Abstract
Background
Dacomitinib is the second‐generation epidermal growth factor receptor (EGFR)‐tyrosine kinase inhibitor (TKI) for mutant non–small cell lung cancer (NSCLC). EGFR‐TKIs are often re‐administered in Japan after the disease progression prior EGFR‐TKI. There is little evidence of dacomitinib in rechallenge setting. This study evaluated clinical outcomes of dacomitinib in rechallenge setting.
Methods
Patients who received dacomitinib for advanced EGFR‐mutant NSCLC who had progressed after EGFR‐TKI in nine institutions in Japan were included in the analyses.
Results
In total, 43 patients were analyzed. The median progression‐free survival (PFS) was 4.3 months (95% confidence interval [CI], 2.5–5.6). The overall survival (OS) was 10.5 months (95% CI, 7.4–not reached). The overall response rate was 25.5% (95% CI, 13.1–33.7). Subset analysis indicated that patients with EGFR exon 21 L858R showed longer PFS than those with EGFR exon 19 deletion (5.8 vs. 4.1 months) (p = 0.018). The most common adverse events leading to dose modification were diarrhea, paronychia, rash, and oral mucositis.
Conclusion
In the real practice in Japan, dacomitinib showed a worthwhile treatment option for NSCLC patients with EGFR mutation after failure of previous EGFR‐TKI. The benefit was especially pronounced in patients with the exon 21 mutation.
Keywords: dacomitinib, epidermal growth factor receptor mutation, exon 21 L858R, non‐small cell lung cancer, rechallenge
Dacomitinib showed a worthwhile treatment option for NSCLC patients with EGFR mutation after failure of previous EGFR‐TKI. The median progression‐free survival was 4.3 months. The overall response rate was 25.5%.
INTRODUCTION
Epidermal growth factor receptor (EGFR)‐tyrosine kinase inhibitors (TKIs) are the established first‐line standard therapy for EGFR mutation‐positive, advanced non–small cell lung cancer (NSCLC). First‐generation reversible EGFR‐TKIs, such as gefitinib and erlotinib, or the second‐generation irreversible ErbB blocker, afatinib, showed longer progression‐free survival (PFS) compared to platinum‐based chemotherapy in randomized phase III clinical trials. 1 , 2 , 3 , 4 , 5 , 6 Recent head‐to‐head randomized phase III trials revealed that second‐ and third‐generation EGFR‐TKIs (dacomitinib and osimertinib) were more effective than first‐generation TKIs in terms of PFS and overall survival (OS), 7 , 8 , 9 , 10 although almost all patients develop acquired resistance and become refractory. Among the different mechanisms of acquired resistance, 11 a secondary mutation, T790M, is the most frequent event, occurring in approximately 50%–60% of cases. 12 Despite the success of osimertinib both in the first‐line treatment setting and as a salvage therapy in the presence of the T790M secondary mutation, acquired resistance inevitably occurs, similar to patients treated with first‐ or second‐generation EGFR‐TKIs. Platinum‐based chemotherapy agents are currently used in patients without the T790M mutation after first‐ or second‐generation EGFR‐TKI treatment failure and in all patients after third‐generation EGFR‐TKI treatment failure.
There have been several reports on rechallenge of EGFR‐TKI. 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 Gefitinib, erlotinib, and afatinib have been used in rechallenge settings, all of which followed first‐ and second‐generation EGFR‐TKI treatment. Considering the efficacy of gefitinib, 13 , 14 , 15 , 16 erlotinib, 17 and afatinib, 18 , 19 , 20 , 21 , 22 , 23 achieved a median PFS of 2.0–8.0 months and overall response rate (ORR) of 7%–25% in rechallenge setting. Some of these studies are retrospective analyses, and some also lack EGFR or T790M mutation analyses. Patients treated initially with third‐generation TKI have also not been analyzed. Overall, data on the EGFR‐TKI rechallenge of dacomitinib in patients with EGFR‐mutated metastatic NSCLC, previously treated with EGFR‐TKIs, are not clear. In Japan, rechallenge of EGFR‐TKI may be selected in clinical practice. In this study, we report real clinical practice data regarding EGFR‐TKI rechallenge of dacomitinib in patients with EGFR mutant NSCLC in Japan.
MATERIALS AND METHODS
Study design
This study was a multicenter retrospective study involving nine institutions from the TOPGAN group in Japan. The objective was to evaluate the clinical outcome of patients with advanced NSCLC harboring the EGFR mutation who received dacomitinib in the EGFR‐TKI rechallenge setting. The study was performed according to protocols approved by the institutional review board of each participating hospital. The requirement for informed consent was waived because of the retrospective nature of the study. This study was registered in the University Hospital Medical Information Network (UMIN) Clinical Trial Registry (Trial number: UMIN000042975).
