Abstract
Objective
To describe the characteristics of high‐frequency hospital users (four or more hospitalizations in a year) and the consequences of including or excluding their data from a readmission‐based measure.
Data sources
2015 and 2016 Massachusetts Medicaid data.
Study design
We compare demographics, morbidity burden, and social risk factors for high‐ and low‐frequency hospital users, and membership in 17 accountable care organizations. We evaluate how excluding hospitalizations of high‐frequency users from a 30‐day readmission measure (with or without risk adjustment) changes its rate and variability and affects performance rankings of accountable care organizations. The outcome is readmission within 30 days; each live discharge from a hospital contributes one observation.
Data collection/extraction methods
We studied 74 706 hospitalizations of 42 794 MassHealth members, 18–64 years old, managed‐care‐eligible, and ever hospitalized in 2016.
Principal findings
Among adult managed‐care‐eligible MassHealth members with at least one acute hospitalization, 8.7% were high‐frequency hospital users; they contributed 30.2% of hospitalizations and 69.4% of readmissions. High‐frequency users were more often male (77.1% vs. 50.0%; P < 0.001) and sicker (mean medical morbidity score was 3.3 vs. 1.9; P < 0.001) than others. They also had significant social risks: 33.1% with housing problems, 44.1% disabled, 83.2% with serious mental illness, and 77.1% with substance abuse disorder (vs. 22.0%, 27.3%, 60.2%, and 50.0%, respectively, for other hospital users [all P values <0.001]). Fully 50.7% of hospitalizations for high‐frequency users led to 30‐day readmissions (vs. 9.7%), contributing 72.0% of the variance in 30‐day readmission, and substantially affecting judgments about the relative performance of accountable care organizations.
Conclusions
A small group of high‐frequency hospital users have a disproportionate effect on 30‐day readmission rates. This negatively affects some Medicaid ACOs, and more broadly is likely to adversely affect safety net hospitals. How these metrics are used should be reconsidered in this context.
Keywords: accountable care organizations, health care, hospitalization, Medicaid, patient readmission, quality indicators
1.
What is known on this topic?
Hospital readmission rates have been used and criticized as a quality measure and have been extended to accountable care organizations (ACOs).
We explore the new concern that an ACO whose members require frequent hospitalization is likely to be harmed by a quality measure driven by high‐frequency users, potentially exacerbating existing health disparities.
What this study adds?
The prevalence of medically and socially complex high‐frequency hospital users in an ACO is the principal driver of its readmission rate.
An “ALL patients” readmission quality measure is highly volatile compared to readmission for only low‐frequency hospital users; including data from high‐frequency users substantially affects judgments about ACOs' performance on this measure.
A good measure should limit the effect of patient‐level variability so that health plan‐level variability may be more plausibly interpreted as a measure of health plan quality.
2. INTRODUCTION
Thirty‐day hospital readmission measures were originally introduced to discourage hospitals from premature discharge and incentivize attentiveness to post‐acute care. 1 As such, the Centers for Medicare and Medicaid Services (CMS) has publicly reported readmission rates for acute heart failure, pneumonia, and myocardial infarction for years, and began penalizing hospitals for excessive unplanned readmissions in 2013. 2 , 3 , 4 While improved hospital care can reduce readmissions, penalties based on readmission measures have been criticized for their arguably unfair, substantial, and negative impact on safety‐net hospitals, whose patients typically have less supportive post‐acute care options. 5 , 6 , 7
All‐cause unplanned readmission as a quality measure has now been extended to accountable care organizations (ACOs). 8 We explore here the new concern that an ACO whose members require frequent hospitalization as a result of social complexity affecting health is likely to be harmed by a quality measure driven by high‐frequency users, potentially exacerbating existing health disparities.
MassHealth, Massachusetts' Medicaid and Children's Health Insurance Program (CHIP), currently includes an ACO‐level 30‐day readmission measure developed by the National Committee for Quality Assurance (NCQA) in its quality measure slate. This paper does not critique this HEDIS measure, which is risk adjusted for various clinical factors describing each discharge, including discharge condition, presence of surgeries, and comorbidities. Nor do we propose an alternate 30‐day readmission measure. Rather, we examine differences between high‐frequency users, defined as those with four or more live discharges from a hospital in a year, 9 and other (less‐frequent) hospital users in MassHealth as to: demographics, medical, and social risk factors, and their contributions to plan‐level readmission rates. We also examine how excluding hospitalizations of high‐frequency users from a 30‐day readmission measure affects the overall readmission rate and its variability, and its effect on ACO performance rankings.
