Abstract
The kidney is a complicated and important internal organ receiving approximately 20% of the cardiac output and mediates numerous pathophysiologic actions. These include selectively filtering macromolecules of the blood, exquisite reclaimation of electrolyctes, urine concentration via an elegant osmotic mechanisms, and excretion of an acid load. In addition, the renal tubules carry out secretory functions and produce hormones and cytokines. The kidney receives innervation and hormonal regulation. Therefore, dysfunction of the kidney leads to retention of metabolic waste products, and/or significant proteinuria and hematuria. In the past several decades, the role of extracellular vesicles (EV) in intercellular communications, and the uptake of extracellular vesicles by recipient cells through phagocytosis and endocytosis have been elucidated. The new knowledge on EVs expands over the classical mechanisms of cellular interaction, and may change our way of thinking of renal pathophysiology in the subcellular scale. Based on some ultrastructural discoveries in the kidney, this review will focus on the role of extracellular vesicles in intercellular communications, their internalization by recipient cells, and their relationship to renal pathology.
Keywords: Excetracellular vesicles, exsomes, microvesicles, apoptosis, phagocytosis, endocytosis, renal disease
1. Introduction
For several decades, medical knowledge of the kidney has expanded. Until the 1970’s, the renal microanatomy field has identified glomerular structures, segments of renal tubules, and the renal microvasculature using light microscopy and electron microscopy (Gordon, 2014; Mezzogiorno et al., 2002). In the second stage, mciropuncture and other techniques have been developed from the 1970’s to study the single nephrons for evaluation of glomerular filtration rate, renal tubular reabsorption and secretion, tubule-glomerular feedback, and hormonal and neural regulation of the kidney (Brenner, 2003; Lytvyn et al., 2019). In the third stage, the extensive understanding of the kidney has been achieved through variants of genetic analysis for identification of the detailed genetic network and their interaction since 1990 (Charlesworth, 2010; McIntosh and Hays, 2016; Rimoin and Hirschhorn, 2004; Singh et al., 2004). Currently, the molecular and genetic analyses of cellular biology and intercellular communications have evolved from basic research to clinical practice for diagnostic and therapeutic applications in various human diseases, including renal diseases.
Cell death is crucial to the pathophysiology of various kidney diseases, including acute tubular necrosis, necrotizing glomerulonephritis, cystic kidney disease, renal autoimmune diseases, delayed graft function, and kidney transplant rejection (Sarhan et al., 2018). Cell death is a natural biological process that occurs under both physiological and pathological conditions (Thery et al., 2018; Wu etah, 2019). Recent studies indicate that extracellular vesicles (EVs) can be released from cells that undergo several types of programmed cell death, including apoptosis, necroptosis, pyroptosis, or NETosis (Luan et al., 2020a; Wu et al., 2019; Liu et al., 2020). In addition, studies have shown that senescent cells may also release membrane EVs into the extracellular space (Thery et al., 2018; Wu et al., 2019). EVs are released through exocytosis or budding from cell surface of their parental cells, and were thought to be extracellular waster or “dusts”. However, accumulating studies have shown that EVs are involved in pathophysiology of various human diseases (Thery et al., 2018). EVs can be divided into 3 subclasses based on their sizes, exosome (10 – 100 nm), ectosome (microvesicles, 100 to 1000 nm), and apoptotic bodies (1000 to 5000 nm) (Li et al., 2020; Liu et al., 2016). EVs are enclosed by bilayer lipid membranes and contain a large range of materials including nucleic acids, miRNA, proteins, and lipid moieties (Li et al., 2020; Liu et al., 2016).
Recent studies have shown that EVs play important roles in intercellular communication, and transfer of genetic information etc (Thery et al., 2018; Wu et al., 2019). EVs may exert their functions through hormonal and paracrine/autocrine effects (Thery et al., 2018; Wu et al., 2019). EVs can act on their parental cell (autocrine effects) or on other cells that are either local (paracrine effects), or remote (hormonal effects) (Li et al., 2020). It is well known that the cardiovascular system and central/peripheral neural system play dominant roles in metabolic transportation, physical coordination and thinking/emotional processes in the body. Based on recent research, EV systems may serve as a newly discovered communication system in the human body. After the EVs are released, they carry information/molecules from their parental cells, and transport them to target cells either local or remote, for intercellular communications under normal physiologic conditions (Liu et al., 2016). The new knowledge may change our way of thinking of renal pathophysiology. This review will discuss how the released EVs interact with variants of renal tubular, glomerular and vascular cells.
2. EVs in transplantation rejection
The knowledge of renal transplant rejection has expanded rapidly in the past 20 years (Haas et al., 2018). Acute cellular rejection is a classic type of rejection, mainly mediated by T lymphocytes, which infiltrate into renal parenchyma and penetrate through tubular basement membranes resulting in tubulitis, and cause vasculitis (Loupy et al., 2020) (Figure 1a). EVs are important in mediating the acute cellular rejection through the release of graft antigens, antigen presentation to dendritic cells, and the activation of T lymphocytes and other inflammatory cells (Benichou et al., 2020; Monguio-Tortajada et al., 2014; Quaglia et al., 2020). Acute and chronic antibody-mediated rejection has been extensively investigated over the past 15 years (Colvin and Smith, 2005; Racusen et al., 2003). Acute antibody-mediated rejection, can occur within days after renal transplantation, results from the development of donor specific antibody against donor human leukocyte antigens. This type of rejection leads to an initial acute tubular injury, peri-capillary neutrophil infiltration, and a thrombotic microangiopathy-like change in glomeruli, as well as vasculitis or parenchymal infarction (Loupy et al., 2020). Complement C4d deposition along peritubular capillaries is a key hallmark for identifying this type of humoral rejection, implying the involvement of complement system in the rejection processes (Colvin and Smith, 2005). Overtime, the chronic antibody-medicated rejection can develop and cause gradual deterioration, leading to renal failure and proteinuria. The rejection process could be mediated by various EVs (Cardinal et al., 2018; Dieude et al., 2020; Jung et al., 2020), which are released from the dead cells of the donor tissue. The EVs from donor tissue may carry autoantigens that trigger the production of autoantibodies, called donor specific antibody, by B cells of the recipients, thus contributing to the antibody-mediated rejection. In addition, BK virus infection, ascending from bladder to kidney, may also cause the release of EVs that trigger inflammatory reactions in the transplant kidney (Handala et al., 2020). Therefore urine/blood testing and renal transplant biopsies are needed to distinguish the acute cellular rejection from BK virus infection associated inflammation.
Figure 1. Schematic illustration of various common renal diseases.
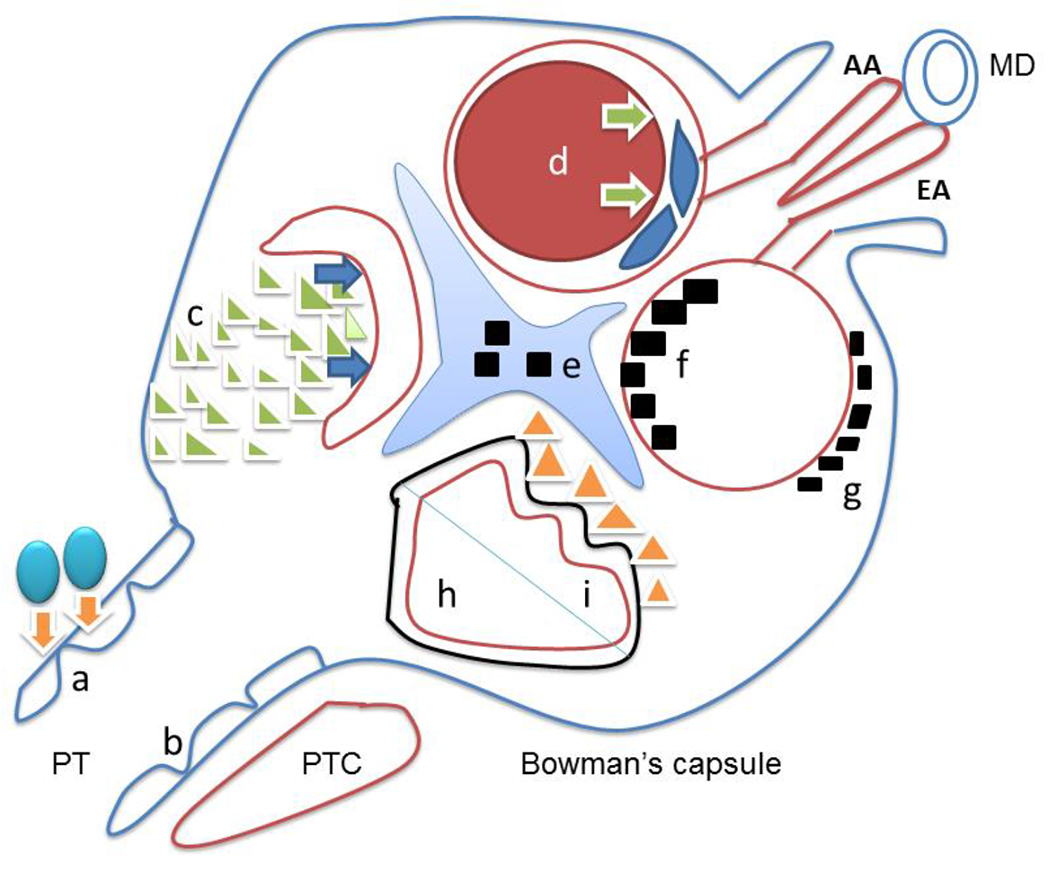
Letters represent following renal diseases: a. acute cellular rejection is due to T lymphocytes infiltration (blue balls) to renal tubules (orange arrows); b. acute tubular injury with flattened epithelium (blue line) usually results from either ischemic or toxic insult to proximal tubules; c. crescentic glomerulonephritis associated with ANCA or anti-glomerular basement antibody results from the proliferation of parietal epithelial cells (green triangles), leading to collapsing glomerular capillary loops (blue arrows) and acute renal failure; d. thrombotic microangiopathy with multiple etiologies, is characterized with thrombosis (red circle) causing luminar obstruction (green arrows) and edematous glomerular endothelial cells (blue spindle endothelial cells); e. mesangial deposits are seen in IgA nephropathy; f. subendothelial deposits are usually found in membranoprolifeative glomerulonephritis and diffuse proliferative lupus nephritis (black squares within red glomerular basement membrane) ; g. subepithelial deposits seen in membranous glomerulopathy are due to primary or secondary etiologies (black squares outside the red glomerular basement membrane); h. minimal change disease and focal segmental glomerulosclerosis (NOS) typically shows diffuse fusion of fusion processes (solid black line outside of red glomerular basement membrane); i, collapsing glomerulopathy is characterized by collapsed glomerular basement membrane (curved red line) with proliferative podocytes (yellow triangles). AA – afferent arteriole, EA – efferent arteriole, MD – macular densa, PT – proximal tubules, PTC – peritubular capillaries.