Data collection
We screened the medical records of patients who were treated with dacomitinib in the rechallenge setting between January 2019 and October 2020. Eligibility criteria were the following: patients with EGFR‐positive NSCLC who were treated with dacomitinib and, in the rechallenge setting, patients whose disease had progressed following at least one EGFR‐TKI therapy. Patient demographics and clinical characteristics were obtained retrospectively from patient files including age, gender, smoking status, performance status (PS), cancer stage, tumor histology, type of EGFR mutation at first diagnosis, T790M status before dacomitinib treatment, treatment history, height, and weight. We also collected data on dacomitinib exposure, including starting dose, dose reduction, and treatment discontinuation. The cut‐off date was April 30, 2021.
Evaluation and statistical analysis
All analyses were performed using JMP 13 (SAS Institute). PFS and OS were estimated using the Kaplan–Meier method, and the log‐rank test was used for intergroup comparisons. Differences were assumed to be significant at p‐values <0.05. PFS was defined as the time from the date of treatment initiation to the date of disease progression, death, or last contact; if none of the events was observed, data were censored from the latest observation date. If post‐treatment was started, data were censored after the date of initiation. If the event was unknown, for example in the case of transfer or nonarrival, data were censored from the last date when patient survival was confirmed. The OS was defined as the time from treatment to death by any cause. Adverse events (AEs) were graded according to the Common Terminology Criteria for Adverse Events (version 5.0). 24 Radiographic tumor responses were defined according to the Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1. 25
RESULTS
Patient characteristics
A total of 43 patients were screened from nine participating institutions of the TOPGAN group. Table 1 shows the characteristics of the eligible patients. Exon 19 deletion (53.5%) and exon 21 L858R (41.9%) were the major subtypes of EGFR mutation. Only two patients (4.6%) had uncommon mutations, which included G719X and L861Q. Thirty‐one patients (72.2%), assessed before dacomitinib treatment, did not have the T790M mutation. Gefitinib, afatinib, and osimertinib were administered frequently as first‐line EGFR‐TKI treatment.
TABLE 1.
Patient characteristics (n = 43)
| No. (%) | |
|---|---|
| Age, median (range), y | 70 (41–83) |
| Gender | |
| Female | 27 (62.8) |
| Male | 16 (37.2) |
| Smoking history | |
| Never smoker | 18 (41.9) |
| Light or former smoker | 25 (58.1) |
| Histology | |
| Adenocarcinoma | 42 (97.7) |
| Non–small cell carcinoma not otherwise specified | 1 (2.3) |
| Clinical stage | |
| IIIB | 1 (2.3) |
| IV | 24 (55.8) |
| Postoperative recurrent disease | 18 (41.9) |
| ECOG performance status | |
| 0–1 | 33 (76.8) |
| 2 | 8 (18.6) |
| 3 | 2 (4.6) |
| CNS metastasis | |
| Present | 21 (48.9) |
| Absent | 22 (51.1) |
| EGFR mutation at the diagnosis | |
| Exon 19 deletion | 23 (53.5) |
| Exon 21 L858R | 18 (41.9) |
| Uncommon mutation | 2 (4.6) |
| T790M at pre‐treatment of dacomitinib | |
| T790M positive | 2 (4.6) |
| T790M negative | 31 (72.2) |
| Unknown | 10 (23.2) |
| Treatment history of osimertinib | 24 (55.8) |
| Treatment history of platinum chemotherapy | 34 (79.1) |
| Treatment history of anti VEGF antibody | 22 (51.2) |
| Previous EGFR‐TKI as first line | |
| Gefitinib | 16 (37.2) |
| Erlotinib | 5 (11.8) |
| Afatinib | 10 (23.2) |
| Erlotinib + bevacizumab | 2 (4.6) |
| Osimertinib | 9 (20.9) |
| Other clinical trial drug | 1 (2.3) |
| Treatment line of dacomitinib | |
| 2 | 6 (13.9) |
| 3 | 4 (9.3) |
| 4 | 12 (27.9) |
| 5 or later | 21 (48.9) |
| BMI | |
| <19 | 11 (25.6) |
| ≥19 | 32 (74.4) |
Abbreviations: BMI, body mass index; CNS, central nervous system; ECOG, Eastern Cooperative Oncology Group; EGFR, epidermal growth factor receptor; PS, performance status; VEGF, vascular endothelial growth factor.
Treatment delivery
Treatment delivery is summarized in Table 2. Starting doses of dacomitinib were 45 mg for 31 patients (72.2%), 30 mg for 10 patients (23.2%), and 15 mg for 2 patients (4.6%), respectively. Clinicians chose starting doses of dacomitinib considering the patient's age or PS. Dose reduction was needed in 26 patients (60.5%). The primary reasons for dose reduction included paronychia, diarrhea, rash, and oral mucositis.
TABLE 2.
Treatment delivery and dose reduction
| No. (%) | |
|---|---|
| Starting dose | |
| 45 mg | 31 (72.2) |
| 30 mg | 10 (23.2) |
| 15 mg | 2 (4.6) |
| Dose reduction | 26 (60.5) |
| Treatment discontinuation for AE | 3 (6.9) |
Abbreviation: AE, adverse events.
