]. NP cells were isolated from this surgical waste as described in our previous studies [45-47, 59]. Briefly, NP tissue fractions were removed from surgical samples and digested for 4 h at 37°C and 5% CO2 in medium containing 0.4% type II collagenase (Worthington Biochemical, Lakewood, NK) and 2% pronase (Roche, Basel, Switzerland). Cells were passed through a 70 μm cell strainer (Thermo Fisher Scientific, Waltham, MA). The isolated cells were then plated in tissue culture flasks until needed for experimentation; cells were maintained up to passage 4 in Ham’s F12 medium (Thermo Fisher Scientific) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin (P/S).
2.5. 2D human nucleus pulposus cell morphological analysis
To study an effect for the combination of cRGD and AG73-conjugated alginate on cell adhesion, sterile polymers were dissolved at 1% w/v in sterile 100 mM HEPES buffer (pH 7.2) overnight at 4 °C with constant stirring. Alginate solutions were quickly mixed with sterile calcium sulfate slurry to a 100 mM final calcium concentration in the gel. The solutions were quickly poured between two glass plates separated by 1 mm spacers and incubated at room temperature for an hour to complete crosslinking of gels. The hydrogels were then punched using a 5 mm biopsy punch and transferred to 16 well chamber slides (Nunc Lab-Tek Chamber Slide Systems™) containing Ham’s F12 medium supplemented with 1% P/S. The gels were incubated at 37 °C in 5% CO2 and the incubation media was refreshed 6 times at regular intervals to remove excess calcium. Primary degenerative adult human NP cells were seeded on the gel at a density of 10,000 cells/well and allowed to attach for 24 h at 37 °C and 5% CO2. Each cell-seeded hydrogel represents an individual sample for analysis.
Following the attachment period, cells were fixed with 4% paraformaldehyde (PFA) supplemented with 2 mM CaCl2 and stained with Alexa Fluor™-633 Phalloidin (Invitrogen™, Carlsbad, CA) and 4′,6-diamidino-2- phenylindole (DAPI, 2 μg/mL, Sigma-Aldrich, St. Louis, MO) as a nuclear counterstain. For each sample (hydrogel condition), a minimum of 3 regions of interest (ROI) were imaged across the hydrogel using confocal microscopy (TCS-SPE with DM6 RGBV confocal microscope; Leica DFC7000T camera; using Leica LAS X core software; Leica Microsystems, Wetzlar, Germany). To minimize the potential variability introduced with random ROI selection in imaging, each single data point represents an average of three ROI measurements per hydrogel. Each data point represents one unique hydrogel. A total of three different donors were used to supply the input cells for the unique gels. Nuclei were counted per ROI and total single cell attachment was calculated by extrapolating the cell number determined from the ROIs to the surface area of the hydrogel. Percent cell attachment was calculated as 100*(calculated cell attachment number/seeded cell number); i.e. 100*(the extrapolated value/10,000 cells). Spread area and cell perimeter were obtained using ImageJ software (NIH, Bethesda, MD) by outlining the cell body from the phalloidin channel. Circularity was calculated as , where A = cell area and P = perimeter. Cell clustering was obtained by counting the number of nuclei in a cluster per ROI. These morphometric measures were stratified into measures obtained for single cells, small clusters (<5 cells), and large clusters (≥5 cells) to reveal differences due to cluster size.
2.6. 3D human nucleus pulposus cell morphological analysis
To study an effect of the combination of cRGD and AG73-conjugated alginate on cell morphology in 3D culture, sterile, freeze-dried polymers were dissolved at 2% w/v in 100 mM HEPES buffer (pH 7.2) overnight at 4 °C with constant stirring, respectively. Primary degenerative adult human NP cells at a density of 2.5 x 106 cells/mL were then mixed with equal volume of alginate solution. This resulted in a final concentration of 1% w/v of alginate in hydrogels, which were crosslinked with calcium sulfate slurry to a final calcium concentration of 100 mM. The solutions were quickly poured between two glass plates separated by 1 mm spacers and incubated at room temperature for an hour to complete crosslinking of gels. The hydrogels were then punched using 5 mm biopsy puncher and transferred to 16 well chamber slides (Nunc Lab-Tek Chamber Slide Systems™) containing F12 + 10% FBS + 1% P/S for 7 days or 21 days at 37 °C and 5% CO2.
Following the culture period, cells were fixed with 4% w/v paraformaldehyde (PFA; in 100 mM HEPES supplemented with 2 mM CaCl2) and stained with Alexa Fluor™-633 Phalloidin (Invitrogen™, Carlsbad, CA) and 4′,6-diamidino-2- phenylindole (DAPI, 2 μg/mL, Sigma-Aldrich, St. Louis, MO) as a nuclear counterstain. For each sample, z-stacks of a minimum of 3 ROIs were imaged across the gel using confocal microscopy (Leica). To minimize the potential variability introduced with random ROI selection in imaging, each single data point represents an average of three ROI measurements per hydrogel. Each data point reported represents one unique hydrogel. A total of three different donors were used to supply the input cells for the unique gels. Spread area, clustering, and circularity were obtained using ImageJ software (NIH, Bethesda, MD) and morphometric data were stratified into single cells, small clusters (<5 cells), and large clusters (≥5 cells) as described for 2D culture experiments above.
2.7. Analysis of 3D cell viability and biosynthetic activity
The encapsulation of primary degenerative adult human NP cells was carried out as described above. Cell viability was surveyed using a LIVE/DEAD cell imaging kit following the manufacturer’s protocol (Invitrogen, Carlsbad, CA). To assay biosynthetic activity, a Functional NonCanonical Amino-acid Tagging (FUNCAT) approach was employed [60]. Briefly, 3D cell-containing hydrogels were made as described above and cultured in L-methionine containing DMEM. Cell-gel constructs were cultured for 7- or 21- days at 37°C and 5% CO2. Four days before each timepoint, medium was replaced with L-methionine free DMEM (Gibco, ThermoFisher, Waltham, MA) supplemented with L-azidohomoalanine (AHA, Click Chemistry Tools, Scottsdale, AZ). After culture timepoints, the media was replaced with L-methionine free DMEM supplemented with 30 μM of the AHA-binding secondary DBCO-488 (Click Chemistry Tools), and incubated for 45 minutes at 37°C and 5% CO2. The constructs were then washed with 100mM HEPES buffer 3 times and fixed for 15 minutes with 4% PFA in 100 mM HEPES supplemented with 2 mM CaCl2. Following, cells were stained with Alexa Fluor™-633 Phalloidin (Invitrogen™) and DAPI (2 μg/mL, Sigma-Aldrich) as a nuclear counterstain. Z-stacks from a minimum of 3 ROIs were imaged across the gel using confocal microscopy (Leica). To minimize the potential variability introduced with random ROI selection in imaging, each single data point represents an average of three ROI measurements per hydrogel. Each data point represents one unique hydrogel. A total of three different donors were used to supply the input cells for the unique gels. Intracellular biosynthetic activity was measured as mean fluorescence intensity by overlaying the phalloidin channel with the AHA channel to capture the integrative density of individual cells (ImageJ software, NIH).
2.8. Immunohistochemistry
2D and 3D hydrogels were created as described above. To analyze phenotypic changes, cells on the hydrogel were incubated for 7 days, and cells encapsulated in the hydrogel were incubated for 7 days and 21 days at 37°C and 5% CO2. Markers were selected following recommendations from the Spine Research Interest Group as published in the 2015 consensus paper [61]. Following incubation, cells were fixed with 4% PFA in 100 mM HEPES supplemented with 2 mM CaCl2, washed with 100 mM HEPES with 2 mM CaCl2, permeabilized by 0.2% v/v triton, and incubated in blocking buffer (5% goat serum with 3.75% BSA) to reduce non-specific binding. The cells were then immunolabeled overnight with healthy juvenile-NP cell markers and cell adhesion specific markers (Supplementary Table 1). Concentration matched isotype controls of the species were also included for each target. After primary staining, all samples were stained with appropriate Alexa-Fluor labeled secondary antibodies and Alexa-Fluor phalloidin (1:200, Invitrogen, Thermo Fisher Scientific, Waltham, MA) for 2 hours for 2D gels or 4 hours for 3D gels. Then, the samples were counter stained with DAPI (2 μg/mL, Sigma-Aldrich, St.Louis, MO) for 15 minutes. For each sample, a minimum of 3 ROIs were imaged across the gel using confocal microscopy (Leica). To minimize the potential variability introduced with random ROI selection in imaging, each single data point represents an average of three ROI measurements per hydrogel. Each data point represents one unique hydrogel. A total of three different donors were used to supply the input cells for the unique gels. Mean fluorescence intensity of protein expression was determined by outlining the cell body using the phalloidin channel and measuring the integrative density using ImageJ software (NIH). Paxillin presence was quantified following protocols outlined by Horzum et al. to identify focal adhesion area [62].
