Abstract
We herein report a case of recurrent multifocal, distal-dominant-sensorimotor neuropathy with ophthalmoplegia, IgM anti-GM1 antibody, and pyrexia-associated relapse. The patient developed sensory disturbance in her limbs after febrile disease at 50 years old. She had experienced several similar episodes and was admitted to the hospital at 56 years old. Based on a pathological study and electrophysiological findings consistent with chronic inflammatory demyelinating polyradiculoneuropathy (CIDP), maintenance IVIg therapy was administered and produced partial improvement with no relapse at one-year follow-up. Immunohistochemical studies suggested the presence of IgG (not IgM) anti-myelin antibodies. Chronic neuropathy with ophthalmoplegia and pyrexia-associated relapse may be a unique variant of CIDP.
Keywords: CIDP, multifocal demyelinating neuropathy, fever, ophthalmoplegia, gangliosides
Introduction
Chronic inflammatory demyelinating polyradiculoneuropathy (CIDP) consists of heterogeneous subtypes, the pathogenesis of which has yet to be elucidated. Cranial nerve involvement is rare in CIDP (1), and IgM or IgG anti-ganglioside antibodies are infrequently found (2). The pathological roles of these antibodies remain unclear. Several cases of chronic sensorimotor neuropathy with cranial nerve involvement, IgM anti-ganglioside antibodies, and pyrexia-associated relapse have been described as unusual cases of CIDP (3-5). These cases may be new subtypes of CIDP, but detailed clinical information, including the treatment responses, has not been described in case reports.
We herein report the clinical and pathological features of a patient with CIDP with transient ophthalmoplegia, anti-ganglioside antibodies, and pyrexia-associated relapse, along with an examination of the possibility of a new subtype of CIDP by reviewing the literature.
Case Report
A 56-year-old woman developed bilateral distal-dominant neuropathy with ophthalmoplegia after upper respiratory infection and was admitted to our hospital. At 50 years old, she had developed a tingling sensation in her fingertips after gastroenteritis. These neurological symptoms had disappeared within two days after resolution of the fever, but she then experienced four or five similar recurrent episodes. Left drop foot without febrile disease developed at 54 years old, and tingling of her fingertips and diplopia occurred following a fever and upper respiratory symptoms at 56 years old. She was referred to our hospital four days after the onset of these neurological symptoms.
On admission, a physical examination was unremarkable, and a sore throat was absent. Diplopia due to right abducens palsy was observed, but other cranial nerves were normal. The patient had asymmetrical upper/lower limb weakness and atrophy of the distal limb muscles, predominantly on the left side (Supplementary material). Her grasp power was 13 kg on the right and 9 kg on the left. Tendon reflexes were diminished in the left upper limb and absent in the lower limbs. The positional sense of the left lower limb and the vibratory sense of the trunk were decreased. Touch and pain sensations were normal in all limbs, but heel/toe walking was not possible. The patient had no family history of peripheral neuropathy.
Routine laboratory examinations, including blood glucose and vitamin B1, B2, and B12 levels, were normal. Anti-nuclear antibodies were positive (homogeneous patterns), and serum IgA (72 mg/dL) was low. Serum IgM antibodies against GM1, Gal-C, and GA1 gangliosides were positive, but other IgM/IgG-anti-ganglioside antibodies (including anti-GQ1b antibodies) were negative. Serum IL-1β, IL-2, and TNF-α levels were in normal ranges, but IL-6 was slightly elevated (5.8 pg/mL; normal <4.3 pg/mL). An IgG subset analysis showed normal levels and proportions of IgG1/2/3. The cerebrospinal fluid (CSF) showed no abnormal findings, except for slight elevation of protein (45 mg/dL). Oligoclonal IgG bands were negative, and the IgG index was 0.45 (normal <0.60).
Brain magnetic resonance imaging (MRI) findings were unremarkable, with no findings indicating cranial nerve hypertrophy. MRI neurography showed asymmetrical fusiform enlargement in the upper trunk of the brachial plexus and lumbosacral roots with contrast enhancement (Figure A). Nerve conduction studies (NCSs) showed partial conduction block or abnormal temporal dispersion in the right median, left ulnar, and left tibial nerves (Table 1, Figure B). F-wave was absent in all examined nerves except for the bilateral median and right ulnar nerve. Sensory nerve studies showed a low amplitude of sensory nerve action potentials (SNAPs) in the bilateral median/ulnar and right sural nerve.
