Abstract
Objective
To describe the National Heart Lung and Blood Institute (NHLBI) sponsored Disparities Elimination through Coordinated Interventions to Prevent and Control Heart and Lung Disease (DECIPHeR) Alliance to support late‐stage implementation research aimed at reducing disparities in communities with high burdens of cardiovascular and/or pulmonary disease.
Study Setting
NHBLI funded seven DECIPHeR studies and a Coordinating Center. Projects target high‐risk diverse populations including racial and ethnic minorities, urban, rural, and low‐income communities, disadvantaged children, and persons with serious mental illness. Two projects address multiple cardiovascular risk factors, three focus on hypertension, one on tobacco use, and one on pediatric asthma.
Study Design
The initial phase supports planning activities for sustainable uptake of evidence‐based interventions in targeted communities. The second phase tests late‐stage evidence‐based implementation strategies.
Data Collection/Extraction Methods
Not applicable.
Principal Findings
We provide an overview of the DECIPHeR Alliance and individual study designs, populations, and settings, implementation strategies, interventions, and outcomes. We describe the Alliance's organizational structure, designed to promote cross‐center partnership and collaboration.
Conclusions
The DECIPHeR Alliance represents an ambitious national effort to develop sustainable implementation of interventions to achieve cardiovascular and pulmonary health equity.
Keywords: cardiovascular disease, community participation, health equity, implementation science, pulmonary disease
What is known on this topic
Marked disparities in heart and lung health exist and persist in the United States across race, ethnicity, sex and/or gender, geography, socioeconomic status, and disability status.
To narrow these gaps and achieve health equity for heart and lung diseases, we need to understand barriers and facilitators to interventions that work and how to implement and sustain them in the community.
The National Heart Lung and Blood Institute sponsored the creation of the Disparities Elimination through Coordinated Interventions to Prevent and Control Heart and Lung Disease (DECIPHeR) Alliance to support research to reduce disparities in communities with high burdens of heart and/or lung disease.
What this study adds
We describe the DECIPHeR Alliance including the seven research projects and a coordinating center to bring the project teams together for the highest impact to narrow health disparities.
1. INTRODUCTION
Despite substantial improvements in cardiovascular and pulmonary health in the United States over recent decades, marked disparities persist. 1 , 2 Significant cardiopulmonary health inequities endure by race, ethnicity, sex and/or gender, geography, socioeconomic status, and disability status, and contribute to the disproportionate burden of preventable mortality in these groups. 3 , 4 Evidence supporting these disparities is compelling and consistent and includes research based on epidemiologic cohorts, and spatial analyses illustrating geographic variation in rates and outcomes for cardiovascular and pulmonary diseases. 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13
To achieve health equity, there is a pressing need to understand how to implement evidence‐based interventions. 14 Dissemination and implementation research methods can tailor programs to address needs at multiple levels as health disparities develop due to complex determinants across the individual, interpersonal, community, and environmental levels. 15 , 16 , 17 , 18 , 19 Late‐stage (T4) implementation research identifies strategies to achieve sustainable uptake of proven‐effective interventions into routine clinical practice and community‐based settings and maximizes impact on population health. 20 In 2020, the National Heart Lung and Blood Institute (NHLBI) created and funded the Disparities Elimination through Coordinated Interventions to Prevent and Control Heart and Lung Disease Risk (DECIPHeR) Alliance to support late‐stage T4 implementation research strategies for optimal implementation and sustainment of evidence‐based multi‐level interventions to reduce disparities in communities with a high burden of cardiovascular and/or pulmonary risk factors. DECIPHeR is funded as a cooperative agreement using the UG3/UH3 administrative mechanism. During an initial 3‐year UG3 planning phase, project teams engage partner communities to identify programs and health areas of most importance for implementation strategies and to identify strategies most appropriate and effective in the community. If milestones for the initial UG3 phase are achieved, a subsequent 4‐year UH3 phase will conduct a prospective study of implementation strategies refined and adapted through the UG3 phase. Here we describe the DECIPHeR Alliance with an overview of study designs, disparities populations, evidence‐based interventions, planned implementation strategies, and outcomes as well as the organizational structure promoting cross‐center partnership and collaboration. At this early stage of the project, the approach to the research planned for the UH3 phase remains flexible and will likely change as information from our communities and their barriers and facilitators expands.
2. METHODS
2.1. The DECIPHeR Alliance
The DECIPHeR Alliance brings together seven Implementation Research Centers (IRCs) and a research coordinating center (RCC). Figure 1 summarizes DECIPHeR Alliance projected outputs and distal outcomes. An advantage of the Alliance is the close collaboration between Alliance members and community partners to improve the quality of the studies and strengthen partnerships. Given the focus on translational research, implementation science, and community engagement, the teams are both multi‐disciplinary and multi‐stakeholder, bringing together academic researchers with leaders from the study communities. Table 1 provides an overview of the seven DECIPHeR IRCs, and we describe the individual projects below.
FIGURE 1.
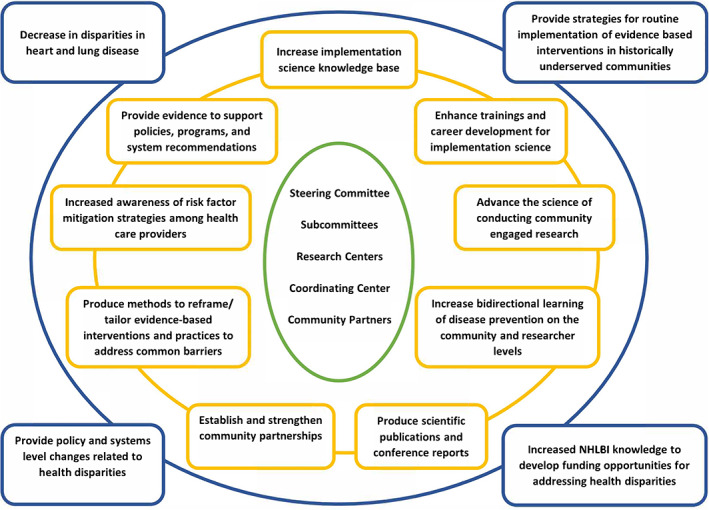
The inputs, outputs, and distal outcomes for the DECIPHeR Alliance. The inner circle lists the inputs, the middle circle lists the outputs, and the outer circle lists the distal outcomes. The DECIPHeR Alliance's organizational structure promotes cross‐center partnership and collaboration and the project's study designs, populations, evidence‐based interventions, planned implementation strategies, and outcomes as currently planned. The DECIPHeR Alliance represents an ambitious national effort to develop and support sustainable implementation of interventions to achieve cardiovascular and pulmonary health equity [Color figure can be viewed at wileyonlinelibrary.com]
TABLE 1.