2.9. Gene expression of human NP cells when cultured in cRGD-and AG73-functionalized alginate in 2D and 3D
NP cells were seeded at density of 100,000 cells/well on unmodified alginate and the combined cRGD-and AG73-coupled alginate (~10 mm diameter) for 2D studies following procedures described above. NP cells were also encapsulated for 3D studies as described above. The cells were incubated at 37 °C and 5% CO2 for 4 days. Following the incubation period, the alginate hydrogels were digested with alginate lyase (0.25 mg/ml in 100 mM HEPES buffer; Sigma-Aldrich) for 50 min at 37 °C to obtain a polymer free cell pellet. The cells were washed in 100 mM HEPES buffer and lysed using RLT buffer (Qiagen, Hilden, Germany) and 1% mercaptoethanol. mRNA isolation was done using a RNeasy Kit with DNase I digestion (Qiagen). Briefly, samples were homogenized with a QIAshredderTM column, passed through a RNeasy spin column for RNA binding, washed and DNA was digested via DNase I. Purified RNA was eluted post-two washes using RNase free water. mRNA quality and quantity were verified by measuring ratio of absorbance at 260/280 nm using NanoDrop One Spectrophotometer (Thermo Fisher Scientific). mRNA was reverse transcribed to cDNA using the iScript cDNA Synthesis Kit (BioRad, Hercules, CA) as per manufacturer instructions. Real time quantitative PCR (rt-PCR) was performed using a Step One Plus Thermal cycler (Thermo Fisher Scientific) for human NP cell and matrix specific primers and 18s and glyceraldehyde 3-phosphate dehydrogenase (Thermo Fisher Scientific) internal controls housekeeping gene (Supplementary Table 2). The samples were set in duplicate using standard conditions of 12.5 μl 2X Universal Master Mix, 1.25 μl TaqMan primer probes, 9.25 μl ddH2O, and 2 μl 10 ng/μl cDNA. Comparative gene expression analysis was done using by the 2-ΔΔCt approach where first fold-change (Δ) was calculated with respect to housekeeping genes and the second fold-change (ΔΔ) was calculated with reference to lysates obtained from NP cells cultured in unmodified alginate.
2.10. Statistical analysis
Replicate samples were used in each assay to ensure that cells from no less than 3 individual patients were obtained per measure while ensuring sufficient power to conduct comparison tests. Each biological replicate is defined as a single hydrogel. Data are presented as mean ± standard deviation of no less than 6 biological replicates for each gel condition. GraphPad Prism 9 (Graph Pad Software, Inc., San Diego, CA, USA) was used to generate plots and performed statistical comparisons. All datasets were tested for normality using Shapiro-Wilk test before performing statistical comparisons. For analyses of hydrogel moduli, one-way ANOVA with Tukey’s multiple comparison’s test was used to compare the moduli between time points within an individual polymer condition and to compare moduli between the modified alginate hydrogels. For cell adhesion, morphological, phenotypic protein expression, and biosynthetic activity analysis, one-way ANOVA (factor (1) = hydrogel, levels (4) = unmodified alginate, cRGD-alginate, AG73-alginate, and combination of cRGD-and AG73-alginate) were performed with Tukey’s multiple comparisons tests with repeated measures (RM). Two-tailed t-tests were performed on gene expression from unmodified alginate and combination of cRGD-and AG73-alginate based on ΔCT values.
3. Results
3.1. Validating peptide coupling density through click reactions.
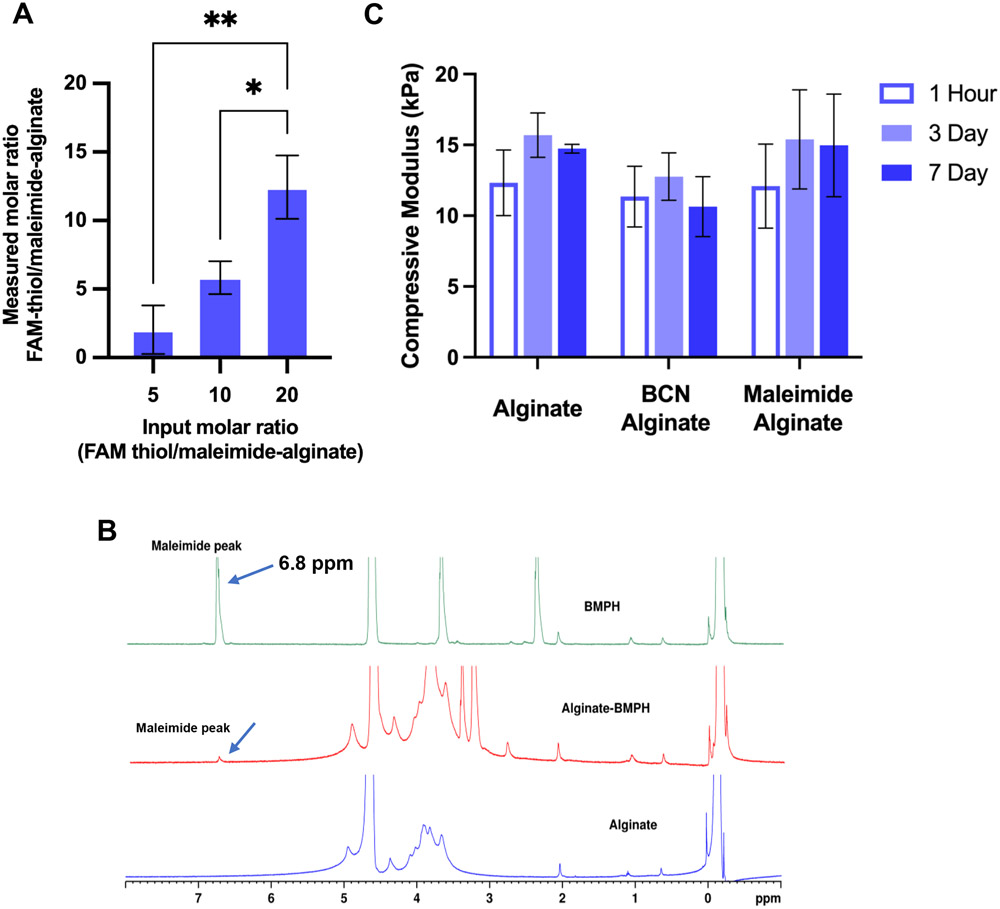
Coupling of FAM-thiol to maleimide-alginate was used as a surrogate conjugation for measuring the degree of coupling of cysteine terminated AG73 (Table 1). Consistent with previous results reported by Madl et al. [57], the efficiency of maleimide-alginate grafting using BMPH was approximately 40%, while the efficiency of click reaction between various input molar ratios of FAM-thiol and maleimides conjugated to alginate was approximately 55% (Fig. 2A). The coupling of maleimide onto alginate was also verified via 1H NMR. Maleimide-coupled alginate showed characteristic peaks of maleimide which appeared at 6.8 ppm (Fig. 2B). Coupling efficiency as determined using the NMR data was approximately 50% for maleimide–alginate conjugation (input of 10 BMPH–polymer). The coupling efficiency as determined by NMR was consistent with that observed via coupling of FAM-thiol to maleimide-alginate. Based on the model reaction between FAM-thiol and maleimide-alginate, and our use of 2-fold molar excess of BMPH for maleimide-modification and 2-fold molar excess of AG73 during peptide click reaction, the final molar density of AG73 coupled to 1% alginate (wt/v) was estimated to be 125 μM depending on the molar input of AG73 (Table 2). The efficiency of BCN-alginate grafting and subsequent FAM-azide to BCN click reaction did not differ from previous results (data not shown; [58]).
Table 1:
Experimental conditions and input molar ratios for studies on conjugation of maleimide to alginate. DS = Degree of substitution.
| Starting alginate (μM)a |
Input molar ratio (maleimide-alginate) |
Coupling efficiency (FAM-thiol Assay) |
Final DS of maleimide (mole of maleimide- alginate) |
|---|---|---|---|
| 40 | 10 | 58.27 ± 12.0 | 6 |
Calculations assume a molecular weight of 250 kDa for Manugel based on the reported molecular weight of alginate formulations with similar viscosity.
Figure 2. Quantification of peptide conjugation efficiency using maleimide–thiol reaction and mechanical property of modified alginate.
A) Conjugation of FAM-thiol to maleimide-alginate (1% w/v) at three different input molar ratios (5, 10, and 20) via Maleimide-thiol click reaction between cysteine and BMPH. FAM-thiol was used as a surrogate for AG73. Fluorescence of the final product was measured to determine the molar ratio of FAM-thiol to maleimide-alginate at the different molar inputs. ~55% coupling efficiency was observed. Error bars are ±SD. *p < 0.05, **p < 0.01, using a one-way ANOVA with Tukey's multiple comparison's test. B) 1H NMR plots of substituted BMPH–alginate (red) with input BMPH molar ratio 10 (final DS: 5) shows the presence of characteristic peaks of maleimide groups at 6.8 ppm (solid arrow) when compared to unmodified alginate (blue) and pure BMPH (green). C) Modification to the backbone of alginate chain and pre-incubation times with media do not impact modulus. Error bars are ±SD. One-way ANOVA with Tukey's multiple comparison's test was used to compare the modulus within individual polymer condition pre-incubated at three different time points and to compare modulus between modified alginate hydrogels.
