Abstract
Objective
To evaluate whether pain management clinic laws and prescription drug monitoring program (PDMP) prescriber check mandates, two state opioid policies with relatively rapid adoption across states, reduced opioid dispensing more or less in Black versus White patients.
Data Sources
Pharmacy claims data, US sample of commercially insured adults, 2007–2018.
Study Design
Stratifying by race, we used generalized estimating equations with an event‐study specification to estimate time‐varying effects of each policy on opioid dispensing, comparing to the four pre‐policy quarters and states without the policy. Outcomes included high‐dosage opioids, overlapping opioid prescriptions, concurrent opioid/benzodiazepines, opioids from >3 prescribers, opioids from >3 pharmacies.
Data Extraction Methods
We identified all prescription opioid dispensing to Black and White adults aged 18–64 without a palliative care or cancer diagnosis code.
Principal Findings
Exactly 7,096,592 White and 1,167,310 Black individuals met inclusion criteria. Pain management clinic laws were associated with reductions in two outcomes; their association with high‐dosage receipt was larger among White patients. In contrast, reductions due to PDMP mandates appeared limited to, or larger in, Black patients compared with White patients in four of five outcomes. For example, PDMP mandates reduced high‐dosage receipt in Black patients by 0.7 percentage points (95% CI: 0.36–1.08 ppt.) over 4 years: an 8.4% decrease from baseline; there was no apparent effect in White patients. Similarly, while there was limited evidence that mandates reduced overlapping opioid receipt in White patients, they appeared to reduce overlapping opioid receipt in Black patients by 1.3 ppt. (95% CI: −1.66–−1.01 ppt.) across post‐policy years—a 14.4% decrease from baseline.
Conclusions
PDMP prescriber check mandates but not pain management clinic laws appeared to reduce opioid dispensing more in Black patients than White patients. Future research should discern the mechanisms underlying these disparities and their consequences for pain management.
Keywords: opioids, policy, prescriptions, racial discrimination, racial disparities, racial inequity
What is known on this topic
Pain management clinic laws and prescription drug monitoring program prescriber check mandates (“PDMP mandates”) can reduce prescription opioid dispensing.
Throughout the ongoing drug overdose crisis, Black Americans have experienced lower rates of overdose attributable to medical and nonmedical use of prescription opioids.
On average, Black patients receive fewer opioids for a given diagnosis than White patients and are more likely to experience dose reductions and opioid discontinuation, and this difference is not explained by relevant clinical factors.
What this study adds
PDMP mandates appeared to reduce opioid dispensing more in Black patients than in White patients, despite lower rates of dispensing at baseline.
Pain management clinic laws appeared to reduce some opioid dispensing outcomes; in one case, the estimated effect was larger in White compared with Black patients.
1. INTRODUCTION
Opioid prescribing, which accelerated dramatically in the mid‐1990s, contributed to the first wave of the modern opioid overdose crisis. 1 This prompted a large policy response to limit the prescription opioid supply, and particularly to curb practices that can increase and indicate risk of opioid‐related harms. These include high‐dose prescribing, overlapping opioid or opioid/benzodiazepine prescribing, and receipt of prescription opioids from multiple prescribers or pharmacies (although each of these practices may be clinically indicated under some circumstances). 2 , 3 , 4 , 5 , 6 While a number of evaluations of these policies have been conducted, their effects have rarely been investigated by race and ethnicity, despite the vast racial and ethnic differences in the trajectory of the opioid crisis. 7 , 8 Through standardization, opioid prescribing policies may reduce racial disparities in opioid prescribing, wherein Black patients tend to receive fewer opioids than White patients for a given diagnosis. 9 , 10 Alternatively, however, prescribing policies may perpetuate or exacerbate racial disparities by raising provider awareness of the dangers of unsafe prescribing and potentially prompting the use of racial stereotypes to assess a patient's “legitimate” need for prescription opioids. 11 , 12
Two state policies that appear to have substantially influenced opioid prescribing include pain management clinic laws and prescription drug monitoring program (PDMP) prescriber check mandates. 3 PDMPs are electronic databases that store patient and prescription information for opioids and other controlled substances. While all states but Missouri had a PDMP as early as 2011, fewer have required prescribers to query the PDMP before prescribing a relevant medication (Table A1). 13 Findings on the overall effects of PDMPs have been mixed, but evidence of an inverse association between PDMP prescriber check mandates and opioid prescribing volume is more consistent. 3 Pain management clinic laws differ from PDMP mandates in that they specifically regulate facilities that primarily treat pain; by 2020, 13 states had adopted a pain management clinic law (Table A1). 14 These laws impose operational, personnel, inspection, and other requirements on pain management clinics toward reducing the existence of the so‐called “pill mills.” 3 , 15 For example, pain management clinic laws often require a clinic to register with the state or obtain a license to operate, and may require clinic owners or medical directors to have particular medical credentials or training. These laws are less well‐studied, but growing evidence suggests they also reduce opioid prescribing, including when paired with PDMPs. 3 , 16 , 17
Given evidence of differential opioid prescribing by patient race, there may be racial disparities in the effects of opioid prescribing policies. Black patients in the United States are less likely than White patients to receive opioids for a given pain‐related diagnosis, particularly when a discrete source of pain is difficult to identify. 9 , 18 Even after adjustment for clinically relevant factors, Black patients may be more likely to experience opioid dose reductions and discontinuation, 19 , 20 as well as precautionary measures such as urinalysis and restricted refills. 21 Yet Black people experience lower rates of prescription opioid overdose and prescription opioid overdose mortality than White people 22 , 23 , 24 , 25 and are equally or less likely to report nonmedical prescription opioid use. 25 , 26 , 27 Together, these findings suggest discriminatory practices by providers, possibly driven by biased perceptions of Black patients as “drug‐seeking,” experiencing less pain than White counterparts, or as otherwise less “legitimate” patients. 28 , 29
By increasing standardization, state laws that aim to reduce excess opioid prescribing could serve to alleviate racial disparities in opioid receipt. This may be particularly likely to result from policies that primarily target individual prescribing decisions, such as PDMP prescriber check mandates (“PDMP mandates”), compared with those that primarily target institutions, like pain management clinic laws. By influencing individual prescriber behavior, PDMP mandates may reduce the potential for racial stereotypes to shape prescriber decision making. However, if state opioid prescribing policies increase provider awareness of higher‐risk practices, and if providers are disproportionately suspicious of Black patients, then these policies may reduce prescribing more in Black compared with White patients, despite lower rates of receipt at baseline. 9 , 18 Indeed, such patterns have been observed in benzodiazepine prescribing, following initiation of New York State's triplicate prescription program. 11 , 12 While some have suggested that racially disparate opioid prescribing could have a protective effect on Black people by creating less risk of prescription opioid addiction and overdose, 8 , 30 the adverse consequences of discrimination in pain management are wide‐reaching, including possible undertreatment of pain and reduced trust in health care providers, a key factor in patients' decisions to seek treatment and preventive care and to adhere to medical recommendations. 28 , 31 , 32 , 33 , 34 , 35 Moreover, efforts to alleviate prescription opioid‐related harms will have limited success if they reduce excess prescribing in populations at lower but not higher risk.
In this study, we estimate the effects of state PDMP prescriber check mandates and pain management clinic laws on indicators of higher‐risk opioid prescribing, by Black/White race. We draw from 2007 to 2018 commercial health care claims data for patients receiving prescription opioids in all 50 states.
2. METHODS
2.1. Study design
We used event‐study design, a quasi‐experimental design, to examine time‐varying associations of pain management clinic laws and PDMP prescriber check mandates with opioid receipt in Black and White patients.
