Abstract
Lenvatinib is a multi-targeted tyrosine kinase inhibitor available for the treatment of unresectable hepatocellular carcinoma (HCC). We herein report an 84-year-old-man with interstitial pneumonia caused by lenvatinib. Four months after the start of lenvatinib administration for HCC, chest computed tomography revealed bilateral ground-glass opacity. However, he continued to take lenvatinib for four more months until he complained of dyspnea on exertion. This is a case of lenvatinib-induced interstitial pneumonia that progressed relatively slowly with a long asymptomatic period despite the appearance of pneumonia on image findings.
Keywords: lenvatinib, interstitial pneumonia, hepatocellular carcinoma
Introduction
Lenvatinib is a multi-targeted tyrosine kinase inhibitor that blocks tumor cell proliferation and angiogenesis mainly by inhibiting vascular endothelial growth factor receptor (VEGFR) 1, 2, and 3; fibroblast growth factor receptor (FGFR) 1, 2, 3, and 4; platelet-derived growth factor receptor (PDGFR) α; RET; and KIT (1,2). In Japan, lenvatinib was approved as a treatment for unresectable thyroid cancer (3,4) and unresectable hepatocellular carcinoma (HCC) (5). Only a few cases among 1,343 patients included in clinical trials presented with lenvatinib-induced interstitial pneumonia (3-5). However, a considerable number of cases of interstitial pneumonia were reported in a post-marketing survey of lenvatinib (6) (Table 1).
Table 1.
Summary of Post-marketing Survey which was Conducted to Hepatocellular Carcinoma (HCC) or Thyroid Cancer (TC) Patients Administered Lenvatinib Cited from Guide to Proper Use of Lenvatinib.
| Age | Sex | Primary disease |
Time from starting lenvatinib to the onset of drug-induced interstitial pneumonia (day) |
Outcome | Time from onset to outcome (day) |
|---|---|---|---|---|---|
| 70s | M | HCC | 28 | death | 3 |
| 70s | M | HCC | 43 | death | 16 |
| 70s | M | HCC | 46 | death | 27 |
| 50s | M | HCC | 9 | death | 3 |
| 70s | M | TC | 16 | death | 3 |
| 60s | M | HCC | 40 | recovered | 6 |
| 70s | F | HCC | 6 | recovered | unknown |
| 70s | M | HCC | 87 | recovered | 2 |
| 80s | M | HCC | 109 | recovered | 17 |
| 70s | M | HCC | 63 | recovered | 103 |
| 70s | F | HCC | 125 | recovered | 11 |
| 60s | M | TC | 51 | recovered | 9 |
| 60s | M | TC | 30 | recovered | 167 |
The characterization of lenvatinib-related interstitial pneumonia is poorly understood at present, and there are only two case reports of lenvatinib-induced interstitial pneumonia (7,8). In these previous reports, lenvatinib administration was immediately discontinued after the appearance of interstitial pneumonia.
We herein report a relatively slowly progressive case of lenvatinib-induced interstitial pneumonia. The patient continued to take lenvatinib for a long time due to the absence of suitable alternative medications.
Case Report
An 84-year-old-man was a former smoker of 1 pack of cigarette per day for 24 years and had a history of hypertension and chronic kidney disease. He was referred to the Department of Gastroenterology in our hospital for the examination of a tumor lesion in the right lobe of the liver and was diagnosed with HCC. Drug-eluting bead transcatheter arterial chemoembolization or transcatheter arterial chemoembolization with cisplatin was performed for HCC a total of six times over the next seven months.
