Abstract
We describe a type III restriction and modification (R/M) system, LlaFI, in Lactococcus lactis. LlaFI is encoded by a 12-kb native plasmid, pND801, harbored in L. lactis LL42-1. Sequencing revealed two adjacent open reading frames (ORFs). One ORF encodes a 680-amino-acid polypeptide, and this ORF is followed by a second ORF which encodes an 873-amino-acid polypeptide. The two ORFs appear to be organized in an operon. A homology search revealed that the two ORFs exhibited significant similarity to type III restriction (Res) and modification (Mod) subunits. The complete amino acid sequence of the Mod subunit of LlaFI was aligned with the amino acid sequences of four previously described type III methyltransferases. Both the N-terminal regions and the C-terminal regions of the Mod proteins are conserved, while the central regions are more variable. An S-adenosyl methionine (Ado-Met) binding motif (present in all adenine methyltransferases) was found in the N-terminal region of the Mod protein. The seven conserved helicase motifs found in the previously described type III R/M systems were found at the same relative positions in the LlaFI Res sequence. LlaFI has cofactor requirements for activity that are characteristic of the previously described type III enzymes. ATP and Mg2+ are required for endonucleolytic activity; however, the activity is not strictly dependent on the presence of Ado-Met but is stimulated by it. To our knowledge, this is the first type III R/M system that has been characterized not just in lactic acid bacteria but also in gram-positive bacteria.
The susceptibility of Lactococcus lactis starter cultures to bacteriophage attack is one of the most enduring problems associated with industrial exploitation of such cultures. The most effective approach for combating bacteriophage infection has been based on a combination of a well-controlled fermentation process and the development of starter strains which are highly resistant to bacteriophage attack (8). A great deal of research on lactococci has been focused on identification and characterization of mechanisms that mediate bacteriophage resistance. Detailed characterization should ultimately allow rational construction of strains that exhibit high levels of bacteriophage resistance (20). Four host-directed bacteriophage resistance mechanisms in lactococci have been described. These mechanisms include adsorption inhibition, prevention of phage DNA penetration, host-controlled restriction and modification (R/M), and abortive infection (20).
R/M is the most common phage resistance mechanism found in bacteria. The infection cycle is halted at an early stage with no affect on the viability of the cells. The role of restriction is to cleave any invading DNA which has not been modified at a specific nucleotide sequence by the host methylation system. It is thought that the restriction enzymes in R/M systems confer phage resistance to the producing strains. Cloning of R/M systems into nonprotected strains should permit the construction of dairy starter cultures that exhibit improved phage resistance (31).
The following three distinct types of R/M systems are recognized based on their subunit compositions, cofactor requirements, and modes of DNA cleavage: types I, II, and III (4). Type III is the smallest class of restriction systems and contains only the following four well-studied members: EcoP1 from prophage P1 (18) and EcoP15 from the prophage P1-related plasmid p15B in Escherichia coli (1); HinfIII from Haemophilus influenzae (19); and StyLTI from Salmonella typhimurium (3). Type III R/M systems require at least two functional genes, res and mod. Mod is responsible for binding the DNA recognition sequence and also methylates DNA regardless of the presence of Res; Res is required for restriction and is not functional without Mod (4).
In a previous study (39) plasmid pND801 was isolated from L. lactis subsp. lactis LL42-1, and it was found that pND801 encoded an R/M system. The presence of pND801 in L. lactis reduced the efficiency of plating (EOP) of isometric phage φ712 to 10−6. In this paper, we describe molecular cloning and characterization of the R/M system encoded by pND801 and show that it is a type III R/M system.
MATERIALS AND METHODS
Bacterial strains, plasmids, and culture conditions.
The strains and plasmids used in this study are listed in Table 1. E. coli cultures were incubated at 37°C in Luria-Bertani medium (35) or in M9 minimal medium (35) supplemented with glucose (0.2%, wt/vol). L. lactis strains were incubated at 30°C in M17 medium (41) supplemented with 0.5% (wt/vol) glucose. When appropriate, the following antibiotics were added: for E. coli, 50 μg of chloramphenicol per ml, 15 μg of tetracycline per ml, and 20 μg of kanamycin per ml; and for L. lactis, 5 μg of erythromycin per ml.
TABLE 1.
Bacterial strains, plasmids, and bacteriophages
| Bacterial strain, plasmid, or phage | Relevant characteristicsa | Source or reference |
|---|---|---|
| Bacterial strains | ||
| L. lactis subsp. lactis | ||
| LL42-1 | Wild type harboring seven plasmids | Gist-Brocades Australia |
| MG1363 | Lac− Prt−, plasmid-free derivative of L. lactis 712 | 14 |
| LM0230 | Lac− Prt−, plasmid-free derivative of L. lactis C2 | 12 |
| E. coli HB101 | Pro− Leu− Thi− RecA−, plasmid-free | 6 |
| Plasmids | ||
| pSA3 | 10.2 kb; hybrid vector encoding Cmr Tcr (E. coli) and Emr (L. lactis) | 9 |
| pND801 | 12 kb; encoding R/M obtained from LL42-1 via coelectroporation | 39 |
| pND805 | 22.2 kb; R/M, pND801 cloned into the pSA3 EcoRI site | This study |
| Bacteriophage φ712 | Small isometric-headed phage propagated on LM0230 and MG1363 | 14 |
Lac−, non-lactose fermenting; Prt−, non-proteinase producing; Emr, erythromycin resistance; Cmr, chloramphenicol resistance; Tcr, tetracycline resistance; Pro−, proline requiring; Leu−, leucine requiring; Thi−, thiamine requiring; RecA−, deficient in recombination protein.