2.2. Data
We used pharmacy claims data from Optum's deidentified Clinformatics Data Mart Database during 2007–2018. The database comprises 12–14 million unique individuals per year enrolled in commercial health plans. 36 These patients are demographically comparable to the US commercially insured population. 36 , 37 We identified all prescription opioid fills by Black and White adults under the age of 65 who did not receive palliative care or a cancer diagnosis code at any time during 2007–2018. We concentrated on adults under the age of 65 because they experience greater risk of nonmedical prescription opioid use and overdose than older adults. 23 , 26
2.3. Variables
2.3.1. Interventions
Exposure variables included binary indicators of the number of years relative to (1, 2, 3, and ≥4 years before and after) policy effective date, if any, of each policy type: (a) pain management clinic law; and (b) PDMP mandate (Table A1). Both sets of policy variables were included in all models. Year 0 was defined as the quarter in which the law came into effect and the three subsequent quarters. A PDMP mandate was considered to be in effect if prescribers were required to check the PDMP based on a set of defined criteria (e.g., first opioid prescription to a new patient, and every 6 months thereafter); in other words, by “objective standard.” PDMP check requirements based on subjective criteria—for example, the prescriber's subjective determination that the patient could be exhibiting signs of misuse—did not qualify in this study as a PDMP prescriber check mandate because they leave checks to prescriber discretion similar to as if there was no such law.
2.3.2. Outcomes
We examined five binary opioid dispensing outcomes considered to increase risk of nonmedical opioid use and overdose, 2 , 38 which pain management clinic laws and PDMP mandates intend to address. Each outcome was measured at the quarter level: (1) receipt of a prescription with average daily dose of ≥90 morphine milligram equivalents (MME); (2) receipt of ≥7 days of overlapping opioid prescriptions; (3) receipt of ≥1 day of concurrent opioid and benzodiazepine prescriptions; (4) receipt of opioid prescriptions from >3 prescribers; (5) receipt of opioid prescriptions from >3 pharmacies. Concurrent prescriptions were ascertained using dispensing date and number of days supplied.
2.3.3. Stratification
We stratified by Black or White race using the race and ethnicity variable in Optum enrollment files, which Optum imputes for some patients based on sociodemographic data. In adults under the age of 65, missingness on this variable was 7%; the proportions of enrolled patients listed as Black and White, respectively, were within one percentage point of Census estimates of the racial breakdown in the commercially insured population. 39 Table A2 compares characteristics of our sample to those with missing race and ethnicity information.
2.3.4. Covariates
In addition to calendar year, we included individual and state‐level demographic covariates that were potential confounders of the relationship between adoption of opioid prescribing policies and opioid dispensing outcomes. Individual‐level covariates available in Optum enrollment files included age (18–34, 35–49, 50–64 years; time‐varying), sex (female, male), and state of residence (time‐varying). State‐level covariates, obtained from American Community Survey 1‐year estimates, 40 included the percentage of the population that is White, the percent female, and mean age: all were time‐varying.
2.4. Statistical analysis
2.4.1. Analyses
Using generalized estimating equations with an event‐study specification, 41 , 42 we modeled each policy's effects by year relative to effective date, stratified by Black/White race. That is, we estimated a distinct coefficient for each year pre‐ and post‐policy; the reference period was the year prior to the quarter the policy came into effect. Patients in states that did not adopt the policy were coded as 0 on each binary lead and lag variable. The identifying assumption was that, in the absence of each policy, differences between treatment and comparison states would have continued along the same trends at each time point relative to policy implementation, regardless of when the policy came into effect. 43 The event‐study approach helps evaluate the credibility of the parallel trends assumption by enabling both visualization of pre‐policy trends and comparison of treatment and comparison states. Small, statistically insignificant pre‐policy estimates would suggest this parallel trends assumption was satisfied. In addition, this design allows for time‐varying policy effects. We included state fixed effects to account for fixed, cross‐sectional differences across states and calendar year fixed effects to account for secular changes that occurred nationally. We used the log link function and binomial distribution. Because nearly half of patients appeared in the dataset only once and due to lack of model convergence with more complex correlation structures, we used the independent correlation structure. Standard errors were clustered at the individual level to account for repeated measures within patients.
To estimate the average effect of each policy on each dispensing outcome over the first 4 years post‐policy, we calculated the mean of the four corresponding marginal effects: in an effort to reduce bias associated with the more commonly used difference‐in‐difference estimates when treatment effects vary over time. 41 To contextualize these estimates, we compared them with the baseline in the treatment states, defined as the mean of the relevant outcome in the four quarters before the policy came into effect. This study was found exempt of oversight by the University of Michigan Institutional Review Board. 44 , 45
3. RESULTS
We identified 7,096,592 White and 1,167,310 Black individuals aged 18–64 who received at least one opioid fill within a given quarter between 2007 and 2018, and who met other inclusion criteria (19,493,261 and 3,287,522 quarter‐level observations, respectively; Table 1). Compared with White patients, a larger percentage of Black patients in the sample lived in a state with a pain management clinic law (White: 45.5%, 95% CI: 45.47%–45.54%; Black: 59.1%, 95% CI: 59.02%–59.20%) and with a PDMP access mandate (White: 71.5%, 95% CI: 71.47%–71.53%; Black: 75.9%, 95% CI: 74.84%–75.99%). The sample was disproportionately female (White: 54.0%, 95% CI: 53.94%–54.01%; Black: 61.1%, 95% CI: 60.96%–61.14%), and in both groups roughly one‐third of patients were in each age group (18–34, 35–49, 50–64 years).
TABLE 1.
Sample characteristics by Black/White race
| White | Black | |||
|---|---|---|---|---|
| Estimate | 95% CI | Estimate | 95% CI | |
| Distinct individuals | 7,096,592 | 1,167,310 | ||
| Quarterly observations | 19,493,261 | 3,287,522 | ||
| Quarters followed (mean) | 2.7 | (2.74–2.75) | 2.8 | (2.81–2.82) |
| Pain management clinic law state (%) | 45.5 | (45.47–45.54) | 59.1 | (59.02–59.20) |
| PDMP mandate state (%) | 71.5 | (71.47–71.53) | 75.9 | (75.84–75.99) |
| Female (%) | 54.0 | (53.94–54.01) | 61.1 | (60.96–61.14) |
| Age (%) | ||||
| 18–34 | 35.0 | (34.95–35.02) | 34.9 | (34.82–34.99) |
| 35–49 | 34.7 | (34.70–34.77) | 35.7 | (35.58–35.76) |
| 50–64 | 30.3 | (30.25–30.32) | 29.4 | (29.34–29.51) |
| Days supplied (mean) | 36.3 | (36.28–36.33) | 34.1 | (34.09–34.20) |
| Daily dose | ||||
| Mean | 43.4 | (41.54–45.27) | 39.0 | (38.94–39.07) |
| >90 MME (%) | 12.2 | (12.18–12.21) | 9.8 | (9.72–9.79) |
| Overlapping opioids (%) | 8.9 | (8.89–8.91) | 7.6 | (7.54–7.59) |
| Concurrent opioids, benzodiazepines (%) | 16.7 | (16.65–16.68) | 12.6 | (12.52–12.59) |
| >3 prescribers (%) | 0.95 | (0.95–0.95) | 1.06 | (1.05–1.07) |
| >3 pharmacies (%) | 0.65 | (0.64–0.65) | 0.62 | (0.61–0.63) |
Note: Pain management clinic law state = percentage of patients who live in a state that adopted a pain management clinic law during the analytic period. PDMP mandate state = percentage of patients who live in a state that adopted a PDMP prescriber check mandate during the analytic period. 95% confidence intervals in parentheses. Sociodemographic characteristics refer to each individual's first observation in the dataset.
With one exception, unadjusted means in each outcome were greater in White patients compared with Black patients. For example, White patients received a mean daily dose of 43.4 MME (95% CI: 41.54–45.27 MME) compared with 39.0 in Black patients (95% CI: 38.94–39.07 MME), and the proportion of concurrent opioid/benzodiazepine prescribing was 16.7% in White patients (95% CI: 16.65%–16.68%) compared with 12.6% in Black patients (95% CI: 12.52%–12.59%). The proportion receiving opioids from >3 prescribers was higher in Black patients (1.06%, 95% CI: 1.05%–1.07%) than in White patients (0.951%, 95% CI: 0.946%–0.955%). Similarly, White patients tended to experience higher or similar rates of receipt in the four‐quarter period before each law came into effect in treatment states (Table A3).
Figures 1, 2, 3 present event‐study plots for each policy, racial group, and outcome; Table A4 provides the corresponding marginal effect estimates. Table 2 presents the mean marginal effects across post‐policy years, in comparison with the mean of the outcome in (a) comparison states and (b) the last pre‐policy year mean in treatment states (“baseline”).