Table 2:
Experimental conditions, input molar ratios, and estimated molar peptide density for maleimide-thiol coupling of FAM-thiol or AG73 to alginate
| Starting alginate (μM) |
Input molar ratio (FAM-thiol or AG73- alginate) |
Output molar ratio (FAM-thiol or AG73- alginate) |
Estimated final molar peptide density in 1% alginate (μM) |
|---|---|---|---|
| 40 | 10 | 6 | 125 |
Unmodified alginate and modified alginate hydrogels were ionically crosslinked using divalent calcium ions. Mechanical testing of the hydrogels at one hour, three days, and seven days pre-incubation in media yielded measures of compressive moduli of ~12 kPa for unmodified, and for both BCN- and maleimide-modified-alginate hydrogels, with no significant changes over time (Fig. 2C). In contrast, hydrogels crosslinked using 50 mM CaSO4 resulted in stiffness of approximately 20 kPa at 3 days of pre-incubation compared to around 50 kPa after one hour crosslinking (data not shown), which was similar to our previous finding [63]. These results suggest that 100 mM CaSO4 is more stable than 50 mM CaSO4 overtime and modification of the alginate backbone and pre-incubation with media did not lead to significant changes in hydrogel stiffness. The value for the modulus of these gels is similar to the rigidity of moderately degenerated NP tissue [64-66], and synthetic matrices based on bisacrylamide or PEG gels from our previous studies [46, 47].
3.2. Integrin- and syndecan-binding peptides lead to morphological differences and promote NP cell adhesion.
Despite the overall peptide density being constant, we observed distinct NP cell morphologies after 7 days depending on whether cRGD alone, AG73 alone, or both peptides were presented from hydrogels (Fig. 3A). cRGD- elicited robust cell spreading and more fibroblast-like, distended cell morphology compared to unmodified alginate (p<10−4, Fig. 3B). AG73 alone and mixture of cRGD and AG73 peptides led to more spreading and similar rounded morphologies compared to unmodified alginate, respectively (p=0.016 and p=0.033, Fig. 3B). In all samples, AG73 alone, along with unmodified alginate, led to a more rounded morphology compared to cRGD alone (p=0.014 and p=0.0052, Fig. 3C). Hydrogel conditions containing peptide regardless of combination promoted similar levels of single cell attachment (Fig. 3D). Interestingly, comparing to previous studies that used linear RGD peptides, we observed robust NP cell adhesion and spreading on substrates presenting the cyclic RGD, which more faithfully mimics the secondary structure of the integrin-binding RGD motif [46, 67, 68]. Finally, we observed that NP cell clustering was enhanced on peptide-conjugated alginates, regardless of the specific combination of peptides (Fig. 3G).
Figure 3. Integrin-binding (cRGD) and syndecan-binding (AG73) peptide lead to morphological differences and improved adhesion in 2D.
A) Representative immunostaining images of primary human NP cells per condition, red is actin, blue is nuclei, scale bar is 50μm. B-C) Cell spreading and circularity behaviors of single cells cultured upon peptide functionalized alginate hydrogel. D) Percent single cell attachment cultured upon hydrogel conditions. E,F) Representative immunostaining images of paxillin and phospho-Myosin Light Chain (pMLC), scale bar is 50 μm. G) Cell clustering behavior upon peptide functionalized alginate hydrogels. H) Quantification of paxillin rich focal adhesions per cell per condition. I) Quantification of pMLC protein expression. n ≥ 6 unique hydrogels from 3 human donors. Error bars are ±SD. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, using a one-way ANOVA with Tukey's multiple comparison's test (B, C, D, H, I).
Matrices presenting cRGD alone and a mixture of cRGD and AG73 significantly enhanced formation of paxillin-rich focal adhesions (FAs) (17.6± 5.7 μm2 per cell and 14.7 ± 9.6 μm2 per cell, respectively) as compared to FA formation per cell elicited by AG73 (5.7 ± 5.3 μm2 per cell; p=0.0043 and p=0.040, respectively; Fig. 3E and H). Cells cultured on hydrogels without adhesive peptides significantly lose the ability to form FA complexes compared to combination of cRGD- and AG73- alginate (p=0.0018, Fig. 3H). In agreement with previous studies, hydrogels presenting cRGD alone also elicited robust assembly of paxillin-rich focal adhesions, similar to the same extent as gels presenting both cRGD and AG73 (p=0.79, [69]).
The relative expression of phospho-Myosin Light Chain 2 (pMLC), a signal downstream of cell adhesion that correlates with cellular contractility, was enhanced both for cRGD alone and the combination of cRGD with AG73. Hydrogels presenting cRGD peptide had higher pMLC expression compared to unmodified alginate (p=0.015) and AG73 alone hydrogel (p=0.0052), while significant enhancement was observed in mixture of cRGD and AG73 compared to AG73 alone (p=0.040; Fig. 3 F and I).
3.3. Combination of integrin- and syndecan-binding peptides regulate protein expression and gene expression in 2D
Quantification of immunohistochemistry revealed significantly higher expression of brain acid soluble protein 1 (BASP-1) and N-Cadherin in NP cells cultured upon a mixture of cRGD- and AG73-conjugated alginate (Fig. 4 A and B) as compared to unmodified (p<10−4), cRGD-alginate (p<10−4), and AG73-alginate (p<10−4), respectively. To further determine the effects of the AG73/cRGD peptide combination on NP cell fate, we measured a panel of genes associated with the juvenile NP cell phenotype. AG73/cRGD mixture led to approximately 6-fold ACAN expression and slight increase in COL2A, CDH2, and GLUT1 compared to unmodified alginate but not significant between the two hydrogels when comparing ∆ CT levels calculated in respect to housekeeping genes. Interestingly, this AG73/cRGD combination also led to relative decrease in COL1A1 and a significant decrease in CTGF (p=0.034), two genes associated with a fibroblast-like, degenerative NP cell phenotype (Fig 4C). Altogether, these protein and gene expression data suggest that presentation of syndecan and integrin ligands allow NP cells from degenerated IVD to re-express a more juvenile-like phenotype than other culture conditions.
Figure 4. Combination of cRGD and AG73-peptide conjugated alginate hydrogel promote a more juvenile-like phenotype in 2D.
A) Representative images of proteins associated with juvenile NP cell phenotype. Scale bar is 50 μm. B) Quantification of protein expression associated with juvenile NP cell phenotype C) qPCR for a panel of genes associated with juvenile NP phenotypes normalized to unmodified alginate. n ≥ 6 unique hydrogels from 3 human donors. Error bars are ±SD. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, using a one-way ANOVA with Tukey's multiple comparison's test (B). Two-tailed t-tests performed on gene expression from unmodified alginate and combination of cRGD-and AG73-alginate based on ΔCT values, *p < 0.05 (C).
3.4. Combinatorial role of cRGD and AG73 in regulating NP cell phenotype in 3D.
Peptide conjugated hydrogels used in these studies promoted NP cell viability and demonstrated negligible cell death at day 7 and 21 days of 3D culture, while unmodified alginate resulted in some cell death (Fig. S2.A and S2.B). Similar to morphologies observed in 2D, at day 7, human NP cells encapsulated in cRGD-alginate exhibited a significant increase in cell area, and assumed a more elongated, less circular cell shape compared to cells within gels presenting AG73 (p<10−4, p=0.0002) or the cRGD/AG73 combination (p<10−4, p=0.0066; Fig. 5A-C). In contrast to the 2D culture condition where the majority of cells (>50%) were part of large clusters (Fig. 3G), cells in 3D culture were much more likely to be isolated from other cells (Fig. 5D). However, amongst the 3D conditions, cells in gels presenting cRGD-only and AG73/cRGD mixture formed a small number of large clusters (≥5 cells; Fig. 5D). Gels presenting the mixture of cRGD- and AG73- elicited higher expression of N-Cadherin compared to unmodified alginate (p<10−4), cRGD alone (p<10−4), or AG73 alone (p<10−4, Fig. 5A and E). N-cadherin protein expression was the most prominent in the alginate gels with the combined peptide condition. Additionally, BASP-1 expression was similar in hydrogels containing peptides regardless of combination, though it was significantly enhanced in hydrogels presented with cRGD compared to unmodified alginate (p=0.042, Fig. 5A and F).
Figure 5. Combination of cRGD and AG73 at equimolar molar concentration promotes juvenile-like NP phenotype at 7 day culture in 3D.
A) Representative images of human NP cells encapsulated in 3D hydrogels. scale bar is 50 μm. B-D) Cell spreading, circularity and clustering behaviors of cells encapsulated in peptide functionalized alginate hydrogel. E-F) Quantification of protein expression associated with juvenile-like phenotype. G) qPCR for a panel of genes associated with juvenile NP phenotypes normalized to unmodified alginate. n ≥ 6 unique hydrogels from 3 human donors. Error bars are ±SD. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, using a one-way ANOVA with Tukey's multiple comparison's test (B-F). Two-tailed t-tests performed on gene expression from unmodified alginate and combination of cRGD-and AG73-alginate based on ΔCT values, *p < 0.05 (G).
