Abstract
We describe a series of patients with COVID-19 who presented with seizures, reported in the Spanish Society of Neurology’s COVID-19 Registry.
This observational, descriptive, multicentre, registry-based study includes patients with confirmed COVID-19 who experienced seizures during active infection. We describe the clinical presentation of COVID-19, seizures, and results of complementary tests. We also describe the suspected aetiology of the seizures.
Of 232 reported cases, 26 (11.2%) presented with seizures; 7 of these patients (26.9%) had prior history of epilepsy, whereas the remaining 19 (73.1%) had no history of seizures. In most cases, seizures presented on days 0 and 7 after onset of COVID-19. By seizure type, 8 patients (30.7%) presented generalised tonic-clonic seizures, 7 (26.9%) status epilepticus, 8 (30.7%) focal impaired-awareness seizures, and 4 (11.7%) secondary generalised seizures. Six patients (23.1%) also presented other neurological symptoms, including altered mental status and decreased level of consciousness. Predisposing factors for seizures (eg, dementia, tumour, cerebrovascular disease) were observed in 10 of the 19 patients with no prior history of epilepsy (52.6%).
Patients with COVID-19 may present with seizures over the course of the disease, either alone or in the context of encephalopathy. Seizures may present in patients with no prior history of epilepsy; however, most of these patients present predisposing factors.
Keywords: COVID-19, Epilepsy, Seizures, Acute symptomatic seizures, Encephalopathy
1. Introduction
The novel coronavirus disease 2019 (COVID-19) has spread worldwide, with Spain being one of the most severely affected countries. The disease, mainly characterised by respiratory and systemic symptoms, is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and the associated immune response [1]. Neurological symptoms are frequently reported in patients with COVID-19, and include olfactory/taste disorders, myalgia, headache, acute stroke, dizziness, and altered mental status [2], [3], [4], [5], [6]. Seizures are among the least frequently reported neurological complications of COVID-19. The incidence of seizures over the course of the infection appears to be low, but it is not well known. Seizures are usually acute and symptomatic, and may present both in epileptic and non-epileptic patients. As most published series include few cases, little information is available on the timing of seizure onset, seizure aetiology, seizure semiology, and the clinical features of patients presenting seizures over the course of COVID-19 [7].
In March 2020, the Spanish Society of Neurology (SEN, for its Spanish initials) created a national registry for reporting of cases of COVID-19 with neurological manifestations [8]. The aim of this study is to describe the clinical characteristics of patients with seizures from the SEN COVID-19 Registry, in order to expand our knowledge of this severe complication. This report is intended to provide as complete a description as possible of the seizures, describing time of onset, risk factors, and causes, and exploring the impact and significance of seizures in COVID-19.
2. Methods
We conducted an observational, descriptive, multicentre study with a case series design. The SEN COVID-19 registry includes 232 patients with confirmed COVID-19 who also presented neurological symptoms or complications potentially associated with COVID-19 during active infection; patients are recorded on the registry by their treating neurologist. Case reporting was not systematic, with neurologists reporting cases at their own discretion. Therefore, this study does not include a consecutive or complete sample of Spanish patients with COVID-19 and seizures. All SEN members were invited to participate in the registry by. Data were collected with a standardised questionnaire hosted on Google Forms. This study includes data gathered between 16 March and 9 July 2020.
2.1. Eligibility
We included patients presenting seizures over the course of COVID-19, ie, from onset of the first clinical symptom to resolution of the infection. Diagnosis of COVID-19 was based on positive polymerase chain reaction (PCR), serological test, or rapid antigen test results, or presence of clinical signs of COVID-19, according to the national guidelines. The Spanish national guidelines establish a diagnosis of probable COVID-19 in patients with high clinical suspicion of the disease and compatible epidemiological data, when symptoms cannot be explained by another condition [9]. We excluded patients for whom complete data were not available. Eligibility criteria for inclusion in the SEN COVID-19 registry are published elsewhere [8].
2.2. Clinical data gathered
We gathered data on clinical presentation of COVID-19 (arthralgia, asthaenia, weakness, diarrhoea, dyspnoea, chest pain, expectoration, fever, odynophagia, skin rash, cough, vomiting, other). Severity of COVID-19 was classified as mild disease, pneumonia, or acute respiratory distress syndrome (ARDS) [10]. Data were also gathered on the need for intensive care unit (ICU) admission or invasive ventilatory support, and clinical outcomes.
Regarding neurological symptoms, we collected data on the type of neurological manifestations, the time of onset, and duration. The participating neurologists described seizures in free-form text, and the reported data were subsequently analysed to classify seizures according to the 2017 ILAE classification (focal onset, generalised onset, and unknown onset), when detailed information was available [11].
We gathered data on neurological complementary tests, including brain imaging (computed tomography [CT], magnetic resonance imaging [MRI]) and cerebrospinal fluid (CSF) analysis (pleocytosis, high protein levels, low glucose levels, elevated CSF opening pressure). Results from SARS-CoV-2 PCR tests of CSF samples were also gathered, when these were reported.