FIGURE 1.
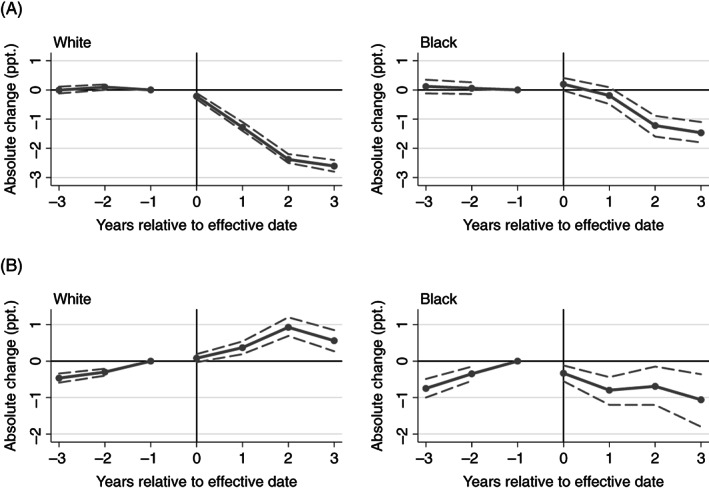
Event‐study estimates for the association of pain management clinic laws and PDMP mandates with high‐dose opioid receipt. (A) High dose: pain management clinic laws. (B) High dose: PDMP mandates. PDMP, PDMP prescriber check mandates; ppt., percentage points. Point estimates refer to the differences in high‐dose opioid receipt associated with each policy in each four‐quarter period relative to effective date, compared with the reference group. State and year fixed effects included, in addition to other covariates. Dashed lines indicate 95% confidence intervals; period −1 lacks a confidence interval because it was the reference period. High dose refers to receipt of at least one prescription with average daily dose of 90+ MME. Year 0 refers to the first four calendar quarters post‐policy. The reference group comprises the year before the law came into effect and patients in comparison states
FIGURE 2.
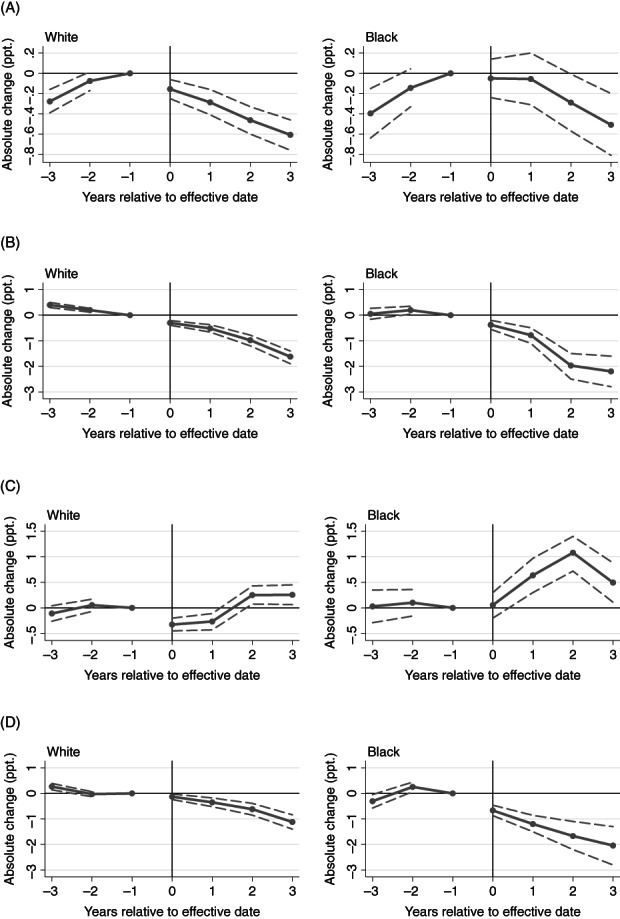
Event‐study estimates for the association of pain management clinic laws and PDMP mandates with overlapping opioid and concurrent opioid/benzodiazepine receipt. (A) Overlapping opioids: pain management clinic laws. (B) Overlapping opioids: PDMP mandates. (C) Concurrent opioids/benzodiazepines: pain management clinic laws. (D) Concurrent opioids/benzodiazepines: PDMP mandates. PDMP, PDMP prescriber check mandates; ppt., percentage points. Point estimates refer to the differences in the outcome associated with each policy in each four‐quarter period relative to effective date, compared to the reference group. State and year fixed effects included, in addition to other covariates. Dashed lines indicate 95% confidence intervals; period −1 lacks a confidence interval because it was the reference period. Year 0 refers to the first four calendar quarters post‐policy. The reference group comprises the year before the law came into effect and patients in comparison states
FIGURE 3.
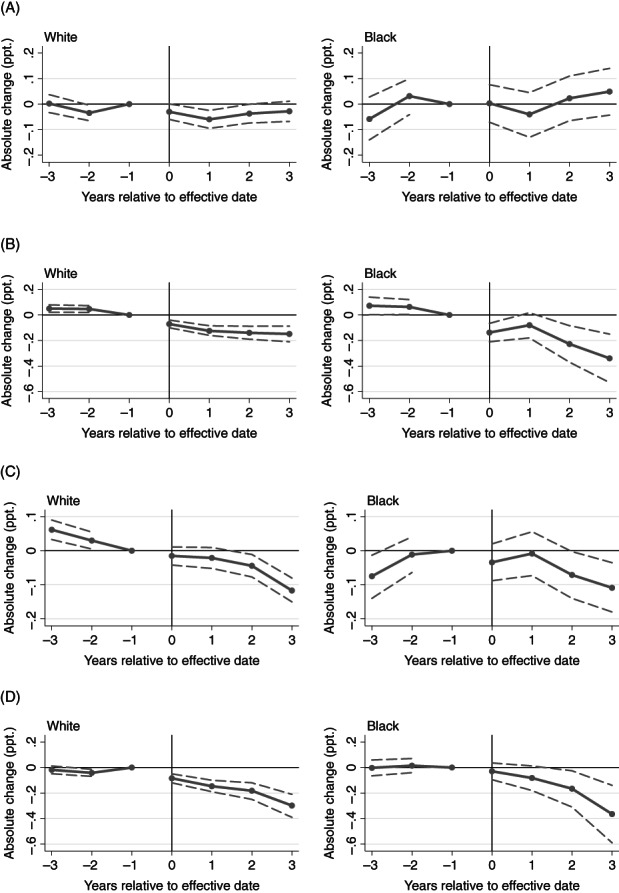
Event‐study estimates for the association of pain management clinic laws and PDMP mandates with opioid receipt from >3 prescribers and >3 pharmacies. (A) >3 prescribers: pain management clinic laws. (B) >3 prescribers: PDMP mandates. (C) >3 pharmacies: pain management clinic laws. (D) >3 pharmacies: PDMP mandates. PDMP, PDMP prescriber check mandates; ppt., percentage points. Point estimates refer to the differences in the outcome associated with each policy in each four‐quarter period relative to effective date, compared with the reference group. State and year fixed effects included, in addition to other covariates. Dashed lines indicate 95% confidence intervals; period −1 lacks a confidence interval because it was the reference period. Year 0 refers to the first four calendar quarters post‐policy. The reference group comprises the year before the law came into effect and patients in comparison states
TABLE 2.
