Abstract
The development of a microbial population and changes in the physicochemical and sensorial characteristics of Mediterranean boque (Boops boops), called gopa in Greece, stored aerobically at 0, 3, 7, and 10°C were studied. Pseudomonads and Shewanella putrefaciens were the dominant bacteria at the end of the storage period, regardless of the temperature tested. Enterobacteria and Brochothrix thermosphacta also grew, but their population density was always 2 to 3 log10 CFU g−1 less than that of pseudomonads. The concentration of potential indicators of spoilage, glucose and lactic acid, decreased while that of the α-amino groups increased during storage. The concentrations of these carbon sources also decreased on sterile fish blocks inoculated with strains isolated from fish microbial flora. The organic acid profile of sterile fish blocks inoculated with the above-mentioned bacteria and that of naturally spoiled fish differed significantly. An excellent correlation (r = −0.96) between log10 counts of S. putrefaciens or Pseudomonas bacteria with freshness was observed in this study.
It is well known from studies with meat that although low in comparison with those of protein and lipids, the concentrations of compounds such as glycogen, glucose, and lactate are all sufficient to support massive microbial growth. These compounds can affect the type (e.g., saccharolytic, proteolytic) and rate of spoilage and, moreover, seem to be the principal precursors of those microbial metabolites that we perceive as spoilage (59). The concentrations of, e.g., glucose and lactate in fish are similar to those reported for meat (43, 60, 64), and their importance has been underestimated (31), although they have been used alternatively for sensory and microbiological analysis to determine fish freshness (68). Among the disadvantages of sensory analysis, which is probably the most appropriate method, and microbiological analysis is that the reliability of the former depends on highly trained panels to minimize subjectivity, which makes it costly and unattractive for routine analysis. For the latter, at least in traditional form (microbial counts, etc.), we get retrospective information and presuppose that the specific spoilage organisms are known and detectable by a chosen technique.
For this reason, both microbiological and sensory analyses can be replaced by biochemical methods (e.g., based on nucleotide catabolism, production of amines, trimethylamine [TMA], and sulfur compounds) or physical methods (Torymeter, K value) which associate findings with microbial growth on fish (29, 30). With respect to spoilage indicators or microbial metabolites, there is a lack of relevant information about fish compared with that about meat. The basis of these methods is that as bacteria grow on fish, they utilize nutrients and produce by-products. Determination of the quantities of these metabolites could provide us with information about the degree of spoilage.
However, fish spoilage depends on specific spoilage organisms, and moreover, these are not the same in every case and are dependent on the climatic and storage conditions, the type of fish, and even the place in which the fish was harvested (19, 31). For example, Shewanella putrefaciens is the only specific spoilage bacterium of marine cold-water fish stored in ice, and the number of S. putrefaciens bacteria is inversely related to remaining shelf life (31). On the other hand, Pseudomonas sp. and S. putrefaciens are the specific spoilage bacteria of marine and freshwater tropical fish stored in ice (31). No report of similar studies with fish from the Mediterranean Sea—temperate waters—is available in the literature.
The aim of this work was to provide information on (i) the microbial attributes of the Mediterranean boque (Boops boops), a marine fish very popular with consumers with emphasis on specific spoilage organisms and (ii) the role of microbial metabolites (e.g., lactate, formic acetate, free amino acids, etc.) as indicators of spoilage in fish stored aerobically at 0, 3, 7, and 10°C.
MATERIALS AND METHODS
Preparation of fish fillets.
Fresh, gutted boque (B. boops) stored in ice after capture was bought from a local fishery shop within 6 to 8 h after being caught. The fish were transported in ice to the laboratory within 30 to 45 min of their purchase. On arrival at the laboratory, they were divided into quarters, and the portions were kept at 0, 3, 7, and 10°C. Three independent storage experiments were conducted, and in each experiment there were 12 sampling times per temperature tested. On each sampling occasion, two fish were analyzed.
Sample preparation.
Fish (25 g) was transferred aseptically to a stomacher bag (Seward Medical, London, United Kingdom), 225 ml of 0.1% peptone water with salt (NaCl, 0.85%, wt/vol) was added, and the mixture was homogenized for 60 s with a stomacher (Lab Blender 400; Seward Medical).
Microbiological media and enumeration.
Samples (0.1 ml) of serial dilutions of treated (either inoculated with the isolates or naturally inoculated) fish homogenates were spread on the surface of the appropriate media in petri dishes for determination of the total viable count on modified Long-and-Hammer agar (66) and incubated at 10°C for 7 days. The medium was composed of the following (grams per liter of distilled water): Proteose Peptone (P 0431; Sigma), 20; gelatin (4070; Merck), 40; K2HPO4, 1; NaCl, 10; agar (L11; Oxoid), 15; ammonium ferric citrate, 0.25. Pseudomonads were determined on cetrimide fusidin cephaloridine agar (Oxoid code CM 559, supplemented with selective supplement SR 103) after incubation at 20°C for 2 days (52). (iii) Brochothrix thermosphacta was determined on streptomycin sulfate-thallous acetate-cycloheximide (actidione) agar (Oxoid code CM 881, supplemented with selective supplement SR 151) after incubation at 20°C for 3 days (22). For members of the family Enterobacteriaceae and hydrogen sulfide-producing bacteria, 1.0 ml was inoculated into 10 ml of molten (45°C) violet red bile glucose agar (Oxoid code CM 485) and iron agar (IA; Oxoid code CM 867), respectively. After setting, a 10-ml overlay of molten medium was added. For the former, incubation was at 30°C for 24 h. The large colonies with purple haloes were counted (54). IA plates were incubated at 20°C for 4 days (34). Black colonies formed by production of H2S were enumerated after 2 to 3 days (23).
Detection of Photobacterium phosphoreum was done by using the direct microscopy method. Thirty colonies were selected randomly from modified Long-and-Hammer medium (66) containing 100 to 200 colonies. Large, gram-negative coccobacilli similar to those described by Dalgaard (8) were counted as P. phosphoreum (11).
Three replicates of at least three appropriate dilutions (1) were enumerated. All plates were examined visually for typical colony types and morphological characteristics associated with each growth medium. In addition, the selectivity of each medium was checked routinely by Gram staining and microscopic examination of smears prepared from randomly selected colonies from all of the media.
Bacterial strains.
Pure cultures of Pseudomonas sp., S. putrefaciens, P. phosphoreum, B. thermosphacta, and lactic acid bacteria were screened in sterile boque fish muscle blocks for spoilage potential, i.e., the ability to produce chemical changes typical of the spoiling product substrate used in inoculation studies of sterile gopa fish tissue. With the exception of P. phosphoreum, all organisms (B. thermosphacta, Shewanella sp., Pseudomonas sp., and lactic acid bacteria) were selected at the latest stage of aerobic storage of boque fish at 3°C. Colonies were isolated from streptomycin sulfate-thallus acetate-cycloheximide (actidione) agar (22), IA, Pseudomonas agar supplemented with cetrimide fusidin cephaloridine agar (52), and MRS medium and characterized further as B. thermosphacta, S. putrefaciens, Pseudomonas sp., and Lactobacillus sp., respectively. The catalase test was used for further confirmation of B. thermosphacta. The isolates from Mead-and-Adams (52) and IA media were examined for colony shape and pigmentation (44), Gram reaction (36), cell morphology (phase-contrast microscopy), flagellar arrangement (51), oxidase reaction (46), and aerobic and anaerobic breakdown of glucose (39). Moreover, the API 20NE System (BioMérieux) was applied for the following tests: nitrite reduction, indole production, arginine dihydrolase, urease, esculin hydrolysis, gelatin hydrolysis, β-galactosidase, and assimilation of the energy sources glucose, arabinose, mannose, mannitol, N-acetylglucosamine, maltose, gluconate, caprate, adipate, malate, citrate, and phenyl acetate. Gram-negative, motile rods with positive catalase and oxidase reactions, oxidative glucose metabolism, and arginine dihydrolase activity were identified as Pseudomonas sp. if they did not reduce trimethylamine oxide (TMAO) or produce H2S. The identity of Shewanella sp. was further confirmed by salmon pink pigment on nutrient agar and copious H2S production in test tubes with soft agar composed of the following (grams per liter of distilled water): tryptone, 10; sodium chloride, 5; Na2S2O3 · 5H2O, 0.5; l-cysteine, 0.6; ferric citrate, 0.3; agar, 4.5 (pH 7.4 ± 0.2). It was also inoculated by stabbing and incubated at 20 to 25°C for 7 days. Copious production of H2S was noted by blackening of the medium after 24 h. For the characterization of Lactobacillus sp., production of CO2 and growth at different temperatures (3, 15, 30, and 45°C), salt concentrations (8 and 10%), and pHs (3, 3.7, and 8.5) were used in combination with the API 50CH tests.