In 3D, expression of specific-NP cell markers was upregulated in gels presenting the cRGD/AG73 mixture as compared to the expression of these genes in cells cultured within unmodified gels (Fig. 5G). The increase in transcript levels for juvenile-like markers was significant for CDH2 (p=0.019) in the cRGD/AG73 functionalized hydrogels compared to the unmodified hydrogel. These data, like data obtained in 2D culture, suggest the ability of the combination of AG73 and cRGD used here to support a shift in phenotype of degenerative NP cells toward a more juvenile-like NP cell state.
3.5. Longitudinal effect of cRGD and AG73 mixture in regulating NP phenotype in 3D.
We observed a non-significant trend toward more cell spreading in cRGD-AG73-mixture alginate compared to AG73-alone and unmodified alginate, while cell spreading was higher in cRGD-alginate compared to AG73-alginate (p=0.0048) and unmodified alginate (p=0.0055; Fig. 6A and B). Cells in cRGD-alginates exhibited a significant decrease in circularity compared to cRGD/AG73 (p=0.038), AG73-alginate (p=0.0074), and unmodified alginate (p=0.0080) while cells in hydrogels with the combined peptides exhibited morphometric features observed in both AG73-alginate and cRGD-alginate hydrogels, where most cells remained circular (Fig. 6C). As at day 7, the majority of cells (>50%) were isolated from other cells within the 3D culture after 21 days, in contrast to 2D culture. The percentage of single cells (as opposed to cells in clusters) did not vary across all conditions. However, there was an increase in large cell clusters observed for cells cultured in gels presenting the cRGD/AG73 mixture. This increase was also observed from day 7 to 21 (Fig. 6D).
Figure 6. 3D Longitudinal culture in cRGD and AG73 mixture enhances juvenile-like phenotype.
A) Representative images of human NP cells encapsulated in 3D hydrogels at 21 days. scale bar is 50 μm. B-D) Cell spreading, circularity and clustering behaviors of cells encapsulated in peptide functionalized alginate hydrogel. E-F) Quantification of protein expression associated with juvenile-like phenotype. n ≥ 6 unique hydrogels from 3 human donors. Error bars are ±SD. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, using a one-way ANOVA with Tukey's multiple comparison's test (B-F).
As on day 7, N-Cadherin were more strongly upregulated by gels presenting the cRGD/AG73 mixture compared to cRGD-alginate (p=0.017), AG73-alginate (p<10−4), and unmodified alginate (p=0.0022; Fig.6A, E, and F). In day 21, there was enhancement of BASP-1 protein expression in mixture of cRGD and AG73 compared to cRGD alone (p=0.035) and AG73 alone (p=0.0078). These results suggest a combinatorial role of integrin and synedecan receptors acting together to promote a more juvenile-like NP cell phenotype to be re-expressed in NP cells derived from degenerative human intervertebral discs, in both 2D and 3D culture.
3.6. The combination of cRGD and AG73 enhances ECM biosynthesis and NP-specific matrix production in 3D.
Total ECM production labeled via functional non-canonical amino acid tagging (FUNCAT), as well as NP-specific matrix proteins, type II collagen and aggrecan, were strongly upregulated by gels presenting the cRGD/AG73 mixture at day 7 and day 21 (Fig. 7A). At day 7, the combined cRGD/AG73 hydrogels enhanced production of ECM protein compared to cRGD-alginate alone (p=0.030) and unmodified alginate (p=0.0035, Fig. 7B). Both type II collagen and aggrecan production were promoted in NP cells encapsulated in hydrogels presenting both cRGD and AG73 compared to cRGD, AG73, or unmodified alginate (p < 10−4 for all comparisons; Fig. 7C and D). Similar to day 7, the level of matrix biosynthesis was significantly increased in NP cells cultured within gels presenting the AG73/cRGD mixture compared to cells cultured within unmodified gels (p<10−4) or gels presenting only one type of peptide (p<10−4; Fig. 7E). Additionally, type II collagen and aggrecan were enhanced in cells encapsulated in the combination of cRGD and AG73 presented hydrogels compared to either cRGD alone (p=0.012, p=0.0007), AG73 alone (p=0.0030, p=0.033), or unmodified alginate (p=0.0041, p=0.0006), respectively (Fig. 7F and G).
Figure 7. Combination of cRGD and AG73 peptides drives NP cell toward a more biosynthetically active state.
A) Representative images of non-specific ECM biosynthesis labeled via functional non-canonical amino acid tagging (FUNCAT) approach and matrix proteins associated with juvenile NP cell phenotype at Day 7 and Day 21 of encapsulation in 3D hydrogels. B-D) Quantification of ECM biosynthesis and NP specific matrix protein expression at Day 7. E-G) Quantification of ECM biosynthesis and NP specific matrix protein expression at Day 21. n ≥ 6 unique hydrogels from 3 human donors. Error bars are ±SD. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, using a one-way ANOVA with Tukey's multiple comparison's test (B-G).
Furthermore, when we screen a panel of gene associated with the biosynthetically active NP cell phenotype, the intensity of increase in transcript levels for NP-like matrix markers was significant for COL2A1 (p=0.048) (Fig. 5G). Altogether, these results demonstrate a benefit of incorporating both cell adhesive domains and syndecan binding domains to allow a more biosynthetically active, juvenile-like NP specific cell phenotype.
4. Discussion
The results of this study demonstrate that presentation of either integrin or syndecan peptides alone elicited favorable effects on NP cell viability, biosynthesis and phenotype compared to what was observed in unmodified alginate. Encapsulation of primary NP cells in alginate functionalized with cRGD or AG73 peptides alone or together led to a high percentage of single cell entrapment in 3D, that was linked to an elevated accumulation of new extracellular and expression of NP-specific protein markers after 7 and 21 days of culture as compared to alginate alone. While cells largely remained isolated as single cells in peptide functionalized alginate, the presentation of cRGD alone or in combination with AG73 supported greater cell spreading in both 2D and 3D cell culture. These behaviors in 2D and 3D hydrogel culture provide new evidence of the bioactivity of these peptides for NP cells and elicit distinct morphologies, phenotypes and biosynthesis patterns from that observed for unmodified alginate hydrogels. The encapsulation of NP cells in alginate microspheres has been established to be the “gold standard” for in vitro culture over a period exceeding two decades [35, 37, 70-73]. Nevertheless, there is some evidence that NP cells may not form a functional matrix in alginate alone with findings that proteoglycan and type II collagen accumulation is relatively constant for NP cells encapsulated in alginate beads over prolonged culture periods up to 4 weeks [37, 72]. The results of the present study affirm the utility of cell encapsulation in alginate hydrogels yet illustrate the potential for cell adhesive peptides to promote increased attachment, viability, and NP-specific phenotypes and protein matrix over that considered to be the “gold standard.”
Just as the cRGD peptide elicited distinct morphologies for NP cells in 2D and 3D alginate hydrogels, the presentation of the syndecan binding AG73 peptide appeared to promote more cell rounding and elevated biosynthesis for NP cells cultured in the functionalized alginate. Utilizing functional noncanonical amino acid tagging to fluorescently label ECM synthesis, our study demonstrated that simultaneous presentation of both integrin-and syndecan-binding peptides on alginate hydrogels promoted a more biosynthetically active, NP cell phenotype compared to single peptide alone and unmodified alginate [60]. In addition to elevated production of non-specific ECM, the combination of cRGD and AG73 peptides strongly upregulated synthesis of type II collagen and aggrecan, which are two main components of NP ECM [61,74]. Furthermore, transcription levels of genes associated with healthy NP tissue ECM (ACAN and COL2A1) were upregulated in NP cells cultured with alginate presenting the cRGD/AG73 combination to that of unmodified alginate in both 2D and 3D. It is of interest to compare morphology and mRNA levels for NP cells cultured on AG73-functionalized PEG hydrogels from a prior report, with those same results for NP cells cultured on AG73-alginate at similar peptide densities as reported here [75]. Nearly 40% of cells attached as single cells and retained a high degree of cellularity with AG73 functionalized to either PEG or alginate, and mRNA levels of COL2A1, COL1A1, ACAN and CDH2 were comparable across studies (ΔCt levels, data not shown). Our work and works from Speer et al. show that AG73 provides some benefits to modulating NP cells toward a more juvenile-like phenotype regardless of cell adhesive domains [75]. Recent reports studying the interplay between 3D microenvironment and early ECM secretion in human mesenchymal stromal cells (hMSCs) hypothesized that hydrogel properties may not completely determine cell fate but rather influence initial pericellular matrix formation which in turn can modulate long-term cell fate [76, 77]. Similarly, our data suggested that cell adhesive cues presented to NP cell in a 3D milieu can elicit distinct behaviors that serve as triggers to produce cell-secreted ECM that can act to maintain a more juvenile, biosynthetically active NP phenotype.
