Abstract
HIV-related neurocognitive impairment can be worsened by cigarette smoking and be more severe in women. Therefore, we analyzed the effects of sex on behavioral function in HIV transgenic (Tg) rats that were exposed to either nicotine alone, to smoke from either nicotine-containing or nicotine-free cigarettes, or non-exposed. The animals were then assessed on the open field test for the total distance traveled and for the fraction of the total distance traveled and the total time spent in the center of the field, and the results then compared to WT rats subjected to the same exposures and testing. Higher total distances indicate greater locomotor activity and a higher center field measures imply a lower anxiety state. Total distances were overall higher for female and for Tg rats exposed to nicotine-free CS. Also, the total distance and both center field measures were overall higher for female rats in the control and nicotine-free CS-exposed groups. This was observed specifically for WT females as compared to WT males and, for the center field measures, for WT females as compared to Tg males. No genotype or sex-related differences were found for rats in the nicotine-free cigarette smoke (CS) and nicotine-containing CS exposed groups. Therefore, nicotine exposure did not impact genotype- and sex-related differences in motor responses and anxiety levels that were found in the control state. However, exposure to the non-nicotine components of CS resulted in locomotor activation in the presence of the HIV genes and was anxiogenic in WT and Tg male animals.
Keywords: HIV, sex differences, transgenic, nicotine, cigarettes
1. Introduction
Individuals with human immunodeficiency virus type-1 (HIV) infection have an elevated risk for developing HIV-associated neurocognitive disorders (HAND), which cause abnormalities of memory, concentration and information processing that can range from mild (asymptomatic neurocognitive impairment) to severe (HIV dementia) [1]. Multiple studies have demonstrated that HAND severity can be worse in individuals who abuse substances such as methamphetamine and cocaine [2, 3]. Tobacco is one of the most commonly abused substances in society, with estimates showing that over 40% of HIV-infected individuals are cigarette smokers [4], which is twice the estimated prevalence of smoking among adults in the general population [5]. Conversely, the rate of quitting smoking among HIV-infected individuals is about 50% less than that in the general population [6]. Studies of the possible role of cigarette smoke (CS) on the risk of cognitive impairment in HIV infection have yielded conflicting results, with some showing no effect [7, 8], others demonstrating an increased association with cognitive impairment and HAND [9, 10], and another showing better cognition in a cohort of HIV-infected women who were smokers as compared to infected non-smokers [11].
We previously published the results of studies whereby we modeled the effects of smoking in HIV infection by exposing F344 wild-type (WT) and HIV-1 transgenic (Tg) rats to smoke from either cigarettes containing 0.7 mg of nicotine (regular cigarettes), cigarettes containing trace amounts of nicotine (“nicotine-free” cigarettes (NF) or to nicotine (NIC) alone [12]. The rats were then subjected to testing, which included an assessment for changes in behavioral function on the open field test (OFT) [12], with an analysis performed of the total time animals from each group spent in the center of the field. For that study, the center field area was 50% of the area of the entire field. On the test, Tg rats that were exposed to smoke from regular cigarettes spent significantly more time in the center of the field than Tg rats exposed to smoke from nicotine-free cigarettes. In contrast, WT rats showed similar OFT responses from the two exposures, which suggests that exposure to regular CS resulted in an overall anxiolytic effect specifically in the Tg rats.
Recent reports describe different susceptibilities for women and men for developing cognitive impairment related to HIV infection [13, 14]. Similarly, gender differences in potential smoking triggers, for the effects of CS and nicotine, and smoking cessation patterns have been also described [15–19]. To gain insight into the potential role of biological sex differences on effects that can be observed from the exposures in our HIV infection model, we analyzed OFT data obtained on the WT and Tg rats for differences related to the sex, as well as the genotype of the animals. For these studies we performed a more stringent assessment of anxiety, with the center area of the open field corresponding to only 25% of the total area. We hypothesized that we would detect differences in performance on the testing in female versus male animals with such findings in our model also linked to genotype.
2. Methods
2.1. Animals and exposures
All the experimental protocols were approved by the University of Maryland School of Medicine Institutional Animal Care and Use Committee and carried out according to the policies and regulations of the National Institutes of Health Office of Laboratory and Animal Welfare. The details of the HIV-1 transgenic rat have been previously described [20]. The rats were maintained on a 12-h light-dark cycle (lights on at 6 AM) and ad libitum access to food and water, except during the exposure and testing periods. WT (N=56; 29 males, 27 females) and Tg (N=52; 34 males, 18 females) F344 rats were exposed to either smoke from regular cigarettes (CIG) (3R4F cigarettes; KTRDC Tobacco Biotechnology Group), which contain 0.7 mg nicotine/cigarette; nicotine-free cigarettes (NF) (Quest 3; Vector Tobacco), which contain 0.05 mg (trace amounts) of nicotine per cigarette; 0.5 mg/kg subcutaneous nicotine (NIC) (nicotine tartrate; Sigma), or, as a control (CON), 0.5 ml subcutaneous saline, as previously described [12]. The nicotine dose utilized has been found to result in levels of the major nicotine metabolite cotinine, which an accepted biomarker for CS exposure, that are similar to that which results from exposure to four cigarettes/day, the number of cigarettes used in our CS exposure protocol (see below) [21]. The number of animals by genotype and biological sex in each exposure group were as follows: CON: 12 WT (5 females, 7 males) and 12 Tg (3 females, 9 males); NIC: 12 WT (3 females, 9 males) and 12 Tg (3 females, 9 males); NF: 8 WT (4 females, 4 males) and 12 Tg (5 females, 7 males); and CIG: 12 WT (5 females, 7 males) and 12 Tg (5 females, 7 males). The ages of the animals were, unfortunately, available for only about 10% of the cohort.
