Abstract
Lupus nephritis (LN) is a major cause for overall morbidity and mortality in patients with systemic lupus erythematosus (SLE), while its pathogenic mechanisms are still not well understood. Extracellular vesicles (EVs) are membrane vesicles that are released from almost all cell types. EVs can be subdivided into exosomes, microvesicles, and apoptotic bodies. Latest studies have shown that EVs can be released during several cellular events, including cell activation, autophagy, and several types of programed cell death, i.e. apoptosis, necroptosis, pyroptosis, and NETosis. Emerging evidence demonstrates that EVs harbor different bioactive molecules, including nucleic acids, proteins, lipids, cytokines, immune complexes (ICs), complements, and other molecules, some of which may contribute to pathogenesis of autoimmune diseases. EVs can serve as novel information shuttle to mediate local autocrine or paracrine signals to nearby cells, and distant endocrine signals to cells located far away. In LN, EVs may have pathogenic effects by transportation of autoantigens or complements, promotion of IC deposition or complement activation, and stimulation of inflammatory responses, renal tissue injury, or microthrombus formation. Additionally, EVs released from kidney cells may serve as specific biomarkers for diagnosis or monitoring of disease activity and therapeutic efficacy. In this review, we will summarize the latest progress about EV generation from basic research, their potential pathologic effects on LN, and their clinical implications. The cutting-edge knowledge about EV research provides insights into novel therapeutic strategy, new tools for diagnosis or prognosis, and evaluation approaches for treatment effectiveness in LN.
Keywords: extracellular vesicles, autoantigen, immune complex, inflammation, lupus nephritis
1. Introduction
Systemic lupus erythematosus (SLE) is a systemic autoimmune disease characterized by presence of autoantibodies, immune complexes (ICs) and complement deposition, and the relevant autoimmune inflammation in different organs/tissues, including kidney [1]. Lupus nephritis (LN) affects 30–60% of adults and up to 70% of children with SLE [2]. LN results in gradual decline of kidney function and renal failure and is a major cause of morbidity and mortality in SLE patients.
Extracellular vesicles (EVs) are a heterogeneous group of membrane vesicles released from cells to extracellular space. Almost all mammalian cell types, even lower eukaryotes, prokaryotes, and plant cells can release EVs. This fact indicates that EV-mediated cell signaling might be essential mechanisms for intercellular communication that emerged in early biological evolution of all living organisms [3]. EVs have been found from nearly all kinds of body fluids and solid organs/tissues, and involved in not only normal physiological events [4, 5], such as immune surveillance, cell-to-cell communication, inflammation and blood coagulation, but also abnormal pathological conditions in various human diseases [6, 7], including autoimmune and cardio-metabolic diseases, as well as cancer development and metastasis.
Cell death is a natural biological process that occurs under both physiological and pathological conditions. To date, over ten types of programmed cell death have been identified [8]. Apoptosis is the most studied type of programmed cell death. Both exosomes and membrane microvesicles can be released in cells undergoing apoptosis, apoptotic bodies are the corpse of apoptotic cells that are formed in the end stage of apoptosis [9]. Recent studies demonstrated that several other types of programmed cell death, i.e. necroptosis, pyroptosis, and NETosis can also cause release of membrane microvesicles [10–14]. Cell death is critical for maintaining homeostasis. Excessive cell death and/or defective clearance of dead cells and their released EVs may break immune tolerance and trigger immune and autoimmune responses in the body [15, 16].
Recent evidence indicates the involvement of EVs in pathogenesis and clinical complications of SLE and LN [17]. Autoantigens generated during apoptosis are clustered, and redistributed into the membrane surface of EVs or apoptotic bodies [18]. The apoptotic EV-associated autoantigens may trigger B cells for adaptive immune response in SLE [19]. EV-associated autoantigens form immune complexes (ICs) with autoantibodies, resulting in formation of EV-ICs [20, 21] which could thus be regarded as large ICs with capacity for deposition in organs/tissues, including kidney [17, 22]. Immune electron microscopy studies have provided the evidence of co-localization of glomerular deposited ICs with microvesicles and galectin-3-binding protein (G3BP) in LN [22]. EV-ICs may activate complements and contribute to endothelial activation, tissue damage, cellular proliferation, and proinflammatory responses in the pathogenesis of LN [23, 24]. In this review, we will summarize the latest progress and recent advances in EV research, potential involvement of EVs in pathogenesis of LN, as well as their clinical implications.
2. Recent progress in generation of Extracellular Vesicles
Based on their origin and physical/biological features, EVs can be subdivided into three main classes , including exosomes (<100 nm), microvesicles (MVs) (<1 μm) and apoptotic bodies (1–5μm) [5]. Exosomes are generated by exocytosis of endosomal derived intracellular membrane vesicles to the extracellular space. Exosomes have been reported to contribute to many aspects of normal physiology, pathological conditions and human diseases [25]. In contrast, microvesicles (MVs, also called microparticles) are larger membrane vesicles derived from cell plasma membrane surface. Since MVs bud from cell membrane surface, the cell membrane-associated molecules are known to be released with MVs [6, 26]. In addition, studies from our and other groups found that cytosolic molecules and even nuclear molecules can also be associated with MV membranes [27–30] or encapsulated in the lumen of MVs [31]. Apoptotic bodies are the largest size membrane vesicles that are generated during apoptosis and can carry nuclear fragments and mitochondria, therefore are important in autoimmune diseases [9]. Here we have briefly illustrated the recent progress in cell death related EV generation (Figs. 1 and 2).
Fig. 1. Schematic illustration of mechanistic generation of exosomes from different cellular events.
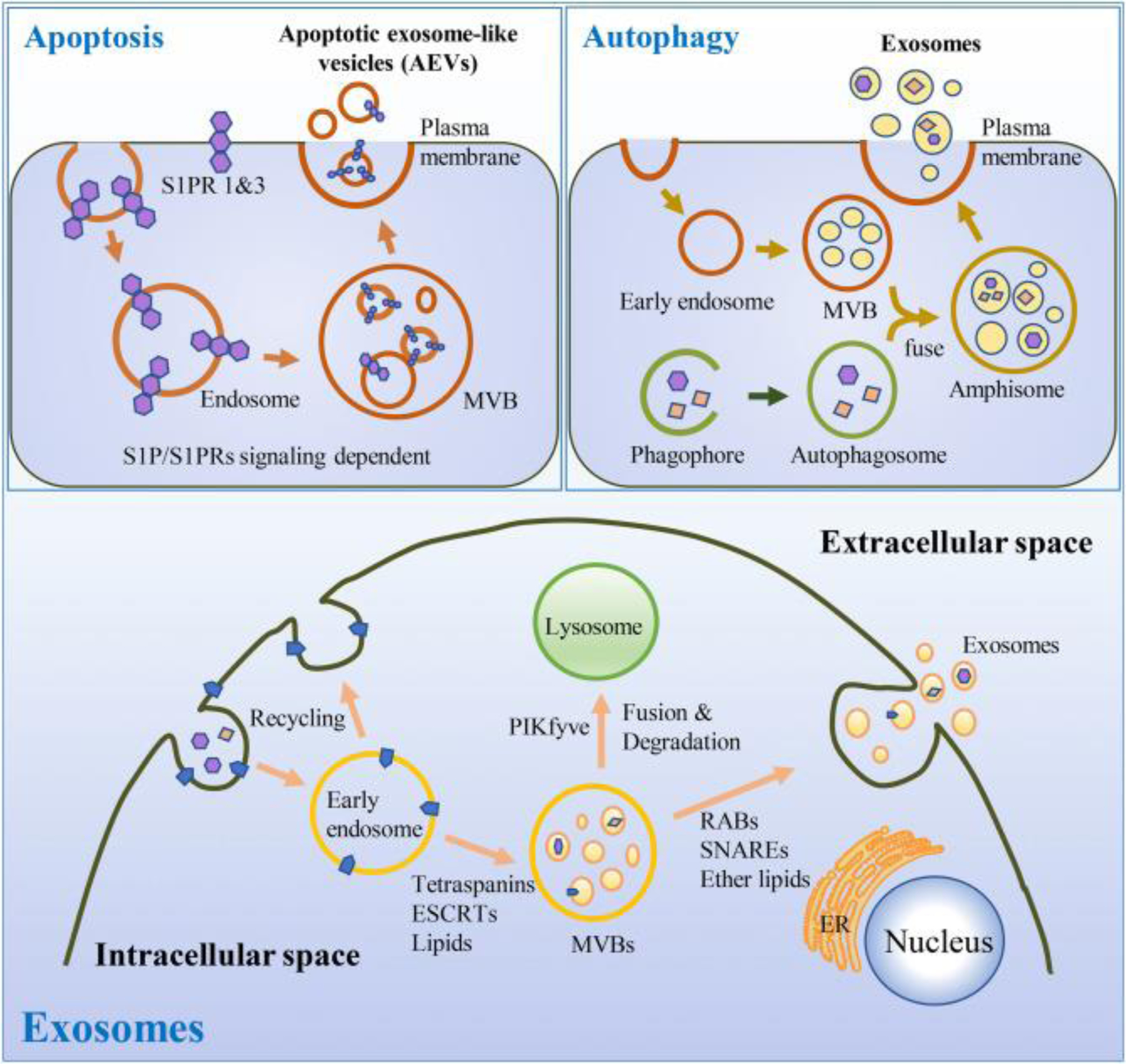
Brief mechanisms for exosome generation in autophagy [41, 42], and apoptotic [26] cells (upper portion). Molecular and cellular mechanisms that regulate exosome biogenesis and/or release. The process can be divided into three steps: exosome biogenesis, transportation of MVBs to the plasma membrane, and fusion of MVBs with plasma membrane. [161–163] (lower portion).
Fig. 2. Schematic illustration of mechanistic generation of MVs from different cellular events.
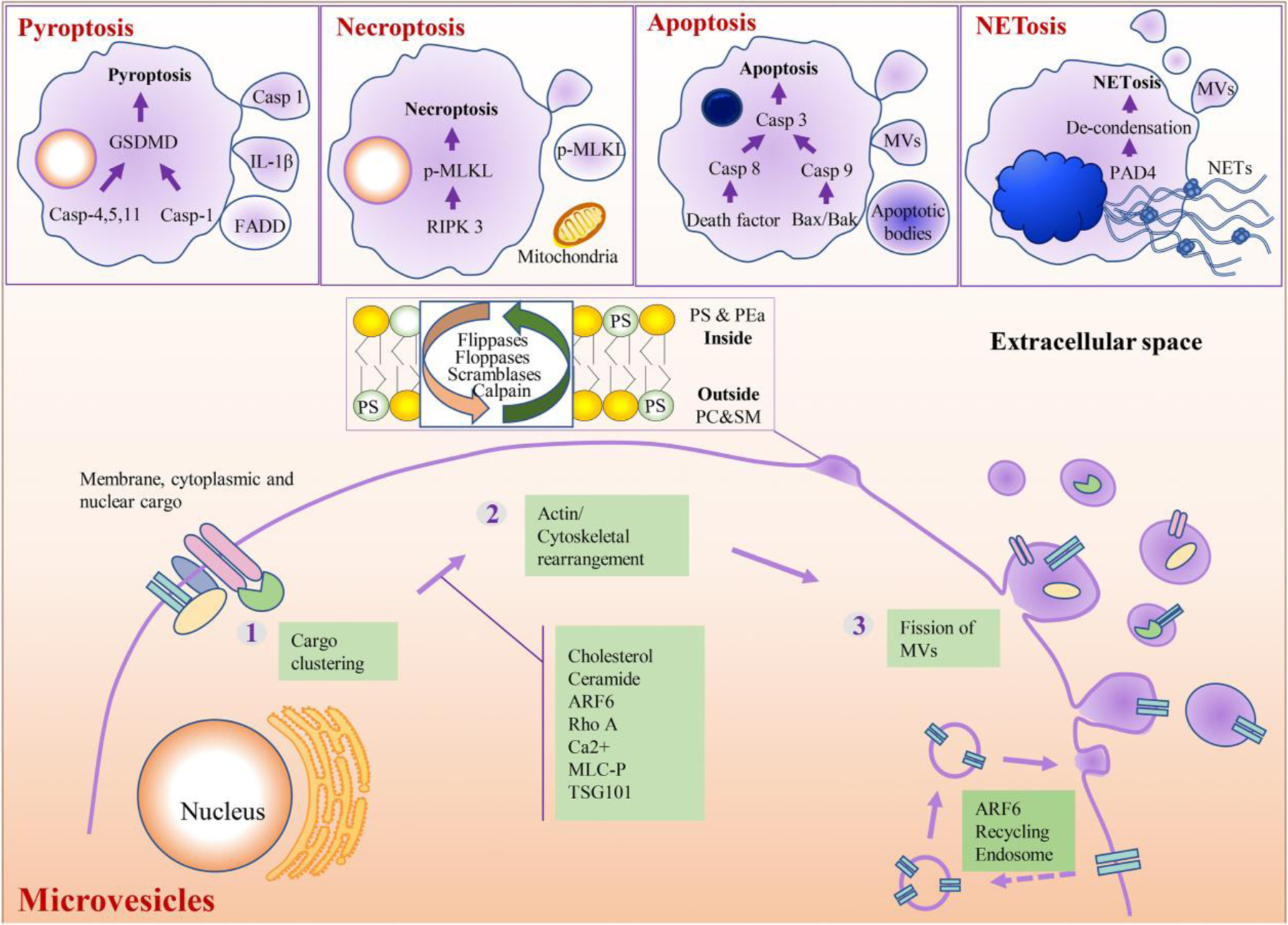
Brief mechanisms of MV generation from pyroptotic [27, 29, 34], necroptotic [30, 38], apoptotic [25], and NETotic [39, 164] cells (upper portion). Molecular and cellular mechanisms that regulate MVs biogenesis and/or release. The process can be divided into three steps. First, membrane-, cytoplasmic- and nuclear- associated cargoes are clustered in specific membrane microdomains of the plasma membrane. Second, the clustered cargoes together with additional machineries promote actin/cytoskeletal rearrangement, phosphatidylserine (PS) exposure, and membrane budding. Then a fission process at the plasma membrane occur. Besides, ARF6 can mediate the secretion of recycling endosome to form MVs. [165–169] (lower portion).