Associations between opioid prescribing policies and opioid dispensing, by Black/White race: (a) pain management clinic laws, (b) PDMP check mandates
| (a) | |||||
|---|---|---|---|---|---|
| High dose | >3 prescribers | >3 pharmacies | Overlapping opioids | Concurrent opioids, benzos | |
| White | |||||
| Mean in comparison states (ppt.) | 12.2 (12.22, 12.26) | 1.0 (0.973, 0.985) | 0.7 (0.659, 0.669) | 8.7 (8.711, 8.745) | 16.3 (16.26, 16.30) |
| Mean at baseline, treatment states (ppt.) | 14.8 (14.73, 14.88) | 1.1 (1.083, 1.128) | 0.7 (0.706, 0.743) | 9.3 (9.243, 9.370) | 15.9 (15.85, 16.01) |
| Mean estimated effect (ppt.) | −1.6 (−1.741, −1.504) | −0.04 (−0.0668, −0.0107) | −0.05 (−0.0745, −0.0241) | −0.4 (−0.485, −0.272) | −0.02 (−0.158, 0.115) |
| Relative change from baseline (%) | −10.8% | −3.6% | −7.1% | −4.3% | −0.1% |
| Black | |||||
| Mean, comparison (ppt.) | 10.1 (10.10, 10.20) | 1.2 (1.171, 1.209) | 0.7 (0.664, 0.692) | 7.7 (7.613, 7.705) | 12.6 (12.57, 12.69) |
| Mean at baseline, treatment (ppt.) | 10.9 (10.77, 11.07) | 1.1 (1.073, 1.174) | 0.7 (0.703, 0.785) | 7.6 (7.454, 7.707) | 10.8 (10.62, 10.91) |
| Mean estimated effect (ppt.) | −0.7 (−0.922, −0.425) | 0.009 (−0.0604, 0.0777) | −0.06 (−0.109, −0.00240) | −0.2 (−0.447, −0.00491) | 0.6 (0.284, 0.851) |
| Relative change from baseline (%) | −6.4% | 0.8% | −8.6% | −2.6% | 5.6% |
| (b) | |||||
|---|---|---|---|---|---|
| High dose | >3 prescribers | >3 pharmacies | Overlapping opioids | Concurrent opioids, benzos | |
| White | |||||
| Mean in comparison states (ppt.) | 11.5 (11.49, 11.54) | 0.9 (0.867, 0.883) | 0.6 (0.584, 0.597) | 8.1 (8.059, 8.105) | 15.4 (15.42, 15.48) |
| Mean at baseline, treatment states (ppt.) | 9.7 (9.595, 9.705) | 0.9 (0.909, 0.945) | 0.4 (0.371, 0.393) | 9.4 (9.385, 9.493) | 19.8 (19.69, 19.84) |
| Mean estimated effect (ppt.) | 0.5 (0.322, 0.643) | −0.1 (−0.153, −0.0884) | −0.2 (−0.217, −0.138) | −0.9 (−0.987, −0.718) | −0.6 (−0.715, −0.395) |
| Relative change from baseline (%) | 5.2% | −11.1% | −50.0% | −9.6% | −3.0% |
| Black | |||||
| Mean, comparison (ppt.) | 8.3 (8.226, 8.347) | 1.0 (0.971, 1.014) | 0.6 (0.573, 0.607) | 7.1 (6.994, 7.106) | 12.5 (12.45, 12.60) |
| Mean at baseline, treatment (ppt.) | 8.3 (8.202, 8.426) | 1.1 (1.085, 1.170) | 0.5 (0.430, 0.485) | 9.0 (8.850, 9.082) | 16.4 (16.28, 16.58) |
| Mean estimated effect (ppt.) | −0.7 (−1.076, −0.364) | −0.2 (−0.284, −0.109) | −0.2 (−0.249, −0.0723) | −1.3 (−1.656, −1.008) | −1.4 (−1.756, −1.030) |
| Relative change from baseline (%) | −8.4% | −18.2% | −40.0% | −14.4% | −8.5% |
Note: “Comparison states” are those that did not adopt the relevant law during the analytic period, and “treatment” are those that did. “Mean at baseline” refers to the mean of the outcome in the four quarters before the relevant law came into effect. “Mean estimated effect” is the mean of the post‐period marginal effects, that is, the percentage point change in the outcome compared to baseline (the four‐quarter period prior to the effective date). “Relative change” refers to the mean estimated effect divided by the mean at baseline in the treatment states. The change estimates should be interpreted in the context of the pre‐period trends visualized in the event‐study plots.
Abbreviation: ppt., percentage points.
3.1. Pain management clinic laws
Overall, pain management clinic laws were associated with lower rates of high‐dose receipt and concurrent opioid receipt. The association between pain management clinic laws and high‐dose receipt was larger in White compared with Black patients, while there was no apparent difference by race in the association with overlapping opioid receipt.
Following flat pre‐policy trends with estimates indistinguishable from zero, pain management clinic laws appeared to reduce the rate of high‐dose receipt in both groups (Figure 1, Table 2). The mean estimated effect across post‐policy years was larger in White patients (−1.6 ppt., 95% CI: −1.74–−1.51 ppt.) compared with Black patients (−0.7 ppt., 95% CI: −0.92–−0.43 ppt.). These effects correspond to an estimated 10.8% decrease from treatment state baseline in White patients and a 6.4% decrease in Black patients. In both groups, the estimated effects grew over the first 3 years post‐policy and then flattened.
Pain management clinic laws appeared to reduce overlapping opioid receipt in both Black and White patients, reversing upward pre‐policy trends (Figure 2). The mean estimated effect across post‐policy years was similar in White patients (−0.4 ppt., 95% CI: −0.485–−0.272 ppt.) and Black patients (−0.2 ppt., 95% CI: −0.447–−0.00491 ppt.). These effects corresponded to an estimated 4.3% decrease from treatment state baseline in White patients and a 2.6% decrease in Black patients. The estimated effects grew over the 4 years post‐policy, although confidence intervals for Black patients were wide.
In the period before the pain management clinic law came into effect, the rate of opioid receipt from >3 pharmacies was decreasing among White patients; post‐policy, this trend flattened for 3 years and then sharpened in the fourth post‐policy year, potentially suggesting that pain management clinic laws slowed the downward trend in receipt from >3 pharmacies in this group (Figure 3). In Black patients, wide confidence intervals limited interpretation, but pain management clinic laws may have reversed an upward trend in receipt from >3 pharmacies; the mean estimated effect across post‐policy years was −0.05 ppt. (95% CI: −0.07–−0.02 ppt.), an approximately 7.1% decrease from baseline.
Pain management clinic laws did not appear to reduce concurrent opioid/benzodiazepine receipt, receipt from >3 prescribers, or receipt from >3 pharmacies. In Black patients, concurrent opioid/benzodiazepine receipt appeared to increase following pain management clinic adoption (Figure 2, Table 2).
3.2. PDMP mandates
Overall, PDMP mandates appeared to reduce rates of high‐dose receipt, overlapping opioids and concurrent opioid/benzodiazepine receipt, and receipt of opioids from >3 prescribers and >3 pharmacies. In four of these five outcomes, however, the effects appeared limited to, or larger in, Black patients compared with White patients.
PDMP mandates appeared to reduce the rate of high‐dose receipt in Black but not White patients (Figure 1, Table 2). In both groups, high‐dose receipt was increasing prior to the PDMP mandate. In White patients, this upward trend continued until 3 years post‐policy and then reversed, remaining higher than at baseline. In Black patients, in contrast, the upward trend reversed post‐mandate; the mean estimated effect across post‐policy years was −0.7 ppt. (95% CI: −1.08–−0.36 ppt.), an estimated 8.4% decrease from baseline.
PDMP mandates appeared to reduce the rate of overlapping opioid receipt in Black patients, and possibly in White patients to a lesser extent (Figure 2, Table 2). White patients experienced a downward pre‐policy trend that may have been accelerated by the PDMP mandate. In Black patients, pre‐policy trends were flat and near zero; after the mandate came into effect, effects were negative and grew over the first three post‐policy years. The mean estimated effect in Black patients across post‐policy years was −1.3 ppt. (95% CI: −1.66–−1.01 ppt.): an estimated 14.4% decrease from baseline.
Similarly, PDMP mandates appeared to reduce the rate of concurrent opioid/benzodiazepine receipt in Black patients and possibly White patients to a lesser extent (Figure 2, Table 2). White patients experienced a downward pre‐policy trend that appeared to continue following the PDMP mandate. In Black patients, pre‐policy estimates were near zero and became increasingly negative post‐policy. The mean estimated effect in Black patients across post‐policy years was −1.4 ppt. (95% CI: −1.76–−1.03 ppt.): an estimated 8.5% decrease from baseline.