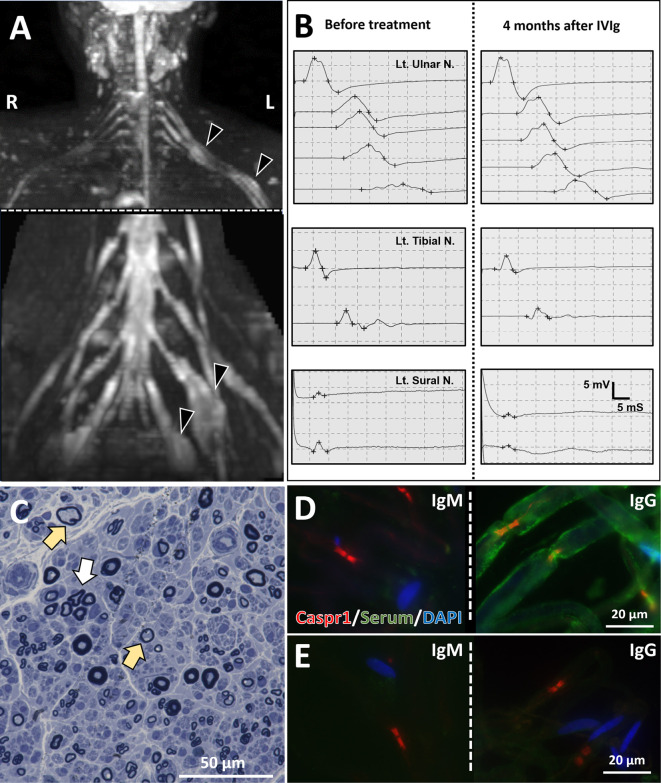
Figure.
Radiological, electrophysiological, and pathological findings. (A) MRI neurography showed brachial and lumbosacral plexus fusiform-enlargement (arrowheads). (B) Serial motor nerve conduction recordings of the left ulnar nerve (upper panel). Compound muscle action potentials (CMAPs) recorded from the left m. abductor digiti minimi after ulnar nerve stimulation at the wrist, below elbow, above elbow, axilla and Erb’s point. On day 1, apparent conduction block and temporal dispersion were seen at most proximal sites. After IVIg treatment (four months after the first recording), conduction block and temporal dispersion improved. Motor and sensory conduction studies (MCS and SCS) in the lower limb showed slowly progressive exacerbation (middle panel: MCS for left tibial nerve, lower panel: SCS for sural nerve). (C) A microscopic examination of the right sural nerve (Toluidine blue stain) revealed reduced myelinated fibers, scattered thinly myelinated fibers (yellow arrows), and regenerating axon clusters (white arrow). (D, E) An immunohistochemical study of rat peripheral nerve tissue. Immunofluorescence staining of teased rat sciatic nerve fibers using anti-contactin-associated protein 1 (Caspr1) antibody (red: nodes of Ranvier), sera obtained from the patient (D) or a healthy control (E) (green), and anti-DAPI antibody (blue: nuclei of Schwann cells) as primary antibodies. When anti-human IgM antibody was used as secondary antibody, sera from the patient and healthy control showed no reactivity for the peripheral nerve (D, E, left panels). In contrast, nodes of Ranvier, paranodes, and myelin sheath were stained with sera from the patient but not that from the control, using anti-human IgG antibody as secondary antibody (D, E, right panels).
Table 1.
Nerve Conduction Study in the Relapse/remission Phase.
