Abstract
We herein report a case of myelin oligodendrocyte glycoprotein (MOG) antibody-associated disease after severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. A 24-year-old woman developed unilateral optic neuritis 3 weeks after contracting coronavirus disease 2019 (COVID-19), followed by intracranial demyelinating lesions and myelitis. Since serum anti-MOG antibody was positive, we diagnosed MOG antibody-associated disease. Immunotherapy with steroids resulted in the rapid improvement of neurological symptoms. This is a suggestive case, as there are no reports of MOG antibody-associated disease with multiple neurological lesions occurring after COVID-19. The response to immunotherapy was favorable. This case suggests that it is important to measure anti-MOG antibodies in patients who develop inflammatory neurological disease after COVID-19.
Keywords: MOG antibody-associated disease, COVID-19, SARS-CoV-2, optic neuritis, myelitis, demyelinating autoimmune disease
Introduction
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has led to the global coronavirus disease 2019 (COVID-19) pandemic (1). SARS-CoV-2 has been mentioned as a potential candidate for synaptic invasion of the central nervous system (CNS) and autoantibody production (2). In fact, SARS-CoV-2 infection has also been reported to induce inflammatory disease in the CNS (3). However, there have been only a few cases of myelin oligodendrocyte glycoprotein (MOG) antibody-associated disease after SARS-CoV-2 infection.
We herein report a case of SARS-CoV-2-related MOG antibody-associated disease, along with a literature review.
Case Report
A 24-year-old woman presented with headache and myalgia in mid-April 2021, followed by a high fever two days later. Nasopharyngeal COVID-19 polymerase chain reaction (PCR) was found to be positive after four days, and she was hospitalized for six days. She had no respiratory symptoms, but a chest computed tomography (CT) showed a pure ground-glass opacity in the lower lobe of the right lung. She was treated with acetaminophen during her hospitalization, and no steroids or other immunosuppressive drugs were used. Even though she was discharged from the hospital, she continued to have intermittent high fevers for about two weeks, but no other systemic or focal symptoms.
In early May, she experienced diminished vision in her left eye, for which she visited an ophthalmologist. An Examination showed that the visual acuity in her left eye had decreased to 0.6, and fundus examination revealed redness of the left optic nerve papilla (Fig. 1A). Critical flicker frequency (CFF) was reduced to 23 Hz in her left optic nerve and Goldman perimetry (GP) showed a decrease in sensitivity around the central area of her left eye (Fig. 1B). Brain CT revealed mild swelling of the left optic nerve (Fig. 1C), suggesting left optic neuritis. The vision loss in her left eye, however, improved spontaneously. In late May, she developed numbness in the soles of both her feet and the perianal area, along with difficulty in urinating and defecating; numbness in the fingertips of both hands also appeared in mid-June. Since fluid-attenuated inversion-recovery (FLAIR) magnetic resonance imaging (MRI) of the brain revealed high-signal lesions, she was referred to our hospital for a further examination and treatment.
