Abstract
Objective
We evaluated the change in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antibody titers from three to six months after the administration of the BNT162b2 vaccine among healthcare workers.
Methods
A total of 337 healthcare workers who received 2 doses of the BNT162b2 vaccine were included in this study. Factors associated with SARS-CoV-2 antibody titers at three and six months and the change in SARS-CoV-2 antibody titers between three and six months after vaccine administration were analyzed using a logistic regression analysis.
Results
The SARS-CoV-2 antibody titer at 3 months was 4,812.1±3,762.9 AU/mL in all subjects and was lower in older workers than in younger ones. The SARS-CoV-2 antibody titer at 6 months was 1,368.9±1,412.3 AU/mL in all subjects. The SARS-CoV-2 antibody titers that were found to be high at three months were also high at six months. The change in SARS-CoV-2 antibody titers from 3 to 6 months was -68.9%±16.1%. The higher SARS-CoV-2 antibody titers at three months showed a more marked decrease from three to six months than lower titers.
Conclusion
This study demonstrates that SARS-CoV-2 antibody titers at three months decreased with age and were associated with the antibody titers at six months and the change in titer from three to six months. Older individuals in particular need to be aware of the declining SARS-CoV-2 antibody titers at six months after the BNT162b2 vaccine. The results of this study may provide insight into COVID-19 vaccine booster strategies.
Keywords: COVID-19 vaccine, Japan, healthcare workers, SARS-CoV-2 antibody titer
Introduction
The coronavirus disease 2019 (COVID-19) pandemic has had serious effects around the world. The BNT162b2 mRNA COVID-19 vaccine (Pfizer-BioNTech) confers high protection against COVID-19 infection. The BNT162b2 vaccine was 95% effective in preventing COVID-19 occurrence (1). However, recently, breakthrough infections of COVID-19 have been reported in vaccine recipients (2,3). A previous study reported concerns about reduced effectiveness of the BNT162b2 vaccine for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants (4). The BNT162b2 vaccine had high effectiveness against COVID-19 infection and led to fewer hospital admissions for up to six months (5,6). However, breakthrough infections have been correlated with SARS-CoV-2 antibody titers during the peri-infection period (3). Therefore, a decline in antibody titers increases the risk of breakthrough infection. Furthermore, breakthrough infection is a concern in Japan, and the government of Japan is considering the administration of a COVID-19 vaccine booster. We believe that understanding the change in SARS-CoV-2 antibody titers after COVID-19 vaccine administration is important managing COVID-19 vaccine distribution.
In Japan, the COVID-19 vaccine was approved for healthcare workers before the general public. In this study, we evaluated the change in SARS-CoV-2 antibody titers from three to six months after the administration of the BNT162b2 vaccine to healthcare workers.
Materials and Methods
In this study, we enrolled 337 healthcare workers in Kamagaya General Hospital who received 2 doses of the BNT162b2 mRNA COVID-19 vaccine in March and April 2021. SARS-CoV-2 antibody titers were measured at three and six months after administration of the vaccine using the SARS-CoV-2 IgG II Quant Reagent Kit (Abbott Japan, Tokyo, Japan); a result of ≥50 AU/mL with this test is considered a positive result. This kit can quantitatively measure immunoglobulin G (IgG) antibodies against the spike receptor-binding domain of SARS-CoV-2 using the chemiluminescent microparticle immunoassay (CMIA) method. There are several methods for testing COVID-19 antibody, such as a CMIA, enzyme-linked Immunosorbent assay (ELISA), and lateral flow immunoassay (LFIA). The CMIA method has higher sensitivity and specificity than the ELISA and LFIA (7).
We investigated factors associated with SARS-CoV-2 antibody titers at three and six months and the change in SARS-CoV-2 antibody titers between three and six months after vaccine administration.