DECIPHeR Alliance implementation research center projects
| University of California Los Angeles | University of Colorado | Tulane University | Northwestern University | University of Illinois at Chicago | New York University Grossman School of Medicine | Johns Hopkins University School of Medicine and University of Michigan | |
|---|---|---|---|---|---|---|---|
| Study | Multi‐ethnic multi‐level strategies and behavioral economics to eliminate hypertension disparities in LA County | Reducing asthma attacks in disadvantaged school children with asthma | Community health worker‐led church‐based intervention for eliminating cardiovascular health disparities in African Americans | Community intervention to reduce cardiovascular disease in Chicago (CIRCL‐Chicago) | Mi QUIT CARE (Mile Square QUIT Community‐Access‐Referral‐Expansion), | Actions to decrease disparities in risk and engage in shared support for blood pressure control (ADRESS‐BP) in Blacks | Achieving cardiovascular health equity in community mental health: optimizing implementation strategies |
| Population | Latino, Black, Korean, Chinese, and Filipino‐American adults | Children living in disadvantaged and under‐resourced communities (rural, mid‐size urban) | Inner city and rural Blacks and African American adults | Black and African American adults | Low‐income racial/ethnic minority adults in clinics | Blacks and African Americans with uncontrolled Hypertension | Adults with serious mental illness |
| Setting | County health services including community agencies and non‐profit organizations | Schools | African American Churches | Faith‐based organizations and Federally Qualified Health Care Centers (FQHCs) | FQHCs | Primary care practices | Outpatient community mental health programs, behavioral health homes |
| City/state | Los Angeles, CA | Colorado | New Orleans, LA | South Side Chicago, IL | Chicago, IL | New York City | Maryland and Michigan |
| Health outcomes | Hypertension management | Asthma in children | Cardiovascular health | Hypertension control | Smoking cessation | Hypertension control | Cardiovascular disease risk |
| Currently proposed study design | Stepped‐wedge cluster randomized design | Stepped‐wedge design | Effectiveness‐implementation hybrid type 2 design—Cluster randomized trial | Effectiveness‐implementation hybrid type 2 design | Effectiveness‐implementation hybrid type 1 design | Stepped‐wedge cluster RCT | Non‐restricted SMART with 2×2 factorial |
| Evidence‐based intervention |
(1) Medication therapy management (2) Home blood pressure monitoring (3) Cultural adaptation of lifestyle modification with support from community health workers |
(1) Colorado School‐Based Asthma Program (Col‐SBAP) (2) Comprehensive Assessment and Case Management of SDOH |
Community‐health workers led church based multifaceted implementation strategy | Adaptation of the Kaiser Hypertension Control Bundle |
(1) An electronically delivered brief smoking cessation intervention (Ask, Advise, Refer, AAR), (2) proactive linkage of smokers to the Illinois Tobacco Quit Line (ITQL) (3) patient navigation to reduce barriers to care. |
(1) Nurse case management (NCM); (2) home blood pressure monitoring (HBPM) (3) community health workers (CHWs) led health education and coaching |
(1) IDEAL: CVD risk factor counseling and education as well as care coordination /management (2) Life goals: self‐management focused intervention for coping with psychiatric symptoms and improving overall health behavior change around CVD risk factors |
| Implementation process and determinants framework(s) | EPIS | EPIS and PRISM | EPIS | EPIS | PRISM | CBPR and CFIR | REP |
| Implementation evaluation framework | RE‐AIM | RE‐AIM | RE‐AIM | RE‐AIM | RE‐AIM | Proctor | Proctor |
2.2. Multi‐ethnic multi‐level strategies and behavioral economics to eliminate hypertension disparities in Los Angeles County—University of California Los Angeles
In Los Angeles County (LAC), the second largest US municipal health system, patient, clinician, health system, and community factors contribute to disparities in hypertension prevalence, control, and outcomes. In the LAC Department of Health Services (DHS) 43% of patients have hypertension, of which 60% are inadequately controlled. The study will focus on racial/ethnic populations within LAC DHS with high rates of hypertension, including Latino (42%), African Americans (48%), Chinese (31%), Filipino (62%), and Korean (34%) populations. Population differences in healthy eating, physical activity, obesity, and antihypertensive pharmacotherapy use, medication adherence, community awareness of hypertension, and community‐level physical and social resources contribute to disparities in blood pressure (BP) control. In partnership with 51 adult primary care clinics in LAC DHS, we plan to significantly reduce hypertension disparities by leveraging our team's expertise in multi‐ethnic, multi‐level evidence‐based strategies, community/stakeholder engagement, public‐private partnerships, implementation science, and behavioral economics. Using the Exploration, Preparation, Implementation, Sustainment (EPIS) framework, we are planning a multi‐level intervention for hypertension control that will complete Exploration/Preparation stages in the UG3 phase and the Implementation/Sustainment stages in the UH3 phase. 21 Through patient and stakeholder interviews, we will first assess multi‐level (patient, clinician, health system leadership, and community) barriers to, facilitators of, and preferences for a menu of culturally tailored evidence‐based practices and sustainable implementation strategies with established efficacy for hypertension control (Aim 1; UG3). Our initial focus will be on medication therapy management, home BP monitoring, and cultural adaptation of lifestyle modification with support from community health workers. We will then link preferred multilevel (patient‐, clinician‐, or community‐directed) implementation strategies to behavioral science approaches, for example, patient incentives to attend initial pharmacy visits or clinician peer comparisons for patient BP control. (Aim 2; UG3) To test the effectiveness of these behavioral‐science linked implementation strategies, we plan a stepped‐wedge cluster randomized trial design using RE‐AIM to guide assessment of uncontrolled hypertension, and BP disparities in comparison to non‐minority LAC populations, and evidence‐based practices. 22 The effectiveness outcomes are BP control in the electronic health record (primary), medication adherence, and costs. The implementation outcomes are acceptability of the intervention components and sustainment of the intervention strategies and BP control.