Efficacy
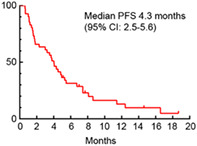
The response to dacomitinib is summarized in Table 3. One patient (2.3%) attained a complete response (CR), 10 (23.2%) patients attained a partial response (PR), and 16 patients (37.2%) had a stable disease (SD). The ORR was 25.5% (95% confidence interval [CI], 13.1–33.7), and disease control rate was 62.7%. Among the 17 patients with brain metastases evaluable by RECIST criteria, 6 patients (35.2%) attained a PR. The median PFS was 4.3 months (95% CI, 2.5–5.6) (Figure 1(a)). The median OS was 10.5 months (95% CI, 7.4–not reached) (Figure 1(b)). Table 4 shows PFS in each subgroup. In the mutation subtype, patients with EGFR exon 21 L858R showed longer PFS than those with EGFR exon 19 deletion (5.8 months vs. 4.1 months, p = 0.018) (Figure 1(c)). In contrast, in the PS 2 or worse group, PFS was statistically shorter than that in the PS 0–1 group (1.8 vs. 5.7 months, p = 0.0003) (Figure 1(d)). In addition, in the low body mass index (BMI) group (BMI < 19), PFS was statistically shorter than that in the high BMI group (BMI ≥ 19) (2.2 vs.5.0 months, p = 0.049). There were no significant differences in other factors. Although 26 patients had to reduce the dose of dacomitinib because of AEs, it did not affect its efficacy in terms of PFS (5.1 months in the reduction group vs. 3.7 months in the no reduction group) (p = 0.29).
TABLE 3.
Response rate
| No. of patients | % | |
|---|---|---|
| Response | ||
| Complete response | 1 | 2.3 |
| Partial response | 10 | 23.2 |
| Stable disease | 16 | 37.2 |
| Progressive disease | 13 | 30.4 |
| Not evaluable | 3 | 6.9 |
| Response rate | 25.5 | |
| Response rate for CNS metastasis | 35.2 |
Abbreviation: CNS, central nervous system.
FIGURE 1.
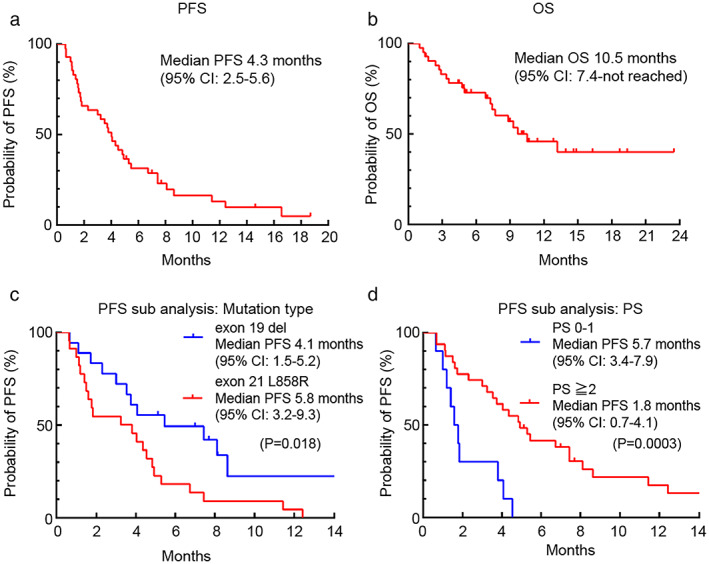
Kaplan–Meier curves of progression‐free survival (a) and overall survival (b) from dacomitinib initiation in all patients. Kaplan–Meier curves of sub group progression‐free survival in patients with EGFR exon 21 L858R and those with EGFR exon 19 deletion (c). Kaplan–Meier curves of sub group progression‐free survival in patients with PS 0–1 and those with PS 2 or more (d)
TABLE 4.