When available, we collected EEG data, including the time between the seizure and the EEG study. The results were classified as normal or abnormal. When EEG results were considered abnormal, findings are classified as nonspecific abnormalities (eg, background or generalised slowing) or specific epileptiform activity (eg, interictal epileptiform discharges, ictal activity).
2.3. Statistical analysis
Qualitative and ordinal data are reported as absolute numbers and percentages; the denominator is given for those variables for which valid responses were not available for the total sample. Continuous quantitative variables are expressed as means and standard deviation (SD). For hypothesis testing, we studied associations between dichotomous qualitative variables with the chi-square test, and between quantitative and qualitative variables with the Mann-Whitney U test. Associations were considered significant when the P value was < 0.05. Statistical analysis was performed with the SPSS statistical software, v26.0 (IBM Corp.; Armonk, NY, USA).
2.4. Ethics
The study protocol was approved by the ethics Review Board of Hospital Clínico Universitario de Valladolid, Spain (project code PI 20–1722). Written informed consent was not required due to the characteristics of the study, the fact that we did not process personal data, and the risk of infection.
3. Results
3.1. Demographic and clinical data
During the study period, 232 cases were included in the registry. Of these cases, 26 (11.2%) presented seizures over the course of COVID-19. The main clinical characteristics of our patients are summarised in Table 1 .
Table 1.
Clinical and demographic characteristics of our sample of patients with COVID-19 and seizures.
| Age (mean, SD) | 71.8 (17.1) |
|---|---|
| Women (n, %) | 13/26 (50%) |
| Systemic comorbidities (n, %) | 23/26 (88.4%) |
| Hypertension (n, %) | 12/26 (46.1%) |
| Diabetes (n, %) | 12/26 (46.1%) |
| Heart disease (n, %) | 7/26 (26.9%) |
| Lung disease (n, %) | 3/26 (11.5%) |
| History of epilepsy (n, %) | 7/26 (26.9%) |
| Chronic antiepileptic treatment (n, %) | 6/26 (23%) |
| Risk factors for epilepsy* (n, %) Dementia Brain tumour Cerebrovascular disease** |
10/26 (38.4%) 5/10 (50%) 1/10 (10%) 5/10 (50%) |
| Severity of COVID-19*** (n, %) Mild disease Pneumonia ARDS |
4/24 (16.6%) 19/24 (79.1%) 1/24 (4.1%) |
*Patients with no history of epilepsy. Patients may present more than one risk factor.
**Chronic lesions of vascular origin detected before or during this study.
***Data unavailable for 2 patients.
ARDS: acute respiratory distress syndrome; SD: standard deviation.
Diagnosis of COVID-19 was based on positive oropharyngeal swab PCR results in 22 patients (84%), compatible clinical/radiological findings in 3 patients (11.5%), and positive serum antibody test results in one (3.5%). Systemic symptoms of COVID-19 are summarised in Table 1.
The most frequent COVID-19 symptoms were the following: fever appeared in 19 of 26 patients (73.1%); cough and dyspnea in 13 of 26 (50%); headache in 3 of 26 (11.5%); asthaenia, weakness and anosmia in 2/26 (7.6%) respectively. Finally, arthralgia, myalgia, diarrhoea, chest pain, vomiting, anorexia and syncope were registered in one case each (3.8% for each symptom). 1 patient had not systemic symptoms (3,8%).
Eight patients (30.7%) had prior history of epilepsy, with all but one receiving chronic antiepileptic treatment (levetiracetam in 4 patients, lamotrigine in 2, and gabapentin in one). Ten patients with no history of epilepsy presented at least one risk factor for seizures.
3.2. Seizure characteristics
Seven patients (26.9%) presented generalised-onset seizures and 12 (46.1%) presented focal-onset seizures. From a semiological perspective, seizures were described as generalised tonic-clonic in 7 patients (26.9%); all focal seizures manifested with impaired awareness. Four patients with focal-onset seizures (33%) presented secondary generalisation.
Seven patients (26.9%) presented status epilepticus. Status epilepticus was focal in 3 patients (43%), generalised in 3 (43%), and of unknown origin in one (14%). Three patients with status epilepticus had prior history of epilepsy. The remaining 4 had no history of epilepsy, although brain CT detected active meningioma with perilesional oedema in one of these patients. Status epilepticus occurred at least 5 days after onset of COVID-19 in all patients but one, in whom the infection manifested with status epilepticus as the initial symptom.