Transposon Tn5 mutagenesis.
A transposon Tn5 mutagenesis analysis of cloned DNA segments was carried out by using the methods of de Bruijn and Lupski (11). Phage λ 476 (λb221 rex::Tn5 cI857 Oam29 Pam80), which was obtained from T. R. Klaenhammer, was used to infect E. coli HB101 containing pND805 (Tcr Cmr R+/M+) at a multiplicity of infection of 10. Transformants were selected on Luria-Bertani medium plates containing tetracycline and kanamycin.
DNA and molecular cloning techniques.
Lactococcal plasmid DNA was isolated by the method of Anderson and McKay (2). Plasmids were isolated from E. coli as described by Birnboim and Doly (5). Plasmid DNA was purified by cesium chloride-ethidium bromide density gradient centrifugation (35) and was desalted by dialysis in 1× TE buffer (10 mM Tris, 1 mM EDTA). Restriction digestion and molecular cloning were performed as described by Sambrook et al. (35). Restriction endonucleases and T4 DNA ligase were purchased from Boehringer, Mannheim, Germany, and were used as recommended by the manufacturer. DNA fragments were recovered from agarose gels with a QIAEX II gel extraction kit (QIAGEN, GmbH, Hilden, Germany). L. lactis was transformed by electroporation as described by Powell et al. (30). For E. coli, the CaCl2 transformation method of Dagert and Ehrlich (7) was used without extended preincubation in CaCl2.
Nucleotide sequencing and analysis.
Both DNA strands were sequenced by using a model 377 DNA sequencer (Applied Biosystems, Foster City, Calif.) as recommended by the manufacturer. Sequencing of the phage-resistant determinant was initiated by using two primers designed on the basis of the sequence of plasmid pACYC184 (GenBank accession no. X06403), which is part of pSA3. Based on the sequences obtained, 20-mer oligonucleotide primers were then synthesized and used to walk along the DNA template. The nucleotide sequence was recorded and analyzed by using the AutoAssembler DNA sequence assembly software (Applied Biosystems) and the ANGIS software system operated by the Australian Genomic Information Center, University of Sydney. Amino acid sequences were compared with all of the sequences in the database by using the BLASTP program. A protein analysis was carried out by using the PEPSTATS program. The nucleotide sequence was searched for −10 and −35 sequences by using the Findpatterns program. A phylogenetic tree was drawn by using the program Growtree in Distances.
Isolation and partial purification of enzyme extracts.
The method used to isolate and partially purify enzyme extracts was modified from the method described by Sugisaki and Kanazawa (40). The cells in a 5- to 10-ml overnight (16-h) culture were centrifuged at 8,000 × g for 5 min at room temperature, washed once with 1 ml of extraction buffer (50 mM Tris HCl [pH 7.6] containing 20 mM MgCl2, 0.1 mM EDTA, and 0.01 M β-mercaptoethanol), and pelleted again by centrifugation at 8,000 × g for 5 min at 4°C. The pellet was resuspended in 1 ml of extraction buffer in a microcentrifuge tube. The cells were lysed with 1 g of acid-washed glass beads (diameters, 212 to 300 μm) by intermittent vortexing for 30 s, followed by 30 s on ice to cool the preparation, for 10 min. The microcentrifuge tube was quick-spun to settle the glass beads, and the upper liquid phase was transferred to a fresh tube. Following cell disruption, the cell debris was removed by centrifugation at 16,000 × g for 5 min at 4°C, and the supernatant was transferred to a new microcentrifuge tube. Streptomycin sulfate was then added to a final concentration of 1% (wt/vol), and the tube was placed in an ice bath for 30 min. The precipitated nucleic acids were removed by centrifugation (18,000 × g, 4°C, 5 min), and the clear supernatant was transferred to a new tube; 5 to 10 μl of this preparation was enough to perform a restriction endonuclease assay. The reaction mixtures (20 μl) contained 0.5 μg of λ DNA, 5 μl of cell extract (in extraction buffer containing 20 mM Mg2+), 10 mM ATP with and without 5 μM S-adenosyl methionine (Ado-Met), and reaction buffer B (Boehringer). The ATP and Ado-Met concentrations used were chosen on the basis of similar assays performed by Kauc and Piekarowicz (19). After incubation at 37°C for the times indicated below, the reactions were stopped by heating the mixtures at 65°C for 5 min or by adding gel loading dye, and each mixture was applied to an 0.8% agarose slab gel for electrophoresis.
Bacteriophage assays.