2.3. Church‐based health intervention to eliminate racial inequalities in cardiovascular health—Tulane
Louisiana residents, especially African Americans, bear a disproportionately high burden of atherosclerotic cardiovascular disease. Age‐adjusted cardiovascular disease (CVD) mortality was 274.0 per 100,000 (the fourth highest in the United States) and stroke mortality was 46.7 per 100,000 (the second highest in the United States) in Louisiana residents in 2016–2018. 23 Church‐based health intervention to eliminate racial inequalities in cardiovascular health (CHERISH) is a cluster randomized trial that aims to test whether a multifaceted strategy for implementing the 2019 American College of Cardiology/American Heart Association primary prevention guideline will reduce cardiovascular health (CVH) disparities in Black populations in Louisiana. 24 CHERISH utilizes an effectiveness‐implementation hybrid design to: (1) test the effectiveness of a community health worker (CHW)‐led church‐based multifaceted implementation strategy for improving CVH over 18 months among Black participants at high risk for CVD, and (2) assess implementation outcomes. The EPIS is used for developing intervention program and implementation strategies and the RE‐AIM for evaluating study outcomes. 25 , 26 Implementation strategies include CHW‐led health coaching on lifestyle changes and medication adherence; church‐based exercise and weight loss programs; self‐monitoring of physical activity, BP, and glucose; and provider education and engagement. The primary effectiveness outcome is the difference in proportion of participants having ≥4 ideal or improved CVH metrics, 27 defined as a healthy diet score of 4–5 components or increase of 2 components from baseline; 150 min/week moderate‐ or 75 min/week vigorous‐intensity physical activity or a combination; never smoking or quitting ≥6 months ago; BMI <25 kg/m2 or weight loss ≥10 pounds; A1c <7.0% (or <8.0% with complications); use of statin therapy as appropriate; and BP <130/80 mmHg or systolic BP reduced by ≥10 mmHg. CHERISH will recruit 1050 Black participants (25 per church) aged ≥40 years who have <4 ideal CVH metrics and randomly assign 21 churches to intervention and 21 to control. Data on effectiveness and implementation outcomes will be collected at 6‐, 12‐, and 18‐month follow‐up visits and a 6‐months post‐intervention follow‐up visit.
2.4. Reducing asthma attacks in disadvantaged school children with asthma—University of Colorado
Asthma is a leading cause of children's hospitalizations, missed school days, and caregivers' missed work days that have a significant impact on low income families. 28 The well‐documented disparities in asthma outcomes for minorities, including death, worse asthma control, greater likelihood of emergency room visits, and high rates of school absenteeism are partly related to unmet social determinants of health (SDOH) for low‐income families. 29 , 30 , 31 Core elements of our existing Colorado school‐based asthma program (Col‐SBAP) are concordant with those identified as effective in a recent Cochrane review, including counseling strategies to improve asthma knowledge and self‐management skills to successfully control asthma. 32 , 33 Although our impact was significant, we recognized that we could do better by addressing SDOH in combination with Col‐SBAP with an asthma navigator. 34 The Colorado program seeks broad‐scale implementation of our effective school‐based asthma program (Col‐SBAP) to improve asthma disparities for children aged 5–12 years in five regional asthma “hot spots” across the state of Colorado. We define asthma “hot spots” as regions with both high rates of uncontrolled asthma and high levels of socioeconomic need. Using community‐based participatory methods with stakeholders representing schools, clinics, families and public health in each region, we will adapt our Col‐SBAP that reduces asthma exacerbations and missed school days to meet the needs, priorities, practices, and resources of these diverse communities. 32 , 35 , 36 , 37 The EPIS framework is being applied during our Planning phase of the UG3 award with community stakeholders to iteratively adapt our current implementation guide into a set of common implementation strategies that meet local community and school setting needs. 26 During the exploration phase, we are conducting meetings with stakeholders in our five targeted regions of Colorado to understand the needs, resources, and outcomes of success. We are using this information in the Preparation Phase to develop implementation strategies for each region tailored for their abilities to conduct the Col‐SBAP as well as access and manage SDOH for their school children with asthma who suffer health disparities. We will use this information to design and conduct a cluster‐randomized trial in participating regions in the Implementation Phase, using a randomized stepped‐wedge study design to compare two interventional elements with a control population: (1) our original evidence‐based Col‐SBAP that addresses a targeted subset of SDOH that limit health care access, and (2) a comprehensive assessment and case management approach to SDOH. This complex, combined program is termed Stop Asthma Attacks (SAA). Meanwhile, for the Sustainment Phase, we intend that the interventional elements will be designed for sustainable delivery by school nurses, in partnership with trained asthma navigators who provide “high‐touch” care coordination with students/families, primary care and community SDOH resources. 38 We will also work with the community stakeholders to identify the appropriate support to sustain the program (Table A1). We will have to determine what capacity each region has for sustaining the Col‐SBAP and/or the SAA Program. The central hypothesis is that SAA will have broad and equitable Reach (primary outcome) and yield important benefits in reducing asthma attacks and symptoms, as compared to schools that have not yet implemented SAA. Cost effectiveness of each program will also be assessed.
2.5. Community intervention to reduce cardiovascular disease in Chicago—Northwestern University
The population in the South Side of Chicago is primarily Non‐Hispanic Black and suffers from hypertension rates that are significantly higher (37%) than the estimated rate of hypertension (28%) of the adult population in Chicago. 39 The community intervention to reduce cardiovascular disease in Chicago (CIRCL‐Chicago) project represents a collaboration between a successful model to engage and train community members of faith‐based organizations in the South Side, a network of local Federally Qualified Health Centers (FQHCs), and a multi‐institutional team of academic investigators across Chicago. 40 , 41 The CIRCL‐Chicago Intervention is based on a model developed and tested by a large integrated health system (Kaiser Permanente) in Northern California which successfully increased BP control rates from 44% to 88% over 8 years through the implementation of an evidence‐based bundle (the Kaiser Hypertension Control Bundle) that included a system‐wide hypertension registry, annual reporting of hypertension control rates, development and promulgation of practice guidelines, medical assistant lead follow‐up, and promotion of single pill combination therapy. 42 Using the Dynamic Adaptation Process, EPIS Framework and community‐engaged implementation research methods based in part on the Pastors for Patient‐Centered Outcomes Research model, CIRCL‐Chicago aims to determine how components of the Kaiser Hypertension Control Bundle can be successfully adapted to the South Side of Chicago for coordinated delivery between FQHCs and faith‐based organizations and demonstrate improvement in a primary clinical outcome of hypertension control. 40 , 43 In addition to our community engagement and community‐academic partnership development strategies, other implementation strategies include implementer training, patient engagement, and practice facilitation. 44 Partnering with FQHCs that are part of practice‐based research networks with shared health information technology infrastructures will facilitate implementation of the Kaiser Bundle components (e.g., patient registry) and the evaluation of our implementation efforts and intervention effectiveness. 45 , 46 , 47 Guided by RE‐AIM, 25 the primary implementation outcomes are to reach into the population, delivery costs, and sustainability of the intervention.