3.3. Time of seizure onset
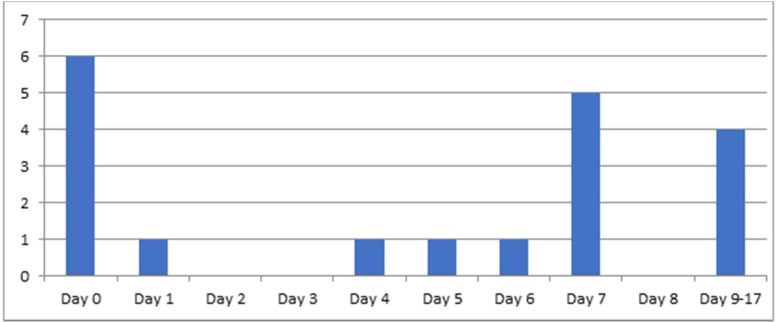
Information on the time of seizure onset was available in 19 patients. Seizures occurred a mean (SD) of 3.91 days (5.07) after onset of general symptoms (Fig. 1 ). They presented before systemic symptoms of COVID-19 in 6 patients (31%): 4 men and 2 women. These 6 patients were all older than 65 years, and none had prior history of epilepsy. None of them displayed acute or chronic epileptogenic lesions in neuroimaging studies. All patients underwent lumbar puncture with CSF analysis, which showed increased protein levels in 2 of them. All but one had focal-onset seizures. EEG findings in these patients were similar to those of the rest of the sample.
Fig. 1.
Time of seizure onset over the course of COVID-19.
In 19 patients, seizures were the only neurological manifestation of COVID-19; in the remaining 7, seizures were accompanied by such other neurological symptoms as altered mental status, decreased level of consciousness, language disturbances, and dizziness with facial palsy in one patient.
3.4. Results of neurological complementary tests
Laboratory analysis returned abnormal results in 23 patients (88.5%). These included lymphopaenia in 17 patients (73.9%), elevated C-reactive protein levels in 17 (73.9%), elevated D-dimer levels in 15 (65.2%), elevated lactate dehydrogenase levels in 9 (39%), elevated ferritin levels in 6 (23%), and decreased glomerular filtration rate in 6 (23%).
CT scans were performed in 22 cases (84%), detecting signs of acute injury in only one (meningioma with oedema and mass effect; Fig. 2 ). CT findings were normal in 11 patients and suggestive of chronic cortical vascular disease in 5. In 4 patients, neuroimaging studies only showed signs of subcortical small vessel disease. Two patients underwent MRI studies, which detected no differences as compared to CT findings. Neuroimaging findings were previously known in 2 patients, and discovered at the time of seizure assessment in the remaining patients.
Fig. 2.
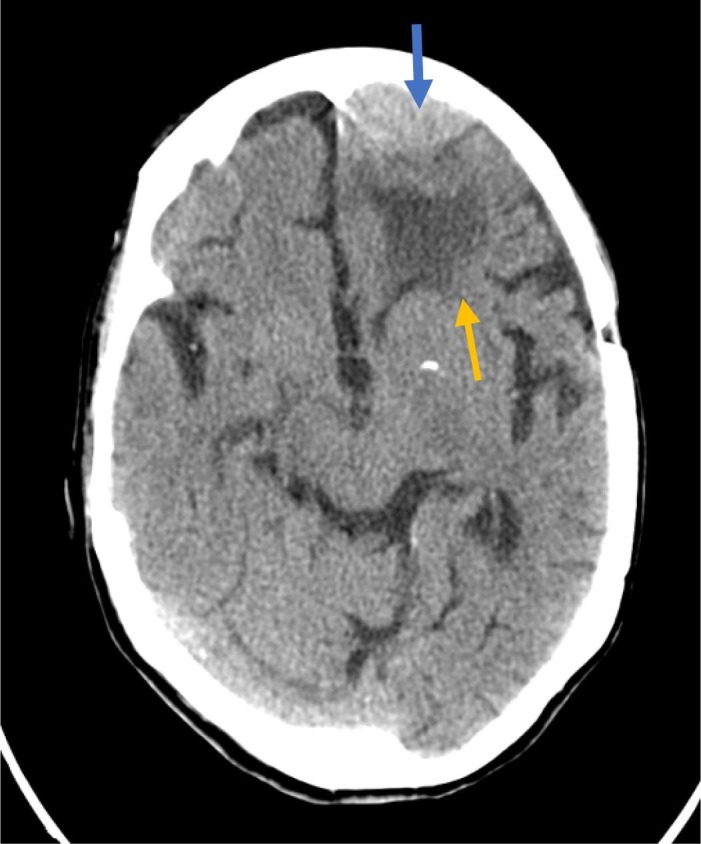
Computed tomography findings compatible with left frontal meningioma (blue arrow) and perilesional oedema (yellow arrow). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Thirteen patients (50%) underwent lumbar puncture, but data were only reported for 11. CSF analysis results were abnormal in 4 patients: 2 presented increased protein levels and the other 2 patients displayed an inflammatory pattern with lymphocytic pleocytosis. One of the latter 2 patients was a 94-year-old man without history of epilepsy, in whom seizure onset coincided with the onset of fever. He presented confusional syndrome with a generalised tonic-clonic seizure that did not resolve spontaneously and was considered status epilepticus. The CT scan showed chronic vascular lesions. The patient did not undergo EEG. He was treated with acyclovir and antiepileptic drugs, but progression of systemic and neurological symptoms was unfavourable, and the patient died. The second patient with lymphocytic pleocytosis was an 85-year-old woman with hypertension and diabetes and no history of epilepsy, who after 7 days of mild respiratory symptoms presented headache, confusion, and a focal impaired-awareness seizure. CSF C-reactive protein testing yielded negative results. EEG was not performed. Brain CT showed non-specific changes; the patient was treated with antiepileptic drugs, progressing favourably. In one of the 2 patients with lymphocytic pleocytosis, SARS-CoV-2 infection was detected by PCR, whereas in the other, PCR results were positive for varicella-zoster virus.