Progression‐free survival for subgroups
| No. | Median PFS (mo) (95% CI) | p Value (log‐rank) | |
|---|---|---|---|
| Age, y | |||
| <75 | 33 | 4.3 (1.9–5.7) | 0.12 |
| ≥75 | 10 | 6.9 (0.7–NR) | |
| Gender | |||
| Male | 16 | 4.6 (1.7–7.9) | 0.97 |
| Female | 27 | 4.3 (1.8–7.2) | |
| Smoking history | |||
| Yes | 18 | 4.6 (1.6–7.9) | 0.74 |
| No | 25 | 4.0 (1.9–7.9) | |
| Clinical stage | |||
| IV | 24 | 4.2 (1.8–7.9) | 0.83 |
| Postoperative recurrent disease | 18 | 4.6 (1.6–7.9) | |
| PS | |||
| 0–1 | 33 | 5.7 (3.4–7.9) | 0.0003 |
| ≥2 | 10 | 1.8 (0.7–4.1) | |
| BMI | |||
| <19 | 11 | 2.2 (1.2–4.3) | 0.049 |
| ≥19 | 32 | 5.0 (3.2–7.9) | |
| Brain metastasis | |||
| Present | 21 | 4.3 (1.9–5.8) | 0.82 |
| Absent | 22 | 4.9 (1.6–8.6) | |
| EGFR mutation | |||
| Exon 19 deletion | 23 | 4.1 (1.5–5.2) | 0.018 |
| Exon 21 L858R | 18 | 5.8 (3.2–9.3) | |
| T790M at pre‐treatment of dacomitinib | |||
| Positive | 2 | 3.2 (1.2–5.3) | 0.37 |
| Negative or unknown | 41 | 4.3 (2.5–5.8) | |
| Treatment history of platinum chemotherapy | |||
| Yes | 34 | 4.3 (2.4–7.2) | 0.81 |
| No | 9 | 5.1 (1.2–8.7) | |
| Treatment history of anti‐VEGF antibody | |||
| Yes | 22 | 4.0 (1.3–7.2) | 0.29 |
| No | 21 | 4.6 (1.8–8.7) | |
| Treatment history of osimertinib (any line) | |||
| Yes | 24 | 4.3 (1.9–5.3) | 0.72 |
| No | 19 | 5.1 (1.5–8.7) | |
| First line treatment | |||
| Osimertinib | 9 | 5.6 (1.6–NR) | 0.31 |
| Other TKI | 34 | 4.0 (1.9–5.3) | |
| Dose reduction of dacomitinib | |||
| Yes | 26 | 5.1 (3.2–7.9) | 0.29 |
| No | 17 | 3.7 (1.1–5.8) | |
| Treatment line of dacomitinib | |||
| <4th | 10 | 5.4 (0.6–13.3) | 0.61 |
| ≥4th or later | 33 | 4.3 (1.9–5.6) |
Abbreviations: BMI, body mass index; IV, intravenous; NR, not reached; PFS, progression‐free survival; PS, performance status; TKI, tyrosine kinase inhibitor; VEGF, vascular endothelial growth factor.
Adverse events
AEs are summarized in Table 5. Grade ≥3 AEs included rash (13.9%), diarrhea (9.3%), anorexia (9.3%), and paronychia (4.6%). The main reasons for dose reduction were paronychia, diarrhea, rash, and oral mucositis. No patients experienced pneumonitis during dacomitinib treatment. Three patients (6.9%) discontinued treatment because of toxicities. One patient developed a grade 3 intestinal obstruction, and this AE led to them discontinuing dacomitinib treatment. The other two patients discontinued treatment because of loss of appetite and diarrhea in one, and prolonged paronychia and oral mucositis in the other.
TABLE 5.
Toxicity in patients treated with dacomitinib
| Adverse events | No. (%) | ||
|---|---|---|---|
| Grade 1–2 | ≥Grade 3 | Any grade | |
| WBC decreased | 2 (4.6) | 0 | 2 (4.6) |
| Neutrophil count decreased | 2 (4.6) | 0 | 2 (4.6) |
| Anemia | 6 (13.9) | 0 | 6 (13.9) |
| Platelet count decreased | 1 (2.3) | 0 | 1 (2.3) |
| Rash | 28 (65.1) | 6 (13.9) | 34 (79.1) |
| Paronychia | 20 (46.5) | 2 (4.6) | 22 (51.7) |
| Dry skin | 18 (41.9) | 0 | 18 (41.9) |
| Diarrhea | 31 (72.2) | 4 (9.3) | 35 (81.4) |
| Oral mucositis | 15 (34.9) | 1 (2.3) | 16 (37.2) |
| Anorexia | 9 (20.9) | 4 (9.3) | 13 (30.4) |
| Nausea | 7 (16.2) | 2 (4.6) | 9 (20.9) |
| Fatigue | 8 (18.6) | 2 (4.6) | 10 (23.3) |
| Infection | 0 | 1 (2.3) | 1 (2.3) |
| Creatinine increased | 6 (13.9) | 0 | 6 (13.9) |
| Pneumonitis | 0 | 0 | 0 |
| Liver dysfunction | 6 (13.9) | 0 | 6 (13.9) |
| Intestinal obstruction | 0 | 1 (2.3) | 1 (2.3) |
| Lung hemorrhage | 1 (2.3) | 0 | 1 (2.3) |
Abbreviation: WBC, white blood cells.