CS-exposed rats were placed in customized plexiglass chambers into which was pumped smoke from two cigarettes per day for six weeks, five days per week with no exposure over weekends [21]. The animals were restrained in the chambers while under constant observation for evidence of distress. The machine puff rate was set so that it required 8–10 min to smoke each cigarette. NIC group rats were treated with once daily subcutaneous injection of 0.5 mg/kg/day of NIC dissolved in sterile 0.9% NaCl. The CON group rats were not exposed to CS or NIC.
2.2. Open Field test
The OFT examines general motor activity as well the willingness of the animal to explore [22]. The testing was performed in a circular open plastic arena with the dimensions 140 centimeters (cm) diameter x 66 cm height. Two trials were performed daily for five days with five minutes allotted between each trial. Two rats were tested simultaneously in two physically separate arenas. During the test, the rats moved freely for 15 minutes with the chambers illuminated at ~35 lux. The test measures were recorded and analyzed with the Top Scan system (Cleversys). The primary outcomes were total distance travelled, a measure of the overall motor activity, the fraction of the total distance traveled in the center field (the center field distance fraction), and the total time spent in the center field (the center field time). Unlike in our previous study, the center field area comprised 25% of the total area, The center field distance fraction was calculated as follows: distance traveled in the center of the open field ÷ the total distance traveled in the field. A decrease in the center field distance fraction and center field time measures indicates an increase in anxiety levels for the animals [22].
2.3. Statistical analyses
Multiple t-tests were performed to compare open field test performance by genotype and sex, both individually and in combination with correction for multiple comparisons using the Holm-Šídák method. Comparisons were first performed for WT versus Tg rats and for female versus male animals overall and then for both genotype and sex. To avoid introducing confounding effects from the different routes of administration that were utilized, the exposure groups were analyzed separately, and no comparisons were performed across exposure groups. A cutoff of p<0.05 was used for determining statistical significance. Since ages were known for only a small number of animals, the analyses could not be adjusted for this variable. The analyses were performed using GraphPad Prism 8 for Windows (GraphPad Software, Inc).
3. RESULTS
3.1. Total distance
Analysis of the total distance for WT versus Tg animals showed no statistically significant differences for control group animals or for those exposed to either subcutaneous nicotine or to smoke from regular cigarettes (figure 1). In contrast, for animals exposed to smoke from nicotine-free cigarettes, the measures were higher for Tg than for WT rats (p=0.036). Analyses performed to identify possible associations between the sex of the animals and performance on the testing showed that, overall, females in the control, nicotine alone, and nicotine-free CS exposure groups had higher levels than for males on those groups (p=0.014, p=0.04 and p=0.027, respectively) (figure 1). There was no difference in the measures for female and male animals exposed to nicotine-containing CS.
Figure 1:
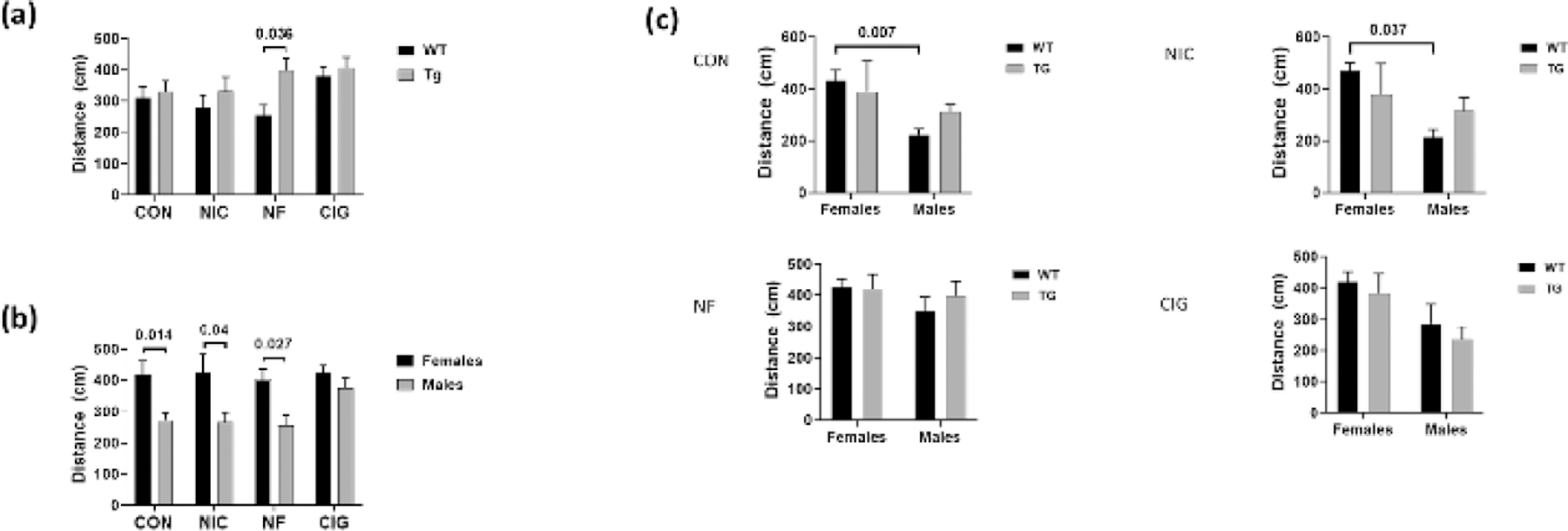
Comparison of total distance by (a) genotype, (b) sex and (c) by both genotype and sex for wild-type WT and HIV-1 transgenic (Tg) rats in the four exposure groups. For rats exposed to NF group, Tg rats showed higher total distance measurements than Tg animals (a), and levels for females in the CON, NIC, and NF groups were higher than for males. Comparison of the total distance measures by both genotype and sex showed higher measures for WT females than for WT males in the CON group and in the NF groups. CON = control; NIC = subcutaneous nicotine; NF = nicotine-free cigarette smoke; CIG = regular cigarette smoke.