The inocula were prepared as follows. Each bacterial strain was maintained on slopes of the appropriate medium agar at 4°C. A loopful of a fresh working subculture (ca. 106 CFU) was used for inoculation of the corresponding broth (100 ml in a 250-ml conical flask). The flask was incubated aerobically without agitation at 25°C for 18 h. Cells were harvested, washed by centrifugation, and washed with sterile saline, and an appropriate dilution in saline (NaCl, 0.85%, wt/vol) was used for inoculation.
For P. phosphoreum (kindly provided by P. Dalgaard, Danish Ministry of Fisheries, Lyngby, Denmark), growth medium broth (13) was used, and it was precultured and incubated at 15°C.
Growth on sterile fish blocks.
Fish tissue blocks were prepared as described by Gram and Melchiorsen (32). Gopa were killed, bled, and iced immediately after being caught and then brought to the laboratory within 5 to 6 h. The skin surface was cleaned with 100% alcohol to sanitize the surface. The skin of the back muscle was removed aseptically, and the sterile tissue below was excised and cut into 15- to 20-g pieces. The pieces were inoculated immediately with the bacterial isolates. To prepare fish extract, fillets were blended with distilled water (1:1.5) for 60 s. The homogenate was heated at 80°C for 3 min, and then it was passed through cheese cloth. The resulting stock was subsequently filtered with 0.45- and 0.22-μm (pore size) microfilters (Millipore) to ensure sterile fish juice. Bacterial isolates were grown at 3 and 10°C in fish extract for 3 to 5 days, and the cultures were diluted in 3 and 10°C sterile physiological saline. Appropriate dilutions were prepared at the above-mentioned temperatures, and then the tissue pieces were dipped in the bacterial suspension for 3 min in chilled, sterile, 17-cm-diameter plastic petri dishes. The inoculated tissue pieces were placed in sterile, 17-cm-diameter plastic petri dishes. Control tissue was dipped in sterile diluent. All inoculations were done in triplicate, and two independent trials were carried out. One 2-g piece was removed from each sample for microbiological and physicochemical analysis.
Curve fitting.
The growth data (12 sampling times) from the enumeration of different groups of microbial association were fitted to estimate the maximum specific growth rate (μmax). The Gompertz function was used (24). The modified Gompertz curve is defined by the equation log10 N(t) = A + C exp{−exp[−B(t − M)]}, where N(t) is the bacterial count at time t, B is the relative maximum growth rate (log10 CFU hour−1), M is the time (hours) at which the absolute growth rate is at a maximum, A is the lowest asymptote of the curve, and C is the upper asymptote of the curve. After fitting of the curves, the parameters B, M, C, and A were obtained by using nonlinear regression with the Fig.P version 2.5 software (2). The parameters were used to calculate μmax with the equation μmax = BC/e.
pH.
The pH value was recorded by a pH meter (Metrohm 691), and the glass electrode was applied directly to the flesh.
Chemical analysis.
Fish flesh (10 g) was transferred to a beaker, 100 ml of perchloric acid (HClO4, 1 M) was added, and the contents were homogenized for 1 min with a blender. The homogenate was centrifuged (15 min at 4,000 × g), and then the supernatant was decanted and stored at −80°C. After thawing, a portion of the deproteinized supernatant was neutralized with KOH (5 M) and diluted with water to 1:20 to give a solution for analysis. l-Lactic acid and d-glucose were assayed enzymatically by the methods described by Noll (58) and Kunst et al. (47), respectively, in which 10 g was reduced to a fine suspension with 100 ml of cold water (3 to 5°C) in an Omni mixer (Waring, New Hartford, United Kingdom). The suspension was centrifuged (5 min at 4,000 × g at 3°C) and filtered, and the clear filtrate was used to determine water-soluble protein and α-amino groups by using the spectrophotometric assays described by Church et al. (6). TMA was quantitatively measured by a colorimetric method (3). TMA content was expressed as milligrams of TMA-N per 100 g of fish.
HPLC analysis of organic acids.
After the microbiological examination, a portion (20 ml) was filtered with Whatman no. 1 paper. The clear filtrate, after addition of trifluoroacetic acid (1% [vol/vol] final concentration) and sodium azide (final concentration, 0.1%), was centrifuged (10 min at 3,000 × g), filtered with a Millipore 0.22-μm-pore-size filter, and stored at −80°C before high-performance liquid chromatography (HPLC) analysis. The profiles of the organic acids of fish were analyzed by HPLC (Spectra Physics P2000 two-pump system with a UV/VIS detector using low-inertia scanning technology—similar to a photodiode array—and software from Spectra Physics, San Jose, Calif.) using a Rheodyne 7125 injector and an Aminex HPX-87H 5-μm column (300 by 7.8 mm; Bio-Rad Laboratories, Richmond, Calif.). The compounds were separated isocratically with 0.009 N H2SO4 in distilled water (flow rate, 0.7 ml min−1). The peak width was 12, the peak threshold was 600, and the attenuation was 32. The whole spectra (190 to 330 nm) of the chromatograms were analyzed. The solvents were HPLC grade, and for identification of peaks, solutions of reference substances (citric, lactic, acetic, tartaric, malic, succinic, formic, and propionic acids) were analyzed by using the same program, and the retention times (RT) and spectra were compared. The contribution of each identified compound was expressed as the peak area eluted in each chromatograph. The precision of the results was always better than ±5%.
Sensory evaluation.
The dorsal half of each fillet was heated (80°C, 15 min) in an unsealed plastic bag, and the quality was assessed by 8 to 10 trained panelists. A scoring scale with three categories was used. Class 1 corresponded to high-quality fillets without any off odor or off flavor, class 2 corresponded to fillets that had slight off odors or flavors but were still acceptable, and class three corresponded to fillets of unacceptable quality. The shelf life limit was defined as the point when 50% of the panelists rejected the fillets (11).
Prediction of remaining shelf life.
At each temperature, the relationship between the remaining shelf life, estimated by sensory methods, and the log of the total viable counts of Pseudomonas and H2S-producing bacteria was calculated by using the following simple linear regression: remaining shelf life (days) = intercept (β0) + slope (β1) × log10(microbial numbers/g). The coefficient of determination (R2) was calculated as a measure of the percentage of the total variability in the remaining shelf life data explained by the model. Fig.P version 2.5 software (2) was used to fit all models and calculate the parameters.
RESULTS
Microbiological analysis.
The changes in the microbial flora of boque fish during storage under aerobic conditions at 0, 3, 7, and 10°C are shown in Table 1. For practical reasons, Table 1 shows only those microbiological changes (7 out of 12 to 15) occurring in samples taken at the same time for all storage conditions, while for the calculation of μmax, all observations were taken into account (Table 2). Total viable counts reached ca. 9 log10 CFU g−1 by the end of the storage period under aerobic conditions, regardless of the storage temperature used. It needs to be noted that there was no statistically significant difference between bacterial numbers on Long-and-Hammer medium and those on IA when all colonies were counted in the latter medium, although in a most cases, IA and Long-and-Hammer medium gave higher counts than plate count agar (results not shown). Pseudomonads were dominant at that time in fish stored at all of the temperatures tested, followed by S. putrefaciens (Table 1). However, it should be noted that the μmax of S. putrefaciens was found to be higher than that of Pseudomonas sp. (Table 2) and, thus, ended up close to the Pseudomonas value, even though the initial counts were lower (1.5-log difference) (Table 1). B. thermosphacta, a bacterium more common in meat products, was found to be the third main member of the microbial association of boque fish (Table 1) and outgrew the Enterobacteriaceae.