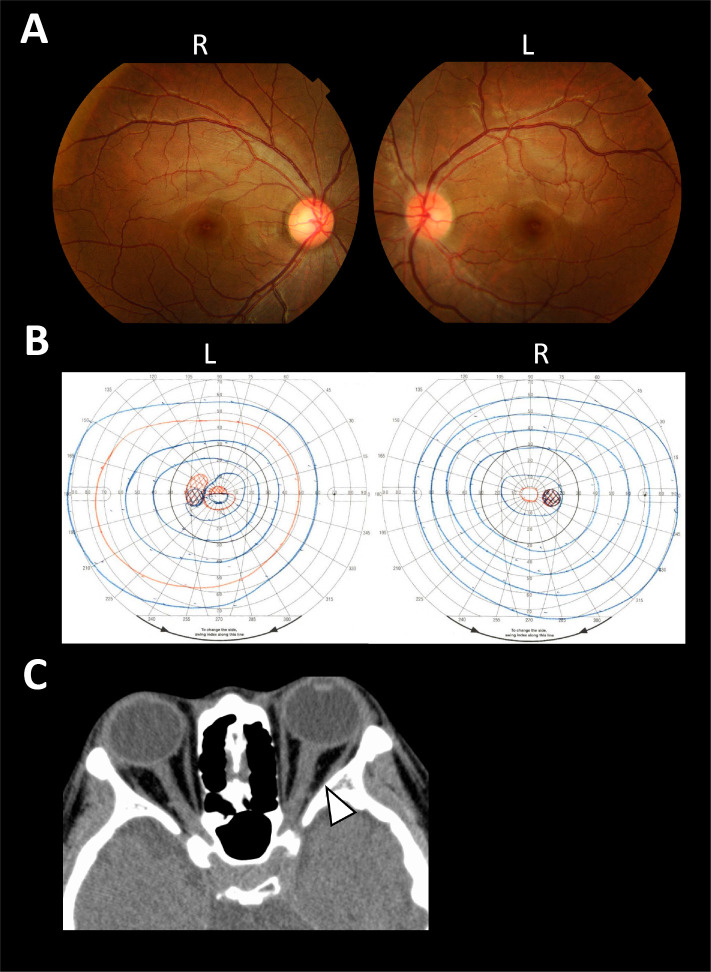
Figure 1.
Ophthalmologic findings and brain CT images. A fundus examination revealed redness of the left optic nerve papilla (A) and GP showed a decreased sensitivity around the central area in her left eye (B) (L: left side, R: right side). Brain CT showed mild swelling of the left optic nerve compared to the right (arrowhead) (C).
On admission, her body temperature was 36.4 °C, pulse was 99 beats/min, blood pressure was 104/66 mmHg, and a general physical examination revealed no abnormalities of note. Visual acuity in her left eye had already improved to 1.2. A neurological examination revealed tactile hyperesthesia in the distal extremities and perineum. The vibration sensation was decreased in her lower limbs. Romberg's sign was positive, and she had difficulty walking. There were no obvious neurological abnormalities in consciousness, higher brain functions, cranial nerves, pyramidal tract, coordination, or deep tendon reflexes.
Blood tests showed no abnormalities in blood counts, the renal function, electrolytes, or the coagulation function. Serum rheumatoid factor, anti-nuclear antibody, anti-SS-A antibody, anti-SS-B antibody, myeloperoxidase-anti-neutrophil cytoplasmic antibody (MPO-ANCA), serine proteinase 3-anti-neutrophil cytoplasmic antibody (PR3-ANCA), and other autoimmune markers were negative. Anti-aquaporin 4-antibody (anti-AQP4 antibody) was negative by both an enzyme-linked immune sorbent assay (ELISA) and cell-based assay.
An electrocardiogram and chest X-ray showed no abnormal findings. Contrast-enhanced MRI of the brain showed faint T2 extension lesions around the trigone and inferior horn of the left lateral ventricle, near the ventricular wall above the trigone of the right ventricle, and bilaterally in the subcortical frontal lobes. There were also small, scattered lesions in the cerebral white matter. Contrast enhanced linear areas were seen at the margins of the lesions in the right superior frontal gyrus (Fig. 2B-H). Spinal cord MRI showed scattered mottled or linear T2 high-signal lesions at the C4-C5, C5-C6, Th3-Th4, Th6-Th7, and Th11-12 levels. Each lesion had a faint contrast effect (Fig. 3). A cerebrospinal fluid (CSF) examination showed a mildly elevated cell count with mononuclear cell predominance (16 cells/μL). CSF protein, β2-microglobulin, IgG, and myelin basic protein (MBP) were elevated at 51 mg/dL, 2.70 μg/mL, 4.2 mg/dL, and 260 pg/mL, respectively. The IgG index was 0.66, and oligoclonal bands were positive. There was no elevation of interleukin-6 (IL-6) in the CSF. The SARS-CoV-2 PCR test of CSF was negative.