For the comparisons of animals grouped by both genotype and sex, levels for WT females in the control group and in the group exposed to nicotine alone were higher than for WT males in those groups (p=0.007 and p=0.037, respectively) (figure 1). No other differences were found with comparing total distance levels by genotype and sex in these groups or in the nicotine-free CS or to nicotine-containing CS exposure groups.
3.2. Center field distance fraction
On the center field distance fraction measures, there were no differences noted between WT and Tg rats in the different exposure groups. However, analysis of the overall effects of sex showed that measures were higher for females as compared to males in the control group (p=0.01) and in animals exposed to nicotine alone (p=0.009) (figure 2). No differences were noted for female versus male animals exposed to either nicotine-free or nicotine-containing CS.
Figure 2:
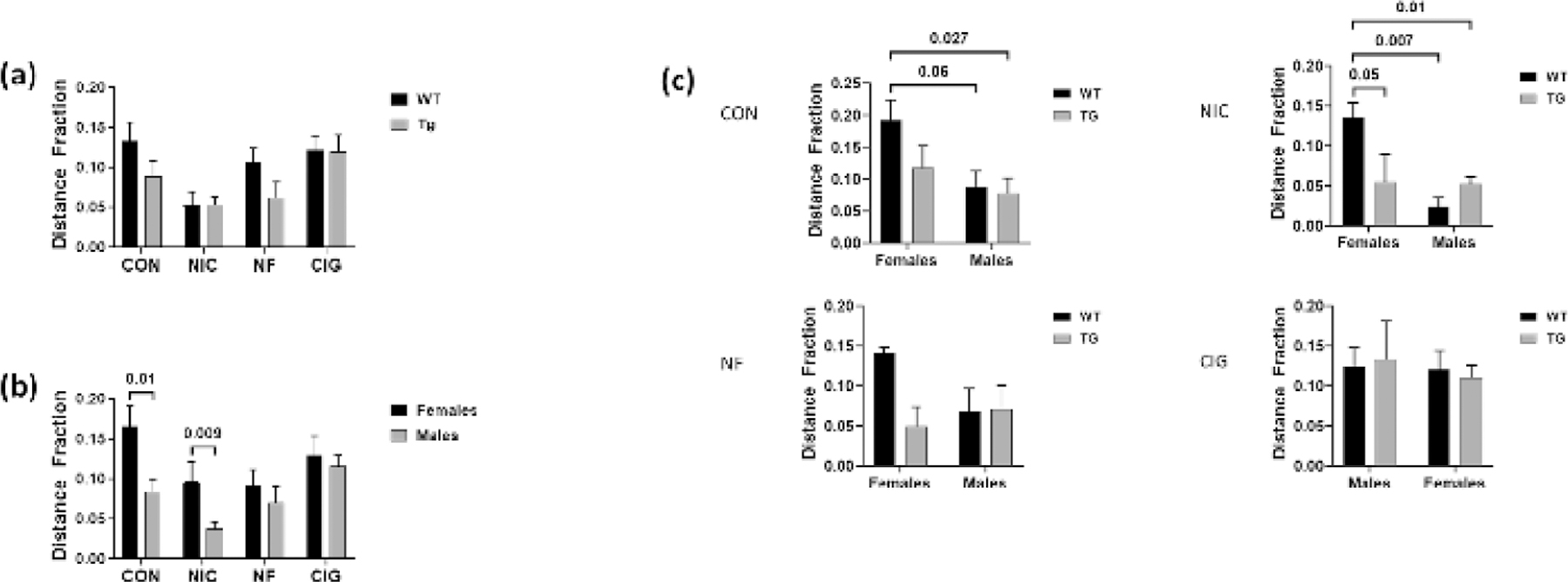
Comparison of small center distance fraction measures by (a) genotype, (b) sex and (C) by both genotype and sex for wild-type WT and HIV-1 transgenic (Tg) rats in the four exposure groups. There were no overall differences in the measures by genotype (a). For females in the CON and NIC groups, the measures were higher for females than for males. Comparison of the measures by both genotype and sex showed higher measures for WT females than for Tg males in the CON group and higher measures for WT females than for WT and Tg males in the NF group. Borderline statistically significant higher levels were found for WT females as compared to WT males in the CON group and for WT females as compared to Tg females in the NF group. CON = control; NIC = subcutaneous nicotine; NF = nicotine-free cigarette smoke; CIG = regular cigarette smoke.
Analysis for differences in center field distance fraction by both genotype and sex showed that, for the control group, levels were significantly higher for WT females than for Tg males (p=0.027). Levels were also higher for control group WT females than for WT males, but this difference was of borderline statistical significance (p=0.06). Also, for animals in the subcutaneous nicotine group, measures for WT females were significantly higher than for both WT and Tg males (p=0.007 and p=0.01, respectively). Levels were also higher for WT females than for Tg females, but this difference was of borderline statistical significance (p=0.05). No genotype-sex differences were noted for the animals in the nicotine-free CS or the regular CS groups.
3.3. Center field time
Comparisons of center field time measures for WT and Tg rats showed similar results by genotype irrespective of the exposure group (figure 3). Analyses by sex showed that times for WT female rats in the control group and in the group exposed to nicotine alone were higher than for males (p=0.002 and p=0.0038, respectively). In contrast, measures for male and female rats were similar for animals in the groups exposed to nicotine-free and nicotine-containing CS.