3. METHODS
3.1. Study data
We used 2016 claims and enrollment data from MassHealth, whose 1.2 million MassHealth members became eligible to choose their health care in March 2018 from among 17 newly organized ACOs, two managed care organizations, or MassHealth's primary care clinician (PCC) plan, for which the State reimburses providers directly. 10 We used the same algorithm that MassHealth used to “default assign” enrollees to 17 virtual ACOs, based on the organizations with which their primary care clinicians later affiliated. We used 2015 data to ensure near‐continuous Medicaid enrollment and to measure morbidity in the year preceding each hospitalization. 11
3.2. Study sample
We used NCQA criteria to identify hospitalizations that contribute to the denominator and numerator for the unadjusted 30‐day readmission measure. 12 We first identified all hospitalizations for members with at least one inpatient stay between January 1 and December 1, 2016 and who had been continuously enrolled in MassHealth for 365 days (with at most one 45‐day gap prior to the discharge date) through 30 days following discharge. 11 , 12 We excluded hospitalizations for females with a principal diagnosis of pregnancy or for a condition originating in the perinatal period as well as hospitalizations followed by planned readmission within 30 days (e.g., transplantation, chemotherapy, rehabilitation). 11 , 12 Finally, we excluded hospitalizations for members younger than 18 or older than 64. Individual members could contribute multiple hospital stays to analyses; each hospital discharge for an eligible member contributed one observation. Our final study sample included 74 706 unique hospitalizations among 42 794 MassHealth members (Figure 1). These hospitalizations (hereafter referred to as index hospitalizations) comprise the denominator for calculating all‐cause 30‐day readmission rates.
FIGURE 1.
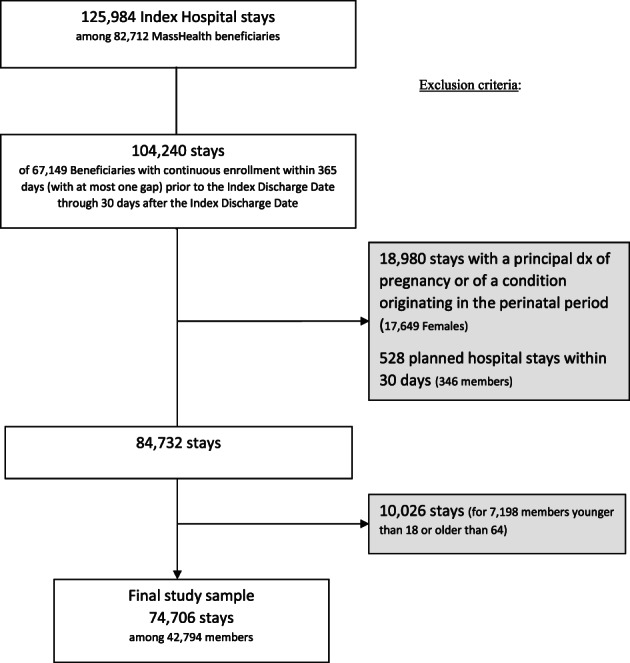
Study population flow chart, hospital stays among MassHealth managed care eligible enrollees
3.3. Outcome measures
Our two key outcomes were (1) frequent use of hospitals (four or more live hospital discharges) for individuals and (2) non‐pregnancy‐related 30‐day hospital readmissions for index hospitalization discharges.
3.4. Covariates
We considered age, gender, and morbidity burden using the CMS's Hierarchical Condition Category (CMS‐HCC) model in the 365 days prior to each index hospitalization. The CMS‐HCC model is calculated from age, sex, and diagnoses organized into condition categories (CCs). 13 While originally developed to predict health care costs, the CMS‐HCC model is widely used as a measure of total morbidity burden. Sicker individuals receive higher scores. 14 , 15 , 16 , 17 , 18 We also considered social determinant of health (SDH) factors including behavioral health issues (i.e., serious mental illness and substance use disorder), disability, housing problems, and a neighborhood‐level stress score. We measured SDH factors during 2016 when available. For the 1.8% of subjects missing 2016 SDH data, we used 2015 information. We used indicators for serious mental illness and substance use disorders based on CCs created with the diagnosis‐based Diagnostic Cost Group Hierarchical Condition Category software (DxCG‐HCC) 19 (Described in Appendix S1). MassHealth routinely adjusts payments to Medicaid managed care organizations using DxCG‐HCC models that organize diagnoses into CCs that are similar to, but more detailed than, those in the CMS‐HCC model. Our marker for disability indicates either Medicaid entitlement due to disability or to having qualified for special services from the state's Departments of Mental Health or Developmental Disabilities. Our “Housing Problem” marker indicates either unstable housing (≥3 addresses within a year) or through the presence of ICD10 code Z59.0 (homelessness) on at least one 2016 claim or encounter record. Neighborhood‐level socioeconomic deprivation was summarized by a neighborhood stress score, calculated at the U.S. Census‐block‐group level from seven variables available through the American Community Survey. 20
3.5. Statistical analyses
First, we used Chi‐squared and Student t tests to assess associations between each covariate and frequent hospitalization use. Second, we compared unadjusted and risk adjusted hospital readmission rates among MassHealth ACOs and their relative performance ranking (1) when all hospitalizations were included and (2) when excluding the hospitalizations of high‐frequency hospital users. Third, we quantified the effect of high‐frequency users' data on the variance of the 30‐day readmission measure.