During apoptosis, procaspase 3 can be cleaved and become active caspase 3, which can activate Rho-associated protein kinase 1 (ROCK1) that is involved in apoptotic membrane blebbing through regulation of actin–myosin contraction [9]. In addition to the release of MVs and apoptotic bodies from apoptotic cells, Park et al reported that a fraction of exosome-like vesicles can also be released from apoptotic cells [32]. Their biogenesis was completely dependent on cellular sphingosine-1-phosphate (S1P)/S1P receptors (S1PRs) signaling [32]. Apoptosis, thus, can release full spectrum of EVs, from very small exosomes, medium sized microvesicles, to large apoptotic bodies. Apoptosis has long been known to be involved in SLE in numerous studies [33]. With the context of clearance deficiency of apoptotic debris, the remnants of apoptotic cells cumulatively challenge immune tolerance and continuously induce autoimmune and inflammatory responses in patients with SLE [34].
Pyroptosis has been shown to be regulated by caspase-1-dependent canonical inflammasome pathway and caspase-1-independent non-canonical inflammasome pathway [35, 36]. Caspase-1-independent pyroptosis is executed by caspase-11 in mice, while regulated by caspase-4/5 in human [35, 36]. Latest studies found that pyroptotic monocytes can release a heterogeneous population of EVs [11]. These EVs encapsulated Gasdermin D (GSDMD), caspases-1 [12] and Fas-associated death domain (FADD) [37]. Furthermore, MVs that contain both cleaved GSDMD and active caspase 1 could induce vascular endothelial cell injury [12].
Furthermore, necroptosis is a form of programmed cell death that critically depends on receptor-interacting serine-threonine kinase 3 (RIPK3) and mixed lineage kinase domain-like (MLKL) and generally manifests with morphological features of necrosis [38]. Necroptotic cells can release EVs and mitochondria to extracellular space [14, 39, 40], under the regulation by RIPK3 and MLKL [14]. Furthermore, release of phospho-MLKL in EVs can downregulate the cellular content of phospho-MLKL in their parental cells, thereby protecting cells from necrotic cell death [14] .Very recently, Thiam et al reported that neutrophils undergoing NETosis can also release membrane MVs before neutrophil extracellular trap (NET) formation [13]. Although the underlying cellular mechanism is unclear, cytoskeletal rearrangement might be involved [13]. In addition, a recent study also reported that autophagy can regulate exosomal release of prions in neuronal cells [41]. Exosomes arise from endosomal-derived multivesicular bodies, and crosstalk between autophagy and the endo/exo-somal vesicular trafficking pathways might be involved in exosome biogenesis during autophagy [42, 43].
In addition to apoptosis, the above discussed other types of programmed cell death have also been associated with pathogenesis of SLE and LN [44]. For instance, activation of necroptosis pathway and the RIPK3 dependent NOD-like receptor family, pyrin domain containing 3 (NLRP3) inflammasome pathway in podocytes have been shown to be involved in LN pathogenesis [45]. B cells from SLE patients shows high expression levels of necroptosis-related genes [46]. Furthermore, autophagosomes have been observed in podocytes in mice and human, and increased autophagy can exert a cytoprotective impact on podocyte injury [47]. Extensive EV research indicates that not only the cells that undergo cell death, but also their released subcellular EVs, can contribute to autoimmune responses and lupus development through various pathogenic functions. However, there is only limited information in the literature regarding the involvement of pyroptosis [48, 49] and necroptosis [45, 46] in lupus. While, there is clue for the potential link between these two types of cell death and SLE, and EVs may be involved in the pathogenesis of LN.
3. Potential pathologic effects of Extracellular Vesicles on Lupus Nephritis
The central paradigm of SLE is loss of immune tolerance to the sustained autoantibody production, while LN is a form of glomerulonephritis with deposition of ICs and complements [50]. In SLE, impaired macrophage phagocytic capacity results in defective clearance of apoptotic bodies, accumulation of autoantigens, and sustained production of autoantibodies with elevated formation of ICs [51, 52]. Nucleic acid-containing ICs drive inflammation through activation of Fcγ receptors (FcγR), complements, and the type I interferon pathway by engaging toll-like receptors (TLRs) [53, 54]. In addition, deposition of ICs and complements in kidney results in endothelial damage, and consequent microthrombi formation, as well as renal tissue damage and cellular proliferation in kidneys of LN patients [24, 53, 55].
EVs have been shown to be involved in pathogenesis of SLE and LN, through different mechanisms. Based on the current understanding of the lupus pathogenesis, we have summarized the potential pathologic effects of EVs on LN (with illustration Fig. 3) in the following aspects: 1) EVs may function as sources of extracellular autoantigens; 2) EV-associated autoantigens may stimulate B cells to generate autoantibodies, and then form EV-ICs, which may deposit in glomeruli; In addition, EVs may 3) either carry complement components or serve as a platform to activate complement system, and 4) cause renal tissue damage; Furthermore, EVs also contribute to 5) autoinflammatory responses and proinflammatory cytokine production, 6) prothrombotic conditions by carrying procoagulant properties. In addition, we also summarized the published clinical studies regarding the involvement of EVs in LN (Table 1).
Fig. 3. Potential pathogenic effects of EVs on Lupus Nephritis.
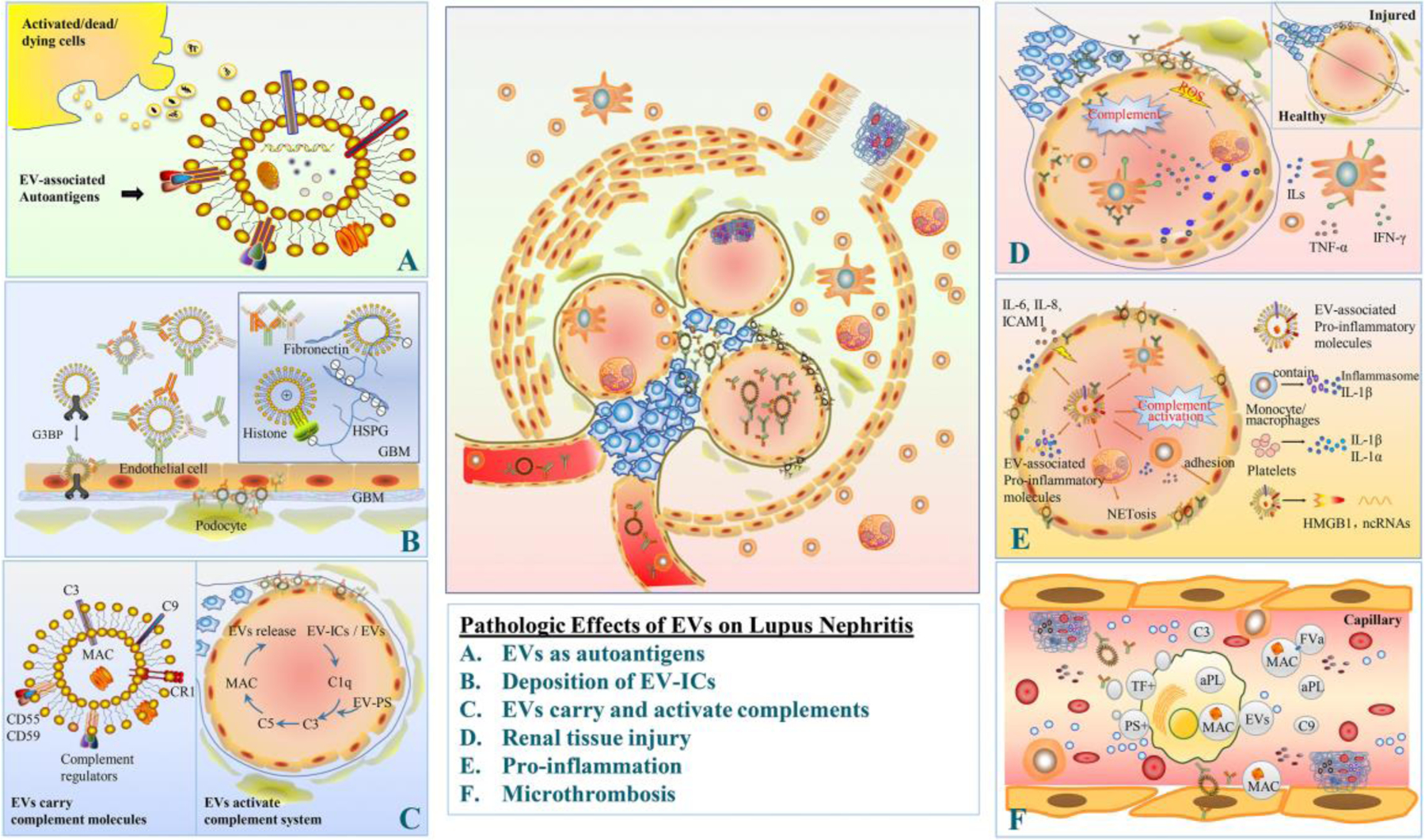
A. EVs: as the source of autoantigens: EVs that are released from activated/dead/dying cells may carry autoantigens, particularly those from nucleus or cytoplasm. B. Deposition of EV-ICs: EVs harbor immunoglobulins and complement, thereby forming EVs containing immune complexes (EV-ICs). These EV-ICs along with immune-active structures and molecules deposit in kidney. EVs molecules, including G3BP, histone, fibronectin, may facilitate glomerular IC deposition. C. EVs carry and activate complements: EVs may carry many complement molecules, like C3, MAC, complement regulators. On the other hand, EVs may activate complement system through either classical or alternative pathways. Some EVs may activate the classical pathway through binding C1q, while other EVs, i.e. PS containing EVs, may also be activator of complements through the alternative pathway. D. Renal tissue damage: EV-associated activated complements and the consequent pro-inflammatory microenvironment causes podocyte injury, foot process effacement, and proliferation of mesangial cells, plus MPO-mediated endothelial damage, therefore leading to proteinuria and glomerular dysfunction. E. Pro-inflammation: EVs may carry pro-inflammatory components (cytokines, inflammasome, adhesion molecules, leukotriene, etc.), which further propagate inflammatory responses by activation of other immune cells. In addition, EVs and EV-ICs may activate complement system. F. Microthrombosis: PS- and TF-positive EVs as well as the EVs associated with complements are procoagulant, thus contributing to microthrombosis in lupus nephritis.