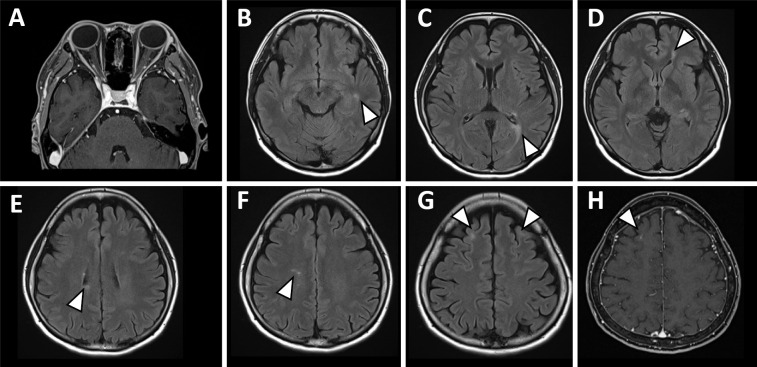
Figure 2.
Brain MRI. Brain MRI on admission showed that the swelling of the left optic neuritis observed on CT had already improved (A). Brain MRI showed faint T2 high-signal lesions (arrowheads) around the trigone and inferior horn of the left lateral cerebral ventricle, near the ventricular wall above the right ventricular trigone, and in the bilateral subcortical frontal lobes. There were also scattered small T2 high-signal lesions (arrowheads) in the cerebral white matter (B-G). Contrast-enhanced MRI showed a linear enhancement area at the limbus of the lesion in the right superior frontal gyrus (H).
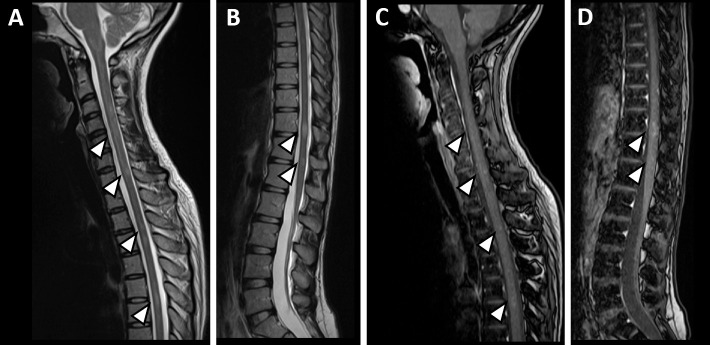
Figure 3.
Spinal cord MRI. Spinal cord MRI showed scattered mottled or linear T2 high-signal lesions (arrowheads) at the C4-C5, C5-C6, Th3-Th4, Th6-Th7, and Th11-12 levels (A, B). Each lesion (arrowheads) had a faint contrast effect (C, D).
Based on the clinical symptoms, imaging findings, and CSF findings, we considered the possibility of inflammatory demyelinating disease of the CNS and decided to treat her with steroids in the acute phase. We started with 2 courses of methylprednisolone pulse therapy (1,000 mg/day, for 5 days) and then switched to oral prednisolone. Her lightheadedness, dysuria, and dyspepsia improved immediately after one course of steroid pulse therapy. We initially assumed the possibility of multiple sclerosis because of the absence of long lesions in the spinal cord, the presence of periventricular demyelinating lesions, and positive oligoclonal bands in CSF. However, during the course of the disease, since serum MOG antibody was found to be positive by a cell-based assay, we ultimately made a diagnosis of MOG antibody-associated disease. Furthermore, since she met the diagnostic criteria of acute disseminated encephalomyelitis (ADEM) (4) and had an episode of optic neuritis, we diagnosed her with ADEM-optic neuritis (ADEMON) as a clinical subtype of MOG antibody-associated disease.
Prednisolone was gradually decreased from 40 mg to 15 mg, and the patient was discharged home in early July. Follow-up MRI of the brain and spinal cord, performed after discharge showed no new lesions, and the known lesions had regressed. Currently, she is continuing to take prednisolone and has shown no recurrence for four months since her discharge from the hospital.