Because each ACO serves a unique set of patients and patients can experience multiple hospitalizations, we used hierarchical generalized linear models to analyze this clustering, as the likelihood that a hospitalization is followed by a readmission may be influenced by the unmodeled patient or ACO characteristics. Indeed, the entire justification for a readmission quality measure relies on the belief that ACOs can exert such effects. We estimated the intraclass correlation coefficient (ICC), which is the ratio of the between‐cluster variance that is accounted for by clustering to the total variance in 30‐day readmission; we then attributed the variance in 30‐day readmissions to three levels: ACO, patient, and hospitalization. We began by fitting logistic regressions with random effects only (unadjusted) to estimate the total variance at each of the ACO‐ and patient‐levels—one including the full population and another excluding all hospitalizations from high‐frequency hospital users. Then, to examine the extent to which variation at the ACO or patient level can be explained by patient characteristics, we re‐fit each model, including fixed effects for 12 age/sex categories, morbidity, serious mental illness, substance use disorder, disability, the neighborhood stress score, and housing problems 21 (see Appendix S2). Analyses used the SAS package version 9.4 (SAS Institute, Cary, NC) and Stata software version 12 (Stata Corporation, College Station, TX).
4. RESULTS
Among the 42 794 unique patients ever‐hospitalized in 2016, only 3728 (8.7%) were high‐frequency hospital users (Table 1). These patients were more often male (77.1% vs. 50.0%; P < 0.001) and were sicker (mean CMS‐HCC morbidity score was 3.3 [standard deviation = 2.2] vs. 1.9 [standard deviation = 1.5]; P < 0.001) than others. They also had significant social risks: 33.1% with housing problems, 44.1% disabled, 83.2% with serious mental illness, and 77.1% with substance abuse disorder (vs. 22.0%, 27.3%, 60.2%, and 50.0% for others, respectively [all P values <0.001]).
TABLE 1.
Characteristics of MassHealth enrollees with at least one acute inpatient stay in 2016
| 4+ Hospitalizations | 1–3 Hospitalizations | ||||
|---|---|---|---|---|---|
| Hospital user type | # | Column % | # | Column % | P‐value a |
| ALL | 3728 | 100.0% | 39 066 | 100.0% | |
| Male | 2873 | 77.1% | 19 533 | 50.0% | <0.001 |
| Age group | |||||
| 18–34 | 1041 | 27.9% | 11 815 | 30.2% | <0.001 |
| 35–54 | 1829 | 49.1% | 17 427 | 44.6% | |
| 55–64 | 858 | 23.0% | 9824 | 25.2% | |
| Race/Ethnicity | |||||
| White/Non‐Hispanic | 1817 | 48.7% | 18 920 | 48.4% | <0.001 |
| Black/Non‐Hispanic | 337 | 9.0% | 3469 | 8.9% | |
| Hispanic | 219 | 5.9% | 2988 | 7.7% | |
| Other/unknown | 1355 | 36.4% | 13 689 | 35.0% | |
| Mean HCC morbidity score (standard deviation) | 3.3 | (2.2) | 1.9 | (1.5) | <0.001 |
| Housing problems b | 1233 | 33.1% | 8614 | 22.0% | <0.001 |
| Disabled c | 1643 | 44.1% | 10 682 | 27.3% | <0.001 |
| Serious mental illness | 3103 | 83.2% | 23 513 | 60.2% | <0.001 |
| Substance use disorder | 2873 | 77.1% | 19 533 | 50.0% | <0.001 |
| Any LTSS use | 1769 | 47.5% | 9265 | 23.7% | <0.001 |
| Mean neighborhood stress score (standard deviation) d | 0.1 | (2.2) | 0.0 | (2.1) | 0.006 |
Note: Authors' calculations using data on 74 706 hospitalizations between January 1 and December 1, 2016 of 42 794 MassHealth managed care eligible adult members.
Abbreviations: HCC, morbidity burden measured using the CMS‐HCC diagnosis‐based Hierarchical Condition Category score; LTSS, long‐term services and supports.