TABLE 1.
Changes in total viable countsa of pseudomonads, B. thermosphacta, S. putrefaciens, Enterobacteriaceae, and P. phosphoreumb on Mediterranean boque stored aerobically at 0, 3, 7, or 10°C
| Temp (°C) and storage time (h) | Mean total viable count ± SD
|
||||
|---|---|---|---|---|---|
| Overall | Pseudomonads | B. thermosphacta | S. putrefaciens | Enterobacteriaceae | |
| NA,c 0 | 5.55 ± 0.67 | 4.37 ± 0.45 | 2.77 ± 0.24 | 3.3 ± 0.32 | 2.34 ± 0.52 |
| 0 | |||||
| 52 | 5.93 ± 0.69 | 4.69 ± 0.37 | 3.00 ± 0.32 | 3.60 ± 0.21 | 2.95 ± 0.64 |
| 99 | 6.75 ± 0.28 | 5.53 ± 0.35 | 3.90 ± 0.41 | 4.80 ± 0.12 | 3.00 ± 0.69 |
| 147 | 7.62 ± 0.46 | 6.33 ± 0.32 | 4.70 ± 0.12 | 5.90 ± 0.05 | 3.47 ± 0.36 |
| 172 | 8.05 ± 1.02 | 7.02 ± 0.25 | 5.00 ± 0.21 | 6.60 ± 1.03 | 4.00 ± 0.45 |
| 220 | 8.46 ± 1.08 | 8.18 ± 0.67 | 5.30 ± 0.11 | 7.60 ± 0.84 | 4.69 ± 0.23 |
| 248 | 8.45 ± 0.98 | 8.36 ± 1.23 | 5.25 ± 0.05 | 7.47 ± 0.75 | 4.60 ± 0.35 |
| 3 | |||||
| 52 | 5.93 ± 0.36 | 4.44 ± 0.32 | 3.07 ± 0.11 | 4.36 ± 0.23 | 2.77 ± 0.51 |
| 99 | 7.14 ± 0.41 | 6.60 ± 0.17 | 4.38 ± 0.23 | 6.78 ± 0.26 | 2.97 ± 0.36 |
| 147 | 7.97 ± 0.11 | 7.26 ± 0.30 | 5.10 ± 0.32 | 6.95 ± 0.13 | 4.35 ± 0.45 |
| 172 | 8.60 ± 0.85 | 8.12 ± 0.27 | 5.40 ± 0.41 | 7.64 ± 0.74 | 4.47 ± 0.23 |
| 220 | 8.95 ± 1.04 | 8.40 ± 0.89 | 5.69 ± 0.21 | 7.83 ± 0.56 | 5.30 ± 0.42 |
| 248 | 8.69 ± 1.10 | 8.58 ± 0.90 | 5.77 ± 0.15 | 7.95 ± 0.99 | 5.69 ± 0.38 |
| 7 | |||||
| 20 | 6.38 ± 0.36 | 5.48 ± 0.32 | 3.32 ± 0.14 | 4.84 ± 0.12 | 3.38 ± 0.63 |
| 30 | 7.17 ± 0.53 | 6.16 ± 0.25 | 4.43 ± 0.32 | 5.39 ± 0.32 | 3.84 ± 0.53 |
| 47 | 7.58 ± 0.34 | 7.06 ± 0.25 | 5.04 ± 0.37 | 6.53 ± 0.38 | 4.61 ± 0.42 |
| 54 | 8.06 ± 0.65 | 7.33 ± 0.30 | 5.59 ± 0.40 | 6.74 ± 0.54 | 4.60 ± 0.36 |
| 71 | 8.98 ± 0.66 | 8.13 ± 0.67 | 5.77 ± 0.65 | 7.87 ± 0.87 | 5.38 ± 0.36 |
| 97 | 8.76 ± 1.04 | 8.43 ± 0.74 | 6.30 ± 0.62 | 7.99 ± 0.80 | 6.00 ± 0.54 |
| 10 | |||||
| 20 | 6.56 ± 0.65 | 5.65 ± 0.45 | 3.54 ± 0.23 | 5.14 ± 0.26 | 3.56 ± 0.48 |
| 30 | 7.33 ± 0.54 | 6.07 ± 0.63 | 4.60 ± 0.34 | 5.71 ± 0.35 | 4.06 ± 0.39 |
| 47 | 8.55 ± 0.35 | 7.00 ± 0.68 | 5.71 ± 0.37 | 7.39 ± 0.37 | 5.11 ± 0.40 |
| 54 | 8.72 ± 0.36 | 7.77 ± 0.32 | 6.00 ± 0.37 | 7.79 ± 0.30 | 5.77 ± 0.30 |
| 71 | 9.29 ± 0.37 | 8.39 ± 1.05 | 6.00 ± 0.30 | 7.90 ± 0.42 | 6.07 ± 0.30 |
| 97 | 9.26 ± 0.30 | 8.84 ± 0.68 | 6.47 ± 0.40 | 8.10 ± 0.64 | 6.25 ± 0.32 |
Log10 CFU gram−1.
P. phosphoreum was less than 1% of the total viable count.
NA, not applicable.
TABLE 2.
Overall μmax hour−1 and those of pseudomonads, B. thermosphacta, S. putrefaciens, and Enterobacteriaceae grown on naturally spoiled boque fish and on sterile fish blocks inoculated with bacteria and stored aerobically at 0, 3, 7, and 10°C
| Fish and bacterial groupa | Mean μmax h−1 ± SD
|
|||
|---|---|---|---|---|
| 0°C | 3°C | 7°C | 10°C | |
| A | ||||
| All | 0.019 ± 0.002 | 0.031 ± 0.001 | 0.064 ± 0.003 | 0.070 ± 0.002 |
| Pseudomonads | 0.023 ± 0.001 | 0.037 ± 0.002 | 0.066 ± 0.001 | 0.080 ± 0.002 |
| B. thermosphacta | 0.019 ± 0.003 | 0.031 ± 0.003 | 0.060 ± 0.001 | 0.079 ± 0.003 |
| S. putrefaciens | 0.030 ± 0.001 | 0.045 ± 0.003 | 0.076 ± 0.002 | 0.102 ± 0.003 |
| Enterobacteriaceae | 0.012 ± 0.001 | 0.022 ± 0.001 | 0.056 ± 0.002 | 0.075 ± 0.002 |
| B | ||||
| Pseudomonads | 0.025 ± 0.005 | NDb | ND | 0.110 ± 0.014 |
| B. thermosphacta | 0.030 ± 0.006 | ND | ND | 0.109 ± 0.012 |
| S. putrefaciens | 0.032 ± 0.004 | ND | ND | 0.173 ± 0.023 |
| Lactic acid bacteria | 0.012 ± 0.001 | ND | ND | 0.105 ± 0.010 |
A, naturally spoiled boque; B, sterile boque inoculated with bacteria.
ND, not determined.
P. phosphoreum was detected only in the initial storage period, and its contribution was less than 1% of the total microflora. In general, the contribution of P. phosphoreum was extremely small and rather unimportant for boque fish stored aerobically (results not shown). At the time of rejection, none of the selected colonies from Long-and-Hammer medium was counted as P. phosphoreum. It needs to be noted that this bacterium did not grow in sterile fish flesh inoculated with strains tested in this study. Their μmax values of the other bacteria used in this study were found to be lower than the μmax of those strains grown individually in sterile boque flesh (Table 2).
Remaining shelf life.
The shelf life of fresh boque fish stored at 0, 3, 7, and 10°C was determined as 174 ± 10, 103 ± 8, 60 ± 5, and 44 ± 7 h, respectively, based on the scoring scale described in Materials and Methods. Excellent correlations (r, >0.96) were observed between the counts (log10 CFU gram−1) of pseudomonads and H2S-producing bacteria with the remaining shelf life at all temperatures (Table 3). The log total viable counts showed less of a correlation with the remaining shelf life than did log numbers of Pseudomonas sp. and H2S-producing bacteria. In addition, total viable count data only ranged from approximately 5.5 to 8.5 log units (Table 1), which limited their usefulness for evaluation of fish freshness.