Table 1.
Summary of the Published Studies about EVs in LN
| EVs origin | Cellular origin | Markers | Associated Specific component | Samples | Techniques for analyzing characteristics | Study findings | References | Year |
|---|---|---|---|---|---|---|---|---|
| MVs | Podocyte | Annexin V/Podocalyxin | NA | Urine | Flow cytometry | Higher podocyte-derived MVs in LN and correlate with high activity indices, and increased proteinuria | [151] | 2019 |
| MVs | Mainly leukocytes | Platelet CD41a; leukocyte CD45; erythrocyte CD235a | HMGB1/HLA-DR/CX3CR1 | PPP/Urine | Flow cytometry | High frequencies of MP-HMGB1+ in circulation and urine of LN patients. Urinary MP-HMGB1+ could discriminate between patients with active and inactive LN. | [117] | 2019 |
| MVs | Mainly platelets | Mitochondria mitoTracker/TOM20/HK1; Platelet CD42a; T cell CD3 | Nucleic acids/IgG | Frozen PPP | Flow cytometry | Patients with signs of ongoing renal lupus activity (BILAG, A-C) had higher levels of mitoMVs and IgG-coated mitoMVs. | [167] | 2019 |
| MVs | Apoptotic endothelial cells | KM-2/LG11–2 | Acetylated chromatin | PPP | Flow cytometry | MVs containing acetylated chromatin drive ROS independent NET release in SLE patients with active LN. | [168] | 2017 |
| MVs | NA | Annexin V/G3BP | G3BP, C1q, immunoglobulins | PPP | Flow cytometry/Co-localization immune electron microscopy(IEM)/Nano-LC-MS/MS | Co-localization of G3BP positive MVs associated with ICs may deposit in the LN kidneys. | [22] | 2015 |
| MVs | Platelet/endothelial cell | Platelet Annexin V/CD41; endothelial cell Annexin V/CD62E | NA | PPP | Flow cytometry | High circulatory platelet microparticles and endothelial microparticles in LN patients can be used as new markers for dysfunctional platelet activation and endothelium. | [169] | 2015 |
| Exo | Tubular renal cells | NA | MiR-31/MiR-107/MiR-135b-5p | Urine | NanoSight/Cryo-TEM/Western blot | Urinary exosomal miR-135b-5p, miR-107, and miR-31 are promising novel markers for clinical outcomes, regulating LN renal recovery by HIF1A inhibition. | [166] | 2020 |
| Exo | NA | CD9/TSG101 | Let-7a/MiR-21 | Urine | NanoSight/Transmission electron microscopy (TEM)/Western blot | Down-regulation of let-7a and miR-21 in urine exosomes from LN patients during disease flare. | [164] | 2018 |
| Exo | NA | CD9/CD81 | MiR-3135b/MiR-654–5p/MiR-146a-5p | Urine | Transmission electron microscopy (TEM)/Western blot | LNIV-CC has a unique miRNA expression profile of urinary exosome and miR-3135b, miR-654–5p and miR-146a-5p in urinary exosomes could predict LNIV-CC. | [170] | 2018 |
| Exo | NA | CD9/AQP2/TSG101 | MiR-29c | Urine | Electron microscopy Western blot | Decreased miR-29c in exosomes in LN is associated with increased renal chronicity and predict early renal fibrosis in LN. | [165] | 2015 |
| Exo | NA | CD9/TSG101 | MiR-146a | Urine | Transmission electron microscopy (TEM)/Western blot | Exosomal miR-146a discriminates the presence of active LN. | [162] | 2015 |
| Exo | NA | NA | MiR-26a | Urine | NA | Increased miR-26a levels in urinary exosomes may serve as a marker of injured podocytes in LN. | [163] | 2014 |
| ABs | NA | NA | NA | Renal tissue | Periodic acid-Schiff methenamine silver | The presence of large numbers of apoptotic bodies in the glomeruli of Clq-deficient mice. | [171] | 1998 |
| ABs | NA | NA | NA | Renal tissue | Electron microscopy | The presence of apoptotic cells and apoptotic bodies in proliferated mesangial areas and within the glomerular capillaries. | [172] | 1995 |
MVs (Microvesicles), mitoMVs (mitochondria MVs), Exo (Exosomes), ABs (Apoptotic Bodies), PPP (platelet poor plasma), LNIV-CC ( Type IV lupus nephritis-cellular crescent), ROS (Reactive oxygen species), LN (Lupus Nephritis), ICs (Immune Complexes), HMGB1 (High-mobility group box 1), G3BP (Galectin-3 binding protein), HK1 (anti-hexokinase 1).
3.1. EVs as the source of autoantigens
Accumulation of autoantigens from dying and dead cells is an important feature in SLE [56]. Certain molecules on nucleus, cytoplasmic, and even plasma membrane, can be clustered as autoantigens in EVs during various cellular events. Nuclear autoantigens are typically not accessible to the immune system [6]. Studies from our and other groups have demonstrated the redistribution of nuclear molecules into cytoplasm and plasma membrane where they may release with MVs during membrane budding or blebbing [29, 57, 58]. It has long been known that nuclear autoantigens (nucleosomal DNA, Ro, La) clustered on the membrane surface in the late stage apoptotic cells [58]. Other nuclear autoantigens, including histone nuclear proteins, lamin B1, and non-histone nuclear protein (like high mobility group box 1 (HMGB1)), can also be released with MVs [29, 59, 60]. In addition, cells undergoing pyroptosis and necroptosis could also release EVs with nuclear or mitochondrial molecules [11, 40] which may also serve as autoantigens.
Furthermore, cytoplasmic molecules may also be loaded into EVs, including mitochondria [61], proteinase 3 (PR3) [62] and myeloperoxidase (MPO) [63, 64]. Neutrophil PR3 or MPO are known to serve as autoantigens in the development of anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis [26]. Both PR3 [62] and MPO [64] have been found to be associated with EVs. This suggests these EV-associated PR3 and MPO may serve as autoantigens that trigger production of the corresponding autoantibodies in LN patients. In line with this hypothesis, Turner-Stokes et al reported that patients with LN have positive ANCA serology [65].
It has long been known that negatively charged plasma membrane PS regulates cellular localization of positively charged proteins [66]. The net positive charge of nuclear HMGB1[29] and cytoplasmic MPO and PR3 [67] provide the chemical basis for their binding with negatively charged PS on plasma membrane [29] during molecule redistribution in activated or apoptotic cells, and thus release with MVs during cell membrane budding. Translocation and association with phospholipids on plasma membrane also contribute to neoantigen properties of those intracellular molecules on EVs [68]. In NETotic cells, histone hypercitrullination modification is the key for chromatin de-condensation and NET formation [69]. Studies have reported that NETs can be the source of citrullinated histone autoantigens that contribute to development of autoimmune diseases [70]. A recent study have found that NETotic neutrophils can also release EVs, however these EVs do not contain nuclear DNA [13]. It does not know if the NETotic EVs also associate with citrullination-modified autoantigens.
In normal healthy cells, the nuclear and cytosolic autoantigenic molecules are located within nucleus or cytoplasm which are surrounded by the nuclear envelope or plasma membrane respectively. Thus, the nuclear and cytosolic autoantigenic molecules cannot be accessed by the immune system [57]. Autoreactive inflammatory responses in lupus require exposure and delivery of autoantigens to antigen presenting cells (APCs) in a proinflammatory context. During apoptosis, membrane translocation of nuclear and cytosolic autoantigens can enhance their exposure and accessibility by APCs [71]. Thus, the self-antigens from nucleus, cytoplasm, or mitochondria may expose on the membrane surface of EVs during the redistribution of autoantigenic molecules and EV budding process, making them accessible by APCs of immune surveillance system. Besides, impaired phagocytic clearance of apoptotic corps by macrophages in SLE patients [52] may enhance extracellular accumulation of EVs with autoantigens [72].
Interestingly, EV-associated autoantigens have potential to stimulate autoreactive inflammatory responses. Ullal et al reported that MVs with DNA and nucleosomal molecules can bind anti-DNA and anti-nucleosomal antibodies, and lupus plasma contains MV-ICs [73]. Binding of nucleosome-containing EVs to B cells can stimulate B proliferation and induce antibody secretion [59]. Furthermore, artificially encapsulating mammalian DNA by transfection reagent (lipofectin) has been shown to increase the immunostimulatory effects in vitro and immune responses in cultured cells [74]. This study suggests that EVs may serve as the endogenous source of encapsulated DNA and exert immunostimulatory properties. All in all, the above discussed findings indicate that EVs may be potential sources of autoantigens by carrying autoantigenic molecules from nucleus, cytoplasm, or cell membrane, therefore contributing to autoimmune responses in lupus.
3.2. EVs and immune complex deposition in renal glomeruli
LN results from glomerular IC deposition and the consequent chronic inflammatory responses. Studies have detected the EV-containing immune complexes (EV-ICs) in SLE patients [21, 22, 75], therefore one may expect to see potential role of EV-ICs in pathogenesis of LN. Fujigaki and colleagues have reported deposition of ICs, complements, and EVs in glomerular basement membrane (GBM) and the nearby epithelial cells in glomerulonephritis rats [76]. G3BP is a multifunctional glycoprotein which has soluble and cell-associated forms [77]. G3BP can be associated with MVs, G3BP-positive MVs and IC-associated MVs are significantly increased in SLE patients [78]. Most interestingly, electron microscopy analysis found the co-localization of G3BP with in vivo-bound IgG in the glomeruli of kidney biopsies from LN patients [22]. Since G3BP is known to mediate cell-cell or cell-matrix adhesion, G3BP-positive MVs may facilitate the deposition of these large EV-ICs in the GBM leading to formation of electron dense structures and nephritis [17].
The GBM is the central and non-cellular layer of the glomerular filtration barrier which is composed primarily of four types of extracellular matrix (ECM) macromoleculelaminin-521, type IV collagen, heparan sulphate (HS) proteoglycan (HSPG), and nidogen [79]. HS provides the molecular source of negative charges in the GBM [79]. Gallo et al found that electrostatic interactions between cationic status of ICs and the fixed anionic sites on GBM may be an important factor in glomerular trapping [80]. Even a small portion of cationic antibodies in ICs may affect their net electrostatic condition [81] resulting in the positive charge of ICs that is sufficient to mediate their deposition in GBM of glomeruli [80, 81]. As an important component of nuclear DNA, positively charged histone has been reported to mediate glomerular deposition of small size DNA and anti-DNA complex[82]. The positively charged histone-associated MVs [83] may mediate the glomerular deposition of EV-ICs by binding to the negatively charged HSPG on GBM, and contribute to development of LN. Furthermore, fibronectin is an ubiquitously distributed glycoprotein in the ECM, and it has been detected on the membrane surface of EVs in various studies [84]. As fibronectin has specific binding sites for HSPGs, it was reported to mediate EV-cell interactions by serving as a bridge between EV-HSPGs and cell membrane-HSPGs in ECM [84]. Elevated levels of plasma fibronectin have been reported in SLE patients [85] and anti-fibronectin antibodies are also detected in portion of SLE patients [86]. However, it’s not clear yet if fibronectin-positive EVs may potentially mediate EV-ICs deposition in glomeruli through molecular interaction between fibronectin and HSPG in GBM.