2.6. Mile Square QUIT Community‐Access‐Referral‐Expansion—University of Illinois at Chicago
Tobacco use is the leading preventable cause of pulmonary health morbidity and mortality in the United States. 48 Despite a decline in the prevalence of smoking, 14.1% of adults—more than 34 million people—continue to smoke. 49 Low‐income populations are disproportionately burdened by tobacco use and carry a greater burden of smoking‐related pulmonary health morbidity. 50 , 51 FQHCs represent an important yet under‐utilized model for reducing smoking‐related health inequalities. Nationally, 25.8% of all patients receiving care at FQHCs are current smokers. 52
Public Health Service guidelines recommend the 5 A's framework (ask patients about their smoking status, advise smokers to quit, assess their readiness to make a quit attempt, assist with accessing treatment, and arrange a follow‐up appointment to monitor progress) for smoking cessation. 53 Although effective, the 5 A's model is time‐consuming and challenging to implement in high‐volume clinical settings, and a simplified version of the framework (Ask‐Advise‐Refer [AAR]) was subsequently developed. 54 , 55
Our multi‐disciplinary team proposes Mile Square QUIT Community‐Access‐Referral‐Expansion (MI QUIT CARE), an innovative strategy for using the patient portal to deliver evidence‐based tobacco treatment (AAR) within an urban FQHC system, Mile Square Health Center. Patient portals are secure online tools linked to an individual's electronic health record designed to help patients access and manage their health information and are increasingly used to deliver educational and health promotion content and interventions. 56 The intervention components include using the patient portal to send a provider message advising current smokers to quit, proactively linking smokers interested in quitting smoking or cutting down to the Illinois Tobacco Quitline, and using patient navigation to address barriers to treatment engagement. The EPIS framework is being used to guide the study. During the exploration phase, we will use clinic data to examine smoking prevalence rates in the patient population and identify patients and clinic sites disproportionately impacted by smoking. In the Planning Phase, we will conduct in‐depth interviews with clinic patients and providers to identify barriers and facilitators to smoking cessation, attitudes regarding the intervention components (patient portal, proactive linkage to the quitline, and patient navigation), and finalize outreach and education materials. In the Implementation Phase, we will conduct a randomized clinical trial comparing the treatment engagement (the receipt of counseling from the tobacco quitline) and smoking cessation outcomes (biochemically verified smoking status at 6 months). The intervention is being developed to maximize sustainability by using low‐touch (sending mass mailings to portal message boxes), automated and population‐based strategies (opt‐in and proactive linkage), and a cost‐effective approach (use of the state‐run quitline). In the Sustainment Phase, we will work with all key stakeholders (patients, providers, patient navigators, and the Illinois Tobacco Quitline) to identify potential barriers to sustainability. The final step of the project will be to translate all materials to Spanish to increase inclusivity and evaluate the program's cost effectiveness.
2.7. New York University Grossman School of Medicine: Actions to decrease disparities in risk and engage in shared support for blood pressure control in Blacks
Black Americans have the highest rate of hypertension in the United States (56.2% prevalence compared to 48% in Whites) and greater rates of fatal stroke (1.5×) and CVD mortality (1.3×). 57 , 58 The most common reason for the mortality gap between Black and White communities is uncontrolled hypertension. 59
The actions to decrease disparities in risk and engage in shared support for blood pressure (ADDRESS‐BP) Project uses practice facilitation to implement three evidence‐based interventions (EBIs) designed to improve hypertension control among Black patients in primary care practices in New York City. 60 , 61 The study integrates (1) nurse case management (NCM); (2) home blood pressure monitoring (HBPM); and (3) CHWs into routine care. These EBIs will be delivered as an integrated community‐clinic linkage model (Practice support And Community Engagement [PACE]) to address patient‐, physician‐, health system‐, and community‐level barriers to hypertension control in Blacks. Community‐clinical linkage models are partnerships facilitating connections among health care providers, community organizations, and public health agencies to improve patients' access to preventive, chronic care, and social services. Employing CHWs to be part of the care team, and to assist patients in navigating complex health systems and facilitate access to community resources is one such strategy. 61 While NCM and HBPM improve HTN provider‐ and self‐management, these strategies do not address community‐level barriers to hypertension control which CHWs are well‐suited to address.
The study planning phase utilizes CFIR to examine barriers and facilitators to implementation of PACE through analysis of secondary data, as well surveys and interviews of practice leadership, providers and staff, and community‐ and faith‐based groups. Using Proctor's Implementation Outcomes Framework, the implementation phase will evaluate the impact of PACE on BP control, adoption and fidelity at 18 months compared to usual care (UC) via a stepped‐wedge cluster RCT of 20 NYU PCPs in 500 Black patients with uncontrolled hypertension. The project includes partnership with a payer (Healthfirst) to assess cost‐effectiveness and inform the sustainability and scalability of integrated community‐clinical linkage models. We hypothesize that rates of BP control and cost‐effectiveness will be higher during the PACE intervention period than during the UC period at 18 months and that rates of BP control will be higher in practices that exhibit higher levels of adoption and implementation fidelity of PACE at 18 months. Payer reimbursement mechanisms will be explored to sustain and scale the model across the NYU health system.