Statistical analyses
A multivariate logistic regression analysis was performed using variables with a p value <0.1 from the univariate logistic regression analysis. These variables included the age, sex, body mass index (BMI), comorbidities of allergic and/or collagen disease, current smoking habit, alcohol intake, exercise habit, and side effects after vaccination. We also included the SARS-CoV-2 antibody titers at three months as a variable in the analyses at six months. A p value of <0.05 was considered significant.
Results
The baseline characteristics of the study subjects at baseline were as follows: the mean age was 36.6±11.7 years, and 71.8% participants were women, with a mean BMI of 22.4±3.4. Comorbidities were present in 9.2%, 11.6% were current smokers, 13.1% had a daily alcohol intake 14.8% exercised regularly, and 91.4% had side effects after the vaccine.
The SARS-CoV-2 antibody titer at 3 months was 4,812.1±3,762.9 AU/mL in all subjects. The only significant factor associated with SARS-CoV-2 antibody titers at 3 months was age (p<0.001) (Table a). The SARS-CoV-2 antibody titers at three months were lower in older workers than in younger ones. The SARS-CoV-2 antibody titer at 6 months was 1,368.9±1,412.3 AU/mL in all subjects. The only significant factor associated with SARS-CoV-2 antibody titers at 6 months was the SARS-CoV-2 antibody titer at 3 months (p<0.001) (Table b). The SARS-CoV-2 antibody titers that were found to be high at three months were also high at six months. Fig. 1 shows the SARS-CoV-2 antibody titer at 3 and 6 months in all subjects and all age groups (20-29, 30-39, 40-49, and ≥50 years old).
Table.
Factors Associated with (a) SARS-CoV-2 Antibody Titers at 3 Months, (b) SARS-CoV-2 Antibody Titers at 6 Months, and (c) the Change in SARS-CoV-2 Antibody Titers from 3 to 6 Months after Receiving the Vaccine.
| (a) | ||
|---|---|---|
| Univariate analysis | Multivariate analysis | |
| Variables | p value | p value |
| Age | <0.001 | <0.001 |
| Sex | 0.667 | |
| Body mass index | 0.599 | |
| Comorbidities of allergic and/or collagen disease | 0.166 | |
| Current smoker | 0.117 | |
| Daily alcohol intake | 0.077 | 0.517 |
| Exercise regularly | 0.876 | |
| Side effects after the vaccine | 0.320 | |
| (b) | ||
| Univariate analysis | Multivariate analysis | |
| Variables | p value | p value |
| Age | <0.001 | 0.984 |
| Sex | 0.741 | |
| Body mass index | 0.617 | |
| Comorbidities of allergic and/or collagen disease | 0.640 | |
| Current smoker | 0.167 | |
| Daily alcohol intake | 0.200 | |
| Exercise regularly | 0.489 | |
| Side effects after the vaccine | 0.287 | |
| SARS-CoV-2 antibody titer at 3 months | <0.001 | <0.001 |
| (c) | ||
| Univariate analysis | Multivariate analysis | |
| Variables | p value | p value |
| Age | 0.376 | |
| Sex | 0.061 | 0.068 |
| Body mass index | 0.440 | |
| Comorbidities of allergic and/or collagen disease | 0.285 | |
| Current smoker | 0.668 | |
| Daily alcohol intake | 0.475 | |
| Exercise regularly | 0.575 | |
| Side effects after the vaccine | 0.705 | |
| SARS-CoV-2 antibody titer at 3 months | <0.001 | <0.001 |
Figure 1.