Furthermore, MVs provide adhesion and costimulatory molecules that may contribute to the ICs deposition when MVs bind to various cells, e.g., to endothelial cells (ECs) in kidney glomeruli. This may explain the presence of autoantibodies at sites where the specific epitopes are absent [75, 87]. In addition, it is not known whether EV-ICs are more difficult to be cleared as compared to conventional ICs. Although PS is a commonly used MV surface marker, portion of MVs are PS negative. Given that PS exposure serves as “eat-me” signal and mediates MVs clearance by macrophages, therefore PS-negative EV-ICs might not be efficiently cleared [21].
3.3. EVs carry or activate complements
Complement system is part of the innate immune system and is involved in the development of LN [24]. In pathologic studies, complements C5b-9 complexes have been detected in sub-epithelial immune deposits and epithelial MVs under electron microscopy in glomerulonephritis rats [76]. Co-presence of EVs and complements in nephritic glomeruli indicates a potential link between the two during immune responses in nephritis, including LN. Ostergaard and colleagues reported that circulating EVs in SLE are enriched with ICs and complements, particularly the complement proteins from the classical pathway [78]. As the common feature of complement activation pathways, conversion of C3 to C3b can activate a stable thioester bond, leading to the covalent attachment of C3b to cell-surface [88], and the downstream cascade molecule activation, as well as formation of MAC on the cell surface that make them possible to be associated with MVs during cell membrane budding process [89]. To assure cell survival and recovery from complement attack, cells may release a subgroup of EVs bearing complement molecules, especially MAC, as a self-protective mechanism to clear complements actively [23]. Such complement-associated EV shedding may result in formation of EVs binding with C3 fragments [90], C9 [90], MAC [91] and complement regulators, like complement receptor 1 (CR1/CD35) [92], CD55, CD59 [93].
Complement activation contributes to a variety of vascular and inflammatory disease states, including LN. Recent studies also demonstrate that EVs may serve as a platform for activation of complement system [23]. ICs are known to be the major activator of classical pathways. Nielsen and colleagues reported that elevated level of immunoglobulins and complements in EVs are correlated with autoantibodies and complement activation [75]. Furthermore, binding of C1q to apoptotic bodies induces activation of the classical complement pathway [94]. Therefore, deposition of ICs, complements, and EVs in GBM in rats with glomerulonephritis detected by electron microscopy [76] suggest their potential interaction in kidney. Interestingly, even without antigen harboring, the phospholipid components of EVs may make complements binding to lipid membranes through electrostatic interactions with surface charge of the phospholipids on EVs [95], and then subsequently leading to deposition and activation of C4 and C3 on EV surface [96]. Therefore, erythrocyte-derived MVs have been reported to fix C1q, following by subsequent activation of the classical pathway complement with binding of C3 fragments [97]. In addition, lipid components, like PS, on EVs could also be activator of complement system through the alternative pathway [98].
On the other hand, APCs-derived exosomes binding with complement regulators, i.e. CD55 and CD59, can functionally inhibit complement activation in vitro by inhibiting complement-mediated lysis [99]. Furthermore, CRl-loaded EVs released by glomerular podocytes [92] may reduce complement-mediated damage by inactivation of C3b in kidney [100]. It’s unclear whether delivering the EV-associated complement regulators/inhibitors can be protective to lupus. However, this could be a new avenue for investigation and development of novel therapeutic strategy for LN treatment. These are still many unanswered questions need to be explored in the future.
3.4. Involvement of EVs in tissue damage in lupus nephritis
As discussed above, deposition of ICs in glomeruli of LN can activate complements that initiate cell damage, generate chemoattractant factors, and activate immune cells (i.e., neutrophils, monocyte, dendritic cells) through FcγR, leading to release of reactive oxygen species (ROS) and proinflammatory cytokines, thus consequently amplifying local immune responses in lupus kidney [53]. In addition, EV-associated pro-inflammatory molecules may directly act on target cells and cause the release of pro-inflammatory cytokines [101–104], thus contribute to inflammation and tissue damage. The inflammatory milieu, induced by EVs and EV-ICs, may induce podocyte injury and foot process effacement, proliferation of mesangial and parietal epithelial cells, leading to leakage of plasma and proteinuria and glomerular dysfunction [51, 105, 106].
Increased circulating ECs and endothelial EVs have been detected in patients with LN [107] or SLE [108], reflecting microvascular injury in these patients. MPO is a positive charged molecule that can easily bind with the negatively charged structures [109], including glomerular ECs, thus may induce endothelial damage [110] and contribute to glomerulonephritis [109]. O’sullivan et al found that many damaged ECs were MPO positive [109]. Regarding the mechanisms by which MPO may be internalized by ECs , it has been reported that β2-integrin-mediated cell-cell contact between neutrophils and ECs has been reported for direct transfer of MPO from neutrophils to ECs [111]. On the other hand, the in vitro experiments have demonstrated that extracellular MPO can be translocated and internalized into ECs and epithelial cells [112]. In addition, neutrophil-derived MPO-positive EVs have been shown to induce vascular endothelial cell damage [63]. Serum levels of anti-MPO have been associated with future LN [113]. The glomerular endothelial damage may trigger vascular microthrombosis [50] that could mechanically obstruct glomerular capillaries, diminishing the blood supply to glomeruli and renal tubules, thereby further causing chronic hypoxic/ischemic injuries on the affected glomeruli and tubules during the development of LN [114].
3.5. EVs and autoimmune inflammation in Lupus Nephritis
Increasing evidence demonstrates that EVs have been associated with autoimmune inflammatory responses in LN. The defective clearance capability of macrophages in SLE [72] results in accumulation of dead cells and their released EVs may contribute to autoimmune inflammation in LN. Cargoes of EVs from nucleus, cytoplasm, or cell membrane of various cellular origins may contribute to pro-inflammatory milieu through EV-associated proinflammatory properties. A recent study demonstrated that many cytokines are released in EV-encapsulated forms in vitro, ex vivo, and in vivo, and these EV-encapsulated cytokines are capable of eliciting biological effects upon contact with target cells [31]. MVs released from monocytes-stimulated by lipopolysaccharide contain IL-1β and inflammasome [115]. These monocytic MVs can also activate ECs to express adhesion molecules, thus further promoting recruitment of inflammatory cells [115]. In turn, EVs shed from activated ECs contain high levels of adhesion molecules, i.e. E-selectin, which can mediate adhesion of monocytes to ECs in vitro [116]. Additionally, we have reported that nuclear HMGB1 can be translocated to plasma membrane and release with EVs to extracellular space [29]. Elevated levels of HMGB1-positive EVs have been found in circulation and urine in SLE patients with LN [117]. Extracellular HMGB1 is an endogenous prototypic damage-associated molecular pattern (DAMP) molecule and has been implicated in several inflammatory disorders [118]. Monocytes and macrophages can recognize HMGB1 through TLR4 receptor, resulting in production of pro-inflammatory cytokines [119].
In addition, EVs may also propagate inflammation indirectly by stimulating other cells to produce proinflammatory materials. EVs or EV-ICs have been reported to activate monocytes to produce pro-inflammatory cytokines, i.e. TNF-α, IL-8, IL-6, and IFN-α [20]. It has been reported that platelet-derived MVs contain both IL-1α and IL-1β that can induce IL-6 and IL-8 production by synoviocytes, indicating their pro-inflammatory potential [120]. Similarly, platelet EV-ICs are highly pro-inflammatory and can elicit leukotriene production from neutrophils [121]. Additionally, EV-associated miRNAs may regulate inflammatory cytokine production in target cells [101]. Recently, Salvi et al. demonstrated that circulating exosome-associated miRNAs from SLE patients can trigger human primary plasmacytoid dendritic cells (pDCs) to produce IFNα, TNF-α and IL-6 through TLR7-dependent activation [122]. Taken together, EVs may contribute to a proinflammatory milieu and autoimmune inflammation in LN either directly with their associated proinflammatory components or indirectly by triggering other cells to produce proinflammatory cytokines or proinflammatory materials.
3.6. EVs and glomerular microthrombosis in Lupus Nephritis
Numerous studies have revealed that presence of microvascular lesions and microthrombus formation could adversely affect the course of renal disease [123]. Prothrombotic function is the first described pathological feature [124] and the mostly studied classical function of EVs [27, 28] . Our own studies have shown that EVs have pro-coagulant properties [27, 28], particularly the monocyte-derived EVs (mEVs) with both tissue factors (TF) and PS exposed on the membrane surface [27, 28]. During the EV formation process, the plasma membrane asymmetry is lost due to membrane and cytoskeleton rearrangements, resulting in membrane exposure of anionic PS on the membrane surface of EVs [125]. In monocytes, the membrane expressed TF can also be released with mEVs during membrane budding [27, 28]. Co-presence of PS with TF on EVs enhances the procoagulant potential as TF can trigger extrinsic coagulation cascade, while the negatively charged PS provides an ideal surface for assembly of coagulation factors [26, 126]. Furthermore, the endothelial derived EVs that express TF might also promote microthrombus formation [127] as well. Interestingly, platelet- and erythrocyte-derived MVs can also initiate thrombin formation in a FXII-dependent manner through intrinsic pathway [128].
Among various renal vasculopathies, thrombotic microangiopathy (TMA), in which complement may be involved [129], represents the most severe vascular manifestations with highest mortality rate and results in renal microvascular thrombosis due to vascular endothelial injury [130]. As described above, EVs may carry complement and serve as a platform for activation of complement system with EV-associated components. Lood et al reported that complement deposition on platelets may be related to thrombosis in SLE patients [131]. Another study with complement C3 or C5 knockout mice have demonstrated the specific roles of C3 in platelet activation and complement-dependent membrane perturbations, leading to prothrombotic TF activation on myeloid cells [132]. Furthermore, it has long been known that MAC can induce vesiculation in endothelial cell membrane and expose catalytic surface for prothrombinase enzyme complex [133]. Additionally, complement activation has been associated with thrombosis in antiphospholipid (aPL) syndrome [134], a common syndrome of SLE. The majority of pathogenic aPL antibodies retain their reactivity with membrane lipids, and induce NADPH-oxidase (NOX)-dependent proinflammatory signaling and TF activation, with assistance of complements [134]. Thus, TF primes monocytes for thrombosis amplification through a crosstalk between complement activation and coagulation signaling [134], EVs may be able to involve in these process with their-associated TF or complements, although there is no direct study has been reported yet.
According to Virchow’s triad, the pathophysiologic thrombosis is achieved by interplay of abnormalities in blood composition, vessel wall injury, and blood flow disturbance [135]. Elevated thrombogenicity by pro-coagulant EVs, while abnormal vessel wall by EV-associated endothelial dysfunction or damage [6, 63, 136], thus EVs may contribute to thrombosis by affecting at least two elements of Virchow’s triad. It has long been known that inflammation and thrombosis are related [137], EVs may play a central role in inflammation-related hypercoagulability state, thus contributing to LN.
In addition to the prothrombotic properties of EVs, they may also carry proteins with coagulation inhibitory properties, such as TF pathway inhibitor [138], protein C [139]. This fact indicates the complexity of contributions of EVs to microthrombus formation. The balance between anti- and pro-thrombotic properties of EVs as well as their contribution to LN still need to be further explored.
4. Potential clinical implications of EVs on Lupus Nephritis
The current available therapeutic strategies in LN primary management still rely heavily on non-specific immunosuppressive treatment. Targeted therapies may offer promising potential for improved therapeutic efficacy with fewer side-effects [140–143]. Although the current clinical trials for emerging therapeutic strategies mainly target on B cells, T cells, or IFNs for lupus treatment, the heterogeneity features of lupus make it very difficult to set ideal targets. As discussed above, recent progress indicates that EVs released by infiltrating immune cells or renal resident cells convey in circulation or spread in local renal tissues by interaction with either innate or adaptive immune cells, involving in almost all aspects of pathogenesis in SLE or LN. Regulation of EV generation or interfering their pathologic involvements in LN may provide insights into novel therapeutic strategies for SLE and LN treatment.