Figure 3:
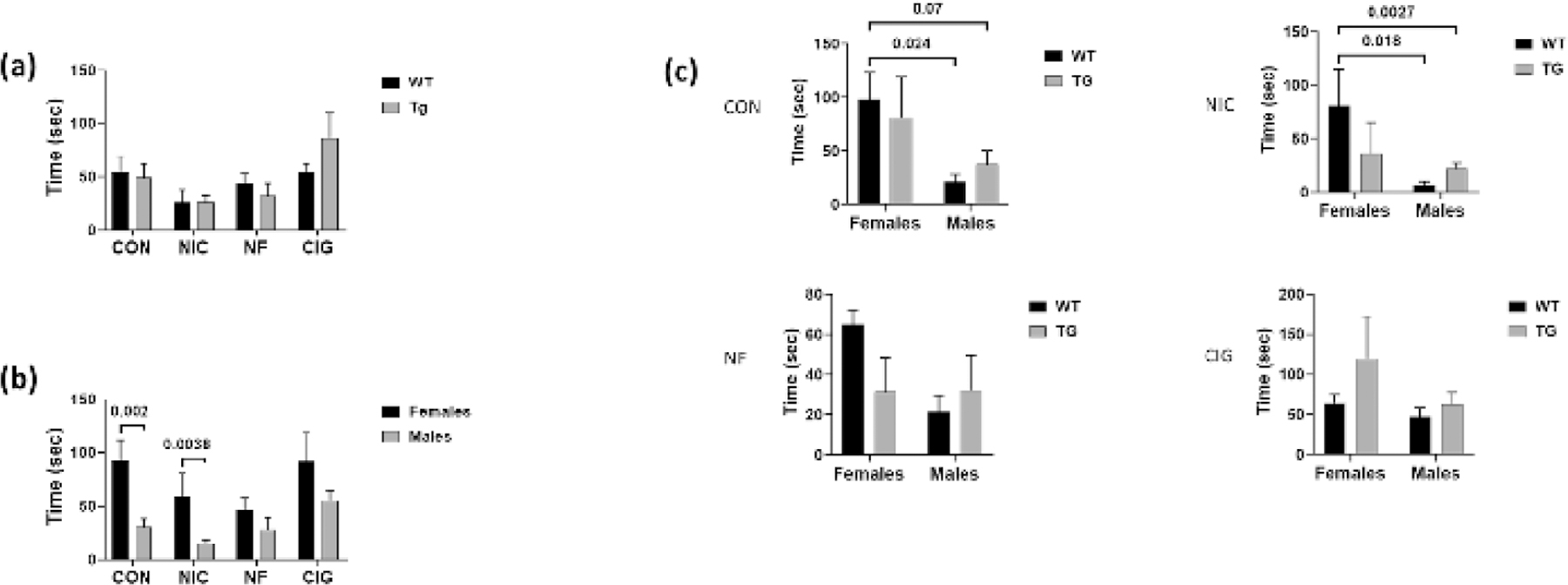
Comparison of small center time measures by (a) genotype, (b) sex and (c) by both genotype and sex for wild-type WT and HIV-1 transgenic (Tg) rats in the four exposure groups. There were no overall differences in the measures by genotype (a). For females in the CON and NIC groups, the measures were higher females than for males. Comparison of the small center time measures by both genotype and sex showed higher measures for WT females than for WT males in the CON group and higher measures for WT females than for WT and Tg males in the NF group. A borderline statistically higher level was found for WT females as compared to Tg males in the CON group. CON = control; NIC = subcutaneous nicotine; NF = nicotine-free cigarette smoke; CIG = regular cigarette smoke.
When the center field time measures were analyzed by both genotype and sex, it was found that, for animals in the control group, measures were higher for WT female than for WT male rats (p=0.024). Center field times were also higher for WT females than for Tg males but this difference was of borderline statistical significance (p=0.07). In addition, for animals in the subcutaneous nicotine-exposed group, the times for WT females were higher than for both WT male (p=0.018) and Tg male (p=0.0027) animals. There were no differences in center field distance times for male and female WT and Tg rats exposed to nicotine-free or nicotine-containing CS.
4. Discussion
The findings from these studies support our hypothesis that both genotypic and sex differences can be associated with significant differential behavioral effects in WT and HIV Tg rats subjected to the various exposures. These genotype and sex effects were observed to occur either independent of the other or concurrently in the animals. In contrast to the findings that we report in this study, in a previous study where the center field area comprised 50% of the total open field area, we found no effects of genotype on open field testing [12]. For this report, it is possible that the smaller center field area that was utilized resulted in more sensitive detection of the effects of genotype, sex, and the exposures on levels of anxiety. Also in addition to analyzing the total time spent in the center of the open field, for this report we added an analysis of the total distance traveled, a measure of locomotor effects from the factors, as well as the fraction of the total distance traveled in the center field.
For the overall effects of genotype on motor activity, measures were found to be higher for Tg than for WT animals in the NF CS group. In contrast, overall effects of sex resulted in higher measures for females in the control, the subcutaneous nicotine, and nicotine-free CS exposure groups as compared to males in those groups. Comparisons of the data when the data were grouped by both genotype and sex factors showed that the higher motor activity that was observed in female animals occurred in WT animals and only in control and subcutaneous nicotine-exposed animals. These findings suggest that both the presence of the HIV transgene and exposure to the non-nicotine components of cigarette smoke, particularly in males, may be associated with greater locomotor activity in Tg and in male animals.
Higher center field measures indicate lower levels of anxiety for tested animals. Analysis of the fraction of the total distance traveled in the center area and the time the animals spent in the center of the field showed no overall effect of genotype. However, for both measures, females in the control group and in the subcutaneous nicotine-exposed group were overall less anxious than males, with no difference noted in the CS-exposed groups. For the comparisons performed by both genotype and sex, WT females in the control group were borderline less anxious than Tg females in that group on center field distance fraction measure, whereas, for both the center field distance and time measures, WT female animals in the control and subcutaneous nicotine-groups were less anxious than both WT and Tg males. Again, no differences between groups were observed for animals in the CS-exposed groups. Therefore, as observed for locomotor activity, differences between male and female animals were not observed in the presences of HIV genes or when the animals were exposed to either nicotine-containing or nicotine-free CS. In addition, the presence of the HIV transgene was associated with slightly anxiogenic effects in female rats exposed to nicotine alone.