Both phage titers and cross-streaking were used to evaluate the phage resistance of cultures. Cross-streaking was performed as follows. A sterile cotton bud was dampened with a high-titer phage preparation (109 PFU ml−1) and streaked in a straight line on plates containing M17 medium supplemented with 0.5% (wt/vol) glucose and 10 mM CaCl2. A sterile stick was then used to streak bacterial colonies across the phage. When more accurate measurements were needed, the EOP was determined by plaque counting. The number of PFU was determined by standard plaque assays in which we used an overlay culture of L. lactis in M17 medium supplemented with 0.5% glucose and 0.6% agar. The plates were incubated for 24 h at 30°C, and the resulting plaques were counted.
Nucleotide sequence accession number.
The GenBank accession number for the DNA sequence of the LlaFI gene encoding the R/M system from L. lactis LL42-1 is AF054600.
RESULTS
Cloning of pND801 into pSA3.
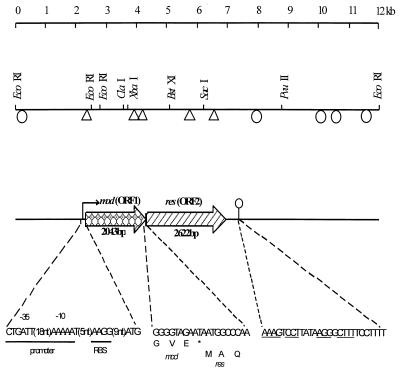
Previous work showed that pND801 conferred phage resistance through an R/M system (39). A physical map of pND801 is shown in Fig. 1. To characterize the genetic determinants of the R/M system, two strategies were used. First, pND801 was digested by EcoRI to generate three fragments. The three fragments were cloned separately into the EcoRI site of pSA3, but none of the fragments expressed phage resistance (Fig. 1). This suggested that the R/M system was inactivated when the DNA was cut with EcoRI. pND801 was then partially digested with EcoRI to linearize the plasmid and ligated into pSA3 digested with the same enzyme. The ligation mixture was transformed directly into L. lactis LM0230, and erythromycin-resistant transformants were screened for phage resistance by cross-streaking against φ712. One of the phage-resistant colonies was purified, and the plasmid harbored by the strain was designated pND805. Restriction analysis of pND805 indicated that the complete pND801 plasmid was inserted into pSA3. Expression of the phage resistance encoded by pND805 in L. lactis was investigated by challenging transformants that harbored pND805 with phage. pND805 and strains harboring the parent plasmid restricted plating of the isometric φ712 to similar extents (EOP, ≈10−6).
FIG. 1.
Genetic organization of the type III R/M system in pND801. The 12-kb plasmid pND801 was cloned into pSA3 to generate pND805, which expressed resistance to φ712. Locations of the Tn5 insertions in pND805 are shown (triangles, Tn5 insertions which inactivated phage resistance; ellipses, Tn5 insertions which did not affect the phage resistance phenotype). The locations of the mod gene and the res gene, the sequences of the putative promoter and the ribosome-binding site (RBS), and the position of the transcriptional terminator (ellipse with vertical line) are shown. The start codon of the res gene overlaps the stop codon of the mod gene.
Localization of the genetic determinants encoding the R/M activity of pND805 by Tn5 mutagenesis.
Plasmid pND805 was introduced into E. coli HB101 and subjected to phage λ 476-mediated Tn5 mutagenesis. Transformants (Tcr Kmr) were harvested, and the plasmid DNA was extracted collectively for electroporation into LM0230. Emr transformants of LM0230 were tested for phage sensitivity by cross-streaking. Of the 64 individual isolates tested, 30 were resistant to φ712 and 34 were sensitive to φ712. Plasmid DNA was then isolated from both phage-resistant and phage-sensitive isolates, transformed back into HB101, reisolated, and analyzed by restriction digestion. The positions of the Tn5 insertions in the cloned fragment of pND805 and the corresponding phage sensitivities of the mutants are shown in Fig. 1. Insertions conferring phage resistance were clustered within a 7-kb region of pND801. We concluded that this region is essential for expression of the R/M phenotype.
Nucleotide sequencing of pND805.
Tn5 mutagenesis indicated that the region encoding phage resistance included the 0.3-kb EcoRI fragment (Fig. 1). This fragment was sequenced first, and based on its sequence more primers were designed and used to sequence the entire DNA region encoding R/M activity. Examination of the sequence revealed the presence of two large open reading frames (ORFs) on the same strand reading in the same direction. The first ORF (ORF1) was 2,043 bp long, began with an ATG start codon, ended at a TAA stop codon, and had the potential to encode a 680-amino-acid protein with a predicted molecular mass of 78,916 Da and an isoelectric point of 4.88. The second ORF (ORF2) began with an ATG codon which overlapped the stop codon of the first ORF by 1 bp (Fig. 1). This ORF was 2,622 bp long and was capable of encoding an 873-amino-acid protein with a predicted molecular mass of 101,630 Da and an isoelectric point of 5.84. The protein encoded by ORF2 contained a higher percentage of basic amino acid residues than the protein encoded by ORF1 contained. Both proteins were hydrophilic, with only a few small hydrophobic regions. No transmembrane regions were found, from which we inferred that the two proteins were located in the cytoplasm.