Through selectively packaging process, EVs harbor or enclose specific DNA, RNA, or proteins thus mediate intercellular communications between neighboring cells or distant cells [144]. This newly discovered approach for old cellular mechanism of intercellular communications could be novel therapeutic targets in various human diseases, including lupus. Cantaluppi et al have reported that EVs derived from endothelial progenitor cells, containing different mRNAs coding for several anti-apoptotic signals and for complement inhibitors, demonstrated protective effects in experimental complement-mediated glomerulonephritis [145]. Other beneficial effects of EVs may also attribute to modulation of fibrosis, tubular and glomerular damage, and angiogenesis by EV-associated contents [146], i.e. mesenchymal stem cell-derived EVs for renal repairment [147]. Furthermore, engineered exosome vector fused with the targeting peptide RVG (rabies viral glycoprotein peptide) has been used to direct exosomes to kidney mediated by acetylcholine receptor [148]. Interestingly, the engineered EVs containing specific contents selectively target to kidney cells for treatment of kidney diseases, including LN, can be expected for future novel treatment. All of the above information shines the light for new era of EV application as emerging therapeutic methods for human diseases, including LN.
Based on the above discussed, EVs contain the information that reflects pathophysiological conditions or disease severity. Therefore, analysis of EVs in urine or plasma may serve as non-invasive “liquid biopsy” [149, 150] approaches for diagnosis and prognosis of LN, in contrast to the classical invasive renal biopsy. Changes in levels of urinary podocyte EVs may reflect ultrastructural pathologic changes of podocytes in patients with active LN in advance of classical albuminuria/nephrin [151]. From the perspective point of view, EVs may have predictive value for monitoring the disease severity and prognosis for future outcomes, although a lot of work needs to be done before the validated methodologies can be established. Furthermore, high levels of cell free DNA (cfDNA) in SLE patients were reported early in 1966 [152]. Since then, there has been increasing interest in applying cfDNA as a potential biomarker for diagnostic purpose of various human diseases, including lupus. Overall, lupus patients show elevated levels of cfDNA that fluctuate concomitantly with disease activity, inflammatory markers and to some extent with therapeutic interventions [153]. A recent study has reported that large portion of plasma cfDNA is associated with EVs [154]. Therefore, detection of EV-associated cell free DNA might be useful for patients with SLE or LN.
Non-coding RNAs (ncRNAs), including long non-coding RNA (lncRNA), circular RNA (circRNA), and micro RNA (miRNA), has been shown to be associated with EVs [155–157]. Recent studies have revealed that the ncRNAs play an important role in the pathogenesis of SLE and/or LN [158–160]. Although studies have shown that EVs can carry ncRNAs, and ncRNAs have been shown to be involved in lupus pathogenesis, while there are no published papers that directly study the presence of lncRNA and circRNA in EVs in SLE patients. In the perspective point of view, this novel area is worthy to be investigated. There are several published papers that have studied EV-associated miRNAs in the context of lupus. Firstly, changes of miRNAs in urinary EVs have been reported in LN [161]. These findings demonstrated that increased miR-146a [162], miR-26a [163] and down-regulation of let-7a, miR-21 [164], miR-29c [165] in urinary exosomes could discriminate the presence of LN. Secondly, changes of miRNA level in EVs could also reflect the injury of podocytes [105] and early renal fibrosis in LN [165]. Thirdly, levels of let-7a and miR-21 in urinary exosomes, which were down-regulated during disease flare, were elevated after complete course of treatment[164]. These findings indicate that miRNAs in EVs can be served as potential biomarkers for disease diagnosis and monitoring. More importantly, uptake of urinary exosomes with high levels of miR-31, miR-107, and miR-135b-5p by mesangial cells regulate LN renal recovery by HIF1A (hypoxia inducible factor 1-alpha) inhibition [166]. In addition, circulating exosome-associated miRNAs from SLE patients have been shown to trigger human primary plasmacytoid dendritic cells (pDCs) to produce IFNα, TNF-α and IL-6 through TLR7-dependent activation [122].
Levels of cfDNA and miRNAs in EVs may have great potential to serve as biomarkers in LN. However, it requires a rigorous evaluation of cfDNA and miRNAs in longitudinal studies with large cohorts of patients and careful comparison with existing inflammatory and clinical markers of disease. The relatively simple and practically applicable assay kits for detection of urinary EVs and their-associated specific biomarkers are expected to be developed for the “precision medicine” for future improvement of clinical management of LN instead of the potentially hazardous and expensive renal biopsy. These efforts are also important for optimization of treatment efficacy for LN patients.
5. Conclusions
In this review, we have summarized the latest progress in cellular mechanisms about the EV generation in several novel types of programmed cell death in addition to apoptosis. The research progress on EV generation may provide insights into new understanding regarding the involvement of these novel types of programmed cell death in SLE and LN. The article also summarized the current literatures and give our interpretation based on our experiences in EV research for over decade. In the article, we summarized the potential pathogenic effects of EVs on several aspects of pathogenesis of LN, i.e. EVs serving as sources of autoantigens, mediating immune complexes renal deposition, harboring or activating complements, inducing renal tissue damage, as well as promoting inflammatory responses and microthrombosis, thus leading to sustained autoimmune inflammation in kidney. The cutting-edge knowledge summarized in the article may provide the insight into novel therapeutic avenues over the current targets of the clinical trials for new treatment in lupus. Furthermore, the current progress in EV research may also be helpful to establish new tools for diagnosis biomarkers, monitoring, prognosis of disease activity, and therapeutic efficacy in patients with autoimmune diseases, including SLE and LN. Whether therapeutic or diagnostic strategies towards EVs can be translated into future clinical practice in LN patients are still not fully understood. A lot of efforts are still needed to find out potential targets on EVs for their crucial roles on LN pathogenesis. And the long-term follow-up studies are also needed to evaluate the possible roles of EVs as clinical biomarkers in LN. To date, further studies are necessary to explore terra incognita about EV generation, regulation and the involvement in human diseases.
Acknowledgements
The authors would like to acknowledge the colleagues in the departments for their help in the preparation of this work.
Funding
This work was supported by the National Institutes of Health [grant numbers R21AI144838]; Lupus Research Alliance [grant numbers 416805].
Footnotes
Competing interests Authors have declared that no conflict interests exist.
References:
- [1].Tsokos GC. Systemic lupus erythematosus. The New England journal of medicine, 2011;365:2110–21. [DOI] [PubMed] [Google Scholar]
- [2].Davidson A. What is damaging the kidney in lupus nephritis? Nature reviews Rheumatology, 2016;12:143–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Margolis L, Sadovsky Y. The biology of extracellular vesicles: The known unknowns. PLoS Biol, 2019;17:e3000363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Stahl PD, Raposo G. Extracellular Vesicles: Exosomes and Microvesicles, Integrators of Homeostasis. Physiology (Bethesda), 2019;34:169–77. [DOI] [PubMed] [Google Scholar]
- [5].E. L. A. S, Mäger I, Breakefield XO, Wood MJ. Extracellular vesicles: biology and emerging therapeutic opportunities. Nat Rev Drug Discov, 2013;12:347–57. [DOI] [PubMed] [Google Scholar]
- [6].Liu ML,Williams KJ, Werth VP. Microvesicles in Autoimmune Diseases. Advances in clinical chemistry, 2016;77:125–75. [DOI] [PubMed] [Google Scholar]
- [7].Shah R, Patel T, Freedman JE. Circulating Extracellular Vesicles in Human Disease. The New England journal of medicine, 2018;379:958–66. [DOI] [PubMed] [Google Scholar]
- [8].Galluzzi L, Vitale I, Aaronson SA, Abrams JM, Adam D, Agostinis P et al. Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell death and differentiation, 2018;25:486–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Nagata S. Apoptosis and Clearance of Apoptotic Cells. Annual review of immunology, 2018;36:489–517. [DOI] [PubMed] [Google Scholar]
- [10].Shi J, Gao W, Shao F. Pyroptosis: Gasdermin-Mediated Programmed Necrotic Cell Death. Trends in biochemical sciences, 2017;42:245–54. [DOI] [PubMed] [Google Scholar]
- [11].Baxter AA, Phan TK, Hanssen E, Liem M, Hulett MD, Mathivanan S et al. Analysis of extracellular vesicles generated from monocytes under conditions of lytic cell death. Scientific reports, 2019;9:7538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Mitra S, Exline M, Habyarimana F, Gavrilin MA, Baker PJ, Masters SL et al. Microparticulate Caspase 1 Regulates Gasdermin D and Pulmonary Vascular Endothelial Cell Injury. Am J Respir Cell Mol Biol, 2018;59:56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Thiam HR, Wong SL, Qiu R, Kittisopikul M, Vahabikashi A, Goldman AE et al. NETosis proceeds by cytoskeleton and endomembrane disassembly and PAD4-mediated chromatin decondensation and nuclear envelope rupture. Proceedings of the National Academy of Sciences of the United States of America, 2020;117:7326–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Yoon S, Kovalenko A, Bogdanov K, Wallach D. MLKL, the Protein that Mediates Necroptosis, Also Regulates Endosomal Trafficking and Extracellular Vesicle Generation. Immunity, 2017;47:51–65.e7. [DOI] [PubMed] [Google Scholar]
- [15].Wallach D, Kang TB, Dillon CP, Green DR. Programmed necrosis in inflammation: Toward identification of the effector molecules. Science, 2016;352:aaf2154. [DOI] [PubMed] [Google Scholar]
- [16].Elkon KB. Review: Cell Death, Nucleic Acids, and Immunity: Inflammation Beyond the Grave. Arthritis & rheumatology (Hoboken, NJ), 2018;70:805–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Nielsen CT, Rasmussen NS, Heegaard NH, Jacobsen S. “Kill” the messenger: Targeting of cell-derived microparticles in lupus nephritis. Autoimmunity reviews, 2016;15:719–25. [DOI] [PubMed] [Google Scholar]
- [18].Utz PJ, Gensler TJ, Anderson P. Death, autoantigen modifications, and tolerance. Arthritis Res, 2000;2:101–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Cocca BA, Cline AM, Radic MZ. Blebs and apoptotic bodies are B cell autoantigens. J Immunol, 2002;169:159–66. [DOI] [PubMed] [Google Scholar]
- [20].Burbano C, Villar-Vesga J, Orejuela J, Muñoz C, Vanegas A, Vásquez G et al. Potential Involvement of Platelet-Derived Microparticles and Microparticles Forming Immune Complexes during Monocyte Activation in Patients with Systemic Lupus Erythematosus. Front Immunol, 2018;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Fortin PR, Cloutier N, Bissonnette V, Aghdassi E, Eder L, Simonyan D et al. Distinct Subtypes of Microparticle-containing Immune Complexes Are Associated with Disease Activity, Damage, and Carotid Intima-media Thickness in Systemic Lupus Erythematosus. The Journal of rheumatology, 2016;43:2019–25. [DOI] [PubMed] [Google Scholar]