DISCUSSION
This is the one of the largest retrospective studies demonstrating that dacomitinib has manageable safety profiles and modest efficacy for patients with advanced NSCLC harboring EGFR mutations in the rechallenge setting. Our study demonstrated PFS of 4.3 months and ORR of 25.5%, which were better than those reported previously with other EGFR‐TKIs. 18 , 19 , 20 , 21 , 22 , 23 Longer PFS was observed in patients with EGFR exon 21 L858R compared with EGFR exon 19 deletion (5.8 months vs. 4.1 months). Two common mutations could predict the clinical response to EGFR‐TKIs, but reported sensitivities to EGFR‐TKIs were different from those in previous reports. Patients with the exon 21 L858R mutation experienced lower drug efficacy than those with the exon 19 deletion. 26 Recent data indicate that approximately 25% of patients with EGFR mutations harbor compound mutations, and the prognosis for patients with compound mutations is worse. 27 Kohsaka et al. 28 reported that compound mutations exist at a high rate in exon 21 L858R‐positive cells and G719X cells following the mixed‐all‐nominated‐mutants‐in‐one method. In the cell‐based compound mutation study, different EGFR‐TKI antitumor activities were investigated. Among them, the antitumor activity of second‐generation EGFR‐TKIs was favorable. Kobayashi et al. 29 reported that second‐generation EGFR‐TKIs were effective in Ba/F3 cells with uncommon mutations in vitro. Our study included patients after fourth‐line or later treatment, and clonal selection was induced via treatment with EGFR‐TKI and cytotoxic chemotherapy. Most patients were treated with first‐ or third‐generation EGFR‐TKIs as the first‐line. In patients with the exon L858R mutation, the compound mutation clone became resistant, and those tumors might be second‐generation TKI responders. Osimertinib is widely used as first‐line treatment in current clinical practice. No treatment has been approved at this time to overcome resistance after osimertinib failure. Recently, a dacomitinib phase 2 trial has been reported in which patients are included if they fail first‐line osimertinib treatment. In this study, the response rate was 17%, and PFS was 1.8 months, which are limited benefits if the disease progresses after initial osimertinib treatment. 30 In our study, the PFS for patients after first‐line osimertinib treatment was 5.6 months (1.6– not reached). Few patients were treated with dacomitinib immediately after failing third‐generation EGFR‐TKI, and many patients had a history of cytotoxic chemotherapy, which might have affected the drug efficacy.
Regarding brain metastasis, 17 of 26 patients were evaluable by RECIST criteria in our study. The ORR of brain metastasis was 35.2%. A phase III study, ARCHER1050, indicated that dacomitinib prolongs PFS compared with gefitinib in patients with EGFR‐mutant NSCLC; however, patients with brain metastases were excluded from the study. 7 The efficacy of dacomitinib in patients with brain metastases has not been clarified. Recently, Peng and colleagues 31 reported a retrospective study that evaluated the efficacy of dacomitinib for 14 patients with treatment‐naive NSCLC and brain metastases. The ORR was 92.9%. There are only two case reports on dacomitinib therapy for cases previously treated for brain metastases, and they indicated successful treatment with dacomitinib for patients with brain metastases who failed previous EGFR‐TKIs. 32 , 33 Although the treatment induction lines for dacomitinib are different, dacomitinib might be effective against brain metastases.
The AEs were manageable, requiring short‐term interruption of dacomitinib or a dose reduction in some patients. New safety signals were not observed in our study, as were observed in the ARCHER1050 study. 7 Nishio et al. 34 reported the data on the ARCHER1050 Japanese subgroup. The proportion of Japanese patients with AEs leading to dose reduction was 85.0%, and dosing interruptions were observed in 67.5%. The main reasons for dose reduction were diarrhea, rash, and paronychia, similar to our study. More recently, an Asian subanalysis was also reported, where the frequency of dose reduction was 67.6%. 35 These two studies indicate that dose modification of dacomitinib helped to manage AEs, enabling patients to continue with dacomitinib. Although our study included patients treated more heavily, the AEs were tolerable with dose reduction and did not affect treatment efficacy (PFS; 5.1 months in patients with dose reduction vs. 3.7 months in those without, p = 0.29). In our study, interstitial lung disease (ILD) was not observed. Possible factors were as follows: most patients did not develop ILD with previous EGFR‐TKI treatment, and the treatment period for dacomitinib in the retreatment setting was shorter than that in the first‐line setting. In our study, patients in the PS 2 or worse group had shorter PFS than those in the PS 0–1 group (1.8 vs. 5.7 months). Previously, Inoue et al. 36 reported EGFR mutation‐positive patients with poor PS benefit from EGFR‐TKI. However, in situations where EGFR‐TKIs have already failed, dacomitinib may be discouraged in patients with poor PS.
The present study has some big limitations. First, because our study was retrospective, the schedule of tumor radiological evaluation was not determined, which might affect PFS estimation. Treatment line of dacomitinib was non‐uniformity. Second, T790M status before dacomitinib treatment was not examined in 10 patients (23.2%), which might affect the PFS subgroup result by T790M status. In our study, no statically significant difference in the efficacy of dacomitinib was observed in patients with or without (and unknown) T790M mutation (3.2 months vs. 4.3 months). The resistance mechanism after third‐generation EGFR‐TKI treatment is complicated 37 and is different from the resistance mechanism after first‐ or second‐generation EGFR‐TKI treatment. It is necessary to investigate the resistance mechanism by next‐generation sequencing or sensitive multiplex polymerase chain reaction assays in future research.
In conclusion, dacomitinib showed a manageable safety and modest efficacy in the retreatment setting. Dacomitinib might be a reasonable option as late‐line salvage treatment for NSCLC patients with EGFR mutations.