N-Cadherin is a key hallmark of the phenotype of healthy, juvenile NP cells, and is an important regulator of cell-cell contacts in many cells [8, 78]. The combination of cRGD and AG73-functionalized alginate promoted the highest level of N-Cadherin protein expression when compared against either single peptide-functionalized alginate or unmodified alginate. Despite observed differences in cell-cell contact formation in the 2D and 3D cell culture conditions, where NP cells attached to 2D alginate largely as clusters, yet remained as isolated cells when encapsulated in 3D alginate, NP cells exhibited robust upregulation of N-Cadherin as measured by both mRNA and protein levels. This suggests cell morphology might not be the dominating factor in controlling NP cell phenotype, and that the engagement and transduction of receptor-ligand signaling might play a more prominent role in determining NP cell fate. This finding has been observed in previous studies using MSCs that showed cell phenotypes expressed in 3D matrix presented with adhesive ligands is not completely dependent on cell morphology, but is strongly dependent on hydrogel stiffness and adhesion ligand presentation [63].
Recent studies on the RGD motif has implicated its role in the modulation of mechanotransduction in NP cells for IVD degeneration and has shown to improve long term cell viability in 3D culture and in in vivo [79, 80]. Interestingly, linear RGD peptides were previously shown to be unsuitable for NP cell adhesion in 2D, but cyclic RGD peptides used here promoted robust adhesion [46]. Indeed, cRGD-elicited cell spreading and fibroblast-like morphologies in 2D culture with attachment at levels higher than that observed for linear RGD peptide conjugated materials, and supported assembly of paxillin-rich focal adhesions and pMLC protein expression alone or in combined presentation with the AG73 peptide, even when cultured in the 3D alginate hydrogels (Fig. 6A-C). The focal adhesion (FA) adapter protein paxillin has been shown to be critical for cytoskeletal organization, cell migration, and recruitment of signaling molecules to adhesive sites downstream of integrin-ECM binding [81, 82]. In natural ECM, integrin-binding and syndecan-binding domains have been shown to interact together to promote cell spreading by enhancing FA assembly [83, 84]. The cooperation of syndecan-4 (AG73-binding) and α5β1 and αvβ3-integrin (cRGD-binding) has shown to trigger paxillin-mediated cytoskeleton rearrangement during adhesion formation [85]. A role for a potential synergistic interaction between the two receptors has been studied in other cell types. For example, Hozumi et al. suggested the interaction of syndecan- (AG73) and integrin-binding (EF1) ligands promoted stronger dermal fibroblast adhesion, cell spreading, and FA phosphorylation than achieved by any single peptide alone [48]. Others have hypothesized that syndecan-matrix adhesion precedes integrin-matrix binding, and that the initial syndecan interaction primes integrin engagement with ECM to promote cytoskeleton activation [84, 86]. Similarly, we observed that a co-presentation of integrin-binding and syndecan-binding peptide or integrin-binding peptide alone led to the formation of paxillin-rich FAs, while FA formation was inhibited on hydrogels conjugated with AG73 alone (Fig. 3H). Phosphorylated myosin light chain (pMLC), which is association with cell migration and actin filament formation downstream of FA assembly, was enhanced in NP cells adherent to gels presenting either cRGD alone or the AG73/cRGD combination (Fig. 3I, [87, 88]). These results strongly suggest paxillin and pMLC are driven by cRGD and in a dose-independent manner, as equivalent expression levels were observed for the cRGD/AG73 combination and cRGD even though the latter is functionalized with twice the molar concentration of cRGD. The bioactivity seen from the presentation of cyclic RGD and AG73 peptide sequence is robust and prominent; however, a potential limitation in our study was that we did not include scrambled AG73 peptides, the control Arg-Gly-Glu (RGE), and scrambled cRGD peptide sequences, which would further validate the bioactivity of the exact peptide sequences used in the current study [89, 90].
Altogether, our data demonstrate there is a combinatorial benefit of integrin and syndecan–binding cell adhesive ligands in modulating degenerative human NP cells to re-express a more biosynthetic, juvenile phenotype. Moreover, while cell morphology and cell-cell interactions are undoubtedly important in controlling NP cell biology, gross changes in either of these parameters do not appear to be required for NP cells to transduce adhesive signals into fate decisions, even those involving expression of a key receptor for cell-cell interactions, N-Cadherin [8, 59, 78]. These data suggest that optimal presentation of adhesive ligands alone may be able to induce expression of healthy, juvenile NP cells, provided that both integrin and syndecan receptors can be engaged. In future studies, a cell-free based biomaterial, and in vivo examination of the biocompatibility, toxicity, and immunogenicity of the developed biomaterial encapsulated with cells following delivery to the IVD defect site will elucidate the regenerative capability of the hydrogel to be used as a carrier to modulate endogenous NP cells and support a degenerative disc. A similar concept has previously been explored by Zhang et al. using MSCs in the disc [91].
5. Conclusions
In the present work, we demonstrated human NP cells cultured upon or within alginate hydrogels presented with cRGD (integrin binding) and AG73 (syndecan binding) peptides contributed to higher cell attachment, viability, biosynthetic activity and NP-specific phenotypic expression over alginate alone. Each cell adhesive peptide contributed uniquely to cell attachment, morphology, focal adhesion formation and downstream effects on biosynthesis in a manner newly revealed for NP cells. The combination of the two peptide motifs was able to elicit markers of the NP-specific cell phenotype, including N-Cadherin, despite differences in cell morphology and the tendency to form multicellular clusters between 2D and 3D culture conditions. These results represent a promising step toward understanding how distinct adhesive peptides can be combined to guide NP cell fate in the gold standard of 3D alginate culture. In the future, these insights may be useful to rationally design hydrogels for NP cell-transplantation based therapies for IVD degeneration.
Supplementary Material
Acknowledgements
This material is based upon work supported by the National Science Foundation Graduate Research Fellowship Program under Grant No. DGE-1745038 and DGE-2139839. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation. This work was further supported by funding from the National Institutes of Health (R01AR077678, R01AR077678-02S1, and R41AR079324). We thank Dr. Chris Madl (Stanford University) for helpful advice regarding Maleimide-Thiol click chemistry, and Barbara Semar (Washington University in St. Louis, Department of Mechanical Engineering and Materials Science) for help with the TA ElectroForce 3200 instrument for mechanical testing.
