Abstract
Spontaneous regression (SR) of thymoma is rare. A 44‐year‐old man with right chest pain underwent computed tomography (CT), which showed an 11.0 cm mass in the anterior mediastinum and right pleural effusion. He refused surgery and was sent home without medication and additional treatment. One year later, the mass had regressed to 5.5 cm, and the right pleural effusion had disappeared. He was then lost to follow‐up. Four years after the initial visit, he presented with diplopia and fatigue. A significant increase in his anti‐acetylcholine receptor antibody levels led to myasthenia gravis (MG) diagnosis. CT revealed a regressed mediastinal mass (3.0 cm). After extended thymectomy, histologic analysis confirmed a thymoma type B2, Masaoka stage IIa. The SR was due to intratumoral infarction. This report is the first to describe MG developing during SR. Anterior mediastinal tumors undergoing SR should be differentiated from thymomas and MG perioperative development should be considered.
Keywords: mediastinum, myasthenia gravis, neoplasm regression, spontaneous, pleural effusion, thymoma
We report a rare case of a thymoma undergoing spontaneous regression (SR) due to intratumoral infarction with myasthenia gravis (MG). This case demonstrates the natural course of SR of thymoma without any medication and treatment for 4 years. Anterior mediastinal tumors undergoing SR should be differentiated from thymomas and MG perioperative development should be considered.
INTRODUCTION
Thymoma is the most common neoplasm of the anterior mediastinum, accounting for 0.2%–1.5% of malignant tumors. 1 Thymomas rarely spontaneously regress. 2 , 3 , 4 Myasthenia gravis (MG), a common autoimmune disease, accounts for 20–30% of all thymomas and occurs as a paraneoplastic disorder. 5 We report a rare case of a thymoma undergoing spontaneous regression (SR) due to intratumoral infarction with MG.
CASE REPORT
A 44‐year‐old man without surgery and trauma history had sudden right chest pain. Chest computed tomography (CT) revealed an 11.0 × 10.0 × 5.2 cm anterior mediastinal mass and right pleural effusion (Figure 1a). A hyperintense multilocular cystic component was evident on T2‐weighted magnetic resonance imaging (Figure 2a). A thymoma was suspected, and a CT‐guided needle biopsy was performed. Only fibrous connective tissue and adipose tissue were obtained. The patient's anti‐acetylcholine‐receptor (anti‐AchR) antibody level was normal, but C‐reactive protein was elevated (2.1 mg/dl). He refused surgery and was sent home without any medication. One year later, the mass had undergone SR to 5.5 × 4.3 × 3.4 cm, and the right pleural effusion had disappeared (Figure 1b). The patient was then lost to follow‐up. Four years after his initial visit, he presented with diplopia and fatigue. An increase in anti‐AchR antibody (20 nmol/L) led to generalized MG diagnosis, and oral doses of 5 mg prednisone and 180 mg pyridostigmine daily were started. CT (Figure 1c) and magnetic resonance imaging (Figure 2b) revealed further mediastinal tumor regression to 3.0 × 3.0 × 1.8 cm, with cystic component resolution.
FIGURE 1.
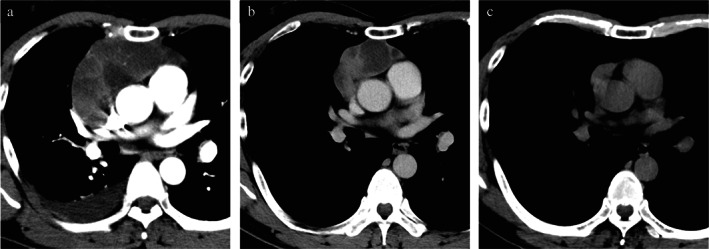
Computed tomography findings. (a) At initial visit: an anterior 11.0 cm mediastinal mass with right pleural effusion. (b) After 1 year of follow‐up: the anterior mediastinal mass has spontaneously regressed to 5.5 cm. The right pleural effusion has disappeared. (c) Four years after the initial visit: the mass has regressed to 3.0 cm. The cystic component is obscure
FIGURE 2.