Chi‐square or Student t test.
Housing problem is defined as 3+ distinct addresses or homelessness (Z59.0) on claims or encounter records during 2016.
Disability status is Medicaid entitlement for disability or qualification for specialized services for mental health or developmental disabilities in 2016.
Neighborhood stress score summarizes seven neighborhood‐level indicators of economic stress using U.S. Census block group data.
High‐frequency hospital users contributed 30.2% of all hospitalizations (Table 2). The overall readmission rate was 22.1%: 50.7% for the admissions of high‐frequency users and 9.7% for those of low‐frequency users.
TABLE 2.
Hospitalizations and readmissions: By hospital use frequency
| ALL | High‐frequency users | Low‐frequency users | |
|---|---|---|---|
| N (Rate) | % (Rate) | % (Rate) | |
| Unique patients | 42 794 | 8.7% | 91.3% |
| Hospitalizations | 74 706 | 30.2% | 69.8% |
| Readmissions | 16 485 | 69.4% | 30.6% |
| Readmission rate | (22.1%) | (50.7%) | (9.7%) |
Note: Authors' calculations using data on 74 706 hospitalizations between January 1 and December 1, 2016 of 42 794 MassHealth managed care eligible adult members.
Table 3 shows that the relative readmission performance of ACOs looked quite different, depending on whether hospitalizations of high‐frequency hospital users were included and/or readmission rates were risk adjusted. For instance, excluding high‐frequency users without risk adjustment worsened the performance ranking of ACO A from best (lowest rate) among 17 to 7th best, while it improved the rank of ACO F from sixth to second best. Excluding hospitalizations of high‐frequency users with risk adjustment resulted in smaller differences in performance ranking compared to no adjustment for most ACOs. However, even with risk adjustment, removing high‐frequency users made a big difference for some ACOs. For instance, excluding hospitalizations of high‐frequency users with risk adjustment worsened the performance ranking of ACO B from 6th to 14th best, while it improved the rank of ACO M from 13th to 7th best.
TABLE 3.
Performance on hospital readmission and relative ranking of accountable care organizations with and without data from high‐frequency users
| Readmission rate (%) | Performance ranking | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Unadjusted | Adjusted b | Unadjusted | Adjusted | ||||||
| ACO a | High‐frequency users (%) | All users | No high‐frequency users | All users | No high‐frequency users | All users | No high‐frequency users | All users | No high‐frequency users |
| All ACOs | 8.7 | 22.1 | 9.7 | 22.1 | 9.7 | — | — | — | — |
| ACO A | 4.9 | 16.7 | 9.1 | 19.4 | 9.2 | 1 | 7 | 2 | 4 |
| ACO B | 5.9 | 18.3 | 7.5 | 20.9 | 9.8 | 2 | 3 | 6 | 14 |
| ACO C | 7.0 | 18.5 | 6.0 | 19.4 | 8.7 | 3 | 1 | 3 | 1 |
| ACO D | 7.8 | 18.7 | 8.3 | 20.3 | 9.4 | 4 | 4 | 4 | 5 |
| ACO E | 8.0 | 18.8 | 8.8 | 20.9 | 9.6 | 5 | 6 | 5 | 8 |
| ACO F | 6.8 | 19.4 | 7.2 | 21.5 | 9.4 | 6 | 2 | 9 | 6 |
| ACO G | 8.0 | 19.7 | 9.2 | 18.3 | 8.8 | 7 | 9 | 1 | 2 |
| ACO H | 8.4 | 21.1 | 9.1 | 21.6 | 9.6 | 8 | 8 | 10 | 11 |
| ACO I | 8.4 | 21.1 | 10.4 | 21.7 | 9.6 | 9 | 12 | 12 | 10 |
| ACO J | 7.9 | 21.6 | 11.0 | 23.7 | 10.2 | 10 | 15 | 16 | 17 |
| ACO K | 8.5 | 22.0 | 9.6 | 21.7 | 9.6 | 11 | 10 | 11 | 12 |
| ACO L | 7.9 | 22.2 | 10.9 | 21.2 | 9.6 | 12 | 14 | 8 | 9 |
| ACO M | 8.2 | 22.3 | 8.7 | 22.2 | 9.4 | 13 | 5 | 13 | 7 |
| ACO N | 9.8 | 23.5 | 11.1 | 22.6 | 9.9 | 14 | 16 | 14 | 15 |
| ACO O | 10.0 | 24.3 | 10.1 | 22.8 | 9.7 | 15 | 11 | 15 | 13 |
| ACO P | 10.6 | 24.6 | 10.5 | 23.9 | 10.0 | 16 | 13 | 17 | 16 |
| ACO Q | 9.2 | 24.8 | 11.4 | 21.0 | 9.1 | 17 | 17 | 7 | 3 |
Note: Authors' calculations using data on 74 706 hospitalizations between January 1 and December 1, 2016 of 42 794 MassHealth managed care eligible adult members.