During cellular- or antibody-mediated rejection, studies have identified that EVs may serve as biomarkers or mediators involved in the rejection processes (Benichou et al., 2020; Quaglia et al., 2020; Rigalli et al., 2020). In 1980’s, morphologic identification of extracellular particles were observed by electron microscopy (Pan and Johnstone, 1983), following by the improved technique of electron microscopy with the gold-labeling antibody (Pan et al., 1985). The following illustrations will introduce the pathologic histology of acute cellular rejection in the human kidney. As shown in the left panel of Figure 2, an electron microscopic image at 10,000 magnifications illustrates a proximal tubule, lined by tubular basement membranes, at the top portion of the image. The bottom shows a lymphocyte with relatively scant cytoplasm before its invasion into the renal tubule. There are sizable foamy particles extruded from the lymphocyte in close connection to leak cytoplasmic materials of proximal tubules through the tubular basement membrane. The extruded excellular materials from the lymphocyte must be fused from numerous different sizes of exosomes and/or microvesicles with a variety of contents – conceivably various cytokines and enzymes. Once the tubular basement membrane shows a broken segment, the activated lymphocyte invades the tubular epithelium. The right panel of Figure 2 reveals that a portion of the lymphocyte cytoplasm has passed through the tubular basement membrane before it fully infiltrating into the renal tubular epithelium as a tubulitis, typical of acute cellular rejection. These ultrastructural images of graft tubules provide evidence of EVs involvement in the acute cell rejection.
Figure 2. Interaction of acute cellular rejection captured by electron microscopy.
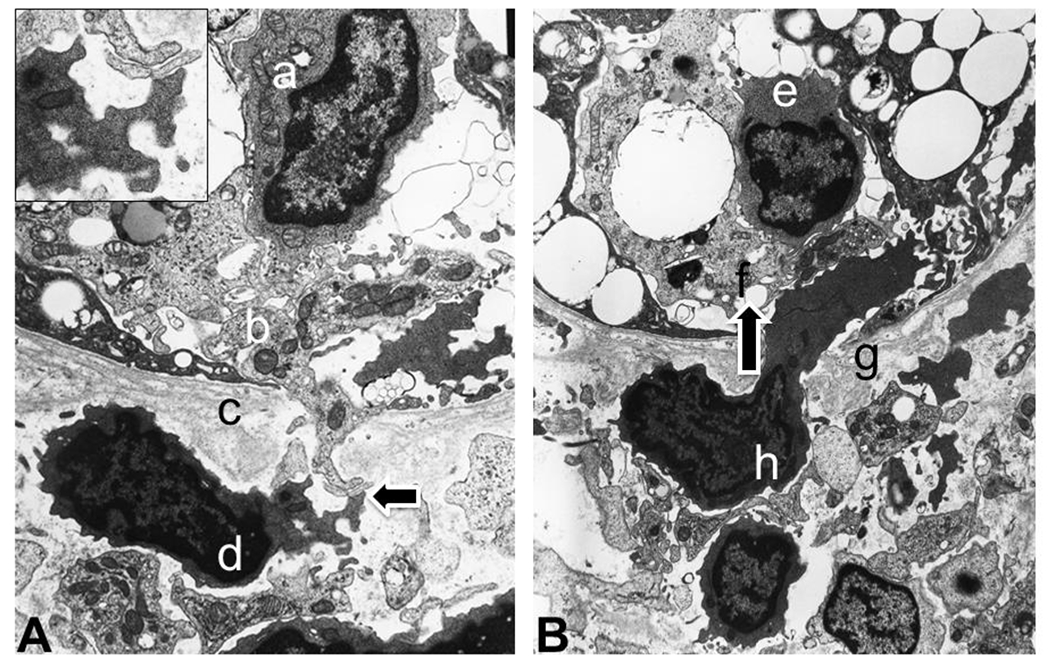
Panel A (left). Leaked proximal tubular cytoplasm, through tubular basement membranes interacts with extracellular materials of lymphocytes as an induction of lymphocytic infiltration. Letters represent following structures: a. an infiltrated lymphocyte in the proximal tubule (tubulitis); b. cytoplasm of proximal tubules with leaking component through tubular basement membrane (c); d. lymphocyte releasing extracellular particles to interact with proximal tubular cytoplasm (indicated by an arrow; details presented in the left upper corner insert). Panel B. Activated lymphocyte physically penetrates through tubular basement membrane into the proximal tubule (infiltration direction is indiacted by an arrow). Letters represent following structures: e. infiltrated lymphocyte (tubulitis); f. proximal tubular epithelium; g. tubular basement membrane; h. lymphocyte extending beyond tubular basement membrane as an action of infiltration.
There are still many unanswered questions remain to be addressed for optimized renal transplant outcome. These may include tissue typing for human leukocyte antigens, donor to recipient tissue matching, and interactions among cellular components including antigen-presenting cells (mainly dendritic cells), T lymphocytes, B lymphocytes and other inflammatory cells. In addition, how antibody-mediated rejection interacts with the acute cellular rejection, and how we can prevent the antibody-mediated rejection are also under investigation, in order to develop better therapeutic strategies. EVs in urine or blood samples of the transplant recipients may be potential biomarkers for distinguishment of the above different conditions. Interestingly, the detection of EVs in the urine may serve as potential non-invasive liquid biopsy for monitoring the pathophysiological conditions of the renal transplant rejection.
3. EVs in acute tubular injury
Eighty percent of the renal cortical parenchyma contain proximal tubules that conduct active reabsorption of electrolytes, and are therefore vulnerable to variants of primary and secondary injury (Brezis and Rosen, 1995). The acute tubular injury in proximal tubules are mainly due to either intrinsic injury of proximal tubules by ischemic or toxic insults, or injuries due to various interstitial nephritis, or obstruction in the distal tubules such as monoclonal cast nephropathy (Figure 1b). A number of mechanisms have been proposed on how injured renal tubules are repaired following acute tubular injury. It is believed that either intratubular progenitor cells or residual tubules play a critical role in repairing the damaged renal tubules by restoring new epithelial cells (Humphreys et al., 2008; Lazzeri et al., 2019).
The parietal epithelium of Bowman’s capsule are progenitor cells stained positively for progenitor marker CD133 (Sagrinati et al., 2006). Subsequently, these CD133 positive progenitor cells can also be found scattered along the entire length of renal tubules (Romagnani, 2011). During acute tubular injury, CD133 becomes positive in all epithelial cells of the proximal tubule, implying a repair process (Andrianova et al., 2019; Huling and Yoo, 2017; Zhang and Hafron, 2014). Although injection of mesenchymal stem cells can reduce acute kidney injury in experimental studies (Nawaz et al., 2016; Qiu et al., 2019), it is not clear how CD133 progenitor cells work and if they can release EVs along the Bowman’s capsule or renal tubules. Some studies have isolated and identified the CD133+ EVs in the urine, suggesting that these EVs can be a potential biomarker. Furthermore, the urinary CD133+ EVs have been detected in normal healthy people, while reduced levels of CD133+ EVs have been found in the urine of patients with end stage renal disease. Importantly, elevated levels of CD133+ EVs have been reported in patients receiving renal transplantation, indicating that CD133+ EVs may be important for maintaining the homeostatic status of the kidney (Dimuccio et al., 2014). Compared to healthy individuals, pediatric patients with acute glomerulonephritis have shown a reduced urinary level of CD133+ EVs, which return to normal levels with recovery from the renal disease (Dimuccio et al., 2020). This study suggests that urinary levels of CD133+ EVs may serve as a biomarker for monitoring the renal disease activity.
Endocytosis is an important step for internalizing EVs. The following example shows how proximal tubules repair the acute tubular injury through a phagocytoic receptor – kidney injury molecule-1 (KIM1-1). In response to severe insults from ischemia or toxicity, proximal tubules release apoptotic EVs. These apoptoic EVs activate one receptor called KIM-1 along the proximal tubules (Ichimura et al., 2008). KIM-1 is a type 1 transmembranous glycoprotein located along the luminal surface of proximal tubules and is upregulated during acute tubular injury (Ichimura et al., 1998). Thus KIM-1 has been used as a specific marker to identify acute tubular injury in human studies (Yin et al., 2019; Yin et al., 2018; Zhang et al., 2008). KIM-1 plays a phagocytotic role in engulfing the apoptotic bodies into residual proximal tubules (Ichimura et al., 2008). The major function of this phagocytosis/endocytosis process is to prevent apoptotic bodies from activation of potential innate inflammation reaction thus preventing further damage to the kidney (Ichimura et al., 2012; Yang et al., 2015). Without KIM-1’s protective phagocytosis/endocytosis during acute tubular injury, the kidney would show more harmful over-reactive inflammation and subsequent interstitial fibrosis.
The key issues for further understanding the acute tubular injury may include several aspects. First, the etiologie related to the acute tubular injury need further investigations, particularly in the vulnerable populations, such as senior citizens with medical history of diabetes and hypertension, and patients with metastatic tumors who receive either immune checkpoint blockade or variour chemotherapy with inhibition of cell proliferative pathways. Second, it is important to investigate what kinds of therapy can truly help patients with acute tubular injury to recover from their renal dysfunction. The third aspect is to focus on how to prevent the acute tubular injury transforming into the interstitial fibrosis. As discussed above, EVs may serve as biomarkers for monitoring the disease activity and renal functional recovery in patients following the acute tubular injury.
4. EVs in vasculitis and primary crescentic glomerulonephritis
We have recently reviewed the involvement of EVs in systematic vasculitis, representing mixed interactions among many elements including endothelial cells, platelets, inflammatory cells, and coagulation factors (Wu et al., 2019). When vasculitis occurs in the kidney, there are two primary mechanisms that primarily present at the glomerular level, namely anti-glomerular basement membrane type of crescentic gloemrunephritis (CGN) (McAdoo and Pusey, 2017) and anti-cytoplamic antibody (ANCA) associated pauci-immune variant of CGN (Jennette and Falk, 2008). We have discussed EVs and the involvement of endocytosis in the interaction of ANCA with complements in the glomerular endothelial cells, and their contribution to the rupture of glomerular basement membranes, and stimulation of parietal epithelial cell proliferation, resulting in cellular crescent formation (Wu et al., 2019).