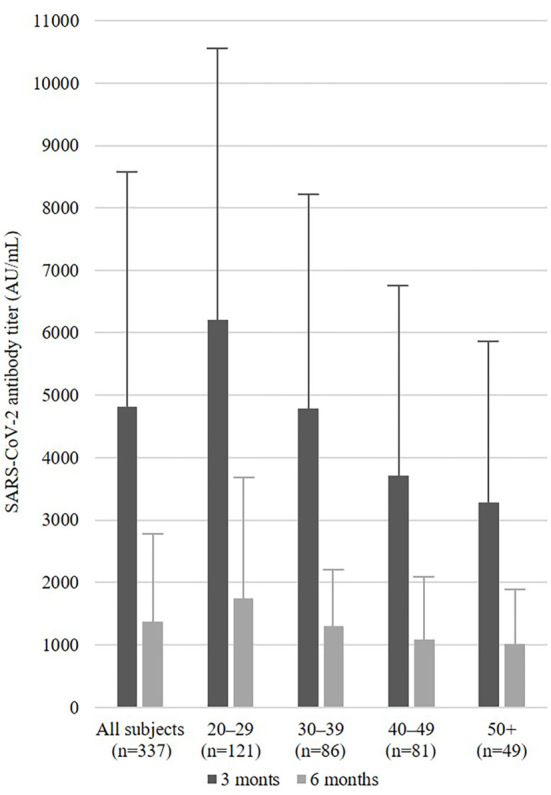
SARS-CoV-2 antibody titer at three and six months in all subjects and age groups (20-29, 30-39, 40-49, and ≥50 years old).
The change in SARS-CoV-2 antibody titers from 3 to 6 months was -68.9±16.1%. The SARS-CoV-2 antibody titer at 3 months was also significantly associated with the change in SARS-CoV-2 antibody titers (p<0.001) (Table c). Those with higher SARS-CoV-2 antibody titers at three months showed a more marked decrease from three to six months than others. Figs. 2 and 3 show the change in SARS-CoV-2 antibody titers from 3 to 6 months by SARS-CoV-2 antibody titers at 3 months groups using tertiles in all subjects and age groups (20-29, 30-39, 40-49, and ≥50 years old).
Figure 2.
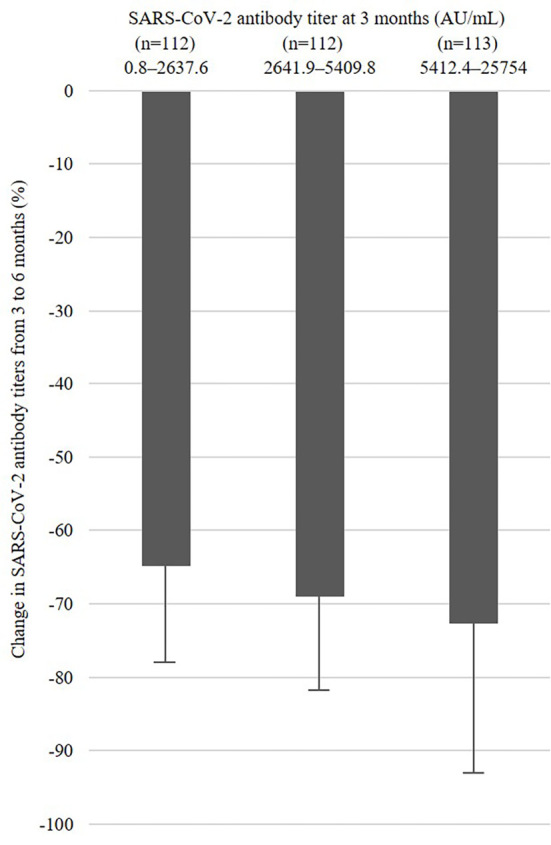
Change in SARS-CoV-2 antibody titers from three to six months by SARS-CoV-2 antibody titers at three months using tertiles.
Figure 3.