Examination of the DNA sequence for transcriptional and translational regulatory sequences revealed a putative promoter region upstream of ORF1 (Fig. 1). Nine base pairs upstream from the ATG codon was a sequence (AAGG) that resembled the Shine-Dalgarno sequences that have been reported for L. lactis (16). This putative ribosome binding site had a free energy of binding of −8.4 kcal mol−1 with the 3′ end of the 16S rRNA of L. lactis. The nucleotide sequence was compared with −10 and −35 sequences found in members of the genus Lactococcus (16). Upstream of the putative Shine-Dalgarno sequence were putative −10 and −35 sequences which were similar to consensus E. coli and Bacillus promoters. Three of six nucleotides in the −35 region and four of the six nucleotides in the −10 region were the same as the nucleotides in consensus sequences. Located 306 bp downstream of the stop codon of ORF2 was a region of dyad symmetry that could form a stem-loop structure up to 20 bp long, followed by a run of T residues (TCCTTTT). This region was similar to a rho-independent transcription terminator. Therefore, we assumed that the two ORFs are arranged in an operon.
Comparative analysis of the amino acid sequences.
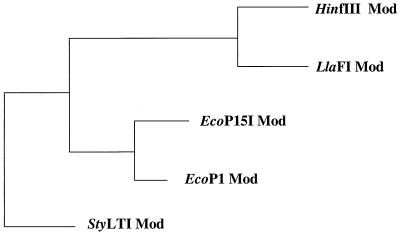
BLASTP analysis of the proteins encoded by ORF1 and ORF2 revealed significant homology to the Mod and Res subunits, respectively, of type III R/M systems encoded by E. coli plasmid p15B (EcoP15), E. coli prophage P1 (EcoP1), H. influenzae Rf (HinfIII), and S. typhimurium (StyLTI). Based on this homology, the R/M system of L. lactis LL42-1 was designated LlaFI in accordance with the nomenclature proposed by Smith and Nathans (38). The complete amino acid sequence of the Mod subunit of LlaFI was aligned with the amino acid sequences of the previously described type III methyltransferases (Fig. 2). Both the N-terminal regions and the C-terminal regions of the five proteins were conserved; certain regions scored as high as 71% (74/104) identity, 90% (94/104) similarity with a high probability (4.2e−140). The central regions were less homologous, which reduced the overall similarity. The overall levels of identity between the LlaFI Mod subunit and the previously described Mod proteins ranged from 25.8 to 38.2%, and the overall levels of similarity ranged from 49.5 to 58.7%. The Asp-Pro-Pro-Tyr motif, a putative Ado-Met-binding motif present in all adenine methyltransferases, was found in the N-terminal conserved region of all five Mod proteins (Fig. 2, box). A phylogenetic tree relating the deduced Mod subunit amino acid sequence to the previously described type III Mod subunit sequences is shown in Fig. 3. LlaFI Mod is most closely related to HinfIII Mod.
FIG. 2.
Multiple alignment of the complete sequences of the Mod proteins of the HinfIII, EcoP15, EcoP1, StyLT, and LlaFI systems. Identity between two or more proteins is indicated by light shading. Darker shading indicates amino acid identity with LlaFI (one-letter code). Asterisks indicate completely conserved amino acids. The numbers on the right indicate the position of the rightmost amino acid of each line. The Ado-Met binding site common to all adenine methyltransferases is enclosed in a box.
FIG. 3.
Evolutionary relationship of the LlaFI Mod subunit sequence to previously described type III Mod subunit sequences: Growtree phylogram of Mod sequences determined by using the distance matrix method and the deduced amino acid sequences. Since the phylogram option was used, the branch lengths reflect the calculated distances.
Using the previously described type III systems, we performed an amino acid homology search by comparing the LlaFI Res protein with the two previously described type III Res proteins (EcoP1 and StyLTI) (SWISS-PROT database). The Res subunit sequences of EcoP15 and HinfIII are not available yet in any of the databases. The levels of similarity between the LlaFI Res protein and the previously described Res proteins were 42 to 45%.
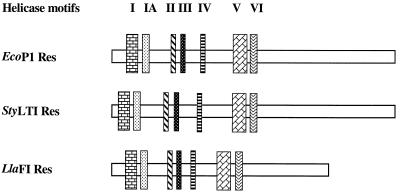
Helicase motifs in LlaFI.
The two previously described type III Res proteins contain seven conserved helicase motifs. These motifs were also identified in the Res protein encoded by LlaFI. The relative location of each motif was determined and compared to the locations of the motifs found in the Res proteins of EcoP1 and StyLTI (Fig. 4). The motifs were searched by performing homology comparisons between the consensus sequences defined by Gorbalenya et al. (15) and the protein encoded by the res gene in LlaFI. Table 2 shows the actual motif sequences found in the Res subunits of LlaFI, EcoP1, and StyLTI together with the consensus sequences. A DEAH motif with invariant D (asparate) and E (glutamate) residues, which is characteristic of the DEAD protein family (34), was found in motif II of all three subunits. Most of the conserved amino acid residues were the same in all three enzymes; in a number of cases amino acids were replaced by related amino acids.