Discussion
MOGs are glycoproteins that exist on the surface of oligodendrocyte myelin, and antibodies to MOG are known to cause demyelination in the CNS (5,6). There are various types of MOG antibody-associated diseases, that have been reported to present with a variety of CNS lesions, such as optic neuritis, myelitis, neuromyelitis optica spectrum disorder, ADEM, brainstem encephalitis, cortical encephalitis, and aseptic meningitis (7). The most common type of MOG antibody-associated disease varies according to age group, with ADEM being more common in children and optic neuritis being more common in adults (8).
In the present case, optic neuritis due to MOG antibody-associated disease developed three weeks after SARS-CoV-2 infection, suggesting a correlation between the two. The relationship between para-infectious or post-infectious demyelinating disease and prior viral infection has been studied for many years, and a report on patients with ADEM indicated that 93% had a history of viral infection within 3 weeks before the onset of neurological symptoms (9). The mechanism of central neuropathy is thought to involve molecular homology, in which various viral antigens induce immune responses directed against CNS myelin proteins, including MOG (10).
It was previously reported that 36.4% of patients had neurological symptoms after SARS-CoV-2 infection, with dizziness and headache appearing in 16.8% and 13.1% of patients, respectively (11). In addition, multiple sclerosis (12), meningoencephalitis (13), ADEM (14), acute hemorrhagic necrotizing encephalitis (3), Guillain-Barré syndrome (14), and Miller Fisher syndrome (15) have been reported as neurological diseases induced after SARS-CoV-2 infection, although there are only a few reports on MOG antibody-associated disease. In a review of 43 cases of inflammatory disease of the CNS following SARS-CoV-2 infection, only 2 were known to be positive for anti-MOG antibodies (3). Table summarizes the reported cases of MOG antibody-associated diseases after SARS-CoV-2 infection, including this case (2,16-19). So far, six such cases (including this case) have been reported, five in adults and one in a child. In five of the cases, neurological symptoms appeared several days to weeks after the onset of infectious symptoms. In one case, a PCR test revealed SARS-CoV-2 infection, although there were no symptoms related to SARS-CoV-2. The most common lesion was optic neuritis alone in three cases. In one case, both optic neuritis and myelitis were present, and in one case, only brain lesions were observed. In our case, the optic nerve, spinal cord, and brain were all affected. Of the four patients presenting with optic neuritis, the condition was bilateral in three cases, and only our patient had unilateral optic neuritis. A CSF examination was performed in all patients. Four patients had mildly elevated CSF cell levels, and three patients had mildly elevated CSF protein levels. SARS-CoV-2 PCR tests in CSF were performed in four cases, all of which were negative. Oligoclonal bands in the spinal fluid were examined in four cases and were positive in the present case and in the cases showing optic neuritis and myelitis. In all cases, steroid pulse therapy and oral steroids were administered in the acute phase, with favorable treatment responses. Only one patient required plasma exchange.
Table.
Clinical Characteristics of Reported Cases of MOG Antibody-associagted Disease after SARS-CoV-2 Infection.
| Case No | Reference | Patient age/gender | Time duration from COVID-19 to onset of neuroligal symptoms | CNS lesions | CSF findings | Treatment | Clinical outcome |
|---|---|---|---|---|---|---|---|