It has been well documented in previously published studies performed in rats that female animals normally show baseline motor activity that is greater than for males [23–25]. At adulthood (2–3 months of age) this difference has been attributed to effects of estrogen [26, 27]. We observed the same differences for male and female animals in our studies. In humans, however, males demonstrate higher levels of motor activity than females [28]. Previous studies have also demonstrated that female rats show evidence of lower anxiety states on the open field test [25, 29], an effect also attributed to estrogen [30] and opposite to what has been found for men versus women, with anxiety disorders occurring about twice as often among women [28, 31, 32]. However, to our knowledge, studies have not been performed that compared premorbid anxiety states for men and women who are subsequently diagnosed with anxiety disorders, which would be more analogous to our study design. In humans, anxiety-inducing effects from nicotine have been observed primarily in men, whereas, in women, nicotine can suppress stress responses [33]. Women are also more likely than men to smoke as a means of coping with stress [16], and it is more difficult for women to quit smoking than for men [17, 34]. This difference could explain why in the study of HIV-infected women reported by Wojna et al, smokers scored better on the neuropsychological testing than the non-smokers. In human studies it has been found that the non-nicotine components of CS can have nicotine-like effects [35, 36]. Notably, women have been found to develop an abstinence syndrome that is worse than for men with discontinuing smoking cigarettes made from this denicotinized tobacco [15]. It is not known whether HIV-infected persons are more sensitive to nicotine effects than seronegative individuals. However, if this is the case it would be consistent with the observed higher smoking and lower smoking cessation rates that have been documented among individuals with HIV-1 infection [6].
The mechanisms that underlie our findings require further study. Of particular interest is to understand the possible role of hormonal effects on our findings. Valle et al, using rats ranging in age from 28 to 46 days, found greater locomotor activity and thigmotaxis among female animals. However, there was no association between motor activity and the pubertal state, although post-puberty was associated with increased body weight [37] which has been associated with increased anxiety-related behavior on open field testing [38]. Ovariectomized rats, on the other hand, may show clear evidence of impairment on open field testing that can be reversed by treatment with estrogen [39, 40], but this may not be the finding in all such studies using ovariectomized animals [41]. In the context of HIV infection, HIV-infected women can develop ovarian failure and an estrogen-deficient state [42]. Conversely, in men, HIV infection can cause decreased levels of testosterone due to either testicular failure or impairment of the hypothalamic-pituitary axis [43, 44]. In hypogonadal individuals, estrogen may be produced in extragonadal tissue by conversion of testosterone which enters from blood to estradiol by the enzyme aromatase. In humans and rodents, aromatase is present in the brain, and at levels that are higher for males than for females [45, 46]. In addition, studies performed in vivo and in vitro show neuroprotective properties for estrogen in HIV infection [47, 48], and the administration of a phytoestrogen was able to mitigate sustained attention deficits that could be demonstrated in HIV Tg rats [48].
It is also possible that age may impact performance on the open field testing, and this question has been addressed in previously published studies. For example, using 6, 12, and 18 month old F344 Fisher rats, Febo et al found an age effect for the total distance traveled with decreased distances observed for older and only male animals [49]. Age and sex, however, were not associated with the amount of time spent in the central regions of the field. Other studies using Sprague-Dawley rats to investigate the effects of prenatal choline supplementation on exploratory behavior and hippocampal plasticity in adulthood, found that, for untreated animals, 24 month old rats overall had a higher latency to enter the center of an open field and spent less time exploring the field center than 1 month old rats [50]. In these studies, the observed differences were among specifically female animals. Male animals in both age groups spent little time in the field center. In two studies utilizing Long Evans hooded rats, one showed that, for 90 day old animals there were worse times on multiple open field test measures, including time in the arena, number of arena visits, and mean visit length, than 30 and 60 day old animals and no main effect of sex [51]. However, the other showed that younger animals and female rats had higher total distance measures and spent less time in the open field center, whereas younger and female animals spent less time in the center field [52]. Finally, Lister hooded rats, a more docile strain of rat, that were up to 109 days of age showed a higher total the total distance moved in an open field among older versus younger animals, increased total locomotion for females, and no age or sex effects on the amount of time spent in the center [51]. These studies demonstrate that locomotor and behavioral function can be variably associated with the age of the animals and more consistently with the sex of the animals. Possible reasons for observed variability in the impact of these factors include differences that may exist in the testing environment and those that may be associated with the experimenter [53, 54].
It would be expected that the effects of genotype and sex that we observed would have been impacted by the expression of acetyl choline receptors (nAChR) in the male and female animals. In studies by Koylu et al of nAChR binding in brain from Sprague Dawley rats injected with nicotine or saline, it was found that, in saline-treated animals, whole brain binding was borderline higher in females than in males [55]. Following chronic exposure to 0.6 mg/kg/day of subcutaneous nicotine, receptor levels were higher in male than females. However, after prolonged discontinuation of the nicotine injections, the female rats were found to have significantly higher levels than the male rats. In studies performed in humans, β2 subunit nAChR levels in thalamus were higher for women than for men non-smokers with no differences in receptor levels for women as compared to men in other brain region [56]. Among individuals with HIV-1 infection, monocyte/macrophage expression of nAChR α7 subunit is increased in association with disruption of anti-inflammatory effects that result from activation of the receptors [57]. In studies performed in HIV-1 transgenic rats, specific regions of brain showed either an increase or decrease in expression of the various nAChR subtypes [58].