PDMP mandates also appeared to reduce the rate of receipt from >3 prescribers in both White and Black patients (Figure 3); the overall estimated effect was larger in Black than White patients but confidence intervals overlapped (Table 2). For both groups, pre‐policy means were stable and decreased post‐policy; this amounted to a level (mean) change in White patients and a level and trend change in Black patients. In White patients, the estimated effect across post‐policy years was −0.1 ppt. (95% CI: −0.15–−0.09 ppt.), corresponding to an estimated 11.1% decrease from baseline. In Black patients, the mean estimated effect across post‐policy years was −0.2 ppt. (95% CI: −0.28–−0.11 ppt.), corresponding to an estimated 18.2% decrease from baseline.
Finally, PDMP mandates appeared to reduce receipt from >3 pharmacies in both Black and White patients, with no clear difference in magnitude of effect. Pre‐policy estimates were flat and similar to zero in both groups and became negative after the law came into effect. In White patients, the mean estimated effect across post‐policy years was −0.2 ppt. (95% CI: −0.22–−0.14 ppt.), corresponding to an estimated 50.0% decrease from baseline. In Black patients, the mean estimated effect across post‐policy years was −0.2 ppt. (95% CI: −0.25–−0.07 ppt.), corresponding to an estimated 40.0% decrease from baseline.
4. DISCUSSION
We found evidence of racially disparate effects of PDMP prescriber check mandates (“PDMP mandates”) on four out of five opioid dispensing outcomes. Despite lower rates of dispensing in Black patients in the year before the law came into effect, the effects of PDMP mandates on (a) receipt of high‐dose opioids, (b) receipt of overlapping opioids, (c) receipt of concurrent opioid/benzodiazepine fills, and (d) receipt of opioids from >3 prescribers appeared limited to, or larger among, Black patients compared with White patients aged 18–64 years. In contrast, we found no clear evidence that pain management clinic laws reduced opioid dispensing to a greater degree among Black patients. Instead, these laws appeared to reduce rates of high‐dose receipt more in White patients, while overlapping opioid receipt declined in Black and White patients to a similar extent.
The magnitude of the estimated effects on higher‐risk opioid dispensing tended to be clinically meaningful but did not reduce the prevalence of these outcomes to zero. In Black patients, for example, PDMP prescriber check mandates appeared to reduce high‐dose opioid receipt by an estimated 8.4% from the four quarters pre‐policy, while they did not appear to reduce high‐dose receipt in White patients. Similarly, PDMP mandates appeared to reduce the rate of opioid receipt from >3 prescribers by an estimated 18.2% in Black patients and 11.1% in White patients, compared with the pre‐policy year; however, the confidence intervals surrounding these effects by group overlapped.
Our estimated race‐disaggregated effects of PDMP mandates are in line with evidence of disparate opioid prescribing to Black compared with White patients, 9 , 19 , 20 and provide the first evidence, to our knowledge, that a leading opioid prescribing policy adopted in response to the ongoing drug overdose crisis may have disproportionately affected Black patients—despite lower risk of nonmedical prescription opioid use and prescription opioid overdose nationally. 22 , 25 , 26 , 27 Disproportionate effects of PDMP mandates on Black patients could lead to a larger reduction in risk of prescription opioid use disorder and overdose in Black compared with White patients. If our findings are explained by prescriber racial bias, however, the adverse implications could be severe and wide‐ranging, including reduced trust in the health care system, reduced medical help‐seeking, and chronic stress due to experienced and anticipated racism, all of which can influence health outcomes. 28 , 31 , 32 , 33 , 34 , 35 , 46 , 47 Future research should examine the mechanisms underlying our findings and the impact on care beyond prescribed opioid receipt.
Moreover, the net benefits of reduced opioid dispensing due to supply‐side policies are unclear. Emerging evidence reveals frequent false positives and limited utility in PDMPs' identification of “doctor and pharmacy shopping”—that is, receipt of opioids from numerous prescribers or pharmacies. 48 In addition, growing evidence suggests that, by reducing prescription opioid access, PDMPs and other policies to curb opioid prescribing could be associated with increased illicit opioid use and overdose. 49 , 50 , 51 , 52 , 53 Our results raise the possibility of this process occurring disproportionately in Black patients. Patients who lose access to prescription opioids and who are at risk of transitioning to illegal opioid use due to opioid use disorder may benefit from transition to medication for opioid use disorder, such as methadone, buprenorphine, or naltrexone; yet the proportion of opioid analgesic prescribers who offer this treatment is small, and access is insufficient. 54 In fact, a recent study also using Optum health care claims data found that Black patients were half as likely as White patients to obtain buprenorphine or naltrexone following an emergency department visit for overdose. 55 Prescribers using PDMPs to inform opioid sparing should ensure that patients not prescribed opioids receive effective alternative forms of pain management, and that they are connected to evidence‐based treatment for opioid use disorder when indicated.
The divergence in results by policy type may relate to the difference in the principal target of pain management clinic laws and PDMP mandates. PDMP mandates target individual‐level prescribing decisions, which research has found to be associated with patient race over and above clinical factors. 9 , 19 , 20 In contrast, pain management clinic laws operate primarily on other factors (e.g., clinic ownership, state oversight, and billing procedures) 14 and may therefore provide less opportunity for discrimination in prescribing. The larger association between pain management clinic laws and high‐dose dispensing in White patients could be related to the racial makeup of pain management clinic patients, which to our knowledge has not been studied and which could differ from the racial makeup of the commercially insured population. While everyone receiving an opioid prescription will in theory be influenced by a PDMP mandate in their state, this is not the case for pain management clinic laws. As a result, we are unable to discern the effects of pain management clinic laws on pain management clinic patients specifically.
Our event‐study approach assumed no variation in policy effects by treatment cohort, that is, the year and quarter in which each state's law came into effect. Although studies have temporally clustered PDMP adoption based on features, 56 , 57 PDMP mandates were adopted continuously over time, 13 and we are not aware of evidence suggesting cohort effects of PDMP mandate queries or PMC laws, or of a likely mechanism for such effects. However, this is an area for future research. Relatedly, in some states, the laws of interest came into effect near the beginning or end of our analytic period (i.e., the years for which we possess data), resulting in an unbalanced panel; we were therefore unable to estimate coefficients for the full pre‐ or post‐period in these states. Anticipation effects, for instance, between policy enactment and effective date, were also possible. However, the event‐study plots provide little evidence for anticipation effects in the year prior to implementation, perhaps with the exception of the models estimating the effect of PDMP query mandates on receipt from >3 prescribers in both Black and White patients. In those models, anticipatory effects would result in conservative coefficient estimates. Given minimal, if any, apparent difference in this potential bias between the Black and White groups, we would not expect this to affect our conclusions.
Our study has additional limitations. We were also unable to capture opioid fills paid out‐of‐pocket, which is particularly common in pain management clinics. 58 The pain management clinic laws in three states—Kentucky, Tennessee, and Wisconsin—included prohibition of cash payments. 14 If patients in those states transitioned from paying cash to billing through insurance following adoption of these laws, it could artificially appear that the patient was newly transitioning to prescribed opioid use. This could bias results toward the null. It is not clear whether any such bias would vary by patient race, however. Second, a strength of our event‐study approach is the ability to assess the credibility of pre‐existing trends. For some outcomes, we observed evidence of pre‐policy trends in treatment states. In many cases, this may not be surprising—these laws were frequently adopted in response to worsening outcomes; still, it complicates the evaluation of causal relationships. We took pre‐policy trends into account in interpreting our findings, but further research is needed to replicate the results. Fourth, while we examined two of the state‐level prescribing policies most consistently shown to influence higher‐risk opioid prescribing, enrollees included in the database may have been subject to other policies and guidelines impacting opioid prescribing beyond those examined here; however, we would expect year and state fixed effects to largely account for this in the models. Finally, and crucially, we were unable to evaluate the appropriateness of reduced opioid dispensing associated with each policy. This should be investigated in future research, along with mechanisms underlying the observed disparities and solutions to address inequitable pain management.