Footnotes
Declaration of Competing Interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- [1].Weber Kathryn T., Jacobsen Timothy D., Maidhof Robert, Virojanapa Justin, Overby Chris, Bloom Ona, Quraishi Shaheda, Levine Mitchell, Chahine NO “Developments in intervertebral disc disease research: pathophysiology, mechanobiology, and therapeutics,” Curr. Rev. Musculoskelet. Med, vol. 8, no. 1, pp. 18–31, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Rodrigues-Pinto R, Richardson SM, and Hoyland JA, “An understanding of intervertebral disc development, maturation and cell phenotype provides clues to direct cell-based tissue regeneration therapies for disc degeneration,” European spine journal : official publication of the European Spine Society, the European Spinal Deformity Society, and the European Section of the Cervical Spine Research Society, vol. 23, no. 9. Springer, pp. 1803–1814, 01-Sep-2014. [DOI] [PubMed] [Google Scholar]
- [3].Kepler CK, Ponnappan RK, Tannoury CA, Risbud MV, and Anderson DG, “The molecular basis of intervertebral disc degeneration,” Spine Journal, vol. 13, no. 3. Elsevier, pp. 318–330, 01-Mar-2013. [DOI] [PubMed] [Google Scholar]
- [4].Feng Y, Egan B, and Wang J, “Genetic factors in intervertebral disc degeneration,” Genes Dis., vol. 3, no. 3, pp. 178–185, Sep. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Adams MA and Roughley PJ, “What is intervertebral disc degeneration, and what causes it?,” Spine (Phila. Pa. 1976)., vol. 31, no. 18, pp. 2151–2161, 2006. [DOI] [PubMed] [Google Scholar]
- [6].Korecki CL, Maclean JJ, and Iatridis JC, “Dynamic Compression Effects on Intervertebral Disc Mechanics and Biology,” Spine J., vol. 33, no. 13, pp. 1403–1409, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Tang X, Jing L, and Chen J, “Changes in the Molecular Phenotype of Nucleus Pulposus Cells with Intervertebral Disc Aging,” PLoS One, vol. 7, no. 12, p. 52020, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Hwang PY, Jing L, Michael KW, Richardson WJ, Chen J, and Setton LA, “N-Cadherin-Mediated Signaling Regulates Cell Phenotype for Nucleus Pulposus Cells of the Intervertebral Disc,” Cell. Mol. Bioeng, vol. 8, no. 1, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Choi K-S, Cohn MJ, and Harfe BD, “Identification of Nucleus Pulposus Precursor Cells and Notochordal Remnants in the Mouse: Implications for Disk Degeneration and Chordoma Formation,” Dev. Dyn, vol. 237, no. 12, pp. 3953–3958, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Boubriak OA, Watson N, Sivan SS, Stubbens N, and Urban JPG, “Factors regulating viable cell density in the intervertebral disc: Blood supply in relation to disc height,” J. Anat, vol. 222, no. 3, pp. 341–348, Mar. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Zhang F, Zhao X, Shen H, and Zhang C, “Molecular mechanisms of cell death in intervertebral disc degeneration (Review),” International Journal of Molecular Medicine, vol. 37, no. 6. pp. 1439–1448, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Roberts S, Evans EH, Kletsas D, Jaffray DC, and Eisenstein SM, “Senescence in human intervertebral discs,” in European Spine Journal, 2006, vol. 15, no. SUPPL. 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Bendtsen M et al. , “Biological challenges for regeneration of the degenerated disc using cellular therapies,” Acta Orthop., vol. 87, pp. 39–46, Dec. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Choi H, Johnson Z, and Risbud M, “Understanding Nucleus Pulposus Cell Phenotype: A Prerequisite for Stem Cell Based Therapies to Treat Intervertebral Disc Degeneration,” Curr. Stem Cell Res. Ther, vol. 10, no. 4, pp. 307–316, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Francisco Aubrey T., Mancino Robert J., Bowles Robby D., Brunger Jonathan M., Tainter David M., Chen Yi-Te, Richardson William J., Guilak Farshid, Setton LA, “Injectable laminin-functionalized hydrogel for nucleus pulposus regeneration,” Biomaterials, vol. 34, no. 30, pp. 7381–7388, Oct. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Huang YC, Leung VYL, Lu WW, and Luk KDK, “The effects of microenvironment in mesenchymal stem cell-based regeneration of intervertebral disc,” Spine Journal, vol. 13, no. 3. pp. 352–362, 2013. [DOI] [PubMed] [Google Scholar]
- [17].Krock E, Rosenzweig D, and Haglund L, “The Inflammatory Milieu of the Degenerate Disc: Is Mesenchymal Stem Cell-based Therapy for Intervertebral Disc Repair a Feasible Approach?,” Curr. Stem Cell Res. Ther, vol. 10, no. 4, pp. 317–328, May 2015. [DOI] [PubMed] [Google Scholar]
- [18].Huebsch N, “Translational mechanobiology: Designing synthetic hydrogel matrices for improved in vitro models and cell-based therapies,” Acta Biomater., vol. 94, pp. 97–111, 2019. [DOI] [PubMed] [Google Scholar]
- [19].Lee Chang Kyu, Heo Dong Hwa, Chung Hungtae, Roh Eun Ji, Darai Anjani, Kyung Jae Won, Choi Hyemin, Kwon Su Yeon, Bhujel Basanta, Han Inbo., “Advances in tissue engineering for disc repair,” Appl. Sci, vol. 11, no. 4, pp. 1–16, Feb. 2021. [Google Scholar]
- [20].Schmitz Tara C., Salzer Elias, Crispim João F., Fabra Georgina Targa, LeVisage Catherine, Pandit Abhay, Tryfonidou Marianna, Le Maitre Christine, Ito Keita., “Characterization of biomaterials intended for use in the nucleus pulposus of degenerated intervertebral discs,” Acta Biomater., vol. 114, pp. 1–15, 2020. [DOI] [PubMed] [Google Scholar]
- [21].Bowles RD and Setton LA, “Biomaterials for intervertebral disc regeneration and repair,” Biomaterials, vol. 129, pp. 54–67, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Bowles RD, Williams RM, Zipfel WR, and Bonassar LJ, “Self-Assembly of Aligned Tissue-Engineered Annulus Fibrosus and Intervertebral Disc Composite Via Collagen Gel Contraction,” Tissue Eng. - Part A, vol. 16, no. 4, pp. 1339–1348, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Woiciechowsky Christian, Abbushi Alexander, Zenclussen Maria L., Casalis Pablo, Krüger Jan Philipp, Freymann Undine, Endres Michaela, Kaps Christian., “Regeneration of nucleus pulposus tissue in an ovine intervertebral disc degeneration model by cell-free resorbable polymer scaffolds,” J. Tissue Eng. Regen. Med, vol. 8, no. 10, pp. 811–820, Oct. 2014. [DOI] [PubMed] [Google Scholar]
- [24].Calderon L, Collin E, Velasco-Bayon D, Murphy M, O’Halloran D, and Pandit A, “Type II Collagen-Hyaluronan Hydrogel - A Step Towards A Scaffold For Intervertebral Disc Tissue Engineering,” Eur. Cells Mater, vol. 20, pp. 134–148, 2010. [DOI] [PubMed] [Google Scholar]
- [25].Bax Daniel V., Davidenko Natalia, Gullberg Donald, Hamaia Samir W., Farndale Richard W., Best Serena M., Cameron Ruth E., “Fundamental insight into the effect of carbodiimide crosslinking on cellular recognition of collagen-based scaffolds,” Acta Biomater., vol. 49, pp. 218–234, Feb. 2017. [DOI] [PubMed] [Google Scholar]
- [26].Haugh MG, Murphy CM, McKiernan RC, Altenbuchner C, and O’Brien FJ, “Crosslinking and mechanical properties significantly influence cell attachment, proliferation, and migration within collagen glycosaminoglycan scaffolds,” Tissue Eng. - Part A, vol. 17, no. 9–10, pp. 1201–1208, May 2011. [DOI] [PubMed] [Google Scholar]
- [27].Sakai Daisuke, Mochida Joji, Yamamoto Yukihiro, Nomura Takeshi, Okuma Masahiko, Nishimura Kazuhiro, Nakai Tomoko, Ando Kiyoshi, Hotta Tomomitsu., “Transplantation of mesenchymal stem cells embedded in Atelocollagen® gel to the intervertebral disc: A potential therapeutic model for disc degeneration,” Biomaterials, vol. 24, no. 20, pp. 3531–3541, 2003. [DOI] [PubMed] [Google Scholar]
- [28].Francisco Aubrey T., Mancino Robert J., Bowles Robby D., Brunger Jonathan M., Tainter David M., Chen Yi-Te, Richardson William J., Guilak Farshid, Setton LA, “Injectable laminin-functionalized hydrogel for nucleus pulposus regeneration,” Biomaterials, vol. 34, no. 30, pp. 7381–7388, Oct. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Cloyd JM, Malhotra NR, Weng L, Chen W, Mauck RL, and Elliott DM, “Material properties in unconfined compression of human nucleus pulposus, injectable hyaluronic acid-based hydrogels and tissue engineering scaffolds,” Eur. Spine J, vol. 16, no. 11, pp. 1892–1898, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Bowles RD, Masuda K, Bonassar LA, and Setton LA, Tissue Engineering for Regeneration and Replacement of the Intervertebral Disc, Fourth Edi. Elsevier, 2013. [Google Scholar]
- [31].Bidarra SJ, Barrias CC, and Granja PL, “Injectable alginate hydrogels for cell delivery in tissue engineering,” Acta Biomaterialia, vol. 10, no. 4. Elsevier BV, pp. 1646–1662, 2014. [DOI] [PubMed] [Google Scholar]