| MCS | ||||||
| Nerve | Side | Phase* |
DL
(ms) |
CMAP
(mV) |
MCV
(m/s) |
F-wave, minimal latency (ms) |
| Median | Rt | Relapse Remission |
2.7 | 12.7-5.5 n.d. |
29.1 n.d. |
46.2 n.d. |
| Lt | Relapse Remission |
2.5 2.8 |
12.1-11.1 15.8-15.3 |
58.9 51.2 |
33.6 34.0 |
|
| Ulnar* | Rt | Relapse Remission |
2.4 | 13.1-8.1-7.9-7.1-5.7 n.d. |
24.0-40.0-41.3-53.8 n.d. |
36.2 n.d. |
| Lt | Relapse Remission |
2.7 | 9.5-7.1-6.7-6.0-2.7 13.2-8.3-8.3-7.3-6.0 |
29.1-58.8-75.0-35.7 30.9-45.5-51.7-34.5 |
Not evoked 41.8 |
|
| Tibial | Rt | Relapse Remission |
4.6 | 3.4-2.9 n.d. |
41.6 n.d. |
Not evoked n.d. |
| Lt | Relapse Remission |
3.3 | 7.6-5.1 5.3-3.2 |
44.2 44.4 |
Not evoked 44.1 |
|
| Peroneal | Rt | Relapse Remission |
5.1 | 0.9-1.1-1.2 n.d. |
44.6-41.4 n.d. |
n.d. n.d. |
| Lt | Relapse Remission |
5.0 | 1.2-1.3-1.3 n.d. |
30.2-60.0 n.d. |
n.d. n.d. |
|
| *To record the CMAPs of the ulnar nerve, the nerve was stimulated at the wrist, below elbow, above elbow, axillar, and Erb’s point. | ||||||
| SCS | ||||||
| Nerve | Side | Phase* |
DL
(ms) |
SNAP
(μV) |
SCV
(m/s) |
|
| Median | Rt | Relapse Remission |
2.1 n.d. |
3.4 n.d. |
61.9 n.d. |
|
| Lt | Relapse Remission |
2.0 n.d. |
5.5 5.9 |
66.3 58.6 |
||
| Ulnar | Rt | Relapse Remission |
1.8 2.2 |
4.5 n.d. |
57.7 n.d. |
|
| Lt | Relapse Remission |
2.2 2.4 |
1.7 2.4 |
46.9 46.2 |
||
| Sural (antidromic) | Rt | Relapse Remission |
2.4 n.d. |
2.1 n.d. |
62 n.d. |
|
| Lt | Relapse Remission |
2.6 3.0 |
7.3 5.9 |
57.7 50.7 |
||
*Relapse phase: day 1 after admission. Remission phase: 2 months after treatment. CMAP: compound motor action potential, DL: distal latency, Lt: left, MCS: motor nerve conduction study, MCV: motor nerve conduction velocity, n.d.: not done, Rt: right, SCS: sensory nerve conduction study, SCV: sensory nerve conduction velocity, SNAP: sensory nerve action potential
A right sural nerve biopsy was performed to make a diagnosis. The pathological findings revealed loss of large myelinated fibers, increased medium and thin myelinated fibers, sparse cluster formation, and myelin ovoid but no myelin phagocytosis by macrophages (Figure C). All of the features described above, including the CSF findings, met the diagnostic categorical criteria for definite CIDP, and the asymmetrical neurological signs met the clinical diagnostic criteria for multifocal acquired demyelinating sensory and motor (MADSAM) neuropathy (6). The patient's IgG reacted with the myelin sheath close to the nodes of Ranvier in peripheral nerve tissue from rats (Figure D, right panel), but her IgM (Figure D, left panel) and IgM and IgG from a healthy control showed no reactivity (Figure E).
After admission, all neurological symptoms, including diplopia, disappeared within a few days despite no treatment. However, the electrophysiological findings of demyelination remained, and lower limb weakness developed after 10-12 weeks of remission. The electrophysiological and clinical features suggested that the disease had remained active, so intravenous immunoglobin (IVIg) was administered (2 g/kg/day, 5 days). The clinical and electrophysiological disorders did not improve immediately after IVIg treatment, but after two months of treatment, the results of NCS were improved. Compound muscle action potentials (CMAPs) increased in the left ulnar nerve, conduction block disappeared, and left sural SNAPs increased (Figure B). An ultrasonographic evaluation of the peripheral nerves was not performed.
After the initial administration of IVIg, oral dexamethasone (40 mg/day, 4 days, every month) was started because of gradual worsening of lower limb weakness during follow-up. Dexamethasone pulses were administered six times, but generalized myalgia occurred after each pulse. We therefore diagnosed her with refractory steroid myopathy and abandoned continuation of oral steroid administration. Subsequently, IVIg-maintenance therapy (500 mg/kg/day, 2 days, every 3 weeks) was initiated, resulting in the maintenance of clinical remission. Lower-extremity weakness and electrophysiological disturbances responded to IVIg and showed improvement with each dose (Figure B, Table 1). Serum IgM-anti GM1, Gal-C, and GA1 antibodies turned negative after IVIg treatment.
Discussion
This case had characteristics of bilateral ophthalmoplegia, neurological symptoms that developed with pyrexia and spontaneously improved without immunotherapy, and IgM anti-GM1 antibodies. The sural nerve pathology supported the diagnosis of chronic demyelinating neuropathy, and the electrophysiological findings were consistent with definite CIDP.