Based on experimental studies of ANCA-associated CGN, activated lymphocytes differentiate into plasma cells to produce circulating ANCA against neutrophils and monocytes, and these ANCA are divided into two subtypes, namely myeloperoxidase (MPO)-ANCA and promteinase-3 (PR-3)-ANCA (Halbwachs and Lesavre, 2012; Jennette et al., 2011; Little et al., 2009). MPO plays a key role in neutrophils, by converting hydrogen peroxidase to hypochloride in the presence of a halide Cl− and amplifies the toxic effects of neutrophils. The glomerular endothelium uptakes EV-associated MPO in the circulation, or obtains MPO through cell-cell direct interaction with neutrophils, by means of endocytosis (Jerke et al., 2013). EV-associated MPO may be involved in the activation of complements C3 and C5, most likely through MPO-generated hypochlorite. Fujigaki and colleagues have also detected epithelial EVs with deposits of complement C5b-9 and immune complexes in sub-epithelial space under electron microscopy in glomerulonephritic rats (Fujigaki et al., 1997). Therefore, EV-associated MPO and complements together may cause endothelial injury (O’Flynn et al., 2014; Pitanga et al., 2014). EV-mediated endothelial injury may also be linked to many pathologic events, including reduced vascular relaxation (Liu et al., 2016), production of oxidants, promotion of atherosclerosis, recruitment of neutrophils and participation in necrotizing vasculitis (Astern et al., 2007; Eiserich et al., 2002; Klinke et al., 2011; McMillen et al., 2005; Xiao et al., 2002). Once the endothelium is broken, the glomerular basement membranes are eroded and ruptured, and the inflammatory cells are extruded into Bowman’s capsule, causing the proliferation of parietal epithelial cells and glomerular necrosis as necrotizing crescentic glomerulonephritis, which compresses glomerular capillary loops and leads to the renal failure (Falk and Jennette, 2010) (Figure 1c). Therefore, EVs and their associated-MPO or complements may contribute to renal vasculitis and primary crescentic glomerulonephritis.
Despite of many investigations conducted to understand a variety of vasculitis, it remains unclear what infectious microorganisms or autoimmune alterations would trigger the development of vasculitis in humans. In addition, it is also critical to find better pharmacologic agents that can maintain therapeutic effects against vasculitis with less side effects. As EVs are involved in the development of ANCA-associated renal vasculitis, EVs might be promising biomarkers for diagnosis and monitoring disease severity of renal vasculitis. Regulation of EV release could also be a potential therapeutic target.
5. EVs in thrombotic microangiopathy (TMA)
TMA has two classic variants, namely hemolytic uremic syndrome (HUS) and thrombotic thrombocytopenic purpura (Namal Rathnayaka et al., 2019). TMA can also be seen in many clinical scenarios such as positive anti-phospholipid antibody in lupus patients, pre-eclampsia, malignant hypertension, and atypical HUS. The renal endothelium of the atypical HUS is injured by the activated alternative complement pathway, triggering the coagulation cascade to form thrombi (Gallan and Chang, 2020).
Prothrombotic function is one of the frequently studied classical functions of EVs (Li et al., 2020; Liu et al., 2007; Wu et al., 2019). We have reported that EVs have pro-coagulant properties (Liu et al., 2007), particularly the monocyte-derived EVs with both tissue factors (TF) and phosphotydelserine (PS) exposed on the EV surface (Liu et al., 2007). Co-presence of PS with TF on EVs surface enhance their procoagulant potential as TF can trigger extrinsic blood coagulation cascade, while the negatively charged PS provides an ideal surface for the assembly of coagulation factors (Liu et al., 2007). As discussed above, EV-associated MPO and complements may induce endothelial damage that contributes to the pathogenesis of thrombosis. In addition, studies from vascular research fields have shown the connection between blood coagulation and angiogenesis/blood vessel development (Mackman and Davis, 2011). TF expression in endothelial cells may stimulate the expression of chemokine ligand 2 (CCL2), which facilitates the recruitment of vascular smooth muscle cells (VSMCs) and the stabilization of EC-VSMC networks, thus paving the way for blood vessel formation (Mackman and Davis, 2011).
In addition, it has long been known that vascular enodothelial growth factor (VEGF) is an angiogenic factor, which exerts multiple functions in stimulating angiogenesis. In the kidney, podocytes produce VEGF and release the EVs for two roles (Eremina and Quaggin, 2004; Foster et al., 2006). VEGF has been found to be associated with EVs (Ko et al., 2019). The EV-associated VEGF can interact with podocytes as an autocrine effect to maintain integrity of slit diaphragm connected by the foot processes of podocytes. The VEGF released by podocytes may also disseminate reversely across the glomerular basement membranes and affect the glomerular endothelial cells via a paracrine mechanism in order to maintain the endothelial integrity and suppress the activation of complements at the endothelial level. In recent years, VEGF inhibitors such as bevacizumab have been used as anti-cancer agents for treatment of metastasis of kidney, colon, lung, ovary and breast carcinoma (Agostino et al., 2010; Cardones and Banez, 2006). It has been reported that renal thrombotic microangiopathy is strongly associated with the anti-cancer treatment (Eremina et al., 2008; Izzedine et al., 2011). Blocking VEGF with an antagonist results in fusion of foot processes with subsequent proteinuria and the activation of the complement system in glomerular endothelial cells, triggering thrombotic microangiopathy (Eremina et al., 2008).
Intravitral injection of VEGF antagonists such as bevacizumab or ramibizumab has been used to treat both macular degeneration and diabetic retinal neovascularization (Rofagha et al., 2013). The majority of patients can tolerate the intravitrial injection well, without significant renal dysfunction and proteinuria (Diabetic Retinopathy Clinical Research et al., 2007). However, occasionally intravitral injection of VEGF can be associated with renal thrombotic microangiopathy (Pelle et al., 2011). An experimental study has extensively investigated how an intravitral injection of VEGF antagonist can cause thrombotic microangiopathy in the murine kidney (Keir et al., 2017). Evidently VEGF antagonists can leak into the circulation within 48 hours after an intravitral injection, with subsequent suppressed VEGF production in the podocytes. The reduced VEGF in the podocytes leads to the fusion of foot processes due to a lack of its autocrine effects, thus causing prointeuira. In addition, the reduced VEGF is associated with the activation of complement factor H due to a deficiency of the paracrine effects on the glomerular endothelium, thus triggering edematous endothelial changes and thrombotic microangiopathy, often leading to renal failure (Keir et al., 2017) (Figure 1d). Therefore, EVs may contribute to TMA through their associated prothrombotic TF and PS, as well as proangiogenic VEGF. Furthermore, EV-associated TF may be important to both thrombosis and angiogenesis.
We are still at an early stage for the understanding of the variants of thrombotic microangiopathy, particularly the atypical HUS (also called complement mediated thrombotic microangiopathy). Many unanswered questions still need to be addressed, i.e. what is the essential triggering event to activate the alternative pathway of complement system, and whether the complement mediated thrombotic microangiopathy has an overlap etiology with the C3 dominant glomerulonephritis and the C3 glomerulopathy (dense deposit disease). Investigations of EVs and their involvement in these renal diseases may provide insight into novel diagnostic and threrapeutic strategies for TMA.
6. EVs in minimal change disease and variants of focal segmental glomerulosclerosis
Minimal change disease and focal segmental glomeruloslerosis (FSGS) represent a spectrum of renal disease, leading to nephrotic range proteinuria, and are ultrastructually characterized by diffuse fusion of foot processes of podocytes, and also called podocytopathies (Suzuki et al., 2020). Minimal change disease usually shows unremarkable glomeruli, which stain negatively for IgG, IgA, IgM, kappa and lambda by immunofluorecent method (Figure 1h). FSGS shows segmental sclerosis areas in glomeruli, and negative immunofluorescent staining, and includes subtypes such as collapsing variants, tip lesion variant, hypercellular variant, perihilar variant and FSGS not otherwise specified (D’Agati et al., 2004). Patients with the collapsing variant of FSGS often present with nephrotic range proteinuria and acute renal failure. Morphologically, the collapsing variant of FSGS is characterized by collapsed glomerular basement membranes, proliferative podocytes in glomeruli and cystic dilation of distal tubules (Markowitz et al., 2001; Valeri et al., 1996). Huang et al reported urinary exosomal miR-193a can be a potential biomarker for FSGS (Huang et al., 2017).
Common etiologies of collapsing FSGS are HIV infection and mutation of APOL1 alleles in individuals of African descent, but other infections and drug reactions can cause the disease as well (Abid et al., 2020; Barisoni et al., 1999; Neyra et al., 2014; Patel et al., 2018) (Figure 1i). Since the COVID-19 pandemic from the beginning of 2020, the Sars-Cov-2 virus (nick.white@covid19crc.org) has infected millions of people and caused many deaths worldwide. The Sars-Cov-2 virus is known to cause lung infection with subsequent infection of other organs, including the kidneys, through viral interaction with cell surface receptors, angiotensin converting enzyme 2 (ACE2) (Batlle et al., 2020), and subsequent endocytosis (Luan et al., 2020b; Tan et al., 2004; Zhou et al., 2020). Electron microscopy has confirmed that the SARS-CoV-2 particles (60-110 nm, with 20 to 40 spikes) are surrounded by double layers of membranes as intra-cytoplasmic vesicles (approximately 600 nm in diameter) in glomerular endothelial cells, podocytes and proximal tubules (Farkash et al., 2020; Kissling, 2020; Su et al., 2020; Varga et al., 2020). These intracellular vesicles may release as EVs that carry Sars-CoV-2 components, including CD9 and ACE2 (Hassanpour et al., 2020). Upon entry into the recipient cells, COVID-19 virus may be directed into the exosomal pathway, and its component may be packaged into exosomes for secretion. The direct infection of COVID19 in renal tissue causes acute tubular injury, and collapsing focal segmental glomeruloslerosis (Farkash et al., 2020; Kissling, 2020; Su et al., 2020). Two recent case reports also confirm the development of collapsing FSGS in patients with positive COVID-19 (Larsen et al., 2020; Peleg et al., 2020). However, the involvement of EVs in COVID-19 infection should be further investigated, as several other recent studies report no definite SARS-CoV virus detected in the renal biopsies or autopsy kidneys from COVID-19 positive patients (Kudose et al., 2020; Santoriello et al., 2020; Sharma et al., 2020; Wu et al., 2020). Interestly, COVID-19 infection has been found to be associated with some coagulative abnormalities in the kidneys, leading to thrombosis and even renal infarction (Mukherjee et al., 2020; Philipponnet et al., 2020; Sardu et al., 2020). This may be due to the endothelial injury and activated coagulative cascade, in which EVs may be involved (Liu et al., 2020; Sardu et al., 2020).
Variants of podocytopathies remain largely mysterious in term of how the genetic vulnerability alters the molecular assembly of podocytes, and their related glomerular basement membrane and glomerular endothelial cells. It is also puzzling if there are some subtle auto-antibodies in the circulation that trigger the development various podocytopathies, as some transplant recipients can develop recurrent FSGS in one or two days following the transplantation. The new view of EVs and their potential involvement in virus infection and FSGS may provide insights into better understanding of the pathophysiology, and stablishment of novel diagnostic and therapeutic strategies.