CONFLICT OF INTEREST
H.T. reports honoraria from AstraZeneca, Chugai Pharmaceutical, Boehringer‐Ingelheim Japan, and Pfizer Japan. F.O. reports honoraria from Astra Zeneca, Chugai Pharmaceutical, Boehringer‐Ingelheim Japan, Pfizer Japan, Bristol‐Myers Squibb Company, Ono Pharmaceutical, Novartis Japan, and Eli Lilly Japan K.K. Y.K. reports honoraria from Taiho Pharmaceutical, Astra Zeneca, and Chugai Pharmaceutical. Y.T. reports honoraria from Astra Zeneca, Chugai Pharmaceutical, Taiho Pharmaceutical, Merck Sharp and Dohme. E.M. reports honoraria from Astra Zeneca, Pfizer Japan, honoraria, advisory fee and grants from Chugai Pharmaceutical, honoraria, advisory fee from Boehringer‐Ingelheim Japan. Y.T.K. reports honoraria from Astra Zeneca, Chugai Pharmaceutical, Taiho Pharmaceutical, Kyowa Kirin, and Ono Pharmaceutical. N.Y. reports honoraria from Astra Zeneca, Chugai Pharmaceutical, Pfizer Japan, Bristol‐Myers Squibb Company, Ono Pharmaceutical, Merck and Co., and Eli Lilly Japan K.K. M.N. reports personal fees and other from Ono Pharmaceuticals, personal fees and other from Chugai Pharmaceutical, personal fees and other from Taiho Pharmaceutical, personal fees and other from Bristol Myers Squibb, personal fees and other from Daiichi Sankyo, personal fees and other from Lilly, grants, personal fees and other from AstraZeneca, personal fees from MSD, personal fees from AbbVie, grants, personal fees and other from Takeda, grants, personal fees and other from Pfizer, personal fees from Boehringer Ingelheim, grants, personal fees and other from Novartis, personal fees from Nippon Kayaku, grants, personal fees and other from Merck, personal fees and other from Janssen, personal fees from Teijin Pharma, outside the submitted work.
CONSENT
Informed consent was waived.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study was performed according to protocols approved by the institutional review board of each participating hospital. The requirement for informed consent was waived because of the retrospective nature of the study. This study was registered at the University Hospital Medical Information Network (UMIN) Clinical Trial Registry (Trial number: UMIN000042975).
ACKNOWLEDGMENTS
We thank the patients and their families and all the investigators.
Tanaka H, Sakamoto H, Akita T, Ohyanagi F, Kawashima Y, Tambo Y, et al. Clinical efficacy of dacomitinib in rechallenge setting for patients with epidermal growth factor receptor mutant non–small cell lung cancer: A multicenter retrospective analysis (TOPGAN2020‐02). Thorac Cancer. 2022;13:1471–1478. 10.1111/1759-7714.14415
DATA AVAILABILITY STATEMENT
All data generated or analyzed during this study are included in this published article.
REFERENCES
- 1. Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, et al. Gefitinib or chemotherapy for non‐small‐cell lung cancer with mutated EGFR. N Engl J Med. 2010;362:2380–8. [DOI] [PubMed] [Google Scholar]
- 2. Mitsudomi T, Morita S, Yatabe Y, Negoro S, Okamoto I, Tsurutani J, et al. Gefitinib versus cisplatin plus docetaxel in patients with non‐small‐cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol. 2010;11:121–8. [DOI] [PubMed] [Google Scholar]
- 3. Zhou C, Wu YL, Chen G, Feng J, Liu XQ, Wang C, et al. Erlotinib versus chemotherapy as first‐line treatment for patients with advanced EGFR mutation‐positive non‐small‐cell lung cancer (OPTIMAL, CTONG‐0802): a multicentre, open‐label, randomised, phase 3 study. Lancet Oncol. 2011;12:735–42. [DOI] [PubMed] [Google Scholar]
- 4. Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E, et al. Erlotinib versus standard chemotherapy as first‐line treatment for European patients with advanced EGFR mutation‐positive non‐small‐cell lung cancer (EURTAC): a multicentre, open‐label, randomised phase 3 trial. Lancet Oncol. 2012;13:239–46. [DOI] [PubMed] [Google Scholar]
- 5. Sequist LV, Yang JC, Yamamoto N, O'Byrne K, Hirsh V, Mok T, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol. 2013;31:3327–34. [DOI] [PubMed] [Google Scholar]
- 6. Wu YL, Zhou C, Hu CP, Feng J, Lu S, Huang Y, et al. Afatinib versus cisplatin plus gemcitabine for first‐line treatment of Asian patients with advanced non‐small‐cell lung cancer harbouring EGFR mutations (LUX‐lung 6): an open‐label, randomised phase 3 trial. Lancet Oncol. 2014;15:213–22. [DOI] [PubMed] [Google Scholar]