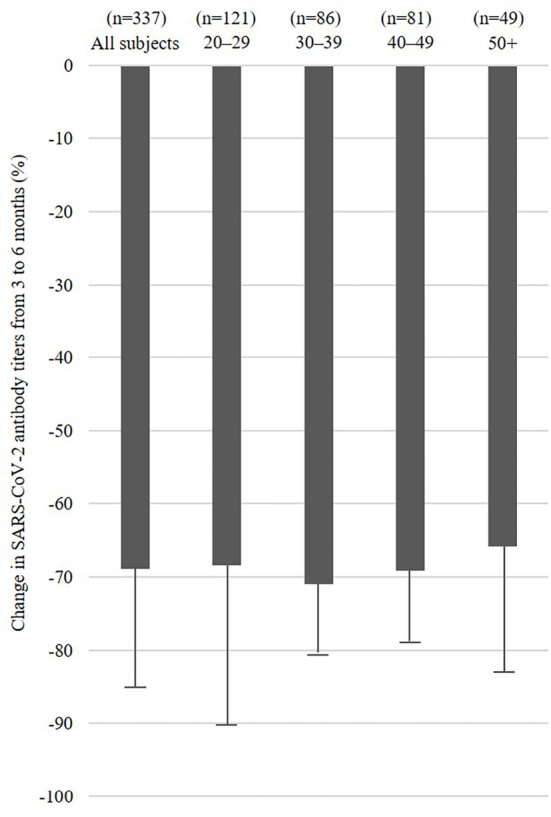
Change in SARS-CoV-2 antibody titers from three to six months in all subjects and age groups (20-29, 30-39, 40-49, and ≥50 years old).
Discussion
This study is a report of the changes in SARS-CoV-2 antibody titers from three to six months after administration of the BNT162b2 vaccine in Japan. This study revealed that the SARS-CoV-2 antibody titers at three months decreased with age. In previous studies, the SARS-CoV-2 antibody titers at seven days after the administration of BNT162b2 vaccine were associated with age and sex (8). SARS-CoV-2 antibody titers within two to five weeks after the BNT162b2 vaccine administration were associated with age, immunosuppressive medication, COVID-19 infection history, sex, duration between doses, glucocorticoids, medication for allergy, and alcohol habit (9). SARS-CoV-2 antibody titers at three months after the BNT162b2 vaccine were associated with age and smoking (10). Age is thus a common factor associated with SARS-CoV-2 antibody titer within three months.
In a previous study, low neutralizing antibody titers were associated with men, older individuals, and receiving immunosuppressive medication for over six months (11). In the present study, SARS-CoV-2 antibody titers showed a rapid decline from three to six months after receiving the COVID-19 vaccine. The SARS-CoV-2 antibody titer at three months was associated with the SARS-CoV-2 antibody titers at six months as well as the change in SARS-CoV-2 antibody titers from three to six months. The SARS-CoV-2 antibody titers at six months in older people tended to be low, although age was not a significant factor at six months, as shown in Fig. 1. Furthermore, subjects with higher values of SARS-CoV-2 antibody titers at three months were more likely to have decreased values at six months, as shown in the Fig. 3. We believe that even subjects with higher values of SARS-CoV-2 antibody titers at three months need to be careful reductions in their titers in the future.
Several limitations associated with the present study warrant mention. First, this study did not record SARS-CoV-2 antibody titers immediately after the BNT162b2 vaccine. In previous studies, the SARS-CoV-2 antibody titers at 1-1.5 months gradually decreased over three months after the BNT162b2 vaccine administration (12,13). However, we still believe that our results are useful, as we measured the six-month data after the BNT162b2 vaccine for future studies. Second, this study did not evaluate the relationship between the SARS-CoV-2 antibody titer and prevention of COVID-19 infection. The change in SARS-CoV-2 antibody titers and neutralizing antibodies at 6 months was -86.8% and -86.4%, respectively (14). We believe that the decrease in SARS-CoV-2 antibody titers led to the decreased neutralizing antibody titers. Finally, the sample size was small, and the study period was short. Therefore, further studies should be performed to evaluate the relationship between the SARS-CoV-2 antibody titer and prevention of COVID-19 infection with larger samples sizes and a longer study period.
In conclusion, this study demonstrates that SARS-CoV-2 antibody titers at three months decreased with age and were associated with antibody titers at six months and the change in titer from three to six months. Increasing age has been shown to be associated with mortality due to and recurrence of COVID-19 infection (15,16). Based on our results and these reports, older people in particular need to be aware of declining SARS-CoV-2 antibody titers at six months after the BNT162b2 vaccine. The results of this study may provide insight into COVID-19 vaccine booster strategies.