Despite the inconsistent effect of age found in other studies, that we were not able to factor the ages of the animals into the analyses we performed is a significant limitation. Also, the analyses were not adjusted for the weights of the animals, which may be altered by the nicotine and cigarette smoke exposures, or for levels of the nicotine metabolite cotinine, which serves as reliable of biomarker of nicotine and nicotine-containing tobacco exposure [59]. These weaknesses, as well as the identified unanswered questions, can be addressed in future studies. Also, we believe that these shortcomings are counterbalanced by the insight related to the effects of smoking and nicotine in persons with and without HIV infection that can be derived from our findings.
Highlights:
Female rats showed overall higher levels of motor activity and lower anxiety levels than males.
Differences in motor activity and anxiety levels observed between female and male animals were decreased by either the presence of the HIV transgene or by exposure to the non-nicotine components of cigarette smoke.
In female animals, the presence of the HIV transgene and exposure nicotine alone was mildly anxiogenic.
Acknowledgments
Supported by: R01 DA044908 (NIH/NIDA; W.R.); 1I01BX003222 (US Department of Veterans Affairs; WR)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
- Walter Royal III, MD has no competing interests with this manuscript
- Joseph Bryant, DVM has no competing interests with this manuscript
- Harry Davis, BA has no competing interests with this manuscript
- Ming Guo, MS has no competing interests with this manuscript
Reference
- [1].Heaton RK, Franklin DR Jr., Deutsch R, Letendre S, Ellis RJ, Casaletto K, Marquine MJ, Woods SP, Vaida F, Atkinson JH, Marcotte TD, McCutchan JA, Collier AC, Marra CM, Clifford DB, Gelman BB, Sacktor N, Morgello S, Simpson DM, Abramson I, Gamst AC, Fennema-Notestine C, Smith DM, Grant I, Group C, Neurocognitive change in the era of HIV combination antiretroviral therapy: the longitudinal CHARTER study, Clin Infect Dis 60(3) (2015) 473–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Chana G, Everall IP, Crews L, Langford D, Adame A, Grant I, Cherner M, Lazzaretto D, Heaton R, Ellis R, Masliah E, Cognitive deficits and degeneration of interneurons in HIV+ methamphetamine users, Neurology. 67(8) (2006) 1486–1489. [DOI] [PubMed] [Google Scholar]
- [3].Chang L, Wang GJ, Volkow ND, Ernst T, Telang F, Logan J, Fowler JS, Decreased brain dopamine transporters are related to cognitive deficits in HIV patients with or without cocaine abuse, Neuroimage. 42(2) (2008) 869–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Lifson AR, Lando HA, Smoking and HIV: prevalence, health risks, and cessation strategies, Curr HIV/AIDS Rep 9(3) (2012) 223–230. [DOI] [PubMed] [Google Scholar]
- [5].CDC, Vital signs: current cigarette smoking among adults aged >/=18 years--United States, 2005–2010, MMWR Morb.Mortal.Wkly.Rep 60(35) (2011) 1207–1212. [PubMed] [Google Scholar]
- [6].Frazier EL, Sutton MY, Brooks JT, Shouse RL, Weiser J, Trends in cigarette smoking among adults with HIV compared with the general adult population, United States - 2009–2014, Prev Med 111 (2018) 231–234. [DOI] [PubMed] [Google Scholar]
- [7].Galai N, Park LP, Wesch J, Visscher B, Riddler S, Margolick JB, Effect of smoking on the clinical progression of HIV-1 infection, J Acquir.Immune.Defic.Syndr.Hum.Retrovirol 14(5) (1997) 451–458. [DOI] [PubMed] [Google Scholar]
- [8].Nieman RB, Fleming J, Coker RJ, Harris JR, Mitchell DM, The effect of cigarette smoking on the development of AIDS in HIV-1-seropositive individuals, AIDS 7(5) (1993) 705–710. [DOI] [PubMed] [Google Scholar]
- [9].Harrison JD, Dochney JA, Blazekovic S, Leone F, Metzger D, Frank I, Gross R, Hole A, Mounzer K, Siegel S, Schnoll RA, Ashare RL, The nature and consequences of cognitive deficits among tobacco smokers with HIV: a comparison to tobacco smokers without HIV, J Neurovirol (2017). [DOI] [PMC free article] [PubMed]
- [10].Liang H, Chang L, Chen R, Oishi K, Ernst T, Independent and Combined Effects of Chronic HIV-Infection and Tobacco Smoking on Brain Microstructure, J Neuroimmune Pharmacol 13(4) (2018) 509–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Wojna V, Robles L, Skolasky RL, Mayo R, Selnes O, de la Torre T, Maldonado E, Nath A, Melendez LM, Lasalde-Dominicci J, Associations of cigarette smoking with viral immune and cognitive function in human immunodeficiency virus-seropositive women, J Neurovirol 13(6) (2007) 561–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Royal W 3rd, Can A, Gould TD, Guo M, Huse J, Jackson M, Davis H, Bryant J, Cigarette smoke and nicotine effects on brain proinflammatory responses and behavioral and motor function in HIV-1 transgenic rats, J Neurovirol 24(2) (2018) 246–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Royal W III, Cherner M, Burdo TH, Umlauf A, Letendre SL, Jumare J, Abimiku A, Alabi P, Alkali N, Bwala S, Okwuasaba K, Eyzaguirre LM, Akolo C, Guo M, Williams KC, Blattner WA, Associations between Cognition, Gender and Monocyte Activation among HIV Infected Individuals in Nigeria, PLoS One 11(2) (2016) e0147182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Maki PM, Rubin LH, Springer G, Seaberg EC, Sacktor N, Miller EN, Valcour V, Young MA, Becker JT, Martin EM, Neuropsychology HIVS Working Groups of the Women’s Interagency, A.C.S. the Multicenter, Differences in Cognitive Function Between Women and Men With HIV, J Acquir Immune Defic Syndr 79(1) (2018) 101–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Barrett SP, The effects of nicotine, denicotinized tobacco, and nicotine-containing tobacco on cigarette craving, withdrawal, and self-administration in male and female smokers, Behav Pharmacol 21(2) (2010) 144–52. [DOI] [PubMed] [Google Scholar]