ACO, accountable care organization. These data are pre‐ACO‐program launch in 2018. For this analysis we attributed members to ACOs by applying MassHealth's algorithm, based on historic member claims and encounters, for default assignment to ACOs. We assigned ACO letters based on unadjusted readmission rates that included all hospitalizations (from A, the best, to Q, the worst).
Adjusted for age, sex, morbidity, serious mental illness, substance use disorder, disability, neighborhood‐level stressors, and housing problems.
Table 4 shows the composition of variance in 30‐day readmission attributed to clustering at the patient‐ and ACO‐levels: 34.99% of the total variance in 30‐day readmission was between patients; multiple hospitalizations within one patient had more similar outcomes than for random hospitalizations. However, this estimate dropped to only 9.84% after excluding high‐frequency users; that is, data from the high‐frequency users contributed most (71.88%) of the variance in 30‐day readmission due to nesting of hospitalizations within patients ([34.99–9.84]/34.99). Furthermore, the proportion of variance explained by differences among ACOs is very small compared to that attributed to the patient level (ICC of 0.28% or less, either before or after excluding high‐frequency users). In either scenario, the ICC was always lower in adjusted models than in unadjusted models. That is, risk adjusting 30‐day readmission by considering patient characteristics decreases variability in this measure from 34.99% to 26.79%, making it more stable. However, even the detailed risk adjustment that we used removed only a small part of the patient‐level variation in the “ALL patients” readmission measure.
TABLE 4.
Variance decomposition statistics for 30‐day readmission
| Patient‐level ICC | ACO a ‐level ICC | |
|---|---|---|
| Unadjusted | ||
| All enrollees | 34.99% (33.73%–36.26%) | 0.24% (0.09%–0.68%) |
| Low‐frequency hospital users | 9.84% (7.46%–12.89%) | 0.28% (0.77%–0.99%) |
| High‐frequency hospital users | 12.94% (11.64%–14.36%) | 0.00% (0.00%–0.00%) |
| Adjusted | ||
| All enrollees | 26.79% (25.55%–28.05%) | 0.10% (0.02%–0.56%) |
| Low‐frequency hospital users | 6.05% (3.80%–9.50%) | 0.22% (0.05%–0.97%) |
| High‐frequency hospital users | 12.63% (11.32%–14.07%) | 0.00% (0.00%–0.00%) |
Source: Authors' calculations using data on 74 706 hospitalizations between January 1 and December 1, 2016 of 42 794 MassHealth managed care eligible adult members. Estimates are based on hierarchical generalized linear models with a logit link and a binomial distribution. Unadjusted models include random effects only. Adjusted models added fixed effects for age, sex, morbidity, serious mental illness, substance use disorder, disability, a neighborhood stress score, and a marker for housing problems.
Abbreviations: ACO, accountable care organization; ICC, intraclass correlation coefficient.
These data are pre‐ACO‐program launch in 2018. For this analysis we attributed members to ACOs by applying MassHealth's algorithm, based on historic member claims and encounters, for default assignment to ACOs.
5. DISCUSSION
We found that the relatively few frequently hospitalized MassHealth beneficiaries have an outsized effect on 30‐day readmissions. These beneficiaries are sicker than other patients, with significant mental illness, substance use disorders, and housing problems. We demonstrated that an “ALL patients” readmission quality measure is highly volatile compared to readmission for only low‐frequency hospital users and may substantially affect judgments about how some ACOs perform on it. We also showed that even risk adjustment (for available clinical and social factors) does not solve this problem with an “ALL patients” readmission measure.