5. CONCLUSION
Our results suggest that PDMP prescriber check mandates, but not pain management clinic laws, were associated with larger declines in opioid dispensing in Black patients compared with White patients. Despite persistently lower rates of nonmedical prescription opioid use and overdose, 22 , 26 , 27 Black patients may experience greater declines in opioid dispensing following adoption of PDMP mandates. That is, rather than influencing both groups equally or primarily the group with greater average risk of opioid‐related harms, PDMP mandates may have disproportionately reduced dispensing in a lower‐risk and already disadvantaged group. Moreover, emerging concerns about unintended consequences of abrupt opioid discontinuation, 59 as well as low access to medication for opioid use disorder (particularly in Black patients), 55 may counterbalance any theoretical benefits of larger reductions in opioid dispensing to Black patients. Future research should discern both the mechanisms underlying these disparities and their consequences for pain management and illicit opioid use. In addition, clinicians should be educated about the baseline disparities in the use of opioids to manage pain and ways in which PDMP mandates may exacerbate these divides.
ACKNOWLEDGMENTS
We would like to acknowledge David Powell and Matthew Myers for their contributions to this research.
APPENDIX A.
TABLE A1.
States adopting pain management clinic laws and PDMP must prescriber check mandates
| Year | Pain management clinic law | PDMP prescriber check mandate |
|---|---|---|
| 2007 | Louisiana | |
| 2008 | ||
| 2009 | Texas | |
| 2010 | Florida | |
| 2011 | Mississippi, Ohio, Tennessee | |
| 2012 | Kentucky, West Virginia | Kentucky, New Mexico |
| 2013 | Alabama, Georgia | New York, Tennessee, Vermont |
| 2014 | Massachusetts, West Virginia | |
| 2015 | Connecticut, Nevada, Ohio, Oklahoma, Pennsylvania, Virginia | |
| 2016 | Wisconsin | New Jersey |
| 2017 | Alaska, Arizona, Arkansas, Louisiana, Maine, New Hampshire, North Carolina, North Dakota, South Carolina, Wisconsin | |
| 2018 | Arizona | California, Colorado, Florida, Georgia, Hawaii, Illinois, Indiana, Iowa, Maryland, Michigan, Rhode Island |
Note: PDMP must access provision effective year is based on the objective standard. Data are from RH's primary legal research and the Prescription Drug Abuse Policy System (http://pdaps.org/datasets/).
TABLE A2.
Comparison of characteristics in patients with missing race information
| Missing | White | p a | Standardized difference b | Black c | p | Standardized difference d | |
|---|---|---|---|---|---|---|---|
| Distinct individuals | 1,441,606 | 7,096,974 | 1,167,446 | ||||
| Total quarterly observations | 3,142,330 | 19,494,397 | 3,288,094 | ||||
| Quarters with opioid fills | 2.2 | 2.7 | <0.001 | −0.281 | 2.8 | <0.001 | −0.265 |
| PMC law state (%) | 46.9 | 45.5 | <0.001 | 0.003 | 59.1 | <0.001 | −0.276 |
| PDMP mandate state (%) | 72.1 | 71.6 | <0.001 | 0.022 | 75.9 | <0.001 | −0.064 |
| Age (%) | |||||||
| 18–34 | 37.0 | 35.0 | <0.001 | 0.070 | 34.9 | <0.001 | 0.094 |
| 35–49 | 33.2 | 34.7 | <0.001 | −0.039 | 35.7 | <0.001 | −0.031 |
| 50–64 | 29.9 | 30.3 | <0.001 | −0.023 | 29.4 | <0.001 | −0.052 |
| Female (%) | 56.6 | 54.0 | <0.001 | 0.040 | 61.1 | <0.001 | −0.115 |
| Dosage | |||||||
| Mean (per day) | 45.2 | 45.2 | 0.87 | −0.001 | 40.5 | <0.001 | 0.109 |
| >90 MME (%) | 12.9 | 12.2 | <0.001 | 0.020 | 9.8 | <0.001 | 0.098 |
| Overlapping opioids (%) | 0.8 | 1.0 | <0.001 | −0.011 | 1.1 | <0.001 | −0.022 |
| Concurrent opioids/benzos (%) | 0.7 | 0.6 | <0.001 | 0.002 | 0.6 | <0.001 | 0.005 |
| >3 prescribers (%) | 8.8 | 8.9 | <0.001 | −0.004 | 7.6 | <0.001 | 0.045 |
| >3 pharmacies | 16.4 | 16.7 | <0.001 | −0.008 | 12.6 | <0.001 | 0.109 |
Note: PMC law state = state in which a pain management clinic law was in effect by 2018. PDMP mandate state = state in which a PDMP prescriber check mandate was in effect by 2018.
Abbreviation: MME, milligram morphine equivalents.
p‐value from Wald test, comparison of means in White patients versus those with missing racial and ethnic information.
Standardized difference between the mean in White patients versus those with missing racial and ethnic information.
p‐value from Wald test, comparison of means in Black patients versus those with missing racial and ethnic information.
Standardized difference between the mean in Black patients versus those with missing racial and ethnic information.
TABLE A3.
Mean outcomes by race at baseline in treatment states
| White | Black | |||
|---|---|---|---|---|
| Pain management clinic laws | ||||
| High‐dose receipt | 12.4 | (12.41–12.45) | 10.2 | (10.19–10.28) |
| Overlapping opioids | 8.8 | (8.75–8.79) | 7.6 | (7.60–7.69) |
| Concurrent opioids, benzodiazepines | 16.3 | (16.23–16.28) | 12.4 | (12.36–12.47) |
| >3 prescribers | 1.0 | (0.98–0.99) | 1.2 | (1.16–1.20) |
| >3 pharmacies | 0.7 | (0.66–0.67) | 0.7 | (0.67–0.70) |
| PDMP mandates | ||||
| High‐dose receipt | 11.2 | (11.17–11.22) | 8.3 | (8.24–8.35) |
| Overlapping opioids | 8.3 | (8.29–8.33) | 7.5 | (7.43–7.54) |
| Concurrent opioids, benzodiazepines | 16.2 | (16.15–16.20) | 13.4 | (13.35–13.48) |
| >3 prescribers | 0.9 | (0.88–0.89) | 1.0 | (1.00–1.04) |
| >3 pharmacies | 0.6 | (0.55–0.56) | 0.6 | (0.55–0.57) |
Note: Baseline refers to the four‐quarter period prior to the law becoming effective. Ninety‐five percent confidence intervals in parentheses.
TABLE A4.
Marginal effect estimates for each year post‐policy: (a) Pain management clinic laws, (b) PDMP query mandates
| (a) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Year | High dose | Overlapping opioids | Concurrent opioids/benzodiazepines | >3 prescribers | >3 pharmacies | |||||
| Est. | 95% CI | Est. | 95% CI | Est. | 95% CI | Est. | 95% CI | Est. | 95% CI | |
| 0 | −0.239 | (−0.322, −0.155) | −0.131 | (−0.205, −0.0565) | −0.342 | (−0.442, −0.242) | −0.0293 | (−0.0536, −0.00489) | −0.0198 | (−0.041, 0.00129) |
| 1 | −1.46 | (−1.57, −1.35) | −0.259 | (−0.357, −0.16) | −0.294 | (−0.423, −0.165) | −0.0564 | (−0.0843, −0.0284) | −0.0134 | (−0.038, 0.0112) |
| 2 | −2.73 | (−2.86, −2.6) | −0.464 | (−0.572, −0.355) | 0.0842 | (−0.0553, 0.224) | −0.0312 | (−0.0603, −0.00199) | −0.0525 | (−0.0791, −0.0258) |
| 3 | −2.95 | (−3.09, −2.8) | −0.609 | (−0.729, −0.489) | 0.0765 | (−0.0748, 0.228) | −0.0178 | (−0.049, 0.0134) | −0.114 | (−0.143, −0.085) |
| 4 | −4.07 | (−4.22, −3.93) | −0.787 | (−0.912, −0.663) | −0.441 | (−0.595, −0.287) | −0.0863 | (−0.116, −0.0569) | −0.144 | (−0.171, −0.116) |
| (b) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Year | High dose | Overlapping opioids | Concurrent opioids/benzodiazepines | >3 prescribers | >3 pharmacies | |||||
| Est. | 95% CI | Est. | 95% CI | Est. | 95% CI | Est. | 95% CI | Est. | 95% CI | |
| 0 | −0.0262 | (−0.113, 0.061) | −0.322 | (−0.39, −0.255) | −0.311 | (−0.395, −0.227) | −0.0808 | (−0.105, −0.0567) | −0.054 | (−0.0811, −0.0269) |
| 1 | 0.115 | (−0.0233, 0.253) | −0.558 | (−0.668, −0.447) | −0.53 | (−0.663, −0.398) | −0.105 | (−0.136, −0.0735) | −0.111 | (−0.148, −0.0735) |
| 2 | 0.57 | (0.382, 0.759) | −1.07 | (−1.23, −0.915) | −0.813 | (−1, −0.627) | −0.139 | (−0.181, −0.0971) | −0.136 | (−0.187, −0.0858) |
| 3 | 0.295 | (0.0624, 0.527) | −1.62 | (−1.81, −1.43) | −1.22 | (−1.44, −0.996) | −0.142 | (−0.192, −0.0929) | −0.232 | (−0.301, −0.163) |
| 4 | 1.55 | (1.24, 1.85) | −2.03 | (−2.3, −1.77) | −2.09 | (−2.39, −1.78) | −0.15 | (−0.21, −0.0891) | −0.194 | (−0.277, −0.111) |
Note: Marginal effect estimates are percentage points relative to the year prior to effective date and states that never enacted the policy of interest. Year 0 refers to the first four‐quarter period during which the policy was in effect. These marginal effects are plotted in Figures 1, 2, 3.