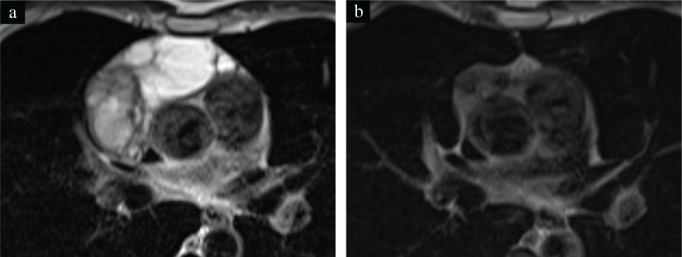
T2‐weighted magnetic resonance imaging findings. (a) At initial visit: the anterior mediastinal mass contains a heterogeneous hypointensity portion, indicating a solid component, and a 6.0 cm homogeneous hyperintensity portion, indicating a multilocular cystic component. (b) Four years after the initial visit: the multilocular cystic component has disappeared and the remaining solid component has regressed
Masaoka stage I thymoma with MG was suspected. A lobular mass was found in the right lobe of the thymus on extended thymectomy performed by median sternotomy. Inflammatory adhesions of tumor to the pericardium and superior vena cava were removed easily. Wedge resection of the right middle lobe of the lung was performed to remove its attachment to the mediastinal tumor. The encapsulated tumor had a white cross‐sectional outer surface and a well‐circumscribed yellowish‐white inner region (Figure 3a). Pathology examination revealed a central yellowish‐white area with necrotic features, cholesterol clefts, and hematoidin deposits (Figure 3b,c) caused by ischemic infarction. Polygonal epithelial cells and abundant lymphocytes indicated a thymoma type B2 (Figure 3d). Partial microscopic invasion of the thymus was observed, with no pulmonary invasion. The diagnosis was thymoma, Masaoka stage IIa. The patient had an unremarkable postoperative course without tumor or MG recurrence for 6 months.
FIGURE 3.
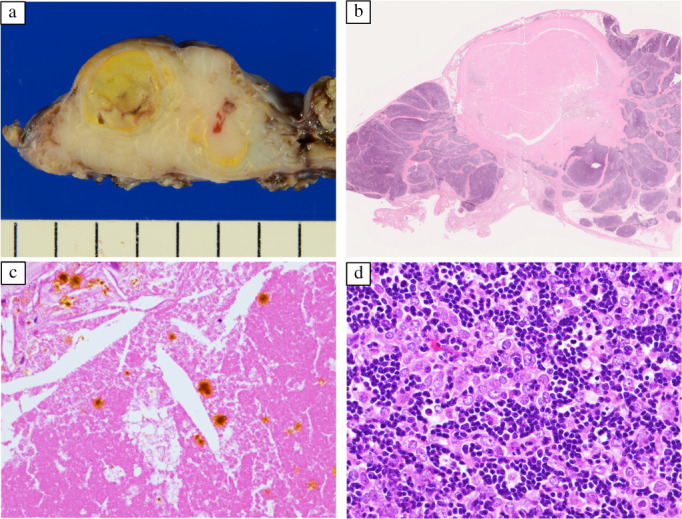
Pathology findings. (a) The tumor is encapsulated, with a white cut surface and a well‐circumscribed yellowish‐white internal area. (b) A low‐power view: the tumor contains a broad necrotic area in the center (hematoxylin–eosin staining). (c) A high‐power view: cholesterol clefts and hematoidin deposits are present in necrotic areas (hematoxylin–eosin staining). (d) A high‐power view: polygonal epithelial cells and abundant lymphocytes suggest thymoma type B2 (hematoxylin–eosin staining)
DISCUSSION
Our report is the first MG development in a patient with a thymoma undergoing SR. Neoplasm SR has been associated with ischemic infarction and thromboembolism caused by sclerosing arteriopathy or occlusive disorders. 2 Most thymomas undergoing SR are encapsulated tumors with intratumoral necrotic features. Ischemic infarction induces an inflammatory reaction presenting as pleural effusion, fever, and chest pain. 2 , 3 , 4 Here, thromboembolism was not observed, hence vascular occlusion caused by the rapid tumor enlargement resulted in ischemic infarction and inflammation. A relationship between multilocular cystic thymoma and inflammation has been reported. The multilocular cystic component observed during the patient's initial visit was consistent with that report. 6
Thymoma SR is commonly reported in Japanese men aged 30–50 years. 3 The predominant histologic subtypes are B2 and B3. Despite an aggressive subtype and large tumor size (median 65 mm, range 30–120 mm), thymomas exhibiting SR are diagnosed as early‐stage tumors, specifically, Masaoka stage I or II. 3 , 4 Because such thymomas are surrounded by a thick capsule, tissue invasion does not occur. The tumor was mostly encapsulated; only the thymus microscopic invasion was found.