A readmission quality measure, even when risk adjusted, could put ACOs with socially and medically complex patients at risk for unwarranted penalties. 2 , 5 , 22 Penalties driven largely by ACOs members' disproportionate needs are unlikely to improve health quality and equity. Other mechanisms, such as targeted incentive payments, are likely needed to improve equity for complex patients and their ACOs. More broadly, the value of a hospitalization‐based readmission measure for ACOs is not obvious. More benefit might be gained by incentivizing reductions in a population‐based measure like avoidable hospitalizations, although such a measure may also be overly influenced by high‐frequency users. ACOs could work to identify members at risk of being frequent hospital users, possibly based on their number of hospitalizations in the prior year, and provide them with individualized patient care plans, coordinated care, and better discharge summaries to guide post‐discharge care. 23 , 24 , 25 Given the high prevalence of mental illness, substance use disorders, and housing problems among those frequently hospitalized, comprehensive social programs might be particularly beneficial. 2 , 26 , 27
This study does not test the properties of a specific alternative 30‐day readmission measure. However, it raises new questions about using readmissions to measure ACO quality. Excluding hospitalizations of high‐frequency users from 30‐day readmission may be fairer and more useful than a measure calculated on all patients, as it would focus attention on patients for whom readmission is a more salient outcome than numbers of hospitalizations. Several questions remain. For one, it is not clear that 4 is the right cutoff to define a high‐frequency user, although sensitivity analyses with cutoffs 3 and 5 yielded qualitatively similar results (see Appendix S3). Also, it is not clear if some form of weighting to reduce the undue influence of hospitalizations from high users is a better way to address the problem we identified. It is not satisfying to employ an after‐the‐fact rule to eliminate data from a quality measure for people who had many hospitalizations, rather than for those who appeared to need them. If the experience of high‐frequency users is removed from a readmission measure, it would be important to retain and report information about how many people and how many hospitalizations were thereby removed. It is unclear whether a reliable additional measure, based only on the experience of high‐frequency users, could be constructed, and what form it would take. Its goal would be to encourage excellent care for the medically and socially complex members who dominate the population of high‐frequency users. However, again, it is unsatisfying to have one more (or one less) hospitalization “bump” a member's data from one measure denominator into another.
The unit of analysis for the 30‐day readmission measure is the hospital discharge or encounter. However, patients may receive care from different providers in different settings, making it unclear which providers are responsible for a readmission. 28 , 29 Moreover, whether a patient is readmitted within 30 days of a discharge may matter less than the overall quality of care involved in managing a specific disease, improving quality of life, or avoiding mortality. 30 An encounter‐based measure of 30‐day readmission is of less obvious utility for judging a health plan than for judging a hospital on its discharge policies. It is not clear how (or even if) a readmission measure can be redesigned to align with the shared accountability that quality measures seek to encourage among doctors, hospitals, and other health care providers in an ACO. It is sobering to observe how much more influence having disproportionate numbers of high‐frequency users has on readmissions than does plan membership. On the contrary, a good measure should reduce as much patient‐level variability to the point that any residual health plan‐level variability may be more plausibly interpreted as a signal for health plan quality. Our approach could be used to analyze the effect of high users of healthcare on hospital readmissions and on other health quality measures in other populations. For instance, in the Medicare population, with beneficiaries older and sicker than Medicaid individuals in our study, one might expect even greater opportunity for mistakenly attributing differences in hospital readmission rates to health plan quality than to population characteristics.
We showed the ranking of ACOs based on point estimates of readmission rates to draw attention to the fact that ACOs' performance changed based on whether we risk adjusted and/or excluded hospitalizations of high‐frequency users. In actual practice, these readmission point estimates are also subject to statistical uncertainty, which CMS does not recognize in penalties and rewards. 31 Although it is beyond the scope of this study, we provide in the technical appendix 95 confidence intervals around unadjusted, partially adjusted, and fully adjusted readmission rates (see Appendix S4).
Our study has limitations. First, we could only account for a limited number of important risks not captured by diagnosis codes. For example, we have no information on social supports, health literacy, English proficiency, and functional status. 32 , 33 , 34 However, while richer data could better address health disparities and promote health equity, they are not widely available. Second, to account for the effect of clustering of hospitalizations at the patient‐ and ACO‐level on readmissions, we used a MassHealth algorithm to assign patients to ACOs based on the primary care physicians they were seeing prior to ACO launch. While our ACO attribution is realistic, it is not real. Actual ACOs, especially over time, may be able to distinguish themselves more than what here appears to be a very modest plan effect. Third, we have not studied the incremental effect of adding social risk factors to the clinical risks that the MassHealth (HEDIS) measure currently adjusts for. Nonetheless, our study suggests that even comprehensive risk adjustment will not solve the problems with an all readmissions measure applied to ACOs. Future research could provide additional insights. Finally, our study included MassHealth members only. It may not generalize to people with other kinds of insurance, nor even to members of Medicaid plans in other states.
High‐frequency hospital users have many medical morbidities and significant psychiatric, substance use, and housing problems. Despite their small numbers, they have a disproportionate effect on 30‐day readmission rates and—we hypothesize—on other utilization outcomes. This negatively affects some Medicaid ACOs, and more broadly is likely to adversely affect safety net hospitals. 2 The use of such metrics should be reconsidered in this context.