- [16].Torres OV, O’Dell LE, Stress is a principal factor that promotes tobacco use in females, Prog Neuropsychopharmacol Biol Psychiatry 65 (2016) 260–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Perkins KA, Gerlach D, Vender J, Grobe J, Meeker J, Hutchison S, Sex differences in the subjective and reinforcing effects of visual and olfactory cigarette smoke stimuli, Nicotine Tob Res 3(2) (2001) 141–50. [DOI] [PubMed] [Google Scholar]
- [18].al’Absi M, Nakajima M, Allen S, Lemieux A, Hatsukami D, Sex differences in hormonal responses to stress and smoking relapse: a prospective examination, Nicotine Tob Res 17(4) (2015) 382–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Saladin ME, Gray KM, Carpenter MJ, LaRowe SD, DeSantis SM, Upadhyaya HP, Gender differences in craving and cue reactivity to smoking and negative affect/stress cues, Am J Addict 21(3) (2012) 210–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Reid W, Sadowska M, Denaro F, Rao S, Foulke J Jr., Hayes N, Jones O, Doodnauth D, Davis H, Sill A, O’Driscoll P, Huso D, Fouts T, Lewis G, Hill M, Kamin-Lewis R, Wei C, Ray P, Gallo RC, Reitz M, Bryant J, An HIV-1 transgenic rat that develops HIV-related pathology and immunologic dysfunction, Proc.Natl.Acad.Sci.U.S.A 98(16) (2001) 9271–9276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Khanna AK, Xu J, Uber PA, Burke AP, Baquet C, Mehra MR, Tobacco smoke exposure in either the donor or recipient before transplantation accelerates cardiac allograft rejection, vascular inflammation, and graft loss, Circulation 120(18) (2009) 1814–1821. [DOI] [PubMed] [Google Scholar]
- [22].Gould TJ, Dao D, Kovacsics CE, The Open Field Test, in: Gould TJ (Ed.), Mood and Anxiety Related Phenotypes in Mice, Humana Press, Totowa, NJ, 2009, pp. 1–20. [Google Scholar]
- [23].Fernandes C, Gonzalez MI, Wilson CA, File SE, Factor analysis shows that female rat behaviour is characterized primarily by activity, male rats are driven by sex and anxiety, Pharmacol Biochem Behav 64(4) (1999) 731–8. [DOI] [PubMed] [Google Scholar]
- [24].Tropp J, Markus EJ, Sex differences in the dynamics of cue utilization and exploratory behavior, Behav Brain Res 119(2) (2001) 143–54. [DOI] [PubMed] [Google Scholar]
- [25].Archer J, Rodent sex differences in emotional and related behavior, Behav Biol 14(4) (1975) 451–79. [DOI] [PubMed] [Google Scholar]
- [26].Domonkos E, Hodosy J, Ostatnikova D, Celec P, On the Role of Testosterone in Anxiety-Like Behavior Across Life in Experimental Rodents, Front Endocrinol (Lausanne) 9 (2018) 441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Scholl JL, Afzal A, Fox LC, Watt MJ, Forster GL, Sex differences in anxiety-like behaviors in rats, Physiol Behav 211 (2019) 112670. [DOI] [PubMed] [Google Scholar]
- [28].Eliot L, Richardson SS, Sex in Context: Limitations of Animal Studies for Addressing Human Sex/Gender Neurobehavioral Health Disparities, J Neurosci 36(47) (2016) 11823–11830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Kokras N, Dalla C, Sex differences in animal models of psychiatric disorders, Br J Pharmacol 171(20) (2014) 4595–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Pellman BA, Schuessler BP, Tellakat M, Kim JJ, Sexually Dimorphic Risk Mitigation Strategies in Rats, eNeuro 4(1) (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE, Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication, Arch Gen Psychiatry 62(6) (2005) 593–602. [DOI] [PubMed] [Google Scholar]
- [32].McLean CP, Asnaani A, Litz BT, Hofmann SG, Gender differences in anxiety disorders: prevalence, course of illness, comorbidity and burden of illness, J Psychiatr Res 45(8) (2011) 1027–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Flucke U, Tops BB, de Saint Aubain Somerhausen N, Bras J, Creytens DH, Kusters B, Groenen PJ, Verdijk MA, Suurmeijer AJ, Mentzel T, Presence of C11orf95-MKL2 fusion is a consistent finding in chondroid lipomas: a study of eight cases, Histopathology 62(6) (2013) 925–30. [DOI] [PubMed] [Google Scholar]
- [34].Piper ME, Cook JW, Schlam TR, Jorenby DE, Smith SS, Bolt DM, Loh WY, Gender, race, and education differences in abstinence rates among participants in two randomized smoking cessation trials, Nicotine Tob Res 12(6) (2010) 647–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Rose JE, Nicotine and nonnicotine factors in cigarette addiction, Psychopharmacology (Berl) 184(3–4) (2006) 274–85. [DOI] [PubMed] [Google Scholar]
- [36].Johnson MW, Bickel WK, Kirshenbaum AP, Substitutes for tobacco smoking: a behavioral economic analysis of nicotine gum, denicotinized cigarettes, and nicotine-containing cigarettes, Drug Alcohol Depend 74(3) (2004) 253–64. [DOI] [PubMed] [Google Scholar]
- [37].Valle FP, Gorzalka B, Open-field sex differences prior to puberty in rats, Bulletin of the Psychonomic Society 16(6) (1980) 429–432. [Google Scholar]