TABLE 3.
Parameter values and statisticsa fitted to the remaining shelf life data of Mediterranean boque stored aerobically at 0, 3, 7, and 10°C
| Bacteria and storage temp (°C) | No. of data points | Intercept (β0) ± 95% CIb | Slope (β1) ± 95% CI | % of total variability explained | Correlation coefficient |
|---|---|---|---|---|---|
| All | |||||
| 0 | 23 | 23.7 ± 3.23 | −3.02 ± 0.23 | 85 | −0.923 |
| 3 | 23 | 19.0 ± 3.08 | −2.42 ± 0.41 | 81 | −0.901 |
| 7 | 19 | 8.6 ± 1.32 | −0.99 ± 0.12 | 80 | −0.897 |
| 10 | 17 | 7.1 ± 1.19 | −0.88 ± 0.14 | 80 | −0.895 |
| Pseudomonads | |||||
| 0 | 23 | 16.2 ± 1.52 | −2.29 ± 0.21 | 95 | −0.978 |
| 3 | 23 | 14.0 ± 1.91 | −1.94 ± 0.24 | 92 | −0.959 |
| 7 | 19 | 7.1 ± 0.67 | −0.89 ± 0.13 | 95 | −0.974 |
| 10 | 17 | 5.6 ± 0.70 | −0.76 ± 0.11 | 93 | −0.965 |
| H2S producers | |||||
| 0 | 23 | 13.9 ± 0.7 | −2.13 ± 0.10 | 98 | −0.992 |
| 3 | 23 | 12.6 ± 0.4 | −1.91 ± 0.11 | 99 | −0.990 |
| 7 | 19 | 5.8 ± 0.4 | −0.75 ± 0.14 | 96 | −0.983 |
| 10 | 17 | 4.6 ± 0.6 | −0.66 ± 0.12 | 91 | −0.957 |
Determined as described in Materials and Methods.
CI, confidence interval.
Changes in physicochemical characteristics.
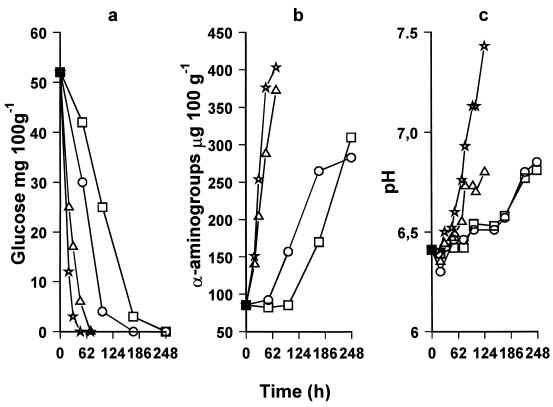
The reduction of glucose in boque fillets and the rise in α-amino groups and pH values are shown in Fig. 1. This decrease was more pronounced in boque fish samples stored at 7 and 10°C than in samples stored at 0 and 3°C (Fig. 1). The glucose decrease was followed by an increase in pH values (Fig. 1c), as well as by an increase in proteolysis and an increase in free amino acids (Fig. 1c). l-Lactate was also found to decrease (Table 4). Thus, the increase in pH (Fig. 1b) can be due to the l-lactic acid decrease (Table 4) and, further, to deamination of amino acids. In this study, the rate of lactate decrease in fresh boque fish was affected by the storage temperature (Table 4). In this study, no TMA was produced during the storage of boque fish under the conditions studied (results not shown).
FIG. 1.
Changes in glucose (a), α-amino groups (equivalent of glycine) (b), and pH (c) of naturally spoiled boque fish during storage at 0°C (□), 3°C (○), 7°C (▵), and 10°C (⋆). Each point is the mean of two samples taken from different experiments (coefficient of variation of the mean of samples taken from different experiments, <5.5%). Each sample was analyzed in duplicate (coefficient of variation of samples from the same experiment, <0.55%).
TABLE 4.
Changes in the areas under lactic, formic, and acetic acid peaks and unknown peaks with RT of 14.8 and 17.6 min during storage of boque fish at 0, 3, 7, and 10°C
| Peak and temp (°C) | Area under peak (103)a at 210 nm
|
|||||||
|---|---|---|---|---|---|---|---|---|
| 0 h | 30 h | 47 h | 52 h | 71 h | 99 h | 172 h | 248 h | |
| Lactic acid | ||||||||
| 0 | 865 | 750 | 616 | 549 | 345 | |||
| 3 | NAb | 683 | NA | 220 | ||||
| 7 | 809 | 461 | 230 | |||||
| 10 | 714 | 388 | 124 | |||||
| Formic acid | ||||||||
| 0 | 13 | NDc | 69 | 67 | 87 | |||
| 3 | NA | 57 | NA | 176 | ||||
| 7 | 93 | 220 | 332 | |||||
| 10 | 130 | 168 | 195 | |||||
| Acetic acid | ||||||||
| 0 | 9 | ND | 7 | 11 | 10 | |||
| 3 | NA | 12 | NA | 38 | ||||
| 7 | 58 | 35 | 81 | |||||
| 10 | 40 | 45 | 91 | |||||
| Bd | ||||||||
| 0 | 22 | 21 | 44 | 60 | 347 | |||
| 3 | NA | 199 | NA | 348 | ||||
| 7 | 81 | 217 | 217 | |||||
| 10 | 294 | 393 | 393 | |||||
| De | ||||||||
| 0 | 5,622 | 5,666 | 6,137 | 5,064 | 1,822 | |||
| 3 | NA | 4,322 | NA | 521 | ||||
| 7 | 3,229 | 1,158 | 3,229 | |||||
| 10 | 316 | 458 | 318 | |||||
Each value is the mean of two samples taken from different experiments (coefficient of variation of the mean of samples taken from different experiments, <5%). Each sample was analyzed in duplicate (coefficient of variation of samples from the same experiment, <0.65%).
ND, not detected.
NA, not analyzed.
RT, 14.8 min.
RT, 17.6 min.
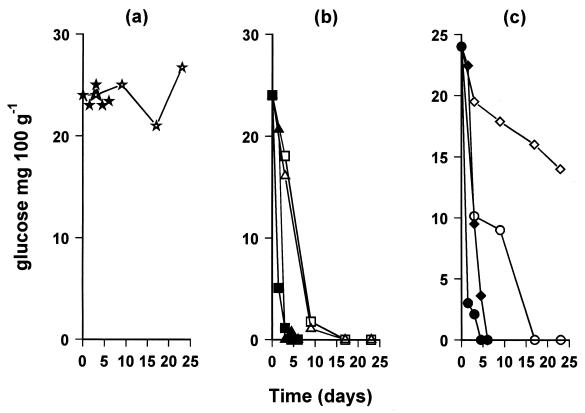
When the physicochemical changes of sterile boque fish were examined, it was found that glucose was used by all of the bacteria tested (Fig. 2). Lactate utilization was more pronounced in sterile blocks inoculated with the test bacteria and stored at 10°C than in samples stored at 0°C. At 0°C, lactate decreased only in sterile blocks inoculated with Pseudomonas sp. and S. putrefaciens (Tables 5 and 6), while it remained at the same level in samples inoculated with B. thermosphacta and lactic acid bacteria. In general, we can say that the organic acid profile observed in sterile fish blocks differed with the bacteria used in this study (Tables 5 and 6). Indeed, for example, the increase of the areas under the formic acid peak and unidentified peak C (Tables 5 and 6) was more pronounced in samples inoculated with S. putrefaciens than in samples inoculated with Pseudomonas sp. or B. thermosphacta. Unidentified peak A (Table 5 and 6) was a characteristic only of samples inoculated with Pseudomonas sp. Surprisingly, these two unidentified peaks were not present in the organic acid profile derived from naturally spoiled boque fish (Table 4). It should be noted that all of the above-mentioned organic acid changes were only evident in natural and deliberately inoculated samples, and thus, it can be concluded that the autolytic enzymes do not contribute to these changes (Tables 5 and 6, uninoculated samples).