EEG was performed in 9 patients (34%). Results are summarised in Table 2 .
Table 2.
EEG findings in our sample of patients with COVID-19 and seizures.
| Patient* | Day of EEG** | Findings |
|---|---|---|
| 1 | 1 | Normal |
| 2 | 3 | Normal |
| 3 | 3 | Normal |
| 4 | 1 | Abnormal: diffuse background slowing |
| 5 | NA | Abnormal: diffuse background slowing |
| 6 | NA | Abnormal: diffuse background slowing |
| 7 | NA | Abnormal: generalised slowing with interictal left temporal spikes |
| 8 | 1 | Abnormal: continuous left frontotemporal sharp waves |
| 9 | 1 | Abnormal: continuous generalised spike–and-wave patterns |
*Only 9 patients underwent EEG.
**Since the first seizure.
EEG: electroencephalography; NA: not available.
3.5. Clinical outcomes
Clinical outcome data were available for 24 patients. Eighteen patients (75%) had favourable outcomes, with resolution of the seizures. Four (16.7%) were admitted to the intensive care unit (ICU) and 3 required orotracheal intubation. Five patients (20.8%) died, all of whom had either pneumonia or ARDS, with 2 presenting status epilepticus. Two patients had not fully recovered at the time of data collection.
Twenty-two patients (84%) were treated with antiepileptic drugs: 17 patients received monotherapy (15 received levetiracetam and 2 lacosamide) and 5 received combination therapy, with the most frequent combination of antiepileptic drugs being levetiracetam plus lacosamide (3 patients).
In the comparative statistical analysis, arterial hypertension was associated with increased mortality risk. Patients with status epilepticus were more likely to be admitted to the ICU, whereas older age was associated with lower likelihood of ICU admission. All other comparisons either were not statistically significant (Table 3, Table 4 ). It is important to note that these comparisons were not appropriately powered to detect some group differences or variable associations due to small sample sizes.
Table 3.
Comparative analysis of risk factors and prognosis (chi-square test). Data are expressed as absolute numbers and percentages.
| Survival (n = 21) |
Death (n = 5) |
P | No ICU (n = 22) |
ICU (n = 4) |
P | |
|---|---|---|---|---|---|---|
| Women (13 [50%]) |
10 (47.6%) | 3 (60%) | 0.192 | 13 (59.1%) | 1 (25%) | 0.208 |
| Hypertension | 10 (47.6%) | 5 (100%) | 0.033 | 15 (68.2%) | 0 | 0.011 |
| Diabetes mellitus | 9 (42.9%) | 3 (60%) | 0.490 | 11 (50%) | 1 (25%) | 0.356 |
| Heart disease | 7 (33.3%) | 0 | 0.131 | 6 (27.3%) | 1 (25%) | 0.925 |
| History of epilepsy | 7 (33.3%) | 1 (20%) | 0.562 | 7 (31.8%) | 1 (25%) | 0.786 |
| History of dementia | 3 (14.3%) | 2 (40%) | 0.190 | 5 (22.7%) | 0 | 0.289 |
| Status epilepticus | 4 (19%) | 3 (60%) | 0.064 | 4 (18.2%) | 3 (75%) | 0.018 |
| LOP/RIT and/or HCQ | 15 (71.4%) | 2 (40%) | 0.184 | 14 (63.6%) | 3 (75%) | 0.660 |
| LEV | 17 (81%) | 2 (40%) | 0.064 | 16 (72.7%) | 3 (75%) | 0.925 |
HCQ: hydroxychloroquine; ICU: intensive care unit; LEV: levetiracetam; LOP: lopinavir; RIT: ritonavir.
Statistical significance was set at P < 0.05.
Table 4.