- [32].Chaudhuri Ovijit, Gu Luo, Klumpers Darinka, Darnell Max, Bencherif Sidi A., Weaver James C., Huebsch Nathaniel, Lee Hong Pyo, Lippens Evi, Duda Georg N., Mooney David J., “Hydrogels with tunable stress relaxation regulate stem cell fate and activity,” Nat. Mater, vol. 15, no. 3, pp. 326–334, Mar. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Bonnevie Edward D., Gullbrand Sarah E., Ashinsky Beth G., Tsinman Tonia K., Elliott Dawn M., Chao Pen-hsiu Grace, Smith Harvey E., Mauck Robert L., “Aberrant mechanosensing in injured intervertebral discs as a result of boundary-constraint disruption and residual-strain loss,” Nat. Biomed. Eng, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Yang X and Li X, “Nucleus pulposus tissue engineering: A brief review,” European Spine Journal, vol. 18, no. 11. pp. 1564–1572, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Maldonado BA and Oegema TR, “Initial characterization of the metabolism of intervertebral disc cells encapsulated in microspheres,” J. Orthop. Res, vol. 10, no. 5, pp. 677–690, 1992. [DOI] [PubMed] [Google Scholar]
- [36].Gruber HE, Fisher EC, Desai B, Stasky AA, Hoelscher G, and Hanley EN, “Human intervertebral disc cells from the annulus: Three-dimensional culture in agarose or alginate and responsiveness to TGF-β1,” Exp. Cell Res, vol. 235, no. 1, pp. 13–21, Aug. 1997. [DOI] [PubMed] [Google Scholar]
- [37].Chiba K, Andersson GBJ, Masuda K, and Thonar EJMA, “Metabolism of the extracellular matrix formed by intervertebral disc cells cultured in alginate,” Spine (Phila. Pa. 1976)., vol. 22, no. 24, pp. 2885–2893, Dec. 1997. [DOI] [PubMed] [Google Scholar]
- [38].Smith Lachlan J., Chiaro Joseph A., Nerurkar Nandan L., Cortes Daniel H., Horava Sarena D., Hebela Nader M., Mauck Robert L., Dodge George R., Elliott Dawn M., “Nucleus pulposus cells synthesize a functional extracellular matrix and respond to inflammatory cytokine challenge following long-term agarose culture.,” Eur. Cell. Mater, vol. 22, pp. 291–301, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Naqvi SM and Buckley CT, “Differential response of encapsulated nucleus pulposus and bone marrow stem cells in isolation and coculture in alginate and chitosan hydrogels,” Tissue Eng. - Part A, vol. 21, no. 1–2, pp. 288–299, Jan. 2015. [DOI] [PubMed] [Google Scholar]
- [40].Chou AI, Akintoye SO, and Nicoll SB, “Photo-crosslinked alginate hydrogels support enhanced matrix accumulation by nucleus pulposus cells in vivo,” Osteoarthr. Cartil, vol. 17, no. 10, pp. 1377–1384, Oct. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Buxboim A, Ivanovska IL, and Discher DE, “Matrix elasticity, cytoskeletal forces and physics of the nucleus: How deeply do cells ‘feel’ outside and in?,” Journal of Cell Science, vol. 123, no. 3. pp. 297–308, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Hwang PY, Chen J, Jing L, Hoffman BD, and Setton LA, “The role of extracellular matrix elasticity and composition in regulating the nucleus pulposus cell phenotype in the intervertebral disc: A narrative review,” J. Biomech. Eng, vol. 136, no. 2, pp. 1–9, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Gilchrist CL, Francisco AT, Plopper GE, Chen J, and Setton LA, “Nucleus pulposus cell-matrix interactions with laminins,” Eur. Cells Mater, vol. 21, pp. 523–532, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Gilchrist CL, Chen J, Richardson WJ, Loeser RF, and Setton LA, “Functional integrin subunits regulating cell–matrix interactions in the intervertebral disc,” J. Orthop. Res, vol. 25, no. 6, pp. 829–840, Jun. 2007. [DOI] [PubMed] [Google Scholar]
- [45].Bridgen DT, Gilchrist CL, Richardson WJ, Isaacs RE, Brown CR, Yang KL, Chen J, Setton LA, “Integrin-mediated interactions with extracellular matrix proteins for nucleus pulposus cells of the human intervertebral disc,” J. Orthop. Res, vol. 31, no. 10, pp. 1661–1667, Oct. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Bridgen Devin T., Fearing Bailey V., Jing Liufang, Sanchez-Adams Johannah, Cohan Megan C., Guilak Farshid, Chen Jun, Setton Lori A., “Regulation of human nucleus pulposus cells by peptide-coupled substrates,” Acta Biomaterialia, vol. 55. pp. 100–108, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Barcellona Marcos N, Speer Julie E, Fearing Bailey V, Jing Liufang, Pathak Amit, Gupta Munish C., Buchowski Jacob M, Kelly Michael, Setton Lori A, “Control of adhesive ligand density for modulation of nucleus pulposus cell phenotype,” Biomaterials, vol. 250, p. 120057, Aug. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Hozumi K, Kobayashi K, Katagiri F, Kikkawa Y, Kadoya Y, and Nomizu M, “Syndecan- and integrin-binding peptides synergistically accelerate cell adhesion,” FEBS Lett., vol. 584, no. 15, pp. 3381–3385, Aug. 2010. [DOI] [PubMed] [Google Scholar]
- [49].Kurakawa Takuto, Kakutani Kenichiro, Morita Yusuke, Kato Yuki, Yurube Takashi, Hirata Hiroaki, Miyazaki Shingo, Terashima Yoshiki, Maeno Koichiro, Takada Toru, Doita Minoru, Kurosaka Masahiro, Inoue Nozomu, Masuda Koichi, Nishida Kotaro, “Functional impact of integrin α5β1 on the homeostasis of intervertebral discs: A study of mechanotransduction pathways using a novel dynamic loading organ culture system,” Spine J., vol. 15, no. 3, pp. 417–426, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Gilbert HTJ, Nagra NS, Freemont AJ, Millward-Sadler SJ, and Hoyland JA, “Integrin - Dependent Mechanotransduction in Mechanically Stimulated Human Annulus Fibrosus Cells: Evidence for an Alternative Mechanotransduction Pathway Operating with Degeneration,” PLoS One, vol. 8, no. 9, p. e72994, Sep. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Lee KY and Mooney DJ, “Alginate: Properties and biomedical applications,” Progress in Polymer Science (Oxford), vol. 37, no. 1. pp. 106–126, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Hsiong SX, Huebsch N, Fischbach C, Kong HJ, and Mooney DJ, “Integrin-Adhesion Ligand Bond Formation of Preosteoblasts and Stem Cells in Three-Dimensional RGD Presenting Matrices,” Biomacromolecules, vol. 9, no. 7, pp. 1843–1851, Jul. 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Baer AE, Wang JY, Kraus VB, and Setton LA, “Collagen gene expression and mechanical properties of intervertebral disc cell-alginate cultures,” J. Orthop. Res, vol. 19, no. 1, pp. 2–10, 2001. [DOI] [PubMed] [Google Scholar]
- [54].Cukierman E, Pankov R, Stevens Daron R., Yamada Kenneth M., “Taking Cell-Matrix Adhesions to the Third Dimension,” Science, vol. 294, no. 5547, pp. 1708–1712, Nov. 2001. [DOI] [PubMed] [Google Scholar]
- [55].Fearing BV, Hernandez PA, Setton LA, and Chahine NO, “Mechanotransduction and cell biomechanics of the intervertebral disc,” JOR Spine, vol. 1, no. 3, p. e1026, Sep. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Griffith LG and Swartz MA, “Capturing complex 3D tissue physiology in vitro,” Nat. Rev. Mol. Cell Biol, vol. 7, no. 3, pp. 211–224, 2006. [DOI] [PubMed] [Google Scholar]
- [57].Madl CM, Mehta M, Duda GN, Heilshorn SC, and Mooney DJ, “Presentation of BMP-2 mimicking peptides in 3D hydrogels directs cell fate commitment in osteoblasts and mesenchymal stem cells,” Biomacromolecules, vol. 15, no. 2, pp. 445–455, Feb. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Jain E, Neal S, Graf H, Tan X, Balasubramaniam R, and Huebsch N, “Copper-Free Azide–Alkyne Cycloaddition for Peptide Modification of Alginate Hydrogels,” ACS Appl. Bio Mater, vol. 4, no. 2, pp. 1229–1237, Feb. 2021. [DOI] [PubMed] [Google Scholar]
- [59].Fearing Bailey V, Jing Liufang, Barcellona Marcos N, Witte Savannah Est, Buchowski Jacob M, Zebala Lukas P, Kelly Michael P, Luhmann Scott, Gupta Munish C, Pathak Amit, Setton Lori A, “Mechanosensitive transcriptional coactivators MRTF-A and YAP/TAZ regulate nucleus pulposus cell phenotype through cell shape,” FASEB J., vol. 33, no. 12, pp. 14022–14035, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Mcleod CM and Mauck RL, “High fidelity visualization of cell-to-cell variation and temporal dynamics in nascent extracellular matrix formation,” Sci. Rep, vol. 6, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Risbud Makarand V., Schoepflin Zachary R., Mwale Fackson, Kandel Rita A., Grad Sibylle, Iatridis James C., Sakai Daisuke, Hoyland Judith A., “Defining the phenotype of young healthy nucleus pulposus cells: Recommendations of the spine research interest group at the 2014 annual ORS meeting,” J. Orthop. Res, vol. 33, no. 3, pp. 283–293, Mar. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Horzum U, Ozdil B, and Pesen-Okvur D, “Step-by-step quantitative analysis of focal adhesions,” MethodsX, vol. 1, pp. 56–59, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Huebsch Nathaniel, Arany Praveen R., Mao Angelo S., Shvartsman Dmitry, Ali Omar A., Bencherif Sidi A., Rivera-Feliciano José, Mooney David J., “Harnessing traction-mediated manipulation of the cell/matrix interface to control stem-cell fate,” Nat. Mater, vol. 9, no. 6, pp. 518–526, Jun. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Iatridis JC, Setton LA, Weidenbaum M, and Mow VC, “Alterations in the mechanical behavior of the human lumbar nucleus pulposus with degeneration and aging,” J. Orthop. Res, vol. 15, no. 2, pp. 318–322, Mar. 1997. [DOI] [PubMed] [Google Scholar]