2.8. Achieving cardiovascular health equity in community mental health: Optimizing implementation strategies—Johns Hopkins University School of Medicine and the University of Michigan
Persons with serious mental illness experience a marked increased prevalence of all cardiovascular risk factors, low control rates, and twice the rate of cardiovascular disease‐related mortality compared to the overall US population. 62 , 63 , 64 , 65 Cardiovascular risk reduction interventions require tailoring for persons with serious mental illness who often have psychiatric symptoms and cognitive impairment. 66 To overcome US general medical and mental health systems fragmentation and behavioral health homes, where mental health organizations coordinate primary care services, have proliferated. While behavioral health homes have shown improved cardiovascular risk factor screening, to date they have not resulted in risk factor improvement, likely because they are not implementing evidence‐based interventions. 67
The overarching project goal is to partner with Michigan and Maryland communities serving persons with serious mental illness to improve uptake of (IDEAL) and Life Goals evidence‐based interventions to reduce cardiovascular risk in behavioral health homes. 68 , 69 , 70 IDEAL is a comprehensive cardiovascular risk reduction intervention consisting of individual education and counseling sessions and care coordination/care management addressing all CVD risk factors. 71 Life Goals is a self‐management‐focused intervention that aims to help individuals cope with mood symptoms and improve health by using healthy behavior changes such as physical activity and eating habits and also incorporates care coordination. 72 Replicating Effective Programs (REP) Framework components will lay the groundwork alongside Coaching and Facilitation implementation strategies, addressing provider and organizational barriers respectively. 72 In the first phase, we are partnering with community mental health programs in Maryland and Michigan to tailor IDEAL/Life Goals and implementation strategies to fit site needs. In the second phase, we plan to conduct a non‐restricted SMART with these community mental health programs to determine the effectiveness of Coaching and Facilitation augmentations to REP on IDEAL/Life Goals delivery, risk factor care quality and control, assessing mechanisms, moderators, and other implementation measures. Uptake of IDEAL/Life Goals at 18 months is the primary outcome and will be measured by the number of evidence‐based practice sessions delivered. This project will inform which combination of strategies lead to optimal uptake of effective cardiovascular risk reduction interventions for persons with serious mental illness, a critical step in reducing their cardiovascular health disparities.
2.9. The Research Coordinating Center at the University of North Carolina at Chapel Hill
The RCC supports and enriches the work of the seven studies by maximizing the synergistic effects of multiple investigators working on separate, but related projects. The RCC coordinated the formation of an alliance structure to stimulate communication and learning among investigators with the goal of promoting increased creativity, dynamic innovation, and outstanding research. A Steering Committee, composed of PIs from each study, the RCC, and NHLBI staff, was convened as the DECIPHeR Alliance governing body. Subcommittees were established to encourage sharing of ideas and approaches among experts with common interests in focused areas: (1) implementation; (2) intervention; (3) community engagement; (4) measurement; (5) design and analysis; (6) publications, presentations, and ancillary studies; and (7) training and mentoring. At least one representative from each IRC is a member of each subcommittee. Table 2 describes the objectives of the subcommittees. Ad hoc working groups are formed as needed to provide in‐depth collaboration and produce products in specific areas, for example, cost measurements and analysis, qualitative measurements and analysis, project coordinators, recruitment and retention, and adverse events. The RCC also provides data‐related and study monitoring functions, supports the convening and activities of a single consortium‐wide Data Safety and Monitoring Board, provides reports to NHLBI, and participates as a scientific partner. The RCC has created a resource section on the DECIPHeR website, https://www.decipheralliance.org/resources with links to other implementation science resources. As the RCC develops products and resources that would be beneficial to other researchers and the general public, they will be added. In addition, at the end of the study the RCC will archive study materials and make them available to investigators outside the DECIPHeR study. They will also construct a de‐identified public use data base that will be available to other investigators in accordance with NIH data sharing policies.
TABLE 2.
Objectives of the DECIPHeR subcommittees
| Subcommittee | Objectives |
|---|---|
| Implementation subcommittee | Discuss all aspects of preparation, planning, and operationalization of implementation research with the goal of optimizing the research by the sharing of ideas including the identification of facilitators and barriers. In collaboration with the Measurement subcommittee select and plan implementation outcome measures with an emphasis on common measures (measurements used by more than one Implementation Research Center). Identify cross‐center opportunities to advance implementation science. |
| Intervention subcommittee | Discuss all aspects of preparation, planning, and operationalization of the evidence‐based effectiveness intervention with the goal of optimizing the research by the sharing of ideas. Develop a list of common process evaluation measures across the studies, including a set of process components that each site will measure (i.e., reach, dose delivered, dose received, fidelity, and secular trends) and, if possible, a common way to measure each process component. |
| Community engagement subcommittee | Support community and participant engagement and identify opportunities to strengthen (and potentially align) study approaches to engagement and community capacity building. Share plans for the development of partnerships and methods for engagement of stakeholders. Also, identify cross center opportunities to advance the science of how best to engage communities/partners/stakeholders in research to implement the multilevel interventions needed to reduce disparities and promote equity in heart and lung disease. |
| Design and analysis subcommittee | Review and enhance study designs and quantitative analytic plans. |
| Measurement subcommittee | In collaboration with other subcommittees, provide recommendations for standardization of common measures. This includes all types of measures—implementation, process variables, clinical/effectiveness measures (intervention, outcome, mediators, and moderators), adverse events, recruitment, retention, engagement, and social determinants of health. |
| Publications, presentations, and ancillary studies subcommittee | Develop policies regarding publications, presentations, ancillary studies, and access to data from the DECIPHeR studies. |
| Training and mentoring subcommittee | Provide cross research center mentorship and training opportunities to trainees and early stage investigators in the DECIPHeR Alliance (post‐doctoral fellows and junior faculty) and to community members and stakeholders. |
3. CONCLUSION
The mission of the DECIPHeR Alliance is to reduce or eliminate disparities in cardiovascular and pulmonary health, disease, and disease risk factors in high‐burden communities in the US through the rigorous study of implementation strategies for proven effective interventions. 73 NHBLI initiated planning for DECIPHeR well before the global Coronavirus 2019 (COVID‐19) pandemic with a focus on health disparities that were prescient. Now the DECIPHeR Alliance is prominently positioned to move the field forward by testing effective and equitable strategies that can be sustained and disseminated to improve population health. The DECIPHeR Alliance is an ambitious national effort to develop and support sustainable implementation of interventions to achieve cardiovascular and pulmonary health equity and maximize the positive impact on population health.