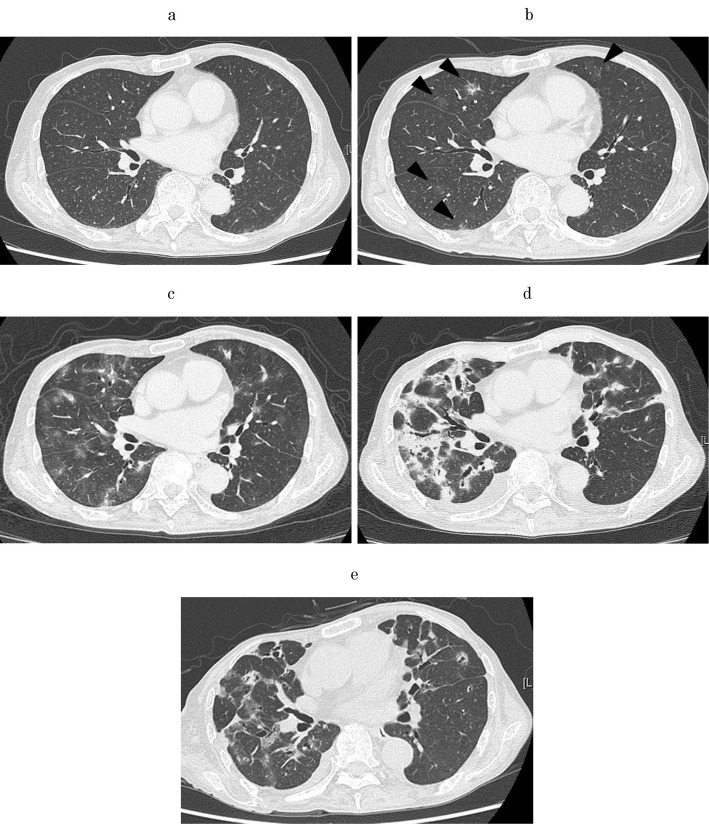
After first-line therapy failed, the treatment for HCC was changed to sorafenib and then regorafenib. Eight months after starting regorafenib, high-resolution computed tomography (HRCT) revealed enlargement of the HCC with worsening of the hepatic function. At this time, HRCT showed no abnormality (Fig. 1a). The chemotherapy was changed to 80 mg/day of lenvatinib. Three months later, the dosage of lenvatinib was decreased to 40 mg/day due to palmar-plantar erythrodysaesthesia. Four months after starting lenvatinib, HRCT showed the appearance of focal ground-glass opacity (GGO) in both lung fields (Fig. 1b). Although the patient had no respiratory symptom, two months later, the spread of GGO on HRCT was observed, with accompanying findings of infiltration shadows. At this point, lenvatinib-induced interstitial pneumonia was assumed, and the pneumonia was considered to be Grade 1 pneumonitis according to the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) v5.0.
Figure 1.
(a) High-resolution chest tomography (HRCT) image at the start of lenvatinib. There was no shadow suggestive of pneumonia in the lung field. (b) HRCT image at four months after starting lenvatinib. Ground-glass opacity (GGO) appeared in both lung fields (arrowheads). (c) HRCT image at six months after starting lenvatinib. The spread of GGO was observed, with the accompaniment of some infiltration shadows. (d) HRCT image at eight months after starting lenvatinib. Patchy infiltration shadow appeared in both lung fields. (e) HRCT image at three months after starting corticosteroid therapy. The infiltration shadow had decreased.
Because there were no other treatments approved for HCC that had never been tried to him, lenvatinib was continued with careful observation under his consent. Two months later, the spread of GGO was observed on HRCT, along with infiltration shadows (Fig. 1c). Nevertheless, because he still had no respiratory symptoms and strongly requested to continue lenvatinib, the administration of lenvatinib was continued under very careful observation. Two months later, he complained of exertional dyspnea. The HRCT findings now showed distinct patchy infiltration shadows in both lung fields (Fig. 1d). He was referred to our department after lenvatinib discontinuation and was admitted.
On admission, he complained dyspnea on exertion, with a Modified British Medical Research Council (mMRC) grade of 2. His initial vital signs were as follows: blood pressure, 103/63 mmHg; body temperature, 36.0 °C; percutaneous oxygen saturation (SpO2), 96%. Resting-state partial pressure of arterial oxygen (PaO2) while breathing room air was 83.1 mmHg. Laboratory test showed: Krebs von den Lungen-6 (KL-6), 1,906 U/mL (normal value: <500 U/mL); surfactant protein D (SP-D). 230 ng/mL (normal value: <100 ng/mL); C-reactive protein (CRP), 1.74 mg/dL (Table 2). No elevated levels of autoantibodies were observed (Table 2). He did not meet the criteria for the diagnosis of any connective tissue diseases. Cytomegalovirus antigens were negative (Table 2). Lung sounds were normal on auscultation. Other physical examination findings were also normal.