- [65].Iatridis JC, Weidenbaum M, Setton LA, and Van Mow C, “Is the nucleus pulposus a solid or a fluid? Mechanical behaviors of the nucleus pulposus of the human intervertebral disc,” in Spine, 1996, vol. 21, no. 10, pp. 1174–1184. [DOI] [PubMed] [Google Scholar]
- [66].Walter Benjamin A., Mageswaran Prasath, Mo Xiaokui, Boulter Daniel J., Mashaly Hazem, Nguyen Xuan V, Prevedello Luciano M, Thoman William, Raterman Brian D, Kalra Prateek, Mendel Ehud, Marras William S, Kolipaka Arunark., “MR Elastography–derived Stiffness: A Biomarker for Intervertebral Disc Degeneration,” Radiology, vol. 285, no. 1, pp. 167–175, Oct. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Hsiong SX, Boontheekul T, Huebsch N, and Mooney DJ, “Cyclic arginine-glycine-aspartate peptides enhance three-dimensional stem cell osteogenic differentiation,” Tissue Eng. - Part A, vol. 15, no. 2, pp. 263–272, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Liu S, “Radiolabeled multimeric cyclic RGD peptides as integrin αvβ3 targeted radiotracers for tumor imaging,” Molecular Pharmaceutics, vol. 3, no. 5. pp. 472–487, 2006. [DOI] [PubMed] [Google Scholar]
- [69].Storrie Hannah, Guler Mustafa O., Abu-Amara Suha N., Volberg Tova, Rao Mukti, Geiger Benjamin, Stupp Samuel I., “Supramolecular crafting of cell adhesion,” Biomaterials, vol. 28, no. 31, pp. 4608–4618, 2007. [DOI] [PubMed] [Google Scholar]
- [70].Wang JY, Baer AE, Kraus VB, and Setton LA, “Intervertebral disc cells exhibit differences in gene expression in alginate and monolayer culture,” Spine (Phila. Pa. 1976)., vol. 26, no. 16, pp. 1747–1752, 2001. [DOI] [PubMed] [Google Scholar]
- [71].Chou AI and Nicoll SB, “Characterization of photocrosslinked alginate hydrogels for nucleus pulposus cell encapsulation,” J. Biomed. Mater. Res. - Part A, vol. 91, no. 1, pp. 187–194, 2009. [DOI] [PubMed] [Google Scholar]
- [72].Chiba K, Andersson GBJ, Masuda K, Momohara S, Williams JM, and Thonar EJMA, “A new culture system to study the metabolism of the intervertebral disc in vitro,” Spine (Phila. Pa. 1976)., vol. 23, no. 17, pp. 1821–1828, 1998. [DOI] [PubMed] [Google Scholar]
- [73].Aguiar DJ, Johnson SL, and Oegema TR, “Notochordal cells interact with nucleus pulposus cells: Regulation of proteoglycan synthesis,” Exp. Cell Res, vol. 246, no. 1, pp. 129–137, Jan. 1999. [DOI] [PubMed] [Google Scholar]
- [74].Guerrero J, Häckel S, Croft AS, Hoppe S, Albers CE, Gantenbein B, “The nucleus pulposus microenvironment in the intervertebral disc: the fountain of youth?,” Eur. Cells Mater, vol. 41, pp. 707–738, 2021. [DOI] [PubMed] [Google Scholar]
- [75].Speer Julie E; Barcellona Marcos N; Lu Michael Y; Zha Zizhen; Jing Liufang; Gupta Munish C; Buchowski Jacob M; Kelly Michael P; Setton Lori A, “Development of a library of laminin-mimetic peptide hydrogels for control of nucleus pulposus cell behaviors,” J. Tissue Eng, vol. 12, p. 204173142110212, Jan. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Ferreira Silvia A., Motwani Meghna S., Faull Peter A., Seymour Alexis J., Yu Tracy T.L., Enayati Marjan, Taheem Dheraj K., Salzlechner Christoph, Haghighi Tabasom, Kania Ewa M., Oommen Oommen P., Ahmed Tarek, Loaiza Sandra, Parzych Katarzyna, Dazzi Francesco, Varghese Oommen P., Festy Frederic, Grigoriadis Agamemnon E., Auner Holger W., Snijders Ambrosius P., Bozec Laurent, Gentleman Eileen, “Bi-directional cell-pericellular matrix interactions direct stem cell fate,” Nat. Commun, vol. 9, no. 1, p. 4049, Dec. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Loebel C, Mauck RL, and Burdick JA, “Local nascent protein deposition and remodelling guide mesenchymal stromal cell mechanosensing and fate in three-dimensional hydrogels,” Nat. Mater, vol. 18, no. 8, pp. 883–891, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Hwang Priscilla Y, Jing Liufang, Chen Jun, Lim Foon Lian, Tang Ruhang, Choi Hyowon, Cheung Kenneth M, Risbud Makarand V, Gersbach Charles A, Guilak Farshid, Leung Victor Y, Setton Lori A, “N-cadherin is key to expression of the nucleus pulposus cell phenotype under selective substrate culture conditions,” Sci. Rep, vol. 6, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Kanda Yutaro, Yurube Takashi, Morita Yusuke, Takeoka Yoshiki, Kurakawa Takuto, Tsujimoto Ryu, Miyazaki Kunihiko, Kakiuchi Yuji, Miyazaki Shingo, Zhang Zhongying, Takada Toru, Hoshino Yuichi, Masuda Koichi, Kuroda Ryosuke, Kakutani Kenichiro, “Delayed notochordal cell disappearance through integrin α5β1 mechanotransduction during ex-vivo dynamic loading-induced intervertebral disc degeneration,” J. Orthop. Res, no. September, 2020. [DOI] [PubMed] [Google Scholar]
- [80].Wang Feng, Nan Li-ping, Zhou Shi-feng, Liu Yang, Wang Ze-yu, Wang Jing-cheng, Feng Xin-min, Zhang Liang, “Injectable Hydrogel Combined with Nucleus Pulposus-Derived Mesenchymal Stem Cells for the Treatment of Degenerative Intervertebral Disc in Rats,” Stem Cells Int., pp. 1–17, Oct. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].López-Colomé AM, Lee-Rivera I, Benavides-Hidalgo R, and López E, “Paxillin: A crossroad in pathological cell migration,” Journal of Hematology and Oncology, vol. 10, no. 1. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Miyamoto S, Akiyama SK, and Yamada KM, “Synergistic Roles for Receptor Occupancy and Aggregation in Integrin Transmembrane Function,” Science (80-. )., vol. 267, no. 5199, pp. 883–885, 1995. [DOI] [PubMed] [Google Scholar]
- [83].Yang N and Friedl A, “Syndecan-1-induced ECM fiber alignment requires integrin αvβ3 and syndecan-1 ectodomain and heparan sulfate chains,” PLoS One, vol. 11, no. 2, Feb. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Morgan MR, Humphries MJ, and Bass MD, “Synergistic control of cell adhesion by integrins and syndecans,” Nat. Rev. Mol. Cell Biol, vol. 8, no. 12, pp. 957–969, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Roper JA, Williamson RC, and Bass MD, “Syndecan and integrin interactomes: Large complexes in small spaces,” Current Opinion in Structural Biology, vol. 22, no. 5. Elsevier Current Trends, pp. 583–590, 01-Oct-2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Galbraith CG, Yamada KM, and Galbraith JA, “Polymerizing actin fibers position integrins primed to probe for adhesion sites,” Science (80-. )., vol. 315, no. 5814, pp. 992–995, 2007. [DOI] [PubMed] [Google Scholar]
- [87].Ikebe M and Hartshorne DJ, “Phosphorylation of smooth muscle myosin at two distinct sites by myosin light chain kinase,” J. Biol. Chem, vol. 260, no. 18, pp. 10027–10031, 1985. [PubMed] [Google Scholar]
- [88].Berger Stephen L., Leo-Macias Alejandra, Yuen Stephanie, …,Delmar Mario, Leterrier Christopher, Salzer James L., “Localized Myosin II Activity Regulates Assembly and Plasticity of the Axon Initial Segment,” Neuron, vol. 97, no. 3, pp. 555–570.e6, Feb. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Cardarelli Pina M., Cobb Ronald R., Nowlin Dawn M., Scholz Wolfgang, Gorcsan Frank, Moscinski Mary, Yasuhara Mikiko, Chiang Shui Lan, Lobl Thomas J., “Cyclic RGD peptide inhibits alpha 4 beta 1 interaction with connecting segment 1 and vascular cell adhesion molecule,” J. Biol. Chem, vol. 269, no. 28, pp. 18668–18673, Jul. 1994. [PubMed] [Google Scholar]
- [90].Pakalns Teika, Haverstick Kraig L., Fields Gregg B., McCarthy James B., Mooradian Daniel L., Tirrell Matthew, “Cellular recognition of synthetic peptide amphiphiles in self-assembled monolayer films,” Biomaterials, vol. 20, no. 23–24, pp. 2265–2279, Dec. 1999. [DOI] [PubMed] [Google Scholar]
- [91].Zhang Chenghao, Gullbrand Sarah E., Schaer Thomas P., Boorman Sophie, Elliott Dawn M., Chen Weiliam, Dodge George R., Mauck Robert L., Malhotra Neil R., Smith Lachlan J., “Combined Hydrogel and Mesenchymal Stem Cell Therapy for Moderate-Severity Disc Degeneration in Goats,” Tissue Eng. Part A, vol. 27, no. 1–2, pp. 117–128, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.