Comparative analysis of quantitative variables using the Mann-Whitney U test.
| Survival | Death | P | No ICU | ICU | P | |
|---|---|---|---|---|---|---|
| Age (years) | 74.5 (60.3–82.8) |
89 (77.5–93) |
0.023 | 79.5 (71.3–85.5) |
56 (47–68) | 0.036 |
| Duration of neurological manifestation (days) | 6 (0–7) | 6 (1.3–9.3) |
0.551 | 6 (0–7) | 5.5 (0–11) | 0.551 |
| Time to neurological manifestation (days)* | 1 (1–8.5) | 1 (1–3.3) | 0.878 | 1 (1–4) | 4 (1–9.3) | 0.946 |
Data are expressed as absolute frequencies and percentages.
*Time from onset of infectious symptoms to onset of seizure.
ICU: intensive care unit.
Statistical significance was set at P < 0.05.
The participating neurologists considered epileptic seizures to be probably related to COVID-19 in 22 patients (84%) and coincidental in the remaining 4 (16%). A definite causal association was not suggested in any case.
3.6. A special case
The only patient in the whole registry with positive CSF PCR results for SARS-CoV-2 and presenting seizures was an 82-year-old woman admitted due to COVID-19–related pneumonia. Five days after admission, her level of consciousness decreased, with mutism and a right focal-onset epileptic seizure (leftward oculocephalic deviation and left-limb hypertonia). The initial CSF analysis detected no abnormalities, but PCR for SARS-CoV-2 yielded positive results. The patient was treated with levetiracetam and remdesivir. A brain MRI scan identified no acute lesions, but revealed chronic parietal stroke. EEG showed non-specific background slowing. An additional CSF analysis performed 10 days later showed slightly elevated leukocyte levels (16 cells/field), with 56% polymorphonuclear cells, and mildly elevated protein levels (0.78 g/L). The patient improved clinically, displaying complete recovery at discharge. The case was classified as meningoencephalitis, but the reporting neurologist was unsure about the role of SARS-CoV-2 in the patient’s neurological complications.
4. Discussion
This study describes the clinical characteristics of 26 patients from the SEN COVID-19 Registry who presented epileptic seizures during the course of COVID-19.
Our results show that epileptic seizures can occur during SARS-CoV-2 infection, both in patients with predisposing risk factors (eg, history of epilepsy, dementia, epileptogenic brain lesions) and in patients without known risk factors. Therefore, we must be alert to the possibility of seizures in patients with COVID-19. Seizures can appear in isolation or in association with other neurological symptoms or syndromes, such as encephalopathy.
Another interesting finding is the time of seizure onset over the course of COVID-19. In some patients, seizures or neurological symptoms presented early. Seizures were found to be more frequent on day 0, as the initial manifestation of the disease, preceding any systemic or respiratory symptoms, and on day 7.
Although only 6 patients presented seizures on day 0, we may hypothesise that seizures presenting as the initial symptom occur in older patients with no prior history of epilepsy, risk factors for epilepsy, or specific CSF alterations. In these patients, seizures are almost always focal. Evidence from other researchers shows that seizures may present as the initial manifestation of COVID-19 [12], [13]. In the study by Anand et al., 3 patients presented seizures but no systemic COVID-19 symptoms. All 3 were older individuals, as in our series, although one of them did have history of epilepsy. Two presented generalised tonic-clonic seizures and one displayed extensive encephalomalacia on brain neuroimaging studies; none of them underwent lumbar puncture. The literature also includes several case reports [14], [15]; these patients present several differences with respect to our patients, but also some similarities, such as the absence of risk factors for seizures. Larger series are needed to better define the characteristics of these patients.
Seizures frequently occurred on day 7, with some patients experiencing seizures thereafter; it has been suggested that the cytokine storm starts after this time point. This raises questions regarding the mechanisms underlying the potential association between COVID-19 and seizures. Vohora et al. [16] reviewed 3 possible mechanisms. Firstly, presence of seizures in some patients with COVID-19 may be a mere coincidence. Secondly, certain non-specific mechanisms common to infections or systemic diseases (eg, sepsis, hypoxia, fever) may increase susceptibility to seizures; in these events, the excessive release of cytokines and other inflammatory molecules can cause neuronal damage. It is therefore plausible that later onset seizures (after day 7) were related to this pathogenic mechanism. And finally, direct invasion of the nervous system may be promoted by the virus’ affinity for angiotensin-converting enzyme 2 receptors or by damage to the blood–brain barrier induced by systemic inflammation.
Most patients in our series had no history of epilepsy before the infection. Some of them presented such risk factors as dementia or cerebrovascular disease, but only one had an acute epileptogenic brain lesion. On the other hand, patients with severe infections, fever, hypoxia, treatment with certain drugs, etc, are known to present increased risk of acute symptomatic seizures, especially those with history of neurological diseases or reduced cognitive reserve [17]. Most of our patients presented these characteristics. Some patients also displayed an inflammatory pattern in the CSF, which suggests that seizure aetiology is usually multifactorial. Other studies have attributed seizures to systemic complications [16], [18], [19]). Thorough assessment is necessary to establish a correct diagnosis. EEG is particularly useful in differentiating encephalopathy, a very common syndrome in COVID-19 patients, from non-convulsive status epilepticus [20], [21], [22], and also in detecting subtle or subclinical seizures.