FIG. 2.
Changes in the concentration of glucose in uninoculated sterile samples (a) and in samples inoculated with Pseudomonas sp. (□) or S. putrefaciens (▵) (b), or with B. thermosphacta (○) or lactic acid bacteria (◊) (c) and stored at 0°C (open symbols) or 10°C (closed symbols). Each point is the mean of two samples taken from different experiments (coefficient of variation of the mean of samples taken from different experiments, <4.5%). Each sample was analyzed in duplicate (coefficient of variation of samples from the same experiment, <0.60%).
TABLE 5.
Changes in the areas under lactic acid, formic acid, and acetic acid peaks and four unknown peaks with RT of 14.05, 14.8, 16.25, and 17.6 min during storage at 0°C of sterile boque fish left uninoculated or inoculated with different microorganisms
| Peak and organism | Area under peak (103)a at 210 nm
|
||||
|---|---|---|---|---|---|
| 0 h | 72 h | 216 h | 408 h | 552 h | |
| Lactic acid | |||||
| Pseudomonas sp. | 1,150 | NAb | 1,115 | 93 | NDc |
| S. putrefaciens | 1,297 | 1,296 | 928 | 240 | |
| B. thermosphacta | 1,250 | 1,194 | 1,172 | 1,239 | |
| Lactobacillus sp. | 1,400 | 1,542 | 1,522 | 1,470 | |
| None (uninoculated) | 1,150 | 1,145 | 1,156 | 1,139 | |
| Formic acid | |||||
| Pseudomonas sp. | 3.5 | NA | ND | 135 | 288 |
| S. putrefaciens | 104 | 400 | 7,781 | 7,955 | |
| B. thermosphacta | 17 | 46 | 60 | 92 | |
| Lactobacillus sp. | 33 | 9 | 13 | ND | |
| None (uninoculated) | 3.7 | 3.6 | 3.3 | 3.4 | |
| Acetic acid | |||||
| Pseudomonas sp. | 1.1 | NA | ND | ND | ND |
| S. putrefaciens | 1.2 | 25 | 118 | 185 | |
| B. thermosphacta | 0.9 | 82 | 66 | 80 | |
| Lactobacillus sp. | 1.0 | 2 | 4.5 | 4.5 | |
| None (uninoculated) | 1.1 | 1.2 | 1.0 | 1.3 | |
| A, Pseudomonas sp. | ND | ND | ND | 379 | 1,335 |
| B | |||||
| Pseudomonas sp. | 41 | NA | 34 | 69 | 198 |
| S. putrefaciens | 34 | 47 | 145 | 205 | |
| B. thermosphacta | 38 | 121 | 61 | 167 | |
| Lactobacillus sp. | 35 | 93 | 131 | 1,028 | |
| None (uninoculated) | 42 | 43 | 40 | 40 | |
| C, S. putrefaciens | ND | ND | 33 | 533 | 717 |
| D | |||||
| Pseudomonas sp. | 4,500 | NA | 11,139 | 6,162 | 4,930 |
| S. putrefaciens | 4,069 | 4,117 | 2,444 | 4,760 | |
| B. thermosphacta | 4,261 | 4,398 | 3,968 | 4,389 | |
| Lactobacillus sp. | 7,330 | 4,106 | 3,572 | 4,100 | |
| None (uninoculated) | 4,534 | 4,527 | 4,478 | 4,502 | |
Each value is the mean of two samples taken from different experiments (coefficient of variation of the mean of samples taken from different experiments, <5%). Each sample was analyzed in duplicate (coefficient of variation of samples from the same experiment, <0.7%).
NA, not analyzed.
ND, not detected.
TABLE 6.
Changes in the areas under lactic acid, formic acid, and acetic acid peaks and four unknown peaks with RT of 14.05, 14.8, 16.25, and 17.6 min during storage at 10°C of sterile boque fish left uninoculated or inoculated with different microorganisms
| Peak and organism | Area under peak (103)a at 210 nm
|
||||
|---|---|---|---|---|---|
| 0 h | 36 h | 72 h | 108 h | 144 h | |
| Lactic acid | |||||
| Pseudomonas sp. | 1,150 | 1,252 | 927 | 288 | 116 |
| S. putrefaciens | 1,116 | NAb | 878 | 564 | |
| B. thermosphacta | 1,219 | 1,181 | 378 | 772 | |
| Lactobacillus sp. | 1,265 | 1,082 | NA | 759 | |
| None (uninoculated) | 1,160 | 1,145 | 1,140 | 1,155 | |
| Formic acid | |||||
| Pseudomonas sp. | 3.5 | 121 | NDc | 48 | 306 |
| S. putrefaciens | 1,552 | NA | 1,955 | 2,706 | |
| B. thermosphacta | 17 | 46 | 60 | 92 | |
| Lactobacillus sp. | 5 | 5.1 | NA | 22 | |
| None (uninoculated) | 3.8 | 3.5 | 3.3 | 4 | |
| Acetic acid | |||||
| Pseudomonas sp. | 1.1 | 0.9 | 0.9 | 0.9 | 111 |
| S. putrefaciens | 82 | NA | 142 | 128 | |
| B. thermosphacta | 23 | 125 | 149 | 157 | |
| Lactobacillus sp. | 8 | 13 | NA | 160 | |
| None (uninoculated) | 1.2 | 1.0 | 1.3 | 1.2 | |
| A, Pseudomonas sp. | ND | ND | 109 | 305 | 912 |
| B, Pseudomonas sp. | 41 | 44 | 62 | 38 | 410 |
| S. putrefaciens | 154 | NA | 126 | 467 | |
| B. thermosphacta | 31 | 34 | 30 | 64 | |
| Lactobacillus sp. | 48 | 153 | NA | 170 | |
| None (uninoculated) | 39 | 40 | 39 | 42 | |
| C, S. putrefaciens | ND | 544 | NA | 875 | 945 |
| D | |||||
| Pseudomonas sp. | 4,500 | 5,900 | 4,612 | 2,377 | 2,400 |
| S. putrefaciens | 4,323 | NA | 4,601 | 3,609 | |
| B. thermosphacta | 4,439 | 4,490 | 5,514 | 3,013 | |
| Lactobacillus sp. | 3,487 | 4,557 | NA | 3,900 | |
| None (uninoculated) | 4,400 | 4,510 | 4,520 | 4,490 | |
Each value is the mean of two samples taken from different experiments (coefficient of variation of the mean of samples taken from different experiments, <5%). Each sample was analyzed in duplicate (coefficient of variation of samples from the same experiment, <0.7%).
NA, not analyzed.
ND, not detected.
DISCUSSION
The initial and final microbial associations of fresh boque fish were found to be similar to those reported in the literature (9, 12, 19, 31, 45, 57, 65). Of P. phosphoreum and B. thermosphacta, two bacteria whose contribution to fish spoilage has been reported only recently (9, 12, 18, 19, 20), the latter was found to be a member of the final microbial association, while the former did not grow in boque fish during aerobic storage (Table 1).