- [38].Alonso-Caraballo Y, Hodgson KJ, Morgan SA, Ferrario CR, Vollbrecht PJ, Enhanced anxiety-like behavior emerges with weight gain in male and female obesity-susceptible rats, Behav Brain Res 360 (2019) 81–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Renczes E, Borbelyova V, Steinhardt M, Hopfner T, Stehle T, Ostatnikova D, Celec P, The Role of Estrogen in Anxiety-Like Behavior and Memory of Middle-Aged Female Rats, Front Endocrinol (Lausanne) 11 (2020) 570560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Djiogue S, Djiyou Djeuda AB, Seke Etet PF, Ketcha Wanda GJM, Djikem Tadah RN, Njamen D, Memory and exploratory behavior impairment in ovariectomized Wistar rats, Behav Brain Funct 14(1) (2018) 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Gogos A, McCarthy M, Walker AJ, Udawela M, Gibbons A, Dean B, Kusljic S, Differential effects of chronic 17beta-oestradiol treatment on rat behaviours relevant to depression, J Neuroendocrinol 30(11) (2018) e12652. [DOI] [PubMed] [Google Scholar]
- [42].Karim R, Mack WJ, Kono N, Tien PC, Anastos K, Lazar J, Young M, Cohen M, Golub E, Greenblatt RM, Kaplan RC, Hodis HN, Gonadotropin and sex steroid levels in HIV-infected premenopausal women and their association with subclinical atherosclerosis in HIV-infected and -uninfected women in the women’s interagency HIV study (WIHS), J Clin Endocrinol Metab 98(4) (2013) E610–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Arver S, Sinha-Hikim I, Beall G, Guerrero M, Shen R, Bhasin S, Serum dihydrotestosterone and testosterone concentrations in human immunodeficiency virus-infected men with and without weight loss, J Androl 20(5) (1999) 611–8. [PubMed] [Google Scholar]
- [44].Rochira V, Guaraldi G, Hypogonadism in the HIV-infected man, Endocrinol Metab Clin North Am 43(3) (2014) 709–30. [DOI] [PubMed] [Google Scholar]
- [45].Shay DA, Vieira-Potter VJ, Rosenfeld CS, Sexually Dimorphic Effects of Aromatase on Neurobehavioral Responses, Front Mol Neurosci 11 (2018) 374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].McTernan PG, Anwar A, Eggo MC, Barnett AH, Stewart PM, Kumar S, Gender differences in the regulation of P450 aromatase expression and activity in human adipose tissue, Int J Obes Relat Metab Disord 24(7) (2000) 875–81. [DOI] [PubMed] [Google Scholar]
- [47].Zemlyak I, Brooke SM, Sapolsky RM, Protection against gp120-induced neurotoxicity by an array of estrogenic steroids, Brain Res 958(2) (2002) 272–6. [DOI] [PubMed] [Google Scholar]
- [48].Moran LM, McLaurin KA, Booze RM, Mactutus CF, Neurorestoration of Sustained Attention in a Model of HIV-1 Associated Neurocognitive Disorders, Front Behav Neurosci 13 (2019) 169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Febo M, Rani A, Yegla B, Barter J, Kumar A, Wolff CA, Esser K, Foster TC, Longitudinal Characterization and Biomarkers of Age and Sex Differences in the Decline of Spatial Memory, Front Aging Neurosci 12 (2020) 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Glenn MJ, Kirby ED, Gibson EM, Wong-Goodrich SJ, Mellott TJ, Blusztajn JK, Williams CL, Age-related declines in exploratory behavior and markers of hippocampal plasticity are attenuated by prenatal choline supplementation in rats, Brain Res 1237 (2008) 110–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Lynn DA, Brown GR, The ontogeny of anxiety-like behavior in rats from adolescence to adulthood, Dev Psychobiol 52(8) (2010) 731–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Lipska BK, Weinberger DR, Genetic variation in vulnerability to the behavioral effects of neonatal hippocampal damage in rats, Proc Natl Acad Sci U S A 92(19) (1995) 8906–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Chesler EJ, Wilson SG, Lariviere WR, Rodriguez-Zas SL, Mogil JS, Influences of laboratory environment on behavior, Nat Neurosci 5(11) (2002) 1101–2. [DOI] [PubMed] [Google Scholar]
- [54].Chesler EJ, Wilson SG, Lariviere WR, Rodriguez-Zas SL, Mogil JS, Identification and ranking of genetic and laboratory environment factors influencing a behavioral trait, thermal nociception, via computational analysis of a large data archive, Neurosci Biobehav Rev 26(8) (2002) 907–23. [DOI] [PubMed] [Google Scholar]
- [55].Koylu E, Demirgoren S, London ED, Pogun S, Sex difference in up-regulation of nicotinic acetylcholine receptors in rat brain, Life Sci 61(12) (1997) PL 185–90. [DOI] [PubMed] [Google Scholar]
- [56].Cosgrove KP, Esterlis I, McKee SA, Bois F, Seibyl JP, Mazure CM, Krishnan-Sarin S, Staley JK, Picciotto MR, O’Malley SS, Sex differences in availability of beta2*-nicotinic acetylcholine receptors in recently abstinent tobacco smokers, Arch Gen Psychiatry 69(4) (2012) 418–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Delgado-Velez M, Baez-Pagan CA, Gerena Y, Quesada O, Santiago-Perez LI, Capo-Velez CM, Wojna V, Melendez L, Leon-Rivera R, Silva W, Lasalde-Dominicci JA, The alpha7-nicotinic receptor is upregulated in immune cells from HIV-seropositive women: consequences to the cholinergic anti-inflammatory response, Clin Transl.Immunology 4(12) (2015) e53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Cao J, Nesil T, Wang S, Chang SL, Li MD, Expression profile of nicotinic acetylcholine receptor subunits in the brain of HIV-1 transgenic rats given chronic nicotine treatment, J Neurovirol 22(5) (2016) 626–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Benowitz NL, The use of biologic fluid samples in assessing tobacco smoke consumption, NIDA Res Monogr 48 (1983) 6–26. [PubMed] [Google Scholar]