FIG. 4.
Relative positions of the seven helicase motifs in the Res proteins of the EcoP1, StyLTI, and LlaFI systems. The sizes of the genes are indicated by the sizes of the horizontal rectangles, and each vertical rectangle represents a helicase motif. 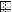 ,
, 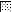 ,
, 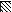 ,
, 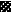 ,
,  ,
,  , and
, and  , motifs I to VI, respectively.
, motifs I to VI, respectively.
TABLE 2.
Seven conserved helicase motifs found in the EcoP1, StyLTI, and LlaFI Res subunits
| R/M subunit | Helicase motifsa
|
||||||
|---|---|---|---|---|---|---|---|
| I | IA | II | III | IV | V | VI | |
| EcoP1 Res | IDVSMETGTGKTYTYTk | NKFIIIVPT1SIKAGT | FIIIDEpH | IIRYGATFS | TLFFIDdIEG | FIFSKWTLREGWDNPNVFQIC | STTSKLQEVGRGLR |
| StyLTI Res | IdVKMETGTGKTYVYTr | FKFVLVVPTpAIKEGA | VVIIDEpH | IIrYgATFS | tLFFIDdIEG | FIFskWTLREGWDnPNVFVIA | SESSKIQEVGRAvR |
| LlaFI Res | LLFNMATGSGKTMVMAS | QNFIFLVnTdAIIKKT | VILGDEAH | LLEFSATIN | TVEsLVkYLK | VIFAvAKLNEGWDVLNLYDIV | ATDSEAQLVGRGAR |
| Consensus | +++ tg GKT + | +++++p r + | ++++DEah | ++++sat | +++f t | ++++t + g + + + ++ | q +GR+ar |
Boldface type indicates amino acids which are either completely conserved or replaced by similar amino acids. Plus signs represent hydrophobic amino acid residues. The consensus residues determined by Gorbalenya et al. (15) are indicated.
Characterization of the restriction activity of LlaFI.
Typically, type III enzymes are ATP and Mg2+ dependent but do not have a stringent requirement for Ado-Met. To investigate the cofactor requirements of LlaFI, partially purified lysates were obtained from 10-ml overnight cultures of L. lactis LM0230 harboring pND805.
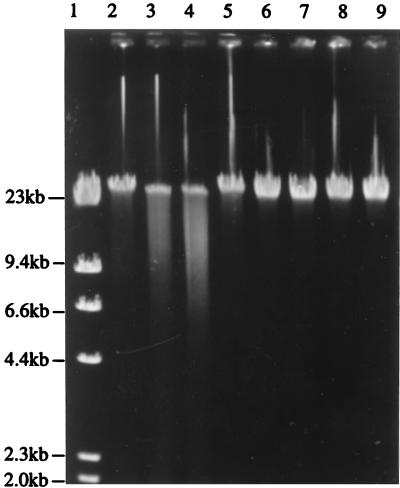
The restriction digests obtained in the presence and absence of cofactors are shown in Fig. 5. The pND805 endonuclease required ATP and Mg2+ but did not require Ado-Met to breakdown DNA, although the reaction was stimulated by the presence of Ado-Met. Cleavage of λ DNA with the endonuclease encoded by pND805 resulted in a smear of fragments. The control reaction confirmed that this cleavage pattern was the result of endonuclease activity alone, which eliminated the possibility that it may have been due to a contaminating nucleases. Also, cleavage and smearing occurred only in the presence of ATP plus Mg2+ and in the presence of ATP plus Mg2+ plus Ado-Met. No effort was made to optimize the ATP, Mg2+, and Ado-Met concentrations in this study. The incomplete digestion observed, which resulted in some DNA remaining almost full length, may be attributed to simultaneous methylase activity (19).
FIG. 5.
Agarose gel electrophoresis of λ DNA digested with partially purified enzyme preparations from LM0230(pND805), showing the effects of Mg2+, ATP, and Ado-Met on pND805 endonuclease activity. Lane 1, λ HindIII standard marker; lanes 2 to 5, preparations containing partially purified enzyme extract from LM0230(pND805); lanes 6 to 9, preparations containing extract from LM0230(pSA3); lanes 2 and 6, extract plus Mg2+; lanes 3 and 7, extract plus Mg2+ plus ATP; lanes 4 and 8, extract plus Mg2+ plus ATP plus Ado-Met; lanes 5 and 9, extract plus Mg2+ plus Ado-Met. The reaction mixtures were incubated for 1 h at 37°C.
The results of the restriction enzyme assays indicated that the properties of the endonuclease encoded by pND805 are different from the properties of type I and type II enzymes and are identical to the properties of type III enzymes.