Concomitant development of thymoma and MG has been observed in 30‐ to 50‐year‐old men, common subtypes being B2 and B3. The clinical presentation resembles a thymoma exhibiting SR. 5 No previous reports have described SR of a thymoma accompanied by MG. However, a thymoma exhibiting SR with elevated anti‐AchR antibody was reported. 3 , 4 The thymus pathology examination was unremarkable. Of thymomas with MG, 65–90% have germinal centers, likely related to MG development in the thymus. 7 Although the relationship between SR and MG development in thymomas remains unclear, anterior mediastinal tumors undergoing SR should be differentiated from thymomas. Moreover, MG perioperative development should be evaluated.
In our case, marked tumor regression (97.2%) was confirmed due to 6.0 cm cystic component disappearance. The mechanism behind SR of thymic cysts reportedly involves the cystic wall breakdown due to inflammation and necrosis or absorption of intracystic components. 6 Oral glucocorticoids sometimes induced radical thymoma regression; 8 however, our patient did not take any medication during regression.
Our case also demonstrates pleural effusion resolution. Reports have described inflammation associated with intratumoral infarction‐induced pleural effusion in thymomas undergoing SR. 3 , 4 Pleural effusion was initially attributed to thymoma dissemination. However, no scars were evident, suggesting parietal and visceral pleura dissemination during surgery. The pleural effusion was probably caused by inflammation.
Solitary anterior mediastinal tumors suspected to be thymomas are resected for immediate diagnosis and treatment. The follow‐up period for SR of a thymoma is ~1 month. 3 , 4 This case demonstrates thymoma SR for 4 years without treatment.
Anterior mediastinal tumors undergoing SR should be differentiated from thymomas and the perioperative development of MG should be considered.
CONFLICT OF INTEREST
The authors have no conflict of interest.
AUTHOR'S CONTRIBUTIONS
All authors had full access to the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Conceptualization, K.N. and M.S.; data curation, K.N., A.N., R.S., T.S., and T.H.; visualization, K.N. and M.S.; writing – original draft, K.N. and M.S.; project administration, M.S.; writing – review and editing, M.S., R.S., T.S., M.H., T.H., and H.H; supervision, H.H.
PATIENT CONSENT
Informed consent was obtained from the patients for this publication.
ACKNOWLEDGMENTS
We would like to thank Editage (www.editage.com) for English language editing.
Nishina K, Suzuki M, Nakamura A, Shimizu R, Shima T, Harada M, et al. Thymoma exhibiting spontaneous regression with developing myasthenia gravis: A case report. Thorac Cancer. 2022;13:1533–1536. 10.1111/1759-7714.14407
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1. Engels EA, Pfeiffer RM. Malignant thymoma in the United States: demographic patterns in incidence and associations with subsequent malignancies. Int J Cancer. 2003;105:546–51. [DOI] [PubMed] [Google Scholar]
- 2. Kuo T. Sclerosing thymoma—a possible phenomenon of regression. Histopathology. 1994;25:289–91. [DOI] [PubMed] [Google Scholar]
- 3. Okagawa T, Uchida T, Suyama M. Thymoma with spontaneous regression and disappearance of pleural effusion. Gen Thorac Cardiovasc Surg. 2007;55:515–7. [DOI] [PubMed] [Google Scholar]
- 4. Furuya K, Isobe K, Sano GO, et al. Thymoma exhibiting spontaneous regression in size, pleural effusion and serum cytokeratin fragment level: a case report. Mol Clin Oncol. 2015;3:1058–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Padda SK, Yao X, Antonicelli A, et al. Paraneoplastic syndromes and thymic malignancies: an examination of the international thymic malignancy interest group retrospective database. J Thorac Oncol. 2018;13:436–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Moran CA, Suster S. Thymoma with prominent cystic and hemorrhagic changes and areas of necrosis and infarction: a clinicopathologic study of 25 cases. Am J Surg Pathol. 2001;25:1086–90. [DOI] [PubMed] [Google Scholar]
- 7. Lefeuvre CM, Payet CA, Fayet OM, et al. Risk factors associated with myasthenia gravis in thymoma patients: the potential role of thymic germinal centers. J Autoimmun. 2020;106:102337. [DOI] [PubMed] [Google Scholar]
- 8. Barratt S, Puthucheary ZA, Plummeridge M. Complete regression of a thymoma to glucocorticoids, commenced for palliation of symptoms. Eur J Cardiothorac Surg. 2007;31:1142–3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