| 1 | 16 | 47/M | No symptoms of infection | Bilateral optic neuritis | CSF normal cell cout and chemistry, RT-PCR negative for SARS-CoV-2 | IVMP, oral steroid | Recoverd |
| 2 | 17 | 11/M | 2 weeks | Bilateral optic neuritis | CSF WBC 55 cells/μL, noraml protein OCB absent | IVMP, oral steroid | Recoverd |
| 3 | 18 | 44/M | 2 weeks | Bilateral optic neuritis | CSF WBC 3 cells/μL, protein 50 mg/dL, OCB absent | IVMP, oral steroid | Recoverd |
| 4 | 2 | 26/M | 2 days | Bilateral optic neuritis, myelitis | CSF WBC 55 cells/μL, protein 31 mg/dL, OCB present RT-PCR negative for SARS-CoV-2 | IVMP, oral steroid | Recoverd |
| 5 | 19 | 44/F | 1 weeks | CNS inflammatory vasculopathy | CSF WBC 13 cells/μL, protein 507 mg/L, RT-PCR negative for SARS-CoV-2 | IVMP, oral steroid, PE | Recoverd |
| 6 | Current case | 24/F | 3 weeks | Unilataral optic neuritis, myelitis, CNS demyelinatining lesions | CSF WBC 16 cells/μL, protein 51 mg/dL, OCB present RT-PCR negative for SARS-CoV-2 | IVMP, oral steroid | Recoverd |
CNS: central nervous system, WBC: white blood count, OCB: oligoclonal band, IVMP: intravenous methylprednisolone, PE: plasma exchange
Various mechanisms might be involved in MOG antibody-associated diseases after SARS-CoV-2 infection, as described below. First, SARS-CoV-2 has been shown to stimulate host immune responses, most notably leading to acute respiratory distress syndrome (ARDS). It has been reported that up to 29% of patients with SARS-CoV-2 infection develop ARDS after SARS-CoV-2 infection (20). Cytokines and inflammatory markers, such as C-reactive protein, D-dimer, interleukin-2 (IL-2), IL-6, interleukin-7 (IL-7), interleukin-10 (IL-10), granulocyte colony stimulating factor, interferon-γ inducible protein 10 kDa (IP10), monocyte chemotactic protein 1 (MCP1), macrophage inflammatory protein 1A (MIP1A), and tumor necrosis factor α (TNFα), have been shown to be activated in patients with severe COVID-19 (2). In addition, a previous report showed that severe CNS inflammatory diseases, such as acute hemorrhagic necrotizing encephalitis, are more likely to occur in severe COVID-19 than in non-severe COVID-19 (3). There have also been reports of autoimmune diseases associated with SARS-CoV-2, such as anti-phospholipid antibody syndrome, Kawasaki disease, Miller Fisher syndrome, and Guillain-Barré syndrome, suggesting that SARS-CoV-2 might trigger autoantibody production (2). In this case, the patient's fever persisted for about three weeks after SARS-CoV-2 infection, which might have triggered a strong immune response and the production of anti-MOG antibodies. The angiotensin-converting enzyme 2 receptor, which is the target of SARS-CoV-2, is expressed on endothelial cells in several organs, including the brain, and a histopathological examination of COVID-19 patients revealed lymphocytic endotheliitis in the lung, heart, kidney, small intestine, and liver (21). In the present case, both brain and spinal cord lesions showed contrast enhancement in the lesions, suggesting disruption of the blood-brain barrier. This disruption of the blood-brain barrier secondary to endotheliitis in the brain might have facilitated the entry of anti-MOG antibodies into the CNS and accelerated the pathogenic process. According to previous reports, oligoclonal band positivity is high in multiple sclerosis, whereas oligoclonal band positivity is thought to occur in less than 15% of patients with anti-MOG antibody-related diseases (22,23). In this case, oligoclonal bands were positive in the CSF, which is atypical for anti-MOG antibody-related diseases, suggesting increased production of anti-MOG antibodies due to SARS-CoV-2 infection, and transfer of antibodies into the CSF due to disruption of the blood-brain barrier.