DISCUSSION
On the basis of the nucleotide and deduced amino acid sequences and the homologies with the other type III mod genes and amino acid sequences, it appears that the R/M system encoded by pND805 is a type III R/M system. The Mod protein from pND805 is similar to the four other type III Mod proteins. Both the N-terminal regions and the C-terminal regions of the five proteins are partially conserved, while the central portions exhibit less homology. This is consistent with the hypothesis (17) that the central domain probably confers sequence specificity, while the two distal conserved blocks presumably are involved in Ado-Met binding interactions and in transmethylation reactions. This type of organization, consisting of alternating conserved and variable regions, is also found in type I and type II methylases (32).
It seems to be a general phenomenon that the genes encoding the two subunits found in type III R/M systems overlap to some extent or are close to each other. Start codon ATG of the type III res gene found in L. lactis overlaps the stop codon of the mod gene, TAA, by 1 bp (A). The genes lie in the same direction of transcription in the order mod-res. EcoP1 and StyLTI, like LlaFI, comprise two close ORFs that are 2 to 12 bp apart in the same order and orientation (37). This observation suggests that the genetic organization of the new R/M system is similar to the genetic organization of other type III R/M systems.
The Res subunits of type I and type III R/M systems exhibit low levels of sequence similarity and contain seven sequence motifs that are characteristic of DNA and RNA helicases belonging to superfamily II (15). The seven helicase motifs described previously for the EcoP1 and StyLTI Res proteins are also found, at the same relative positions, in the LlaFI Res sequence. Helicase motifs play a classical role in unwinding of DNA during repair and recombination. Recently, several researchers have suggested that the helicase motifs identified in both type I and type III R/M systems are also involved in translocation of DNA (21, 23, 25).
The endonuclease encoded by LlaFI required ATP and Mg2+, which is a characteristic of other type III restriction endonucleases (19). Like other type III systems, the R/M system encoded by pND805 did not require Ado-Met to break down DNA, but the reaction was stimulated by the presence Ado-Met (33, 44). Restriction reactions and modification reactions have been found to be competing reactions in the presence of ATP and Ado-Met because once a recognition site has been modified, it can no longer be cleaved (19). The role of Ado-Met in methylase binding is not clear, but it is thought that Ado-Met plays a role in the affinity of the enzyme for binding to a specific DNA sequence. The methylase binds to specific and nonspecific sequences, and it has been suggested that the presence of ATP greatly helps in discrimination of these sequences (1). Type III systems characteristically recognize DNA sequences that are asymmetric, uninterrupted, and five or six nucleotides long. Cleavage occurs approximately 25 to 27 nucleotides downstream from the recognition sequence. Only one strand of the recognition site is methylated (44). Cleavage takes place only when two unmodified sites are present in the DNA in inverse orientations (4).
Cleavage and smearing were observed only in the presence of ATP plus Mg2+ and in the presence of ATP plus Mg2+ plus Ado-Met. The smearing, which implied that incomplete digestion occurred, and the finding that some DNA remained nearly full length may be attributed to simultaneous methylase activity (19). Type III enzymes rarely completely digest DNA, even in the absence of Ado-Met, for reasons that are not clear (4).
The existence of most R/M systems in lactococci has been deduced from the results of phage restriction studies. However, four systems have been characterized by enzyme purification and characterization of the DNA target (13, 22, 26, 27) or by cloning and sequencing of the corresponding genes (10, 24, 27–29, 42, 43). Three of these systems are type II systems, and one (29), comprising three genes associated with restriction activity and a type IIs methylase, has not been classified. Recently, type I R/M systems have been found on lactococcal plasmids (36). Here we describe an example of the least common class of R/M systems (type III) in L. lactis. The existence of all three types of R/M systems in lactococci demonstrates that many lactococci have developed collections of defense mechanisms, which presumably resulted from constant exposure to phages. The variety of defense mechanisms provides excellent biological material for constructing phage-resistant strains for the dairy industry.
ACKNOWLEDGMENTS
This work was supported by the Australian Cooperative Research Center for Food Industry Innovation and by Gist-Brocades Australia.
We thank Gwen E. Allison for critical reading of the manuscript.