7. EVs in monoclonal protein associated renal diseases
There are a number of new developments in paraprotein-related renal diseases. The key concept has been changed from monoclonal gammopathy of undetermined significance (MGUS) to monoclonal gammopathy of renal significance (MGRS) if the monoclonal protein deposits in the kidney cause acute renal failure and/or significant proteinuria despite bone marrow showing a small amount of monoclonal plasma cells (< 10%) (Bridoux et al., 2015; Leung et al., 2019; Leung et al., 2012). Based on this concept, MGRS can be treated with chemotherapy targeting the small B cell clone in the bone marrow to reduce further burden of monoclonal protein deposition in the kidney (Fermand et al., 2013; Sethi et al., 2018). The entities of MGRS includes amyloidosis AL or AH type, monoclonal light chain or heavy chain deposition disease, monoclonal proximal tubulopathy, proliferative glomerulonephritis with monoclonal immunoglobulin deposits, type 1 cryogluboulinemic glomerulopathy, monoclonoal fibrillary glomerulopathy, and immunotactoid glomerulopathy (Bridoux et al., 2015; Herrera, 2014; Leung et al., 2019). However, any of above entities can become a heavy burden group if monoclonal plasma cells progress to malignant myeloma amount (usually > 10 to 30 % of bone marrow). In addition, monoclonal cast nephropathy and type 2 monoclonal cryglobulinemic glomerulopathy (related to Waldenstrom macroglobulinemia) belong to the heavy burden group, as they are usually associated with a high rate of malignant myeloma and acute renal failure at the time of renal biopsy (Bridoux et al., 2015; Leung et al., 2019).
Studies have reported that malignant plasma cells produce large amounts of EV-containing monoclonal heavy chains, monoclonal light chains and other lipid and enzyme products (De Luca et al., 2019; Di Noto et al., 2014; Morandi et al., 2018). Monoclonal light chains are cytotoxic (Sanders et al., 1988), therefore their deposition in different renal compartments can cause various kidney injuries, leading to the renal failure (such as monoclonal cast nephropathy, monoclonal proximal tubulopathy and monoclonal light chain deposition disease), and nephrotic proteinuria (such as AL amyloidosis and proliferative glomerulopathy with monoclonal immunoglobulin deposits). Many in vitro and in vivo models have been created to study monoclonal light chain deposition in the kidneys, but not all variants of paraprotein-associated kidney diseases having their corresponding experimental models (Lai et al., 2019; Sirac et al., 2018). Monoclonal proximal tubulopathy is a relatively new entity among many varieties of paraprotein-associated kidney diseases (Decourt et al., 2003; Herrera, 2014). It is also a good example of how monoclonal light chains interact with their receptors along proximal tubules, leading to proximal tubular injury. The light chains are small molecules that can be freely filtered through the glomerular filtration barrier. When they reach the proximal tubules, they interact with two receptors; megalin and tubilin, and then undergo endocytosis into the proximal tubular cytoplasm (Batuman et al., 1998; Klassen et al., 2005). Due to the large quantity of monoclonal light chains present in the proximal tubules, the receptors readily become saturated (Nakhoul and Batuman, 2011). Once they are internalized into proximal epithelial cells through their receptors, they cause increased cytoplasmic free oxygen radicals to activate c-Src and NF-kB, resulting in the release of pro-inflammatory cytokines and leading to the proximal tubular injury (Sanders, 2011; Ying et al., 2019). Therefore, the acute proximal tubular injury due to monoclonal light chain deposition is a type of cytotoxic injury. Morphologically, there are four types of monoclonal proximal tubulopathy that have been observed (Herrera, 2014). Monoclonal light chains can be crystalized in the proximal tubular cytoplasm, often leading to Fanconi syndrome, called monoclonal proximal tubulopathy with cytoplasmic inclusions. Second, monoclonal light chains can trigger a surrounding inflammatory reaction causing an interstitial nephritis variant of monoclonal proximal tubulopathy. In addition, monoclonal light chains may occasionally be detained in lysosomes of proximal tubular cytoplasm, causing swelling of proximal tubular epithelium (also called lysosomal ingestion/constipation variant of monoclonal proximal tubulopathy). Finally, the monoclonal light chains can randomly distribute along proximal tubules, which can be identified by immunofluorescent staining, but there is no specific appearance by electron microscopy, thus called monoclonal proximal tubulopathy without cytoplasmic inclusions. The monoclonal proximal tubules can also co-exist with other variants of paraprotein-associated kidney diseases such as monoclonal cast nephropathy or monoclonal light chain deposition disease (Parasuraman et al., 2013).
Future studies can further investigate what type of monoclonal proteins can be associated with any particular type of monoclonal protein-related deposition in the kidney. So that, identifying a specific type of monoclonal protein based on the molecular structures, can be predictable for the disease type and its associated disease progression. It is also intriguing to develop animal models that are related to paraprotein deposition in the kidney, as these animal models will be very valuable for investigation of therapeutic effects. Further investigation of EVs will provide an opportunity to understand MGRS pathophysiology at cellular, subcellular, and molecular levels, which may help to develop novel diagnostic and therapeutic strategies for paraprotein-related renal diseases.
8. EVs in immune complex mediated glomerulonephritis and complement mediated glomerulopathy
Generally, immune complex mediated glomerulonephropathies include 1) membranous glomerulopathy that presents with nephrotic syndrome, 2) IgA nephropathy that presents with significant hematuria, and 3) lupus nephritis that is featured with different degrees of renal failure, proteinuria and/or hematuria (Bajema et al., 2018; Rodrigues et al., 2017; Ronco and Debiec, 2010) (Figure 1e–g). Conventional type 1, type 2 and type 3 membranoproliferative glomerulonephritis (MPGN) have been largely modified due to the expanding knowledge of the activated alternative pathway in the complement activation cascade (Pickering et al., 2013; Sethi et al., 2012). Classic type 2 MPGN (also called dense deposit disease) becomes a prototype of C3 glomerulopathy, which has dominant C3 positive immunofluorescent staining in glomeruli and rainbow-like aggregated complements along the glomerular basement membranes by electron microscopy (Sethi et al., 2012). In addition, classic type 1 MPGN is divided into two types. Some are called MPGN type 1 if immunoflourescent study shows some IgG, kappa and lambda stains, while others are now called C3 dominant glomerulonephritis when C3 staining is 2 to 3 + strong in the glomeruli in the absence of other staining (De Vriese et al., 2015; Sethi et al., 2012; Thurman and Nester, 2016).
Membranous nephropathy can be either primary type or secondary to various other medial conditions such as infection (i.e. hepatitis B infection), or autoimmune disease (i.e. lupus nephritis), or malignancy (Debiec and Ronco, 2011). The majority of primary membranous glomerulopathy result from phospholipase A2 receptor (PLA2R) mutation (a protein along glomerular basement membrane), which sloughed antigen component triggers the production of an antibody, resulting in antigen-antibody immune complex deposition at the subepithelial spaces of glomeruli (Bech et al., 2014; Bomback, 2018). In addition, thrombospondin type 1 domain containing 7A (THSD7A), another antibody, has been found in the minority of cases of primary membranous glomerulopathy (Beck, 2017). Importantly, deposition of immune complex and complements with EVs in glomerular basement membranes have been detected in a rat model of glomerulonephritis (Fujigaki et al., 1997). In addition, G3BP-positive microvesicles and immune complex-associated microvesicles are significantly increased in lupus patients (Luan et al., 2020a; Zhao et al., 2020). Here, we illustrate the potential interaction of immune complex deposition and EV of different blood components in a glomerular capillary loop (Figure 3). Electronic microscopy shows an ultrastructural image at 6,000 magnifications of a glomerular capillary loop full of immune complex deposits at the subepithelial spaces (Figure 3). Within the capillary loop, there are multiple blood components including platelets, one neutrophil, one lymphocyte and other glomerular cells (Figure 3, left panel). Platelets are broken into fragments (Figure 3, the right upper insert), while the neutrophil reveals numerous membranous hair-like extensions as EVs (Figure 3, the right lower insert). Although specific proteins can not be identified on the electron microscopic image, the ultrastructural image further provides the morphologic evidence for the existence of EVs around cellular components in membranous glomerulopathy, thus turning the invisible network of EVs into an action reality of EV communication in the microstructural world of kidney.
Figure 3. Shedding platelet fragments and releasing extracellular vesicles of neutrophil as early sign of thrombosis in membranous glomerulopathy.
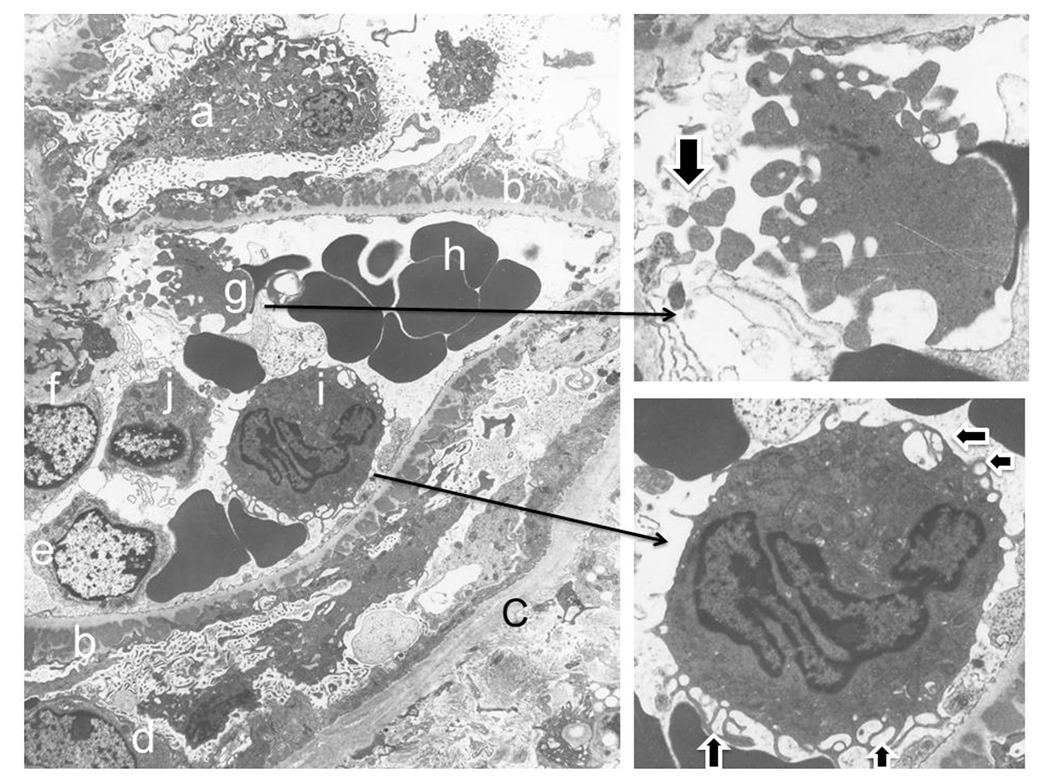
The left large panel of image captures shedding platelet fragments with a glomerular capillary loop, which details can be further seen in the right upper insert (fragments indicated by an arrow). Meanwhile, an activated neutrophil within the same loop demonstrates its exosome vesicles in the cytoplasm surface (indicated by two horizontal arrows in right lower panel insert) and releasing exosomes and/or microparticles from its cell surface membranes (two vertical arrows in the right lower panel inset). Letters represent following structures: a. podocyte; b. subpeithelial immune complex deposits above the glomerular basement membrane; c. Bowman’s capsule, d, parietal epithelial cell; e. glomerular endothelial cell; f. mesangial cell; g. platelet with fragmentation (also see in the insert in right upper panel); h. red blood cells; i. activated neutrophil (also see in the insert in right lower panel); j. lymphocyte.