Table 2.
The Serum Test Results at the Time of Admission to Our Department.
| WBC | 6,500 | /µL | Na | 140 | mEq/dL |
| Neut | 71.6 | % | K | 4.8 | mEq/dL |
| Eo | 3 | % | TP | 5.5 | g/dL |
| Baso | 0.3 | % | Alb | 2.5 | g/dL |
| Mono | 9.8 | % | CRP | 5.33 | mg/dL |
| Lymp | 12.4 | % | βD-Glucan | <6.0 | pg/mL |
| RBC | 3.27×104 | /µL | KL-6 | 1,906 | U/mL |
| Hb | 9.5 | g/dL | SP-D | 230 | ng/mL |
| Plt | 25.9×104 | /µL | RF | <10 | IU/mL |
| AST | 46 | U/L | ANA | <40 | times |
| ALT | 18 | U/L | anti-SS-A antibody | (-) | |
| ALP | 659 | U/L | PR3-ANCA | <1.0 | IU/mL |
| LDH | 189 | U/L | MPO-ANCA | <1.0 | IU/mL |
| γ-GTP | 104 | U/L | CMV anitigen* | 0 | cells/2 slides |
| BUN | 22 | mg/dL | T-SPOT | (-) | |
| Cre | 1.29 | mg/dL |
WBC: white blood cell, Neut: neutrophil, Eo: eosinophil, Baso: basophil, Mono: monocyte, Lymp: lymphocyte, RBC: red blood cell, Hb: hemoglobin, Plt: platelet count, AST: aspartate aminotransferase, ALT: alanine aminotransferase, ALP: alkaline phosphatase, LDH: lactate dehydrogenase, γ-GTP: γ-glutamyl transpeptidase, BUN: blood urea nitrogen, Cre: creatinine, TP: total protein, Alb: albumin, CRP: C-reactive protein, βD-Glucan: beta-d-glucan , KL-6: krebs von den lugen-6, SP-D: surfactant protein D, RF: rheumatoid factor, ANA: antinuclear antibody, anti-SS-A antibody: anti-Sjögren’s-syndrome-related antigen A autoantibodies, PR3-ANCA: proteinase3 antineutrophil cytoplasmic antibody, MPO-ANCA: myeroperoxidase antineutrophil cytoplasmic antibody, CMV antigen: cytomegalovirus antigen, T-SPOT: T-SPOT®-TB
* cytomegalovirus, viral antigen (pp65. C10, C11)
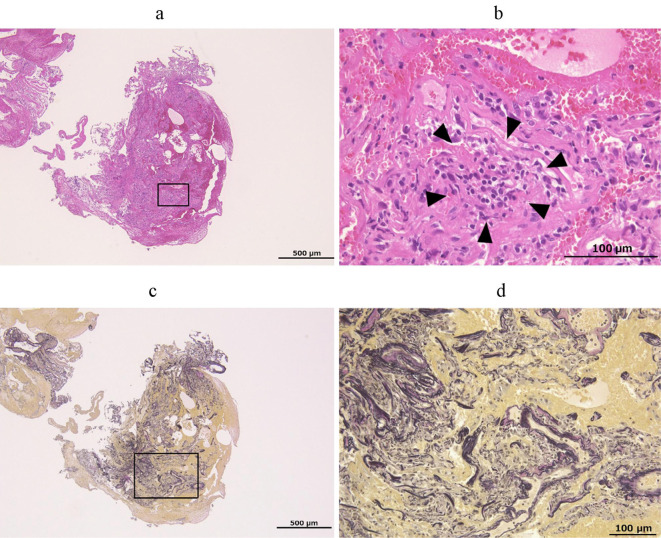
Bronchoalveolar lavage (BAL) of the left middle lobe showed a mild increase in the lymphocyte rate (lymphocyte: 20.8%) (Table 3). A transbronchial lung biopsy (TBLB) of the left upper lobe with Hematoxylin and Eosin (H & E) staining showed infiltration of lymphocytes into the alveolar interstitium but no thickening of the alveolar wall or Masson's body (Fig. 2a, b). Fibrosis was not evident on Elastica van Gieson (EVG) staining (Fig. 2c, d). DLST was positive (599 cpm, stimulation index 2.5; normal range: lower than 1.8), suggesting that the present case had an allergic reaction to lenvatinib. Given these results, a diagnosis of drug-induced interstitial pneumonia caused by lenvatinib was made.