In summary, we experienced a case of anti-MOG antibody-associated disease after SARS-CoV-2 infection. This report is important, as there have been no reports of multiple CNS lesions after SARS-CoV-2 infection. The response to immunotherapy in our patient was favorable. Our experience suggests that it is important to measure anti-MOG antibodies in patients who develop CNS inflammatory disease after SARS-CoV-2 infection.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Wu F, Zhao S, Yu B, et al. A new coronavirus associated with human respiratory disease in China. Nature 579: 265-269, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhou S, Jones-Lopez EC, Soneji DJ, Azevedo CJ, Patel VR. Myelin oligodendrocyte glycoprotein antibody-associated optic neuritis and myelitis in COVID-19. J Neuroophthalmol 40: 398-402, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sriwastava S, Tandon M, Podury S, et al. COVID-19 and neuroinflammation: a literature review of relevant neuroimaging and CSF markers in central nervous system inflammatory disorders from SARS-COV2. J Neurol 268: 4448-4478, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cole J, Evans E, Mwangi M, Mar S. Acute disseminated encephalomyelitis in children: an updated review based on current diagnostic criteria. Pediatr Neurol 100: 26-34, 2019. [DOI] [PubMed] [Google Scholar]
- 5. Fernandez-Carbonell C, Vargas-Lowy D, Musallam A, et al. Clinical and MRI phenotype of children with MOG antibodies. Mult Scler 22: 174-184, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Waters P, Fadda G, Woodhall M, et al. Serial anti-myelin oligodendrocyte glycoprotein antibody analyses and outcomes in children with demyelinating syndromes. JAMA Neurol 77: 82-93, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Netravathi M, Holla VV, Nalini A, et al. Myelin oligodendrocyte glycoprotein-antibody-associated disorder: a new inflammatory CNS demyelinating disorder. J Neurol 268: 1419-1433, 2021. [DOI] [PubMed] [Google Scholar]
- 8. Sepúlveda M, Armangue T, Martinez-Hernandez E, et al. Clinical spectrum associated with MOG autoimmunity in adults: significance of sharing rodent MOG epitopes. J Neurol 263: 1349-1360, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Leake JA, Albani S, Kao AS, et al. Acute disseminated encephalomyelitis in childhood: epidemiologic, clinical and laboratory features. Pediatr Infect Dis J 23: 756-764, 2004. [DOI] [PubMed] [Google Scholar]
- 10. Kuerten S, Lichtenegger FS, Faas S, Angelov DN, Tary-Lehmann M, Lehmann PV. MBP-PLP fusion protein-induced EAE in C57BL/6 mice. J Neuroimmunol 177: 99-111, 2006. [DOI] [PubMed] [Google Scholar]
- 11. Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol 77: 683-690, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Palao M, Fernández-Díaz E, Gracia-Gil J, Romero-Sanchez CM, Diaz-Maroto I, Segura T. Multiple sclerosis following SARS-CoV-2 infection. Mult Scler Relat Disord 45: 102377, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Moriguchi T, Harii N, Goto J, et al. A first case of meningitis/encephalitis associated with SARS-coronavirus-2. Int J Infect Dis 94: 55-58, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Toscano G, Palmerini F, Ravaglia S, et al. Guillain-Barré syndrome associated with SARS-CoV-2. N Engl J Med 382: 2574-2576, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gutiérrez-Ortiz C, Méndez-Guerrero A, Rodrigo-Rey S, et al. Miller Fisher syndrome and polyneuritis cranialis in COVID-19. Neurology 95: e601-e605, 2020. [DOI] [PubMed] [Google Scholar]
- 16. Kogure C, Kikushima W, Fukuda Y, et al. Myelin oligodendrocyte glycoprotein antibody-associated optic neuritis in a COVID-19 patient: a case report. Medicine (Baltimore) 100: e25865, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Khan A, Panwala H, Ramadoss D, Khubchandani R. Myelin oligodendrocyte glycoprotein (MOG) antibody disease in a 11 year old with COVID-19 infection. Indian J Pediatr 88: 488-489, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sawalha K, Adeodokun S, Kamoga GR. COVID-19-induced acute bilateral optic neuritis. J Investig Med High Impact Case Rep 8: 2324709620976018, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pinto AA, Carroll LS, Nair V, Varatharaj A, Galea I. CNS inflammatory vasculopathy with antimyelin oligodendrocyte glycoprotein antibodies in COVID-19. Neurol Neuroimmunol Neuroinflamm 7: e813, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395: 497-506, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet 395: 1417-1418, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jurynczyk M, Messina S, Woodhall MR, et al. Clinical presentation and prognosis in MOG-antibody disease: a UK study. Brain 140: 3128-3138, 2017. [DOI] [PubMed] [Google Scholar]
- 23. Jarius S, Ruprecht K, Kleiter I, et al. MOG-IgG in NMO and related disorders: a multicenter study of 50 patients. Part 2: epidemiology, clinical presentation, radiological and laboratory features, treatment responses, and long-term outcome. J Neuroinflammation 13: 280, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]