The remaining bacterial groups examined in the present study, primarily Pseudomonas sp. and S. putrefaciens, a late spoiler in the temperature range of −1.4 to 15°C, have been reported to be the specific spoilage bacteria in temperate and tropical waters (31, 33, 35, 49). That the presence of these two bacteria correlated better with remaining shelf life than did the total viable count (Table 3) could be explained by the fact that spoilage is more often a result of the production of off odors and flavors caused by specific spoilage organisms, which are only a fraction of the total microflora (40). On the basis of numerous data reported in the literature, it is concluded that spoilage of fresh fish is due to the activity of more than one specific spoilage organism. Similar results have been reported for other fresh fish stored aerobically (5, 18, 19, 23, 34, 38, 42). However, it should be noted that although a number of data concerning the correlation between H2S-producing bacteria and freshness have been collected, Pseudomonas sp. has not received the appropriate attention for the effect of microbial interaction on spoilage (42). This can be important in understanding spoilage, as it was found that there is an interaction between the above-mentioned bacteria. Indeed, it was reported that Pseudomonas sp. can inhibit the growth of S. putrefaciens due to the ability of the former to produce siderophores, and this interaction can be the major factor governing the development of spoilage flora (32). On the other hand, studies with fresh meat have shown that pseudomonads predominated over the other meat bacteria because of their faster growth rates, their greater affinity for oxygen, and, as a consequence, their greater catabolism of glucose and lactate (26, 27). In general, the microbe-microbe interaction can also be influenced by factors such as oxygen and substrate limitation (14), and thus, microbial competition (21, 55) not only affects the development of microbial association in a fish ecosystem but could also influence the chemical changes which are essentially an expression of the development of such an ecosystem. Indeed, this can be seen in our findings. Both of the bacteria mentioned above were present in spoiled boque fish and were also studied individually in sterile blocks under comparable aerobic conditions. There was no similarity among the organic acid profiles of Pseudomonas sp.-inoculated, S. putrefaciens-inoculated, and naturally spoiled samples, regardless of the storage temperature (Tables 4, 5, and 6). This can possibly be attributed to the fact that in the latter case, the decreasing availability of the carbon and energy substrate may cause competition between bacteria. This competition usually forces the microorganisms to regulate their enzymatic and nutrient uptake systems appropriately. Bacteria belonging to different genera seem to differ in substrate or oxygen affinity (25), since Drosinos (15) has reported a minor contribution of glucose and oxygen affinity to the domination of P. fragi over the pseudomonads P. fluorescens and P. lundensis on chilled meat. Thus, it was suggested that other key physiological properties may contribute to the succession of Pseudomonas spp. in meat ecosystems (15). Among these, the metabolic versatility of pseudomonads that allows them to grow on a wide range of substrates can be considered a competitive advantage over more specialized species with greater substrate specificity (14). Thus, organisms belonging to this genus grew both in the protein-free fraction of fish press juice containing soluble components and in the protein fraction devoid of soluble compounds (67), although their growth was faster in the former case. In a medium consisting of a mixture of both of the fractions, prolific growth of the organisms was noticed, which was accompanied by protein breakdown (67). An increase in the concentration of α-amino groups (Fig. 1) in naturally spoiled fresh fish was evident in this study.
As far as substrate specificity is concerned, several studies with meat and fish have shown that bacteria can grow on flesh at the expense of one or more of the low-molecular-weight soluble components such as glucose, lactic acid, certain amino acids, nucleotides, TMAO, and water-soluble proteins (18, 20, 50). Glucose and lactate seem to be the substrates which are attacked first by the various groups of spoilage bacteria under aerobic and anaerobic conditions (25). Although these compounds are also present in fish muscle (18, 20, 43, 50, 53, 60, 68), their role as intrinsic determinants of spoilage has been discussed in detail only in fresh-meat ecosystems (59). These two substrates initially affect the composition of microflora developing during storage, the metabolic products produced by the flora (switch from saccharolytic to amino acid-degrading metabolism), and the cell density attained at the onset of spoilage (16, 17, 18, 20, 25, 48). Glucose and lactate have been proposed as potential spoilage indicators for fish and meat (4, 56, 63, 68).
Since none of the bacteria ceased growing because of substrate exhaustion under aerobic conditions, oxygen availability was suggested to be the limiting factor mainly due to massive growth of bacteria (61). Under such conditions, those bacteria which can switch to using readily available TMAO as an electron acceptor must have a great ecological advantage over bacteria lacking this ability. This could be the case for S. putrefaciens, a nonfermentative bacterium (62), growing on the exposed areas of the fish, where it gets energy for growth by oxidation of easily diffusible extractives (such as glucose, lactate, and free amino acids). By the time a significant number of bacteria can penetrate the muscle due to proteolysis (28, 37, 61, 67), the fish is largely unacceptable for human consumption due to the formation of off odors. Indeed, S. putrefaciens produces large amounts of TMA (11), while Pseudomonas cannot use TMAO to produce TMA (35). We should mention that TMA was not found during the storage period in this study. This is in agreement with results related to other Mediterranean fish (12, 18, 19), where no TMA was produced or extremely small quantities of TMA were produced. Of course, the absence of TMA in our study can be due either to the fact that it is not detectable because the S. putrefaciens count never reached 108 CFU g−1 (10, 11) or to the low concentration or absence of TMAO in boque fish. The former can be of great importance because of the potential use of TMA as a spoilage indicator. Reasons include the varying content of the precursor TMAO in different fish species and seasonal variations within species (7).
Other microbial metabolites found in this study (e.g., formic and acetic acids; Tables 4, 5, and 6) were produced either by the microbial association or as a result of microbial interaction with the environment and showed good correlations with the microbial numbers (results not shown), but there is a lack of information about their effect on the sensory characteristics of fish. It is generally accepted that the use of microbial metabolites as potential indicators should meet, among others, the following criteria (41): (i) the compound should be absent or at least present at low levels initially, (ii) it should increase with storage, and (iii) it should be produced by the dominant microbial flora and have a good correlation with sensory characteristics. Determination of the quantities of metabolites could provide us with information about the degree of spoilage. The identification of the ideal metabolite that can be used for spoilage assessment has proved a difficult task for the following reasons. (i) Most metabolites are specific to certain organisms (e.g., gluconate is specific to pseudomonads), and the absence of these organisms or their inhibition naturally or due to food ecology measures imposed by humans provides incorrect spoilage information. (ii) The metabolites are the result of the consumption of a specific substrate, but the absence of a given substrate or its presence in small quantities does not preclude spoilage. (iii) The rate of microbial metabolite production and the metabolic pathways of these bacteria are affected by the environmental conditions imposed (e.g., pH, oxygen tension, temperature, etc.). (iv) Accurate detection and measurement require sophisticated procedures, highly educated personnel, time, and equipment. (v) Retrospective information about many of these metabolites is not satisfactory. More research is needed in this field.
ACKNOWLEDGMENTS
This study was a part of two research projects on fish safety and quality (EPET II and fair-1090) funded by the Greek Ministry of Development (General Secretariat of Research and Technology) and the European Union (DGXIV), respectively.
We thank C. Genigeorgis for reviewing the manuscript.
REFERENCES
- 1.Anonymous. Microorganisms in foods. 1. Their significance and methods of enumeration. 2nd ed. Toronto, Ontario, Canada: University of Toronto Press; 1978. p. 45. [Google Scholar]
- 2.Anonymous. Fig.P for windows. User’s manual, version 2.5. Cambridge, United Kingdom: Fig.P Biosoft Software; 1995. [Google Scholar]
- 3.Association of Official Analytical Chemists. Trimethylamine nitrogen in seafood. In: Sidney W, editor. Official methods of analysis. 15th ed. Arlington, Va: Association of Official Analytical Chemists; 1990. p. 123. [Google Scholar]
- 4.Boers R H, Dijkmann K E, Wijngaards G. Shelf-life of vacuum-packaged wild boar meat in relation to that of vacuum-packaged pork: relevance of intrinsic factors. Meat Sci. 1994;37:91–102. doi: 10.1016/0309-1740(94)90147-3. [DOI] [PubMed] [Google Scholar]
- 5.Chai T, Chen C, Rossen A, Levin R F. Detection and incidence of specific species of spoilage bacteria on fish. II. Relative incidence of Pseudomonas putrefaciens and fluorescent pseudomonads on haddock fillets. Appl Microbiol. 1968;16:1738–1741. doi: 10.1128/am.16.11.1738-1741.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Church C F, Swaisgood H E, Porter D H, Catignani L G. Spectrophotometric assay using o-phthaldialdehyde on determination of proteolysis in milk and isolated milk proteins. J Dairy Sci. 1983;66:1219–1227. [Google Scholar]
- 7.Dainty R H. Chemical/biochemical detection of spoilage. Int J Food Microbiol. 1996;33:19–34. doi: 10.1016/0168-1605(96)01137-3. [DOI] [PubMed] [Google Scholar]
- 8.Dalgaard, P. 1997. Personal communication.