One of our patients tested positive for SARS-CoV-2 in CSF PCR; however, the reporting neurologists could not establish whether meningoencephalitis was caused by SARS-CoV-2 due to the lack of a standardised CSF PCR protocol, the absence of specific inflammatory lesions on MRI, and the patient’s nonspecific clinical picture. Few cases have been published of SARS-CoV-2 detection in the CSF [23], [24], which suggests that direct central nervous system invasion by SARS-CoV-2 is very infrequent [25]. Our results and available literature suggest that CSF is sometimes positive for SARS-CoV-2 when tested by PCR, as has been reported for rare cases, although the true prevalence of this remains unknown due to limited study to date.
To our knowledge, this study reports one of the largest series to date of patients with seizures associated with acute COVID-19. Several series have been published of patients with neurological complications during COVID-19 [2], [3], [4], [5], [6]. All of them report low or very low incidence rates of epileptic seizures. For example, the ALBACOVID registry [2] reported an incidence of neurological complications of 0.7%, and did not include any case of status epilepticus. In another series of 4491 patients with COVID-19, the authors report a prevalence rate of 1.6% [26]. In a multicentre study aiming to determine the incidence of acute symptomatic seizures, including 304 patients hospitalised due to COVID-19, no cases were found of seizures or status epilepticus [27]. In a study by Xiong et al. [28], no cases of epileptic seizures were reported. Hepburn et al. [29] described 2 patients admitted to the ICU with severe COVID-19 who developed acute symptomatic seizures, and the largest series published to date reported an incidence rate of 1.49% among 1550 patients hospitalised with COVID-19 (0.84% were new-onset seizures and 0.65% were recurrent seizures) [12]. Our sample highlights a significant number of patients who experience seizures during COVID-19, some of whom developed status epilepticus, even though they had no prior history of seizures. These data implies that series are more common in COVID-19 than previously reported in the mentioned literature, although we do not have a clear hypothesis to explain these differences.
Some studies have also evaluated the impact of the COVID-19 pandemic on patients with epilepsy. Some studies report worsening of pre-existing epilepsy in some patients. This has been attributed to stress [30], [31], [32], poor sleep quality and use of many antiepileptic drugs [30] tumour-related epilepsy, insomnia, reduced income, and drug resistance [31], [32]. Interestingly, in a cohort of 255 patients with epilepsy, 25 patients were found to have presented an increase in seizure frequency, but none of the 5 patients who presented COVID-19 reported epilepsy worsening [31], Conde et al. [33] distributed an online survey to 312 patients during lockdown in Spain, finding that 31.12% reported seizure worsening, especially those with mood and sleep disorders. Therefore, according to published evidence, this worsening seems to be triggered by the collateral effects of the pandemic, rather than by the virus itself. Although this variable is not directly analysed in our study, the fact that a significant percentage of our patients (almost 30%) had history of epilepsy suggests that, in addition to the collateral effects of the pandemic, SARS-CoV-2 infection may itself trigger seizures in patients diagnosed with epilepsy.
This study has several limitations. Its design inevitably introduces a selection bias, as participation in the registry was voluntary. Therefore, only a small percentage of all cases of COVID-19 have been included, as a total of 1 125 222 patients were hospitalised from the beginning of the pandemic to 30 June 2020. Cases were selected at the discretion of the reporting neurologists. However, many Spanish regions are represented in the registry, especially those most affected by the pandemic, such as Madrid, the Basque Country, and Catalonia. Furthermore, the information provided is incomplete in some cases, and neurological work-up studies were not systematically performed. Our series is not sufficiently large to draw conclusions about risk or prognostic factors. Physicians must be encouraged to report more cases of seizures in the context of COVID-19, and to provide as much information as possible.
5. Conclusions
COVID-19 may be associated with epileptic seizures, either in isolation or in the context of encephalopathy. Seizures may present in patients with and without known epilepsy. In patients with no history of epilepsy, seizures generally appear in individuals with predisposing factors. Seizures often occur within a week, but sometimes occurs thereafter.
The incidence of seizures in patients with COVID-19 may be higher than reported or expected. Given that most patients will present acute symptomatic seizures, we recommend performing complementary tests to rule out frequent causes of seizures, including SARS-CoV-2 infection, a possible trigger factor during the COVID-19 pandemic.
Data sharing policy.
The authors confirm that the data supporting the findings of this study are provided in the article and/or its supplementary materials.
Ethical publication statement.
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
The authors wish to thank the Spanish Society of Neurology’s translation department for their language editing services.