ACKNOWLEDGMENTS
We acknowledge funding from the National Heart Lung and Blood Institute grants numbers: UCLA Grant Number: UG3 HL154302, University of Colorado Grant Number: UG3 HL151297, University of Illinois at Chicago Grant Number: UG3 HL151302, Northwestern University Grant number: UG3 HL154297, Tulane Grant Number: UG3 HL151309, NYU Grant Number: UG3 HL151310, Johns Hopkins/University of Michigan Grant number: UG3 HL154280, and University of North Carolina Grant Number: U24 HL151308. The views expressed are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs.
APPENDIX A.
A.1.
TABLE A1.
DECIPHeR Alliance implementation research center project stakeholders
| University of California Los Angeles | University of Colorado | Tulane University | Northwestern University | University of Illinois at Chicago | New York University Grossman School of Medicine | Johns Hopkins University School of Medicine and University of Michigan | |
|---|---|---|---|---|---|---|---|
| Study | Multi‐ethnic multi‐level strategies and behavioral economics to eliminate hypertension disparities in LA County | Reducing asthma attacks in disadvantaged school children with asthma | Community health worker‐led church‐based intervention for eliminating cardiovascular health disparities in African Americans | Community intervention to reduce cardiovascular disease in Chicago (CIRCL‐Chicago) | Mi QUIT CARE (Mile Square QUIT Community‐Access‐Referral‐Expansion), | Actions to decrease disparities in risk and engage in shared support for blood pressure control (ADRESS‐BP) In Blacks | Achieving cardiovascular health equity in community mental health: optimizing implementation strategies |
| Stakeholders | |||||||
| Faith‐based organizations | X | X | X | ||||
| Schools | X | ||||||
| Community‐based service providing organizations | X | X | X | X | X | X | X |
| Health systems | X | X | X | X | X | X | X |
| Payer organizations | X | X | X | ||||
| Government | X | X | X | X | X | X | X |
| Community health workers | X | X | X | X | X | ||
| Advocacy organizations | X | X | X | ||||
| Large non‐profit organizations | X | X | X | ||||
| Institutional partners | X | X | X | X | |||
| Persons with mental illness and family members | X |
Kho A, Daumit GL, Truesdale KP, et al. The National Heart Lung and Blood Institute Disparities Elimination through Coordinated Interventions to Prevent and Control Heart and Lung Disease Alliance. Health Serv Res. 2022;57(Suppl. 1):20‐31. doi: 10.1111/1475-6773.13983
Abel Kho and Gail Daumit are joint first authors of this paper.
Funding information National Heart, Lung, and Blood Institute, Grant/Award Numbers: UG3 HL154302, UG3HL154280‐01; University of North Carolina, Grant/Award Number: U24 HL151308; Johns Hopkins/University of Michigan, Grant/Award Number: UG3 HL154280; NYU, Grant/Award Number: UG3 HL151310; Tulane, Grant/Award Number: UG3 HL151309; Northwestern University, Grant/Award Number: UG3 HL154297; University of Illinois at Chicago, Grant/Award Number: UG3 HL151302; University of Colorado, Grant/Award Number: UG3 HL151297
Contributor Information
Abel Kho, Email: akho@nm.org.
Gail L. Daumit, Email: gdaumit@jhmi.edu.
REFERENCES
- 1. Heckler MM. Report of the Secretary's Task Force on Black and Minority Health; 1985. [PubMed]
- 2. Cooper R, Cutler J, Desvigne‐Nickens P, et al. Trends and disparities in coronary heart disease, stroke, and other cardiovascular diseases in the United States: findings of the national conference on cardiovascular disease prevention. Circulation. 2000;102(25):3137‐3147. [DOI] [PubMed] [Google Scholar]
- 3. Graham G. Disparities in cardiovascular disease risk in the United States. Curr Cardiol Rev. 2015;11(3):238‐245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mensah GA, Mokdad AH, Ford ES, Greenlund KJ, Croft JB. State of disparities in cardiovascular health in the United States. Circulation. 2005;111(10):1233‐1241. [DOI] [PubMed] [Google Scholar]
- 5. Jackson Heart Study . https://www.jacksonheartstudy.org/
- 6. Hispanic Community Health Study / Study of Latinos. https://sites.cscc.unc.edu/hchs/
- 7.Coronary Artery Risk Development in Young Adults (CARDIA). https://www.cardia.dopm.uab.edu/
- 8. Balfour PC, Rodriguez CJ, Ferdinand KC. The role of hypertension in race‐ethnic disparities in cardiovascular disease. Curr Cardiovasc Risk Rep. 2015;9(4):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Diez Roux AV, Mair C. Neighborhoods and health. Ann N Y Acad Sci. 2010;1186:125‐145. [DOI] [PubMed] [Google Scholar]
- 10. Forde AT, Sims M, Muntner P, et al. Discrimination and hypertension risk among African Americans in the Jackson Heart Study. Hypertension. 2020;76(3):715‐723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kaiser P, Diez Roux AV, Mujahid M, et al. Neighborhood environments and incident hypertension in the multi‐ethnic study of atherosclerosis. Am J Epidemiol. 2016;183(11):988‐997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mujahid MS, Diez Roux AV, Morenoff JD, et al. Neighborhood characteristics and hypertension. Epidemiology. 2008;19:590‐598. [DOI] [PubMed] [Google Scholar]
- 13. Roux AVD, Merkin SS, Arnett D, et al. Neighborhood of residence and incidence of coronary heart disease. N Engl J Med. 2001;345(2):99‐106. [DOI] [PubMed] [Google Scholar]
- 14. Mensah GA, Boyce CA, Price LSN, Mishoe HO, Engelgau MM. Perspective: late‐stage (T4) translation research and implementation science: the National Heart, Lung, and Blood Institute strategic vision. Ethn Dis. 2017;27(4):367‐370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sallis JF, Owen N, Fisher E. Ecological models of health behavior. Health Behavior: Theory, Research, and Practice. Vol 5. Jossey‐Bass/Wiley; 2015:43‐64. [Google Scholar]
- 16. Stokols D. Establishing and maintaining healthy environments: toward a social ecology of health promotion. Am Psychol. 1992;47(1):6‐22. [DOI] [PubMed] [Google Scholar]