Table 3.
The Results Of Bronchoalveolar Lavage Fluid (balf).
| Total cell counts | 22.5×104 | /mL |
| cell fractionation | ||
| alveolar macrophages | 74.3 | % |
| lymphocytes | 20.8 | % |
| neutrophils | 4.1 | % |
| eosinophils | 0.8 | % |
| basophils | 0 | % |
| CD4/8 ratio* | 0.59 | |
| Cytodiagnosis | Class II | |
| BALF cultures | negative |
*CD4+/CD8+T-lymphocyte ratio
Figure 2.
Histological findings of the transbronchial lung biopsy specimen. A histological examination was performed with Hematoxylin and Eosin staining (a, b) and the Elastica van Gieson staining (c, d). The square area of the low-magnification photomicrograph (a, c) is shown as a high-magnification photomicrograph (b, d) with each stain. The arrowheads indicate the infiltration of lymphocyte into the alveolar interstitium. A scale bar is shown in each figure.
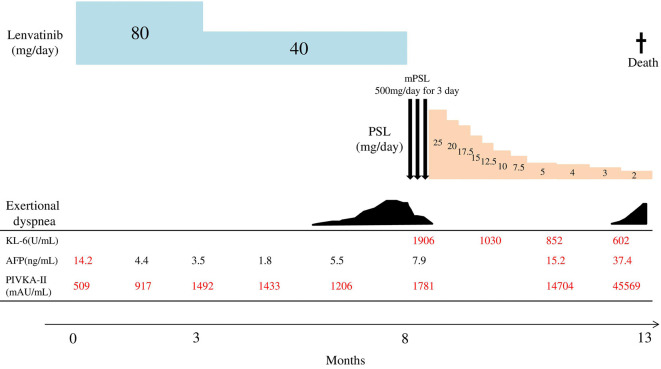
Following intravenous pulse steroid therapy with 500 mg of methylprednisolone (mPSL) for 3 days, we started the oral administration of 25 mg/day (0.5 mg/kg) of prednisolone (PSL). After starting corticosteroid treatment, his respiratory condition improved with a reduction in the KL-6 value (Fig. 3). Three months after starting corticosteroid therapy, the radiological findings gradually improved (Fig. 1e). However, after he stopped the administration of lenvatinib, his HCC gradually worsened with an increase in the alpha-fetoprotein (AFP) and protein induced by vitamin K absence or antagonist (PIVKAII) values. He ultimately passed away four months after starting corticosteroid therapy due to exacerbation of HCC.
Figure 3.
Clinical course of the patient after starting lenvatinib. AFP: alpha-fetoprotein, KL-6: Krebs von den Lugen-6, mPSL: methylprednisolone, PIVKA-II: protein induced by vitamin K absence or antagonist II, PSL: prednisolone
Discussion
The mechanism underlying lenvatinib-induced interstitial pneumonia has not been clarified yet. In general, direct toxicity of the causative drug can be responsible for drug-induced pneumonia (9). Some previous reports have suggested that the induction of alveolar epithelial cell apoptosis by VEGF receptor inhibition might be involved in the development of interstitial pneumonia (10-12). Similarly, one previous case report of lenvatinib-induced interstitial pneumonia stated that it was induced by alveolar epithelial cell apoptosis following VEGF receptor inhibition (8). However, drug-induced pneumonia is often caused by immune-mediated reaction (9). The DLST of lenvatinib was positive in both the present case and a previous case (Table 4), suggesting that an allergic reaction to lenvatinib may also cause drug-induced interstitial pneumonia.
Table 4.