REFERENCES
- 1.Ahmad I, Krishnamurthy V, Rao D N. DNA recognition by the EcoP15I and EcoP1 modification methyltransferases. Gene. 1995;157:143–147. doi: 10.1016/0378-1119(95)00671-r. [DOI] [PubMed] [Google Scholar]
- 2.Anderson D G, McKay L L. Simple and rapid method for isolating large plasmid DNA from lactic streptococci. Appl Environ Microbiol. 1983;46:549–552. doi: 10.1128/aem.46.3.549-552.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Backer O, Colson C. Two-step cloning and expression in Escherichia coli of the DNA restriction-modification system StyLTI from Salmonella typhimurium. J Bacteriol. 1991;173:1321–1327. doi: 10.1128/jb.173.3.1321-1327.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bickle T A, Kruger D H. Biology of DNA restriction. Microbiol Rev. 1993;57:434–450. doi: 10.1128/mr.57.2.434-450.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Birnboim H C, Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979;7:1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boyer H W, Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1968;41:459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- 7.Dagert M, Erhlich S D. Prolonged incubation in calcium chloride improves competence of Escherichia coli cells. Gene. 1979;6:23–28. doi: 10.1016/0378-1119(79)90082-9. [DOI] [PubMed] [Google Scholar]
- 8.Daly C, Fitzgerald G F, Davis R. Biotechnology of lactic acid bacteria with special reference to bacteriophage resistance. Antonie Leeuwenhoek. 1996;70:99–110. doi: 10.1007/BF00395928. [DOI] [PubMed] [Google Scholar]
- 9.Dao M L, Ferretti J J. Streptococcus-Escherichia coli shuttle vector pSA3 and its use in the cloning of streptococcal genes. Appl Environ Microbiol. 1985;49:115–119. doi: 10.1128/aem.49.1.115-119.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davis R, van der Lelie D, Mercenier A, Daly C, Fitzgerald G F. ScrFI restriction-modification system of Lactococcus lactis subsp. cremoris UC503: cloning and characterization of two ScrFI methylase genes. Appl Environ Microbiol. 1993;59:777–785. doi: 10.1128/aem.59.3.777-785.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Bruijn F N, Lupski J R. The use of transposon Tn5 mutagenesis in the rapid generation of correlated physical and genetic maps of Tn5 segments cloned into multicopy plasmids—a review. Gene. 1984;27:131–149. doi: 10.1016/0378-1119(84)90135-5. [DOI] [PubMed] [Google Scholar]
- 12.Efstathiou J D, McKay L L. Inorganic salts resistance associated with a lactose-fermenting plasmid in Streptococcus lactis. J Bacteriol. 1977;130:257–265. doi: 10.1128/jb.130.1.257-265.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fitzgerald G F, Daly C, Brown L R, Gingeras T R. ScrF1: a new sequence specific endonuclease from Streptococcus cremoris. Nucleic Acids Res. 1982;10:8171–8179. doi: 10.1093/nar/10.24.8171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gasson M J. Plasmid complements of Streptococcus lactis NCDO 712 and other lactic streptococci after protoplast-induced curing. J Bacteriol. 1983;154:1–9. doi: 10.1128/jb.154.1.1-9.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gorbalenya A E, Koonin E V, Donchenko A P, Blinov V M. Two related superfamilies of putative helicases involved in replication, recombination, repair and expression of DNA and RNA genomes. Nucleic Acids Res. 1989;17:4713–4730. doi: 10.1093/nar/17.12.4713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guchte M, Kok J, Venema G. Gene expression in Lactococcus lactis. FEMS Microbiol Rev. 1992;88:73–92. doi: 10.1111/j.1574-6968.1992.tb04958.x. [DOI] [PubMed] [Google Scholar]
- 17.Humbelin M, Suri B, Rao D N, Hornby D P, Eberle H, Pripfi T, Kenel S, Bickle T A. Type III DNA restriction and modification systems EcoP1 and EcoP15. J Mol Biol. 1988;200:23–29. doi: 10.1016/0022-2836(88)90330-0. [DOI] [PubMed] [Google Scholar]
- 18.Iida S, Meyer J, Bachi B, Carlemalm M S, Schrickel S, Bickle T A, Arber W. DNA restriction-modification gene of phage P1 and plasmid p15B. J Mol Biol. 1983;165:1–18. doi: 10.1016/s0022-2836(83)80239-3. [DOI] [PubMed] [Google Scholar]
- 19.Kauc L, Piekarowicz A. Purification and properties of a new restriction endonuclease from Haemophilus influenzae Rf. Eur J Biochem. 1978;92:417–426. doi: 10.1111/j.1432-1033.1978.tb12762.x. [DOI] [PubMed] [Google Scholar]
- 20.Klaenhammer T R, Fitzgerald G F. Bacteriophage and bacteriophage resistance. In: Gasson M J, de Vos W M, editors. Genetics and biotechnology of lactic acid bacteria. Glasgow, Scotland: Blackie Academic and Professional, Chapman and Hall; 1994. pp. 106–168. [Google Scholar]
- 21.Matson S W, Bean D W, George J W. DNA helicases: enzymes with essential roles in all aspects of DNA metabolism. Bioessays. 1994;16:13–20. doi: 10.1002/bies.950160103. [DOI] [PubMed] [Google Scholar]
- 22.Mayo B, Hardisson C, Brana A F. Nucleolytic activities in Lactococcus lactis subsp. lactis NCDO 497. FEMS Microbiol Lett. 1991;79:195–198. [Google Scholar]