- 7. Wu YL, Cheng Y, Zhou X, Lee KH, Nakagawa K, Niho S, et al. Dacomitinib versus gefitinib as first‐line treatment for patients with EGFRmutation‐positive non‐small‐cell lung cancer (ARCHER1050): a randomised, open‐label, phase 3 trial. Lancet Oncol. 2017;18:1454–66. [DOI] [PubMed] [Google Scholar]
- 8. Mok TS, Cheng Y, Zhou X, Lee KH, Nakagawa K, Niho S, et al. Improvement in overall survival in a randomized study that compared dacomitinib with gefitinib in patients with advanced non‐small‐cell lung cancer and EGFR‐activating mutations. J Clin Oncol. 2018;36:2244–50. [DOI] [PubMed] [Google Scholar]
- 9. Soria JC, Ohe Y, Vansteenkiste J, Reungwetwattana T, Chewaskulyong B, Lee KH, et al. Osimertinib in untreated EGFR‐mutated advanced non–small‐cell lung cancer. N Engl J Med. 2018;378:113–25. [DOI] [PubMed] [Google Scholar]
- 10. Ramalingam SS, Vansteenkiste J, Planchard D, Cho BC, Gray JE, Ohe Y, et al. Overall survival with osimertinib in untreated, EGFR mutated advanced NSCLC. N Engl J Med. 2020;382:41–50. [DOI] [PubMed] [Google Scholar]
- 11. Lim SM, Syn NL, Cho BC, Soo RA. Acquired resistance to EGFR targeted therapy in non‐small cell lung cancer: mechanisms and therapeutic strategies. Cancer Treat Rev. 2018;65:1–10. [DOI] [PubMed] [Google Scholar]
- 12. Yu HA, Arcila ME, Rekhtman N, Sima CS, Zakowski MF, Pao W, et al. Analysis of tumor specimens at the time of acquired resistance to EGFR‐TKI therapy in 155 patients with EGFR‐mutant lung cancers. Clin Cancer Res. 2013;19:2240–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Asahina H, Oizumi S, Inoue A, Kinoshita I, Ishida T, Fujita Y, et al. Phase II study of gefitinib re‐administration in patients with advanced non‐small cell lung cancer and previous response to gefitinib. Oncology. 2010;79:423–9. [DOI] [PubMed] [Google Scholar]
- 14. Koizumi T, Agatsuma T, Ikegami K, Suzuki T, Kobayashi T, Kanda S, et al. Prospective study of gefitinib re‐administration after chemotherapy in patients with advanced non‐small‐cell lung cancer who previously responded to gefitinib. Clin Lung Cancer. 2012;13:458–63. [DOI] [PubMed] [Google Scholar]
- 15. Oh IJ, Ban HJ, Kim KS, Kim YG. Retreatment of gefitinib in patients with non‐small cell lung cancer who previously controlled to gefitinib: a single‐arm, open‐label, phase II study. Lung Cancer. 2012;77:121–7. [DOI] [PubMed] [Google Scholar]
- 16. Tomizawa Y, Fujita Y, Tamura A, Shirai M, Shibata S, Kawabata T, et al. Effect of gefitinib re‐challenge to initial gefitinib responder with non‐small cell lung cancer followed by chemotherapy. Lung Cancer. 2010;68:269–72. [DOI] [PubMed] [Google Scholar]
- 17. Horiike A, Yamamoto N, Tanaka H, Yanagitani N, Kudo K, Ohyanagi F, et al. Phase II study of erlotinib for acquired resistance to gefitinib in patients with advanced non‐small cell lung cancer. Anticancer Res. 2014;34:1975–81. [PubMed] [Google Scholar]
- 18. Miller VA, Hirsh V, Cadranel J, Chen YM, Park K, Kim SW, et al. Afatinib versus placebo for patients with advanced, metastatic non‐small‐cell lung cancer after failure of erlotinib, gefitinib, or both, and one or two lines of chemotherapy (LUX‐lung 1): a phase 2b/3 randomised trial. Lancet Oncol. 2012;13:528–38. [DOI] [PubMed] [Google Scholar]
- 19. Katakami N, Atagi S, Goto K, Hida T, Horai T, Inoue A, et al. LUX‐lung 4: a phase II trial of afatinib in patients with advanced non‐small‐cell lung cancer who progressed during prior treatment with erlotinib, gefitinib, or both. J Clin Oncol. 2013;31:3335–41. [DOI] [PubMed] [Google Scholar]
- 20. Lee VH, Leung DK, Choy TS, Lam KO, Lam PM, Leung TW, et al. Efficacy and safety of afatinib in Chinese patients with EGFR‐mutated metastatic non‐small‐cell lung cancer (NSCLC) previously responsive to first‐generation tyrosine‐kinase inhibitors (TKI) and chemotherapy: comparison with historical cohort using erlotinib. BMC Cancer. 2016;16:147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Oda N, Hotta K, Ninomiya K, Minami D, Ichihara E, Murakami T, et al. A phase II trial of EGFR‐TKI re‐administration with afatinib in advanced non‐small‐cell lung cancer harboring a sensitive non‐T790M EGFR mutation: Okayama lung cancer study group trial 1403. Cancer Chemother Pharmacol. 2019;83:817–25. [DOI] [PubMed] [Google Scholar]