Supporting information
Appendix S1 Indicators for serious mental illness and substance use disorders were based on the diagnosis‐based Hierarchical Condition Category (DxCG‐HCC) model that organizes diagnoses into condition categories:
Appendix S2: Risk adjusted odds ratios for 30‐day readmission
Appendix S3: Hospitalizations and Readmissions by hospital use frequency using different definitions
Appendix S4: Performance on hospital readmission and relative ranking of accountable care organizations with and without data from high‐frequency users
ACKNOWLEDGEMENTS
This study received no external funding.
Fouayzi H, Ash AS. High‐frequency hospital users: The tail that wags the readmissions dog. Health Serv Res. 2022;57(3):579–586. 10.1111/1475-6773.13677
REFERENCES
- 1. Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee‐for‐service program. N Engl J Med. 2009;360(14):1418‐1428. 10.1056/nejmsa0803563. [DOI] [PubMed] [Google Scholar]
- 2. Burgess JF, Hockenberry JM. Can all cause readmission policy improve quality or lower expenditures? A historical perspective on current initiatives. Heal Econ Policy Law. 2014;9(2):193‐213. 10.1017/S1744133113000340. [DOI] [PubMed] [Google Scholar]
- 3. Hospital Readmissions Reduction Program (HRRP) | CMS. https://www.cms.gov/medicare/medicare-fee-for-service-payment/acuteinpatientpps/readmissions-reduction-program. Accessed April 8, 2021.
- 4. Thompson MP, Waters TM, Kaplan CM, Cao Y, Bazzoli GJ. Most hospitals received annual penalties for excess readmissions, but some fared better than others. Health Aff. 2017;36:893‐901. 10.1377/hlthaff.2016.1204. [DOI] [PubMed] [Google Scholar]
- 5. Joynt KE, Jha AK. Characteristics of hospitals receiving penalties under the hospital readmissions reduction program. J Am Med Assoc. 2013;309:342‐343. 10.1001/jama.2012.94856. [DOI] [PubMed] [Google Scholar]
- 6. Gilman M, Hockenberry JM, Adams EK, Milstein AS, Wilson IB, Becker ER. The financial effect of value‐based purchasing and the hospital readmissions reduction program on safety‐net hospitals in 2014: a cohort study. Ann Intern Med. 2015;163:427‐436. 10.7326/M14-2813. [DOI] [PubMed] [Google Scholar]
- 7. McCarthy CP, Vaduganathan M, Patel KV, et al. Association of the new peer group‐stratified method with the reclassification of penalty status in the hospital readmission reduction program. JAMA Netw Open. 2019;2:e192987. 10.1001/jamanetworkopen.2019.2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mask A, Adepoju OE. Relationship between accountable care organization status and 30‐day hospital‐wide readmissions: are all accountable care organizations created equal? J Healthc Qual. 2019;41(1):10‐16. 10.1097/JHQ.0000000000000132. [DOI] [PubMed] [Google Scholar]
- 9. Jiang HJ, Weiss AJ, Barrett ML, et al. Characteristics of Hospital Stays for Super‐Utilizers by Payer, 2012: Statistical Brief #190; 2006. [PubMed]
- 10. MassHealth Launches Restructuring To Improve Health Outcomes for 1.2 Million Members | Mass.gov. https://www.mass.gov/news/masshealth-launches-restructuring-to-improve-health-outcomes-for-12-million-members. Accessed April 22, 2021.
- 11. Joynt Maddox KE, Reidhead M, Hu J, et al. Adjusting for social risk factors impacts performance and penalties in the hospital readmissions reduction program. Health Serv Res. 2019;54:327‐336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. HEDIS Measures and Technical Resources—NCQA. https://www.ncqa.org/hedis/measures/. Accessed April 22, 2021.
- 13. Risk Adjustment | CMS. https://www.cms.gov/Medicare/Health-Plans/MedicareAdvtgSpecRateStats/Risk-Adjustors. Accessed April 22, 2021.