Summary of the Patient with the Three Lenvatinib-induced Interstitial Pneumonia Reported in the Literature.
| Case of Study [referene] |
Age | Sex | Cancer type | PS | Smoking history (pack year) | Complication of interstitial pneumonia before the start of lenvatinib | Time from first administration to the onset of drug-induced interstitial pneumonitis (month) | Pattern of HRCT | KL-6 (U/mL) |
Lymphocyte ration of BAL(%) | TBLB | DLST | treatment | outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case Rep Oncol 11:75-80, 2018 [7]. |
67 | M | Cncer of unknown primary (Squamous cell carcinoma) | 0 | 15 | no complication | 1 | bilateral GGO | 582 | 61.10% | no data | positive | discontinuation of lenvatinib | recoverd |
| Clin J Gastroenterol 12: 355-360, 2019 [8]. |
59 | M | HCC | 0 | 45 | complication of mild interstitial pneumonitis induced by regorafenib | 1 | bilateral GGO | 1283 | no data | no data | negative | discontinuation of lenvatinib steroid therapy | recoverd |
| present case | 84 | M | HCC | 1 | 24 | no complication | 4 | bilateral GGO ⇒bilateral infiltration shadow |
1906 | 20.80% | infiltration of lymphocyte into alveolar interstitium | positive | discontinuation of lenvatinib steroid therapy | recoverd |
BAL: bronchoalveolar lavage, DLST: drug induced lymphocyte stimulation test, GGO: ground-glass opacity, HCC: hepatic cell carcinoma, HRCT: high-resolution chest tomography, KL-6: Krebs von den Lugen-6, PS: performance status, TBLB: transbronchial lung biopsy
The typical imaging findings of lenvatinib-induced interstitial pneumonia are unclear. In two previous reports, HRCT showed GGO (Table 2) (7,8). In the present case as well, the shadow of GGO was initially the main component. However, the HRCT findings changed to distinctive patchy alveolar opacities resembling a fibrosing organizing pneumonia pattern when the lenvatinib-induced interstitial pneumonia worsened. After the corticosteroid therapy, abnormal interstitial thickening remained despite the bilateral alveolar opacities gradually disappearing, suggesting that progressive fibrosis might have occurred in the lungs (Fig. 1e). Although the fibrosis was not evident on EVG staining, this might have been due to the small size of specimen collected by the TBLB. Therefore, if lenvatinib had been discontinued earlier, the abnormal shadow might have disappeared without leaving scars in the lung.
However, in the present case, lenvatinib was continued when drug-induced pneumonitis was suspected six months after the initiation of therapy. Consequently, the progression-free survival (PFS) of lenvatinib for HCC was about 8 months, and the overall survival (OS) for HCC was about 12 months. These data are compatible with the results in a phase 3 study of lenvatinib for unresectable HCC, showing that the PFS and OS for first-line chemotherapy were 7.4 and 12.5 months, respectively (5). However, in the guide to the proper use of lenvatinib, drug discontinuation and a close examination, including laboratory and imaging tests, are recommended when drug-induced pneumonia is suspected (6). Therefore, in the present case, the discontinuation of lenvatinib at least six months after starting its therapy should have been selected.
In 9 out of 13 cases reported in the post-marketing survey, lenvatinib-induced interstitial pneumonia was observed within 2 months after starting the administration of lenvatinib (Table 1). Among these nine acute or subacute progressive cases, five died within one month after the onset of pneumonia. In contrast, the other four cases that took more than two months to develop pneumonia eventually recovered (Table 1). In the present case, fortunately, the lenvatinib-induced interstitial pneumonia in our patient recovered with corticosteroid treatment. This good responsiveness to treatment may be related to the slow progression of pneumonia in the present case.