There are still a lot of work to be done for better understanding how immune complex diseases are initiated. Although some blood tests for lupus nephritis are available, the triggering events leading to other renal diseases such as the membranous glomerulopathy, IgA nephropathy and membranoproliferative glomerulonephritis are largely unclear. Many challenges and improvements are still needed, i.e. better approaches to suppress and even alter the over-reactive status of the immune system in patients, and strategies to prevent the development of the chronic kidney disease from its active (acute) stage. As discussed above, and in our recently published review article about the role of EVs in pathophysiology of lupus nephritis (Zhao et al., 2020), EVs may be involved in immune complex-related renal diseases in different ways. Basic and clinical studies in the context will not only gain our knowledge in pathophysiology, but also provide an opportunity for the establishment of novel diagnostic and therapeutic strategies in the future.
9. Conclusions
In this review article, we have summarized the recent advancement in the knowledge of nephropathologic changes in terms of new etiologies, progression in pathologic diagnosis, and potential therapeutic strategies for many intrisinc renal diseases. There is growing evidence about EV release in human tissue/organs, including the kidney as we have discussed. The uptake of foreign EVs by recipient cells may happen under pathologic conditions through phagocytosis and/or endocytosis. Almost all renal epithelial and endothelial cells can be either “donor cells” or “recipient cells”, making the invisible ultrastructural world mysterious but meaningful for active EV release and internalization among cells in the kidney. The introduction of EVs alters and expands our way of thinking of the interactions between cells at physiologic and pathologic levels. The existing literature reveals that several areas of nephropathology, such as FSGS and paraprotein-related renal diseases, still require further investigation regarding EVs, phagocytosis and endocytosis in order to shed light on better understanding of the intercellular communications.
In the basic science aspects, more investigations are needed to study the involvement of EVs in the antibody-mediated rejection, and their interaction with complements and other elements in thrombotic microangiopathy, as well as the deposition of monoclonal IgG in paraprotein-associated kidney diseases. In the clinical point of view, urine biomarkers, such as CD133 and KIM-1, should be further investigated in the acute phase of kidney injury, such as detecting the early rejection associated kidney injury, and monitoring chemotherapy associated acute kidney injury. In addition, detection of EVs in urinary samples can be regarded as a convenient liquid biopsy to determine whether the biomarkers can be used cost-effectively for monitoring the early kidney injury in patients with other chronic diseases, i.e. diabetes and/or hypertension. Certainly, the clinical ramifications of a specific pathologic diagnosis and clinical treatment can be advanced with the expansion of knowledge in these fields.
Acknowledgment
Authors thank Mr. Joseph Roszka for his providing the precious electron microscopic images in Figure 2 and Figure 3, and Mrs. Karen L. Lewinski for her excellent technical assistance to convert the printed images into the digital figures in Electron Microscopy Laboratory, Beaumont Hospital, Royal Oak, MI. Authors also thank senior nephrologist Dr. Oalf Kroneman of Beaumont Hospital for his critical review and comments of the manuscript. The work was supported by Lupus Research Alliance (416805) and NIH R21AI144838 (to MLL).
References
- Abid Q, Best Rocha A, Larsen CP, Schulert G, Marsh R, Yasin S, Patty-Resk C, Valentini RP, Adams M, and Baracco R. 2020. APOL1-Associated Collapsing Focal Segmental Glomerulosclerosis in a Patient With Stimulator of Interferon Genes (STING)-Associated Vasculopathy With Onset in Infancy (SAVI). Am J Kidney Dis. 75:287–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agostino NM, Gingrich R, and Drabick JJ. 2010. Bevacizumab demonstrates prolonged disease stabilization in patients with heavily pretreated metastatic renal cell carcinoma: a case series and review of the literature. Adv Urol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrianova NV, Buyan MI, Zorova LD, Pevzner IB, Popkov VA, Babenko VA, Silachev DN, Plotnikov EY, and Zorov DB. 2019. Kidney Cells Regeneration: Dedifferentiation of Tubular Epithelium, Resident Stem Cells and Possible Niches for Renal Progenitors. Int J Mol Sci. 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astern JM, Pendergraft WF 3rd, Falk RJ, Jennette JC, Schmaier AH, Mahdi F, and Preston GA. 2007. Myeloperoxidase interacts with endothelial cell-surface cytokeratin 1 and modulates bradykinin production by the plasma Kallikrein-Kinin system. Am J Pathol. 171:349–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajema IM, Wilhelmus S, Alpers CE, Bruijn JA, Colvin RB, Cook HT, D’Agati VD, Ferrario F, Haas M, Jennette JC, Joh K, Nast CC, Noel LH, Rijnink EC, Roberts ISD, Seshan SV, Sethi S, and Fogo AB. 2018. Revision of the International Society of Nephrology/Renal Pathology Society classification for lupus nephritis: clarification of definitions, and modified National Institutes of Health activity and chronicity indices. Kidney Int. 93:789–796. [DOI] [PubMed] [Google Scholar]
- Barisoni L, Kriz W, Mundel P, and D’Agati V. 1999. The dysregulated podocyte phenotype: a novel concept in the pathogenesis of collapsing idiopathic focal segmental glomerulosclerosis and HIV-associated nephropathy. J Am Soc Nephrol. 10:51–61. [DOI] [PubMed] [Google Scholar]
- Batuman V, Verroust PJ, Navar GL, Kaysen JH, Goda FO, Campbell WC, Simon E, Pontillon F, Lyles M, Bruno J, and Hammond TG. 1998. Myeloma light chains are ligands for cubilin (gp280). Am J Physiol. 275:F246–254. [DOI] [PubMed] [Google Scholar]
- Bech AP, Hofstra JM, Brenchley PE, and Wetzels JF. 2014. Association of anti-PLA(2)R antibodies with outcomes after immunosuppressive therapy in idiopathic membranous nephropathy. Clin J Am Soc Nephrol. 9:1386–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck LH Jr. 2017. PLA2R and THSD7A: Disparate Paths to the Same Disease? J Am Soc Nephrol. 28:2579–2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benichou G, Wang M, Ahrens K, and Madsen JC. 2020. Extracellular vesicles in allograft rejection and tolerance. Cell Immunol. 349:104063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bomback AS 2018. Management of Membranous Nephropathy in the PLA2R Era. Clin J Am Soc Nephrol. 13:784–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner BM 2003. AMGEN International Prize: the history and future of renoprotection. Kidney Int. 64:1163–1168. [DOI] [PubMed] [Google Scholar]
- Brezis M, and Rosen S. 1995. Hypoxia of the renal medulla--its implications for disease. N Engl J Med. 332:647–655. [DOI] [PubMed] [Google Scholar]
- Bridoux F, Leung N, Hutchison CA, Touchard G, Sethi S, Fermand JP, Picken MM, Herrera GA, Kastritis E, Merlini G, Roussel M, Fervenza FC, Dispenzieri A, Kyle RA, Nasr SH, International K, and Monoclonal Gammopathy Research G. 2015. Diagnosis of monoclonal gammopathy of renal significance. Kidney Int. 87:698–711. [DOI] [PubMed] [Google Scholar]
- Cardinal H, Dieude M, and Hebert MJ. 2018. Endothelial Dysfunction in Kidney Transplantation. Front Immunol. 9:1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardones AR, and Banez LL. 2006. VEGF inhibitors in cancer therapy. Curr Pharm Des. 12:387–394. [DOI] [PubMed] [Google Scholar]
- Charlesworth B 2010. Molecular population genomics: a short history. Genet Res (Camb). 92:397–411. [DOI] [PubMed] [Google Scholar]
- Colvin RB, and Smith RN. 2005. Antibody-mediated organ-allograft rejection. Nat Rev Immunol. 5:807–817. [DOI] [PubMed] [Google Scholar]
- D’Agati VD, Fogo AB, Bruijn JA, and Jennette JC. 2004. Pathologic classification of focal segmental glomerulosclerosis: a working proposal. Am J Kidney Dis. 43:368–382. [DOI] [PubMed] [Google Scholar]
- De Luca L, Laurenzana I, Trino S, Lamorte D, Caivano A, and Musto P. 2019. An update on extracellular vesicles in multiple myeloma: a focus on their role in cell-to-cell cross-talk and as potential liquid biopsy biomarkers. Expert Rev Mol Diagn. 19:249–258. [DOI] [PubMed] [Google Scholar]
- De Vriese AS, Sethi S, Van Praet J, Nath KA, and Fervenza FC. 2015. Kidney Disease Caused by Dysregulation of the Complement Alternative Pathway: An Etiologic Approach. J Am Soc Nephrol. 26:2917–2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debiec H, and Ronco P. 2011. PLA2R autoantibodies and PLA2R glomerular deposits in membranous nephropathy. N Engl J Med. 364:689–690. [DOI] [PubMed] [Google Scholar]
- Decourt C, Bridoux F, Touchard G, and Cogne M. 2003. A monoclonal V kappa l light chain responsible for incomplete proximal tubulopathy. Am J Kidney Dis. 41:497–504. [DOI] [PubMed] [Google Scholar]
- Di Noto G, Chiarini M, Paolini L, Mazzoldi EL, Giustini V, Radeghieri A, Caimi L, and Ricotta D. 2014. Immunoglobulin Free Light Chains and GAGs Mediate Multiple Myeloma Extracellular Vesicles Uptake and Secondary NfkappaB Nuclear Translocation. Front Immunol. 5:517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diabetic Retinopathy Clinical Research, N., Scott IU, Edwards AR, Beck RW, Bressler NM, Chan CK, Elman MJ, Friedman SM, Greven CM, Maturi RK, Pieramici DJ, Shami M, Singerman LJ, and Stockdale CR. 2007. A phase II randomized clinical trial of intravitreal bevacizumab for diabetic macular edema. Ophthalmology. 114:1860–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieude M, Turgeon J, Karakeussian Rimbaud A, Beillevaire D, Qi S, Patey N, Gaboury LA, Boilard E, and Hebert MJ. 2020. Extracellular vesicles derived from injured vascular tissue promote the formation of tertiary lymphoid structures in vascular allografts. Am J Transplant. 20:726–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimuccio V, Peruzzi L, Brizzi MF, Cocchi E, Fop F, Boido A, Gili M, Gallo S, Biancone L, Camussi G, and Bussolati B. 2020. Acute and chronic glomerular damage is associated with reduced CD133 expression in urinary extracellular vesicles. Am J Physiol Renal Physiol. 318:F486–F495. [DOI] [PubMed] [Google Scholar]