- 14. Shea DG, Terza JV, Stuart BC, Briesacher B. Estimating the effects of prescription drug coverage for medicare beneficiaries. Health Serv Res. 2007;42:933‐949. 10.1111/j.1475-6773.2006.00659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ash AS, Posner MA, Speckman J, Franco S, Yacht AC, Bramwell L. Using claims data to examine mortality trends following hospitalization for heart attack in Medicare. Health Serv Res. 2003;38:1253‐1262. 10.1111/1475-6773.00175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Briesacher BA, Andrade SE, Fouayzi H, et al. Medication adherence and use of generic drug therapies. Am J Manag Care. 2009;15(7):450‐456. [PMC free article] [PubMed] [Google Scholar]
- 17. Briesacher BA, Quittner AL, Saiman L, Sacco P, Fouayzi H, Quittell LM. Adherence with tobramycin inhaled solution and health care utilization. BMC Pulm Med. 2011;11(1):1‐6. 10.1186/1471-2466-11-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Briesacher BA, Andrade SE, Fouayzi H, Chan KA. Comparison of drug adherence rates among patients with seven different medical conditions. Pharmacotherapy. 2008;28:437‐443. 10.1592/phco.28.4.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ash AS, Ellis RP, Pope GC, et al. Using diagnoses to describe populations and predict costs. Health Care Financ Rev. 2000;21(3):7‐28. [PMC free article] [PubMed] [Google Scholar]
- 20. Ash AS, Mick EO, Ellis RP, Kiefe CI, Allison JJ, Clark MA. Social determinants of health in managed care payment formulas. JAMA Intern Med. 2017;177:1424‐1430. 10.1001/jamainternmed.2017.3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sall L, Hayward RD, Fessler MM, Edhayan E. Between‐hospital and between‐neighbourhood variance in trauma outcomes: cross‐sectional observational evidence from the Detroit metropolitan area. BMJ Open. 2018;8(11):e022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Joynt KE, Jha AK. Thirty‐day readmissions—truth and consequences. N Engl J Med. 2012;366:1366‐1369. 10.1056/nejmp1201598. [DOI] [PubMed] [Google Scholar]
- 23. Kripalani S, Theobald CN, Anctil B, Vasilevskis EE. Reducing hospital readmission rates: current strategies and future directions. Annu Rev Med. 2013;65:471‐485. 10.1146/annurev-med-022613-090415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Naylor MD, Brooten D, Campbell R, et al. Comprehensive discharge planning and home follow‐up of hospitalized elders: a randomized clinical trial. J Am Med Assoc. 1999;281:613‐620. 10.1001/jama.281.7.613. [DOI] [PubMed] [Google Scholar]
- 25. Naylor M, Brooten D, Jones R, Lavizzo‐Mourey R, Mezey M, Pauly M. Comprehensive discharge planning for the hospitalized elderly: a randomized clinical trial. Ann Intern Med. 1994;120:999‐1006. 10.7326/0003-4819-120-12-199406150-00005. [DOI] [PubMed] [Google Scholar]
- 26. Carroll KM, Onken LS. Behavioral therapies for drug abuse. Am J Psychiatry. 2005;162:1452‐1460. 10.1176/appi.ajp.162.8.1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Corrigan PW, Shapiro JR. Measuring the impact of programs that challenge the public stigma of mental illness. Clin Psychol Rev. 2010;30:907‐922. 10.1016/j.cpr.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hussey PS, Sorbero ME, Mehrotra A, et al. Using episodes of care as a basis for performance measurement and payment: moving from concept to practice. Health Aff. 2009;28(5):1406‐1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pham HH, Schrag D, O'Malley AS, et al. Care patterns in medicare and their implications for pay for performance. N Engl J Med. 2007;356:1130‐1139. 10.1056/NEJMsa063979. [DOI] [PubMed] [Google Scholar]
- 30. Rosen AB, Cutler DM. Challenges in building disease‐based national health accounts. Med Care. 2009;47:S7‐S13. 10.1097/MLR.0b013e3181a23e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shen C, Wadhera RK, Yeh RW. Misclassification of hospital performance under the hospital readmissions reduction program: implications for value‐based programs. JAMA Cardiol. 2021;6(3):332‐335. 10.1001/jamacardio.2020.4746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kansagara D, Englander H, Salanitro A, et al. Risk prediction models for hospital readmission: a systematic review. J Am Med Assoc. 2011;306:1688‐1698. 10.1001/jama.2011.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Joynt KE, Jha AK. A path forward on Medicare readmissions. N Engl J Med. 2013;368:1175‐1177. 10.1056/NEJMp1300122. [DOI] [PubMed] [Google Scholar]
- 34. Calvillo‐King L, Arnold D, Eubank KJ, et al. Impact of social factors on risk of readmission or mortality in pneumonia and heart failure: systematic review. J Gen Intern Med. 2013;28:269‐282. 10.1007/s11606-012-2235-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Indicators for serious mental illness and substance use disorders were based on the diagnosis‐based Hierarchical Condition Category (DxCG‐HCC) model that organizes diagnoses into condition categories:
Appendix S2: Risk adjusted odds ratios for 30‐day readmission
Appendix S3: Hospitalizations and Readmissions by hospital use frequency using different definitions
Appendix S4: Performance on hospital readmission and relative ranking of accountable care organizations with and without data from high‐frequency users