This study was approved by the independent ethics committee of Kamagaya General Hospital (approval number: TGE01730-064) and was undertaken following the principles of the Declaration of Helsinki.
Informed consent was obtained from the patients after explaining the study protocol.
Author's disclosure of potential Conflicts of Interest (COI).
Takeshi Mochizuki: Honoraria, Astellas, Bristol-Myers, Chugai, Daiichi Sankyo, Eli Lilly, Janssen, Mochida and UCB. Koichiro Yano: Honoraria, AbbVie, Astellas, Ayumi, Bristol-Meyers, Eisai, Hisamitsu, Mochida and Takeda. Katsunori Ikari: Honoraria, AbbVie, Asahi Kasei, Astellas, Bristol-Myers, Chugai, Eisai, Eli Lilly, Janssen, Takeda, Tanabe-Mitsubishi, Pfizer and UCB.
References
- 1. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med 383: 2603-2615, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hacisuleyman E, Hale C, Saito Y, et al. Vaccine breakthrough infections with SARS-CoV-2 variants. N Engl J Med 384: 2212-2218, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bergwerk M, Gonen T, Lustig Y, et al. COVID-19 Breakthrough infections in vaccinated health care workers. N Engl J Med 385: 1474-1484, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kustin T, Harel N, Finkel U, et al. Evidence for increased breakthrough rates of SARS-CoV-2 variants of concern in BNT162b2-mRNA-vaccinated individuals. Nat Med 27: 1379-1384, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Thomas SJ, Moreira ED Jr, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine through 6 months. N Engl J Med 385: 1761-1733, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tartof SY, Slezak JM, Fischer H, et al. Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: a retrospective cohort study. Lancet 398: 1407-1416, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lisboa Bastos M, Tavaziva G, Abidi SK, et al. Diagnostic accuracy of serological tests for COVID-19: systematic review and meta-analysis. BMJ 370: m2516, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pellini R, Venuti A, Pimpinelli F, et al. Initial observations on age, gender, BMI and hypertension in antibody responses to SARS-CoV-2 BNT162b2 vaccine. EClinicalMedicine 36: 100928, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kageyama T, Ikeda K, Tanaka S, et al. Antibody responses to BNT162b2 mRNA COVID-19 vaccine and their predictors among healthcare workers in a tertiary referral hospital in Japan. Clin Microbiol Infect 27: 186.e1-186.e5, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nomura Y, Sawahata M, Nakamura Y, et al. Age and smoking predict antibody titres at 3 months after the second dose of the BNT162b2 COVID-19 vaccine. Vaccines (Basel) 9: 1042, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Levin EG, Lustig Y, Cohen C, et al. Waning immune humoral response to BNT162b2 COVID-19 vaccine over 6 months. N Engl J Med 385: e84, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Erice A, Varillas-Delgado D, Caballero C. Decline of antibody titres 3 months after two doses of BNT162b2 in non-immunocompromised adults. Clin Microbiol Infect 28: 139.e1-139.e4, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Favresse J, Bayart JL, Mullier F, et al. Antibody titres decline 3-month post-vaccination with BNT162b2. Emerg Microbes Infect 10: 1495-1498, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Padoan A, Cosma C, Bonfante F, et al. Neutralizing antibody titers six months after Comirnaty vaccination: kinetics and comparison with SARS-CoV-2 immunoassays. Clin Chem Lab Med 60: 456-463, 2022. [DOI] [PubMed] [Google Scholar]
- 15. Gómez-Belda AB, Fernández-Garcés M, Mateo-Sanchis E, et al. COVID-19 in older adults: what are the differences with younger patients? Geriatr Gerontol Int 21: 60-65, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ramos-Martínez A, Parra-Ramírez LM, Morrás I, et al. Frequency, risk factors, and outcomes of hospital readmissions of COVID-19 patients. Sci Rep 11: 13733, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]