- 23.Meisel A, Mackeldanz P, Bickle T A, Kruger D H, Schroeder D. Type III restriction endonucleases translocate DNA in a reaction driven by recognition site-specific ATP hydrolysis. EMBO J. 1995;14:2958–2966. doi: 10.1002/j.1460-2075.1995.tb07296.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moineau S, Walker S A, Vedamuthu E R, Vandenbergh P A. Cloning and sequencing of LlaII restriction/modification genes from Lactococcus lactis and relatedness of this system to the Streptococcus pneumoniae DpnII system. Appl Environ Microbiol. 1995;61:2193–2202. doi: 10.1128/aem.61.6.2193-2202.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murray N E, Daniel A S, Cowan G M, Sharp P M. Conservation of motifs within the unusually variable polypeptide sequences of type I restriction and modification enzymes. Mol Microbiol. 1993;9:133–143. doi: 10.1111/j.1365-2958.1993.tb01675.x. [DOI] [PubMed] [Google Scholar]
- 26.Nyengaard N, Vogensen F K, Josephsen J. LlaAI and LlaBI, two type-II restriction endonucleases from Lactococcus lactis subp. cremoris W9 and W56 recognizing, respectively, 5′-/GATC-3′ and 5′-C/TRYAG-3′. Gene. 1993;136:371–372. doi: 10.1016/0378-1119(93)90499-s. [DOI] [PubMed] [Google Scholar]
- 27.Nyengaard N, Vogensen F K, Josephsen J. Restriction-modification systems in Lactococcus lactis. Gene. 1995;157:13–18. doi: 10.1016/0378-1119(95)91235-r. [DOI] [PubMed] [Google Scholar]
- 28.Nyengaard N R, Falkenberg-Klok J, Josephsen J. Cloning and analysis of the restriction-modification system LlaBI, a bacteriophage resistance system from Lactococcus lactis subsp. cremoris W56. Appl Environ Microbiol. 1996;62:3494–3498. doi: 10.1128/aem.62.9.3494-3498.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O’Sullivan D J, Zagula K, Klaenhammer T R. In vivo restriction by LlaI is encoded by three genes, arranged in an operon with llaIM, on the conjugative Lactococcus plasmid pTR2030. J Bacteriol. 1995;177:134–143. doi: 10.1128/jb.177.1.134-143.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Powell B I, Achen G M, Hillier J A, Davidson E B. A simple and rapid method for genetic transformation of lactic streptococci by electroporation. Appl Environ Microbiol. 1988;54:655–660. doi: 10.1128/aem.54.3.655-660.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Price C, Bickle T A. A possible role for DNA restriction in bacterial evolution. Microbiol Sci. 1986;3:296–299. [PubMed] [Google Scholar]
- 32.Rao D N, Eberle H, Bickle T A. Characterization of mutations of the bacteriophage P1 mod gene encoding the recognition subunit of the EcoP1 restriction and modification system. J Bacteriol. 1989;171:2347–2352. doi: 10.1128/jb.171.5.2347-2352.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saha S, Rao D N. ATP hydrolysis is required for DNA cleavage by EcoP1 restriction enzyme. J Mol Biol. 1995;247:559–567. doi: 10.1016/s0022-2836(05)80137-8. [DOI] [PubMed] [Google Scholar]
- 34.Saha S, Rao D N. Mutations in the res subunit of the EcoP1 restriction enzyme that affect ATP-dependent reactions. J Mol Biol. 1997;269:342–354. doi: 10.1006/jmbi.1997.1045. [DOI] [PubMed] [Google Scholar]
- 35.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 36.Schouler C, Clier F, Lerayer A L, Ehrlich S D, Chopin M C. A type I Ic restriction-modification system in Lactococcus lactis. J Bacteriol. 1998;180:407–411. doi: 10.1128/jb.180.2.407-411.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sharrocks A D, Hornby D P. Transcription analysis of the restriction and modification genes of bacteriophage P1. Mol Microbiol. 1991;5:685–694. doi: 10.1111/j.1365-2958.1991.tb00740.x. [DOI] [PubMed] [Google Scholar]
- 38.Smith H O, Nathans D. A suggested nomenclature for bacterial host modification systems and their enzymes. J Mol Biol. 1973;81:419–423. doi: 10.1016/0022-2836(73)90152-6. [DOI] [PubMed] [Google Scholar]
- 39.Su P, Ng A, Kennelly V, Costello M, Havey M, Dunn N. Additive effects of two different plasmid-linked restriction and modification systems in Lactococcus. Biotechnol Lett. 1998;20:515–518. [Google Scholar]
- 40.Sugisaki H, Kanazawa S. New restriction endonucleases from Flavobacterium okeanokoites (FokI) and Micrococcus luteus (MluI) Gene. 1981;16:73–78. doi: 10.1016/0378-1119(81)90062-7. [DOI] [PubMed] [Google Scholar]
- 41.Terzaghi B E, Sandine W E. Improved medium for lactic streptococci and their bacteriophages. Appl Microbiol. 1975;29:807–813. doi: 10.1128/am.29.6.807-813.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Twomey D P, Davis R, Daly C, Fitzgerald G F. Sequence of the gene encoding a second ScrFI m5C methyltransferase of Lactococcus lactis. Gene. 1993;136:205–209. [Google Scholar]
- 43.Twomey D P, Gabillet N, Daly C, Fitzgerald G F. Molecular characterization of the restriction endonuclease gene (scrFIR) associated with the ScrFI restriction/modification system from Lactococcus lactis subsp. cremoris UC503. Microbiology. 1997;143:2277–2286. doi: 10.1099/00221287-143-7-2277. [DOI] [PubMed] [Google Scholar]
- 44.Wilson G G. Organization of restriction-modification systems. Nucleic Acids Res. 1991;19:2539–2566. doi: 10.1093/nar/19.10.2539. [DOI] [PMC free article] [PubMed] [Google Scholar]