- 9.Dalgaard P. Qualitative and quantitative characterization of spoilage bacteria from packed fish. Int J Food Microbiol. 1995;26:319–333. doi: 10.1016/0168-1605(94)00137-u. [DOI] [PubMed] [Google Scholar]
- 10.Dalgaard P. The effect of anaerobic conditions and carbon dioxide. FAO Fish Tech Pap. 1995;348:68–76. [Google Scholar]
- 11.Dalgaard P, Gram L, Huss H H. Spoilage and shelf life of cod fillets packed in vacuum or modified atmospheres. Int J Food Microbiol. 1993;19:283–294. doi: 10.1016/0168-1605(93)90020-h. [DOI] [PubMed] [Google Scholar]
- 12.Dalgaard P, Mejlholm O, Christiansen T J, Huss H H. Importance of Photobacterium phosphoreum in relation to spoilage of modified atmosphere-packaged fish products. Lett Appl Microbiol. 1997;24:373–378. [Google Scholar]
- 13.Dalgaard P, Ross T, Kamperman L, Neumeyer K, McMeekin T A. Estimation of bacterial growth rates from turbidimetric and viable count data. Int J Food Microbiol. 1994;23:391–404. doi: 10.1016/0168-1605(94)90165-1. [DOI] [PubMed] [Google Scholar]
- 14.Dolfing J, Gottschal J C. Microbe-microbe interactions. In: Mackie R I, White B A, Isaacson R E, editors. Gastrointestinal microbiology. 2. Gastrointestinal microbes and host interactions. New York, N.Y: Chapman & Hall; 1996. pp. 373–433. [Google Scholar]
- 15.Drosinos E H. Microbial associations of minced lamb and their ecophysiological attributes. Ph.D. thesis. Bath, United Kingdom: University of Bath; 1994. [Google Scholar]
- 16.Drosinos E H, Board R G. A survey of minced lamb packaged in modified atmospheres. Fleischwirtschaft. 1995;75:281–284. [Google Scholar]
- 17.Drosinos E H, Board R G. Attributes of microbial associations of meat growing as xenic batch cultures in a meat juice at 4°C. Int J Food Microbiol. 1995;26:279–293. doi: 10.1016/0168-1605(94)00131-o. [DOI] [PubMed] [Google Scholar]
- 18.Drosinos E H, Lambropoulou K, Mitre E, Nychas G-J E. Attributes of fresh gilt-head seabream (Sparus aurata) fillets treated with potassium sorbate, sodium gluconate and stored under a modified atmosphere at 0 ± 1°C. J Appl Microbiol. 1997;83:569–575. [Google Scholar]
- 19.Drosinos E H, Nychas G-J E. Brochothrix thermosphacta, a dominant organism in Mediterranean fresh fish (Sparus aurata) stored under modified atmosphere. Ital J Food Sci. 1996;4:323–329. [Google Scholar]
- 20.Drosinos E H, Nychas G-J E. Production of acetic acid in relation to the content of glucose during modified atmosphere storage of gilt-head seabream (Sparus aurata) at 0 ± 1°C. Food Res Int. 1998;9:711–717. [Google Scholar]
- 21.Fredrickson A G, Stephanopoulos G. Microbial competition. Science. 1981;213:972–979. doi: 10.1126/science.7268409. [DOI] [PubMed] [Google Scholar]
- 22.Gardner G A. A selective medium for the enumeration of Microbacterium thermosphactum in meat and meat products. J Appl Bacteriol. 1966;29:455–460. doi: 10.1111/j.1365-2672.1966.tb03497.x. [DOI] [PubMed] [Google Scholar]
- 23.Gennari M, Campanini R. Isolamento e caratterizzazione di Shewanella putrefaciens da pesce fresco e alterato, carni fresche e alterate, prodotti lattiero-caseari, acqua e suolo. Ind Aliment. 1991;30:965–976. , 988. [Google Scholar]
- 24.Gibson A M, Bratchell N, Roberts T A. Predicting microbial growth: growth responses of salmonellae in a laboratory medium as affected by pH, sodium chloride and storage temperature. Int J Food Microbiol. 1988;6:155–178. doi: 10.1016/0168-1605(88)90051-7. [DOI] [PubMed] [Google Scholar]
- 25.Gill C O. The control of microbial spoilage in fresh meats. In: Pearson A M, Dutson T R, editors. Advances in meat research: meat and poultry microbiology. T. L. New York, N.Y: Macmillan; 1986. pp. 49–88. [Google Scholar]
- 26.Gill C O, Molin G. Modified atmospheres and vacuum packaging. In: Russell N J, Gould G W, editors. Food preservatives. Glasgow, Scotland: Blackie; 1991. pp. 172–199. [Google Scholar]
- 27.Gill C O, Newton K G. The development of aerobic spoilage flora on meat stored at chill temperatures. J Appl Bacteriol. 1977;43:189–195. doi: 10.1111/j.1365-2672.1977.tb00742.x. [DOI] [PubMed] [Google Scholar]
- 28.Gill C O, Penney N. Penetration of bacteria into meat. Appl Environ Microbiol. 1977;33:1284–1286. doi: 10.1128/aem.33.6.1284-1286.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gill T A. Chemical and biochemical indices in seafood quality. In: Huss H H, Jacobsen M, Liston J, editors. Quality assurance in the fish industry. Amsterdam, The Netherlands: Elsevier; 1992. pp. 377–387. [Google Scholar]
- 30.Gill T A. Advanced analytical tools in seafood science. In: Luten J B, Børresen T, Oehlenschläger J, editors. Developments in food science. 38. Seafood from producer to consumer. Integrated approach to quality. Amsterdam, The Netherlands: Elsevier Science; 1997. pp. 479–490. [Google Scholar]
- 31.Gram L, Huss H H. Microbiological spoilage of fish and fish products. Int J Food Microbiol. 1996;33:121–137. doi: 10.1016/0168-1605(96)01134-8. [DOI] [PubMed] [Google Scholar]
- 32.Gram L, Melchiorsen J. Interaction between fish spoilage bacteria Pseudomonas sp. and S. putrefaciens in fish extracts and on fish tissue. J Appl Bacteriol. 1996;80:589–595. doi: 10.1111/j.1365-2672.1996.tb03262.x. [DOI] [PubMed] [Google Scholar]
- 33.Gram L, Oundo J O, Bon J. Storage life of Nile perch (Lates niloticus) in relation to temperature and initial bacterial load. Trop Sci. 1989;29:221–236. [Google Scholar]
- 34.Gram L, Trolle G, Huss H H. Detection of specific spoilage bacteria from fish stored at low (0°C) and high (20°C) temperatures. Int J Food Microbiol. 1987;4:65–72. [Google Scholar]
- 35.Gram L, Webell-Neergaard C, Huss H H. Bacteriology of fresh and spoiling Lake Victorian Nile perch (Lates nilotocus) Int J Food Microbiol. 1990;10:303–316. doi: 10.1016/0168-1605(90)90077-i. [DOI] [PubMed] [Google Scholar]
- 36.Gregersen T. Rapid method for distinction of Gram-negative and Gram-positive bacteria. Eur J Appl Microbiol. 1978;5:123–127. [Google Scholar]
- 37.Gupta L K, Nagamohini Y. Penetration of poultry meat by Pseudomonas and Lactobacillus spp. World J Microbiol Biotechnol. 1992;8:212–213. doi: 10.1007/BF01195852. [DOI] [PubMed] [Google Scholar]
- 38.Herbert R A, Hendrie M S, Gibson D M, Shewan J M. Bacteria active in the spoilage of certain sea foods. J Appl Bacteriol. 1971;34:41–50. doi: 10.1111/j.1365-2672.1971.tb02267.x. [DOI] [PubMed] [Google Scholar]
- 39.Hugh R, Leifson E. The taxonomic significance of fermentative versus oxidative metabolism of carbohydrates by various gram negative bacteria. J Bacteriol. 1953;66:24–26. doi: 10.1128/jb.66.1.24-26.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huss H H, Dalgaard P, Hansen L, Ladefoged H, Pedersen A, Zittan L. The influence of hygiene in catch handling on the storage life of iced cod and plaice. J Food Technol. 1974;9:213–221. [Google Scholar]