- 17. Kilbourne AM, Switzer G, Hyman K, Crowley‐Matoka M, Fine MJ. Advancing health disparities research within the health care system: a conceptual framework. Am J Public Health. 2006;96(12):2113‐2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nooraie RY, Kwan BM, Cohn E, et al. Advancing health equity through CTSA programs: opportunities for interaction between health equity, dissemination and implementation, and translational science. J Clin Transl Sci. 2020;4(3):168‐175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shelton RC, Chambers DA, Glasgow RE. An extension of RE‐AIM to enhance sustainability: addressing dynamic context and promoting health equity over time. Front Public Health. 2020;8:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yoong SL, Clinton‐McHarg T, Wolfenden L. Systematic reviews examining implementation of research into practice and impact on population health are needed. J Clin Epidemiol. 2015;68(7):788‐791. [DOI] [PubMed] [Google Scholar]
- 21. Aarons GA, Hurlburt M, Horwitz SM. Advancing a conceptual model of evidence‐based practice implementation in public service sectors. Adm Policy Ment Health Ment Health Serv Res. 2011;38(1):4‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Glasgow RE, Vogt TM, Boles SM. Evaluating the public health impact of health promotion interventions: the RE‐AIM framework. Am J Public Health. 1999;89(9):1322‐1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Virani SS, Alonso A, Aparicio HJ, et al. Heart disease and stroke statistics—2021 update: a report from the American Heart Association. Circulation. 2021;143(8):e254‐e743. [DOI] [PubMed] [Google Scholar]
- 24. Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;74(10):e177‐e232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Glasgow RE, Harden SM, Gaglio B, et al. RE‐AIM planning and evaluation framework: adapting to new science and practice with a 20‐year review. Front Public Health. 2019;7:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Moullin JC, Dickson KS, Stadnick NA, Rabin B, Aarons GA. Systematic review of the exploration, preparation, implementation, sustainment (EPIS) framework. Implementation Sci. 2019;14(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lloyd‐Jones DM, Hong Y, Labarthe D, et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association's strategic impact goal through 2020 and beyond. Circulation. 2010;121(4):586‐613. [DOI] [PubMed] [Google Scholar]
- 28. Akinbami OJ, Moorman JE, Bailey C, et al. Trends in asthma prevalence, health care use, and mortality in the United States, 2001‐2010. NCHS Data Brief. 2012;94:1‐8. [PubMed] [Google Scholar]
- 29. Akinbami LJ, Moorman JE, Garbe PL, Sondik EJ. Status of childhood asthma in the United States, 1980–2007. Pediatrics. 2009;123(Suppl 3):S131‐S145. [DOI] [PubMed] [Google Scholar]
- 30. Noyes K, Bajorska A, Fisher S, Sauer J, Fagnano M, Halterman JS. Cost‐effectiveness of the school‐based asthma therapy (SBAT) program. Pediatrics. 2013;131(3):e709‐e717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lieu TA, Lozano P, Finkelstein JA, et al. Racial/ethnic variation in asthma status and management practices among children in managed Medicaid. Pediatrics. 2002;109(5):857‐865. [DOI] [PubMed] [Google Scholar]
- 32. Kneale D, Harris K, McDonald VM, Thomas J, Grigg J. Effectiveness of school‐based self‐management interventions for asthma among children and adolescents: findings from a Cochrane systematic review and meta‐analysis. Thorax. 2019;74(5):432‐438. [DOI] [PubMed] [Google Scholar]
- 33. Harris K, Kneale D, Lasserson TJ, et al. School‐based self‐management interventions for asthma in children and adolescents: a mixed methods systematic review. Cochrane Database Syst Rev. 2019;1:CD011651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Federico MJ, McFarlane AE 2nd, Szefler SJ, Abrams EM. The impact of social determinants of health on children with asthma. J Allergy Clin Immunol Pract. 2020;8(6):1808‐1814. [DOI] [PubMed] [Google Scholar]
- 35. Cicutto L, Gleason M, Haas‐Howard C, et al. Building bridges for asthma care program: a school‐centered program connecting schools, families, and community health‐care providers. J Sch Nurs. 2020;36(3):168‐180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gottlieb LM, Hessler D, Long D, et al. Effects of social needs screening and in‐person service navigation on child health: a randomized clinical trial. JAMA Pediatr. 2016;170(11):e162521. [DOI] [PubMed] [Google Scholar]
- 37. Szefler SJ, Cloutier MM, Villarreal M, et al. Building bridges for asthma care: reducing school absence for inner‐city children with health disparities. J Allergy Clin Immunol. 2019;143(2):746‐754.e2. [DOI] [PubMed] [Google Scholar]
- 38. Hussey MA, Hughes JP. Design and analysis of stepped wedge cluster randomized trials. Contemp Clin Trials. 2007;28(2):182‐191. [DOI] [PubMed] [Google Scholar]
- 39. Chicago Health Atlas . Average hypertension rate. https://chicagohealthatlas.org/indicators/HCSHYTP?topic=hypertension‐rate
- 40. Johnson R, Ingram D, Gordon BS, Davis P, Greer‐Smith R. Community‐initiated research engagement: equitable partnership delivering research‐ready faith‐based ambassadors. Prog Community Health Partnersh. 2020;14(2):197‐206. [DOI] [PubMed] [Google Scholar]
- 41. Smith JD, Davis P, Kho AN. Community‐driven health solutions on Chicago's South Side. Stanf Soc Innov Rev. 2021;19(3):A27–A29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jaffe MG, Lee GA, Young JD, Sidney S, Go AS. Improved blood pressure control associated with a large‐scale hypertension program. JAMA. 2013;310(7):699‐705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Aarons GA, Green AE, Palinkas LA, et al. Dynamic adaptation process to implement an evidence‐based child maltreatment intervention. Implementation Sci. 2012;7(1):1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wang A, Pollack T, Kadziel LA, et al. Impact of practice facilitation in primary care on chronic disease care processes and outcomes: a systematic review. J Gen Intern Med. 2018;33(11):1968‐1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Liss DT, Peprah YA, Brown T, et al. Using electronic health records to measure quality improvement efforts: findings from a large practice facilitation initiative. Jt Comm J Qual Patient Saf. 2020;46(1):11‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Persell SD, Liss DT, Walunas TL, et al. Effects of 2 forms of practice facilitation on cardiovascular prevention in primary care: a practice‐randomized, comparative effectiveness trial. Med Care. 2020;58(4):344‐351. [DOI] [PubMed] [Google Scholar]