References
- 1.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y.i., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Romero-Sánchez C.M., Díaz-Maroto I., Fernández-Díaz E., Sánchez-Larsen Á., Layos-Romero A., García-García J., et al. Neurologic manifestations in hospitalized patients with COVID-19: The ALBACOVID registry. Neurology. 2020;95(8):e1060–e1070. doi: 10.1212/WNL.0000000000009937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Helms J., Kremer S., Merdji H., Clere-Jehl R., Schenck M., Kummerlen C., et al. Neurologic Features in Severe SARS-CoV-2 Infection. N Engl J Med. 2020;382(23):2268–2270. doi: 10.1056/NEJMc2008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ellul M.A., Benjamin L., Singh B., Lant S., Michael B.D., Easton A., et al. Neurological associations of COVID-19. Lancet Neurol. 2020;19(9):767–783. doi: 10.1016/S1474-4422(20)30221-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen X, Laurent S, Onur OA, et al. A systematic review of neurological symptoms and complications of COVID-19 [published online ahead of print, 2020 Jul 20]. J Neurol. 2020;1-11. doi:10.1007/s00415-020-10067. [DOI] [PMC free article] [PubMed]
- 6.Chuang D.T., Aydemir S., Magda P., Thomas C., Zarnegar R. Neurological manifestations as primary presentation of COVID‐19 in hospitalized patients. Acta Neurol Scand. 2021;143(5):569–574. doi: 10.1111/ane.13399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuroda N. Epilepsy and COVID-19: Updated evidence and narrative review. Epilepsy Behav. 2021;116:107785. doi: 10.1016/j.yebeh.2021.107785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.García-Azorín D., Abenza Abildúa M.J., Erro Aguirre M.E., Fernández Fernández S., García Moncó J.C., Guijarro C., et al. Neurological presentations of COVID-19: findings from the Spanish Society of Neurology NeuroCOVID-19 Registry. J Neurol Sci. 2020 doi: 10.1016/j.jns.2020.117283. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.https://www.mscbs.gob.es/profesionales/saludPublica/ccayes/alertasActual/nCovChina/documentos/COVID19_Estrategia_vigilancia_y_control_e_indicadores.pdf.
- 10.Metlay J.P., Waterer G.W., Long A.C., Anzueto A., Brozek J., Crothers K., et al. Diagnosis and treatment of adults with community-acquired pneumonia: an official clinical practice guideline of the American Thoracic Society and infectious disease Society of AmericaAm. J Respir Crit Care Med. 2019;200(7):e45–e67. doi: 10.1164/rccm.201908-1581ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fisher R.S., Cross J.H., French J.A., Higurashi N., Hirsch E., Jansen F.E., et al. Operational classification of seizure types by the international league against epilepsy: position paper of the ILAE commission for classification and terminology. Epilepsia. 2017;58(4):522–530. doi: 10.1111/epi.13670. [DOI] [PubMed] [Google Scholar]
- 12.Sun M., Ruan X., Li Y., Wang P., Zheng S., Shui G., et al. Clinical characteristics of 30 COVID-19 patients with epilepsy: A retrospective study in Wuhan. Int J Infectious Diseases. 2021;103:647–653. doi: 10.1016/j.ijid.2020.09.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anand P., Al-Faraj A., Sader E., Dashkoff J., Abdennadher M., Murugesan R., et al. Seizure as the presenting symptom of COVID-19: A retrospective case series. Epilepsy Behav. 2020;112:107335. doi: 10.1016/j.yebeh.2020.107335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abdulsalam M.A., Abdulsalam A.J., Shehab D. Generalized status epilepticus as a possible manifestation of COVID-19. Acta Neurol Scand. 2020 Oct;142(4):297–298. doi: 10.1111/ane.13321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lyons S., O’Kelly B., Woods S., Rowan C., Brady D., Sheehan G., et al. Seizure with CSF lymphocytosis as a presenting feature of COVID-19 in an otherwise healthy young man. Seizure. 2020;80:113–114. doi: 10.1016/j.seizure.2020.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vohora D, Jain S, Tripathi M, Potschka H. COVID-19 and seizures: Is there a link? Epilepsia. 2020 Sep 17:10.1111/epi.16656. doi: 10.1111/epi.16656. Epub ahead of print. PMID: 32944929; PMCID: PMC7537056. [DOI] [PMC free article] [PubMed]
- 17.Sen A., Jette N., Husain M., Sander J.W. Epilepsy in older people. Lancet. 2020 Feb 29;395(10225):735–748. doi: 10.1016/S0140-6736(19)33064-8. PMID: 32113502. [DOI] [PubMed] [Google Scholar]
- 18.Asadi-Pooya A.A. Seizures associated with coronavirus infections. Seizure. 2020;79:49–52. doi: 10.1016/j.seizure.2020.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Asadi-Pooya AA, Simani L, Shahisavandi M, Barzegar Z. COVID-19, de novo seizures, and epilepsy: a systematic review. Neurol Sci. 2020 Nov 25:1–17. doi: 10.1007/s10072-020-04932-2. Epub ahead of print. PMID: 33237493; PMCID: PMC7686454. [DOI] [PMC free article] [PubMed]