Several similar cases have been reported (Table 2). Mazzucco et al. (4) and Ueda et al. (5) described patients with CIDP with pyrexia-triggered relapse who had ophthalmoplegia and IgM anti-GM1 antibodies. Likosky et al. reported three cases of CIDP in which ophthalmoplegia was characterized by fever-induced relapse, and IgG subset deficiency (low IgG1/3) was present (3). Cases of CIDP with ophthalmoplegia and IgM-antiGM1 antibodies have also been described, but these did not show fever-induced relapse or spontaneous remission (7,8). The five cases above (3-5) and our case were asymmetrical CIDP and differed from MADSAM neuropathy or focal CIDP to some extent. These cases suggest that there is a CIDP subtype characterized by ophthalmoplegia, pyrexia-associated relapse, and autoantibodies.
Table 2.
Demographic Data of Reported Cases of CIDP with Pyrexia-associated Relapse and Ocular Palsy (Including the Present Case).
| Case | Onset age, y | Sex | Clinical subtype | Clinical characteristics | Interval of fever to neurological signs | Anti-ganglioside antibodies | Therapy | Clinical characteristics after treatment | Reference No. |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 65 | F | Focal CIDP | Pyrexia-associated relapse, unilateral ptosis, diplopia, focal limb weakness | 1 day | None | None | Minimal to no neurological deficit without specific treatment | 3 |
| 2 | 76 | F | MADSAM | Pyrexia-associated relapse, unilateral ptosis, diplopia, multifocal weakness/sensory deficit | 2 days | GM1* | IVIg | Distal dominant asymmetrical limb weakness and mild sensory deficit. | |
| 3 | 53 | M | Focal CIDP | Pyrexia-associated relapse, unilateral ptosis, focal limb weakness and numbness | n. a. | None | None | Minimal to no neurological deficit without specific treatment | |
| 4 | 48 | M | MADSAM | Pyrexia-associated relapse, dysphagia, bilateral facial palsy, sensory ataxia, multifocal limb weakness/sensory deficit | 2 days | None | IVIg | Slightly ataxic gait, but the patient was able to walk unassisted up to 3 km | 4 |
| 5 | 56 | F | MADSAM | Pyrexia-associated relapse, unilateral abducens palsy, asymmetric distal-dominant weakness/paresthesia of limbs | 2 days | IgG-GalNac-GD1a | None | Paresthesia on unilateral foot and remaining multifocal electrophysiological deficit | 5 |
| Present case | 56 | F | MADSAM | Pyrexia-associated relapse, unilateral oculomotor palsy, asymmetric distal-dominant muscle weakness and paresthesia of limbs | 1-2 days | IgM-GM1, Gal-C, and GA1 | IVIg | Partial amelioration of CB in upper extremity, but limited degree of improvement | This case |
*Immunoglobulin subclass was not shown in the report.
CB: conduction block, CIDP: chronic inflammatory demyelinating polyradiculoneuropathy, F: female, M: male, MADSAM: multifocal acquired demyelinating sensory and motor, MRC: Medical Research Council, n.a.: not available
The pathophysiological role of IgM-anti-ganglioside antibodies in such cases and the mechanism underlying the pyrexia-related relapse are unknown. Neurological symptoms in CIDP may remit spontaneously. Two of the five previously reported cases received immunotherapy, while 3 received no treatment. Two of the five cases showed obvious neurological deficits after a long disease course (Table 2, cases 4, 5) (3-5). In our case, based on the sural nerve pathology and abnormal electrophysiological findings, IVIg treatment was started, followed by maintenance IVIg every three weeks. The full dose of IVIg (400 mg/kg/day for 5 days) achieved partial amelioration of conduction block in the upper extremity, and periodic IVIg-maintenance therapy resulted in clinical remission and no relapse. However, there was limited improvement in muscle weakness and atrophy in the lower limbs, without recovery to the level before onset of the disease. This clinical course suggests that early treatment is needed to prevent treatment resistance.
IgM anti-ganglioside antibodies presumably have a relationship with the pathogenesis of CIDP with cranial nerve involvement (7-10), but the exact details remain unclear. IgM anti-GM1 antibody was positive on an enzyme-linked immunosorbent assay, but immunohistochemical studies suggested the presence of IgG antibodies that reacted with myelin, and these IgG antibodies may be more closely related to the pathology. This finding indicates that it is necessary to search carefully for IgG-class autoantibodies, even in cases of chronic neuropathy such as CIDP. In the future, we plan to perform an analysis aimed at identifying the target antigen.