Abbreviation: Est., estimate.
Townsend TN, Bohnert ASB, Lagisetty P, Haffajee RL. Did prescribing laws disproportionately affect opioid dispensing to Black patients? Health Serv Res. 2022;57(3):482-496. doi: 10.1111/1475-6773.13968
This article was conceived and drafted when Dr. Haffajee was employed at the RAND Corporation, and the findings and views in this article do not necessarily reflect the official views or policy of her current employer, the U.S. Department of Health and Human Services, or the U.S. Government.
Funding information National Institute on Drug Abuse, Grant/Award Number: 5T32DA007233‐37
REFERENCES
- 1. Ciccarone D. The triple wave epidemic: supply and demand drivers of the US opioid overdose crisis. Int J Drug Policy. 2019;71:183‐188. doi: 10.1016/j.drugpo.2019.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. JAMA. 2016;315(15):1624‐1645. doi: 10.1001/jama.2016.1464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mauri AI, Townsend TN, Haffajee RL. The association of state opioid misuse prevention policies with patient‐ and provider‐related outcomes: a scoping review. Milbank Q. 2019;98(1):57‐105. doi: 10.1111/1468-0009.12436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hall AJ. Patterns of abuse among unintentional pharmaceutical overdose fatalities. JAMA. 2008;300(22):2613‐2620. doi: 10.1001/jama.2008.802 [DOI] [PubMed] [Google Scholar]
- 5. Sun EC, Dixit A, Humphreys K, Darnall BD, Baker LC, Mackey S. Association between concurrent use of prescription opioids and benzodiazepines and overdose: retrospective analysis. BMJ. Published online March 14, 2017. j760. doi: 10.1136/bmj.j760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jena AB, Goldman D, Weaver L, Karaca‐Mandic P. Opioid prescribing by multiple providers in Medicare: retrospective observational study of insurance claims. BMJ. 2014;348:g1393. doi: 10.1136/bmj.g1393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kaiser Family Foundation . Opioid Overdose Deaths by Race/Ethnicity. Opioid Overdose Deaths by Race/Ethnicity (1999‐2018). 2020. https://www.kff.org/other/state-indicator/opioid-overdose-deaths-by-raceethnicity
- 8. Alexander MJ, Kiang MV, Barbieri M. Trends in Black and White opioid mortality in the United States, 1979–2015. Epidemiology. 2018;29(5):707‐715. doi: 10.1097/EDE.0000000000000858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Meghani SH, Byun E, Gallagher RM. Time to take stock: a meta‐analysis and systematic review of analgesic treatment disparities for pain in the United States. Pain Med. 2012;13:150‐174. [DOI] [PubMed] [Google Scholar]
- 10. Harrison JM, Lagisetty P, Sites BD, Guo C, Davis MA. Trends in prescription pain medication use by race/ethnicity among US adults with noncancer pain, 2000–2015. Am J Public Health. 2018;108(6):788‐790. doi: 10.2105/AJPH.2018.304349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pearson SA, Soumerai S, Mah C, et al. Racial disparities in access after regulatory surveillance of benzodiazepines. Arch Intern Med. 2006;166(5):572‐579. doi: 10.1001/archinte.166.5.572 [DOI] [PubMed] [Google Scholar]
- 12. Ross‐Degnan D, Simoni‐Wastila L, Brown JS, et al. A controlled study of the effects of state surveillance on indicators of problematic and non‐problematic benzodiazepine use in a Medicaid population. Int J Psychiatry Med. 2004;34(2):103‐123. doi: 10.2190/8FR4-QYY1-7MYG-2AGJ [DOI] [PubMed] [Google Scholar]
- 13. Haffajee RL. Prescription drug monitoring programs—friend or folly in addressing the opioid‐overdose crisis? N Engl J Med. 2019;381(8):699‐701. doi: 10.1056/NEJMp1904714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Temple University Beasley School of Law . Prescription Drug Abuse Policy System (PDAPS); 2020. http://pdaps.org
- 15. Centers for Disease and Prevention Office for Tribal, Local, and Territorial Support . Menu of Pain Management Clinic Regulation; 2012:7. https://www.cdc.gov/phlp/docs/menu-pmcr.pdf
- 16. Frizzell LC, Vuolo M, Kelly BC. State pain management clinic policies and county opioid prescribing: a fixed effects analysis. Drug Alcohol Depend. 2020;216:108239. doi: 10.1016/j.drugalcdep.2020.108239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liang D, Shi Y. The association between pain clinic laws and prescription opioid exposures: new evidence from multi‐state comparisons. Drug Alcohol Depend. 2020;206:107754. doi: 10.1016/j.drugalcdep.2019.107754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Singhal A, Tien YY, Hsia RY. Racial‐ethnic disparities in opioid prescriptions at emergency department visits for conditions commonly associated with prescription drug abuse. PLoS One. 2016;11(8):e0159224. doi: 10.1371/journal.pone.0159224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gaither JR, Gordon K, Crystal S, et al. Racial disparities in discontinuation of long‐term opioid therapy following illicit drug use among black and white patients. Drug Alcohol Depend. 2018;192:371‐376. doi: 10.1016/j.drugalcdep.2018.05.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Buonora M, Perez HR, Heo M, Cunningham CO, Starrels JL. Race and gender are associated with opioid dose reduction among patients on chronic opioid therapy. Pain Med. Published online July 18, 2018. doi: 10.1093/pm/pny137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Becker WC, Starrels JL, Heo M, Li X, Weiner MG, Turner BJ. Racial differences in primary care opioid risk reduction strategies. Ann Fam Med. 2011;9(3):219‐225. doi: 10.1370/afm.1242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wilson N, Kariisa M, Seth P, Iv HS, Davis NL. Drug and opioid‐involved overdose deaths – United States, 2017–2018. MMWR Morb Mortal Wkly Rep. 2020;69(11):8‐297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bohnert ASB, Valenstein M, Bair MJ, et al. Association between opioid prescribing patterns and opioid overdose‐related deaths. JAMA. 2011;305(13):7. [DOI] [PubMed] [Google Scholar]
- 24. Paulozzi LJ. Prescription drug overdoses: a review. J Safety Res. 2012;43(4):283‐289. doi: 10.1016/j.jsr.2012.08.009 [DOI] [PubMed] [Google Scholar]
- 25. Centers for Disease Control, and Prevention . 2019 Annual Surveillance Report of Drug‐Related Risks and Outcomes—United States Surveillance Special Report. Centers for Disease Control and Prevention, U.S. Department of Health and Human Services; 2019:128. Accessed December 11, 2021. www.cdc.gov/drugoverdose/pdf/pubs/2019-cdc-drug-surveillancereport.pdf
- 26. Han B, Compton WM, Blanco C, Crane E, Lee J, Jones CM. Prescription opioid use, misuse, and use disorders in U.S. adults: 2015 National Survey on Drug Use and Health. Ann Intern Med. Published online 2017. 10. [DOI] [PubMed] [Google Scholar]
- 27. Schuler MS, Schell TL, Wong EC. Racial/ethnic differences in prescription opioid misuse and heroin use among a national sample, 1999‐2018. Drug Alcohol Depend. Published online February 2021. 108588. doi: 10.1016/j.drugalcdep.2021.108588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shavers VL, Bakos A, Sheppard VB. Race, ethnicity, and pain among the U.S. adult population. J Health Care Poor Underserved. 2010;21(1):177‐220. doi: 10.1353/hpu.0.0255 [DOI] [PubMed] [Google Scholar]
- 29. Hoffman KM, Trawalter S, Axt JR, Oliver MN. Racial bias in pain assessment and treatment recommendations, and false beliefs about biological differences between blacks and whites. Proc Natl Acad Sci U S A. 2016;113(16):4296‐4301. doi: 10.1073/pnas.1516047113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Frakt A, Monkovic T. A ‘rare case where racial biases’ protected African‐Americans. The New York Times. Published December 2, 2019. https://www.nytimes.com/2019/11/25/upshot/opioid-epidemic-blacks.html
- 31. Anderson KO, Green CR, Payne R. Racial and ethnic disparities in pain: causes and consequences of unequal care. J Pain. 2009;10(12):1187‐1204. doi: 10.1016/j.jpain.2009.10.002 [DOI] [PubMed] [Google Scholar]
- 32. Benkert R, Peters RM, Clark R, Keves‐Foster K. Effects of perceived racism, cultural mistrust and trust in providers on satisfaction with care. J Natl Med Assoc. 2006;98(9):1532‐1540. [PMC free article] [PubMed] [Google Scholar]
- 33. Williams DR, Mohammed SA. Discrimination and racial disparities in health: evidence and needed research. J Behav Med. 2009;32(1):20‐47. doi: 10.1007/s10865-008-9185-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cintron A, Morrison RS. Pain and ethnicity in the United States: a systematic review. J Palliat Med. 2006;9(6):1454‐1473. doi: 10.1089/jpm.2006.9.1454 [DOI] [PubMed] [Google Scholar]
- 35. Boulware LE, Cooper LA, Ratner LE, LaVeist TA, Powe NR. Race and trust in the health care system. Public Health Rep. 2003;118:358‐365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. University of Michigan Institute for Healthcare Policy and Innovation . OptumInsight Data Seminar. Presented at: 2016.