- [22].Nielsen CT, Ostergaard O, Rekvig OP, Sturfelt G, Jacobsen S, Heegaard NH. Galectin-3 binding protein links circulating microparticles with electron dense glomerular deposits in lupus nephritis. Lupus, 2015;24:1150–60. [DOI] [PubMed] [Google Scholar]
- [23].Karasu E, Eisenhardt SU, Harant J, Huber-Lang M. Extracellular Vesicles: Packages Sent With Complement. Frontiers in immunology, 2018;9:721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Leffler J, Bengtsson AA, Blom AM. The complement system in systemic lupus erythematosus: an update. Annals of the rheumatic diseases, 2014;73:1601–6. [DOI] [PubMed] [Google Scholar]
- [25].Harding CV, Heuser JE, Stahl PD. Exosomes: looking back three decades and into the future. J Cell Biol, 2013;200:367–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Wu X, Liu Y, Wei W, Liu ML. Extracellular vesicles in autoimmune vasculitis - Little dirts light the fire in blood vessels. Autoimmunity reviews, 2019;18:593–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Liu ML, Reilly MP, Casasanto P, McKenzie SE, Williams KJ. Cholesterol enrichment of human monocyte/macrophages induces surface exposure of phosphatidylserine and the release of biologically-active tissue factor-positive microvesicles. Arteriosclerosis, thrombosis, and vascular biology, 2007;27:430–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Li M, Yu D, Williams KJ, Liu ML. Tobacco smoke induces the generation of procoagulant microvesicles from human monocytes/macrophages. Arteriosclerosis, thrombosis, and vascular biology, 2010;30:1818–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Chen Y, Li G, Liu Y, Werth VP, Williams KJ, Liu ML. Translocation of Endogenous Danger Signal HMGB1 From Nucleus to Membrane Microvesicles in Macrophages. Journal of cellular physiology, 2016;231:2319–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Li Y, Zeidi M, Bashir M, Werth V, Liu M-L. 054 Extracellular MAVS associates with microvesicles that can actively trigger IFNβ production. J Invest Dermatol, 2019;139:S9. [Google Scholar]
- [31].Fitzgerald W, Freeman ML, Lederman MM, Vasilieva E, Romero R, Margolis L. A System of Cytokines Encapsulated in ExtraCellular Vesicles. Scientific reports, 2018;8:8973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Park SJ, Kim JM, Kim J, Hur J, Park S, Kim K et al. Molecular mechanisms of biogenesis of apoptotic exosome-like vesicles and their roles as damage-associated molecular patterns. Proceedings of the National Academy of Sciences of the United States of America, 2018;115:E11721–E30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Favaloro B, Allocati N, Graziano V, Di Ilio C, De Laurenzi V. Role of apoptosis in disease. Aging (Albany NY), 2012;4:330–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Mahajan A, Herrmann M, Muñoz LE. Clearance Deficiency and Cell Death Pathways: A Model for the Pathogenesis of SLE. Frontiers in immunology, 2016;7:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Man SM, Karki R, Kanneganti TD. Molecular mechanisms and functions of pyroptosis, inflammatory caspases and inflammasomes in infectious diseases. Immunological reviews, 2017;277:61–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Jorgensen I, Miao EA. Pyroptotic cell death defends against intracellular pathogens. Immunol Rev, 2015;265:130–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Mouasni S, Gonzalez V, Schmitt A, Bennana E, Guillonneau F, Mistou S et al. The classical NLRP3 inflammasome controls FADD unconventional secretion through microvesicle shedding. Cell Death Dis, 2019;10:190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Galluzzi L, Kepp O, Chan FK, Kroemer G. Necroptosis: Mechanisms and Relevance to Disease. Annu Rev Pathol, 2017;12:103–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Zargarian S, Shlomovitz I, Erlich Z, Hourizadeh A, Ofir-Birin Y, Croker BA et al. Phosphatidylserine externalization, “necroptotic bodies” release, and phagocytosis during necroptosis. PLoS biology, 2017;15:e2002711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Spencer DM, Dye JR, Piantadosi CA, Pisetsky DS. The release of microparticles and mitochondria from RAW 264.7 murine macrophage cells undergoing necroptotic cell death in vitro. Exp Cell Res, 2018;363:151–9. [DOI] [PubMed] [Google Scholar]
- [41].Abdulrahman BA, Abdelaziz DH, Schatzl HM. Autophagy regulates exosomal release of prions in neuronal cells. The Journal of biological chemistry, 2018;293:8956–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Xu J, Camfield R, Gorski SM. The interplay between exosomes and autophagy - partners in crime. Journal of cell science, 2018;131. [DOI] [PubMed] [Google Scholar]
- [43].Minakaki G, Menges S, Kittel A, Emmanouilidou E, Schaeffner I, Barkovits K et al. Autophagy inhibition promotes SNCA/alpha-synuclein release and transfer via extracellular vesicles with a hybrid autophagosome-exosome-like phenotype. Autophagy, 2018;14:98–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Mistry P, Kaplan MJ. Cell death in the pathogenesis of systemic lupus erythematosus and lupus nephritis. Clinical immunology (Orlando, Fla), 2017;185:59–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Guo C, Fu R, Zhou M, Wang S, Huang Y, Hu H et al. Pathogenesis of lupus nephritis: RIP3 dependent necroptosis and NLRP3 inflammasome activation. Journal of autoimmunity, 2019;103:102286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Fan H, Liu F, Dong G, Ren D, Xu Y, Dou J et al. Activation-induced necroptosis contributes to B-cell lymphopenia in active systemic lupus erythematosus. Cell Death Dis, 2014;5:e1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Qi YY, Zhou XJ, Cheng FJ, Hou P, Ren YL, Wang SX et al. Increased autophagy is cytoprotective against podocyte injury induced by antibody and interferon-alpha in lupus nephritis. Ann Rheum Dis, 2018;77:1799–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Magna M, Pisetsky DS. The Role of Cell Death in the Pathogenesis of SLE: Is Pyroptosis the Missing Link? Scandinavian journal of immunology, 2015;82:218–24. [DOI] [PubMed] [Google Scholar]
- [49].Faliti CE, Gualtierotti R, Rottoli E, Gerosa M, Perruzza L, Romagnani A et al. P2X7 receptor restrains pathogenic Tfh cell generation in systemic lupus erythematosus. The Journal of experimental medicine, 2019;216:317–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Anders HJ, Saxena R, Zhao MH, Parodis I, Salmon JE, Mohan C. Lupus nephritis. Nature reviews Disease primers, 2020;6:7. [DOI] [PubMed] [Google Scholar]
- [51].Lech M, Anders HJ. The pathogenesis of lupus nephritis. Journal of the American Society of Nephrology : JASN, 2013;24:1357–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Tsokos GC, Lo MS, Costa Reis P, Sullivan KE. New insights into the immunopathogenesis of systemic lupus erythematosus. Nature reviews Rheumatology, 2016;12:716–30. [DOI] [PubMed] [Google Scholar]
- [53].Flores-Mendoza G, Sansón SP, Rodríguez-Castro S, Crispín JC, Rosetti F. Mechanisms of Tissue Injury in Lupus Nephritis. Trends Mol Med, 2018;24:364–78. [DOI] [PubMed] [Google Scholar]
- [54].Crow MK, Olferiev M, Kirou KA. Type I Interferons in Autoimmune Disease. Annu Rev Pathol, 2019;14:369–93. [DOI] [PubMed] [Google Scholar]
- [55].Birmingham DJ, Hebert LA. The Complement System in Lupus Nephritis. Seminars in nephrology, 2015;35:444–54. [DOI] [PubMed] [Google Scholar]
- [56].Boeltz S, Hagen M, Knopf J, Mahajan A, Schick M, Zhao Y et al. Towards a pro-resolving concept in systemic lupus erythematosus. Seminars in immunopathology, 2019;41:681–97. [DOI] [PubMed] [Google Scholar]
- [57].Schiller M, Bekeredjian-Ding I, Heyder P, Blank N, Ho AD, Lorenz HM. Autoantigens are translocated into small apoptotic bodies during early stages of apoptosis. Cell death and differentiation, 2008;15:183–91. [DOI] [PubMed] [Google Scholar]
- [58].Casciola-Rosen LA, Anhalt G, Rosen A. Autoantigens targeted in systemic lupus erythematosus are clustered in two populations of surface structures on apoptotic keratinocytes. The Journal of experimental medicine, 1994;179:1317–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Radic M, Marion T, Monestier M. Nucleosomes are exposed at the cell surface in apoptosis. J Immunol, 2004;172:6692–700. [DOI] [PubMed] [Google Scholar]
- [60].Klein B, Lutz-Meindl U, Kerschbaum HH. From the nucleus to the plasma membrane: translocation of the nuclear proteins histone H3 and lamin B1 in apoptotic microglia. Apoptosis : an international journal on programmed cell death, 2014;19:759–75. [DOI] [PubMed] [Google Scholar]
- [61].Morrison TJ, Jackson MV, Cunningham EK, Kissenpfennig A, McAuley DF, O’Kane CM et al. Mesenchymal Stromal Cells Modulate Macrophages in Clinically Relevant Lung Injury Models by Extracellular Vesicle Mitochondrial Transfer. Am J Respir Crit Care Med, 2017;196:1275–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Martin KR, Kantari-Mimoun C, Yin M, Pederzoli-Ribeil M, Angelot-Delettre F, Ceroi A et al. Proteinase 3 Is a Phosphatidylserine-binding Protein That Affects the Production and Function of Microvesicles. The Journal of biological chemistry, 2016;291:10476–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Pitanga TN, de Aragao Franca L, Rocha VC, Meirelles T, Borges VM, Goncalves MS et al. Neutrophil-derived microparticles induce myeloperoxidase-mediated damage of vascular endothelial cells. BMC cell biology, 2014;15:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Slater TW, Finkielsztein A, Mascarenhas LA, Mehl LC, Butin-Israeli V, Sumagin R. Neutrophil Microparticles Deliver Active Myeloperoxidase to Injured Mucosa To Inhibit Epithelial Wound Healing. J Immunol, 2017;198:2886–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Turner-Stokes T, Wilson HR, Morreale M, Nunes A, Cairns T, Cook HT et al. Positive antineutrophil cytoplasmic antibody serology in patients with lupus nephritis is associated with distinct histopathologic features on renal biopsy. Kidney international, 2017;92:1223–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Yeung T, Gilbert GE, Shi J, Silvius J, Kapus A, Grinstein S. Membrane phosphatidylserine regulates surface charge and protein localization. Science, 2008;319:210–3. [DOI] [PubMed] [Google Scholar]
- [67].Korkmaz B, Horwitz MS, Jenne DE, Gauthier F. Neutrophil elastase, proteinase 3, and cathepsin G as therapeutic targets in human diseases. Pharmacol Rev, 2010;62:726–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Van Rhijn I, van Berlo T, Hilmenyuk T, Cheng TY, Wolf BJ, Tatituri RV et al. Human autoreactive T cells recognize CD1b and phospholipids. Proceedings of the National Academy of Sciences of the United States of America, 2016;113:380–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Wang Y, Li M, Stadler S, Correll S, Li P, Wang D et al. Histone hypercitrullination mediates chromatin decondensation and neutrophil extracellular trap formation. J Cell Biol, 2009;184:205–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Khandpur R, Carmona-Rivera C, Vivekanandan-Giri A, Gizinski A, Yalavarthi S, Knight JS et al. NETs are a source of citrullinated autoantigens and stimulate inflammatory responses in rheumatoid arthritis. Science translational medicine, 2013;5:178ra40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Frisoni L, McPhie L, Colonna L, Sriram U, Monestier M, Gallucci S et al. Nuclear autoantigen translocation and autoantibody opsonization lead to increased dendritic cell phagocytosis and presentation of nuclear antigens: a novel pathogenic pathway for autoimmunity? J Immunol, 2005;175:2692–701. [DOI] [PubMed] [Google Scholar]
- [72].Licht R, Dieker JW, Jacobs CW, Tax WJ, Berden JH. Decreased phagocytosis of apoptotic cells in diseased SLE mice. Journal of autoimmunity, 2004;22:139–45. [DOI] [PubMed] [Google Scholar]