The presence of pyrexia-associated exacerbation and disappearance of symptoms followed by improvement after cooling is similar to Uhthoff's phenomenon. Such a phenomenon in our case might have been caused by fever-induced reversible conduction block in demyelinated fibers due to unknown autoantibodies. Another mechanism may be the vulnerability of the demyelinating nerve to external factors, such as cytokines produced by infection. There was no worsening of neurological symptoms with increased body temperature, except for infection, which suggests that cytokines and other factors may be involved in the pathogenesis of these symptoms.
In conclusion, this case may be a distinct subtype of CIDP with cranial nerve involvement, anti-IgM ganglioside antibody, and pyrexia-associated relapse. This subtype is prone to improve spontaneously but may not fully recover if there is a delay in the initial treatment.
The authors state that they have no Conflict of Interest (COI).
Financial Support
This study was supported in part by the Japan Agency for Medical Research and Development (AMED) under grant numbers JP19lk0201080j0102, JP19ek0109376h0002, and JP20ek0109376h0003, and by JSPS KAKENHI under grant number JP18K07403, and JP21K15688.
Supplementary Material
Results of manual muscle testing (MMT) on admission.
Acknowledgements
We particularly thank Dr. Jun Shimizu for the pathological study of the biopsy specimen of the peripheral nerve.
References
- 1. Kuwabara S, Isose S, Mori M, et al. Different electrophysiological profiles and treatment response in ‘typical’ and ‘atypical’ chronic inflammatory demyelinating polyneuropathy. J Neurol Neurosurg Psychiatr 86: 1054-1059, 2015. [DOI] [PubMed] [Google Scholar]
- 2. Martinez-Thompson JM, Snyder MR, Ettore M, et al. Composite ganglioside autoantibodies and immune treatment response in MMN and MADSAM. Muscle Nerve 57: 1000-1005, 2018. [DOI] [PubMed] [Google Scholar]
- 3. Likosky DJ, Kraus EE, Yuen EC. Recurrent multifocal demyelinating neuropathy with febrile illness and IgG subset deficiency. Neurology 52: 1902-1905, 1999. [DOI] [PubMed] [Google Scholar]
- 4. Mazzucco S, Ferrari S, Mezzina C, Tomelleri G, Bertolasi L, Rizzuto N. Hyperpyrexia-triggered relapses in an unusual case of ataxic chronic inflammatory demyelinating polyradiculoneuropathy. Neurol Sci 27: 176-179, 2006. [DOI] [PubMed] [Google Scholar]
- 5. Ueda J, Yoshimura H, Kohara N. Pyrexia-associated relapse in chronic inflammatory demyelinating polyradiculoneuropathy. Intern Med 57: 2723-2726, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Joint Task Force of the E; the PNS. European Federation of Neurological Societies/Peripheral Nerve Society guideline on the use of skin biopsy in the diagnosis of small fiber neuropathy. Report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society. J Peripher Nerv Syst 15: 79-92, 2010. [DOI] [PubMed] [Google Scholar]
- 7. Ryo M, Saito T, Kunii N, Hasegawa H, Kowa H. [A case of chronic inflammatory demyelinating polyneuropathy with recurrent ophthalmoplegia, persistent conduction block, antibody activity against gangliosides GM1]. Rinsho Shinkeigaku (Clin Neurol) 34: 702-706, 1994. [PubMed] [Google Scholar]
- 8. Ozaki I, Baba M, Kurihara A, Saitoh T. Chronic inflammatory demyelinating polyneuropathy (CIDP) with ophthalmoplegia: a case with asymmetric limb weakness and high titers of anti-GM1 antibody. Eur J Neurol 3: 457-461, 1996. [Google Scholar]
- 9. Teramoto H, Morita A, Hara M, et al. Relapse with dysphagia in a case of chronic inflammatory demyelinating polyradiculoneuropathy. Intern Med 54: 1791-1793, 2015. [DOI] [PubMed] [Google Scholar]
- 10. Hickman SJ, Allen JA, Baisre A, et al. Neuro-ophthalmological complications of chronic inflammatory demyelinating polyradiculoneuropathy. Neuroophthalmology 37: 146-156, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Results of manual muscle testing (MMT) on admission.