- 37. Jeffery MM, Hooten WM, Henk HJ, et al. Trends in opioid use in commercially insured and Medicare Advantage populations in 2007‐16: retrospective cohort study. BMJ. Published online August 1, 2018. k2833. doi: 10.1136/bmj.k2833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rose AJ, Bernson D, Chui KKH, et al. Potentially inappropriate opioid prescribing, overdose, and mortality in Massachusetts, 2011–2015. J Gen Intern Med. 2018;33(9):1512‐1519. doi: 10.1007/s11606-018-4532-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Berchick ER, Hood E, Barnett JC. Health Insurance Coverage in the United States: 2017. U.S. Census Bureau; 2018:44. Accessed January 10, 2020. https://www.census.gov/content/dam/Census/library/publications/2018/demo/p60-264.pdf
- 40. U.S. Census Bureau . American Community Survey Data. U.S. Census Bureau. Published October 11, 2018. Accessed July 12, 2019. https://www.census.gov/programs-surveys/acs/data.html
- 41. Goodman‐Bacon A. Difference‐in‐Differences with Variation in Treatment Timing. National Bureau of Economic Research; 2018:w25018. doi: 10.3386/w25018 [DOI] [Google Scholar]
- 42. Goodman‐Bacon A, Marcus J. Using difference‐in‐differences to identify causal effects of COVID‐19 policies. SSRN J. Published online 2020. doi: 10.2139/ssrn.3603970 [DOI] [Google Scholar]
- 43. Marcus M, Sant'Anna PHC. The role of parallel trends in event study settings: an application to environmental economics. J Assoc Environ Resour Econ. 2021;8(2):235‐275. doi: 10.1086/711509 [DOI] [Google Scholar]
- 44. University of Michigan Research Ethics and Compliance . Does my project require IRB review? Accessed February 10, 2020. https://research-compliance.umich.edu/faqs/does-my-project-require-irb-review
- 45. NYU Langone Health . Getting Started with the Institutional Review Board Submission Process. Published online 2020. Accessed November 29, 2020. https://med.nyu.edu/research/office-science-research/clinical-research/resources-researchers-study-teams/institutional-review-board-operations/getting-started-the-irb-submission-process#determine-if-your-project-is-human-subjects-research
- 46. Himmelstein MS, Young DM, Sanchez DT, Jackson JS. Vigilance in the discrimination‐stress model for Black Americans. Psychol Health. 2015;30(3):253‐267. doi: 10.1080/08870446.2014.966104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hicken MT, Lee H, Ailshire J, Burgard SA, Williams DR. “Every shut eye, ain't sleep”: the role of racism‐related vigilance in racial/ethnic disparities in sleep difficulty. Race Soc Probl. Published online 2014. 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Delcher C, Harris DR, Park C, Strickler G, Talbert J, Freeman PR. “Doctor and pharmacy shopping”: a fading signal for prescription opioid use monitoring? Drug Alcohol Depend. Published online February 2021. 108618. doi: 10.1016/j.drugalcdep.2021.108618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Martins SS, Ponicki W, Smith N, et al. Prescription drug monitoring programs operational characteristics and fatal heroin poisoning. Int J Drug Policy. 2019;74:174‐180. doi: 10.1016/j.drugpo.2019.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Alpert A, Powell D, Pacula RL. Supply‐Side Drug Policy in the Presence of Substitutes: Evidence from the Introduction of Abuse‐Deterrent Opiods:60. [DOI] [PMC free article] [PubMed]
- 51. Evans WN, Lieber EMJ, Power P. How the reformulation of OxyContin ignited the heroin epidemic. Rev Econ Stat. 2019;101(1):1‐15. doi: 10.1162/rest_a_00755 [DOI] [Google Scholar]
- 52. Ali MM, Dowd WN, Classen T, Mutter R, Novak SP. Prescription drug monitoring programs, nonmedical use of prescription drugs, and heroin use: evidence from the National Survey of Drug Use and Health. Addict Behav. 2017;69:65‐77. doi: 10.1016/j.addbeh.2017.01.011 [DOI] [PubMed] [Google Scholar]
- 53. Beheshti D. Adverse health effects of abuse‐deterrent opioids: evidence from the reformulation of OxyContin. Health Econ. 2019;28(12):1449‐1461. doi: 10.1002/hec.3944 [DOI] [PubMed] [Google Scholar]
- 54. Jones CM, Campopiano M, Baldwin G, McCance‐Katz E. National and state treatment need and capacity for opioid agonist medication‐assisted treatment. Am J Public Health. 2015;105(8):e55‐e63. doi: 10.2105/AJPH.2015.302664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kilaru AS, Xiong A, Lowenstein M, et al. Incidence of treatment for opioid use disorder following nonfatal overdose in commercially insured patients. JAMA Netw Open. 2020;3(5):e205852. doi: 10.1001/jamanetworkopen.2020.5852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Haffajee RL, Mello MM, Zhang F, Zaslavsky AM, Larochelle MR, Wharam JF. Four states with robust prescription drug monitoring programs reduced opioid dosages. Health Aff. 2018;37(6):964‐974. doi: 10.1377/hlthaff.2017.1321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Smith N, Martins SS, Kim J, et al. A typology of prescription drug monitoring programs: a latent transition analysis of the evolution of programs from 1999 to 2016: PDMP typologies. Addiction. 2019;114(2):248‐258. doi: 10.1111/add.14440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Rigg J. Moving lives: migration and livelihoods in the Lao PDR. Popul Space Place. 2007;13(3):163‐178. doi: 10.1002/psp.438 [DOI] [Google Scholar]
- 59. Dowell D, Haegerich T, Chou R. No shortcuts to safer opioid prescribing. N Engl J Med. 2019;380(24):2285‐2287. doi: 10.1056/NEJMp1904190 [DOI] [PMC free article] [PubMed] [Google Scholar]