- [73].Ullal AJ, Reich CF 3rd, Clowse M, Criscione-Schreiber LG, Tochacek M, Monestier M et al. Microparticles as antigenic targets of antibodies to DNA and nucleosomes in systemic lupus erythematosus. Journal of autoimmunity, 2011;36:173–80. [DOI] [PubMed] [Google Scholar]
- [74].Zhu FG, Reich CF, Pisetsky DS. Effect of cytofectins on the immune response of murine macrophages to mammalian DNA. Immunology, 2003;109:255–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Nielsen CT, Østergaard O, Stener L, Iversen LV, Truedsson L, Gullstrand B et al. Increased IgG on cell-derived plasma microparticles in systemic lupus erythematosus is associated with autoantibodies and complement activation. Arthritis and rheumatism, 2012;64:1227–36. [DOI] [PubMed] [Google Scholar]
- [76].Fujigaki Y, Nagase M, Kojima K, Yamamoto T, Hishida A. Glomerular handling of immune complex in the acute phase of active in situ immune complex glomerulonephritis employing cationized ferritin in rats. Ultrastructural localization of immune complex, complements and inflammatory cells. Virchows Arch, 1997;431:53–61. [DOI] [PubMed] [Google Scholar]
- [77].Loimaranta V, Hepojoki J, Laaksoaho O, Pulliainen AT. Galectin-3-binding protein: A multitask glycoprotein with innate immunity functions in viral and bacterial infections. Journal of leukocyte biology, 2018;104:777–86. [DOI] [PubMed] [Google Scholar]
- [78].Ostergaard O, Nielsen CT, Iversen LV, Tanassi JT, Knudsen S, Jacobsen S et al. Unique protein signature of circulating microparticles in systemic lupus erythematosus. Arthritis and rheumatism, 2013;65:2680–90. [DOI] [PubMed] [Google Scholar]
- [79].Suh JH, Miner JH. The glomerular basement membrane as a barrier to albumin. Nature reviews Nephrology, 2013;9:470–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Gallo GR, Caulin-Glaser T, Lamm ME. Charge of circulating immune complexes as a factor in glomerular basement membrane localization in mice. The Journal of clinical investigation, 1981;67:1305–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Gauthier VJ, Mannik M. A small proportion of cationic antibodies in immune complexes is sufficient to mediate their deposition in glomeruli. Journal of immunology (Baltimore, Md : 1950), 1990;145:3348–52. [PubMed] [Google Scholar]
- [82].Morioka T, Woitas R, Fujigaki Y, Batsford SR, Vogt A. Histone mediates glomerular deposition of small size DNA anti-DNA complex. Kidney international, 1994;45:991–7. [DOI] [PubMed] [Google Scholar]
- [83].Nair RR, Mazza D, Brambilla F, Gorzanelli A, Agresti A, Bianchi ME. LPS-Challenged Macrophages Release Microvesicles Coated With Histones. Frontiers in immunology, 2018;9:1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Purushothaman A, Bandari SK, Liu J, Mobley JA, Brown EE, Sanderson RD. Fibronectin on the Surface of Myeloma Cell-derived Exosomes Mediates Exosome-Cell Interactions. The Journal of biological chemistry, 2016;291:1652–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Nishinarita S, Yamamoto M, Takizawa T, Hayakawa J, Karasaki M, Sawada S. Increased plasma fibronectin in patients with systemic lupus erythematosus. Clinical rheumatology, 1990;9:214–9. [DOI] [PubMed] [Google Scholar]
- [86].Atta MS, Lim KL, Ala’deen DA, Powell RJ, Todd I. Investigation of the prevalence and clinical associations of antibodies to human fibronectin in systemic lupus erythematosus. Ann Rheum Dis, 1995;54:117–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].van Bavel CC, Fenton KA, Rekvig OP, van der Vlag J, Berden JH. Glomerular targets of nephritogenic autoantibodies in systemic lupus erythematosus. Arthritis and rheumatism, 2008;58:1892–9. [DOI] [PubMed] [Google Scholar]
- [88].Abdul Ajees A, Gunasekaran K, Volanakis JE, Narayana SV, Kotwal GJ, Murthy HM. The structure of complement C3b provides insights into complement activation and regulation. Nature, 2006;444:221–5. [DOI] [PubMed] [Google Scholar]
- [89].Walport MJ. Complement. First of two parts. The New England journal of medicine, 2001;344:1058–66. [DOI] [PubMed] [Google Scholar]
- [90].Stahl AL, Sartz L, Karpman D. Complement activation on platelet-leukocyte complexes and microparticles in enterohemorrhagic Escherichia coli-induced hemolytic uremic syndrome. Blood, 2011;117:5503–13. [DOI] [PubMed] [Google Scholar]
- [91].Scolding NJ, Morgan BP, Houston WA, Linington C, Campbell AK, Compston DA. Vesicular removal by oligodendrocytes of membrane attack complexes formed by activated complement. Nature, 1989;339:620–2. [DOI] [PubMed] [Google Scholar]
- [92].Pascual M, Steiger G, Sadallah S, Paccaud JP, Carpentier JL, James R et al. Identification of membrane-bound CR1 (CD35) in human urine: evidence for its release by glomerular podocytes. The Journal of experimental medicine, 1994;179:889–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Clayton A, Harris CL, Court J, Mason MD, Morgan BP. Antigen-presenting cell exosomes are protected from complement-mediated lysis by expression of CD55 and CD59. European journal of immunology, 2003;33:522–31. [DOI] [PubMed] [Google Scholar]
- [94].Nauta AJ, Trouw LA, Daha MR, Tijsma O, Nieuwland R, Schwaeble WJ et al. Direct binding of C1q to apoptotic cells and cell blebs induces complement activation. European journal of immunology, 2002;32:1726–36. [DOI] [PubMed] [Google Scholar]
- [95].Bradley AJ, Brooks DE, Norrisjones R, Devine DV. C1q Binding to liposomes is surface charge dependent and is inhibited by peptides consisting of residues 14–26 of the human C1qA chain in a sequence independent manner, 1999;1418:19–30. [DOI] [PubMed] [Google Scholar]
- [96].Yin W, Ghebrehiwet B, Peerschke EI. Expression of complement components and inhibitors on platelet microparticles. Platelets, 2008;19:225–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Sadallah S, Eken C, Schifferli JA. Erythrocyte-derived ectosomes have immunosuppressive properties. Journal of leukocyte biology, 2008;84:1316–25. [DOI] [PubMed] [Google Scholar]
- [98].Liu D, Feng L, Song YK. Recognition and clearance of liposomes containing phosphatidylserine are mediated by serum opsonin, 1995;1235:140–6. [DOI] [PubMed] [Google Scholar]
- [99].Clayton A, Harris CL, Court J, Mason MD, Morgan BP. Antigen-presenting cell exosomes are protected from complement-mediated lysis by expression of CD55 and CD59. Eur J Immunol;33:522–31. [DOI] [PubMed] [Google Scholar]
- [100].Role of complement receptor 1 (CR1; CD35) on epithelial cells: A model for understanding complement-mediated damage in the kidney. Mol Immunol;67:S0161589015300249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Salvi V, Gianello V, Tiberio L, Sozzani S, Bosisio D. Cytokine Targeting by miRNAs in Autoimmune Diseases. Frontiers in immunology, 2019;10:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Wang JG, Williams JC, Davis BK, Jacobson K, Doerschuk CM, Ting JP et al. Monocytic microparticles activate endothelial cells in an IL-1β-dependent manner. Blood, 2011;118:2366–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Rasmussen NS, Jacobsen S. Microparticles - culprits in the pathogenesis of systemic lupus erythematosus? Expert review of clinical immunology, 2018;14:443–5. [DOI] [PubMed] [Google Scholar]
- [104].Cloutier N, Tan S, Boudreau LH, Cramb C, Subbaiah R, Lahey L et al. The exposure of autoantigens by microparticles underlies the formation of potent inflammatory components: the microparticle-associated immune complexes. EMBO Mol Med, 2013;5:235–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Sir Elkhatim R, Li JY, Yong TY, Gleadle JM. Dipping your feet in the water: podocytes in urine. Expert review of molecular diagnostics, 2014;14:423–37. [DOI] [PubMed] [Google Scholar]
- [106].Devarapu SK, Lorenz G, Kulkarni OP, Anders HJ, Mulay SR. Cellular and Molecular Mechanisms of Autoimmunity and Lupus Nephritis. International review of cell and molecular biology, 2017;332:43–154. [DOI] [PubMed] [Google Scholar]
- [107].Yao G, Liu ZH, Zheng C, Zhang X, Chen H, Zeng C et al. Evaluation of renal vascular lesions using circulating endothelial cells in patients with lupus nephritis. Rheumatology (Oxford, England), 2008;47:432–6. [DOI] [PubMed] [Google Scholar]
- [108].Nielsen CT, Østergaard O, Johnsen C, Jacobsen S, Heegaard NH. Distinct features of circulating microparticles and their relationship to clinical manifestations in systemic lupus erythematosus. Arthritis and rheumatism, 2011;63:3067–77. [DOI] [PubMed] [Google Scholar]
- [109].O’Sullivan KM, Lo CY, Summers SA, Elgass KD, McMillan PJ, Longano A et al. Renal participation of myeloperoxidase in antineutrophil cytoplasmic antibody (ANCA)-associated glomerulonephritis. Kidney international, 2015;88:1030–46. [DOI] [PubMed] [Google Scholar]
- [110].Cheng D, Talib J, Stanley CP, Rashid I, Michaëlsson E, Lindstedt EL et al. Inhibition of MPO (Myeloperoxidase) Attenuates Endothelial Dysfunction in Mouse Models of Vascular Inflammation and Atherosclerosis. Arteriosclerosis, thrombosis, and vascular biology, 2019;39:1448–57. [DOI] [PubMed] [Google Scholar]
- [111].Jerke U, Rolle S, Purfürst B, Luft FC, Nauseef WM, Kettritz R. β2 integrin-mediated cell-cell contact transfers active myeloperoxidase from neutrophils to endothelial cells. The Journal of biological chemistry, 2013;288:12910–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Yang JJ, Preston GA, Pendergraft WF, Segelmark M, Heeringa P, Hogan SL et al. Internalization of proteinase 3 is concomitant with endothelial cell apoptosis and internalization of myeloperoxidase with generation of intracellular oxidants. The American journal of pathology, 2001;158:581–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Olson SW, Lee JJ, Poirier M, Little DJ, Prince LK, Baker TP et al. Anti-Myeloperoxidase Antibodies Associate with Future Proliferative Lupus Nephritis. Autoimmune Dis, 2017;2017:1872846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Zheng H, Chen Y, Ao W, Shen Y, Chen XW, Dai M et al. Antiphospholipid antibody profiles in lupus nephritis with glomerular microthrombosis: a prospective study of 124 cases. Arthritis research & therapy, 2009;11:R93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Wang JG, Williams JC, Davis BK, Jacobson K, Doerschuk CM, Ting JP et al. Monocytic microparticles activate endothelial cells in an IL-1beta-dependent manner. Blood, 2011;118:2366–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Lee SK, Yang SH, Kwon I, Lee OH, Heo JH. Role of tumour necrosis factor receptor-1 and nuclear factor-kappaB in production of TNF-alpha-induced pro-inflammatory microparticles in endothelial cells. Thrombosis and haemostasis, 2014;112:580–8. [DOI] [PubMed] [Google Scholar]
- [117].Burbano C, Gomez-Puerta JA, Munoz-Vahos C, Vanegas-Garcia A, Rojas M, Vasquez G et al. HMGB1(+) microparticles present in urine are hallmarks of nephritis in patients with systemic lupus erythematosus. European journal of immunology, 2019;49:323–35. [DOI] [PubMed] [Google Scholar]
- [118].Sims GP, Rowe DC, Rietdijk ST, Herbst R, Coyle AJ. HMGB1 and RAGE in inflammation and cancer. Annual review of immunology, 2010;28:367–88. [DOI] [PubMed] [Google Scholar]
- [119].Kim S, Kim SY, Pribis JP, Lotze M, Mollen KP, Shapiro R et al. Signaling of high mobility group box 1 (HMGB1) through toll-like receptor 4 in macrophages requires CD14. Mol Med, 2013;19:88–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Boilard E, Nigrovic PA, Larabee K, Watts GFM, Coblyn JS, Weinblatt ME et al. Platelets amplify inflammation in arthritis via collagen-dependent microparticle production. Science (New York, NY), 2010;327:580–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Cloutier N, Tan S, Boudreau LH, Cramb C, Subbaiah R, Lahey L et al. The exposure of autoantigens by microparticles underlies the formation of potent inflammatory components: the microparticle-associated immune complexes. EMBO Mol Med, 2013;5:235–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Salvi V, Gianello V, Busatto S, Bergese P, Andreoli L, D’Oro U et al. Exosome-delivered microRNAs promote IFN-alpha secretion by human plasmacytoid DCs via TLR7. JCI insight, 2018;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Appel GB, Pirani CL, D’Agati V. Renal vascular complications of systemic lupus erythematosus. Journal of the American Society of Nephrology : JASN, 1994;4:1499–515. [DOI] [PubMed] [Google Scholar]