- Dimuccio V, Ranghino A, Pratico Barbato L, Fop F, Biancone L, Camussi G, and Bussolati B. 2014. Urinary CD133+ extracellular vesicles are decreased in kidney transplanted patients with slow graft function and vascular damage. PLoS One. 9:e104490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiserich JP, Baldus S, Brennan ML, Ma W, Zhang C, Tousson A, Castro L, Lusis AJ, Nauseef WM, White CR, and Freeman BA. 2002. Myeloperoxidase, a leukocyte-derived vascular NO oxidase. Science. 296:2391–2394. [DOI] [PubMed] [Google Scholar]
- Eremina V, Jefferson JA, Kowalewska J, Hochster H, Haas M, Weisstuch J, Richardson C, Kopp JB, Kabir MG, Backx PH, Gerber HP, Ferrara N, Barisoni L, Alpers CE, and Quaggin SE. 2008. VEGF inhibition and renal thrombotic microangiopathy. N Engl J Med. 358:1129–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eremina V, and Quaggin SE. 2004. The role of VEGF-A in glomerular development and function. Curr Opin Nephrol Hypertens. 13:9–15. [DOI] [PubMed] [Google Scholar]
- Falk RJ, and Jennette JC. 2010. ANCA disease: where is this field heading? J Am Soc Nephrol. 21:745–752. [DOI] [PubMed] [Google Scholar]
- Farkash EA, Wilson AM, and Jentzen JM. 2020. Ultrastructural Evidence for Direct Renal Infection with SARS-CoV-2. J Am Soc Nephrol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fermand JP, Bridoux F, Kyle RA, Kastritis E, Weiss BM, Cook MA, Drayson MT, Dispenzieri A, Leung N, K. International, and G. Monoclonal Gammopathy Research. 2013. How I treat monoclonal gammopathy of renal significance (MGRS). Blood. 122:3583–3590. [DOI] [PubMed] [Google Scholar]
- Foster RR, Satchell SC, Seckley J, Emmett MS, Joory K, Xing CY, Saleem MA, Mathieson PW, Bates DO, and Harper SJ. 2006. VEGF-C promotes survival in podocytes. Am J Physiol Renal Physiol. 291:F196–207. [DOI] [PubMed] [Google Scholar]
- Fujigaki Y, Nagase M, Kojima K, Yamamoto T, and Hishida A. 1997. Glomerular handling of immune complex in the acute phase of active in situ immune complex glomerulonephritis employing cationized ferritin in rats. Ultrastructural localization of immune complex, complements and inflammatory cells. Virchows Arch. 431:53–61. [DOI] [PubMed] [Google Scholar]
- Gallan AJ, and Chang A. 2020. A New Paradigm for Renal Thrombotic Microangiopathy. Semin Diagn Pathol. 37:121–126. [DOI] [PubMed] [Google Scholar]
- Gordon RE 2014. Electron microscopy: a brief history and review of current clinical application. Methods Mol Biol. 1180:119–135. [DOI] [PubMed] [Google Scholar]
- Haas M, Loupy A, Lefaucheur C, Roufosse C, Glotz D, Seron D, Nankivell BJ, Halloran PF, Colvin RB, Akalin E, Alachkar N, Bagnasco S, Bouatou Y, Becker JU, Cornell LD, van Huyen JPD, Gibson IW, Kraus ES, Mannon RB, Naesens M, Nickeleit V, Nickerson P, Segev DL, Singh HK, Stegall M, Randhawa P, Racusen L, Solez K, and Mengel M. 2018. The Banff 2017 Kidney Meeting Report: Revised diagnostic criteria for chronic active T cell-mediated rejection, antibody-mediated rejection, and prospects for integrative endpoints for next-generation clinical trials. Am J Transplant. 18:293–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halbwachs L, and Lesavre P. 2012. Endothelium-neutrophil interactions in ANCA-associated diseases. J Am Soc Nephrol. 23:1449–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handala L, Blanchard E, Raynal PI, Roingeard P, Morel V, Descamps V, Castelain S, Francois C, Duverlie G, Brochot E, and Helle F. 2020. BK Polyomavirus Hijacks Extracellular Vesicles for En Bloc Transmission. J Virol. 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassanpour M, Rezaie J, Nouri M, and Panahi Y. 2020. The role of extracellular vesicles in COVID-19 virus infection. Infect Genet Evol. 85:104422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera GA 2014. Proximal tubulopathies associated with monoclonal light chains: the spectrum of clinicopathologic manifestations and molecular pathogenesis. Arch Pathol Lab Med. 138:1365–1380. [DOI] [PubMed] [Google Scholar]
- Huang Z, Zhang Y, Zhou J, and Zhang Y. 2017. Urinary Exosomal miR-193a Can Be a Potential Biomarker for the Diagnosis of Primary Focal Segmental Glomerulosclerosis in Children. Biomed Res Int. 2017:7298160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huling J, and Yoo JJ. 2017. Comparing adult renal stem cell identification, characterization and applications. J Biomed Sci. 24:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys BD, Valerius MT, Kobayashi A, Mugford JW, Soeung S, Duffield JS, McMahon AP, and Bonventre JV. 2008. Intrinsic epithelial cells repair the kidney after injury. Cell Stem Cell. 2:284–291. [DOI] [PubMed] [Google Scholar]
- Ichimura T, Asseldonk EJ, Humphreys BD, Gunaratnam L, Duffield JS, and Bonventre JV. 2008. Kidney injury molecule-1 is a phosphatidylserine receptor that confers a phagocytic phenotype on epithelial cells. J Clin Invest. 118:1657–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichimura T, Bonventre JV, Bailly V, Wei H, Hession CA, Cate RL, and Sanicola M. 1998. Kidney injury molecule-1 (KIM-1), a putative epithelial cell adhesion molecule containing a novel immunoglobulin domain, is up-regulated in renal cells after injury. J Biol Chem. 273:4135–4142. [DOI] [PubMed] [Google Scholar]
- Ichimura T, Brooks CR, and Bonventre JV. 2012. Kim-1/Tim-1 and immune cells: shifting sands. Kidney Int. 81:809–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izzedine H, Sene D, Hadoux J, Gharbi C, Bourry E, Massard C, and Soria JC. 2011. Thrombotic microangiopathy related to anti-VEGF agents: intensive versus conservative treatment? Ann Oncol. 22:487–490. [DOI] [PubMed] [Google Scholar]
- Jennette JC, and Falk RJ. 2008. New insight into the pathogenesis of vasculitis associated with antineutrophil cytoplasmic autoantibodies. Curr Opin Rheumatol. 20:55–60. [DOI] [PubMed] [Google Scholar]
- Jennette JC, Xiao H, Falk R, and Gasim AMH. 2011. Experimental models of vasculitis and glomerulonephritis induced by antineutrophil cytoplasmic autoantibodies. Contrib Nephrol. 169:211–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerke U, Rolle S, Purfurst B, Luft FC, Nauseef WM, and Kettritz R. 2013. beta2 integrin-mediated cell-cell contact transfers active myeloperoxidase from neutrophils to endothelial cells. J Biol Chem. 288:12910–12919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung HY, Lee CH, Choi JY, Cho JH, Park SH, Kim YL, Moon PG, Baek MC, Berm Park J, Hoon Kim Y, Ha Chung B, Lee SH, and Kim CD. 2020. Potential urinary extracellular vesicle protein biomarkers of chronic active antibody-mediated rejection in kidney transplant recipients. J Chromatogr B Analyt Technol Biomed Life Sci. 1138:121958. [DOI] [PubMed] [Google Scholar]
- Keir LS, Firth R, Aponik L, Feitelberg D, Sakimoto S, Aguilar E, Welsh GI, Richards A, Usui Y, Satchell SC, Kuzmuk V, Coward RJ, Goult J, Bull KR, Sharma R, Bharti K, Westenskow PD, Michael IP, Saleem MA, and Friedlander M. 2017. VEGF regulates local inhibitory complement proteins in the eye and kidney. J Clin Invest. 127:199–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kissling S, Rotman S, Gerber C, Halfron M, Lamoth F, Sadallah S, Fakhouri F. 2020. Collapsing glomerulopathy in a COVID-19 patient. Kidney International. doi: 10.1016/j.kint.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klassen RB, Allen PL, Batuman V, Crenshaw K, and Hammond TG. 2005. Light chains are a ligand for megalin. J Appl Physiol (1985). 98:257–263. [DOI] [PubMed] [Google Scholar]
- Klinke A, Nussbaum C, Kubala L, Friedrichs K, Rudolph TK, Rudolph V, Paust HJ, Schroder C, Benten D, Lau D, Szocs K, Furtmuller PG, Heeringa P, Sydow K, Duchstein HJ, Ehmke H, Schumacher U, Meinertz T, Sperandio M, and Baldus S. 2011. Myeloperoxidase attracts neutrophils by physical forces. Blood. 117:1350–1358. [DOI] [PubMed] [Google Scholar]
- Ko SY, Lee W, Kenny HA, Dang LH, Ellis LM, Jonasch E, Lengyel E, and Naora H. 2019. Cancer-derived small extracellular vesicles promote angiogenesis by heparin-bound, bevacizumab-insensitive VEGF, independent of vesicle uptake. Commun Biol. 2:386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudose S, Batal I, Santoriello D, Xu K, Barasch J, Peleg Y, Canetta P, Ratner LE, Marasa M, Gharavi AG, Stokes MB, Markowitz GS, and D’Agati VD. 2020. Kidney Biopsy Findings in Patients with COVID-19. J Am Soc Nephrol. 31:1959–1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai Z, Kumar T, Zhao R, Li W, Kanaan HD, Zhang PL, and Liu B. 2019. Monoclonal Gammopathy of Renal Significance and its Associated Experimental Models. Ann Clin Lab Sci. 49:439–447. [PubMed] [Google Scholar]
- Larsen CP, Bourne TD, Wilson JD, Saqqa O, and Sharshir MA. 2020. Collapsing Glomerulopathy in a Patient With Coronavirus Disease 2019 (COVID-19). Kidney Int Rep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazzeri E, Angelotti ML, Conte C, Anders HJ, and Romagnani P. 2019. Surviving Acute Organ Failure: Cell Polyploidization and Progenitor Proliferation. Trends Mol Med. 25:366–381. [DOI] [PubMed] [Google Scholar]