- 22. Yamaguchi O, Kaira K, Mouri A, Shiono A, Hashimoto K, Miura Y, et al. Re‐challenge of afatinib after 1st generation EGFR‐TKI failure in patients with previously treated non‐small cell lung cancer harboring EGFR mutation. Cancer Chemother Pharmacol. 2019;83:817–25. [DOI] [PubMed] [Google Scholar]
- 23. Tanaka H, Taima K, Itoga M, Ishoka Y, Baba K, Shiratori T, et al. Real‐world study of afatinib in first‐line or re‐challenge settings for patients with EGFR mutant non‐small cell lung cancer. Med Oncol. 2019;36:57. [DOI] [PubMed] [Google Scholar]
- 24. Freites‐Martinez A, Santana N, Arias‐Santiago S, Viera A. Using the common terminology criteria for adverse events (CTCAE ‐ version 5.0) to evaluate the severity of adverse events of anticancer therapies. Actas Dermosifiliogr (Engl Ed). 2021;112:90–2. [DOI] [PubMed] [Google Scholar]
- 25. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47. [DOI] [PubMed] [Google Scholar]
- 26. Lee CK, Wu YL, Ding PN, Lord SJ, Inoue A, Zhou C, et al. Impact of specific epidermal growth factor receptor (EGFR) mutations and clinical characteristics on outcomes after treatment with EGFR tyrosine kinase inhibitors versus chemotherapy in EGFR‐mutant lung cancer: a meta‐analysis. J Clin Oncol. 2015;33:1958–65. [DOI] [PubMed] [Google Scholar]
- 27. Kim EY, Cho EN, Park HS, Hong JY, Lim S, Youn JP, et al. Compound EGFR mutation is frequently detected with co‐mutations of actionable genes and associated with poor clinical outcome in lung adenocarcinoma. Cancer Biol Ther. 2016;17:237–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kohsaka S, Nagano M, Ueno T, Suehara Y, Hayashi T, Shimada N, et al. A method of highthroughput functional evaluation of EGFR gene variants of unknown significance in cancer. Sci Transl Med. 2017;9:eaan 6566. [DOI] [PubMed] [Google Scholar]
- 29. Kobayashi Y, Mitsudomi T. Not all epidermal growth factor receptor mutations in lung cancer are created equal: perspectives for individualized treatment strategy. Cancer Sci. 2016;107:1179–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Choudhury NJ, Makhnin A, Tobi YY, Daly RM, Preeshagul IR, Iqbal AN, et al. Pilot study of dacomitinib for patients with metastatic EGFR‐mutant lung cancers with disease progression after initial treatment with osimertinib. JCO Precis Oncol. 2021;5:PO.21.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Peng W, Pu X, Jiang M, Wang J, Li J, Xu Y, et al. Dacomitinib induces objective responses in metastatic brain lesions of patients with EGFR‐mutant non‐small‐cell lung cancer: a brief report. Lung Cancer. 2021;152:66–70. [DOI] [PubMed] [Google Scholar]
- 32. Kudo K, Kawakado K, Kawajiri T, Nishi T, Makimoto G, Tamura T, et al. Dramatic response of brain metastasis from EGFR‐mutation‐positive NSCLC to dacomitinib. Intern Med. 2020;59:1739–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mizusaki S, Otsubo K, Ninomiya T, Arimura H, Tsuchiya‐Kawano Y, Inoue K, et al. Remarkable response to dacomitinib in a patient with leptomeningeal carcinomatosis due to EGFR‐mutant non‐small cell lung cancer. Thorac Cancer. 2021;12:114–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nishio M, Kato T, Niho S, Yamamoto N, Takahashi T, Nogami N, et al. Safety and efficacy of first‐line dacomitinib in Japanese patients with advanced non‐small cell lung cancer. Cancer Sci. 2020;111:1724–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cheng Y, Mok TS, Zhou X, Lu S, Zhou Q, Zhou J, et al. Safety and efficacy of first‐line dacomitinib in Asian patients with EGFR mutation‐positive non‐small cell lung cancer: results from a randomized, open‐label, phase 3 trial (ARCHER 1050). Lung Cancer. 2021;154:176–85. [DOI] [PubMed] [Google Scholar]
- 36. Inoue A, Kobayashi K, Usui K, Maemondo M, Okinaga S, Mikami I, et al. First‐line gefitinib for patients with advanced non‐small‐cell lung cancer harboring epidermal growth factor receptor mutations without indication for chemotherapy. J Clin Oncol. 2009;27:1394–400. [DOI] [PubMed] [Google Scholar]
- 37. Leonetti A, Sharma S, Minari R, Perego P, Giovannetti E, Tiseo M, et al. Resistance mechanisms to osimertinib in EGFR‐mutated non‐small cell lung cancer. Br J Cancer. 2019;121:725–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.