- 20.Besnard S., Nardin C., Lyon E., Debroucker T., Arjmand R., Moretti R., et al. Electroencephalographic Abnormalites in SARS-CoV-2 Patients. Front Neurol. 2020 Nov;26(11) doi: 10.3389/fneur.2020.582794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Galanopoulou A.S., Ferastraoaru V., Correa D.J., Cherian K., Duberstein S., Gursky J., et al. EEG findings in acutely ill patients investigated for SARS-CoV-2/COVID-19: A small case series preliminary report. Epilepsia Open. 2020 May 17;5(2):314–324. doi: 10.1002/epi4.12399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen W., Toprani S., Werbaneth K., Falco-Walter J. Status epilepticus and other EEG findings in patients with COVID-19: A case series. Seizure. 2020;81:198–200. doi: 10.1016/j.seizure.2020.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moriguchi T., Harii N., Goto J., Harada D., Sugawara H., Takamino J., et al. A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. Int J Infectious Diseases. 2020;94:55–58. doi: 10.1016/j.ijid.2020.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang Y.H., Jiang D., Huang J.T. SARS-CoV-2 Detected in Cerebrospinal Fluid by PCR in a Case of COVID-19 Encephalitis. Brain Behav Immun. 2020;87:149. doi: 10.1016/j.bbi.2020.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou Z., Kang H., Li S., Zhao X. Understanding the neurotropic characteristics of SARS-CoV-2: from neurological manifestations of COVID-19 to potential neurotropic mechanisms. J Neurol. 2020;267(8):2179–2184. doi: 10.1007/s00415-020-09929-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frontera JA, Sabadia S, Lalchan R, Fang T, Flusty B, Millar-Vernetti P, Snyder T, Berger S, Yang D, Granger A, Morgan N, Patel P, Gutman J, Melmed K, Agarwal S, Bokhari M, Andino A, Valdes E, Omari M, Kvernland A, Lillemoe K, Chou SH, McNett M, Helbok R, Mainali S, Fink EL, Robertson C, Schober M, Suarez JI, Ziai W, Menon D, Friedman D, Friedman D, Holmes M, Huang J, Thawani S, Howard J, Abou-Fayssal N, Krieger P, Lewis A, Lord AS, Zhou T, Kahn DE, Czeisler BM, Torres J, Yaghi S, Ishida K, Scher E, de Havenon A, Placantonakis D, Liu M, Wisniewski T, Troxel AB, Balcer L, Galetta S. A Prospective Study of Neurologic Disorders in Hospitalized COVID-19 Patients in New York City. Neurology. 2020 Oct 5:10.1212/WNL.0000000000010979. doi: 10.1212/WNL.0000000000010979. Epub ahead of print. PMID: 33020166.
- 27.Lu L.u., Xiong W., Liu D., Liu J., Yang D., Li N., et al. New onset acute symptomatic seizure and risk factors in coronavirus disease 2019: A retrospective multicenter study. Epilepsia. 2020;61(6) doi: 10.1111/epi.16524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xiong W, Mu J, Guo J, et al. New onset neurologic events in people with COVID-19 infection in three regions in China [published online ahead of print, 2020 Jun 17]. Neurology. 2020;10.1212/WNL.0000000000010034. doi:10.1212/WNL.0000000000010034.
- 29.Hepburn M., Mullaguri N., George P., Hantus S., Punia V., Bhimraj A., et al. Acute Symptomatic Seizures in Critically Ill Patients with COVID-19: Is There an Association? Neurocrit Care. 2021;34(1):139–143. doi: 10.1007/s12028-020-01006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang S., Wu C., Jia Y., Li G., Zhu Z., Lu K., et al. COVID‐19 outbreak: The impact of stress on seizures in patients with epilepsy. Epilepsia. 2020;61(9):1884–1893. doi: 10.1111/epi.16635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fonseca E., Quintana M., Lallana S., Luis Restrepo J., Abraira L., Santamarina E., et al. Epilepsy in time of COVID‐19: A survey‐based study. Acta Neurol Scand. 2020;142(6):545–554. doi: 10.1111/ane.13335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sanchez-Larsen A., Gonzalez-Villar E., Díaz-Maroto I., Layos-Romero A., Martínez-Martín Á., Alcahut-Rodriguez C., et al. Influence of the COVID-19 outbreak in people with epilepsy: Analysis of a Spanish population (EPICOVID registry) Epilepsy Behav. 2020;112:107396. doi: 10.1016/j.yebeh.2020.107396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Conde Blanco E, Manzanares I, Centeno M, Khawaja M, Betrán O, Donaire A, Carreño M. Epilepsy and lockdown: A survey of patients normally attending a Spanish centre. Acta Neurol Scand. 2020 Sep 29:10.1111/ane.13354. doi: 10.1111/ane.13354. Epub ahead of print. PMID: 32990951; PMCID: PMC7646661. [DOI] [PMC free article] [PubMed]