- Leung N, Bridoux F, Batuman V, Chaidos A, Cockwell P, D’Agati VD, Dispenzieri A, Fervenza FC, Fermand JP, Gibbs S, Gillmore JD, Herrera GA, Jaccard A, Jevremovic D, Kastritis E, Kukreti V, Kyle RA, Lachmann HJ, Larsen CP, Ludwig H, Markowitz GS, Merlini G, Mollee P, Picken MM, Rajkumar VS, Royal V, Sanders PW, Sethi S, Venner CP, Voorhees PM, Wechalekar AD, Weiss BM, and Nasr SH. 2019. The evaluation of monoclonal gammopathy of renal significance: a consensus report of the International Kidney and Monoclonal Gammopathy Research Group. Nat Rev Nephrol. 15:45–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung N, Bridoux F, Hutchison CA, Nasr SH, Cockwell P, Fermand JP, Dispenzieri A, Song KW, Kyle RA, K. International, and G. Monoclonal Gammopathy Research. 2012. Monoclonal gammopathy of renal significance: when MGUS is no longer undetermined or insignificant. Blood. 120:4292–4295. [DOI] [PubMed] [Google Scholar]
- Li CJ, Fang QH, Liu ML, and Lin JN. 2020. Current understanding of the role of Adipose-derived Extracellular Vesicles in Metabolic Homeostasis and Diseases: Communication from the distance between cells/tissues. Theranostics. 10:7422–7435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little MA, Smyth L, Salama AD, Mukherjee S, Smith J, Haskard D, Nourshargh S, Cook HT, and Pusey CD. 2009. Experimental autoimmune vasculitis: an animal model of anti-neutrophil cytoplasmic autoantibody-associated systemic vasculitis. Am J Pathol. 174:1212–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu ML, Lyu X, and Werth VP. 2020. NETotic neutrophils release extracellular vesicles that may contribute to the pro-thrombotic conditions in patients with COVID-19. J Clin Invest. 10.1172/JCI141374. [DOI] [Google Scholar]
- Liu ML, Reilly MP, Casasanto P, McKenzie SE, and Williams KJ. 2007. Cholesterol enrichment of human monocyte/macrophages induces surface exposure of phosphatidylserine and the release of biologically-active tissue factor-positive microvesicles. Arteriosclerosis, thrombosis, and vascular biology. 27:430–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu ML, Williams KJ, and Werth VP. 2016. Microvesicles in Autoimmune Diseases. Adv Clin Chem. 77:125–175. [DOI] [PubMed] [Google Scholar]
- Loupy A, Haas M, Roufosse C, Naesens M, Adam B, Afrouzian M, Akalin E, Alachkar N, Bagnasco S, Becker JU, Cornell LD, Clahsen-van Groningen MC, Demetris AJ, Dragun D, Duong van Huyen JP, Farris AB, Fogo AB, Gibson IW, Glotz D, Gueguen J, Kikic Z, Kozakowski N, Kraus E, Lefaucheur C, Liapis H, Mannon RB, Montgomery RA, Nankivell BJ, Nickeleit V, Nickerson P, Rabant M, Racusen L, Randhawa P, Robin B, Rosales IA, Sapir-Pichhadze R, Schinstock CA, Seron D, Singh HK, Smith RN, Stegall MD, Zeevi A, Solez K, Colvin RB, and Mengel M. 2020. The Banff 2019 Kidney Meeting Report (I): Updates on and clarification of criteria for T cell- and antibody-mediated rejection. Am J Transplant. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luan J, Chen W, Fan J, Wang S, Zhang X, Zai W, Jin X, Wang Y, Feng Z, Zhang J, Liu ML, and Ju D. 2020a. GSDMD membrane pore is critical for IL-1beta release and antagonizing IL-1beta by hepatocyte-specific nanobiologics is a promising therapeutics for murine alcoholic steatohepatitis. Biomaterials. 227:119570. [DOI] [PubMed] [Google Scholar]
- Luan J, Lu Y, Jin X, and Zhang L. 2020b. Spike protein recognition of mammalian ACE2 predicts the host range and an optimized ACE2 for SARS-CoV-2 infection. Biochem Biophys Res Commun. 526:165–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lytvyn Y, Bjornstad P, Lovshin JA, Boulet G, Farooqi MA, Lai V, Tse J, Cham L, Lovblom LE, Weisman A, Keenan HA, Brent MH, Paul N, Bril V, Advani A, Sochett E, Perkins BA, and Cherney DZI. 2019. Renal Hemodynamic Function and RAAS Activation Over the Natural History of Type 1 Diabetes. Am J Kidney Dis. 73:786–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackman N, and Davis GE. 2011. Blood coagulation and blood vessel development: is tissue factor the missing link? Arterioscler Thromb Vasc Biol. 31:2364–2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowitz GS, Appel GB, Fine PL, Fenves AZ, Loon NR, Jagannath S, Kuhn JA, Dratch AD, and D’Agati VD. 2001. Collapsing focal segmental glomerulosclerosis following treatment with high-dose pamidronate. JAm Soc Nephrol. 12:1164–1172. [DOI] [PubMed] [Google Scholar]
- McAdoo SP, and Pusey CD. 2017. Anti-Glomerular Basement Membrane Disease. Clin J Am Soc Nephrol. 12:1162–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh JR, and Hays T. 2016. A Brief History of Research on Mitotic Mechanisms. Biology (Basel). 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillen TS, Heinecke JW, and LeBoeuf RC. 2005. Expression of human myeloperoxidase by macrophages promotes atherosclerosis in mice. Circulation. 111:2798–2804. [DOI] [PubMed] [Google Scholar]
- Mezzogiorno A, Mezzogiorno V, and Esposito V. 2002. History of the nephron. Am J Nephrol. 22:213–219. [DOI] [PubMed] [Google Scholar]
- Monguio-Tortajada M, Lauzurica-Valdemoros R, and Borras FE. 2014. Tolerance in organ transplantation: from conventional immunosuppression to extracellular vesicles. Front Immunol. 5:416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morandi F, Marimpietri D, Horenstein AL, Bolzoni M, Toscani D, Costa F, Castella B, Faini AC, Massaia M, Pistoia V, Giuliani N, and Malavasi F. 2018. Microvesicles released from multiple myeloma cells are equipped with ectoenzymes belonging to canonical and non-canonical adenosinergic pathways and produce adenosine from ATP and NAD(). Oncoimmunology. 7:e1458809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee A, Ghosh R, and Furment MM. 2020. Case Report: COVID-19 Associated Renal Infarction and Ascending Aortic Thrombosis. Am J Trop Med Hyg. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakhoul N, and Batuman V. 2011. Role of proximal tubules in the pathogenesis of kidney disease. Contrib Nephrol. 169:37–50. [DOI] [PubMed] [Google Scholar]
- Namal Rathnayaka R, Ranathunga PAN, and Kularatne SA. 2019. Thrombotic Microangiopathy, Hemolytic Uremic Syndrome, and Thrombotic Thrombocytopenic Purpura Following Hump-nosed Pit Viper (Genus: Hypnale) Envenoming in Sri Lanka. Wilderness Environ Med. 30:66–78. [DOI] [PubMed] [Google Scholar]
- Nawaz M, Fatima F, Vallabhaneni KC, Penfornis P, Valadi H, Ekstrom K, Kholia S, Whitt JD, Fernandes JD, Pochampally R, Squire JA, and Camussi G. 2016. Extracellular Vesicles: Evolving Factors in Stem Cell Biology. Stem Cells Int. 2016:1073140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neyra JA, Vaidya OU, Hendricks A, and Sambandam KK. 2014. Collapsing focal segmental glomerulosclerosis resulting from a single dose of zoledronate. Nephron Extra. 4:168–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- nick.white@covid19crc.org, C.-C.R.C.E.a. 2020. Global coalition to accelerate COVID-19 clinical research in resource-limited settings. Lancet. 395:1322–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Flynn J, Dixon KO, Faber Krol MC, Daha MR, and van Kooten C. 2014. Myeloperoxidase directs properdin-mediated complement activation. J Innate Immun. 6:417–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan BT, and Johnstone RM. 1983. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: selective externalization of the receptor. Cell. 33:967–978. [DOI] [PubMed] [Google Scholar]
- Pan BT, Teng K, Wu C, Adam M, and Johnstone RM. 1985. Electron microscopic evidence for externalization of the transferrin receptor in vesicular form in sheep reticulocytes. J Cell Biol. 101:942–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parasuraman R, Wolforth SC, Wiesend WN, Dumler F, Rooney MT, Li W, and Zhang PL. 2013. Contribution of polyclonal free light chain deposition to tubular injury. Am J Nephrol. 38:465–474. [DOI] [PubMed] [Google Scholar]
- Patel AM, Zenenberg RD, and Goldberg RJ. 2018. De novo CMV-associated collapsing focal segmental glomerulosclerosis in a kidney transplant recipient. Transpl Infect Dis. 20:e12884. [DOI] [PubMed] [Google Scholar]
- Peleg Y, Kudose S, D’Agati V, Siddall E, Ahmad S, Kisselev S, Gharavi A, and Canetta P. 2020. Acute Kidney Injury Due to Collapsing Glomerulopathy Following COVID-19 Infection. Kidney Int Rep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelle G, Shweke N, Duong Van Huyen JP, Tricot L, Hessaine S, Fremeaux-Bacchi V, Hiesse C, and Delahousse M. 2011. Systemic and kidney toxicity of intraocular administration of vascular endothelial growth factor inhibitors. Am J Kidney Dis. 57:756–759. [DOI] [PubMed] [Google Scholar]
- Philipponnet C, Aniort J, Chabrot P, Souweine B, and Heng AE. 2020. Renal artery thrombosis induced by COVID-19. Clin Kidney J. 13:713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickering MC, D’Agati VD, Nester CM, Smith RJ, Haas M, Appel GB, Alpers CE, Bajema IM, Bedrosian C, Braun M, Doyle M, Fakhouri F, Fervenza FC, Fogo AB, Fremeaux-Bacchi V, Gale DP, Goicoechea de Jorge E, Griffin G, Harris CL, Holers VM, Johnson S, Lavin PJ, Medjeral-Thomas N, Paul Morgan B, Nast CC, Noel LH, Peters DK, Rodriguez de Cordoba S, Servais A, Sethi S, Song WC, Tamburini P, Thurman JM, Zavros M, and Cook HT. 2013. C3 glomerulopathy: consensus report. Kidney Int. 84:1079–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitanga TN, de Aragao Franca L, Rocha VC, Meirelles T, Borges VM, Goncalves MS, Pontes-de-Carvalho LC, Noronha-Dutra AA, and dos-Santos WL. 2014. Neutrophil-derived microparticles induce myeloperoxidase-mediated damage of vascular endothelial cells. BMC cell biology. 15:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu G, Zheng G, Ge M, Wang J, Huang R, Shu Q, and Xu J. 2019. Functional proteins of mesenchymal stem cell-derived extracellular vesicles. Stem Cell Res Ther. 10:359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaglia M, Dellepiane S, Guglielmetti G, Merlotti G, Castellano G, and Cantaluppi V. 2020. Extracellular Vesicles as Mediators of Cellular Crosstalk Between Immune System and Kidney Graft. Front Immunol. 11:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racusen LC, Colvin RB, Solez K, Mihatsch MJ, Halloran PF, Campbell PM, Cecka MJ, Cosyns JP, Demetris AJ, Fishbein MC, Fogo A, Furness P, Gibson IW, Glotz D, Hayry P, Hunsickern L, Kashgarian M, Kerman R, Magil AJ, Montgomery R, Morozumi K, Nickeleit V, Randhawa P, Regele H, Seron D, Seshan S, Sund S, and Trpkov K. 2003. Antibody-mediated rejection criteria - an addition to the Banff 97 classification of renal allograft rejection. Am J Transplant. 3:708–714. [DOI] [PubMed] [Google Scholar]