- 47. Westfall JM, Roper R, Gaglioti A, Nease, Jr DE. Practice‐based research networks: strategic opportunities to advance implementation research for health equity. Ethn Dis. 2019;29(Suppl 1):113‐118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health . The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. US Department of Health and Human Services, Centers for Disease Control and Prevention; 2014. [PubMed] [Google Scholar]
- 49. Wang TW, Asman K, Gentzke AS, et al. Tobacco product use among adults—United States, 2017. Morb Mortal Wkly Rep. 2018;67(44):1225‐1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Substance Abuse and Mental Health Services Administration . Results from the 2013 National Survey on Drug Use and Health: Mental Health Findings; 2013. 2, p. 013. [PubMed]
- 51. Singh GK, Williams SD, Siahpush M, Mulhollen A. Socioeconomic, rural‐urban, and racial inequalities in US cancer mortality: part I—all cancers and lung cancer and part II—colorectal, prostate, breast, and cervical cancers. J Cancer Epidemiol. 2011;2011:107497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Flocke SA, Hoffman R, Eberth JM, et al. The prevalence of tobacco use at federally qualified health centers in the United States, 2013. Prev Chronic Dis. 2017;14:E29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Fiore MC, Jaén CR, Baker TB, et al. Treating Tobacco Use and Dependence: 2008 Update. US Department of Health and Human Services; 2008. [Google Scholar]
- 54. Bernstein SL, Boudreaux ED, Cydulka RK, et al. Tobacco control interventions in the emergency department: a joint statement of emergency medicine organizations. J Emerg Nurs. 2006;32(5):370‐381. [DOI] [PubMed] [Google Scholar]
- 55. Roski J, Jeddeloh R, An L, et al. The impact of financial incentives and a patient registry on preventive care quality: increasing provider adherence to evidence‐based smoking cessation practice guidelines. Prev Med. 2003;36(3):291‐299. [DOI] [PubMed] [Google Scholar]
- 56. Han H‐R, Gleason KT, Sun CA, et al. Using patient portals to improve patient outcomes: systematic review. JMIR Hum Factors. 2019;6(4):e15038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Control CFD and Prevention . Hypertension cascade: hypertension prevalence, treatment and control estimates among US adults aged 18 years and older applying the criteria from the American College of Cardiology and American Heart Association's 2017 Hypertension Guideline—NHANES 2013–2016. US Department of Health and Human Services; 2019.
- 58. Murphy SL, Xu J, Kochanek KD, Arias E, Tejada‐Vera B. Deaths: final data for 2018. Natl Vital Stat Rep. 2021;69:1‐83. [PubMed] [Google Scholar]
- 59. Saeed A, Dixon D, Yang E. Racial Disparities in Hypertension Prevalence and Management: A crisis control. American College of Cardiology; 2020. [Google Scholar]
- 60. Ogedegbe G, Schoenthaler A. A systematic review of the effects of home blood pressure monitoring on medication adherence. J Clin Hypertens. 2006;8(3):174‐180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Islam N, Rogers ES, Schoenthaler A, Thorpe LE, Shelley D. A Cross‐Cutting Workforce Solution for Implementing Community–Clinical Linkage Models. American Public Health Association; 2020:S191‐S193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Colton CW, Manderscheid RW. PEER REVIEWED: congruencies in increased mortality rates, years of potential life lost, and causes of death among public mental health clients in eight states. Prev Chronic Dis. 2006;3(2):A42. [PMC free article] [PubMed] [Google Scholar]
- 63. McEvoy JP, Meyer JM, Goff DC, et al. Prevalence of the metabolic syndrome in patients with schizophrenia: baseline results from the clinical antipsychotic trials of intervention effectiveness (CATIE) schizophrenia trial and comparison with national estimates from NHANES III. Schizophr Res. 2005;80(1):19‐32. [DOI] [PubMed] [Google Scholar]
- 64. Newcomer JW, Hennekens CH. Severe mental illness and risk of cardiovascular disease. JAMA. 2007;298(15):1794‐1796. [DOI] [PubMed] [Google Scholar]
- 65. Saha S, Chant D, McGrath J. A systematic review of mortality in schizophrenia: is the differential mortality gap worsening over time? Arch Gen Psychiatry. 2007;64(10):1123‐1131. [DOI] [PubMed] [Google Scholar]
- 66. Mueser KT, McGurk SR. Schizophrenia. Lancet. 2004;363(9426):2063‐2072. [DOI] [PubMed] [Google Scholar]
- 67. Murphy KA, Daumit GL, Stone E, McGinty EE. Physical health outcomes and implementation of behavioural health homes: a comprehensive review. Int Rev Psychiatry. 2018;30(6):224‐241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Daumit GL, Dalcin AT, Dickerson FB, et al. Effect of a comprehensive cardiovascular risk reduction intervention in persons with serious mental illness: a randomized clinical trial. JAMA Netw Open. 2020;3(6):e207247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kilbourne AM, Barbaresso MM, Lai Z, et al. Improving physical health in patients with chronic mental disorders: twelve‐month results from a randomized controlled collaborative care trial. J Clin Psychiatry. 2017;78(1):129‐137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Kilbourne AM, Goodrich DE, Lai Z, et al. Randomized controlled trial to assess reduction of cardiovascular disease risk in patients with bipolar disorder: the Self‐Management Addressing Heart Risk Trial (SMAHRT). J Clin Psychiatry. 2013;74(7):e655‐e662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Dalcin AT, Jerome GJ, Appel LJ, et al. Need for cardiovascular risk reduction in persons with serious mental illness: design of a comprehensive intervention. Front Psychiatry. 2019;9:786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Kilbourne AM, Neumann MS, Pincus HA, Bauer MS, Stall R. Implementing evidence‐based interventions in health care: application of the replicating effective programs framework. Implementation Sci. 2007;2(1):1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Mensah GA, Cooper RS, Siega‐Riz AM, et al. Reducing cardiovascular disparities through community‐engaged implementation research: a National Heart, Lung, and Blood Institute workshop report. Circ Res. 2018;122(2):213‐230. [DOI] [PMC free article] [PubMed] [Google Scholar]