- [124].Wolf P. The nature and significance of platelet products in human plasma. British journal of haematology, 1967;13:269–88. [DOI] [PubMed] [Google Scholar]
- [125].Morel O, Jesel L, Freyssinet JM, Toti F. Cellular mechanisms underlying the formation of circulating microparticles. Arteriosclerosis, thrombosis, and vascular biology, 2011;31:15–26. [DOI] [PubMed] [Google Scholar]
- [126].Owens AP 3rd,Mackman N.Microparticles in hemostasis and thrombosis. Circulation research, 2011;108:1284–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Combes V, Simon AC, Grau GE, Arnoux D, Camoin L, Sabatier F et al. In vitro generation of endothelial microparticles and possible prothrombotic activity in patients with lupus anticoagulant. The Journal of clinical investigation, 1999;104:93–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Van Der Meijden PE, Van Schilfgaarde M, Van Oerle R, Renne T, ten Cate H, Spronk HM. Platelet- and erythrocyte-derived microparticles trigger thrombin generation via factor XIIa. Journal of thrombosis and haemostasis : JTH, 2012;10:1355–62. [DOI] [PubMed] [Google Scholar]
- [129].Gavriilaki E, Anagnostopoulos A, Mastellos DC. Complement in Thrombotic Microangiopathies: Unraveling Ariadne’s Thread Into the Labyrinth of Complement Therapeutics. Frontiers in immunology, 2019;10:337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Song D, Wu LH, Wang FM, Yang XW, Zhu D, Chen M et al. The spectrum of renal thrombotic microangiopathy in lupus nephritis. Arthritis research & therapy, 2013;15:R12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Lood C, Eriksson S, Gullstrand B, Jönsen A, Sturfelt G, Truedsson L et al. Increased C1q, C4 and C3 deposition on platelets in patients with systemic lupus erythematosus--a possible link to venous thrombosis? Lupus, 2012;21:1423–32. [DOI] [PubMed] [Google Scholar]
- [132].Subramaniam S, Jurk K, Hobohm L, Jäckel S, Saffarzadeh M, Schwierczek K et al. Distinct contributions of complement factors to platelet activation and fibrin formation in venous thrombus development. Blood, 2017;129:2291–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Hamilton KK, Hattori R, Esmon CT, Sims PJ. Complement proteins C5b-9 induce vesiculation of the endothelial plasma membrane and expose catalytic surface for assembly of the prothrombinase enzyme complex. The Journal of biological chemistry, 1990;265:3809–14. [PubMed] [Google Scholar]
- [134].Muller-Calleja N, Hollerbach A, Ritter S, Pedrosa DG, Strand D, Graf C et al. Tissue factor pathway inhibitor primes monocytes for antiphospholipid antibody-induced thrombosis. Blood, 2019;134:1119–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Bagot CN, Arya R. Virchow and his triad: a question of attribution. British journal of haematology, 2008;143:180–90. [DOI] [PubMed] [Google Scholar]
- [136].Liu ML, Williams KJ. Microvesicles: potential markers and mediators of endothelial dysfunction. Curr Opin Endocrinol Diabetes Obes, 2012;19:121–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Iba T, Levy JH. Inflammation and thrombosis: roles of neutrophils, platelets and endothelial cells and their interactions in thrombus formation during sepsis. Journal of thrombosis and haemostasis : JTH, 2018;16:231–41. [DOI] [PubMed] [Google Scholar]
- [138].Steppich B, Mattisek C, Sobczyk D, Kastrati A, Schomig A, Ott I. Tissue factor pathway inhibitor on circulating microparticles in acute myocardial infarction. Thrombosis and haemostasis, 2005;93:35–9. [DOI] [PubMed] [Google Scholar]
- [139].Perez-Casal M, Downey C, Fukudome K, Marx G, Toh CH. Activated protein C induces the release of microparticle-associated endothelial protein C receptor. Blood, 2005;105:1515–22. [DOI] [PubMed] [Google Scholar]
- [140].Tunnicliffe DJ, Palmer SC, Henderson L, Masson P, Craig JC, Tong A et al. Immunosuppressive treatment for proliferative lupus nephritis. The Cochrane database of systematic reviews, 2018;6:Cd002922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Tamirou F, D’Cruz D, Sangle S, Remy P, Vasconcelos C, Fiehn C et al. Long-term follow-up of the MAINTAIN Nephritis Trial, comparing azathioprine and mycophenolate mofetil as maintenance therapy of lupus nephritis. Ann Rheum Dis, 2016;75:526–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Liu Z, Zhang H, Liu Z, Xing C, Fu P, Ni Z et al. Multitarget therapy for induction treatment of lupus nephritis: a randomized trial. Ann Intern Med, 2015;162:18–26. [DOI] [PubMed] [Google Scholar]
- [143].Mok CC, Ying KY, Yim CW, Siu YP, Tong KH, To CH et al. Tacrolimus versus mycophenolate mofetil for induction therapy of lupus nephritis: a randomised controlled trial and long-term follow-up. Ann Rheum Dis, 2016;75:30–6. [DOI] [PubMed] [Google Scholar]
- [144].Tkach M, Thery C. Communication by Extracellular Vesicles: Where We Are and Where We Need to Go. Cell, 2016;164:1226–32. [DOI] [PubMed] [Google Scholar]
- [145].Cantaluppi V, Medica D, Mannari C, Stiaccini G, Figliolini F, Dellepiane S et al. Endothelial progenitor cell-derived extracellular vesicles protect from complement-mediated mesangial injury in experimental anti-Thy1.1 glomerulonephritis. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association, 2015;30:410–22. [DOI] [PubMed] [Google Scholar]
- [146].Pomatto MAC, Gai C, Bussolati B, Camussi G. Extracellular Vesicles in Renal Pathophysiology. Frontiers in molecular biosciences, 2017;4:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Nargesi AA, Lerman LO, Eirin A. Mesenchymal Stem Cell-derived Extracellular Vesicles for Renal Repair. Curr Gene Ther, 2017;17:29–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Wang H, Wang B, Zhang A, Hassounah F, Seow Y, Wood M et al. Exosome-Mediated miR-29 Transfer Reduces Muscle Atrophy and Kidney Fibrosis in Mice. Mol Ther, 2019;27:571–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Liu ML, Werth VP, Williams KJ. Blood plasma versus serum: which is right for sampling circulating membrane microvesicles in human subjects? Ann Rheum Dis, 2020;79:e73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Chen Y, Li G, Liu ML. Microvesicles as Emerging Biomarkers and Therapeutic Targets in Cardiometabolic Diseases. Genomics, proteomics & bioinformatics, 2018;16:50–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Lu J, Hu ZB, Chen PP, Lu CC, Zhang JX, Li XQ et al. Urinary podocyte microparticles are associated with disease activity and renal injury in systemic lupus erythematosus. BMC Nephrol, 2019;20:303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Tan EM, Kunkel HG. Characteristics of a soluble nuclear antigen precipitating with sera of patients with systemic lupus erythematosus. Journal of immunology (Baltimore, Md : 1950), 1966;96:464–71. [PubMed] [Google Scholar]
- [153].Duvvuri B, Lood C. Cell-Free DNA as a Biomarker in Autoimmune Rheumatic Diseases. Frontiers in immunology, 2019;10:502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Fernando MR, Jiang C, Krzyzanowski GD, Ryan WL. New evidence that a large proportion of human blood plasma cell-free DNA is localized in exosomes. PloS one, 2017;12:e0183915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Fan Q, Yang L, Zhang X, Peng X, Wei S, Su D et al. The emerging role of exosome-derived non-coding RNAs in cancer biology. Cancer Lett, 2018;414:107–15. [DOI] [PubMed] [Google Scholar]
- [156].Li Y, Zheng Q, Bao C, Li S, Guo W, Zhao J et al. Circular RNA is enriched and stable in exosomes: a promising biomarker for cancer diagnosis. Cell Res, 2015;25:981–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Hinger SA, Cha DJ, Franklin JL, Higginbotham JN, Dou Y, Ping J et al. Diverse Long RNAs Are Differentially Sorted into Extracellular Vesicles Secreted by Colorectal Cancer Cells. Cell reports, 2018;25:715–25.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Zhao CN, Mao YM, Liu LN, Li XM, Wang DG, Pan HF. Emerging role of lncRNAs in systemic lupus erythematosus. Biomed Pharmacother, 2018;106:584–92. [DOI] [PubMed] [Google Scholar]
- [159].Jin J, Sun H, Shi C, Yang H, Wu Y, Li W et al. Circular RNA in renal diseases. J Cell Mol Med, 2020;24:6523–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Long H, Wang X, Chen Y, Wang L, Zhao M, Lu Q. Dysregulation of microRNAs in autoimmune diseases: Pathogenesis, biomarkers and potential therapeutic targets. Cancer Lett, 2018;428:90–103. [DOI] [PubMed] [Google Scholar]
- [161].Wang G, Tam LS, Li EK, Kwan BC, Chow KM, Luk CC et al. Serum and urinary free microRNA level in patients with systemic lupus erythematosus. Lupus, 2011;20:493–500. [DOI] [PubMed] [Google Scholar]
- [162].Perez-Hernandez J, Forner MJ, Pinto C, Chaves FJ, Cortes R, Redon J. Increased Urinary Exosomal MicroRNAs in Patients with Systemic Lupus Erythematosus. PloS one, 2015;10:e0138618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Ichii O, Otsuka-Kanazawa S, Horino T, Kimura J, Nakamura T, Matsumoto M et al. Decreased miR-26a expression correlates with the progression of podocyte injury in autoimmune glomerulonephritis. PloS one, 2014;9:e110383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Tangtanatakul P, Klinchanhom S, Sodsai P, Sutichet T, Promjeen C, Avihingsanon Y et al. Down-regulation of let-7a and miR-21 in urine exosomes from lupus nephritis patients during disease flare. Asian Pac J Allergy Immunol, 2018. [DOI] [PubMed] [Google Scholar]
- [165].Sole C, Cortes-Hernandez J, Felip ML, Vidal M, Ordi-Ros J. miR-29c in urinary exosomes as predictor of early renal fibrosis in lupus nephritis. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association, 2015;30:1488–96. [DOI] [PubMed] [Google Scholar]
- [166].Garcia-Vives E, Solé C, Moliné T, Vidal M, Agraz I, Ordi-Ros J et al. The Urinary Exosomal miRNA Expression Profile is Predictive of Clinical Response in Lupus Nephritis. International journal of molecular sciences, 2020;21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Mobarrez F, Fuzzi E, Gunnarsson I, Larsson A, Eketjall S, Pisetsky DS et al. Microparticles in the blood of patients with SLE: Size, content of mitochondria and role in circulating immune complexes. Journal of autoimmunity, 2019;102:142–9. [DOI] [PubMed] [Google Scholar]
- [168].Rother N, Pieterse E, Lubbers J, Hilbrands L, van der Vlag J. Acetylated Histones in Apoptotic Microparticles Drive the Formation of Neutrophil Extracellular Traps in Active Lupus Nephritis. Frontiers in immunology, 2017;8:1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Lu GY, Xu RJ, Zhang SH, Qiao Q, Shen L, Li M et al. Alteration of circulatory platelet microparticles and endothelial microparticles in patients with chronic kidney disease. Int J Clin Exp Med, 2015;8:16704–8. [PMC free article] [PubMed] [Google Scholar]
- [170].Li Y, Xu X, Tang X, Bian X, Shen B, Zhao H et al. MicroRNA expression profile of urinary exosomes in Type IV lupus nephritis complicated by cellular crescent. J Biol Res (Thessalon), 2018;25:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Botto M, Dell’Agnola C, Bygrave AE, Thompson EM, Cook HT, Petry F et al. Homozygous C1q deficiency causes glomerulonephritis associated with multiple apoptotic bodies. Nature genetics, 1998;19:56–9. [DOI] [PubMed] [Google Scholar]
- [172].Takemura T, Murakami K, Miyazato H, Yagi K, Yoshioka K. Expression of Fas antigen and Bcl-2 in human glomerulonephritis. Kidney international, 1995;48:1886–92. [DOI] [PubMed] [Google Scholar]