In summary, we described a case of slowly progressive interstitial pneumonia induced by lenvatinib. The Japanese Respiratory Socicty (JRS) guidelines for the management of drug-induced lung disease recommends the suspected drug be discontinued soon after the pneumonia is determined to have been drug-induced, as a principle (13). However, according to the characteristics of interstitial pneumonia, several drugs, such as sirolimus, everolimus, pembrolizumab, atezolizumab, and durvalumab, can be continued when the grade of drug-induced pneumonia is Grade 1 (asymptomatic) (14-18). In the case of lenvatinib, although the discontinuation is generally recommended when drug-induced interstitial pneumonia is suspected, further information on more cases would enable the development of a precise management strategy depending on each case.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Matsui J, Yamamoto Y, Funahashi Y, et al. E7080, a novel inhibitor that targets multiple kinases, has potent antitumor activities against stem cell factor producing human small cell lung cancer H146, based on angiogenesis inhibition. Int J Cancer 122: 664-671, 2008. [DOI] [PubMed] [Google Scholar]
- 2. Matsui J, Funahashi Y, Uenaka T, et al. Multikinase inhibitor E7080 suppresses lymph node and lung metastases of human mammary breast tumor MDA-MB-231 via inhibition of vascular endothelial growth factor-receptor (VEGF-R) 2 and VEGF-R3 kinase. Clin Cancer Res 14: 5459-5465, 2008. [DOI] [PubMed] [Google Scholar]
- 3. Schlumberger M, Tahara M, Wirth LJ, et al. Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N Engl J Med 372: 621-630, 2015. [DOI] [PubMed] [Google Scholar]
- 4. Kiyota N, Schlumberger M, Muro K, et al. Subgroup analysis of Japanese patients in a phase 3 study of lenvatinib in radioiodine-refractory differentiated thyroid cancer. Cancer Sci 106: 1714-1721, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomized phase 3 non-inferiority trial. Lancet 391: 1163-1173, 2018. [DOI] [PubMed] [Google Scholar]
- 6. Eisai. Guide to proper use of lenvatinib for HCC [Internet]. 2021 [cited 2021 Feb 1]. Available from: https://www.info.pmda.go.jp/go/rmp/material/4f065c69-5cb2-4ee4-af58-d58940575d29 (in Japanese)
- 7. Kimura-Tsuchiya R, Sasaki E, Nakamura I, et al. A case of squamous cell carcinoma of unknown primary that responded to the multi-tyrosine kinase inhibitor lenvatinib. Case Rep Oncol 11: 75-80, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kotani K, Enomoto M, Okada M, et al. Interstitial pneumonia suspected during regorafenib administration and exacerbated by subsequent therapy with lenvatinib for unresectable hepatocellular carcinoma. Clin J Gastroenterol 12: 355-360, 2019. [DOI] [PubMed] [Google Scholar]
- 9. Matuo O. Drug-induced interstitial lung disease: mechanisms and best diagnostic approaches. Respir Res 13: 39, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Myung HJ, Jeong SH, Kim JW, et al. Sorafenib-induced interstitial pneumonitis in a patient with hepatocellular carcinoma: a case report. Gut Liver 4: 543-546, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yamaguchi T, Seki T, Miyasaka C, et al. Interstitial pneumonia induced by sorafenib in a patient with hepatocellular carcinoma: an autopsy case report. Oncol Lett 9: 1633-1636, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Takeda H, Nishikawa H, Iguchi E, et al. Sorafenib-induced acute interstitial pneumonia in patients with advanced hepatocellular carcinoma: report of three cases. Clin J Gastroenterol 5: 407-412, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Committee for the JRS Guidelines for the Management of Drug-induced Lung Disease. The JRS guidelines for the management of drug-induced lung disease (the second edition). Jpn Respir Soc, Tokyo, 2018(in Japanese). [Google Scholar]
- 14. Nobelpharma. Guide to proper use of sirolimus for lymphangioleiomyomatosis [Internet]. 2020 [cited 2021 Feb 1]. Available from: https://nobelpark.jp/product/pdf/rapalimus_gm.pdf (in Japanese).
- 15. Novartis Pharma. Guide to proper use of everolimus [Internet]. 2010. [cited 2021 Feb 1]. Available from: https://www.jrs.or.jp/uploads/uploads/files/photos/615.pdf (in Japanese)
- 16. MSD. Guide to proper use of pembrolizumab [Internet]. 2021. [cited 2021 Feb 1]. Available from: https://www.msdconnect.jp/static/mcijapan/images/properuse_guide_keytruda.pdf (in Japanese)
- 17. Chugai Pharmaceutical. Guide to proper use of Atezolizumab [Internet]. 2020. [cited 2021 Feb 1]. Available from: https://chugai-pharm.jp/content/dam/chugai/product/tec/div/guide-lg/doc/tec_guide_lg.pdf (in Japanese)
- 18. AstraZeneca. Guide to proper use of durvalumab for non-small cell lung cancer or extensive small cell lung cancer [Internet]. 2020. [cited 2021 Feb 1]. Available from: https://med2.astrazeneca.co.jp/safety/download/IMF03.pdf (in Japanese)