- 41.Jay J M. Microbial spoilage indicators and metabolites. In: Pierson M D, Sterm N J, editors. Foodborne microorganisms and their toxins: developing methodology. Basel, Switzerland: Marcel Dekker, Inc.; 1986. pp. 219–240. [Google Scholar]
- 42.Jøorgensen B R, Gibson D M, Huss H H. Microbiological quality and shelf life prediction of chilled fish. Int J Food Microbiol. 1988;6:295–307. doi: 10.1016/0168-1605(88)90023-2. [DOI] [PubMed] [Google Scholar]
- 43.Kakouri A, Drosinos E H, Nychas G-J E. Storage of Mediterranean fresh fish (Boops boops and Sparus aurata) under modified atmospheres or vacuum at 3 and 10°C. In: Luten J B, Børresen T, Oehlenschläger J, editors. Developments in food science. 38. Seafood from producer to consumer. Integrated approach to quality. Amsterdam, The Netherlands: Elsevier Science; 1997. pp. 171–178. [Google Scholar]
- 44.King E D, Ward M K, Roney D E. Two simple media for the demonstration of pyocyanin and fluorescein. J Lab Clin Med. 1954;44:301–307. [PubMed] [Google Scholar]
- 45.Koutsoumanis K, Taoukis P, Drosinos E H, Nychas G-J E. Proceedings of the Final Meeting of the Concerted Action “Evaluation of Fish Freshness.”. Paris, France: International Institute of Refrigeration; 1998. Lactic acid bacteria and Brochothrix thermosphacta—the dominant spoilage microflora of Mediterranean fresh sea fish stored under modified atmosphere packaging conditions; pp. 158–165. [Google Scholar]
- 46.Kovacs N. Identification of Pseudomonas pyocyanea by oxidase reaction. Nature. 1956;178:703–707. doi: 10.1038/178703a0. [DOI] [PubMed] [Google Scholar]
- 47.Kunst A, Draeger B, Ziegenhorn J. Colorimetric methods with glucose oxidase and peroxidase. In: Bergmeyer H U, Bergmeyer J, Graßl M, editors. Methods of enzymatic analysis. 3rd ed. 6. Metabolites 1: carbohydrates. Weinheim, Germany: Verlag Chemie; 1984. pp. 178–185. [Google Scholar]
- 48.Lambropoulou K A, Drosinos E H, Nychas G-J E. The effect of glucose supplementation on the spoilage microflora and chemical composition of minced beef stored aerobically or under a modified atmosphere at 4°C. Int J Food Microbiol. 1996;30:281–291. doi: 10.1016/0168-1605(96)00954-3. [DOI] [PubMed] [Google Scholar]
- 49.Lima dos Santos C A M. The storage life of tropical fish in ice—a review. Trop Sci. 1981;23:97–127. [Google Scholar]
- 50.Liston J. Recent advances in the chemistry of iced fish spoilage. In: Martin R E, Flicks G J, Bebard C E, Ward D E, editors. Chemistry and biochemistry of marine products. Conn: AVI Publishing Co.; 1982. pp. 27–36. [Google Scholar]
- 51.Mayfield C I, Inniss W E. A rapid, simple method for staining bacterial flagella. Can J Microbiol. 1977;23:1311–1313. doi: 10.1139/m77-198. [DOI] [PubMed] [Google Scholar]
- 52.Mead G C, Adams B W. A selective medium for the rapid isolation of Pseudomonas associated with poultry meat spoilage. Br Poultry Sci. 1977;18:661–670. doi: 10.1080/00071667708416418. [DOI] [PubMed] [Google Scholar]
- 53.Montero P, Mackie I M. Changes in intramuscular collagen of cod (Gadus morhua) during post mortem storage in ice. J Sci Food Agric. 1992;59:89–96. [Google Scholar]
- 54.Mossel D A A, Eelderink L, Koopmans M, Rossem F V. Influence of carbon source, bile salts and incubation temperature on recovery of Enterobacteriaceae from foods using MacConkey-type agars. J Food Prot. 1979;42:470–475. doi: 10.4315/0362-028X-42.6.470. [DOI] [PubMed] [Google Scholar]
- 55.Mossel D A A, Ingram M. The physiology of the microbial spoilage of foods. J Appl Bacteriol. 1955;18:232–268. [Google Scholar]
- 56.Nassos P S, King A D, Jr, Stafford A E. Lactic acid concentration and microbial spoilage in anaerobically and aerobically stored ground beef. J Food Sci. 1985;50:710–712. , 715. [Google Scholar]
- 57.Nickelson R, Finne G, Hanna M O, Vandezant C. Minced fish flesh from nontraditional Gulf of Mexico finfish species: bacteriology. J Food Sci. 1980;45:1321–1326. [Google Scholar]
- 58.Noll F. l-(+)-Lactate. In: Bergmeyer H U, Bergmeyer J, Graßl M, editors. Methods of enzymatic analysis. 3rd ed. 6. Metabolites 1: carbohydrates. Weinheim, Germany: Verlag Chemie; 1984. pp. 582–588. [Google Scholar]
- 59.Nychas G-J E, Drosinos E H, Board R G. Chemical changes in meat. In: Board R G, Davies A, editors. Microbiology of meat and poultry. London, England: Blackie Academic & Professional; 1998. pp. 288–326. [Google Scholar]
- 60.Parisi G, Geri G, Mecati M, Guidotti P, Franci O. Proceedings of the Conference of IIR Commission C2, Preservation Techniques Applied to Biodiversity Management in Fish Farming. Paris, France: International Institute of Refrigeration; 1996. Changes in some chemical and rheological parameters and in the indices of rigor in the muscle of rainbow trout and sea bass in the 24 hour post mortem period; pp. 159–166. [Google Scholar]
- 61.Ringø E, Stenberg E, Strøm A R. Amino acids and lactate catabolism in trimethylamine oxide respiration of Alteromonas putrefaciens NCMB 1735. Appl Environ Microbiol. 1984;47:1084–1089. doi: 10.1128/aem.47.5.1084-1089.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Scott J H, Nealson K H. A biochemical study of the intermediary carbon metabolism of Shewanella putrefaciens. J Bacteriol. 1994;176:3408–3411. doi: 10.1128/jb.176.11.3408-3411.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Seymour I J, Cole M B, Coote P J. A substrate-mediated assay of bacterial proton effux/influx to predict the degree of spoilage of beef mince stored at chill temperatures. J Appl Bacteriol. 1994;76:608–615. doi: 10.1111/j.1365-2672.1994.tb01659.x. [DOI] [PubMed] [Google Scholar]
- 64.Tarr H L A. Post mortem changes in glycogen, nucleotides sugar phosphates and sugars in fish muscles—a review. J Food Sci. 1966;31:846–855. [Google Scholar]
- 65.Tassou C C, Drosinos E H, Nychas G-J E. Inhibition of resident microbial flora and pathogen inocula on cold fresh fish fillets in olive oil, oregano and lemon juice stored under a modified atmosphere or air. J Food Prot. 1996;59:31–34. doi: 10.4315/0362-028X-59.1.31. [DOI] [PubMed] [Google Scholar]
- 66.Van Spreekens K J A. The suitability of a modification of Long and Hammer’s medium for the enumeration of more fastidious bacteria from fresh fishery products. Arch Lebensm Hyg. 1974;25:213–219. [Google Scholar]
- 67.Venugopal V. Extracellular proteases of contaminant bacteria in fish spoilage: a review. J Food Prot. 1990;53:341–350. doi: 10.4315/0362-028X-53.4.341. [DOI] [PubMed] [Google Scholar]
- 68.Watanabe E, Endo H, Ikeda Y, Shibamoto N, Toyama K. Determination of glucose in fish muscle and serum with an enzyme sensor. Bull Jpn Soc Sci Fish. 1986;52:711–717. [Google Scholar]