- Rigalli JP, Barros ER, Sommers V, Bindels RJM, and Hoenderop JGJ. 2020. Novel Aspects of Extracellular Vesicles in the Regulation of Renal Physiological and Pathophysiological Processes. Front Cell Dev Biol. 8:244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimoin DL, and Hirschhorn K. 2004. A history of medical genetics in pediatrics. Pediatr Res. 56:150–159. [DOI] [PubMed] [Google Scholar]
- Rodrigues JC, Haas M, and Reich HN. 2017. IgA Nephropathy. Clin J Am Soc Nephrol. 12:677–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rofagha S, Bhisitkul RB, Boyer DS, Sadda SR, Zhang K, and Group S-US. 2013. Seven-year outcomes in ranibizumab-treated patients in ANCHOR, MARINA, and HORIZON: a multicenter cohort study (SEVEN-UP). Ophthalmology. 120:2292–2299. [DOI] [PubMed] [Google Scholar]
- Romagnani P 2011. Family portrait: renal progenitor of Bowman’s capsule and its tubular brothers. Am J Pathol. 178:490–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronco P, and Debiec H. 2010. Membranous glomerulopathy: the evolving story. Curr Opin Nephrol Hypertens. 19:254–259. [DOI] [PubMed] [Google Scholar]
- Sagrinati C, Netti GS, Mazzinghi B, Lazzeri E, Liotta F, Frosali F, Ronconi E, Meini C, Gacci M, Squecco R, Carini M, Gesualdo L, Francini F, Maggi E, Annunziato F, Lasagni L, Serio M, Romagnani S, and Romagnani P. 2006. Isolation and characterization of multipotent progenitor cells from the Bowman’s capsule of adult human kidneys. J Am Soc Nephrol. 17:2443–2456. [DOI] [PubMed] [Google Scholar]
- Sanders PW 2011. Light chain-mediated tubulopathies. Contrib Nephrol. 169:262–269. [DOI] [PubMed] [Google Scholar]
- Sanders PW, Herrera GA, Chen A, Booker BB, and Galla JH. 1988. Differential nephrotoxicity of low molecular weight proteins including Bence Jones proteins in the perfused rat nephron in vivo. J Clin Invest. 82:2086–2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoriello D, Khairallah P, Bomback AS, Xu K, Kudose S, Batal I, Barasch J, Radhakrishnan J, D’Agati V, and Markowitz G. 2020. Postmortem Kidney Pathology Findings in Patients with COVID-19. J Am Soc Nephrol. 31:2158–2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sardu C, Gambardella J, Morelli MB, Wang X, Marfella R, and Santulli G. 2020. Hypertension, Thrombosis, Kidney Failure, and Diabetes: Is COVID-19 an Endothelial Disease? A Comprehensive Evaluation of Clinical and Basic Evidence. J Clin Med. 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarhan M, von Massenhausen A, Hugo C, Oberbauer R, and Linkermann A. 2018. Immunological consequences of kidney cell death. Cell death & disease. 9:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethi S, Nester CM, and Smith RJ. 2012. Membranoproliferative glomerulonephritis and C3 glomerulopathy: resolving the confusion. Kidney Int. 81:434–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethi S, Rajkumar SV, and D’Agati VD. 2018. The Complexity and Heterogeneity of Monoclonal Immunoglobulin-Associated Renal Diseases. J Am Soc Nephrol. 29:1810–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma P, Uppal NN, Wanchoo R, Shah HH, Yang Y, Parikh R, Khanin Y, Madireddy V, Larsen CP, Jhaveri KD, Bijol V, and Northwell Nephrology C-RC. 2020. COVID-19-Associated Kidney Injury: A Case Series of Kidney Biopsy Findings. J Am Soc Nephrol. 31:1948–1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh P, Batra HS, and Naithani M. 2004. History of biochemistry. Bull Indian Inst Hist Med Hyderabad. 34:75–86. [PubMed] [Google Scholar]
- Sirac C, Herrera GA, Sanders PW, Batuman V, Bender S, Ayala MV, Javaugue V, Teng J, Turbat-Herrera EA, Cogne M, Touchard G, Leung N, and Bridoux F. 2018. Animal models of monoclonal immunoglobulin-related renal diseases. Nat Rev Nephrol. 14:246–264. [DOI] [PubMed] [Google Scholar]
- Su H, Yang M, Wan C, Yi LX, Tang F, Zhu HY, Yi F, Yang HC, Fogo AB, Nie X, and Zhang C. 2020. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T, Kohatsu K, Han W, Watanabe S, Yahagi K, Nakata M, Ueno T, Ichikawa D, Imai N, Shirai S, Koike J, and Shibagaki Y. 2020. Morphological Features of Minimal Change Disease and Focal Segmental Glomerulosclerosis Using Repeat Biopsy and Parietal Epithelial Cell Marker. Kidney Dis (Basel). 6:119–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan YJ, Teng E, Shen S, Tan TH, Goh PY, Fielding BC, Ooi EE, Tan HC, Lim SG, and Hong W. 2004. A novel severe acute respiratory syndrome coronavirus protein, U274, is transported to the cell surface and undergoes endocytosis. J Virol. 78:6723–6734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thery C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, Antoniou A, Arab T, Archer F, Atkin-Smith GK, Ayre DC, Bach JM, Bachurski D, Baharvand H, Balaj L, Baldacchino S, Bauer NN, Baxter AA, Bebawy M, Beckham C, Bedina Zavec A, Benmoussa A, Berardi AC, Bergese P, Bielska E, Blenkiron C, Bobis-Wozowicz S, Boilard E, Boireau W, Bongiovanni A, Borras FE, Bosch S, Boulanger CM, Breakefield X, Breglio AM, Brennan MA, Brigstock DR, Brisson A, Broekman ML, Bromberg JF, Bryl-Gorecka P, Buch S, Buck AH, Burger D, Busatto S, Buschmann D, Bussolati B, Buzas EI, Byrd JB, Camussi G, Carter DR, Caruso S, Chamley LW, Chang YT, Chen C, Chen S, Cheng L, Chin AR, Clayton A, Clerici SP, Cocks A, Cocucci E, Coffey RJ, Cordeiro-da-Silva A, Couch Y, Coumans FA, Coyle B, Crescitelli R, Criado MF, D’Souza-Schorey C, Das S, Datta Chaudhuri A, de Candia P, De Santana EF, De Wever O, Del Portillo HA, Demaret T, Deville S, Devitt A, Dhondt B, Di Vizio D, Dieterich LC, Dolo V, Dominguez Rubio AP, Dominici M, Dourado MR, Driedonks TA, Duarte FV, Duncan HM, Eichenberger RM, Ekstrom K, El Andaloussi S, Elie-Caille C, Erdbrugger U, Falcon-Perez JM, Fatima F, Fish JE, Flores-Bellver M, Forsonits A, Frelet-Barrand A, et al. 2018. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 7:1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurman JM, and Nester CM. 2016. All Things Complement. Clin J Am Soc Nephrol. 11:1856–1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valeri A, Barisoni L, Appel GB, Seigle R, and D’Agati V. 1996. Idiopathic collapsing focal segmental glomerulosclerosis: a clinicopathologic study. Kidney Int. 50:1734–1746. [DOI] [PubMed] [Google Scholar]
- Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, Mehra MR, Schuepbach RA, Ruschitzka F, and Moch H. 2020. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 395:1417–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Larsen CP, Hernandez-Arroyo CF, Mohamed MMB, Caza T, Sharshir M, Chughtai A, Xie L, Gimenez JM, Sandow TA, Lusco MA, Yang H, Acheampong E, Rosales IA, Colvin RB, Fogo AB, and Velez JCQ. 2020. AKI and Collapsing Glomerulopathy Associated with COVID-19 and APOL 1 High-Risk Genotype. J Am Soc Nephrol. 31:1688–1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Liu Y, Wei W, and Liu ML. 2019. Extracellular vesicles in autoimmune vasculitis - Little dirts light the fire in blood vessels. Autoimmun Rev. 18:593–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao H, Heeringa P, Hu P, Liu Z, Zhao M, Aratani Y, Maeda N, Falk RJ, and Jennette JC. 2002. Antineutrophil cytoplasmic autoantibodies specific for myeloperoxidase cause glomerulonephritis and vasculitis in mice. J Clin Invest. 110:955–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Brooks CR, Xiao S, Sabbisetti V, Yeung MY, Hsiao LL, Ichimura T, Kuchroo V, and Bonventre JV. 2015. KIM-1-mediated phagocytosis reduces acute injury to the kidney. J Clin Invest. 125:1620–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin W, Kumar T, Lai Z, Zeng X, Kanaan HD, Li W, and Zhang PL. 2019. Kidney injury molecule-1, a sensitive and specific marker for identifying acute proximal tubular injury, can be used to predict renal functional recovery in native renal biopsies. Int Urol Nephrol. 51:2255–2265. [DOI] [PubMed] [Google Scholar]
- Yin W, Zhang PL, Macknis JK, Lin F, and Bonventre JV. 2018. Kidney injury molecule-1 identifies antemortem injury in postmortem adult and fetal kidney. Am J Physiol Renal Physiol. 315:F1637–F1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying WZ, Li X, Rangarajan S, Feng W, Curtis LM, and Sanders PW. 2019. Immunoglobulin light chains generate proinflammatory and profibrotic kidney injury. J Clin Invest. 129:2792–2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang PL, and Hafron JM. 2014. Progenitor/stem cells in renal regeneration and mass lesions. Int Urol Nephrol. 46:2227–2236. [DOI] [PubMed] [Google Scholar]
- Zhang PL, Rothblum LI, Han WK, Blasick TM, Potdar S, and Bonventre JV. 2008. Kidney injury molecule-1 expression in transplant biopsies is a sensitive measure of cell injury. Kidney Int. 73:608–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Wei W, and Liu ML. 2020. Extracellular vesicles and lupus nephritis - New insights into pathophysiology and clinical implications. Journal of autoimmunity :102540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, Si HR, Zhu Y, Li B, Huang CL, Chen HD, Chen J, Luo Y, Guo H, Jiang RD, Liu MQ, Chen Y, Shen XR, Wang X, Zheng XS, Zhao K, Chen QJ, Deng F, Liu LL, Yan B, Zhan FX, Wang YY, Xiao GF, and Shi ZL. 2020. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 579:270–273. [DOI] [PMC free article] [PubMed] [Google Scholar]


